Abstract
Human metapneumovirus (HMPV) is a leading cause of lower respiratory tract infection (LRTI) in pediatric and geriatric populations. We recently found that two PDZ-binding motifs of the M2–2 protein, 29-DEMI-32 and 39-KEALSDGI-46, play a significant role in mediating HMPV immune evasion in airway epithelial cells (AECs). However, their role in the overall pulmonary responses to HMPV infection has not been investigated. In this study, we found that two recombinant HMPVs (rHMPV) lacking the individual M2–2 PDZ-binding motif are attenuated in mouse lungs. Mice infected with mutants produce more cytokines/chemokines in bronchoalveolar lavage (BAL) fluid compared to mice infected with wild-type rHMPV. In addition, both mutants are able to enhance the pulmonary recruitment of dendritic cells (DCs) and T cells and induce effective protections against the HMPV challenge. The DC maturation is also significantly improved by the motif mutation. Taken together, our data provide proof-of-principle for two live-attenuated M2–2 mutants to be promising HMPV vaccine candidates that are effective in inducing higher pulmonary innate immunity and generating protection against HMPV infection.
Keywords: HMPV, PDZ-binding motif, innate immunity, pulmonary innate response
1. Introduction
Human metapneumovirus (HMPV) is a respiratory pathogen belonging to the Metapneumovirus genus in the Pneumoviridae family1. It was first isolated in 2001 and was quickly recognized to be a major cause of lower respiratory tract infection (LRTI) in children, geriatric, and immunocompromised patients worldwide2–4. Reinfection with HMPV occurs repeatedly throughout life without significant antigenic changes, suggesting the induction of insufficient or short-lived immunity5. There are currently no effective vaccines or specific therapeutic agents available for individuals infected with HMPV.
To date, various HMPV vaccine candidates have been developed and tested in cell lines, animals, and humans. Among them, heat- or formalin-inactivated HMPV vaccines result in enhanced disease and higher mortality in immunized animals rather than unimmunized animals6,7, suggesting that these vaccines are not safe. Some vaccine candidates, such as subunit vaccines that consist of viral protein(s) in vectors or virus-like particles, do not result in enhanced disease in cotton rats and non-human primates after the induction of protective immunity. However, the immunity does not last long and multiple doses are needed to maintain antiviral immunity8–10, suggesting the ineffectiveness of these candidates. On the other hand, some live-attenuated recombinant HMPV (rHMPV) lacking specific viral protein(s) have been shown as promising vaccine candidates, because they induce strong immunogenicity in animal models11,12. In addition, an rHMPV, which has an introduced mutation in SH to keep the overall HMPV genome stable, and a live-attenuated rHMPV with its P protein replaced with avian MPV P protein, have been shown as safe in immunized human volunteers13,14, which suggests that live-attenuated rHMPV could be a promising vaccine candidate. However, the molecular mechanisms underlying the attenuation of the live-attenuated rHMPV are largely unknown.
HMPV, with the genome length of 13Kb, encodes nine proteins: N, P, M, F, M2–1, M2–2, SH, G, and L. Some proteins, such as G, P, SH, and M2–2 have been shown to modulate host innate immunity to favor HMPV infection via different mechanisms15–19. We have recently shown that the M2–2 protein of HMPV facilitates immune evasion by targeting the mitochondrial antiviral-signaling protein (MAVS) and myeloid differentiation primary response 88 (MyD88), two central antiviral signaling molecules in airway epithelial cells (AECs) and dendritic cells (DCs), respectively18,20. Furthermore, we identified that two putative PDZ-binding motifs of M2–2, 29-DEMI-32 and 39-KEALSDGI-46, contribute significantly to M2–2-mediated immune evasion in cells21. However, their impacts on pulmonary responses to HMPV infection have not been explored.
To evaluate in vivo functions of these two motifs, we compared the virus replication, immune mediator induction, and immune cell recruitment/activation in the lungs between wild type (WT)-rHMPV-infected mice and mice infected with mutants lacking the individual motif. Overall, the data were consistent with our previous findings in the suppressive role of two M2–2 PDZ-binding motifs in cellular innate immunity21,22. Attenuated replication and enhanced host innate immune responses to mutant infections also suggested that rHMPV lacking either motif could serve as live-attenuated vaccine candidates.
2. Materials and Methods
2.1. rHMPV preparation and titration
WT-rHMPV or recombinant mutants with mutations in the motif 29-DEMI-32 (Mut-1) or the motif 39-KEALSDGI-46 (Mut-2), were generated and propagated in LLC-MK2 cells (ATCC, Manassas, VA, USA), as previously described21,23,24. Viral titers of purified recombinant viruses or viruses from the infected mouse lungs were determined by immunostaining in LLC-MK2 cells, as described previously21,25.
2.2. Mice
6–8 week-old female BALB/c mice were purchased from The Jackson Laboratory and maintained under specific-pathogen-free conditions at the University of Texas Medical Branch (UTMB) at Galveston. To investigate the innate response of mice to the primary infection, mice were anesthetized and administered intranasally with 5×106 pfu of rHMPV, diluted in Dulbecco’s PBS (D-PBS) (Invitrogen, Carlsbad, CA, USA), or with an equivalent volume of sucrose diluted in D-PBS (mock-infection), as previously described26. To study whether mice with primary inoculation of rHMPV develop protection, mice were challenged with 107 pfu of HMPV/CAN/97/83/A2 on day 28 post-primary infection. Lungs at day 5 post-primary infection or post-challenge were harvested to determine the HMPV titers in the lungs. All of the studies were approved by the UTMB Institutional Animal Care and Use Committee.
