Abstract
Introduction
Venous thromboembolism (VTE) is a common and serious complication of systemic anticancer therapies. Delays in presentation increase risk of death or long‐term morbidity.
Background
A patient charity developed an information video for patients receiving systemic anticancer therapy including what to do if they developed symptoms of VTE. This was introduced into clinical practice in a regional cancer center and its impact compared with a district general hospital where the video was not used.
Methods
A mixed‐methods approach was used, comprising clinical audit data, patient surveys, and key informant interviews. The time between development of VTE symptoms and seeking medical evaluation was routinely recorded on patients attending a regional cancer‐associated thrombosis service with systemic anticancer therapy–provoked VTE. The video was then embedded into clinical practice at the regional cancer center for 3 months. The primary outcome was the difference in time to presentation with VTE symptoms, between patients attending the regional cancer center and the district general hospital (which acted as control). Other outcomes included impact on radiology resources, patient knowledge, and perspectives of chemotherapy nurses.
Results
Addition of the video was associated with a lower mean time to presentation from 8.9 to 2.9 days (0.33 hazard ratio; 95% confidence interval, 4.5‐7.4; P < .0001). This may reflect greater awareness of VTE, resulting in earlier clinical presentation when they developed attributable symptoms.
Conclusion
The video was associated with reduced delays in diagnosis of systemic anticancer therapy–associated VTE by 6 days, thereby reducing long‐term complications.
Keywords: cancer‐associated thrombosis, mixed methods, patient information, qualitative, venous thromboembolism
Essentials.
Patients should be aware of the risks of venous thromboembolism (VTE) provoked by chemotherapy.
A patient‐developed video was introduced into clinical practice at a regional cancer center.
Patients undergoing chemotherapy who watched the video sought help for VTE three times more quickly.
Multiple formats of patient information may improve patient understanding and care.
1. INTRODUCTION
The prothrombotic state induced by the presence of cancer is well documented. Venous thromboembolism (VTE) comprising deep vein thrombosis (DVT) and pulmonary embolism (PE), is a common complication of cancer and its treatments, and may occur during the lifetime of 20% of patients with cancer.1 The risk of developing cancer‐associated thrombosis (CAT) will vary according to cancer primary, disease stage, comorbidities, and the use of systemic anticancer therapies. Systemic anticancer therapies, which include cytotoxic chemotherapy, immunotherapy, monoclonal antibodies, and hormonal agents, are arguably one of the most significant risk factors for CAT and have been associated with up to 84% of cases.2 CAT is a cause of significant mortality and morbidity; it is the most common cause of chemotherapy‐related death, as well as being associated with long‐term physical and psychological sequelae.3, 4, 5 Recent clinical guidelines have recognized the importance of identifying patients receiving ambulant systemic anticancer therapy at risk of CAT who should be considered for primary thromboprophylaxis. This may be through targeting particular cancer types (eg, pancreatic cancer or myeloma) or through the use of a validated risk assessment tool, such as the Khorana score.6, 7, 8 Despite these developments, adherence to CAT‐specific clinical guidelines remains poor, and the majority of systemic anticancer therapy–induced CAT cases presenting to clinical services have not received thromboprophylaxis.2, 9, 10, 11, 12
There is a growing body of data recognizing the benefit of a dedicated CAT service.2, 13 While this approach leads to improvements in care through consistency of practice and prescribing safety, it has no influence on how or when patients will present with symptoms of VTE. Previous qualitative data suggest that patients embarking on systemic anticancer therapy receive little information regarding the risks of CAT or red flag symptoms that would warrant urgent medical attention.4 Consequently, many cases of CAT have a delayed presentation, with an increased risk of fatal PE, extensive DVT, postthrombotic syndrome, and long‐term psychological distress.14, 15, 16
2. DEVELOPMENT OF “BLOOD CLOTS, CANCER AND YOU” VIDEO
In 2017, the charity Anticoagulation UK (formerly Anticoagulation Europe) developed a patient information video “Blood Clots, Cancer and You.” This was in response to a UK Parliamentary report suggesting that <15% of hospitals offered verbal or written information about the risks of VTE associated with chemotherapy.17
The content of the video was developed by trustees of the charity through a series of focus groups with participants of mixed ethnicity and social levels. Groups included patients with cancer (both receiving chemotherapy and in remission), family members of patients with cancer, and members of the public with no cancer experience. Themes from focus groups were collated and used to formulate a draft video board and script, which was drafted by trustees and clinical experts affiliated with the charity. The charity engaged Just Health Comms, a media company, to make the video. Full control of contents remained with the charity through regular meetings and phone calls at the development stage. A “long‐term” advisory group, derived from focus group members, continued to advise throughout and help as necessary. Funding was provided through an unrestricted educational grant provided by Leo Pharma, which had no input into the development or construction of the video.
The video, which is available online https://www.youtube.com/watch?v=dSIwFwhoFA4, was short‐listed for a national patient engagement award in 2018 but had not been formally evaluated. We therefore sought to evaluate the impact of the video on patient care and health care resource usage.
3. AIMS
The overarching aim of the EMPOWER study was to evaluate the impact of the patient information video “Blood Clots, Cancer and You” on the care of patients with cancer receiving systemic anticancer therapy. Specifically, the study aimed to evaluate the impact of the video as evidenced by:
Time taken for patients to seek medical attention from development of symptoms suggestive of CAT.
Use of radiology resources.
Patient awareness of CAT‐related symptoms.
Views of oncology nurses regarding need for, and utility of, the video in the clinical workplace.
