Abstract
Objectives
Long working hours are linked to an increased risk of exposure to work safety hazards that threaten the health of workers. To date, only a few cross‐sectional studies regarding the relationship between working characteristics, such as over‐workload and chronic kidney disease (CKD) have been reported. Therefore, in this longitudinal study, we aimed to examine the direct relationship between long working hours and the incidence of CKD.
Methods
We included 97 856 participants without CKD in the Kangbuk Samsung Health Study. Using a self‐report questionnaire, we evaluated weekly working hours, which were categorized into 35‐40, 41‐52, and >52 hours. CKD was defined as estimated glomerular filtration rate <60 mL/min/1.73 m2. Hazard ratios (HRs) and 95% confidence intervals (CIs) for incident CKD were estimated using Cox proportional hazards regression analyses with weekly working 35‐40 hours as the reference.
Results
During a median follow‐up of 4.0 years, 185 participants developed incident CKD (incidence density, 4.83 per 104 person‐years). Multivariable‐adjusted HRs (95% CI) of incident CKD for weekly working >52 hours compared with working 35‐40 hours were 1.99 (1.22‐3.25). In subgroup analyses, the significant association between working >52 hours and incident CKD was consistently observed in groups of age ≥40 years, men, and obesity with no interaction.
Conclusions
Our large‐scale cohort study of young‐ to middle‐aged men and women demonstrated a significant association between long working hours and an increased risk of incident CKD.
Keywords: chronic kidney disease, glomerular filtration rate, long working hours, longitudinal study, overwork
1. INTRODUCTION
Chronic kidney disease (CKD) has become an increasing global public health concern, which can progress to end‐stage renal disease, increase the risk of developing cardiovascular complications, and increase all‐cause mortality.1 The global prevalence of CKD is estimated at 11%‐13%, and CKD affects approximately 8% of adults in Korea.2, 3 Risk factors for CKD include cardiovascular and metabolic diseases such as hypertension, diabetes mellitus (DM), and obesity, the prevalence of which are increasing with that of CKD.1
Working characteristics, such as shift work and working hours, have a significant impact on the occupational health of workers.4 Long working hours are linked to an increased risk of exposure to work safety hazards, thereby threatening the health of workers.5 In 2019, South Korea was ranked as one of the top countries for the longest annual working hours per worker (1967 hours),6 making workers vulnerable to the harmful effects of long working hours. Epidemiological studies have shown that long working hours adversely affect the incidence of cardiovascular disease,7 obesity,8 hypertension,9 and DM.10
Previous studies have demonstrated that the aforementioned risk factors for CKD are related to long working hours. In this regard, long working hours might be associated with incident CKD. However, only a few studies have reported the relationship between working characteristics and CKD.11, 12 A cross‐sectional study showed that long working hours are associated with decreased estimated glomerular filtration rate (eGFR).11 Therefore, our longitudinal study aimed to further examine the direct relationship between long working hours and incident CKD. We assessed the effects of weekly working hours on the risk of decreased kidney function determined by eGFR in a large cohort of CKD‐free Korean men and women participated in a health screening program.
2. METHODS
2.1. Study population
The Kangbuk Samsung Health Study is a cohort study of South Korean men and women aged at least 18 years who underwent a comprehensive annual or biennial health examination at the Kangbuk Samsung Hospital Total Healthcare Center in Seoul and Suwon, South Korea.13 More than 80% of the examinees were employees of various companies and local governmental organizations and their spouses. In South Korea, the Industrial Safety and Health Law requires annual or biennial health screening examinations of all employees without charge. Other participants voluntarily underwent health checkups at the healthcare center.
The present study included a total of 218 904 participants who underwent comprehensive health examinations from January 1, 2012 to December 31, 2017 and had undergone at least one other screening examination before December 31, 2018. We excluded 74 099 participants with missing study variables at baseline or at any follow‐up visit (Figure 1): work‐related factor, eGFR, proteinuria, and any other relevant covariate required for adjustment. Among potential participants (n = 144 805), we further excluded 46 949 participants who had any of the following conditions at baseline: history of malignancy or medication use for malignancy, history of renal disease or medication use for renal disease, history of hypertension or medication use for hypertension, history of DM or medication use for DM, eGFR <60 mL/min/1.73 m2, proteinuria, and working less than 35 hours per week. Finally, 97 856 participants were eligible for this study at baseline. This study was approved by the Institutional Review Board of Kangbuk Samsung Hospital, which waived the requirement for informed consent because we accessed only de‐identified data routinely collected as part of health screening examinations (IRB No: KBSMC2021‐01‐032).
