Abstract
Purpose
To determine whether Macklin effect (a linear collection of air contiguous to the bronchovascular sheath) on baseline CT imaging is an accurate predictor for subsequent pneumomediastinum (PMD)/pneumothorax (PNX) development in invasively ventilated patients with COVID-19-related acute respiratory distress syndrome (ARDS).
Materials and methods
This is an observational, case-control study. From a prospectively acquired database, all consecutive invasively ventilated COVID-19 ARDS patients who underwent at least one baseline chest CT scan during the study time period (February 25th, 2020–December 31st, 2020) were identified; those who had tracheal lesion or already had PMD/PNX at the time of the first available chest imaging were excluded.
Results
37/173 (21.4%) patients enrolled had PMD/PNX; specifically, 20 (11.5%) had PMD, 10 (5.8%) PNX, 7 (4%) both. 33/37 patients with subsequent PMD/PNX had Macklin effect on baseline CT (89.2%, true positives) 8.5 days [range, 1–18] before the first actual radiological evidence of PMD/PNX. Conversely, 6/136 patients without PMD/PNX (4.4%, false positives) demonstrated Macklin effect (p < 0.001). Macklin effect yielded a sensitivity of 89.2% (95% confidence interval [CI]: 74.6–96.9), a specificity of 95.6% (95% CI: 90.6–98.4), a positive predictive value (PV) of 84.5% (95% CI: 71.3–92.3), a negative PV of 97.1% (95% CI: 74.6–96.9) and an accuracy of 94.2% (95% CI: 89.6–97.2) in predicting PMD/PNX (AUC:0.924).
Conclusions
Macklin effect accurately predicts, 8.5 days in advance, PMD/PNX development in COVID-19 ARDS patients.
Keywords: Acute respiratory distress syndrome; Mechanical ventilation; Tomography, X-ray computed; Pneumothorax; Pneumomediastinum; COVID-19
Abbreviations: ARDS, acute respiratory distress syndrome; COVID-19, coronavirus disease 2019; LR, likelihood ratio; NPV, negative predictive value; PMD, pneumomediastinum; PNX, pneumothorax; PPV, positive predictive value
1. Introduction
Pneumomediastinum (PMD) and pneumothorax (PNX) occur frequently in mechanically ventilated patients with coronavirus disease 2019 (COVID-19)-related acute respiratory distress syndrome (ARDS) [1], with a reported incidence up to 24% in the first Italian pandemic wave [2].
COVID-19 ARDS patients are thought to be indeed more vulnerable to PMD/PNX occurrence as compared with patients with ARDS due to other than COVID-19 causes [1,3]; a higher than expected incidence of PMD/PNX was also observed in COVID-19 patients not requiring invasive mechanical ventilation [[3], [4], [5], [6]]. Furthermore, despite employment of lung protective ventilation strategies, these complications have difficult, non-standardized management, and are associated with increased risk of mortality [2,7,8]. Taken together, these findings corroborate the existence of a virus-induced frailty of airways tissue, resulting in an extensive, COVID-19-specific diffuse alveolar damage; microvascular thrombosis [9], interstitial inflammation, as well as endothelial barrier disruption have been suggested as main contributors for such a condition [10].
There is indeed an urgent need for a tool able to objectively assess this lung frailty and therefore provide early risk stratification in terms of barotrauma susceptibility amongst mechanically ventilated COVID-19 ARDS patients. In this setting, we recently suggested a radiological predictor of spontaneous PMD/PNX, the so-called Macklin effect [2], which is defined, on lung parenchyma windowed CT images, as a linear collection of air contiguous to the bronchovascular sheath [11]: 20 out of 21 patients with subsequent occurrence of spontaneous PMD/PNX were found to have Macklin effect on baseline computed tomography (CT) imaging, about ten days in advance of symptoms onset.
Accordingly, we designed a larger study with the aim to define, on a broader cohort of patients, the exact accuracy of Macklin effect in predicting subsequent occurrence of overt PMD/PNX in mechanically ventilated COVID-19 ARDS patients. Our secondary objective was to describe topographical distribution of the Macklin effect within the lungs and to assess eventual associations with temporal interval before PMD/PNX occurrence.