4. SETTING
The evaluation of the video occurred within a UK regional cancer service, serving 7000 new patients with cancer per annum from across a diverse mix of urban/rural, socioeconomic, ethnic, and cultural backgrounds. It is standard practice for all patients planned for systemic anticancer therapy to attend a patient information session that is delivered by chemotherapy specialist nurses. Patients will then receive an individualized information/consent session comprising both written and verbal information. Systemic anticancer therapy is then administered at the regional cancer center or a local district general hospital. For the purpose of this evaluation, the video was introduced to the regional cancer center but not the district general hospital, which acted as a control. The video was incorporated as part of the information session, in addition to standard written and verbal information. The video was made available as individual video cards within the chemotherapy suites and outpatient clinics for patients and their significant others to watch repeatedly as desired. As described below, data were collected before and 3 months following the implementation of the video.
5. METHODS
This was a mixed‐methods study comprising four components, which are summarized in Figure 1.
FIGURE 1.
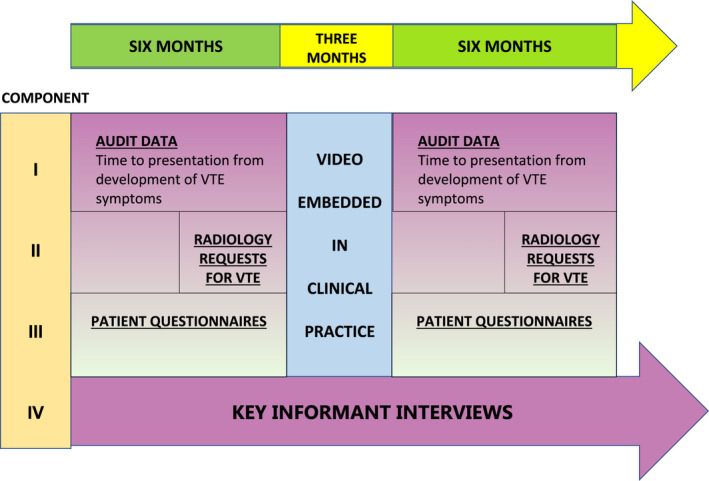
Summary of methods
There is no universally agreed definition for systemic anticancer therapy–associated CAT, and it is impossible to accurately quantify how much a VTE event is attributable to such therapies or whether they are merely an associated risk factor. Therefore, the following pragmatic was used: “Any radiologically confirmed cancer‐associated thrombosis occurring while the patient is receiving systemic anticancer therapy or 3 weeks post completion of treatment.”
For sections involving patients, the following inclusion/ exclusion criteria were applied:
Inclusion criteria:
Aged ≥18 years.
Histologically proven cancer.
Radiologically proven VTE.
Currently receiving systemic anticancer therapy or completed treatment within 3 weeks.
Exclusion criteria:
Past history of VTE.
Receiving anticoagulation (full or prophylactic dose) for any reason.
5.1. Component I: Clinical audit data of time to presentation
It is standard practice that all patients diagnosed with CAT are reviewed at the regional CAT specialist clinic and are asked about symptoms attributable to VTE. Data are routinely collected for time taken to seek medical attention after developing symptoms. A pragmatic decision was made to collect initial data over a 6‐month period to ensure all prospective data collection and analysis could be completed within the study’s 12‐month funding period. Consequently, data were collected on 50 sequential patients with systemic anticancer therapy–associated VTE before the introduction of the video, and then repeated 6 months after the video was embedded in clinical practice. An unpaired (independent) t test was used to test the hypothesis that there was no difference between the means of the two groups. SPSS Statistics for Windows, version 27.0 (IBM, Armonk, NY, USA) was used for statistical analysis.
5.2. Component II: Clinical audit data of radiology resource usage
While it is hoped that raised awareness of the symptoms of VTE will lead to earlier presentation, diagnosis, and treatment, the corollary is that increased symptom vigilance will generate a rise in radiology requests with subsequent health economic implications. It was therefore important to evaluate whether the introduction of the video impacted on radiology resource usage, with particular focus on increases in the number of negative scans.
Radiology reports for leg Doppler ultrasounds and computed tomography pulmonary angiograms (CTPAs) covering a 3‐month period before introduction of the video were reviewed and cross referenced with pharmacy systemic anticancer therapy prescriptions administered within the previous 4 weeks. Hand searches of radiology requests were undertaken to establish which investigations were undertaken to investigate suspected VTE. This process was repeated for a further 3 months of radiology reports after the video had been established in practice for 6 months.
5.3. Component III: Patient questionnaires
A patient questionnaire was developed by a multidisciplinary team working in CAT. Questions were devised to explore how much patients who were due to embark on systemic anti‐cancer therapy understood about VTE and the associated risks with cancer treatments. Questions were cross referenced with the factual content of the video and reviewed by patient and public involvement representatives from Anticoagulation UK and Thrombosis UK. The questionnaire underwent two rounds of field testing with patients and health care professionals. Cognitive interviews were undertaken after each round for relevance, content validity, and usability, with refinements made as necessary. The questionnaire was composed of 12 questions and explored four main areas of patient awareness (see Appendix 1):
Common side effects of chemotherapy.
Awareness of DVT and PE.
Risk factors for VTE.
Signs and symptoms of VTE and what patients should do if they develop them.
Questionnaires were distributed to patients receiving systemic anticancer therapy who had attended the information sessions before the introduction of the video. This exercise was repeated 3 months after the video was embedded in clinical practice.