FIGURE 1.
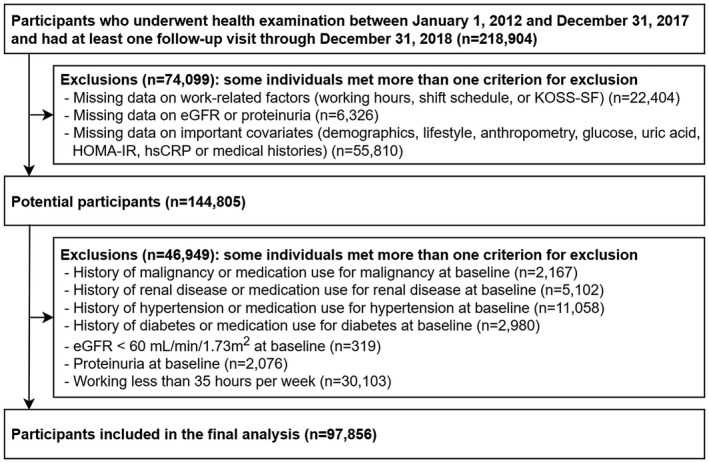
Flowchart of study participants. eGFR, estimated glomerular filtration rate; HOMA‐IR, homeostasis model assessment of insulin resistance; hsCRP. High‐sensitivity C‐reactive protein; KOSS‐SF, Korean Occupational Stress Scale‐Short form
2.2. Measurements
All examinations were conducted at the Kangbuk Samsung Hospital Total Healthcare Screening Center in Seoul and Suwon. At each visit, demographic characteristics, working characteristics, smoking status, alcohol consumption, regular exercise, medical history, and medication use were collected using standardized, self‐administered questionnaires. Smoking status was categorized as non‐, former, and current smokers. Alcohol consumption was categorized as ≥10 and <10 g/day. We also assessed the weekly frequencies of moderate‐ and vigorous‐intensity physical activity, which were categorized as <3 or ≥3 times per week, respectively. Education level was categorized as less than college graduate, college graduate, and higher.
According to the Labor Standards Act of Korea, working hours of adults should not exceed 40 hours per week, excluding recess time (12 additional hours per week are allowed with workers’ permission), and working hours of adolescents should not exceed 35 hours per week (five additional hours per week are allowed with workers’ permission). Based on this standard, we evaluated the working characteristics using a self‐report questionnaire. The weekly working hour was assessed using the following question: “How many hours did you work in a week on average in your job for the past year, including overtime?” and successively categorized them as 35‐40, 41‐52, and >52 hours per week. The work shift schedule was assessed using the following question: “In the past year, during which time of the day did you work the most?” Daytime work was defined as work performed mostly during the day (between 6 AM and 6 PM), and shift work was defined as work performed during other hours. Occupational stress was assessed using the Korean Occupational Stress Scale‐Short Form (KOSS‐SF), a 24‐item self‐report questionnaire scored on a 4‐point Likert scale from 1 to 4. A previous nationwide epidemiological study showed a high internal consistency and validity of the KOSS‐SF.14 The KOSS‐SF consists of the following seven subscales of work‐related stress factors: job demands, insufficient job control, interpersonal conflict, job insecurity, organizational injustice, lack of reward, and occupational climate. The total KOSS‐SF score was calculated using a 100‐point system proposed by the developers. In this study, we used the total KOSS‐SF score, and we dichotomized participants into high or low occupational stress groups based on the median of the converted scores.14
Blood pressure (BP), height, and weight were measured by trained nurses. Obesity was defined as a body mass index (BMI) ≥25 kg/m2.15 Hypertension was defined as a systolic BP ≥140 mm Hg, a diastolic BP ≥90 mm Hg, a self‐reported history of hypertension, or current use of anti‐hypertensive medications. The fasting blood sample measurements included glucose, total cholesterol, low‐density lipoprotein cholesterol (LDL‐C), high‐density lipoprotein cholesterol (HDL‐C), triglycerides, blood urea nitrogen (BUN), creatinine, uric acid, and high‐sensitivity C‐reactive protein (hsCRP). DM was defined as a fasting serum glucose level of ≥126 mg/dL, a hemoglobin A1c level ≥6.5%, a self‐reported history of DM, or current use of anti‐diabetic medications. Insulin resistance was assessed using the homeostasis model assessment of insulin resistance (HOMA‐IR) equation as follows: fasting insulin (μU/mL) × fasting glucose (mg/dL)/405.16 Serum creatinine was measured using the kinetic alkaline picrate (Jaffe) method in an automated chemistry analyzer (a Modular D2400 from Roche AG). Estimated GFR was calculated using the Modification of Diet in Renal Disease Equation 17 In this study, CKD was defined as eGFR <60 mL/min/1.73 m2.18
2.3. Statistical analysis
The chi‐squared test and one‐way ANOVA were used to compare the characteristics of participants categorized by working hours at baseline. The study endpoint was the development of incident CKD. Participants were followed from the baseline visit to the visit wherein CKD was diagnosed or to the last available visit before December 31, 2018, whichever came first. Incidence density was calculated as the number of incident cases divided by person‐years of the follow‐up period.