2. Materials and methods
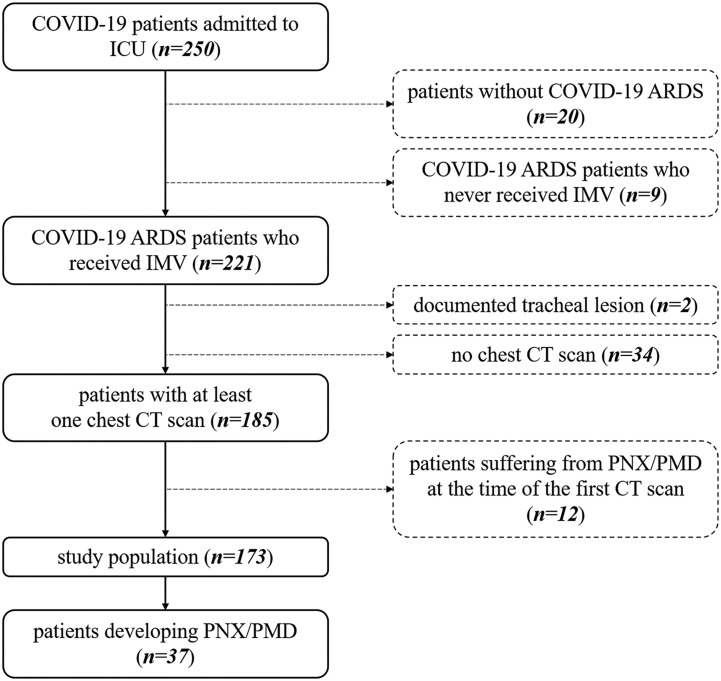
Under our Institutional Review Board-approved protocol (protocol number 34/int/2020; ClinicalTrials.gov number NCT04318366), we retrospectively identified all consecutive adult patients admitted to one of several intensive care units (ICUs) of San Raffaele Scientific Institute (Milan, Italy) for SARS-CoV-2 (Severe Acute Respiratory Syndrome Coronavirus 2) infection and ARDS requiring invasive mechanical ventilation between February 25, 2020, and December 31, 2020 (n = 221). Patients who underwent at least one chest CT scan during their ICU stay, irrespective of contrast medium administration, were finally enrolled; of note, those who had documented tracheal lesion (n = 2) or already had PMD/PNX at the time of the first available chest CT imaging (n = 12) were excluded (Fig. 1 ). In this cohort, occurrence of radiologically proven PMD/PNX was systematically recorded. Details on hospital organization and clinical management have been previously published [[12], [13], [14]].
Fig. 1.
Inclusion and exclusion criteria flowchart. COVID-19: coronavirus disease 2019; ARDS: Acute Respiratory Distress Syndrome; IMV: invasive mechanical ventilation; CT: Computed Tomography; PMD: pneumomediastinum; PNX: pneumothorax.
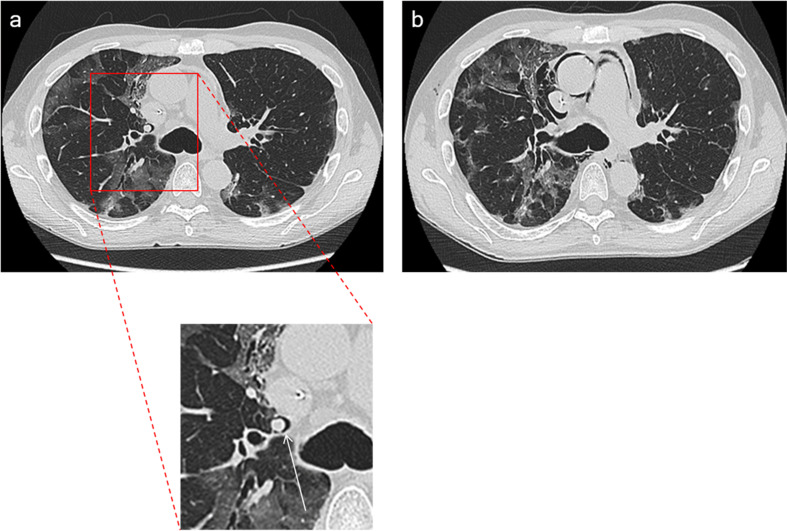
Chest imaging studies (x-ray, CT scan), as well as contrast medium administration, were performed at the discretion of the attending physicians, according to clinical needs. Two experienced radiologists (D.P., G.G.) independently reviewed all selected baseline CT images searching for Macklin effect (Fig. 2 ); they were blinded to patients' symptoms and eventual outcome. Macklin effect was analysed in terms of presence/absence and topographical distribution within the lungs (adjacent to peripheral [segmental/subsegmental] vs. central [lobar] bronchial branches).
