JGP modeling study suggests that selectivity filter constriction is a plausible mechanism for C-type inactivation of the Shaker voltage-gated potassium channel.
Abstract
JGP modeling study suggests that selectivity filter constriction is a plausible mechanism for C-type inactivation of the Shaker voltage-gated potassium channel.
In response to prolonged activation, many K+ channels spontaneously reduce the membrane conductance by undergoing C-type inactivation, a kinetic process crucial for the pacing of cardiac action potentials and the modulation of neuronal firing patterns. In the pH-activated bacterial channel KcsA, C-type inactivation appears to involve constriction of the channel’s selectivity filer that prohibits ion conduction, but whether voltage-gated channels like Drosophila Shaker use a similar mechanism is controversial (1). In this issue of JGP, a computational study by Li et al. suggests that filter constriction is indeed a plausible mechanism for the C-type inactivation of Shaker (2).
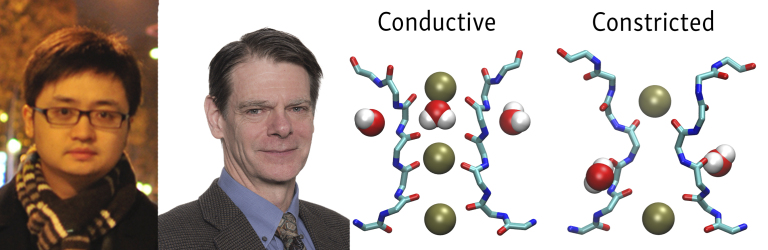
(Left to right) Jing Li, Benoît Roux, and colleagues use computational modeling to show that selectivity filter constriction, allosterically promoted by opening of the intracellular activation gate, is a plausible mechanism for the C-type inactivation of voltage-gated K+ channels such as Drosophila Shaker. The selectivity filter is conductive (left) when the intracellular gate is partially open, but adopts a constricted conformation (right) when the gate is open wide.
Various structural approaches have shown that C-type inactivation of KcsA channels is associated with the symmetrical constriction of all four channel subunits at the level of the central glycine residue in the selectivity filter. Benoît Roux and colleagues at The University of Chicago used MD simulations to show that the KcsA pore can transition from the conductive to the constricted conformation on an appropriate timescale, and that this transition is allosterically promoted by the wide opening of the pore’s intracellular gate (3). Modeling by Roux and colleagues suggests that C-type inactivation of cardiac hERG channels could also involve selectivity filter constriction, though in this case it appears to be an asymmetric process in which only two of the channel’s subunits move closer together (4).
“In view of the high similarity between the pore domains of Shaker and KcsA (almost 40% sequence identity), we wanted to examine if it’s possible for the Shaker selectivity filter to constrict and, if so, how similar it is to KcsA,” Roux explains. Led by first author Jing Li—now an assistant professor at the University of Mississippi—Roux and colleagues developed several homology models of the Shaker pore domain with the intracellular gate open to various degrees (2).
MD simulations and free energy calculations revealed that the Shaker selectivity filter can dynamically transition from a conductive to a constricted conformation, and that this transition is allosterically coupled to the intracellular gate; the constricted conformation is stable when the gate is wide open. “Our computations strongly suggest that constriction is a plausible mechanism for the C-type inactivation of Shaker,” Roux says. “There’s no reason based on the currently available information to reject the existence of a constricted state in Shaker channels.”
As with KcsA, Shaker channels appear to constrict symmetrically at the level of the selectivity filter’s central glycine. But Li et al.’s simulations revealed some small variations between the two channels, including differences in the number of water molecules bound to each channel subunit and the arrangement of the hydrogen-bond network they form to stabilize the constricted state.
Li et al. also modeled the pore domain of the Shaker W434F mutant, which is widely assumed to be trapped in a C-type inactivated state. The simulation suggests that the mutant channel’s filter adopts a stable constricted conformation even when the intracellular gate is only partially open, although the constriction is asymmetric and occurs at the level of a different filter residue (2).
Constriction may therefore be a universal mechanism of C-type inactivation, even if the exact conformation varies from channel to channel. But, says Roux, confirming this will require more experimental work using the right conditions and mutations to capture the structure of inactivated channels.
References
- 1.Hoshi, T., and Armstrong C.M.. 2013. J. Gen. Physiol. 10.1085/jgp.201210888 [DOI] [Google Scholar]
- 2.Li, J., et al. 2021. J. Gen. Physiol. 10.1085/jgp.202112875 [DOI] [Google Scholar]
- 3.Li, J., et al. 2018. J. Gen. Physiol. 10.1085/jgp.201812082 [DOI] [Google Scholar]
- 4.Li, J., et al. 2021. Sci. Adv. 10.1126/sciadv.abd6203 [DOI] [Google Scholar]