2.3. Bronchoalveolar lavage (BAL) analysis
BAL fluid was collected by washing the lungs two times with 1 ml D-PBS at different days post-infection (p.i.). After centrifuging at 1,500 rpm for 5 min at 4 °C, the supernatants were used for quantification of cytokines/chemokines using Luminex-based Bio-Plex Pro™ Mouse Cytokine 23-plex Assay (Bio-Rad Laboratory, Hercules, CA, USA, Cat #M60009RDPD) according to the manufacturer’s instructions. The examined cytokines include Eotaxin, G-CSFm GM-CSF, IFN-γ, IL-1α, IL-1β, IL-2, IL-3, Il-4, Il-5, IL-6, IL-9, IL-10, IL-12p40, IL-12p70, IL-13, IL-17A, KC, MCP-1, MIP-1α, MIP-1β, RANTES, and TNF-α. The detection limit for all cytokines by the Bioplex assay is 3 pg/ml. The IFN-β was quantified by ELISA (PBL Biomedical Laboratories, Piscataway, NJ) with the detection limit of 50 pg/ml.
2.4. Airway hyperresponsiveness (AHR)
AHR was assessed in unrestrained conscious mice by non-invasive non-ventilated enhanced pause (Penh) measurement, as described previously26,27. Freely moving mice were placed in whole-body barometric plethysmography (Buxco Electronics, Troy, NY, USA) and respiratory activity was recorded for 4 min to measure baseline Penh values.
2.5. Fluorescence-activated cell sorting (FACS) analysis of immune cells
Lung samples of mice, mock-inoculated or inoculated with WT-rHMPV or mutants, were harvested at days 5 and 7 p.i. Total lung cells were prepared, as previously described25,26. Isolated cells were incubated with anti-FcγRII/RcγRIIImAb (24G2, BD Biosciences, San Jose, CA, USA) followed by cell-surface marker staining. For cell-surface marker staining, isolated cells were stained with the following antibodies: anti-CD11c in combination with anti-CD80, anti-CD86, or anti-MHCII (all from BD Pharmingen, San Jose, CA, USA) for DCs and anti-CD4 or anti-CD8 in combination with anti-CD3 (all from BD Pharmingen) for T cells. Samples were stained at 4 °C in PBS with 1% FBS and analyzed with a BD FACSCanto flow cytometer equipped with BD FACSDiva software (both from Becton Dickinson Immunocytometry Systems, Franklin Lakes, NJ, USA). The analysis was performed using Cyflogic (Cyflo Ltd, Turku, Finland).
2.6. Statistical analysis
Statistical significance was determined by the Mann-Whitney U test. Values of P<0.05 were considered significant. Values for viral replication, cell numbers, and cytokine/chemokine production experiments were presented as mean ± standard error of the mean (SEM).
3. Results
3.1. M2–2 PDZ-binding motifs are important to pulmonary HMPV replication and the modulation of pulmonary cytokine/chemokine induction
To determine whether the motifs 29-DEMI-32 and 39-KEALSDGI-46 are important in mediating pulmonary innate responses to HMPV, we first compared pulmonary viral loads in WT-, Mut-1-, or Mut-2-infected mice, at day 5 p.i., the replication peak of primary HMPV infection 26,27. In brief, BALB/c mice were intranasally inoculated with WT, Mut-1, or Mut-2 at 5×106 pfu/mice, followed by lung harvesting and viral titration. The mock-inoculation was used as a negative control. As shown in Fig. 1A, mice infected with Mut-1 and Mut-2 exhibited more than a log reduction in lung viral titer than those infected with WT, indicating the replication attenuation of mutants. To confirm the mutations after infection, we isolated total RNAs from infected lungs at day 5 p.i. The DNA sequencing of reverse transcription PCR products demonstrated that mutations of each motif were kept intact (data not shown), supporting the importance of motifs in the regulation of HMPV replication. The sequencing was done in the Molecular Genomics Core at UTMB.
Figure 1.
The effect of M2–2 PDZ-binding motifs on pulmonary viral replication and cytokine/chemokine induction. BALB/c mice were intranasally inoculated with 5 × 106pfu of WT, Mut-1, or Mut-2. (A) Total lungs were harvested at day 5 p.i. and infectious viral particles were titrated by the immunostaining in LLC-MK2 cells. n=4 mice/group from three independent experiments. On day 1 (B, C) and day 5 p.i. (D), BAL samples were collected from mock-, WT-, Mut-1-, or Mut-2-inoculated mice. The cytokines/chemokines were measured by the Luminex-based Bio-Plex system and IFN-β was measured by ELISA. n=3 mice/group from three independent experiments. Data are presented as means ± SEM. *P<0.05 and **P<0.01 for comparison to WT-infected samples.