Fig. 2.
Macklin effect in a mechanically ventilated COVID-19 patient. Lung parenchyma windowed CT images demonstrate [a] a crescent collection of air contiguous to the right upper lobar bronchovascular sheath (that is, Macklin effect – white arrow). Two days later [b], pneumomediastinum occurred.
The reliability of agreement between the two readers was assessed using Fleiss' kappa (Fleiss' kappa values higher than 0.80 were deemed to be almost perfect). Macklin effect accuracy in terms of prediction of spontaneous PMD/PNX was measured in terms of sensitivity, specificity, positive and negative likelihood ratios (positive LR and negative LR, respectively), positive and negative predictive values (PPV and NPV, respectively) (Supplementary Appendix). Receiver operating characteristics (ROC) curve analysis was also performed and the area under the curve (AUC) computed. Dichotomous data were compared using χ2 test; comparison of continuous variables between patients with peripheral and central distributed Macklin effect was evaluated using a Students' t-test. A p-value of less than 0.05 was considered statistically significant. Statistical analyses were performed with SPSS 25 (SPSS Inc./IBM, Armonk, NY).
3. Results
3.1. Patients' characteristics
250 ARDS COVID-19 patients required invasive mechanical ventilation over the time period. After exclusions, 173 patients (31 female [17.9%], 142 male [82.1%]; median age: 64.8 years [range, 41–79]) were included in our study. Overall, 37 patients (21.3%) suffered from spontaneous PMD/PNX during their ICU stay; specifically, 20 patients (11.5%) had spontaneous PMD, 10 spontaneous PNX (5.8%), whereas 7 (4%) experienced both. Fourteen patients (37.8%) required drainage tube placement, whereas the other 23 had conservative management.
3.2. Macklin effect – inter reader agreement
The inter reader agreement for Macklin effect detection was almost perfect (k value: 0.82) between the two radiologists.
3.3. Macklin effect – diagnostic accuracy and predictive value
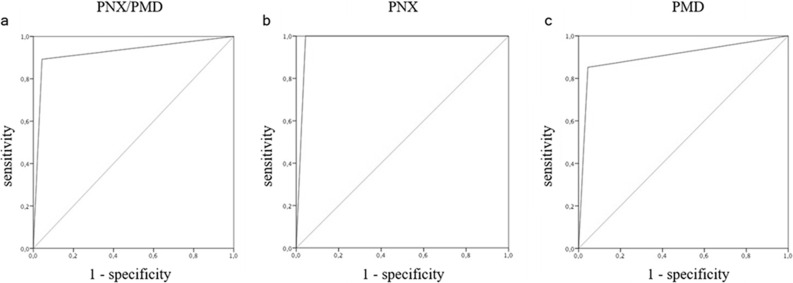
Thirty-three out of the 37 patients with subsequent occurrence of spontaneous PMD/PNX were found to have Macklin effect on baseline CT scan (89.2%, true positive patients), a median 8.5 days [range, 1–18] before the first radiological evidence of PMD/PNX. Conversely, 6 patients out of the 136 without PMD/PNX (4.4%, false positive patients) had the Macklin effect (p < 0.001). True and false negative rates were 95.6% (130/136) and 10.8% (4/37), respectively (Table 1 ). As a consequence, Macklin effect yielded a sensitivity of 89.2% (95% CI: 74.6–96.9), a specificity of 95.6% (95% CI: 90.6–98.4), a positive LR of 20.2 (95% CI: 9.2–44.5), a negative LR of 0.11 (95% CI: 0.04–0.29), a PPV of 84.5% (95% CI: 71.3–92.3), a NPV of 97.1% (95% CI: 74.6–96.9) and an overall accuracy of 94.2% (95% CI: 89.6–97.2) in predicting spontaneous PMD/PNX (AUC: 0.924, Fig. 3a).
