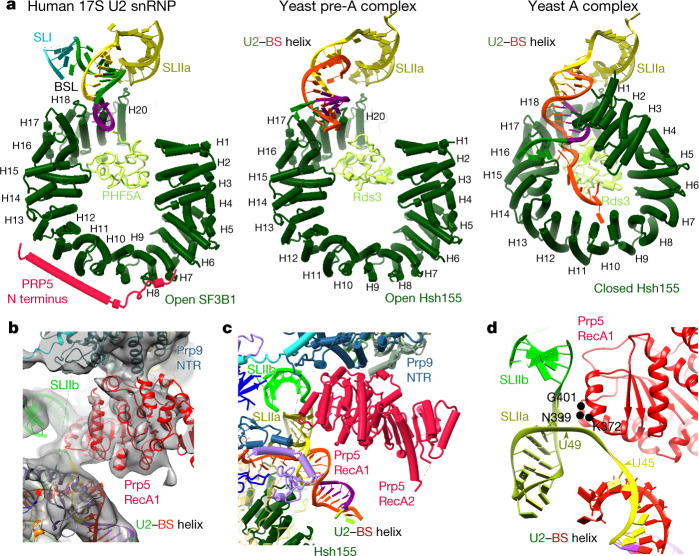
Fig. 2. The Hsh155 HEAT domain has an open conformation in the pre-A complex.
a, Conformation of the SF3B1 and Hsh155 HEAT domains and position of the U2–BS helix and U2 snRNA SLI and SLIIa in human 17S U2 snRNP (Protein DataBank (PDB) (https://www.rcsb.org) accession number 6Y5Q) and in the S. cerevisiae pre-A and A complexes (PDB 6G90). These domains were aligned via Hsh155 heat repeats 19–20, Rse1BPA and U2 SLIIa. Olive green, SLIIa nucleotides; reddish orange, pre-mRNA branch-site nucleotides; purple, BSL nucleotides that later form the U2–BS helix; yellow, BSL nucleotides forming the extended part of the U2–BS helix; dark green, remaining BSL nucleotides; blue, SLI. b, Fit of Prp5RecA1 into the pre-A EM density. c, Location of the Prp5 RecA1 and RecA2 domains in the pre-A complex. d, Prp5RecA1 contacts U2 snRNA nucleotides that connect the U2–BS helix to U2 SLIIa. The positions of Prp5 amino acids (located outside of the SAT motif) that when mutated suppress branch-site mutations are indicated in black.