We then investigated the pulmonary cytokine/chemokine induction by infection, WT or mutants. In brief, we inoculated mice with WT or mutants, using mock-inoculation as a control, and measured the abundance of cytokines/chemokines in pulmonary BAL fluid at day 1 and 5 p.i by Bio-Plex Pro™ Mouse Cytokine 23-plex Assay (Bio-Rad, Hercules, CA, USA). Generally speaking, the induction of most cytokines/chemokines by HMPV infection peaks at day 1 p.i., and goes back to the basal level at day 5 p.i.28. Herein, Figs. 1B and Fig. 1C show the induction at day 1 p.i. Mediators with the induction >5000 pg/ml are listed in Fig. 1B, while the mediators with the induction <2500 pg/ml are shown in Fig. 1C. Compared with the cytokine/chemokine induction by WT-rHMPV, the induction of these molecules was significantly enhanced by Mut-1 or Mut-2 infection. Pulmonary IFN-β induction was comparable between WT- and Mut-2 infected lungs. At day 5 p.i., the abundance of pulmonary cytokines/chemokines became significantly less in all groups, compared with that at day 1 p.i. The ones detected are shown in Fig. 1D. Our Bio-plex assay did not detect any induction of Th2 cytokines including IL-4, IL5, and IL-13 in all experimental groups. Overall, these results demonstrated that both motifs 29-DEMI-32 and 39-KEALSDGI-46 of M2–2 suppress the induction of pulmonary innate immune mediators after HMPV infection.
3.2. Mutant infection of mice provides protection against viral challenge with a clinical isolate
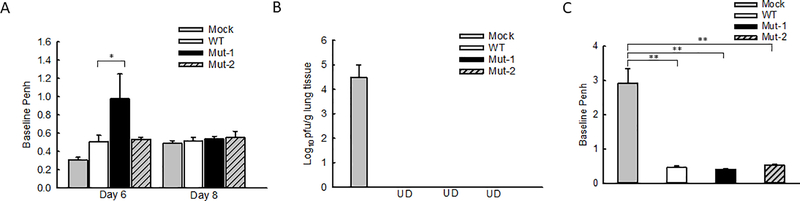
To investigate the impact of motifs on the lung function in response to HMPV infection, we compared the AHR in WT- and mutant-infected mice by Penh measurement, a method using a non-invasive and non-ventilated unrestrained whole-body plethysmography to correlate changes in airway physiology by the infection29–31. Mock-inoculation was used as a negative control. The basal Penh in response to HMPV infection usually peaks between day 5 and day 7 p.i.27. Therefore, we chose day 6 p.i. for the Penh investigation. We found that, at day 6 p.i., there were no significant Penh differences between WT- and Mut-2-infected mice, while Mut-1 induced moderately higher Penh than WT and Mut-2 (Fig. 2A). At day 8 p.i., the Penh in response to WT, Mut-1, and Mut-2 infections became comparable among the groups.
Figure 2.
The impact of motifs on pulmonary function. (A) Mice were mock-inoculated or inoculated with WT-, Mut-1-, or Mut-2 as described in Fig. 1. At days 6 and 8 p.i., lung function was determined by Penh measurement. (B) Mice, mock-inoculated or inoculated with recombinant viruses, were challenged with HMPV/CAN/97/83/A2 at 107pfu at day 28 post-primary infection. The lung samples were harvested at day 5 post-challenge followed by the titration. UD; Undetected. (C) Mice were challenged as (B) and the base Penh was measured at day 5 post-challenge. Data are presented as means ± SEM. n=5 mice/group from two independent experiments. *P<0.05 and **P<0.01 for comparison to WT-infected samples.
Innate inflammatory cytokines and viral loads are important determinants of lung pathogenesis. Both are generally suggested to be associated with the disease severity of HMPV and RSV32–40. Although the attenuation of mutant replication (Fig. 1A) and enhanced induction of protective immune mediators, such as IL12-p4041, could prevent severe lung injury from possible adverse effects of mutant-enhanced inflammatory cytokines, there are three recent publications from different groups, who supported that higher innate cytokines triggered by an essential amount of infectious particles are necessary to prevent severe RSV diseases42–44. Viruses with a low inoculation indeed can cause more severe disease because of their failure to induce sufficient innate immune responses, demonstrating complicated balance mechanisms underlying disease severity42–44. Although no such reports are available for HMPV infection, and we do not know whether mutant-enhanced cytokine/chemokine induction is beneficial or deleterious to lung functions, our Penh results did not show uncontrollable pathogenesis by mutant infections, compared with WT infection. No animal death was observed after the primary infection of WT or mutants either.
Next, to evaluate the protective capacity of rHMPV mutants, we challenged mice with HMPV/CAN/97/83/A2 at 107 pfu/mice on day 28 post-initial inoculation with WT or mutant rHMPV. The mock-inoculation was used as a negative control. The lungs were harvested at day 5 post-challenge for viral titration. No pulmonary viral particles could be detected in WT- and mutant rHMPV-inoculated mice, while mock-inoculated mice had significant virus replication (Fig. 2B), suggesting that mutants could generate protection. We also did not observe the difference in Penh at day 5 post-challenge among mice which were immunized with WT, Mut-1, and Mut-2 (Fig. 2C). No animal death and detectable Th2 cytokines (IL-4, IL-5, and IL-13) were observed after the challenge. The Th2 cytokine quantification was done by ELISA kits (R&D Systems, Minneapolis, MN).