Table 1.
Cross tabulations of Macklin effect and pneumomediastinum and/or pneumothorax. Data are number of patients.
| Macklin effect | Present | Absent | TOTAL |
|---|---|---|---|
| Pneumomediastinum and/or Pneumothorax | |||
| Present | 33 (true positives) | 6 (false positives) | 39 |
| Absent | 4 (false negatives) | 130 (true negatives) | 134 |
| TOTAL | 37 | 136 | 173 |
| Pneumothorax | |||
| Present | 17 (true positives) | 6 (false positives) | 23 |
| Absent | 0 (false negatives) | 130 (true negatives) | 130 |
| TOTAL | 17 | 136 | 153 |
| Pneumomediastinum | |||
| Present | 23 (true positives) | 6 (false positives) | 29 |
| Absent | 4 (false negatives) | 130 (true negatives) | 134 |
| TOTAL | 27 | 136 | 163 |
Fig. 3.
Macklin effect accuracy. ROC curve analysis showing overall accuracy of the Macklin effect in predicting pneumothorax and/or pneumomediastinum [a] (AUC: 0.924), pneumothorax alone [b] (AUC: 0.978) and pneumomediastinum alone [c] (AUC: 0.904).
Of note, the four false negative patients underwent baseline CT imaging significantly earlier when compared to true positive ones (23.2 ± 2.1 days before PMD/PNX occurrence vs. 7.5 days ±5.2, p < 0.001).
Sub cohort analysis considering separately PNX and PMD demonstrated different performances of the Macklin effect in predicting these events. Specifically, amongst the 17 patients with PNX (alone or in association with PMD), no false negatives were detected (sensitivity: 100% [95% CI: 80.5–100], specificity: 95.6% [95% CI: 90.6–98.4], positive LR: 22.7 [95%CI: 10.4–49.6], negative LR: 0, PPV: 71.1% [95% CI: 52.9–84.3], NPV: 100%, overall accuracy: 96% [95% CI: 91.6–98.5]) (AUC: 0.978, Fig. 3b) (Table 1). On the contrary, amongst the 27 patients with PMD (alone or in association with PNX), four false negatives were identified (sensitivity: 85.7 [95% CI: 67.3–95.9], specificity: 95.6% [95% CI: 90.6–98.4], positive LR: 19.3 (95% CI: 8.7–42.3), negative LR: 0.15 (95% CI: 0.06–0.38), PPV: 77% [95% CI: 60.1–88.1], NPV: 97.5 [95% CI: 94–98.9], overall accuracy: 94.1% [95% CI: 89.4–97.2]) (AUC: 0.904, Fig. 3c) (Table 1).
Finally, each patient requiring drainage tube placement was found to have Macklin effect on baseline CT imaging (14/14, 100%).
3.4. Macklin effect – topographical distribution
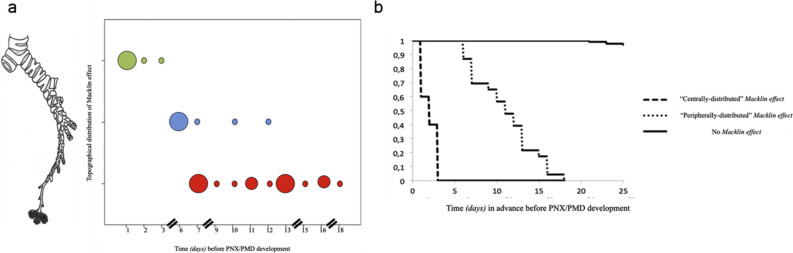
The exact topographical distribution within the lung of Macklin effect was found to be peripheral in the majority of patients (25/33 [75.7%]). Peripheral distribution of Macklin effect was moreover associated with significantly higher temporal interval to the first radiological evidence of PMD/PNX when compared to central distribution (9.6 days ±6.4 vs. 1.4 days ±0.7, p < 0.001). Furthermore, the smaller the bronchovascular sheath involved, the longer the temporal advance (p < 0.001, R = − 0.896) (Fig. 4 ).
