Abstract
Biomarkers that predict symptom trajectories after trauma can facilitate early detection or intervention for posttraumatic stress disorder (PTSD) and may also advance our understanding of its biology. Here, we aimed to identify trajectory-based biomarkers using blood transcriptomes collected in the immediate aftermath of trauma exposure. Participants were recruited from an Emergency Department in the immediate aftermath of trauma exposure and assessed for PTSD symptoms at baseline, 1, 3, 6, and 12 months. Three empirical symptom trajectories (chronic-PTSD, remitting, and resilient) were identified in 377 individuals based on longitudinal symptoms across four data points (1, 3, 6, and 12 months), using latent growth mixture modeling. Blood transcriptomes were examined for association with longitudinal symptom trajectories, followed by expression quantitative trait locus analysis. GRIN3B and AMOTL1 blood mRNA levels were associated with chronic vs. resilient post-trauma symptom trajectories at a transcriptome-wide significant level (N = 153, FDR-corrected p value = 0.0063 and 0.0253, respectively). We identified four genetic variants that regulate mRNA blood expression levels of GRIN3B. Among these, GRIN3B rs10401454 was associated with PTSD in an independent dataset (N = 3521, p = 0.04). Examination of the BrainCloud and GTEx databases revealed that rs10401454 was associated with brain mRNA expression levels of GRIN3B. While further replication and validation studies are needed, our data suggest that GRIN3B, a glutamate ionotropic receptor NMDA type subunit-3B, may be involved in the manifestation of PTSD. In addition, the blood mRNA level of GRIN3B may be a promising early biomarker for the PTSD manifestation and development.
Subject terms: Gene expression, Risk factors
Introduction
Posttraumatic stress disorder (PTSD) is a severe and impairing psychiatric illness occurring in susceptible individuals exposed to traumatic life experiences. PTSD is characterized by fear, avoidance, hyperarousal, hypervigilance, and reliving the trauma through intrusive memories, nightmares, and flashbacks. Early interventions have been shown to significantly reduce the prevalence of PTSD [1–3]. However, these interventions could require significant system resources, which are not always available, and might be unnecessary for resilient individuals whose symptoms may fade over time on their own. Hence, early risk biomarkers that can predict the manifestation of PTSD may facilitate both early interventions and targeted treatments, increasing positive outcomes for patients known to be at risk.
Many individuals exhibit early symptoms after experiencing a traumatic life experience, with varying degrees of severity and persistence. Only a minority of those remain highly symptomatic and manifest the full symptoms of PTSD [4]. A combination of genetic and environmental factors influence susceptibility to develop PTSD after trauma exposure. Latent class analysis has successfully identified the dissociative subtype of PTSD [5], uncovered qualitative differences between the classes, and categorized homogeneous classes of individuals based on broad characteristics not directly measurable. Moreover, longitudinal studies of psychological response to trauma have identified a range of outcomes over time that are not adequately captured by PTSD diagnostic criteria [6]. Thus, clusters of these symptoms over time, which can be grouped into empirical trajectories, can better reflect different outcomes (e.g., chronic-PTSD vs. resilient individuals). These symptom trajectories represent different biological dimensions and have a distinct genetic basis, facilitating the identification of biomarkers and risk genes [7]. Thus, investigating symptom trajectories leading to chronic-PTSD or resilience may play a central role in understanding this disorder. The genetic biomarkers associated with these trajectories may predict who among trauma-exposed individuals may develop PTSD [8, 9].
In human populations, blood transcriptomic profiling, which requires smaller sample sizes than traditional genome-wide association studies (GWAS), has been successfully used as a discovery tool for genes associated with PTSD [10–12]. So far, only a few blood transcriptome-wide association studies (TWAS) associated with PTSD core symptom trajectories have been reported [13]. This study design may facilitate identifying genes involved in the course of PTSD and biomarkers for early interventions.
Of note, the primary tissue of interest for the pathophysiology of PTSD is the brain, with traumatic experiences activating specific regions, including the amygdala [14, 15], hippocampus, and the prefrontal cortex [16–19]. The degree of correlation between blood and brain gene expression is difficult to establish [20]. Still, the increasing number of accessible sources, such as brain biobanks and transcriptome databases (e.g., GTEx and BrainCloud), has facilitated the understanding of these body–brain correlations.
The current prospective Emergency Department (ED) study recruited participants in the aftermath of trauma exposure and followed them longitudinally over 1 year. In this study, we aimed to investigate early biomarkers that could predict the course of trauma-related symptoms, using blood transcriptomes. Symptom trajectories were extrapolated using posttraumatic symptoms assessed at 1, 3, 6, and 12 months after trauma exposure [21–23]; such patterns provide more information than dichotomous PTSD case–control or individual PTSD symptom scale (PSS) analyses in the identification of genetic factors [24, 25]. Based on this approach, three distinct trajectories (chronic-PTSD, remitting, and resilient) were identified, as previously described in the papers above [21–23].
In this study, we aimed to investigate possible genes that could predict the course of trauma-related symptoms by performing a transcriptomic analysis of these empirical trajectories. Furthermore, the genes identified using this method were further investigated using several tools, including analyses of methylation loci and genetic variants. We used public databases to evaluate the direct association between genetic variants and brain gene expression. We then explored the association between these DNA variants and PTSD, using a larger independent cohort of similar demographics (the Grady Trauma Project).
Materials and methods
Participants
Participants were patients in the ED of Grady Memorial Hospital in Atlanta (GA) who had experienced a traumatic event within the past 24 h. Participants were included if they spoke English, were 18–65 years of age, endorsed a criterion A trauma as defined by the DSM-IV-TR [26], and provided contact information for follow-up visits. Exclusion criteria included previous hospitalization for mental health reasons, current suicidal ideation, attempted suicide in the past 3 months, current intoxication, or altered mental status during the ED visit. Informed consent was obtained from all research subjects, and all procedures were approved by the Institutional Review Board of Emory University School of Medicine and the Grady Health Systems Research Oversight Committee.
Emergency Department assessments at the time of trauma exposure
Demographic information and information about the index trauma were gathered in the ED using the Standardized Trauma Interview [27]. PTSD symptoms were assessed at baseline, 1, 3, 6, and 12 months following the ED visit. PTSD symptom severity (PSS) was measured using the 17-item self-report scale [21–23, 28, 29].
Drug and alcohol use were assessed using a ten-item version of the Drug Abuse Screening Test (DAST) [30, 31] and the Alcohol Use Disorders Identification Test (AUDIT) [30, 32], respectively. Depressive symptoms were assessed using the Beck Depression Inventory BDI [33].
Symptom trajectory
Latent growth mixture modeling (LGMM) was employed to identify PTSD symptom trajectories based on PSS total scores across 1, 3, 6, and 12 months using MPlus 7.2 [34], as previously described [21–23]. Subjects were excluded if they were missing two or more assessments, resulting in a sample size of 377. To identify the best fitting number of trajectory classes that best describe the PTSD symptom severity, we applied the LGMM, starting incrementally from one to six classes. We examined both a linear and quadratic slope to identify the best fitting trajectory shape. We used a nested model approach, testing a progressive number of classes until the model fit indices no longer favor the addition of any more classes. To determine the number of classes, we used a confluence of evidence across conventional indices, including reductions in the Bayesian Information Criterion (BIC), sample-size adjusted Bayesian Information Criterion (SSBIC), Aikaike Information Criterion (AIC) indices, and significance indicated by the Lo-Mendell-Rubin likelihood ratio test (LRT), the Vuong-Lo-Mendell-Rubin likelihood ratio test (VLRT), and the Bootstrap likelihood ratio test, along with parsimony and interpretability equally weighed consistent with recommendations from the literature[35]. We found that the best fitting LGMM model was a three-class solution (resilient, remitting, and chronic-PTSD) with a linear slope (AIC = 8,111.160, BIC = 8,158.347, SSBIC = 8,120.274, VLRT = 0.0036, LRT = 0.0046, and entropy of 83%) [23, 36].
RNA collection and RNA sequencing
Venous blood was collected in Tempus Tubes (RNA, Applied Biosystems) in the ED in the immediate aftermath of trauma exposure. After RNA extraction, mRNA libraries were created using the TrueSeq preparation kit and sequenced on the Illumina HiSeq 2000 by the Translational Genomics Research Institute (TGEN). Reads were aligned to GRCh37 using STAR v2.5.2b [37]. Gene counts were computed with FeatureCounts v1.5.1 [38], and then the counts were processed with DESeq2 [39]. The Limma-Voom R-package (version 3.6.2) [40, 41] was used for normalization, converting raw counts to log-CPM (count per million), adjusting for library sizes, and reducing heteroscedasticity. We kept genes with CPM values of 1 in at least a third of the samples (13,951 genes for the primary analysis).