3.3. The motifs inhibit lung DC maturation in response to HMPV
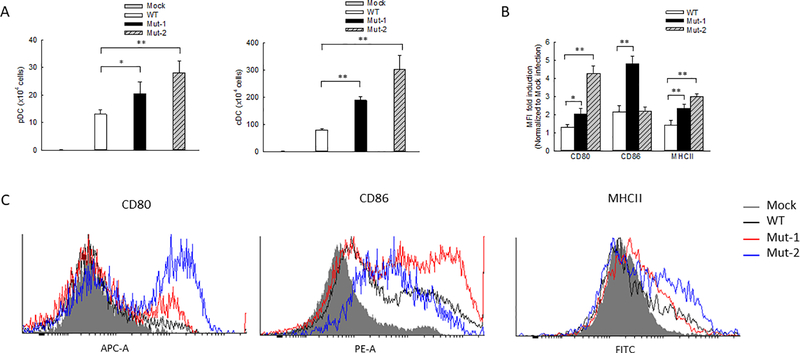
It has been previously shown that HMPV infection leads to the pulmonary recruitment of plasmacytoid DCs (pDCs) and conventional DCs (cDCs) with different kinetics after the primary infection of mice45. Recently, we also demonstrated that monocyte-derived DCs (moDCs), an appropriate model for lung cDCs46,47, are regulated by the M2–2 protein in response to HMPV infection20. Herein, we investigated whether M2–2 uses its putative PDZ-binding motifs to regulate the DCs in rHMPV-infected lungs. FACS analysis revealed that the number of lung pDCs and cDCs was increased after mutant inoculation compared with WT inoculation and this increase was most significant in Mut-2-infected mice at day 7 p.i. (Fig. 3A). In addition to enhanced cDC infiltration, we also found the cDC maturation was affected by the motifs. In brief, lung cells isolated from WT- or mutant-infected mice were stained with DC maturation markers of CD80, CD86, or MHCII and followed by FACS analysis. At day 7 p.i., CD11c+ lung cells from Mut-1- and Mut-2-infected mice had a significant increase in the expression of CD80 and MHCII (Figs. 3B and 3C) and Mut-1 had more impact on CD86 expression than WT-infected lungs. These results supported that M2–2 PDZ-binding motifs have an inhibitory effect on DC maturation and recruitment in HMPV infection.
Figure 3.
The influence of M2–2 motifs on lung DC recruitment and maturation. Mice were mock-inoculated or inoculated with 5 × 106pfu of WT or mutants. (A) At day 7 p.i., pDCs and cDCs were quantified by FACS. (B) The mean fluorescence intensity (MFI) of cell surface markers CD80, CD86, and MHCII was also determined for lung CD11c+ cells. (C) Representative histograms to elucidate the expression of DC maturation markers were also shown. Data are presented as means ± SEM. n=3 mice/group from three independent experiments. *P<0.05 and **P<0.01 for comparison to WT-infected samples.
3.4. The motifs suppress pulmonary T cell infiltration and activation in response to HMPV
DCs play an essential role in bridging innate and adaptive immunity48. DC maturation identified by costimulatory molecules including CD80 and CD86 is important for T cell function49,50. Improved DC maturation by mutant infection, therefore, likely leads to enhanced T cell responses. To test this hypothesis, pulmonary T cells were assessed at day 7 p.i. in mice infected with WT or mutants through FACS analysis. Compared with WT infection, the number of lung CD4 and CD8 cells was increased by Mut-1 infection (Fig. 4A). Mut-2 infection also led to significant enhancement in CD4 cells, but not in CD8 cells. As shown in Fig. 4B, the percentage of both CD4 and CD8 T cells was significantly enhanced by mutants, compared with pulmonary T cells of WT-infected lungs. Collectively, these results suggest that M2–2 PDZ-binding motifs are important in regulating T cell responses to HMPV infection.
Figure 4.
The effect of motifs on pulmonary T cell responses. Lung cells from mock-, WT-, Mut-1-, or Mut-2-inoculated mice were isolated at day 7 p.i. and stained with the indicated antibodies. Total CD4 and CD8 T cells (A) and the percentage of CD4 and CD8 T cells (B) were analyzed by FACS. The percentage of T cells was calculated from total lung cells. Data are presented as means ± SEM. n=3 mice/group from three independent experiments. *P<0.05 and **P<0.01 for comparison to WT-infected samples.