Fig. 4.
Topographical distribution of Macklin effect and temporal interval before pneumothorax/pneumomediastinum occurrence.
[a] According to the bronchial order (lobar [green dots, upper line], segmental [blue dots, mid line] or subsegmental [red dots, lower line]) most adjacent to the Macklin effect observed, the smaller the bronchovascular sheath involved, the longer the temporal advance before pneumothorax/pneumomediastinum (p < 0.001, R = − 0.896). Each dot represents a patient with Macklin effect on baseline CT scan; larger dots means that multiple patients with Macklin effect share the same temporal advance before pneumothorax/pneumomediastinum development.
[b] Kaplan Meier curve demonstrating statistically significant difference between patients with “centrally-distributed” Macklin effect and those with “peripherally-distributed” Macklin effect in terms of temporal interval before pneumothorax/pneumomediastinum occurrence. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
4. Discussion
We found that mechanically ventilated COVID-19 ARDS patients with Macklin effect on baseline CT imaging are to be considered at very high risk to develop clinically overt PMD/PNX (OR = 54.3). We also provided precise assessment of the accuracy of the Macklin effect in predicting subsequent PMD/PNX development, showing very high sensitivity and almost perfect specificity. Furthermore, Macklin effect demonstrated slightly different accuracy in predicting, separately, PNX and PMD, with the former having greater sensitivity values (no false negative results). Finally, we found a relationship between topographical distribution of Macklin effect on chest CT imaging and temporal interval to the first radiological evidence of PMD/PNX: the smaller the bronchovascular sheath involved, the longer the temporal advance.
A possible explanation to these findings lies in the pathophysiology of Macklin effect [[15], [16], [17]]. This is a three-step process: whenever a significant pressure gradient exists between air spaces and adjacent bronchovascular sheath (both in terms of increased alveolar and/or decreased interstitial pressure), alveolar rupture may occur [first step], resulting in air dissection along the pulmonary interstitium [second step] and air spreading into the mediastinum [third step]. Once the gas is in the mediastinum, it can then track to the cervical soft tissues (subcutaneous emphysema) or into the abdomen (pneumoperitoneum). A broad alveolar collapse might significantly reduce pulmonary compliance, possibly resulting in lung parenchyma rupture (PNX), not necessarily at the level of the alveoli primarily affected by the pressure gradient issue [16]. It follows that PNX represents the macroscopic outcome of a severe Macklin effect, and this is consistent with our data since we have observed no false positive results in patients with later PNX development (100% sensitivity, 100% NPV): a higher number of alveoli involved causes a larger air leakage along the pulmonary interstitium, resulting in a better recognizable Macklin effect on CT images. This observation also provides a compelling explanation for another finding: when dealing with clinically significant (namely, requiring intervention) PMD/PNX, Macklin effect was always detected on baseline CT scan.