5.4. Component IV: Key informant interviews
Key informant interviews are qualitative, in‐depth interviews of people selected for their first‐hand knowledge about a topic of interest.18 Interviews are loosely structured, following a list of issues to be discussed to allow a free flow of ideas and information. Purposive sampling of systemic anticancer therapy nurses was undertaken at two hospital sites that administered chemotherapy. Semistructured interviews were conducted with nurses working at the study site, who had seen the video, and those at the control site, who had not viewed the video. Participants were interviewed by an experienced qualitative researcher who was also a clinician with a specialist interest in CAT (SN or NP) in a quiet location at their participating hospital at a time convenient to themselves. The clinicians adhered to a semistructured interview guide (see Appendix 2). Interviews lasted no more than 30 minutes and were audio‐recorded, transcribed verbatim, and anonymized. Transcript data were then managed using NVivo 12 computer software (QSR International, Melbourne, Australia). The qualitative data were first analyzed by the lead author (EB) using thematic analysis as described by Braun and Clarke.19 She familiarized herself with the transcripts, noting initial thoughts on the content of the interviews. Interesting features were then initially coded systematically, ensuring that relevant data were collated across all transcripts. The codes were then grouped into potential overarching themes, followed by a review of each specific code within the themes and in the wider context of the transcripts.19 The themes were then further defined and reviewed, including dual coding by a second researcher (ATB) and meetings to address any conflicting themes/ideas that emerged. Themes were derived in a deductive manner, due to the specific topic of the interviews undertaken.
6. RESULTS
The results reflect data collected for four components of the mixed‐methods study, some collected prospectively and some derived from preexisting databases. The timing of data collections through databases was dependent on respective governance systems and application processes. This inevitably led to a discordance between the data collection time points, before video introduction and the time points chosen once embedded into clinical practice. For the purpose of this paper, the results are presented in the order they were described in the Methods section and shall be discussed as a whole data corpus in the Discussion section.
6.1. Component I: Clinical audit data of time to presentation
Before the introduction of the video, data were collected from 50 sequential patients presenting to the CAT service with symptomatic systemic anticancer therapy–associated VTE. Patient characteristics are summarized in Table 1. I am not able to add a comment for some reason, but the title of table 1 seems wrong ‐ doesn't match the headers. if the title was right the headers would be watched video, did not watch video. please check with authors.
TABLE 1.
Characteristics of patients who had not watched video and those who had
| Not watched video | Watched video | |
|---|---|---|
| Mean age, y (range) | 67.6 (38‐82) | 66.54 (36‐83) |
| Sex, n (%) | ||
| Male | 22 (44) | 23 (46) |
| Female | 28 (56) | 27 (54) |
| Ethnicity, n (%) | ||
| White | 45 (90) | 46 (92) |
| Asian | 3 (6) | 2 (4) |
| Black | 2 (4) | 2 (4) |
| Systemic anticancer therapy purpose, n (%) | ||
| Radical/Curative intent | 1 (2) | 1 (2) |
| Adjuvant/Neoadjuvant | 14 (28) | 13 (26) |
| Palliative | 35 (70) | 36 (72) |
| Not watched video | F:M | Watched video | F:M | |
|---|---|---|---|---|
| Tumor primary, n (%) | ||||
| Lung | 7 (14) | 4:3 | 6 (12) | 3:3 |
| Gastroesophageal | 3 (6) | 1:2 | 4 (8) | 1:3 |
| Hepatobiliary/Pancreatic | 3 (6) | 1:3 | 5 (10) | 1:4 |
| Colorectal | 11 (22) | 5:6 | 10 (20) | 5:5 |
| Gynecologic | 6 (12) | 6:0 | 7 (14) | 7:0 |
| Male genitourinary | 8 (16) | 0:8 | 7 (14) | 0:7 |
| Breast | 10 (20) | 10:0 | 9 (18) | 9:0 |
| Brain | 1 (2) | 0:1 | 0 (0) | 0:0 |
| Unknown primary | 1 (2) | 1:0 | 2 (4) | 1:1 |
| Total, n (%) | 50 (100) | 50 (100) | ||
The time taken for patients to seek medical attention from the development of symptoms ranged from 1 to 21 days (median, 9 days; mode, 10; mean, 8.9).
This prospective data collection of 50 sequential patients was repeated 6 months after the video was embedded into clinical practice. On this occasion, the time to presentation with symptomatic systemic anticancer therapy–associated VTE ranged from 12 hours to 14 days (median, 2 days; mode, 2; mean, 2.9).
This suggests the video was associated with a 0.67 relative risk reduction (RRR) (0.33 HR, 95% confidence interval [CI], 4.50‐7.47; P < .0001) in time to presentation of symptoms from 8.9 to 2.9 days.
6.2. Component II: Clinical audit data of radiology resource usage
The results are summarized in Table 2. During the first 3‐month cohort, 18 of 50 (36%) investigations were positive for VTE, comprising 0 of 11 CTPA positive for PE and 18 of 39 Doppler ultrasounds positive for DVT. Data for the second cohort were collected for a 3‐month period, commencing 6 months after the video was embedded into practice. During this time, 23 of 71 (32.4%) investigations were positive for VTE, comprising 3 of 17 CTPAs and 20 of 54 Doppler ultrasounds of the leg.
TABLE 2.
Radiology resource usage over 3 month period before and after video was embedded in clinical practice
| Scan requested | Number undertaken | Positive for VTE | Negative for VTE | |
|---|---|---|---|---|
|
Cohort 1 Before introduction of video | ||||
| 50 patients | CTPA | 11 | 0 | 11 |
| Doppler | 39 | 18 | 21 | |
|
Cohort 2 6 months after introduction of video | ||||
| 71 patients | CTPA | 17 | 3 | 14 |
| Doppler | 54 | 20 | 34 | |
CTPA, computer tomography pulmonary arteriogram; VTE, venous thromboembolism.