4. Discussion
As mentioned, the PDZ domains 29-DEMI-32 and 39-KEALSDGI-46 of M2–2 play an important role in host innate immune response in vitro18,21,22. However, the overall regulatory role in pulmonary innate responses to HMPV has not been investigated, although such studies are of significant importance to the development of therapeutic and preventive methods against HMPV infection51. Herein, we demonstrated these two motifs are important in regulating HMPV replication and HMPV-induced pulmonary innate immunity. The attenuated motif mutants not only enhanced pulmonary innate immunity but protected immunized animals from naïve HMPV challenge without enhanced diseases. The enhanced innate immunity by motif mutants includes improved induction of many pulmonary immune mediators, better pulmonary recruitment of DCs and T cells, and more mature pulmonary DCs. Unlike heat-inactivated HMPV52, Th2 cytokines including IL-4, IL-5, and IL-13, were not detectable following the primary mutant infection. Formalin-inactivated HMPV induces hypersensitivity in immunized animals following HMPV challenge7. However, we did not observe enhanced Penh following HMPV challenge in mutant immunized mice (Fig. 2C). All of these suggest that modification of M2–2 PDZ-binding motifs could be an approach to overcome poor mucosal innate immunity induction by HMPV. We also found that, following the primary infection, the effects of Mut-1 and Mut-2 on the induction of DC maturation markers, pulmonary function (Penh), and the production of cytokines/chemokines were distinct, suggesting the regulation of HMPV-induced innate immunity is motif-specific. In additional studies, we will investigate whether non-PDZ motifs, if there are any, also play a role in the immune evasion upon HMPV infection.
We also found that motifs differentially regulate DCs (Fig. 3). In addition to the distinct induction of chemoattractants by two mutants, there are several other possibilities contributing to the motif-dependent regulation of DCs. Mutants may produce different pathogen-derived antigens, which may stimulate DCs differently. Motifs may control cellular signaling and cytoskeleton reorganization in DCs differently, leading to a distinct expression of their maturation markers in response to mutant infections50,53. AECs, our proteomics data have revealed that there are many other changes than motif-controlled immunity, such as pathways involved in cell signaling, chromatin remodeling, and oxidative responses24. Overall, our finding demonstrates that M2–2 motif mutations are beneficial to induce a robust pulmonary innate immune response to HMPV infection.
In DCs, toll-like receptors (TLRs) and helicase melanoma differentiation-associated gene 5 (MDA5), two major pattern recognition receptors (PRRs), are essential for HMPV-induced innate immune responses18,20,54–57. MDA5 uses MAVS as a central adaptor for antiviral signaling, while most TLRs, except TLR-3, use MyD88 to carry out antiviral responses56,58. We have recently shown that MAVS and MyD88 are critical to pulmonary immune responses to HMPV25,26. We also discovered that M2–2 hijacks MAVS and MyD88 to evade the immune response, and two M2–2 PDZ-binding motifs are responsible for such immune inhibition in AECs by suppressing the interaction between MAVS and its downstream effectors18,20,21. Whether M2–2 also uses these two motifs to suppress MyD88-mediated immune responses in DCs remains to be investigated.
The study also demonstrated the importance of M2–2 PDZ-binding motifs in T cell responses. In the context of HMPV infection, T cells play essential roles in immune surveillance, protection, and viral clearance27,59. A defective CD8 T cell response delays HMPV clearance60. In addition, memory CD8 T cells are capable of providing protection against a secondary infection61. The induction of CD8 T cells by peptide immunization reduces viral load, but induces lung pathogenesis upon the HMPV challenge59. Although CD8 T cells have been suggested to cause pathogenesis upon HMPV challenge, mutant-enhanced T cell response seemed not to impact disease severity following challenge, based on Penh in WT- and mutant-infected mice (Fig. 2C), suggesting that viral- and peptide-induced CD8 T cells may function differently from live virus-induced CD8 T cell response upon HMPV challenge.
A promising aspect of our mutants is their attenuated pulmonary replication. A recent study has identified the existence of defective interfering RNAs (DIs) in HMPV particles which are generated upon in vitro passage and able to induce type I interferon (IFN)62. Since HMPV replication is sensitive to the IFN, the attenuation of mutants might result from more DI production upon mutant infection. However, this is doubtful, as DIs are accumulated when virus stocks are prepared at multiple passages (of 5) with a high MOI (of 3). HMPV stocks used in this study were prepared at low passage (no more than 4–5 passages but passage 2 was mostly used) with a low MOI (approximately 0.01)21, suggesting that DIs are not likely to accumulate. Furthermore, Mut-2 did not produce more type I IFN than WT (Fig. 1C). This result is somewhat different from what we found for the role of 39-KEALSDGI-46 in IFN-β induction in AECs. The difference between in vitro and in vivo settings may result from the involvement of other pulmonary cells in IFN-β induction and possible cell-type-dependent responses to Mut-2 infection. Our previous report also showed that mutant viruses are attenuated in Vero cells, which area cell line deficient of type I IFN signaling21, which suggests that attenuation of mutant viral replication could be independent of DI production.
Several studies have demonstrated that the HMPV mutant with the deletion of M2–2 is a promising live-attenuated vaccine candidate12,63,64. The M2–2 deletion mutant is highly attenuated and immunogenic in animals, however, the underlying mechanisms are not clear. Compared with the M2–2 deletion mutant, our motif mutants may have additional advantages. For example, our mutants have an intact H-2D-restricted cytotoxic T-lymphocyte (CTL) epitope which contributes to the clearance of HMPV-infected cells by T cells59. In addition, the deletion of the whole M2–2 leads to a higher mutation frequency of HMPV63. We found our motif mutants did not produce additional mutations at passages 2 or 3 (data not shown). In summary, our M2–2 PDZ-binding motif mutants were attenuated, elicited higher innate immune responses, and induced higher DC maturation and the recruitment of T cells and DCs in mouse lungs.