Construction of weighted co-expression modules from RNA-sequencing data (WGCA)
Module eigengenes were used for summarizing clusters of highly correlated genes using weighted correlation network analyses (WGCNA) [42]. Briefly, raw RNA-sequencing data were first filtered keeping only genes with >50 reads across the samples (26,193 genes); then, the data were normalized and transformed using DESeq2 R-package [39]. Two outliers were removed by using hclust function. Clustering modules were defined with Blockwisemodules using the following parameters: soft-thresholding power 5 (chosen using pickSoftThreshold), correlation type (corType “bicor”), the network type (networkType “signed”), the type of Topological Overlap Matrix (TOMType “signed”), a relatively large minimum module size (minModuleSize 30), p value ratio threshold for reassigning proteins between modules (reassignThreshold 0.05), a low propensity to merge modules (mergeCutHeight 0.07), and high sensitivity to cluster splitting (deepSplit = 4). Among 26,193 genes 15,382 were assigned to 38 modules.
Cell type proportion determination
White blood cell composition was assessed to investigate whether cell type distribution was a potential confound for gene expression measures. The frequencies of PBMC cell types were estimated based on genome-wide gene expression using CIBERSORT cell type deconvolution method at the default setting [43]. Normalized expression data and the LM22 signature matrix were used as input, providing the frequencies of 22 immune cell types. Monocytes, T cell, and neutrophils were used as covariates in the analyses.
DNA genotyping
Extracted DNA from whole blood was genotyped by TGEN using the Illumina Infinium PsychArray BeadChip (v.1.0, v.1.1., and v.1.2) in three different batches. Standard quality control (QC) of the genome-wide genotyping was performed per batch with PLINK1.9 [44]: individuals with >5% missing data, and one in each pair of related individuals (cousins or a closer relation) based on identity by descent proportion were removed. Single-nucleotide polymorphisms (SNPs) with call rates <95%, minor allele frequency < 0.05, and deviation from Hardy-Weinberg proportions (p < 1 × 10−6 in controls and p < 1 × 10−10 in PTSD) were also removed. The three batches were merged, and further QC was performed. To determine population ancestry, we calculate genomic principal components (PCs) from autosomal independent genomic markers (pairwise r2 < 0.25) to infer axes of ancestry and to remove outliers. One GRIN3B variant (rs10666583), associated with schizophrenia in previous studies [45, 46], was imputed with BC-Gene (https://bcgene.emory.edu/), using IMPUTE2 (version 2.3.0) and 1000 Genomes Phase I (December 2013, NCBI build 37) for haplotype reference.
DNA methylation
DNA was extracted from ED-collected blood by AKESOgen (http://www.akesogen.com). Genomic DNA was bisulfite converted using EZ-96 DNA Methylation Kit (Zymo Research), and DNA methylation (DNAm) levels were interrogated either with HumanMethylation450 BeadChip (>480,000 CpG sites, N = 120) or MethylationEPIC BeadChip (>850,000 CpG sites, N = 36). Hybridization and processing were performed according to manufacturer’s instructions, followed by beta generation (Bead Studio) and standard QC (detection call rates <95% and an average intensity value of either <50% of the experiment-wide sample mean or <2000 arbitrary units; CpG sites with missing data for >10% of samples were excluded from analysis). Normalization was conducted using Beta Mixture Quantile Normalization [47], and ComBat was used to account for batch and positional effects [48].
Validation sample
The Grady Trauma Project is a comprehensive study of a predominantly African ancestry (AA) population of low socioeconomic status, exposed to stressful life events [49]. Similar to the ED, current PTSD symptoms were assessed using the PSS, and subjects were considered chronic-PTSD if they had a PSS score of 19 or above (third quartile cutoff) and resilient if they had a PSS of 7 or below.
DNA extraction and genome-wide genotyping using the Omni-Quad 1 M BeadChip has been described elsewhere [50]. QC was performed by using the Psychiatric Genomics Consortium PTSD Workgroup guidelines [51].
Statistical analyses
A transcriptome-wide association study (TWAS) of chronic-PTSD vs. resilient class was performed by fitting a linear model from each gene given a series of arrays using a standard function (lmfit) from the Limma R-package [40], followed by empirical Bayes moderation [52], to rank genes in order of evidence for differential expression. We included in the matrix the two-class trajectories, sex, age, baseline PTSD symptoms, cell type proportions, three ancestry PCs, DNA batch, and RNA-sequencing batch; we also added education, DAST or AUDIT score in the secondary analyses. Genes were considered associated with trajectory if they reached an FDR < 0.05. Association between GRIN3b blood mRNA level and PSS at baseline and at each subsequent follow-up assessment was examined using linear regression, adjusting for potential confounding factors.
To establish if GRIN3B expression levels were associated with the course of the disease, we repeated the analysis for the three-class trajectories (chronic-PTSD vs. remitting vs. resilient).
We performed a genetic association between PTSD and GRIN3B genetic variants using logistic regression in PLINK1.9 [44], assuming an additive model of association. We applied a Bonferroni correction to define statistical significance (0.05/4 variants = 0.01). To examine association between co-expression modules and the chronic vs. resilient trajectory, we performed linear regression using each eigengene as the outcome, the class trajectory as predictor, adjusting for the same covariates as mentioned above.
Methylation quantitative trait loci
Twenty GRIN3B-CpGs were selected as being present in both EPIC and 450 K. GRIN3B expression values were regressed for beta values, covarying for age, sex, three ancestry PCs, cell types, and array type. A Bonferroni corrected p value of 0.0025 (0.05/20) was used to define significance.
Expression quantitative trait loci (eQTL)
eQTL analyses were performed in R (version 3.5.2) using the package MatrixEQTL [53]. Expression levels of GRIN3B were regressed with the 23 variants in the gene, spanning from 974 to 1040 Kb, (including promotors, 5′ and 3′ region), assuming an additive model, and adjusting for sex, age, and the top three ancestry PCs. Variants with FDR < 0.05 were deemed eQTLs.
Brain expression eQTLs
To evaluate if the top GRIN3B blood eQTL variant could affect mRNA brain expression, we interrogated two publicly available databases: GTEx (https://gtexportal.org) [54] and BrainCloud (http://eqtl.brainseq.org/phase1/eqtl/) [55]. The BrainCloud used the expression values from dorsolateral prefrontal cortices (DLPFC) to identify eQTLs in a total of 412 subjects (N = 175 patients with schizophrenia, and N = 237 unaffected controls) or in DLPF-controls (N = 237).
Results
Emergency Department study participants
In the ED cohort, a total of 377 subjects with reported post-trauma symptoms were used for inferring symptom trajectory. Participants were mostly from AA (75.5%), while more than half had some college or higher education (59.3%); the most common trauma was road accidents (70.3%), which include motor vehicle crashes and bikes or pedestrian accidents; the majority of the participants showed low to moderate baseline PTSD symptoms (PSS-median score of 6; Table S1).
Three distinct symptom trajectories emerged in the 12 months post-trauma
In the ED cohort, longitudinal trajectories of symptoms after exposure to traumatic events were identified based on the PSS score across 1, 3, 6, and 12 months [21–23, 36]. LGMM yielded three trajectories: resilient (56.2%), remitting (32.9%), and chronic-PTSD (10.9%; Fig. S1). Individuals were assigned to one of these trajectories based on their most likely class membership. The trajectory algorithm was externally validated and demonstrated ability to discriminate PTSD risk with high precision [23].
Baseline PSS was excluded from the growth curve trajectory. Overall, individuals in the chronic-PTSD class tended to have lower educational attainment, a higher percentage of sexual-assault history, and a higher representation of females (61%, Table 1) compared to the other two categories. PSS symptoms of the three-class trajectories partially overlap before the 6-month marker (Fig. S1). When considering only chronic-PTSD vs. resilient, a clear separation of symptoms was already evident after one month. Hence, we decided to perform the TWAS analyses using the two extreme phenotypes (chronic-PTSD vs. resilient).
Table 1.
Sociodemographic characteristics of the subset ED cohort and their prospective trajectory classes adopted for transcriptome analyses.
| Total | Chronic | Remitting | Resilience | |
|---|---|---|---|---|
| Number of subjects (%) | 224 | 23 | 71 | 130 |
| Sex (% females) | 50.9% | 60.9% | 53.5% | 47.7% |
| Age (SD) | 35.8 (12.5) | 34.6 (11.2) | 36.0 (13.2) | 35.9 (12.4) |
| Race | ||||
| African ancestry | 71.4% | 73.9% | 77.5% | 67.7% |
| European ancestry | 19.6% | 13.0% | 16.9% | 22.3% |
| Other | 8.9% | 13.0% | 5.6% | 10.0% |
| Trauma | ||||
| No sexual assault | 6.7% | 0.0% | 11.3% | 5.4% |
| Sexual assault | 6.3% | 17.4% | 7.0% | 3.8% |
| Road accidentsa | 73.2% | 69.6% | 70.4% | 75.4% |
| Gun accidents | 1.8% | 4.3% | 1.4% | 1.5% |
| Stabbing | 1.8% | 0.0% | 4.2% | 0.8% |
| Animal bites | 1.3% | 4.3% | 1.4% | 0.8% |
| Other | 8.9% | 4.3% | 4.2% | 12.3% |
| Education | ||||
| Some HS or less | 14.3% | 30.4% | 15.5% | 10.8% |
| HS graduate | 27.2% | 34.8% | 26.8% | 26.2% |
| AA/some college | 39.3% | 30.4% | 39.4% | 40.8% |
| BS/BA | 15.2% | 4.3% | 14.1% | 17.7% |
| Master/Ph.D. | 4.0% | 0.0% | 4.2% | 4.6% |
| Baseline PTSD symptoms (SD) | 9.3 (9.9) | 19.7 (12.7) | 12.1 (9.14) | 5.92 (7.7) |
There were no significant differences in gender, age, or class trajectory representation between this subgroup and the entire ED cohort.
aRoad accidents includes motor vehicles crashes, and pedestrians and bike accidents.