Hazard ratios (HRs) and 95% confidence intervals (CIs) for incident CKD were estimated using Cox proportional hazards regression analyses. We initially adjusted for age, sex, and year of screening examination in Model 1. To adjust the potential confounders that might affect both exposure and outcome,11, 13 Model 2 was adjusted for age, sex, year of screening examination, center (Seoul and Suwon), alcohol intake, smoking status, regular exercise, education level, history of dyslipidemia, and medication for dyslipidemia. To explore whether clinical factors mediated the association between working hours and CKD,11, 19, 20 Model 3 was further adjusted for BMI, systolic blood pressure, fasting blood glucose, HOMA‐IR, uric acid, and hsCRP. To clearly elucidate the effect of working hours on CKD,12, 21 Model 4 was further adjusted for work‐related factors, such as work shift schedule and total KOSS‐SF score. A proportional hazards assumption was tested by examining the graphs of estimated log (−log) survival and using “estat phtest” command based on Schoenfeld residuals. There was no violation of the proportional hazards assumption. To determine the linear trend of incidence, the number of categories was used as a continuous variable and tested in each model. To evaluate the effects of changes in covariates during follow‐up, we conducted additional analyses using covariates (alcohol intake, smoking status, regular exercise, BMI, systolic BP, fasting blood glucose, HOMA‐IR, uric acid, hsCRP, and total KOSS‐SF score) as time‐varying covariates in the models.
To explore the mechanism underlying the observed associations between working hours and CKD risk, stratified analyses in predefined subgroups were performed by age (<40 vs ≥40 years), sex (women vs men), and BMI (<25 vs ≥25 kg/m2). Interactions between working hours categories and subgroup characteristics were tested using likelihood ratio tests, which compared models with and without multiplicative interaction terms.
Statistical analyses were performed using stata version 16.1 (StataCorp LP). All reported P values were two‐tailed. A P < .05 was considered to be statistically significant.
3. RESULTS
In Table 1, the mean (standard deviation) age and serum creatinine of 97 856 participants at baseline were 36.4 (6.5) years and 0.91 (0.17) mg/dL, respectively. Weekly working hours were positively associated with men, current smoker, alcohol intake, high education level, history of dyslipidemia, obesity, BMI, systolic and diastolic BP, fasting blood glucose, HOMA‐IR, total cholesterol, LDL‐C, triglycerides, BUN, creatinine, uric acid, and hsCRP, high occupational stress, and daytime work. However, the weekly working hours were inversely associated with age, regular exercise, and HDL‐C.
TABLE 1.