Acknowledgments
Authors thank Cynthia Tribble for assistance with manuscript editing.
Funding: This work was supported by grants from the National Institutes of Health-National Institute of Allergy and Infectious Diseases 1R01AI116812 and R21 AI113771, and the Clinical Innovator Award from the Flight Attendant Medical Research Institute to X.B.
Footnotes
Conflicts of Interest: All authors concur there are no conflicts of interest associated with this published work.
References
- 1.Afonso CL, Amarasinghe GK, Banyai K, et al. Taxonomy of the order Mononegavirales: update 2016. Archives of virology. 2016;161(8):2351–2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.van den Hoogen BG, de Jong JC, Groen J, et al. A newly discovered human pneumovirus isolated from young children with respiratory tract disease. Nature medicine. 2001;7(6):719–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Falsey AR, Erdman D, Anderson LJ, Walsh EE. Human metapneumovirus infections in young and elderly adults. The Journal of infectious diseases. 2003;187(5):785–790. [DOI] [PubMed] [Google Scholar]
- 4.Cane PA, van den Hoogen BG, Chakrabarti S, Fegan CD, Osterhaus AD. Human metapneumovirus in a haematopoietic stem cell transplant recipient with fatal lower respiratory tract disease. Bone marrow transplantation. 2003;31(4):309–310. [DOI] [PubMed] [Google Scholar]
- 5.Shafagati N, Williams J. Human metapneumovirus - what we know now. F1000Res. 2018;7:135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hamelin ME, Couture C, Sackett MK, Boivin G. Enhanced lung disease and Th2 response following human metapneumovirus infection in mice immunized with the inactivated virus. J Gen Virol. 2007;88(Pt 12):3391–3400. [DOI] [PubMed] [Google Scholar]
- 7.de Swart RL, van den Hoogen BG, Kuiken T, et al. Immunization of macaques with formalin-inactivated human metapneumovirus induces hypersensitivity to hMPV infection. Vaccine. 2007;25(51):8518–8528. [DOI] [PubMed] [Google Scholar]
- 8.Mok H, Tollefson SJ, Podsiad AB, et al. An alphavirus replicon-based human metapneumovirus vaccine is immunogenic and protective in mice and cotton rats. Journal of virology. 2008;82(22):11410–11418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tang RS, Mahmood K, Macphail M, et al. A host-range restricted parainfluenza virus type 3 (PIV3) expressing the human metapneumovirus (hMPV) fusion protein elicits protective immunity in African green monkeys. Vaccine. 2005;23(14):1657–1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Soto JA, Galvez NMS, Rivera CA, et al. Recombinant BCG Vaccines Reduce Pneumovirus-Caused Airway Pathology by Inducing Protective Humoral Immunity. Frontiers in immunology. 2018;9:2875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Skiadopoulos MH, Biacchesi S, Buchholz UJ, et al. Individual contributions of the human metapneumovirus F, G, and SH surface glycoproteins to the induction of neutralizing antibodies and protective immunity. Virology. 2006;345(2):492–501. [DOI] [PubMed] [Google Scholar]
- 12.Biacchesi S, Pham QN, Skiadopoulos MH, Murphy BR, Collins PL, Buchholz UJ. Infection of nonhuman primates with recombinant human metapneumovirus lacking the SH, G, or M2–2 protein categorizes each as a nonessential accessory protein and identifies vaccine candidates. Journal of virology. 2005;79(19):12608–12613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Talaat KR, Karron RA, Thumar B, et al. Experimental infection of adults with recombinant wild-type human metapneumovirus. The Journal of infectious diseases. 2013;208(10):1669–1678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Karron RA, San Mateo J, Wanionek K, Collins PL, Buchholz UJ. Evaluation of a Live Attenuated Human Metapneumovirus Vaccine in Adults and Children. Journal of the Pediatric Infectious Diseases Society. 2018;7(1):86–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Soto JA, Galvez NMS, Benavente FM, et al. Human Metapneumovirus: Mechanisms and Molecular Targets Used by the Virus to Avoid the Immune System. Frontiers in immunology. 2018;9:2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goutagny N, Jiang Z, Tian J, et al. Cell type-specific recognition of human metapneumoviruses (HMPVs) by retinoic acid-inducible gene I (RIG-I) and TLR7 and viral interference of RIG-I ligand recognition by HMPV-B1 phosphoprotein. J Immunol. 2010;184(3):1168–1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hastings AK, Amato KR, Wen SC, Peterson LS, Williams JV. Human metapneumovirus small hydrophobic (SH) protein downregulates type I IFN pathway signaling by affecting STAT1 expression and phosphorylation. Virology. 2016;494:248–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ren J, Wang Q, Kolli D, et al. Human metapneumovirus M2–2 protein inhibits innate cellular signaling by targeting MAVS. Journal of virology. 2012;86(23):13049–13061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bao X, Liu T, Shan Y, Li K, Garofalo RP, Casola A. Human metapneumovirus glycoprotein G inhibits innate immune responses. PLoS pathogens. 2008;4(5):e1000077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ren J, Liu G, Go J, Kolli D, Zhang G, Bao X. Human metapneumovirus M2–2 protein inhibits innate immune response in monocyte-derived dendritic cells. PloS one. 2014;9(3):e91865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen Y, Deng X, Deng J, et al. Functional motifs responsible for human metapneumovirus M2–2-mediated innate immune evasion. Virology. 