Transcriptome-wide association study of symptom trajectory suggests that low blood GRIN3B mRNA expression after trauma exposure predicted resilience
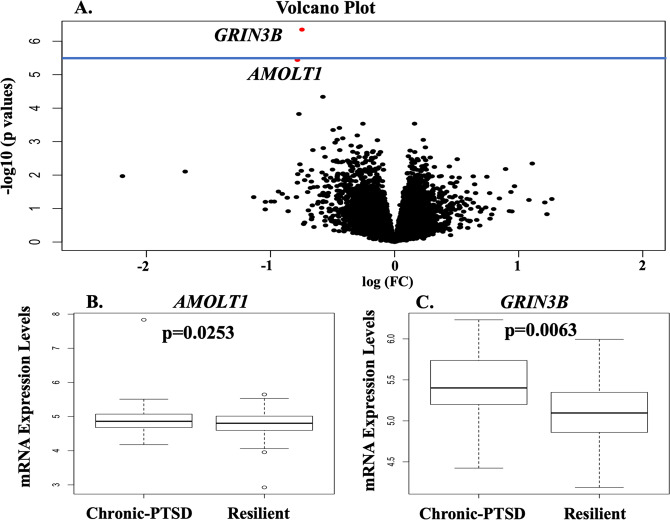
To investigate blood mRNAs that could predict resilient vs. chronic symptom trajectories, we performed a transcriptome-wide differential analysis of the resilient vs. chronic-PTSD group, adjusting for potential confounding variables. Our analysis identified two genes, GRIN3B (p = 4.50 × 10−7, FDR = 6.3 × 10−3) and AMOTL1 (p = 3.63 × 10−6, FDR = 0.0253) whose low expression after trauma predicted the resilient trajectory (Fig. 1, and Tables S2 and S3). To note, GRIN3B also survived a stricter Bonferroni correction adjustment (Fig. 1A). GRIN3B, but no AMOLT1, remained significant after adjusting for drug use (N = 130; DAST score FDR = 6.4 × 10−4), alcohol (N = 126; AUDIT score FDR = 2.0 × 10−4), or education (N = 153, FDR = 0.017). Depression and PTSD are often comorbid; in our datasets, they were high correlated at baseline (n = 147, correlation = 0.6; p value = 1.7 × 10−15); consequently, we did not include depression in our model. A closer examination of AMOLT1 mRNA levels in the chronic and resilience trajectories revealed a small difference in the median mRNA level (Fig. 1B). We found that the median mRNA level of GRIN3B was significantly higher in the chronic compared to the resilience trajectory (Fig. 1C).
Fig. 1. Blood mRNA expressions after trauma predict resilience.
A Volcano plot showing changes in gene expressions in resilient vs. chronic trajectories. The horizontal axis of the volcano plot is effect size (log2 foldchange) for the differentially expressed genes. The vertical axis is the negative of log10 of the p values. Each dot represents a gene, with red dots showing genes reaching an FDR-corrected p value of 0.05, and with black dots representing genes with similar expression levels in resilience and chronic clusters. The blue horizontal blue line indicates a strict Bonferroni correction p values threshold [p = 3.58 × 10−6 (0.05/13,951 tests). B, C Box and whiskers plots of blood mRNA expressions for B AMOTL1 and C GRIN3B in resilient vs. chronic-PTSD trajectory. Y-axis show gene expression values. The box represents their quartiles; whiskers indicate the variability outside the upper and lower quartiles; the horizontal line represents the median.
We then evaluated the difference in GRIN3B and AMOLT1 expression levels in the three trajectories. We observed a gradual decrease of GRIN3B mRNA expression (p = 1.04 × 10−5; t = −4.5) from chronic to remitting to resilient categories (Fig. S2A). In contrast, AMOLT1 expression did not declined steadily (t = −2.7; p = 0.01; Fig. S2B).
Finally, we examined the association between baseline GRIN3B expression level and PSS score at baseline and each subsequent follow-up assessment. We found that baseline GRIN3B expression level was significantly associated with PSS score at 1, 3, 6, and 12-month follow-up, but not at baseline (Table S4).
Using the transcriptomic profile and weighted gene correlation network analysis, we identified 38 gene co-expression modules. The number of genes in each module ranged between 52 and 2362. Neither GRIN3B nor AMOLT1 was a member of these modules. We did not observe a significant association between the module eigengenes and class trajectory (chronic vs. resilient; Table S5).
DNA methylation loci associated with blood expression levels
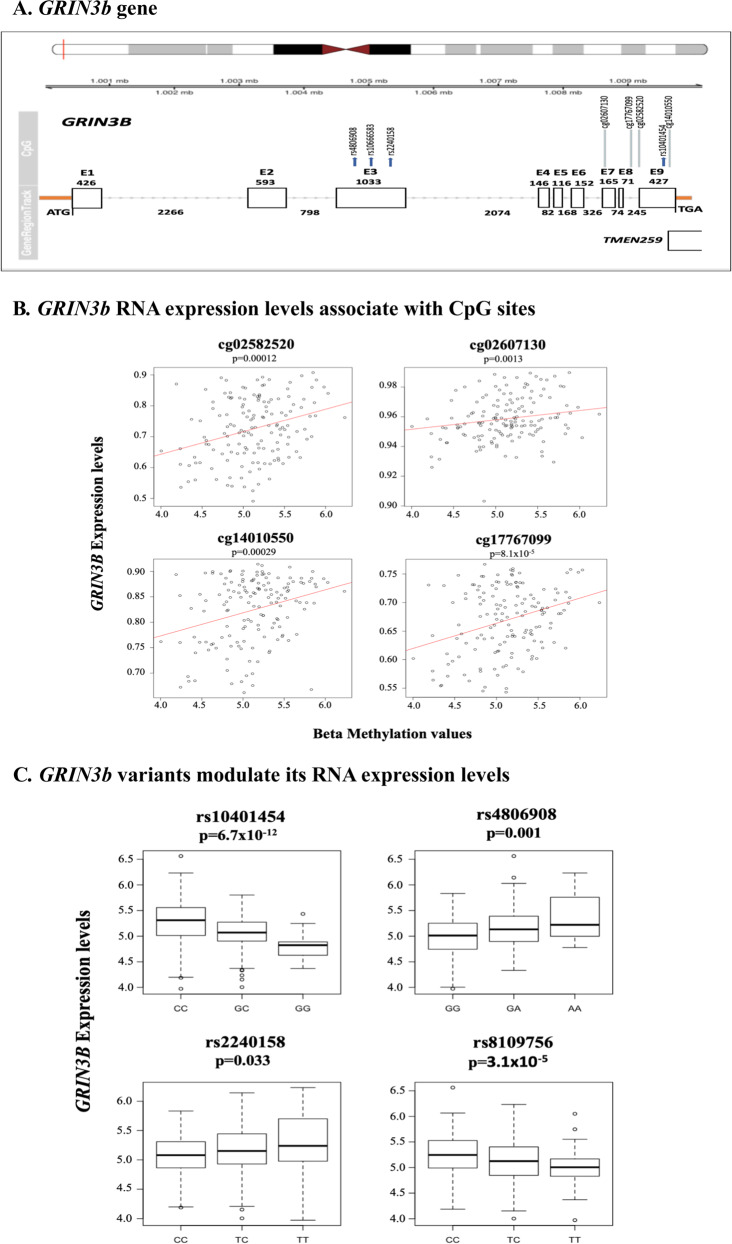
As DNAm in specific genomic loci can affect mRNA expression, we evaluated whether DNAm can explain the observed decrease in GRIN3B mRNA levels in resilience. In a subset of patients (N = 151), we identified 20 CpG sites in the GRINB3 gene using EPIC and 450 BeadChip data. After regressing beta values with mRNA expression levels, we found four CpGs sites located in the genic and intragenic region of GRINB3 (Fig. 2A), whose DNAm levels significantly increased with higher gene expression (p < 0.0025; Fig. 2B). None of these four loci was associated with class trajectory (chronic N = 14; resilience N = 56), possibly due to limited statistical power.
Fig. 2. GRIN3B gene: its RNA expression levels are modulated by CpG loci and DNA variants.