Baseline Characteristics of study participants by weekly working hours
| Characteristics | Overall | Weekly working hours | P value for trend | ||
|---|---|---|---|---|---|
| 35‐40 | 41‐52 | >52 | |||
| Number | 97 856 | 18 315 | 52 952 | 26 589 | |
| Men (%) | 75.3 | 54.4 | 77.4 | 85.6 | <.001 |
| Current smoker (%) | 25.3 | 19.4 | 24.5 | 31.1 | <.001 |
| Alcohol intake (%)a | 45.9 | 39.2 | 45.8 | 50.8 | <.001 |
| Regular exercise (%)b | 12.2 | 13.5 | 12.5 | 10.7 | <.001 |
| High education level (%)c | 88.5 | 81.0 | 89.3 | 92.2 | <.001 |
| History of dyslipidemia (%) | 12.0 | 10.6 | 12.0 | 12.9 | <.001 |
| Medication for dyslipidemia (%) | 1.30 | 1.36 | 1.23 | 1.39 | .57 |
| Obesity (%)d | 31.0 | 26.4 | 30.7 | 34.5 | <.001 |
| High occupational stress (%)e | 16.6 | 11.8 | 15.0 | 22.8 | <.001 |
| Daytime work (%)f | 91.3 | 89.9 | 91.3 | 92.1 | <.001 |
| Age (years)* | 36.4 (6.5) | 37.7 (7.2) | 36.0 (6.4) | 36.2 (6.1) | <.001 |
| BMI (kg/m2)* | 23.6 (3.2) | 23.1 (3.2) | 23.6 (3.2) | 24.0 (3.2) | <.001 |
| Systolic BP (mm Hg)* | 108.8 (11.7) | 106.6 (12.2) | 109.0 (11.6) | 109.9 (11.2) | <.001 |
| Diastolic BP (mm Hg)* | 70.3 (9.4) | 69.1 (9.8) | 70.4 (9.3) | 71.1 (9.1) | <.001 |
| Glucose (mg/dL)# | 93 (88‐98) | 92 (87‐98) | 93 (88‐98) | 93 (88‐98) | <.001 |
| HOMA‐IR# | 1.27 (0.85‐1.87) | 1.23 (0.82‐1.81) | 1.27 (0.85‐1.87) | 1.30 (0.86‐1.91) | <.001 |
| Total cholesterol (mg/dL)* | 194.9 (33.6) | 193.0 (33.9) | 194.8 (33.6) | 196.4 (33.2) | <.001 |
| LDL‐C (mg/dL)* | 122.7 (31.5) | 119.7 (31.7) | 122.7 (31.6) | 124.7 (31.2) | <.001 |
| HDL‐C (mg/dL)* | 56.7 (14.6) | 59.1 (15.3) | 56.6 (14.6) | 55.2 (14.0) | <.001 |
| Triglycerides (mg/dL)# | 96 (68‐142) | 88 (62‐133) | 96 (68‐142) | 100 (71‐148) | <.001 |
| BUN (mg/dL)* | 12.4 (2.9) | 12.2 (3.0) | 12.4 (2.9) | 12.6 (2.9) | <.001 |
| Creatinine (mg/dL)* | 0.91 (0.17) | 0.84 (0.18) | 0.91 (0.16) | 0.94 (0.15) | <.001 |
| Uric acid (mg/dL)* | 5.71 (1.41) | 5.25 (1.45) | 5.76 (1.39) | 5.94 (1.35) | <.001 |
| hsCRP (mg/L)# | 0.04 (0.03‐0.09) | 0.04 (0.02‐0.08) | 0.04 (0.03‐0.09) | 0.05 (0.03‐0.09) | .01 |
Data are expressed as *mean (standard deviation), #median (interquartile range), or percentage.
Abbreviations: BMI, body mass index; BP, blood pressure; HOMA‐IR, homeostasis model assessment of insulin resistance; LDL‐C, low‐density lipoprotein cholesterol; HDL‐C, high‐density lipoprotein cholesterol; BUN, blood urea nitrogen; hsCRP, high‐sensitivity C‐reactive protein.
≥10 g/day.
≥3 times per week.
≥College graduate.
BMI ≥25 kg/m2.
Median cutoff values of total KOSS‐SF score: 50.1.
Participants who answered "I work mostly during the day (between 6 AM and 6 PM)".
The relationship between weekly working hours and the incidence of CKD was shown in Table 2. A total of 185 participants developed CKD (incidence density, 4.83 per 104 person‐years) over 383 360.4 person‐years of follow‐up period (median follow‐up, 4.0 years; interquartile range, 2.3‐5.4 years). Participants with longer working hours had a higher incidence of CKD. All models showed that weekly working >52 hours were associated with a significantly higher risk of incident CKD than working 35‐40 hours. In Model 4, multivariable‐adjusted HR (95% CI) of incident CKD for working >52 hours to compared with working 35‐40 hours was 1.99 (1.22‐3.25). Even after introducing confounders as time‐varying covariates, the association between working >52 hours and CKD was still observed in the time‐dependent model.
TABLE 2.