The topographical distribution (mainly peripheral) of Macklin effect observed in our population broadly differs from that previously reported in the literature [11,18,19]. In a cohort of 20 symptomatic patients with spontaneous (non COVID-19 related) PMD, for example, Okada and colleagues [19] pointed out a central distribution of Macklin effect in all patients. A possible reason for such a substantial discrepancy could lie in the different, quite novel design of the present study as compared with previous works, rather than in the different etiologies considered (COVID-19 vs. non COVID-19). To the best of our knowledge, this is indeed the first study to systematically explore a possible prediction role for Macklin effect, looking for this sign before the actual occurrence of clinically overt PMD/PNX. Conversely, the existing, mainly radiological literature [11,[18], [19], [20]] suggests Macklin effect as a diagnostic tool only (when PMD/PNX already occurred, with symptoms development), able to allow proper, non-invasive differentiation between respiratory PMD and other causes such as tracheobronchial/oesophageal injury. In this setting, pulmonary interstitial emphysema already dissected towards pulmonary hila, justifying a predominantly central Macklin effect. Timing of CT imaging was the main issue: the longer the time after the onset of Macklin effect, the less frequently it was detected peripherally; and this is consistent with our data, showing a relationship between topographical distribution of Macklin effect and temporal advance before the first radiological evidence of PMD/PNX. This assumption also provide a possible explanation for the four false negative patients we observed, since they all had baseline CT imaging three weeks before PMD/PNX occurrence, possibly before Macklin effect development. Taken together, these findings corroborate our novel hypothesis of Macklin effect as a sort of “radiological countdown” for PMD/PNX development, capturing air leakage centripetal movement along the pulmonary interstitium.
We believe that our findings may have potentially relevant research and clinical application. We recently completed a systematic literature review that confirmed the high mortality (greater than 60%) associated with development of barotrauma in COVID-19 ARDS patients [21]. In this context, early detection of Macklin effect could help to identify patients at high risk for overt/clinically relevant barotrauma. It is possible that this complication may be avoided by applying different ventilation strategies as compared with standard practice, for example by further reducing tidal volumes/airways pressures [22], and considering early application of extracorporeal techniques if maintaining safer ventilatory parameters does not allow adequate gas exchange [23]. Use of extracorporeal membrane oxygenation (ECMO) without intubation has already been described in case reports, and may be a potentially interesting approach [[23], [24], [25]]. However, further studies are required to confirm the effectiveness of ultraprotective ventilation or ECMO in preventing overt barotrauma development in Macklin-positive patients, and that prevention of barotrauma actually translates in improved outcome.
Of note, our findings may also be applied in non-COVID-19 ARDS, although this also requires further confirmation studies.
The present study has several limitations, the most important ones being the relatively small sample size, and the lack of standardization regarding the timing of radiological examinations; external validation is also warranted. Moreover, one may argue that Macklin effect accounts for local peribronchiolar air leakage itself and that, once it is detected, subsequent development of clinically overt PMD/PNX is unavoidable. On that note, it is worth emphasizing that Macklin effect cannot be regarded as a full predictor of each pulmonary air leakage, but rather, in strict sense, as an early detector of lung frailty (following for example COVID-19 related diffuse alveolar damage). Accordingly, Macklin effect can be instead regarded as a predictor of clinically threatening occurrences like PMD/PNX since it allows early risk stratification in terms of barotrauma susceptibility amongst mechanically ventilated COVID-19 ARDS patients and, therefore, prompt deployment of ultraprotective ventilation strategies and ECMO. In doing so, the centripetal movement of any air leakage could be possibly “frozen” until full reabsorption, thus avoiding the occurrence of extensive lung damage (clinically overt PMD/PNX). In these terms, identification of Macklin effect may have a substantial clinical significance.
5. Conclusions
In conclusion, Macklin effect is a reproducible, very accurate radiological sign able to predict clinically overt PMD/PNX development in mechanically ventilated COVID-19 ARDS patients with almost perfect specificity and very good sensitivity: patients with Macklin effect on baseline CT imaging are to be regarded extremely vulnerable to subsequent PMD/PNX occurrence. Further studies are needed to confirm these results in other (non COVID-19) clinical settings.
Funding
None.
Author statement
Dr. Palumbo, prof. De Cobelli and prof. Landoni had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Conceptualization and methodology design: Palumbo, Landoni, Belletti, Guazzarotti, Zangrillo, De Cobelli. Acquisition, analysis, or interpretation of data: Palumbo, Landoni, Belletti, Guazzarotti, Pennella, Gritti, Marmiere. Drafting of the manuscript: Palumbo, Landoni, Belletti. Critical revision of the manuscript for important intellectual content: All authors. Statistical analysis: Palumbo. Supervision: Landoni, De Cobelli.
Declaration of Competing Interest
None.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jcrc.2021.07.022.