A Schematic representation of the GRIN3B gene and its localization on: chromosome 19 (top of the figure). Each rectangle represents an exon. Numbers above the rectangles are the base pair of each exon; intronic base pair numbers are also indicated. Four variants (rs4806908, rs10666583, rs2240158, and rs1041454) and their localization within the gene and exon are shown; rs8109756 is located near the CNN2 gene and not shown in the figures. None of these SNPs are in LD with each other, despite the close localization. The CpG methylation loci and their localization in the gene are also shown. B Four CpG sites are positive correlated with GRIN3b expression levels. Y-axis shows gene expression values; X-axis represents beta-methylation values. The red line indicates the predicted regression line. C Box and whisker plots of GRIN3b blood RNA expression levels changing with the genotype of the four different variants. Y-axis shows the mRNA expression levels at baseline; X-axis shows the three different genotypes.
SNP variants in GRIN3B associated with blood expression levels (eQTLs)
We next assessed if any genomic locus in the GRIN3B gene could explain the change in blood mRNA levels. Among the 23 identified GWAS variants, four SNPs (rs10401454, rs8109756, rs4806908, and rs2240158) were associated with significant change in blood expression (FDR < 0.05; Table 2 and Fig. 1A–C).
Table 2.
Genetic variants associated with mRNA expression levels (eQTLs).
| SNPs | Gene | Beta | p Value | FDR | Location |
|---|---|---|---|---|---|
| rs10401454 | GRIN3B | −0.23 | 2.90 × 10−13 | 6.7 × 10−12 | Exon missense variant |
| rs8109756 | CNN2 | −0.14 | 2.71 × 10−6 | 3.1 × 10−5 | Intron |
| rs4806908 | GRIN3B | 0.19 | 0.0001 | 0.001 | Exon synonymous variant |
| rs2240158 | GRIN3B | 0.08 | 0.0058 | 0.033 | Exon missense variant |
A GRIN3B SNP associated with PTSD phenotype in the validation dataset
To fully evaluate if any genetic eQTL variants identified in the ED cohort were associated with developing PTSD, we used the GTP cohort, a cross-sectional dataset of similar ancestry (Table S6 displays the GTP demographics).
Out of these four eQTLs, rs10401454 was nominally significantly associated with PTSD case–control phenotypes, based on PSS symptoms (N = 3519, p = 0.036, OR = 1.13) with the minor allele (GG) predicting resilience (Table S7 and Fig. 3); a similar result was obtained after adjusting for DAST score (N = 1176, p = 0.045, OR = 1.24). The direction of association was consistent with the initial ED findings, as GRIN3B expression was lower in the resilient class than the other two classes (p = 1.04 × 10−5; t = −4.5; Fig. S2). Similarly, rs10401454 minor allele was associated with lower expression levels in the ED cohort, and low PTSD symptoms in the GTP cohort. rs10401454 was not associated with resilience in the ED cohort (Table S8), possibly due to limited statistical power.
Fig. 3. GRIN3B Genetic Association with PTSD symptoms.
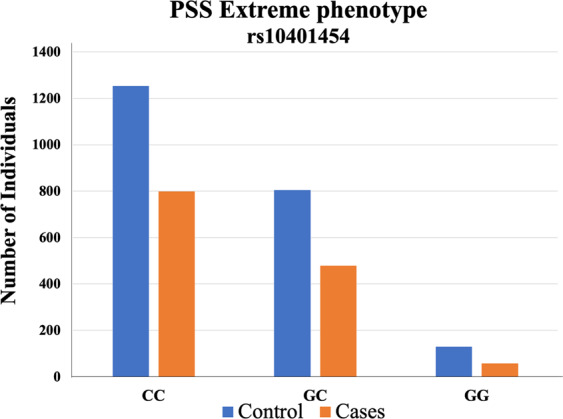
Bar plot of number of cases (PSS ≥ 19) and controls (PSS ≤ 7) for GRIN3B genotype (CC, GC, and GG). The Y-axis represents the number of subjects.
GRIN3B rs10401454 associated with mRNA expression in the brain
To further corroborate the relevance of rs10401454 for gene expression in the brain, we resorted to the BrainCloud and the GTEx databases. In the BrainCloud dataset, we found that rs10401454 was an eQTL in DLPFC group (FDR = 6.5 × 10−7, beta = −0.04) and in the DLPFC controls (FDR = 2.8 × 10−4, beta = −0.04; Fig. S3A, B). In GTEx, rs10401454 genotypes affected mRNA levels expressed in the brain cortex (p = 2 × 10−10) and brain frontal cortex BA9 (p = 6.2 × 10−6), with the homozygote for the minor allele (GG) having less expression than CG and CC genotypes (Fig. S3C, D). The patterns of expression observed in BrainCloud and GTEx were consistent with the one in the ED-blood TWAS (Fig. 2C). Notably, in the GTEx, this SNP is also an eQTL in whole blood (p = 1.2 × 10−10) and several other tissues, including brain cerebellum and basal ganglia with an m value > 0.9 in 39 out 49 tissues (Fig. S4).
Discussion
Identifying novel biomarkers of PTSD risk may facilitate early intervention, mitigating both individual suffering and public health concerns. Blood biomarkers could be particularly advantageous, being an objective measure for future symptom manifestation, and when interview for PSS symptoms and trauma type might not be feasible in the aftermath of a trauma exposure. In our study, using transcriptomes profiled in the immediate aftermath of trauma exposure, we observed that the expression levels of GRIN3B at baseline predicted resilience vs. PTSD. One eQTL variant in GRIN3B was also nominally associated with PTSD in a larger independent cohort of similar backgrounds.
Trajectory modeling analyses previously led to important findings in complex genetic traits, including psychiatric diseases (e.g., PTSD) [6, 56]. Thus, we utilized empirical trajectories, a best LGMM fitting model with a three-class solution, which statistically describes symptom progression over 12 months after trauma, as the primary outcome for gene discovery rather than diagnostic categories [23]. In prior studies, these trajectory membership showed a significant association with plasma proinflammatory tumor necrosis factor α and interferon-γ [21], with increased skin conductance response among subjects in the chronic trajectory [22] and emotion dysregulation [36].
The characteristics of the chronic-PTSD trajectory are in concordance with several other studies, in which female patients with low education were associated with a higher likelihood of developing PTSD [57]. Furthermore, in the ED, most sexual-assault victims were clustering in the chronic-PTSD category, suggesting that PTSD risk varies by type of trauma. Indeed, sexual assaults (e.g., rape) associate with both the highest probability of developing PTSD, and a longer duration of PTSD symptoms [58, 59]. The trajectory also indicates that PSS symptoms could predict outcomes, but a clear distinction among trajectories emerged after 6 months.
The TWAS of class trajectories in our study identified GRIN3B baseline mRNA expression as informative of PTSD course and outcomes.
We observed a significant gradual decrease of GRIN3B mRNA levels in chronic-PTSD vs. recovery vs. resilient trajectory, indicating a steady reduction of expression with the disease’s etiology. Although the sample size is limited, the finding seems well-founded for several reasons: (a) adding either alcohol or drug use, or education, did not change the results (b) we observed a dose–response in the three-trajectory analysis. Contrary, AMOLT1analyses did not show analogous results.
GRIN3B encodes a subunit (NR3B) of an N-methyl-d-aspartate (NMDA) receptor, a glutamate receptor, that is widely distributed in neurons throughout the central nervous system (CNS). In rodents, exposure to stress causes an increase in glutamate release, the excitatory neurotransmitter for NMDA receptors, with associated increased NMDAR expression in brain regions linked to emotional behavior [60, 61], including fear conditioning [62]. In a mouse model, we did not find a significant correlation between blood and amygdala expression level of GRIN3B [20]. Nevertheless, we cannot rule out significant correlations of GRIN3B level between blood and other brain regions implicated in PTSD, such as hippocampus or ventral medial prefrontal cortex. Furthermore, we cannot exclude the possibility of an indirect effect of GRIN3B through the blood immune system as leukocytes express functional NMDA receptors [63], which might be involved in the brain–body signal transmission and given the role of immune system in the etiology of PTSD [64–66]. Finally, the aim of the study was to identify peripheral biomarker for manifestation of PTSD symptoms. Thus, NMDA receptors are particularly interesting in the pathophysiology of PTSD. They also seem to be involved in plastic changes within the CNS responsible for learning and memory, development, and synapse formation [67]. Interestingly, NMDA receptor antagonists (e.g., ketamine) have been used to reduce symptom severity in patients with chronic-PTSD [68, 69]. This accumulating evidence in human and animal models of NMDA receptors’ role in PTSD suggests the GRIN3B gene as an exciting candidate for predicting PTSD course shortly after trauma.
In human populations, GRIN3B has been linked to opioid addiction and schizophrenia [45, 46, 70–72], although expression of GRIN3B mRNA was not associated with computer game addiction [73]. Chronic opioid abuse, but also abstinence and methadone treatment led to the significant upregulation of GRIN3B mRNA [71]. Intriguingly, the downregulation of mRNA expression may have been caused by methadone’s ability to antagonize NMDA receptors [71]. In our cohort, we observed an upregulation of GRIN3B expression in subjects with future chronic-PTSD symptoms. Furthermore, we observed a stronger association after including a variable for drug use, suggesting that addiction may contribute to GRIN3B expression changes and possibly increased PTSD risk. While no association was observed between GRIN3b expression levels and PSS symptoms at baseline, GRIN3b expression was associated with PSS symptoms during the follow-up visits, with the highest significant observed at 6 months. As a corollary, some NMDA antagonists, such as ketamine, may downregulate GRIN3B expression levels, indicating a point of intervention to improve outcome and disease course in post-trauma subjects with higher GRIN3B levels.