Development of CKD according to weekly working hours
| Weekly working hours | Person‐years (PY) | Incident cases | Incidence density (per 104 PY) (95% CI) | Multivariable‐adjusted HR (95% CI)a | HR (95% CI)b in model using time‐dependent variables | |||
|---|---|---|---|---|---|---|---|---|
| Model 1 | Model 2 | Model 3 | Model 4 | |||||
| 35‐40 | 67 954.0 | 24 | 3.53 (2.37‐5.27) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| 41‐52 | 209 011.2 | 95 | 4.55 (3.72‐5.56) | 1.55 (0.98‐2.46) | 1.54 (0.97‐2.45) | 1.51 (0.95‐2.40) | 1.51 (0.95‐2.40) | 1.50 (0.95‐2.39) |
| >52 | 106 395.2 | 66 | 6.20 (4.87‐7.90) | 2.06 (1.27‐3.34) | 2.07 (1.28‐3.36) | 1.98 (1.22‐3.22) | 1.99 (1.22‐3.25) | 1.95 (1.20‐3.18) |
| P for trend | .002 | .002 | .004 | .005 | .006 | |||
Model 1 was adjusted for age, sex, and year of screening examination.
Model 2: model 1 plus adjustment for center, alcohol intake, smoking status, regular exercise, education level, history of dyslipidemia, and medication for dyslipidemia.
Model 3: model 2 plus adjustment for BMI, systolic blood pressure, fasting blood glucose, HOMA‐IR, uric acid, and hsCRP.
Model 4: model 3 plus adjustment for work shift schedule and total KOSS‐SF score.
Abbreviations: BMI, body mass index; CI, confidence interval; CKD, chronic kidney disease; HOMA‐IR, homeostasis model assessment of insulin resistance; HR, hazard ratio; hsCRP, high‐sensitivity C‐reactive protein; KOSS‐SF, Korean Occupational Stress Scale‐Short Form.
Estimated from Cox proportional hazard models.
Estimated from Cox proportional hazard models with alcohol intake, smoking status, regular exercise, BMI, systolic blood pressure, fasting blood glucose, HOMA‐IR, uric acid, hsCRP and total KOSS‐SF score as time‐dependent variables and baseline age, sex, center, year of screening examination, education level, weekly working hours, work shift schedule, history of dyslipidemia, and medication for dyslipidemia as time‐fixed variables.
In Table 3, subgroup analyses showed consistent and significant associations between working >52 hours and incident CKD compared with working 35‐40 hours among participants with age ≥40 years, men, and BMI ≥25 kg/m2. There was no significant interaction with predetermined subgroups.
TABLE 3.
Hazard ratiosa (95% CI) for CKD by weekly working hours in clinically relevant subgroups
| Subgroup | Weekly working hours | P for interaction | ||
|---|---|---|---|---|
| 35‐40 | 41‐52 | >52 | ||
| Age | ||||
| <40 years (n = 68 215) | 1.00 (reference) | 0.96 (0.41‐2.24) | 1.36 (0.56‐3.28) | .465 |
| ≥40 years (n = 29 641) | 1.00 (reference) | 1.66 (0.96‐2.87) | 2.06 (1.14‐3.71) | |
| Sex | ||||
| Women (n = 24 166) | 1.00 (reference) | 0.74 (0.17‐3.26) | 2.15 (0.49‐9.45) | .571 |
| Men (n = 73 690) | 1.00 (reference) | 1.59 (0.97‐2.62) | 2.04 (1.21‐3.44) | |
| BMI | ||||
| <25 kg/m2 (n = 67 569) | 1.00 (reference) | 1.22 (0.66‐2.22) | 1.84 (0.98‐3.48) | .549 |
| ≥25 kg/m2 (n = 30 287) | 1.00 (reference) | 1.97 (0.95‐4.11) | 2.24 (1.03‐4.86) | |
Abbreviations: BMI, body mass index; CI, confidence interval; CKD, chronic kidney disease; HOMA‐IR, homeostasis model assessment of insulin resistance; hsCRP, high‐sensitivity C‐reactive protein; KOSS‐SF, Korean Occupational Stress Scale‐Short Form.
Estimated from Cox proportional hazard models adjusted for age, sex, year of screening examination, center, alcohol intake, smoking status, regular exercise, education level, history of dyslipidemia, medication for dyslipidemia, BMI, systolic blood pressure, fasting blood glucose, HOMA‐IR, uric acid, hsCRP, work shift schedule, and total KOSS‐SF score.