Contributor Information
for the COVID-BioB Study Group:
Carolina Faustini, Nicolò Maimeri, Rosalba Lembo, Giuseppe Di Lucca, Raffaella Scotti, Maria Vittoria Lavorato, Alessandro Tomellieri, Corrado Campochiaro, Fatemeh Darvizeh, Francesca Calabrese, Roberto Mapelli, Nicola Pasculli, Giovanni Borghi, Antonella Cipriani, Maria Grazia Calabrò, Martina Crivellari, Annalisa Franco, Marina Pieri, Evgeny V. Fominskiy, Stefano Franchini, Antonio Dell'Acqua, Alessandro Marinosci, Giordano Vitali, and Nicola Compagnone
Appendix A. Supplementary data
Supplementary material
References
- 1.Lemmers D.H.L., Abu Hilal M., Bna C., et al. Pneumomediastinum and subcutaneous emphysema in COVID-19: barotrauma or lung frailty? ERJ Open Res. 2020;6(4) doi: 10.1183/23120541.00385-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Belletti A., Palumbo D., Zangrillo A., et al. COVID-BioB Study Group Predictors of pneumothorax/pneumomediastinum in mechanically ventilated COVID-19 patients. J Cardiothorac Vasc Anesth. 2021 doi: 10.1053/j.jvca.2021.02.008. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McGuinness G., Zhan C., Rosenberg N., et al. Increased incidence of barotrauma in patients with COVID-19 on invasive mechanical ventilation. Radiology. 2020;297(2):E252–E262. doi: 10.1148/radiol.2020202352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Palumbo D., Belletti A., Campochiaro C., et al. COVID-BioB Study Group Pneumothorax/pneumomediastinum in non-intubated COVID-19 patients: differences between first and second Italian pandemic wave. Eur J Intern Med. 2021;88:144–146. doi: 10.1016/j.ejim.2021.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Martinelli A.W., Ingle T., Newman J., et al. COVID-19 and pneumothorax: a multicentre retrospective case series. Eur Respir J. 2020;56(5) doi: 10.1183/13993003.02697-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Manna S., Maron S.Z., Cedillo M.A., et al. Spontaneous subcutaneous emphysema and pneumomediastinum in non-intubated patients with COVID-19. Clin Imaging. 2020;67:207–213. doi: 10.1016/j.clinimag.2020.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kahn M.R., Watson R.L., Thetford J.T., Wong J.I., Kamangar N. High incidence of Barotrauma in patients with severe coronavirus disease 2019. J Intensive Care Med. 2021;36(6):646–654. doi: 10.1177/0885066621989959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Al-Azzawi M., Douedi S., Alshami A., Al-Saoudi G., Mikhail J. Spontaneous subcutaneous emphysema and pneumomediastinum in COVID-19 patients: An indicator of poor prognosis? Am J Case Rep. 2020;21 doi: 10.12659/AJCR.925557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.De Cobelli F., Palumbo D., Ciceri F., et al. Pulmonary Vascular Thrombosis in COVID-19 Pneumonia. J Cardiothorac Vasc Anesth. 2021 doi: 10.1053/j.jvca.2021.01.011. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ciceri F., Beretta L., Scandroglio A.M., et al. Microvascular COVID-19 lung vessels obstructive thromboinflammatory syndrome (MicroCLOTS): an atypical acute respiratory distress syndrome working hypothesis. Crit Care Resusc. 2020;22(2):95–97. doi: 10.51893/2020.2.pov2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Murayama S., Gibo S. Spontaneous pneumomediastinum and Macklin effect: overview and appearance on computed tomography. World J Radiol. 2014;6(11):850–854. doi: 10.4329/wjr.v6.i11.850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zangrillo A., Beretta L., Scandroglio A.M., et al. COVID-BioB Study Group Characteristics, treatment, outcomes and cause of death of invasively ventilated patients with COVID-19 ARDS in Milan, Italy. Crit Care Resusc. 