Similarly, expression of glutamate receptor subunits in peripheral blood was also shown to be altered by sex, pregnancy, or depression during pregnancy, with the lowest GRIN3B mRNA levels in healthy pregnant women [74]. Healthy volunteers evaluated for working memory showed no difference in NMDA receptor expression, denoting a different role of NMDARs in stressful situations (e.g., trauma exposure) [75].
Our results are not in contrast with previous PTSD transcriptome studies, in which immune and inflammatory genes and pathways had been identified, as we examined the blood transcriptomic profiles collected at the time of trauma exposure, which can be different from transcriptomic profiles from postmortem brain tissue [76] or from blood [12] at the time of having PTSD.
To explore possible mechanisms that may account for significant differences in GRIN3B expression in PTSD trajectories, we evaluated DNAm loci and genetic variants. Four CpG sites were hypermethylated, and associated with increased mRNA blood levels. Higher levels of GRIN3B methylation levels in mice brain had been associated with increased mRNA levels [77]. Furthermore, a locus located ∼10 kb upstream of the GRIN3B gene was found to be hypomethylated in the brains of schizophrenic and major psychosis [78]. Notably, often gene promotors are hypomethylated, while intragenic and coding regions are hypermethylated [77, 79], both of which mechanisms may result in increased mRNA expression. Four variants (two missense mutations, a synonymous SNP, and an intronic variant) in the GRIN3B gene were associated with baseline blood expression levels of GRIN3B. While how these variants regulated mRNA expression is currently unknown, we surmise that their genomic locations could be cryptic transcription factors binding loci.
We then investigated if any of the GRIN3B variants could be a risk factor for PTSD in the GTP cohort [49]. rs10401454 was associated with PTSD case–control extreme phenotype. The exon 9 location of this SNP is particularly intriguing, as GRIN3B-Exon 9 encodes two putative Ca2+/calmodulin-dependent kinase sites, important regulating the calcium-permeable channel and activation/deactivation of the NMDA receptor. High Ca2+ permeability of NMDA receptors also play a critical role in neurodevelopment, synaptic plasticity, and neuronal death, suggesting that variations in this exon, such as rs10401454, may confer particular vulnerability and pathological consequences.
Using data from two eQTL brain databases, we observed that rs10401454 regulates mRNA expression in the brain cortex, a region of the brain that is important for the pathophysiology of PTSD [80, 81]. Furthermore, in the GTEx dataset, rs10401454 seemed to regulate several other tissues, implying that this variant could ubiquitously affect expression levels in different organs/tissue.
Genetic variants in GRIN3B have been associated with an increased risk of developing schizophrenia [45, 46, 72], auditory mismatch negativity [70], and heroin addiction [82]. Specifically, the GRIN3B SNP rs2240158 was associated with heroin addiction in a Han Chinese population [82] and mismatch negativity in healthy subjects [70]. Our study found that this variant was a GRIN3B eQTL, was associated with trajectories in the ED cohort, but not with PTSD phenotype in the GTP cohort. A four-base pair insertion variant, rs10666583, has been associated with increased schizophrenia risk in two independent studies [45, 46]. This same null variant was associated with extreme case–control phenotype in the GTP cohort (Table S5). Given that PTSD shares genetic risk factors with other psychiatric disorders, including schizophrenia, [83–85], the same variants may contribute to increased risk across diseases.
The major limitation of this study is the lack of an independent cohort with a similar study design to replicate our findings. We hope to replicate and expand these results in the future, using the AURORA study [86], which has been enrolling discharge ED subjects after trauma exposures, and followed longitudinally with a target sample size of over 5000 samples. Another limitation of this study was the relatively modest sample size in the transcriptome analysis, although in line with other studies [10, 65, 66, 87–89]. On the other hand, this work relied on growth curve trajectories, which may represent separate genetic clusters less heterogeneous than the classical PTSD phenotype, and more suitable for gene discovery. The ED also offers a primary point of contact for individuals immediately after exposure to trauma, providing an ideal foundation to (i) evaluate subjects longitudinally, (ii) assess PTSD symptoms shortly after the trauma, and (iii) appraise early individual PTSD risk factors, which may be used toward systematic prevention. Although, given the study designed, we could not analyze the patient’s blood prior to the trauma exposure, we did not observe any association of GRIN3b levels at baseline with PSS symptoms, inferring a possible direct or indirect involvement of GRIN3b gene in the etiology of PTSD.
In summary, the current study provides evidence that peripheral gene expression signatures following trauma are informative of PTSD key clinical features and outcomes. Specifically, GRIN3B expression seems to predict post-trauma symptom trajectory and might be a promising early biomarker for PTSD manifestation and outcome, and a possible intervention and prevention point.
Funding
This work was supported by the Howard Hughes Medical Institute, the National Institute for Health (R01 MH094757 to K.J.R.; U01 HG009807 to A.W. and K.J.R.; R01 MH094759 to C.B.N.; R21 MH106902 to A.K.S. and T.J.), the Library Information Technology Services (UL1 TR000424), the German Research Foundation (SCHU 3259/1-1 to K.S.), and the Brain and Behavior Research Foundation. The Genotype-Tissue Expression (GTEx) Project was supported by the Common Fund of the Office of the Director of the National Institutes of Health, and by NCI, NHGRI, NHLBI, NIDA, NIMH, and NINDS.
Supplementary information
Acknowledgements
We are grateful to Kimberly Kerley, Becky Hinrichs, Alex O. Rothbaum, Andrew Kimmel, Kammarauche Asuzu, and Raquel Kirmse for their technical assistance in performing experiments, collecting behavioral data, data management, and recruiting. We are grateful to the nurses, physicians, staff, and Emergency Department participants for their time and effort in supporting this research.
Author contributions
A.L. performed the statistical analyses, analyzed and interpreted the data, and contributed to writing the manuscript. K.S. modeled the growth curve trajectories based on longitudinal PSS symptoms, helped with the statistical analyses, and contributed to editing the manuscript. I.G.-L. modeled the growth curve trajectories based on longitudinal PSS symptoms and contributed to the writing of the manuscript. N.P.D. inferred the cell type proportion, performed statistical analyses, and contributed to the manuscript’s writing. S.K. performed the methylation analyses and contributed to the manuscript’s writing. A.K.S. led the methylation analyses, including collection and processing of the samples, and contributed to the manuscript’s writing. A.J.M. helped to design the experiment, recruit the patients, and edit the manuscript. R.R. and M.H. were involved in processing the blood for RNA analyses, including QC and statistical analyses, and they contributed to the editing of the manuscript. G.G. helped with the expression analyses, and contributed to the manuscript’s editing. S.W. helped to design the experiment, recruit the patients, and write the manuscript. F.G. and P.D.H. helped to design the experiment, recruit the patients, and edit the manuscript. C.B.N. helped to design the experiment, and write the manuscript. T.J., J.L.M.-K., J.S.S., and V.M. helped to design the experiment, recruit the patients, and write the manuscript. B.O.R. helped to design the experiment and write the manuscript. A.P.W. and K.J.R. obtained funding, helped to design the experiment, analyze and interpret the data, supervise other contributors, write the initial manuscript, and edit the final manuscript. E.G. contributed to analyses and writing.
Competing interests
The data for the analyses described in this manuscript (GRIN3B eQTL variants) were obtained from the GTEx Portal on 12/13/2019. C.B.N discloses the following: Research/Grants: National Institutes of Health (NIH). Consulting (past 12 months): ANeuroTech (division of Anima BV), Signant Health, Sunovion Pharmaceuticals, Inc., Janssen Research and Development LLC, Magstim, Inc., Navitor Pharmaceuticals, Inc., Intra-Cellular Therapies, Inc., EMA Wellness, Acadia Pharmaceuticals, Axsome, Sage, BioXcel Therapeutics, Silo Pharma, XW Pharma, Neuritek, Engrail Therapeutics, and Corcept Therapeutics Pharmaceuticals Company. Stockholder: Xhale, Seattle Genetics, Antares, BI Gen Holdings, Inc., Corcept Therapeutics Pharmaceuticals Company, and EMA Wellness. Scientific Advisory Boards: ANeuroTech (division of Anima BV), Brain and Behavior Research Foundation (BBRF), Anxiety and Depression Association of America (ADAA), Skyland Trail, Signant Health, Laureate Institute for Brain Research (LIBR), Inc., Magnolia CNS, and Heading Health. Board of Directors: Gratitude America, ADAA, and Xhale Smart, Inc. Patents: method and devices for transdermal delivery of lithium (US 6,375,990B1). Method of assessing antidepressant drug therapy via transport inhibition of monoamine neurotransmitters by ex vivo assay (US 7,148,027B2). Speakers Bureau: none. K.J.R. provides fee-for-service consultation for Biogen, Alkermes, and Resilience Therapeutics. He also holds patents for a number of targets related to improving extinction of fear; however, he has received no equity or income within the past 3 years related to these. He receives or has received research funding from NIMH, HHMI, NARSAD, and the Burroughs Wellcome Foundation. B.O.R has funding from Wounded Warrior Project, Department of Defense, National Institute of Mental Health, and McCormick Foundation. B.O.R. receives royalties from Oxford University Press, Guilford, APPI, and Emory University, and has served on recent advisory boards for Aptinyx, Sandoz, and Nobilis. N.P.D. has held a part-time paid position at Cohen Veteran Biosciences. N.P.D. has been a consultant for Sunovion Pharmaceuticals and is on the scientific advisory board for Sentio Solutions, Inc. All remaining authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Aliza P. Wingo, Email: awingo@emory.edu
Kerry J. Ressler, Email: kressler@mclean.harvard.edu
Supplementary information
The online version contains supplementary material available at 10.1038/s41386-021-01073-8.