6.3. Component III: Patient questionnaires
In the interest of brevity, patients receiving systemic anticancer therapy who had not seen the video will be referred to as video naïve (n = 51), and those who had seen the video as part of their chemotherapy education session will be called video familiar (n = 53). While most video‐naïve patients were familiar with the terms DVT and PE, only 6% identified chemotherapy as a risk factor, with the majority (60%) naming long‐haul air travel as the main precipitant (Figure 2). Furthermore, they considered VTE to be neither a common consequence of systemic anticancer therapy (2%) nor a serious side effect (4%). In contrast, they considered sepsis to be the most serious/life threatening of complications (Figures 3 and 4). Systemic anticancer therapy was recognized as a risk factor for CAT in 60% of video‐familiar participants, with 80% identifying it as one of the most serious complications.
FIGURE 2.
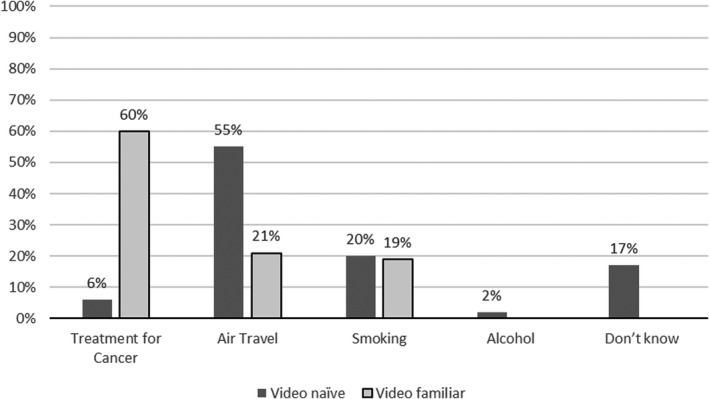
Risk factors for VTE. VTE, venous thromboembolism
FIGURE 3.
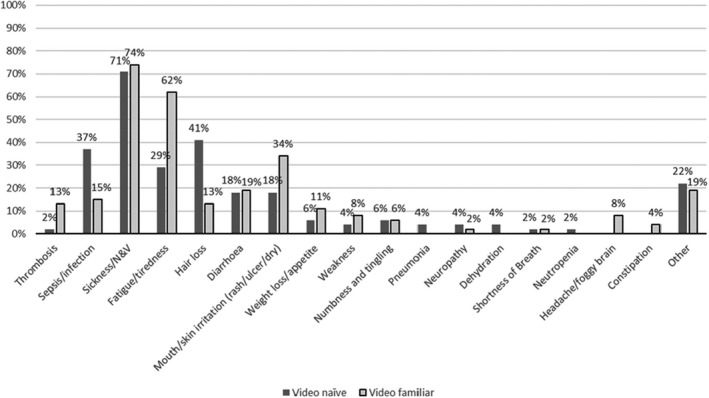
Top three side effects patients report they associate with systemic anticancer therapy. N&V, nausea and vomiting
FIGURE 4.
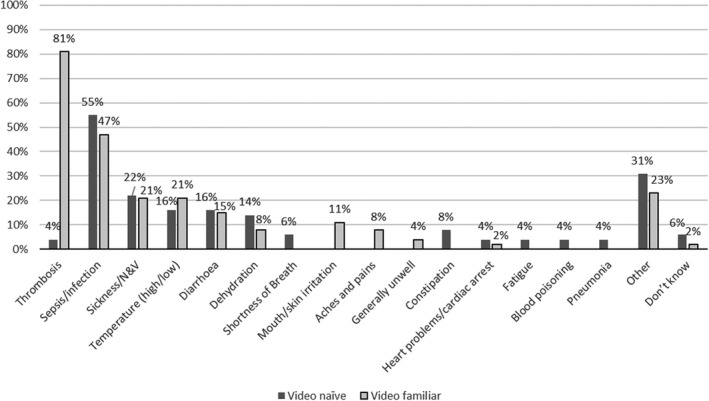
Severe side effects associated with systemic anticancer therapy reported by patient participants. N&V, nausea and vomiting
The majority of video‐naïve patients (76%) had heard of DVT, but 33% were unable to identify a single associated sign or symptom. Similarly, of the 67% video‐naïve patients familiar with PE, 43% could not suggest any clinical characteristics that would necessitate medical attention. Familiarity with the video was associated with a higher level of knowledge of the signs and symptoms of VTE; only 3% and 4% of video‐familiar patients were unable to describe any features of DVT and PE, respectively.
Regarding what a patient should do on development of symptoms attributable to VTE, 100% of video‐familiar patients would seek immediate medical attention via the chemotherapy help line or emergency services. Fewer video‐naïve patients (80%) stated that they would seek medical advice, and only 69% would do so as a matter of urgency (Table 3).
TABLE 3.
Actions and timing of evaluation for patients developing symptoms of VTE
| What to do when symptoms develop? | When? | ||||
|---|---|---|---|---|---|
| Seek medical assistance | Don’t know | Immediately | Schedule appointment | Don’t know | |
|
Video naïve (n = 51) |
80% (n = 41) | 20% (n = 10) | 69% (n = 35) | 6% (n = 3) | 25% (n = 13) |
|
Video familiar (n = 53) |
100% (n = 53) | 0% (n = 0) | 100% (n = 53) | 0% (n = 0) | 0% (n = 0) |
VTE, venous thromboembolism.