2016;499:361–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ren Y, Choi E, Zhang K, et al. Detection of Nuclear Protein Profile Changes by Human Metapneumovirus M2–2 Protein Using Quantitative Differential Proteomics. Vaccines (Basel). 2017;5(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bao X, Sinha M, Liu T, et al. Identification of human metapneumovirus-induced gene networks in airway epithelial cells by microarray analysis. Virology. 2008;374(1):114–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ren Y, Choi E, Zhang K, et al. Detection of Nuclear Protein Profile Changes by Human Metapneumovirus M2–2 Protein Using Quantitative Differential Proteomics. Vaccines. 2017;5(4):45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Deng J, Chen Y, Liu G, et al. Mitochondrial antiviral-signalling protein plays an essential role in host immunity against human metapneumovirus. The Journal of general virology. 2015;96(8):2104–2113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ren J, Kolli D, Deng J, et al. MyD88 controls human metapneumovirus-induced pulmonary immune responses and disease pathogenesis. Virus research. 2013;176(1–2):241–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kolli D, Bataki EL, Spetch L, et al. T lymphocytes contribute to antiviral immunity and pathogenesis in experimental human metapneumovirus infection. Journal of virology. 2008;82(17):8560–8569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Guerrero-Plata A, Casola A, Garofalo RP. Human metapneumovirus induces a profile of lung cytokines distinct from that of respiratory syncytial virus. Journal of virology. 2005;79(23):14992–14997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Swartz A, Collier T, Young CA, et al. The effect of early child care attendance on childhood asthma and wheezing: A meta-analysis. Journal of Asthma. 2018:1–11. [DOI] [PubMed] [Google Scholar]
- 30.Hamelin M-È, Boivin G. Human metapneumovirus: a ubiquitous and long-standing respiratory pathogen. The Pediatric infectious disease journal. 2005;24(11):S203–S207. [DOI] [PubMed] [Google Scholar]
- 31.Wang L, Li J, Zhang J, et al. Inhibition of TRPC3 downregulates airway hyperresponsiveness, remodeling of OVA-sensitized mouse. Biochemical and biophysical research communications. 2017;484(1):209–217. [DOI] [PubMed] [Google Scholar]
- 32.Malmo J, Moe N, Krokstad S, et al. Cytokine Profiles in Human Metapneumovirus Infected Children: Identification of Genes Involved in the Antiviral Response and Pathogenesis. PLoS One. 2016;11(5):e0155484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chakraborty K, Zhou Z, Wakamatsu N, Guerrero-Plata A. Interleukin-12p40 modulates human metapneumovirus-induced pulmonary disease in an acute mouse model of infection. PloS one. 2012;7(5):e37173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hornsleth A, Loland L, Larsen LB. Cytokines and chemokines in respiratory secretion and severity of disease in infants with respiratory syncytial virus (RSV) infection. Journal of clinical virology : the official publication of the Pan American Society for Clinical Virology. 2001;21(2):163–170. [DOI] [PubMed] [Google Scholar]
- 35.Bohmwald K, Galvez NMS, Canedo-Marroquin G, et al. Contribution of Cytokines to Tissue Damage During Human Respiratory Syncytial Virus Infection. Frontiers in immunology. 2019;10:452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vazquez Y, Gonzalez L, Noguera L, et al. Cytokines in the Respiratory Airway as Biomarkers of Severity and Prognosis for Respiratory Syncytial Virus Infection: An Update. Frontiers in immunology. 2019;10:1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bosis S, Esposito S, Osterhaus AD, et al. Association between high nasopharyngeal viral load and disease severity in children with human metapneumovirus infection. Journal of clinical virology : the official publication of the Pan American Society for Clinical Virology. 2008;42(3):286–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Roussy JF, Carbonneau J, Ouakki M, et al. Human metapneumovirus viral load is an important risk factor for disease severity in young children. Journal of clinical virology : the official publication of the Pan American Society for Clinical Virology. 2014;60(2):133–140. [DOI] [PubMed] [Google Scholar]
- 39.Hasegawa K, Jartti T, Mansbach JM, et al. Respiratory syncytial virus genomic load and disease severity among children hospitalized with bronchiolitis: multicenter cohort studies in the United States and Finland. The Journal of infectious diseases. 2015;211(10):1550–1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.DeVincenzo JP, El Saleeby CM, Bush AJ. Respiratory syncytial virus load predicts disease severity in previously healthy infants. The Journal of infectious diseases. 2005;191(11):1861–1868. [DOI] [PubMed] [Google Scholar]