4. DISCUSSION
Our large‐scale cohort study, in which participants had normal eGFR and fewer risk factors of CKD at baseline, showed an association between long working hours and the risk of incident CKD. In particular, participants who worked more than 52 hours per week had a significantly increased risk of incident CKD compared with those who worked 35‐40 hours per week. Even after adjusting for a wide range of relevant covariates, the association between longer working hours per week and the incidence of CKD remained statistically significant. To minimize the effect of a change in working hours caused by a decrease in renal function, we excluded participants who had factors that may influence renal function at baseline. This study is the first longitudinal study minimizing the probability of reverse causation.
To the best of our knowledge, the direct mechanisms underlying the association between long working hours and CKD are not clear. It is difficult to establish experimental evidence for the effect of long working hours, as randomized controlled trials and animal studies are difficult to design for working hours. Only a few previous studies have demonstrated the association between CKD and work‐related factors such as working hour, shift work, and job stress.11, 12, 21 A cohort study of Chinese steelworkers reported that long‐term night shift work was associated with a higher risk of early stages of renal disease mediated by BP.12 Furthermore, shift workers who worked in irregular, split, and rotating shifts tended to work for longer duration due to less work schedule flexibility.22, 23 In our study, the relationship between working hours and CKD was significantly maintained, even after a statistical adjustment of work shift schedule as a confounder. In addition, the association remained significant after excluding shift workers at baseline to clarify the influence of long working hours on the development of CKD (data not shown). Therefore, we found that long working hours showed association with CKD independently of shift work.
On the other hand, working hours constitute a measurable work stressor, and longer working hours can lead to higher job strain.24, 25, 26 In a cross‐sectional study on 1433 Japanese White‐collar workers, there was a significant interaction effect of high job stress on the relationship between eGFR and metabolic risk factors such as BP and serum triglycerides.21 Consequently, we considered that job stress played a role as a mediator in the relationship between long working hours and CKD. However, the effect of long working hours on CKD almost remained unchanged when adjusting for job stress in our study. Therefore, there might be other mechanisms that can explain the association between long working hours and incident CKD.
Previous studies have shown that long working hours were associated with increased hsCRP in middle‐ and old‐aged workers.27, 28 In a cross‐sectional study of 7470 Korean workers, there was an interaction between long working hours and shift work in terms of elevated hsCRP, which was explained by an induction of systemic inflammation by circadian misalignment.28 A recent study of individuals with cardiovascular disease reported that elevated hsCRP was associated with lower eGFR.20 Meanwhile, a study of telecommunication workers demonstrated that shift work was associated with hyperuricemia.29 A possible mechanism of the abovementioned association is the role of stress‐induced elevation of serum uric acid, which can also apply to the effects of long working hours on health.29 Although the relationship between long working hours and hyperuricemia was not reported, it is reasonable to theorize that stress due to long working hours could induce hyperuricemia, which is a well‐known aggravating factor of renal function.19 In our study, hsCRP and uric acid were statistically adjusted to examine whether they acted as mediators between working hours and CKD. Consequently, the HR was slightly attenuated, and statistical significance was maintained, which appeared to be partially mediated by hsCRP and uric acid.
In subgroup analyses, participants with older than 40 years, men, and obesity consistently presented significant associations although the interactions were insignificant. Renal function begins to decrease by an eGFR of approximately 10 mL/min/1.73 m2 per decade after 40 years of age30; thus, we set the cutoff value of subgroup by age. Additionally, it is well‐known that obesity is independently associated with a greater prevalence of CKD,31 which was corroborated with the results of our study. However, the prevalence of CKD, including early stages, tended to be higher in women, whereas end‐stage renal disease was more prevalent in men, suggesting that CKD in men had a higher risk of progression and mortality.32 The results of our study demonstrated that the association between working hours and CKD was only significant among men, which was inconsistent with previous studies. The significant association in men might be explained because sex hormone is a major inducer of gender discrepancy.32 Animal studies have shown that estrogen has a protective effect on renal injury, while the effect decreases with a progression of renal disease in postmenopausal women.33, 34 As our study participants were young‐ and middle‐aged individuals, the association might be insignificant due to the protective effect of estrogen.
Although we adjusted for various covariates, there are still some other explanations of the relationship between working hours and incident CKD. Artazcoz et al demonstrated that weekly working more than 50 hours were associated with no leisure‐time physical activity.35 Another study of office and service workers reported that longer working hours prolonged sedentary time.36 Moreover, recent studies have shown that more time spent in sedentary behaviors with a low level of physical activity affects the risk of renal damage mediated by metabolic risk factors.37, 38 With respect to sleep profiles, Artazcoz et al also reported that working more than 50 hours per week was related to short sleep duration.35 A cohort study illustrated that long working hours might be a risk factor for shortened sleep duration with poor sleep quality.39 In addition, a large‐scale longitudinal study indicated that poor sleep quality and less than 6 h of sleep duration were associated with an increased risk of incident CKD.40 Therefore, no physical activity, increased sedentary time, and poor sleep quality caused by long working hours could be other mechanisms explaining our study findings.