References
- 1.Kar N. Cognitive behavioral therapy for the treatment of post-traumatic stress disorder: a review. Neuropsychiatr Dis Treat. 2011;7:167–81. doi: 10.2147/NDT.S10389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shalev AY, Ankri Y, Israeli-Shalev Y, Peleg T, Adessky R, Freedman S. Prevention of posttraumatic stress disorder by early treatment: results from the Jerusalem Trauma Outreach And Prevention study. Arch Gen Psychiatry. 2012;69:166–76. doi: 10.1001/archgenpsychiatry.2011.127. [DOI] [PubMed] [Google Scholar]
- 3.Rothbaum BO, Kearns MC, Reiser E, Davis JS, Kerley KA, Rothbaum AO, et al. Early intervention following trauma may mitigate genetic risk for PTSD in civilians: a pilot prospective emergency department study. J Clin Psychiatry. 2014;75:1380–7. doi: 10.4088/JCP.13m08715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62:593–602. doi: 10.1001/archpsyc.62.6.593. [DOI] [PubMed] [Google Scholar]
- 5.Wolf EJ, Miller MW, Reardon AF, Ryabchenko KA, Castillo D, Freund R. A latent class analysis of dissociation and posttraumatic stress disorder: evidence for a dissociative subtype. Arch Gen Psychiatry. 2012;69:698–705. doi: 10.1001/archgenpsychiatry.2011.1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Galatzer-Levy IR, Huang SH, Bonanno GA. Trajectories of resilience and dysfunction following potential trauma: a review and statistical evaluation. Clin Psychol Rev. 2018;63:41–55. doi: 10.1016/j.cpr.2018.05.008. [DOI] [PubMed] [Google Scholar]
- 7.True WR, Rice J, Eisen SA, Heath AC, Goldberg J, Lyons MJ, et al. A twin study of genetic and environmental contributions to liability for posttraumatic stress symptoms. Arch Gen Psychiatry. 1993;50:257–64. doi: 10.1001/archpsyc.1993.01820160019002. [DOI] [PubMed] [Google Scholar]
- 8.Schultebraucks K, Qian M, Abu-Amara D, Dean K, Laska E, Siegel C, et al. Pre-deployment risk factors for PTSD in active-duty personnel deployed to Afghanistan: a machine-learning approach for analyzing multivariate predictors. Mol Psychiatry. 2020. 10.1038/s41380-020-0789-2. [DOI] [PMC free article] [PubMed]
- 9.Schultebraucks K, Galatzer-Levy IR. Machine learning for prediction of posttraumatic stress and resilience following trauma: an overview of basic concepts and recent advances. J Trauma Stress. 2019;32:215–25. doi: 10.1002/jts.22384. [DOI] [PubMed] [Google Scholar]
- 10.Logue MW, Smith AK, Baldwin C, Wolf EJ, Guffanti G, Ratanatharathorn A, et al. An analysis of gene expression in PTSD implicates genes involved in the glucocorticoid receptor pathway and neural responses to stress. Psychoneuroendocrinology. 2015;57:1–13. doi: 10.1016/j.psyneuen.2015.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wingo AP, Almli LM, Stevens JS, Klengel T, Uddin M, Li Y, et al. Corrigendum: DICER1 and microRNA regulation in post-traumatic stress disorder with comorbid depression. Nat Commun. 2016;7:10958. doi: 10.1038/ncomms10958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Breen MS, Tylee DS, Maihofer AX, Neylan TC, Mehta D, Binder EB, et al. PTSD blood transcriptome mega-analysis: shared inflammatory pathways across biological sex and modes of trauma. Neuropsychopharmacology. 2018;43:469–81. doi: 10.1038/npp.2017.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Segman RH, Shefi N, Goltser-Dubner T, Friedman N, Kaminski N, Shalev AY. Peripheral blood mononuclear cell gene expression profiles identify emergent post-traumatic stress disorder among trauma survivors. Mol Psychiatry. 2005;10:500–13. doi: 10.1038/sj.mp.4001636. [DOI] [PubMed] [Google Scholar]
- 14.Fanselow MS, LeDoux JE. Why we think plasticity underlying Pavlovian fear conditioning occurs in the basolateral amygdala. Neuron. 1999;23:229–32. doi: 10.1016/S0896-6273(00)80775-8. [DOI] [PubMed] [Google Scholar]
- 15.Fenster RJ, Lebois LAM, Ressler KJ, Suh J. Brain circuit dysfunction in post-traumatic stress disorder: from mouse to man. Nat Rev Neurosci. 2018;19:535–51. doi: 10.1038/s41583-018-0039-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Quirk GJ, Russo GK, Barron JL, Lebron K. The role of ventromedial prefrontal cortex in the recovery of extinguished fear. J Neurosci. 2000;20:6225–31. doi: 10.1523/JNEUROSCI.20-16-06225.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Milad MR, Quirk GJ. Neurons in medial prefrontal cortex signal memory for fear extinction. Nature. 2002;420:70–4. doi: 10.1038/nature01138. [DOI] [PubMed] [Google Scholar]
- 18.Milad MR, Wright CI, Orr SP, Pitman RK, Quirk GJ, Rauch SL. Recall of fear extinction in humans activates the ventromedial prefrontal cortex and hippocampus in concert. Biol Psychiatry. 2007;62:446–54. doi: 10.1016/j.biopsych.2006.10.011. [DOI] [PubMed] [Google Scholar]
- 19.Corcoran KA, Desmond TJ, Frey KA, Maren S. Hippocampal inactivation disrupts the acquisition and contextual encoding of fear extinction. J Neurosci. 2005;25:8978–87. doi: 10.1523/JNEUROSCI.2246-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lori A, Maddox SA, Sharma S, Andero R, Ressler KJ, Smith AK. Dynamic patterns of threat-associated gene expression in the amygdala and blood. Front Psychiatry. 2018;9:778. doi: 10.3389/fpsyt.2018.00778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Michopoulos V, Beurel E, Gould F, Dhabhar FS, Schultebraucks K, Galatzer-Levy I, et al. Association of prospective risk for chronic PTSD symptoms with low TNFalpha and IFNgamma concentrations in the immediate aftermath of trauma exposure. Am J Psychiatry. 2020;177:58–65. doi: 10.1176/appi.ajp.2019.19010039. [DOI] [PubMed] [Google Scholar]
- 22.Hinrichs R, van Rooij SJ, Michopoulos V, Schultebraucks K, Winters S, Maples-Keller J, et al. Increased skin conductance response in the immediate aftermath of trauma predicts PTSD risk. Chronic Stress. 2019;3:2470547019844441. 10.1177/2470547019844441. [DOI] [PMC free article] [PubMed]
- 23.Schultebraucks K, Shalev AY, Michopoulos V, Grudzen CR, Shin SM, Stevens JS, et al. A validated predictive algorithm of post-traumatic stress course following emergency department admission after a traumatic stressor. Nat Med. 2020;26:1084–8. doi: 10.1038/s41591-020-0951-z. [DOI] [PubMed] [Google Scholar]
- 24.Galatzer-Levy IR, Ankri Y, Freedman S, Israeli-Shalev Y, Roitman P, Gilad M, et al. Early PTSD symptom trajectories: persistence, recovery, and response to treatment: results from the Jerusalem Trauma Outreach and Prevention Study (J-TOPS) PLoS ONE. 2013;8:e70084. doi: 10.1371/journal.pone.0070084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bryant RA, Nickerson A, Creamer M, O’Donnell M, Forbes D, Galatzer-Levy I, et al. Trajectory of post-traumatic stress following traumatic injury: 6-year follow-up. Br J Psychiatry. 2015;114:145516. doi: 10.1192/bjp.bp.114.145516. [DOI] [PubMed] [Google Scholar]
- 26.Association AP diagnostic and statistical manual-text revision (DSM-IV-TRim, 2000). (American Psychiatric Association; 2000).
- 27.Foa EB, Rothbaum BO. Treating the trauma of rape: cognitive-behavioral therapy for PTSD. Guilford; 2001.