2020;22(3):200–211. doi: 10.1016/S1441-2772(23)00387-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zangrillo A., Beretta L., Silvani P., et al. Fast reshaping of intensive care unit facilities in a large metropolitan hospital in Milan, Italy: facing the COVID-19 pandemic emergency. Crit Care Resusc. 2020;22(2):91–94. doi: 10.51893/2020.2.pov1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rovere-Querini P., Tresoldi C., Conte C., et al. COVID-BioB Study Group Biobanking for COVID-19 research. Panminerva Med. 2020 doi: 10.23736/S0031-0808.20.04168-3. In press. [DOI] [PubMed] [Google Scholar]
- 15.Macklin C.C. Transport of air along sheaths of pulmonic blood vessels from alveoli to mediastinum: clinical implications. Arch Intern Med. 1939;64(5):913–926. doi: 10.1001/archinte.1939.00190050019003. [DOI] [Google Scholar]
- 16.Romero K.J., Trujillo M.H. Spontaneous pneumomediastinum and subcutaneous emphysema in asthma exacerbation: the Macklin effect. Heart Lung. 2010;39(5):444–447. doi: 10.1016/j.hrtlng.2009.10.001. [DOI] [PubMed] [Google Scholar]
- 17.Wintermark M., Schnyder P. The Macklin effect: a frequent etiology for pneumomediastinum in severe blunt chest trauma. Chest. 2001;120(2):543–547. doi: 10.1378/chest.120.2.543. [DOI] [PubMed] [Google Scholar]
- 18.Sakai M., Murayama S., Gibo M., Akamine T., Nagata O. Frequent cause of the Macklin effect in spontaneous pneumomediastinum: demonstration by multidetector-row computed tomography. J Comput Assist Tomogr. 2006;30(1):92–94. doi: 10.1097/01.rct.0000187416.07698.8d. [DOI] [PubMed] [Google Scholar]
- 19.Okada M., Adachi H., Shibuya Y., Ishikawa S., Hamabe Y. Diagnosis and treatment of patients with spontaneous pneumomediastinum. Respir Investig. 2014;52(1):36–40. doi: 10.1016/j.resinv.2013.06.001. [DOI] [PubMed] [Google Scholar]
- 20.Russell D.W., Watts J.R., Powers T.A. Searching for the source of the leak: PIE and the Macklin effect. Ann Am Thorac Soc. 2018;15(11):1354–1356. doi: 10.1513/AnnalsATS.201803-200CC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Belletti A., Todaro G., Valsecchi G., Losiggio R., Palumbo D., Landoni G., et al. Barotrauma in COVID-19 patients undergoing invasive mechanical ventilation: a systematic literature review. Crit Care Med. 2021 doi: 10.1097/CCM.0000000000005283. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rozencwajg S., Guihot A., Franchineau G., Lescroat M., Bréchot N., Hékimian G., et al. Ultra-protective ventilation reduces biotrauma in patients on Venovenous extracorporeal membrane oxygenation for severe acute respiratory distress syndrome. Crit Care Med. 2019;47(11):1505–1512. doi: 10.1097/CCM.0000000000003894. [DOI] [PubMed] [Google Scholar]
- 23.Schmidt M., Pineton de Chambrun M., Lebreton G., Hékimian G., Chommeloux J., Bréchot N., et al. Extracorporeal membrane oxygenation instead of invasive mechanical ventilation in a patient with severe COVID-19-associated acute respiratory distress syndrome. Am J Respir Crit Care Med. 2021;203(12):1571–1573. doi: 10.1164/rccm.202102-0259le. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Loyalka P., Cheema F.H., Rao H., Rame J.E., Rajagopal K. Early usage of extracorporeal membrane oxygenation in the absence of invasive mechanical ventilation to treat COVID-19-related hypoxemic respiratory failure. ASAIO J. 2021;67(4):392–394. doi: 10.1097/MAT.0000000000001393. [DOI] [PubMed] [Google Scholar]
- 25.Azzam M.H., Mufti H.N., Bahaudden H., Ragab A.Z., Othman M.M., Tashkandi W.A. Awake extracorporeal membrane oxygenation in coronavirus disease 2019 patients without invasive mechanical ventilation. Crit Care Explor. 2021;3(7) doi: 10.1097/CCE.0000000000000454. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material