Consequently, despite the adjustment for relevant covariates, the results in our study showed that long working hours were independently associated with the development of CKD. Furthermore, how strong a potential unmeasured confounder was to nullify the observed association between long working hours and incident CKD could be evaluated by measuring the E‐value.41 Based on a multivariable‐adjusted HR in Model 4 (HR = 1.99), the calculated E‐value estimate of 3.39 suggests that our observed HR could be explained away by an unmeasured confounder that associated with both our exposure and outcome by a HR of 3.39‐fold each, above and beyond the measured confounders, but weaker confounding could not do so.41, 42 In other words, the E‐value indicates that the observed association is unlikely to be invalidated by an unmeasured confounder. Several unknown pathways could be involved in this association. Therefore, further studies are required to demonstrate the possible mechanisms explaining the direct association.
Several limitations in this study should be considered. First, we defined CKD using only eGFR measurement. The exact definition of CKD includes the presence of at least an evidence of kidney damage, such as structural abnormality of kidney, albuminuria, or history of kidney transplantation; or eGFR <60 mL/min/1.73 m2 for more than 3 months. However, our study data did not fully satisfy the exact definition of CKD. Furthermore, most participants were examined annually or biennially, and thus it was difficult to analyze the parameters in a 3‐month duration. Therefore, CKD might have been misclassified. Second, because other work‐related factors, such as types of collar workers, were not classified, the differences between occupations could not be analyzed and occupational characteristics were not fully examined. In addition, other variables, such as sleep‐related factors or sitting time, were not considered in this study; further research is necessary to elucidate the different mechanisms underlying the association between long working hours and CKD. Third, since our prospective cohort data were obtained from participants who first visited from 2012 to 2017, subjects registered in 2017 could be followed‐up for up to a maximum of 1 year, suggesting that right censoring bias might occur. However, when analyzing 36 194 participants who first visited in 2012 with relatively long follow‐up period (median follow‐up, 5.77 years; interquartile range, 4.57‐6.02) by the same method, the risk of incident CKD was still significantly higher in those with weekly working more than 52 hours (HR = 1.95, 95% CI 1.07‐3.55, Table S1) than with working 35‐40 hours. Hence, our censoring of later cohorts could be considered as non‐informative. Lastly, our study population consisted of young‐ to middle‐aged Korean workers with relatively good health and high education level. Therefore, our findings need to be generalized carefully to other populations of age, race, and ethnicity.
Notwithstanding such limitations, our study had several notable strengths. To the best of our knowledge, our cohort study is the first longitudinal study to demonstrate the temporal relationship between overwork and the development of CKD. Moreover, our study had a large sample size, refined cohort design, and standardized study data, and therefore our statistical results were reasonably reliable. Lastly, our findings derived from a relatively healthy young‐ and middle‐aged population might not be affected by survivor bias from comorbidities or unpredictable health conditions.
5. CONCLUSION
Our large‐scale cohort study of young‐ to middle‐aged men and women showed that long working hours were associated with an increased risk of incident CKD. Further studies are required to elucidate implicit mechanisms underlying such association.
DISCLOSURE
Approval of the research protocol: The study was approved by the Institutional Review Board of Kangbuk Samsung Hospital (IRB No: KBSMC2021‐01‐032), which waived the requirement for informed consent as only de‐identified data obtained as part of routine health screening examinations were used. Informed consent: N/A. Registry and Registration number of the study/trial: N/A. Animal studies: N/A. Conflict of interest: The authors have no conflict of interest to disclose.
AUTHOR CONTRIBUTIONS
Conceptualization: Yesung Lee and Woncheol Lee; Methodology: Yesung Lee and Woncheol Lee; Formal analysis and investigation: Yesung Lee and Woncheol Lee; Writing––original draft preparation: Yesung Lee; Writing––review and editing: Yesung Lee, Eunhye Seo, Eunchan Mun, and Woncheol Lee.
Supporting information
Table S1.
ACKNOWLEDGMENTS
This study was conducted based on data provided by the Kangbuk Samsung Health Study. The authors thank all study participants and the study personnel for their dedication and continuing support.