- 28.Falsetti SA, Resnick HS, Resick PA, Kilpatrick DG. The Modified PTSD Symptom Scale: a brief self-report measure of posttraumatic stress disorder. Behav Ther. 1993;16:161–2.
- 29.Foa EB, Tolin DF. Comparison of the PTSD symptom scale-interview version and the clinician-administered PTSD scale. J Trauma Stress. 2000;13:181–91. doi: 10.1023/A:1007781909213. [DOI] [PubMed] [Google Scholar]
- 30.Stevens JS, Ely TD, Sawamura T, Guzman D, Bradley B, Ressler KJ, et al. Childhood maltreatment predicts reduced inhibition-related activity in the rostral anterior cingulate in Ptsd, but not trauma-exposed controls. Depress Anxiety. 2016;33:614–22. doi: 10.1002/da.22506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yudko E, Lozhkina O, Fouts A. A comprehensive review of the psychometric properties of the Drug Abuse Screening Test. J Subst Abus Treat. 2007;32:189–98. doi: 10.1016/j.jsat.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 32.Saunders JB, Aasland OG, Babor TF, de la Fuente JR, Grant M. Development of the alcohol use disorders identification test (AUDIT): WHO collaborative project on early detection of persons with harmful alcohol consumption-II. Addiction. 1993;88:791–804. doi: 10.1111/j.1360-0443.1993.tb02093.x. [DOI] [PubMed] [Google Scholar]
- 33.Beck AT, Steer RA, Garbin MG. Psychometric properties of the Beck Depression Inventory: twenty-five years of evaluation. Clin Psychol Rev. 1988;8:77–100. doi: 10.1016/0272-7358(88)90050-5. [DOI] [Google Scholar]
- 34.Muthen LK, & Muthen, BO. Mplus user’s guide. 3 ed. Muthen & Muthen; 1998.
- 35.Nylund KL, Asparouhov T, Muthen BO. Deciding on the number of classes in latent class analysis and growth mixture modeling: a Monte Carlo simulation study. Struct Equ Modeling. 2007;14:535–69. doi: 10.1080/10705510701575396. [DOI] [Google Scholar]
- 36.Pencea I, Munoz AP, Maples-Keller JL, Fiorillo D, Schultebraucks K, Galatzer-Levy I, et al. Emotion dysregulation is associated with increased prospective risk for chronic PTSD development. J Psychiatr Res. 2020;121:222–8. doi: 10.1016/j.jpsychires.2019.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dobin A, Davis CA, Schlesinger F, Drenkow J, Zaleski C, Jha S, et al. STAR: ultrafast universal RNA-seq aligner. Bioinformatics. 2013;29:15–21. doi: 10.1093/bioinformatics/bts635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liao Y, Smyth GK, Shi W. featureCounts: an efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics. 2014;30:923–30. doi: 10.1093/bioinformatics/btt656. [DOI] [PubMed] [Google Scholar]
- 39.Love MI, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014;15:550. doi: 10.1186/s13059-014-0550-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ritchie ME, Phipson B, Wu D, Hu Y, Law CW, Shi W, et al. limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic acids Res. 2015;43:e47. doi: 10.1093/nar/gkv007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Law CW, Chen Y, Shi W, Smyth GK. voom: Precision weights unlock linear model analysis tools for RNA-seq read counts. Genome Biol. 2014;15:R29. doi: 10.1186/gb-2014-15-2-r29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Langfelder P, Horvath S. WGCNA: an R package for weighted correlation network analysis. BMC Bioinformatics. 2008;9:559. doi: 10.1186/1471-2105-9-559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Newman AM, Liu CL, Green MR, Gentles AJ, Feng W, Xu Y, et al. Robust enumeration of cell subsets from tissue expression profiles. Nat Methods. 2015;12:453–7. doi: 10.1038/nmeth.3337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Purcell S, Neale B, Todd-Brown K, Thomas L, Ferreira MA, Bender D, et al. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet. 2007;81:559–75. doi: 10.1086/519795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Matsuno H, Ohi K, Hashimoto R, Yamamori H, Yasuda Y, Fujimoto M, et al. A naturally occurring null variant of the NMDA type glutamate receptor NR3B subunit is a risk factor of schizophrenia. PLoS ONE. 2015;10:e0116319. doi: 10.1371/journal.pone.0116319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hornig T, Gruning B, Kundu K, Houwaart T, Backofen R, Biber K, et al. GRIN3B missense mutation as an inherited risk factor for schizophrenia: whole-exome sequencing in a family with a familiar history of psychotic disorders. Genet Res. 2017;99:e1. doi: 10.1017/S0016672316000148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Teschendorff AE, Marabita F, Lechner M, Bartlett T, Tegner J, Gomez-Cabrero D, et al. A beta-mixture quantile normalization method for correcting probe design bias in Illumina Infinium 450 k DNA methylation data. Bioinformatics. 2013;29:189–96. doi: 10.1093/bioinformatics/bts680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Johnson WE, Li C, Rabinovic A. Adjusting batch effects in microarray expression data using empirical Bayes methods. Biostatistics. 2007;8:118–27. doi: 10.1093/biostatistics/kxj037. [DOI] [PubMed] [Google Scholar]
- 49.Gillespie CF, Bradley B, Mercer K, Smith AK, Conneely K, Gapen M, et al. Trauma exposure and stress-related disorders in inner city primary care patients. Gen Hosp Psychiatry. 2009;31:505–14. doi: 10.1016/j.genhosppsych.2009.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Almli LM, Lori A, Meyers JL, Shin J, Fani N, Maihofer AX, et al. Problematic alcohol use associates with sodium channel and clathrin linker 1 (SCLT1) in trauma-exposed populations. Addict Biol. 2017. 10.1111/adb.12569. [DOI] [PMC free article] [PubMed]
- 51.Nievergelt C,. Maihofer AX, Koenen KC. International meta-analysis of PTSD genome-wide association studies identifies sex- and ancestry- specific genetic risk loci. Nat Commun. 2019;10. 10.1038/s41467-019-12576-w. [DOI] [PMC free article] [PubMed]
- 52.Smyth GK. Linear models and empirical bayes methods for assessing differential expression in microarray experiments. Stat Appl Genet Mol Biol. 2004;3:Article3. 10.2202/1544-6115.1027. [DOI] [PubMed]
- 53.Shabalin AA. Matrix eQTL: ultra fast eQTL analysis via large matrix operations. Bioinformatics. 2012;28:1353–8. doi: 10.1093/bioinformatics/bts163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Consortium GT. The Genotype-Tissue Expression (GTEx) project. Nat Genet. 2013;45:580–5. doi: 10.1038/ng.2653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Collado-Torres L, Burke EE, Peterson A, Shin J, Straub RE, Rajpurohit A, et al. Regional heterogeneity in gene expression, regulation, and coherence in the frontal cortex and hippocampus across development and schizophrenia. Neuron. 2019;103:203–16. doi: 10.1016/j.neuron.2019.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.McGiffin JN, Galatzer-Levy IR, Bonanno GA. Socioeconomic resources predict trajectories of depression and resilience following disability. Rehabil Psychol. 2019;64:98–103. doi: 10.1037/rep0000254. [DOI] [PubMed] [Google Scholar]
- 57.Shalev AY, Gevonden M, Ratanatharathorn A, Laska E, van der Mei WF, Qi W.et al; International Consortium to Predict P. Estimating the risk of PTSD in recent trauma survivors: results of the International Consortium to Predict PTSD (ICPP). World Psychiatry. 2019;18:77–87. 10.1002/wps.20608. [DOI] [PMC free article] [PubMed]
- 58.McLaughlin KA, Koenen KC, Hill ED, Petukhova M, Sampson NA, Zaslavsky AM, et al. Trauma exposure and posttraumatic stress disorder in a national sample of adolescents. J Am Acad Child Adolesc Psychiatry. 2013;52:815–30. doi: 10.1016/j.jaac.2013.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kessler RC, Aguilar-Gaxiola S, Alonso J, Benjet C, Bromet EJ, Cardoso G, et al. Trauma and PTSD in the WHO World Mental Health Surveys. Eur J Psychotraumatol. 2017;8:1353383. doi: 10.1080/20008198.2017.1353383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Niemann S, Kanki H, Fukui Y, Takao K, Fukaya M, Hynynen MN, et al. Genetic ablation of NMDA receptor subunit NR3B in mouse reveals motoneuronal and nonmotoneuronal phenotypes. Eur J Neurosci. 2007;26:1407–20. doi: 10.1111/j.1460-9568.2007.05774.x. [DOI] [PubMed] [Google Scholar]