- 41.Chakraborty K, Zhou Z, Wakamatsu N, Guerrero-Plata A. Interleukin-12p40 modulates human metapneumovirus-induced pulmonary disease in an acute mouse model of infection. PLoS ONE. 2012;7(5):e37173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Piedra FA, Mei M, Avadhanula V, et al. The interdependencies of viral load, the innate immune response, and clinical outcome in children presenting to the emergency department with respiratory syncytial virus-associated bronchiolitis. PLoS One. 2017;12(3):e0172953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Thwaites RS, Coates M, Ito K, et al. Reduced Nasal Viral Load and IFN Responses in Infants with Respiratory Syncytial Virus Bronchiolitis and Respiratory Failure. Am J Respir Crit Care Med. 2018;198(8):1074–1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hijano DR, Vu LD, Kauvar LM, Tripp RA, Polack FP, Cormier SA. Role of Type I Interferon (IFN) in the Respiratory Syncytial Virus (RSV) Immune Response and Disease Severity. Front Immunol. 2019;10:566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Guerrero-Plata A, Kolli D, Hong C, Casola A, Garofalo RP. Subversion of pulmonary dendritic cell function by paramyxovirus infections. J Immunol. 2009;182(5):3072–3083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jakubzick C, Tacke F, Llodra J, van Rooijen N, Randolph GJ. Modulation of dendritic cell trafficking to and from the airways. J Immunol. 2006;176(6):3578–3584. [DOI] [PubMed] [Google Scholar]
- 47.Varol C, Landsman L, Fogg DK, et al. Monocytes give rise to mucosal, but not splenic, conventional dendritic cells. The Journal of experimental medicine. 2007;204(1):171–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Steinman RM. The dendritic cell system and its role in immunogenicity. Annual review of immunology. 1991;9:271–296. [DOI] [PubMed] [Google Scholar]
- 49.Tseng SY, Otsuji M, Gorski K, et al. B7-DC, a new dendritic cell molecule with potent costimulatory properties for T cells. The Journal of experimental medicine. 2001;193(7):839–846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Verdijk P, van Veelen PA, de Ru AH, et al. Morphological changes during dendritic cell maturation correlate with cofilin activation and translocation to the cell membrane. European journal of immunology. 2004;34(1):156–164. [DOI] [PubMed] [Google Scholar]
- 51.Schuster JE, Williams JV. Human Metapneumovirus. Microbiology spectrum. 2014;2(5). [DOI] [PubMed] [Google Scholar]
- 52.Hamelin ME, Couture C, Sackett MK, Boivin G. Enhanced lung disease and Th2 response following human metapneumovirus infection in mice immunized with the inactivated virus. J Gen Virol. 2007;88(Pt 12):3391–3400. [DOI] [PubMed] [Google Scholar]
- 53.Dalod M, Chelbi R, Malissen B, Lawrence T. Dendritic cell maturation: functional specialization through signaling specificity and transcriptional programming. The EMBO journal. 2014;33(10):1104–1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kolli D, Bao X, Liu T, et al. Human metapneumovirus glycoprotein G inhibits TLR4-dependent signaling in monocyte-derived dendritic cells. J Immunol. 2011;187(1):47–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Liao S, Bao X, Liu T, et al. Role of retinoic acid inducible gene-I in human metapneumovirus-induced cellular signalling. The Journal of general virology. 2008;89(Pt 8):1978–1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Loo YM, Fornek J, Crochet N, et al. Distinct RIG-I and MDA5 signaling by RNA viruses in innate immunity. Journal of virology. 2008;82(1):335–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Banos-Lara Mdel R, Ghosh A, Guerrero-Plata A. Critical role of MDA5 in the interferon response induced by human metapneumovirus infection in dendritic cells and in vivo. Journal of virology. 2013;87(2):1242–1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.von Bernuth H, Picard C, Puel A, Casanova JL. Experimental and natural infections in MyD88- and IRAK-4-deficient mice and humans. European journal of immunology. 2012;42(12):3126–3135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Herd KA, Mahalingam S, Mackay IM, Nissen M, Sloots TP, Tindle RW. Cytotoxic T-lymphocyte epitope vaccination protects against human metapneumovirus infection and disease in mice. Journal of virology. 2006;80(4):2034–2044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wen SC, Schuster JE, Gilchuk P, Boyd KL, Joyce S, Williams JV. Lung CD8+ T Cell Impairment Occurs during Human Metapneumovirus Infection despite Virus-Like Particle Induction of Functional CD8+ T Cells. Journal of virology. 2015;89(17):8713–8726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Schmidt ME, Varga SM. The CD8 T Cell Response to Respiratory Virus Infections. Frontiers in immunology. 2018;9:678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.van den Hoogen BG, van Boheemen S, de Rijck J, et al. Excessive production and extreme editing of human metapneumovirus defective interfering RNA is associated with type I IFN induction. The Journal of general virology. 2014;95(Pt 8):1625–1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Schickli JH, Kaur J, Macphail M, Guzzetta JM, Spaete RR, Tang RS. Deletion of human metapneumovirus M2–2 increases mutation frequency and attenuates growth in hamsters. Virology journal. 2008;5:69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Buchholz UJ, Biacchesi S, Pham QN, et al. Deletion of M2 gene open reading frames 1 and 2 of human metapneumovirus: effects on RNA synthesis, attenuation, and immunogenicity. Journal of virology. 2005;79(11):6588–6597. [DOI] [PMC free article] [PubMed] [Google Scholar]