6.4. Component IV: Key informant interviews
Nine chemotherapy nurses, all women, were interviewed (see Table 4). Six were working in a hospital where the video was embedded in clinical practice, and three were at the control site. Nurses were highly experienced, with the majority having worked in the chemotherapy unit for >8 years.
TABLE 4.
How long qualified and how much experience in chemotherapy/cancer care
| Demographic information | Video naïve | Video familiar | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Participant No. | 1 | 2 | 3 | 1 | 2 | 3 | 4 | 5 | 6 |
| How long qualified (y) | 28 | 21 | 5 | 3.5 | 10 | 18 | 30 | 30 | 19 |
| How many years chemotherapy | 25 | 8 | 5 | 8 mo | 5.5 | 14 | 30 | 25 | 19 |
Thematic analysis identified two major themes—low prioritization of thrombosis and impact of the video—which had three and two associated subthemes, respectively. These are summarized in Figure 5 and discussed below. Themes are supported by participant quotes, which have been chosen to illustrate the theme being discussed, and to represent a breadth of data across as many participants as possible.
FIGURE 5.
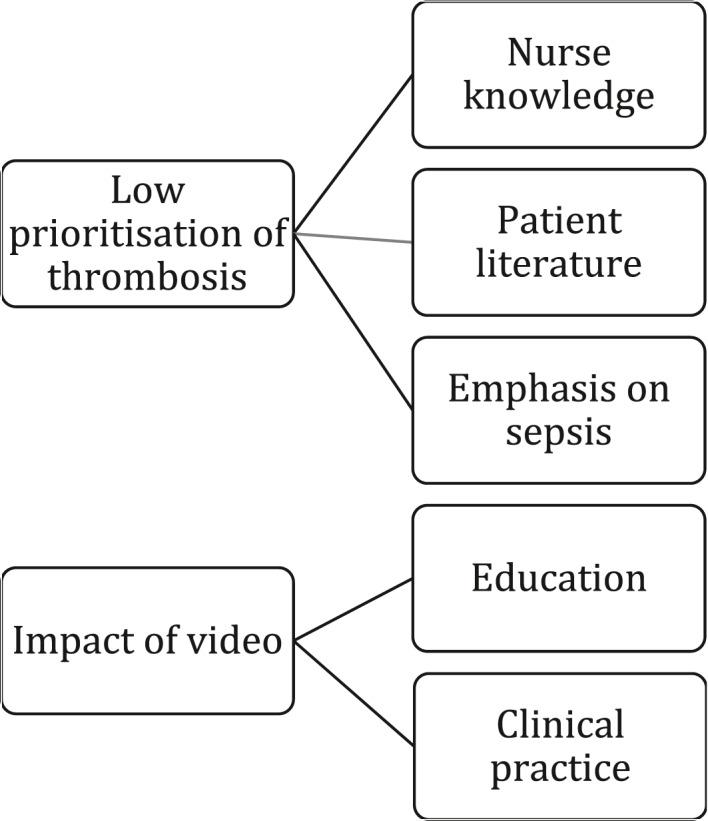
Themes and subthemes from key informant interview
6.4.1. Major Theme: Low prioritization of thrombosis
Before the administration of systemic anticancer therapy, it is usual practice for patients to be given information regarding their planned treatment, common side effects, and what to do if they develop symptoms of serious complications. While patients would be told they were at risk of CAT, they were given minimal additional information.
I think I just say a risk of erm blood clots, yeah. (Vn03)
I just say that they are more at risk of blood clots. I don’t really tell them why… I only say probably a sentence to patients. (Vf06)
It was common for conditions such as neutropenic sepsis to be prioritized over CAT,
I don’t think it’s given the same priority as sepsis. I mean it is something that I do try to discuss but I wouldn’t give it the same priority, in all honesty. (Vn02)
There appeared to be three key factors that led to low prioritization of thrombosis. These are outlined below.
Subtheme: Nurse knowledge
While nurses knew that systemic anticancer therapy increased the risk of CAT, reasons for this were poorly understood.
If they were asking a general question, I would say, well you are more at risk, but I wouldn’t be able to say how much more at risk. (Vn02)
Participants subsequently avoided further detail and spent more time on complications they understood.
I don’t really know why they’re getting increased risk. … I don’t understand the mechanisms of that. So I guess that’s probably a lot to do with why we don’t go into so much detail. (Vn02)
Subtheme: Emphasis on sepsis
Participants universally considered sepsis the most important complication of systemic anticancer therapy and prioritized it in their education sessions. It would be the first condition they described because it was life threatening.
My first ones are always life‐threatening infection… (Vn03)
So I think it’s a priority for them to be aware about the sepsis. (Vf05)
Participants were wary of overwhelming patients with too much information, so they would discuss issues they felt were most important first.
Obviously, infection is right up there, and I think the reason I do that is when you’re working on a busy unit, not saying that you should not ever give all of the information to a patient but I think giving the most important ones first is what they will take away with them and even if they don’t get past like the risk of infection, at least you’ve got that one.… (Vf04)
Subtheme: Patient literature
The delivery of patient information and the subsequent consent process was usually supported by a standard document that listed what the document authors considered the most common and important side effects. Participants would usually follow this in their clinical practice.
It’s usually within that leaflet so it’s, it’s laid out there. … There are specific ones but I mean most of the ones we, you want to prioritize are within the leaflet … it's easier for your patients to recall isn’t it, so I think if you’re, if you just go down that as it is in there it is easier. … (Vf03)
However, not all literature included CAT and it sometimes went unmentioned.