- 61.Bartanusz V, Aubry JM, Pagliusi S, Jezova D, Baffi J, Kiss JZ. Stress-induced changes in messenger RNA levels of N-methyl-D-aspartate and AMPA receptor subunits in selected regions of the rat hippocampus and hypothalamus. Neuroscience. 1995;66:247–52. doi: 10.1016/0306-4522(95)00084-v. [DOI] [PubMed] [Google Scholar]
- 62.Riaza Bermudo-Soriano C, Perez-Rodriguez MM, Vaquero-Lorenzo C, Baca-Garcia E. New perspectives in glutamate and anxiety. Pharm Biochem Behav. 2012;100:752–74. doi: 10.1016/j.pbb.2011.04.010. [DOI] [PubMed] [Google Scholar]
- 63.Miglio G, Varsaldi F, Lombardi G. Human T lymphocytes express N-methyl-D-aspartate receptors functionally active in controlling T cell activation. Biochem Biophys Res Commun. 2005;338:1875–83. doi: 10.1016/j.bbrc.2005.10.164. [DOI] [PubMed] [Google Scholar]
- 64.Nievergelt CM, Maihofer AX, Klengel T, Atkinson EG, Chen CY, Choi KW, et al. International meta-analysis of PTSD genome-wide association studies identifies sex- and ancestry-specific genetic risk loci. Nat Commun. 2019;2019:4558. doi: 10.1038/s41467-019-12576-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bam M, Yang X, Zumbrun EE, Zhong Y, Zhou J, Ginsberg JP, et al. Dysregulated immune system networks in war veterans with PTSD is an outcome of altered miRNA expression and DNA methylation. Sci Rep. 2016;6:31209. doi: 10.1038/srep31209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mehta D, Voisey J, Bruenig D, Harvey W, Morris CP, Lawford B, et al. Transcriptome analysis reveals novel genes and immune networks dysregulated in veterans with PTSD. Brain Behav Immun. 2018;74:133–42. doi: 10.1016/j.bbi.2018.08.014. [DOI] [PubMed] [Google Scholar]
- 67.Andersson O, Stenqvist A, Attersand A, von Euler G. Nucleotide sequence, genomic organization, and chromosomal localization of genes encoding the human NMDA receptor subunits NR3A and NR3B. Genomics. 2001;78:178–84. doi: 10.1006/geno.2001.6666. [DOI] [PubMed] [Google Scholar]
- 68.Feder A, Parides MK, Murrough JW, Perez AM, Morgan JE, Saxena S, et al. Efficacy of intravenous ketamine for treatment of chronic posttraumatic stress disorder: a randomized clinical trial. JAMA Psychiatry. 2014;71:681–8. doi: 10.1001/jamapsychiatry.2014.62. [DOI] [PubMed] [Google Scholar]
- 69.Nair J, Singh, Ajit S. The role of the glutamatergic system in posttraumatic stress disorder. CNS Spectr. 2008;13:585–91. doi: 10.1017/s1092852900016862. [DOI] [PubMed] [Google Scholar]
- 70.Lin YT, Hsieh MH, Liu CC, Hwang TJ, Chien YL, Hwu HG, et al. A recently-discovered NMDA receptor gene, GRIN3B, is associated with duration mismatch negativity. Psychiatry Res. 2014;218:356–8. doi: 10.1016/j.psychres.2014.04.032. [DOI] [PubMed] [Google Scholar]
- 71.Sedaghati M, Vousooghi N, Goodarzi A, Yaghmaei P, Mokri A, Zarrindast MR. Expression of NR3B but not NR2D subunit of NMDA receptor in human blood lymphocytes can serve as a suitable peripheral marker for opioid addiction studies. Eur J Pharmacol. 2010;633:50–4. doi: 10.1016/j.ejphar.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 72.Yu Y, Lin Y, Takasaki Y, Wang C, Kimura H, Xing J, et al. Rare loss of function mutations in N-methyl-D-aspartate glutamate receptors and their contributions to schizophrenia susceptibility. Transl Psychiatry. 2018;8:12. doi: 10.1038/s41398-017-0061-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sadat-Shirazi MS, Vousooghi N, Alizadeh B, Makki SM, Zarei SZ, Nazari S, et al. Expression of NMDA receptor subunits in human blood lymphocytes: a peripheral biomarker in online computer game addiction. J Behav Addict. 2018;7:260–8. doi: 10.1556/2006.7.2018.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bhandage AK, Jin Z, Hellgren C, Korol SV, Nowak K, Williamsson L, et al. NMDA and kainate glutamate receptor subunits are expressed in human peripheral blood mononuclear cells (PBMCs) where the expression of GluK4 is altered by pregnancy and GluN2D by depression in pregnant women. J Neuroimmunol. 2017;305:51–8. doi: 10.1016/j.jneuroim.2017.01.013. [DOI] [PubMed] [Google Scholar]
- 75.Sadat-Shirazi MS, Ashabi G, Hessari MB, Khalifeh S, Neirizi NM, Matloub M, et al. NMDA receptors of blood lymphocytes anticipate cognitive performance variations in healthy volunteers. Physiol Behav. 2019;201:53–8. doi: 10.1016/j.physbeh.2018.12.015. [DOI] [PubMed] [Google Scholar]
- 76.Girgenti MJ, Wang J, Ji D, Cruz DA, Traumatic Stress Brain Research G, Stein MB, et al. Transcriptomic organization of the human brain in post-traumatic stress disorder. Nat Neurosci. 2021;24:24–33. doi: 10.1038/s41593-020-00748-7. [DOI] [PubMed] [Google Scholar]
- 77.Irwin RE, Thakur A, ON KM, et al. CP. 5-Hydroxymethylation marks a class of neuronal gene regulated by intragenic methylcytosine levels. Genomics. 2014;104:383–92. doi: 10.1016/j.ygeno.2014.08.013. [DOI] [PubMed] [Google Scholar]
- 78.Mill J, Tang T, Kaminsky Z, Khare T, Yazdanpanah S, Bouchard L, et al. Epigenomic profiling reveals DNA-methylation changes associated with major psychosis. Am J Hum Genet. 2008;82:696–711. doi: 10.1016/j.ajhg.2008.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Maunakea AK, Nagarajan RP, Bilenky M, Ballinger TJ, D’Souza C, Fouse SD, et al. Conserved role of intragenic DNA methylation in regulating alternative promoters. Nature. 2010;466:253–7. doi: 10.1038/nature09165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Shin LM, Rauch SL, Pitman RK. Amygdala, medial prefrontal cortex, and hippocampal function in PTSD. Ann N Y Acad Sci. 2006;1071:67–79. doi: 10.1196/annals.1364.007. [DOI] [PubMed] [Google Scholar]
- 81.Dahlgren MK, Laifer LM, VanElzakker MB, Offringa R, Hughes KC, Staples-Bradley LK, et al. Diminished medial prefrontal cortex activation during the recollection of stressful events is an acquired characteristic of PTSD. Psychol Med. 2018;48:1128–38. doi: 10.1017/S003329171700263X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Xie X, Liu H, Zhang J, Chen W, Zhuang D, Duan S, et al. Association between genetic variations of NMDA receptor NR3 subfamily genes and heroin addiction in male Han Chinese. Neurosci Lett. 2016;631:122–5. doi: 10.1016/j.neulet.2016.08.025. [DOI] [PubMed] [Google Scholar]
- 83.Chantarujikapong SI, Scherrer JF, Xian H, Eisen SA, Lyons MJ, Goldberg J, et al. A twin study of generalized anxiety disorder symptoms, panic disorder symptoms and post-traumatic stress disorder in men. Psychiatry Res. 2001;103:133–45. doi: 10.1016/s0165-1781(01)00285-2. [DOI] [PubMed] [Google Scholar]
- 84.Duncan LE, Ratanatharathorn A, Aiello AE, Almli LM, Amstadter AB, Ashley-Koch AE, et al. Largest GWAS of PTSD (N=20 070) yields genetic overlap with schizophrenia and sex differences in heritability. Mol Psychiatry. 2018;23:666–73. doi: 10.1038/mp.2017.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Misganaw B, Guffanti G, Lori A, Abu-Amara D, Flory JD, Muller S, et al. Polygenic risk associated with post-traumatic stress disorder onset and severity. Transl Psychiatry. 2019;9:165. doi: 10.1038/s41398-019-0497-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.McLean SA, Ressler K, Koenen KC, Neylan T, Germine L, Jovanovic T, et al. The AURORA Study: a longitudinal, multimodal library of brain biology and function after traumatic stress exposure. Mol Psychiatry. 2020;25:283–96. 10.1038/s41380-019-0581-3. [DOI] [PMC free article] [PubMed]
- 87.Mehta D, Klengel T, Conneely KN, Smith AK, Altmann A, Pace TW, et al. Childhood maltreatment is associated with distinct genomic and epigenetic profiles in posttraumatic stress disorder. Proc Natl Acad Sci USA. 2013;110:8302–7. doi: 10.1073/pnas.1217750110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Breen MS, Maihofer AX, Glatt SJ, Tylee DS, Chandler SD, Tsuang MT, et al. Gene networks specific for innate immunity define post-traumatic stress disorder. Mol Psychiatry. 2015;20:1538–45. 10.1038/mp.2015.9. [DOI] [PMC free article] [PubMed]
- 89.Kuan PF, Waszczuk MA, Kotov R, Clouston S, Yang X, Singh PK, et al. Gene expression associated with PTSD in World Trade Center responders: an RNA sequencing study. Transl Psychiatry. 2017;7:1297. doi: 10.1038/s41398-017-0050-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.