It doesn’t actually have clots on there, does it? So that one isn’t in our checklist … I don’t know because this is a national booklet so it’s not something that we have developed. (Vn02)
Where CAT was included in the literature, it was usually at the end of documents.
I think they are in there … I think they’re at the end … as the patient is going through I would imagine they, they think that the most important bits are at the beginning and not at the end. They are more likely to switch off probably towards the end. … (Vn02)
6.4.2. Major Theme: Impact of the video
Participants found the video to be very informative and responses suggested attitudinal change toward the prioritization of CAT in their discussions.
I do think that there should be more importance put on blood clots and I think we should educate patients better, and it’s a shame that we haven’t done it up until this point given the risk. (Vf02)
Subtheme: Education
While participants readily conceded that they had limited understanding around CAT, they were surprised by some of the statistics presented in the video. These reinforced their interest in learning more about the impact of chemotherapy on thrombosis risk.
It is the second most, you know aside from disease progression. I didn’t realize that as actually the biggest fatality for patients. (Vf02)
Education for nurses around clots, maybe quite useful because I think we’re all very much aware of PE and DVT but, you know, why it happens, how it happens, and numbers and things like that would be quite useful update I think for a lot of the nurses. (Vn02)
Some participants mentioned that sepsis was much easier to describe and talk to patients about compared with describing blood clots. Further training on CAT would allow them to explain more to patients and answer any questions that may arise.
For nurses (it) would be good because we can then relay that information or reassure a patient when those questions come up … so I think like a grounding of that information would be useful for nurses. (Vf04)
Subtheme: Clinical practice
The introduction of the video led to changes in the management of patient care. Participants reported giving greater priority to discussing thrombosis risk as well as changes in departmental procedure. On an individual basis, participants reported that they would give more attention to CAT.
Everyone I educate I always include it in my, in my pre‐chemo education. (Vf06)
In addition to the video being incorporated into the patient education sessions, the increased knowledge regarding thrombosis resulted in changes in the patient information leaflet.
The education now, which has changed, it does say in the leaflet now that they are at higher risk of getting clots. So it is there … initially it wasn’t and we probably didn’t back in the day but now, it is prominent… it isn’t on the alert card at the moment… it’s within the patient information leaflet, but not on the alert card, but yeah I think it probably should be. (Vf03)
7. DISCUSSION
The fact that this study has identified a low patient awareness of VTE should come as no surprise. Numerous surveys and qualitative studies suggest that this is a global issue, unaffected by differences in culture, language, models of health care, or access to the Internet.4, 14, 20, 21, 22, 23 A particularly informative survey was commissioned by the ISTH in 2014, in which they explored the views of 800 members of the public from across nine countries.20 While participants were familiar with thrombotic conditions such as heart attack and stroke, <50% of them were aware of VTE. Furthermore, only 45% of respondents were aware that blood clots were preventable, and awareness of cancer, hospitalization, and surgery as risk factors of VTE was low (16%, 25%, and 36%, respectively). Specific to patients with cancer, an online survey in the United States suggested only 24% and 15% of the 500 patients, respectively, had heard of the terms DVT/PE; 19% and 17% could name signs/symptoms of DVT/PE.24
It is of considerable concern that, despite being the cause of significant morbidity and mortality, VTE is also a low priority to health care professionals, responsible for obtaining consent from patients embarking on systemic anticancer therapy.4 Conversely, sepsis appears to be universally prioritized in patient education, information leaflets, and national quality improvement programs.25
The primary aim of the EMPOWER study was to assess the impact of the video on the time taken for patients to seek medical attention from the development of symptoms of VTE. Its introduction was associated with a 0.67 RRR (0.33 HR, 95% CI, 4.50‐7.47; P < .0001) from 9 to 3 days.
The study also identified factors that may determine which complications of systemic anticancer therapy are prioritized in patient education sessions. These predominantly reflected nurses’ awareness of, and their confidence in, discussing each condition. It highlighted a considerable knowledge gap around CAT and subsequent education need. However, the lack of attention afforded to CAT is not solely due to the knowledge and confidence of nurses; the fact that local and national consent checklists give only a cursory mention of VTE suggests a more global and systemic challenge. It is encouraging that embedding the video into practice improved nurses’ self‐reported knowledge of CAT and led to changes in their practice. It also created a desire for ongoing education on the topic. Nonetheless, a wider change in practice is unlikely to occur unless there is engagement with national bodies responsible for publishing systemic anticancer therapy literature and consent checklists. The video should therefore be viewed as an effective adjunct to patient care but not a solution to a wider organizational issue.
The reduction in time to present following the development of symptoms of VTE, while clinically significant, may impact on radiology services. Introduction of the video was associated with a reduction in positive scan requests from 36% to 32.4%. While at face value these figures may be seen as a slight increase in requests for negative scans, these data need to be considered in the context of other important parameters. Arguably, this increase is an acceptable trade‐off in the face of a 67% reduction in time to investigation and treatment of VTE. However, it is difficult to demonstrate this definitively without formal health economic evaluation and adjustment for confounding variables. Furthermore, given the short time frame over which the video was appraised, additional gains such as reduction in postthrombotic syndrome and chronic psychological sequelae would be yet to manifest and be factored into such an assessment. Furthermore, it would be challenging to evaluate which variables would be attributed to the impact of VTE and which to the cancer itself. In the absence of paired controls to compare against, health economic data would be of limited use.
It is important that these results and their conclusions are considered within the context of the study limitations. First, the evaluation of the video was undertaken in a single cancer center and the practice, knowledge, and attitudes of staff may not be representative of all UK cancer services. A wider rollout of the video across more sites would help clarify its wider utility. Second, it is a matter of debate as to whether such a video would be of use more widely, since patients’ desire for information about CAT varies between countries, health care models, and cultures. For example, qualitative data suggest a greater desire for information from UK, Spanish, and Canadian patients, compared with those from France, Singapore, and New Zealand..14, 15, 16, 21, 22 Nevertheless, patient education delivered through the medium of an information video appears to be acceptable to patients and staff and is a straightforward and inexpensive way of informing those who choose to engage with it.
It is also important to recognize that patients differ in how they best receive information. A survey of 421 US stakeholders suggested that patients are amenable to various learning methods; most prefer to receive education in the context of a doctor‐patient encounter.26 Interestingly, the majority prefer to learn about VTE in the context of how much harm it may cause them. Several VTE education programs around patients with cancer embarking on systemic anticancer therapy have been published. Some have focused on system‐based changes measuring improvements in risk assessment and provision of primary thromboprophylaxis as a primary outcome.27 Others have focused on nurse education, evaluating improvement according to self‐reported learning reports at 90 days.28 We believe this is the first time an end point directly linked to clinical outcomes has been reported within the context of a CAT education tool.
For some, verbal information is most effective, while others prefer to engage with written information. The medium of video engages those most receptive to concurrent audiovisual stimuli. However, it is likely that all three modes of information delivery have their role and may act as adjuncts to each other. So, rather than a video being considered “the best” or “most effective” way to communicate information, we feel it should be viewed as another effective available resource. It is also important to be mindful that increasing awareness of one complication may inadvertently decrease the prominence of another equally important complication. In our study, only 4% of patients considered VTE to be an important complication, rising to 81% after watching the video. However, this was associated with a reduction in attributed importance to sepsis from 55% to 47%. The increased awareness of VTE is encouraging, but ongoing work should ensure that other important complications remain prioritized.
From this evaluation, a video highlighting the risks of VTE from systemic anticancer therapy helps reduce the time taken for patients to seek medical attention on development of symptoms. Additional benefits include increasing the knowledge of health care professionals and changing attitudes with respect to prioritizing complications of systemic anticancer therapy when delivering prechemotherapy education. While this video does not represent a vade mecum of patient education, it represents an acceptable and effective medium for those patients who are less likely to engage with written literature.
AUTHOR CONTRIBUTIONS
All authors made a substantial contribution to the development, conduct, and writing of the manuscript. EB undertook the primary data collection, analysis, and first draft of the manuscript. SN conceived the idea, developed the protocol, contributed to data analysis, and oversaw the project. RN developed the protocol and contributed to the manuscript. AN and ATB contributed to data analysis and writing. NP co‐conceived the idea and contributed to data collection, analysis, and writing. AN contributed to protocol development, data analysis, and writing.
RELATIONSHIP DISCLOSURE
SN: speakers bureau for Leo Pharma, Pfizer, and Bristol‐Myers Squib; and advisory board for Daichi Sankyo and Bristol‐Myers Squib. The other authors declare no conflicts of interest.
Supporting information
Appendix S1
Appendix S2
ACKNOWLEDGMENTS
The authors are grateful to Eve Knight (Anticoagulation UK) and Annya Stephens Boal (Thrombosis UK) for their invaluable input and advice. The posts of Simon Noble, Annmarie Nelson, and Alisha Newman are supported by core funding from a Marie Curie Programme Grant.
Baddeley E, Torrens‐Burton A, Newman A, et al. A mixed‐methods study to evaluate a patient‐designed tool to reduce harm from cancer‐associated thrombosis: The EMPOWER study. Res Pract Thromb Haemost. 2021;5:e12545. 10.1002/rth2.12545
Handling Editor: Cihan Ay
Funding information
The EMPOWER Study was funded by Tenovus Cancer Care Grant Ref: TIG2017‐31. The video “Blood Clots, Cancer and You” was developed by Anticoagulation UK through an educational grant from Leo Pharma. The cost of video cards of “Blood Clots, Cancer and You” were provided by Friends of Velindre Cancer Centre
Contributor Information
Anna Torrens‐Burton, @AnnaTB12.
Alisha Newman, @alishaannnewman.
Annmarie Nelson, @annmarienelson0.
Nikki Pease, @Pease2013.
Rosie Nelson, @roropanolo.
Simon Noble, Email: NobleSI1@cardiff.ac.uk, @simonnoble.
REFERENCES
- 1.Timp JF, Braekkan SK, Versteeg HH, Cannegieter SC. Epidemiology of cancer‐associated venous thrombosis. Blood. 2013;122(10):1712‐1723. [DOI] [PubMed] [Google Scholar]
- 2.Noble S, Pease N, Sui J, et al. Impact of a dedicated cancer‐associated thrombosis service on clinical outcomes: a mixed‐methods evaluation of a clinical improvement exercise. BMJ Open. 2016;6(11):e013321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Khorana AA, Francis CW, Culakova E, Kuderer NM, Lyman GH. Thromboembolism is a leading cause of death in cancer patients receiving outpatient chemotherapy. J Thromb Haemost. 2007;5(3):632‐634. [DOI] [PubMed] [Google Scholar]
- 4.Seaman S, Nelson A, Noble S. Cancer‐associated thrombosis, low‐molecular‐weight heparin, and the patient experience: a qualitative study. Patient Prefer Adherence. 2014;8:453‐461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Noble S, Nelson A, Scott J, et al. Patient experience of living with cancer‐associated thrombosis in Canada (PELICANADA). Res Pract Thromb Haemost. 2019;4(1):154‐160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Key NS, Khorana AA, Kuderer NM et al. Venous thromboembolism prophylaxis and treatment in patients with cancer: ASCO clinical practice guideline update. J Clin Oncol. 2019; 38(5):JCO1901461. [DOI] [PubMed] [Google Scholar]
- 7.Farge D, Frere C, Connors JM, et al. 2019 international clinical practice guidelines for the treatment and prophylaxis of venous thromboembolism in patients with cancer. Lancet Oncol. 2019;10:566‐581. [DOI] [PubMed] [Google Scholar]
- 8.NICE . Venous Thromboembolism in Over 16s: Reducing the Risk of Hospital‐Acquired Deep Vein Thrombosis or Pulmonary Embolism. NICE Guideline [NG89]. London, UK: NICE; 2018. [PubMed] [Google Scholar]
- 9.Mahe I, Chidiac J, Helfer H, Noble S. Factors influencing adherence to clinical guidelines in the management of cancer‐associated thrombosis. J Thromb Haemost. 2016;14(11):2107‐2113. [DOI] [PubMed] [Google Scholar]
- 10.Cajfinger F, Debourdeau P, Lamblin A, et al. Low‐molecular‐weight heparins for cancer‐associated thrombosis: adherence to clinical practice guidelines and patient perception in TROPIQUE, a 409‐patient prospective observational study. Thromb Res. 2016;144:85‐92. [DOI] [PubMed] [Google Scholar]
- 11.Guo JD, Hlavacek P, Poretta T, et al. Inpatient and outpatient treatment patterns of cancer‐associated thrombosis in the United States. J Thromb Thrombolysis. 2020;50(2):386‐394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Matzdorff A, Ledig B, Stuecker M, Riess H. Practice patterns for prophylaxis and treatment of venous thromboembolism in German cancer patients. Oncol Res Treat. 2016;39(4):194‐201. [DOI] [PubMed] [Google Scholar]
- 13.Rabinovich E, Bartholomew JR, Wilks ML, Tripp BL, McCrae KR, Khorana AA. Centralizing care of cancer‐associated thromboembolism: the Cleveland Clinic experience. Thromb Res. 2016;147:102‐103. [DOI] [PubMed] [Google Scholar]
- 14.Font C, Nelson A, Garcia‐Fernandez T, Prout H, Gee P, Noble S. Patients' experience of living with cancer‐associated thrombosis in Spain (PELICANOS). Support Care Cancer. 2018;26:3233‐3239. [DOI] [PubMed] [Google Scholar]
- 15.Noble S, Nelson A, Scott J, et al. Patient experience of living with cancer‐associated thrombosis in Canada (PELICANADA). Res Pract Thromb Haemost. 2020;4(1):154‐160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Noble S, Prout H, Nelson A. Patients' Experiences of LIving with CANcer‐associated thrombosis: the PELICAN study. Patient Prefer Adherence. 2015;9:337‐345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Venous Thromboembolism (VTE) in Cancer Patients. All Party Parliamentary Thrombosis Group; 2016. [Google Scholar]
- 18.Marshall MN. The key informant technique. Fam Pract. 1996;13(1):92‐97. [DOI] [PubMed] [Google Scholar]
- 19.Braun V, Clarke V. Using thematic analysis in psychology. Qual Res Psychol. 2006;3(2):77‐101. [Google Scholar]
- 20.Wendelboe AM, McCumber M, Hylek EM, et al. Global public awareness of venous thromboembolism. J Thromb Haemost. 2015;13(8):1365‐1371. [DOI] [PubMed] [Google Scholar]
- 21.Mahe I, Chidiac J, Pinson M, et al. Patients experience of living with cancer associated thrombosis in France (Le PELICAN). Thromb Res. 2020;194:66‐71. [DOI] [PubMed] [Google Scholar]
- 22.Woulfe T, Mann K, Pollack D, et al. “Wolverine, I think it's called: blood thinners but in tablets.” Patients experience of living with cancer associated thrombosis in New Zealand (PELICANZ). Thromb Res. 2020;189:35‐38. [DOI] [PubMed] [Google Scholar]
- 23.Almodaimegh H, Alfehaid L, Alsuhebany N, et al. Awareness of venous thromboembolism and thromboprophylaxis among hospitalized patients: a cross‐sectional study. Thromb J. 2017;15:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Aggarwal A, Fullam L, Brownstein AP, et al. Deep vein thrombosis (DVT) and pulmonary embolism (PE): awareness and prophylaxis practices reported by patients with cancer. Cancer Invest. 2015;33(9):405‐410. [DOI] [PubMed] [Google Scholar]
- 25.Robson WP, Daniel R. The Sepsis Six: helping patients to survive sepsis. Br J Nurs. 2008;17(1):16‐21. [DOI] [PubMed] [Google Scholar]
- 26.Popoola VO, Lau BD, Shihab HM, et al. Patient preferences for receiving education on venous thromboembolism prevention ‐ a survey of stakeholder organizations. PLoS One. 2016;11(3):e0152084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Holmes CE, Ades S, Gilchrist S, et al. Successful model for guideline implementation to prevent cancer‐associated thrombosis: venous thromboembolism prevention in the ambulatory cancer clinic. JCO Oncol Pract. 2020;16(9):e868‐e874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Leong L, Mendelsohn M, Saavedra C, Morgan RJ. Quality improvement education for venous thromboembolism (VTE) prevention in cancer. J Contin Educ Health Prof. 2015;35(Suppl 1):S29‐S30. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1
Appendix S2