Lee Y, Seo E, Mun E, Lee W. A longitudinal study of working hours and chronic kidney disease in healthy workers: The Kangbuk Samsung Health Study. J Occup Health. 2021;63:e12266. 10.1002/1348-9585.12266
DATA AVAILABILITY STATEMENT
The data are not available to be shared publicly, because we do not have permission from the Institutional Review Board to distribute the data. However, data can be available from the Kangbuk Samsung Health Study upon reasonable request, whose authors may be contacted through the corresponding author of this manuscript.
REFERENCES
- 1.Jha V, Garcia‐Garcia G, Iseki K, et al. Chronic kidney disease: global dimension and perspectives. Lancet. 2013;382(9888):260‐272. [DOI] [PubMed] [Google Scholar]
- 2.Hill NR, Fatoba ST, Oke JL, et al. Global prevalence of chronic kidney disease – a systematic review and meta‐analysis. PLoS One. 2016;11(7):e0158765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Park JI, Baek H, Jung HH. Prevalence of chronic kidney disease in Korea: the Korean National Health and Nutritional Examination Survey 2011–2013. J Korean Med Sci. 2016;31(6):915‐923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Isahak M, Loh MY, Susilowati IH, et al. The association of workplace exposures on quality of life in small and medium enterprises workers: a cross‐sectional study in four ASEAN Countries. Asia Pac J Public Health. 2017;29(4):315‐327. [DOI] [PubMed] [Google Scholar]
- 5.Wong K, Chan AHS, Ngan SC. The effect of long working hours and overtime on occupational health: a meta‐analysis of evidence from 1998 to 2018. Int J Environ Res Public Health. 2019;16(12):2102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.OECD . Hours worked (indicator). Organization for economic cooperation and development. https://data.oecd.org/emp/hours–worked.htm. Accessed January 18, 2021.
- 7.Virtanen M, Heikkila K, Jokela M, et al. Long working hours and coronary heart disease: a systematic review and meta‐analysis. Am J Epidemiol. 2012;176(7):586‐596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Virtanen M, Jokela M, Lallukka T, et al. Long working hours and change in body weight: analysis of individual‐participant data from 19 cohort studies. Int J Obes (Lond). 2020;44(6):1368‐1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Trudel X, Brisson C, Gilbert‐Ouimet M, Vézina M, Talbot D, Milot A. Long working hours and the prevalence of masked and sustained hypertension. Hypertension. 2020;75(2):532‐538. [DOI] [PubMed] [Google Scholar]
- 10.Bannai A, Yoshioka E, Saijo Y, Sasaki S, Kishi R, Tamakoshi A. The risk of developing diabetes in association with long working hours differs by shift work schedules. J Epidemiol. 2016;26(9):481‐487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee D‐W, Lee J, Kim H‐R, Jun KY, Kang M‐Y. Long work hours and decreased glomerular filtration rate in the Korean working population. Occup Environ Med. 2020;77(10):699‐705. [DOI] [PubMed] [Google Scholar]
- 12.Zhang S, Wang Y, Zhu Y, Li X, Song Y, Yuan J. Rotating night shift work, exposure to light at night, and glomerular filtration rate: baseline results from a Chinese occupational cohort. Int J Environ Res Public Health. 2020;17(23):9035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang Y, Chang Y, Ryu S, et al. Thyroid hormone levels and incident chronic kidney disease in euthyroid individuals: the Kangbuk Samsung Health Study. Int J Epidemiol. 2014;43(5):1624‐1632. [DOI] [PubMed] [Google Scholar]
- 14.Chang SJ, Koh SB, Kang D, et al. Developing an Occupational Stress Scale for Korean Employees. Korean J Occup Environ Med. 2005;17(4):297‐317. [Google Scholar]
- 15.Chang Y, Jung H‐S, Cho J, et al. Metabolically healthy obesity and the development of nonalcoholic fatty liver disease. Am J Gastroenterol. 2016;111(8):1133‐1140. [DOI] [PubMed] [Google Scholar]
- 16.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta‐cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28(7):412‐419. [DOI] [PubMed] [Google Scholar]
- 17.Manjunath G, Sarnak MJ, Levey AS. Prediction equations to estimate glomerular filtration rate: an update. Curr Opin Nephrol Hypertens. 2001;10(6):785‐792. [DOI] [PubMed] [Google Scholar]
- 18.Stevens PE, Levin A. Evaluation and management of chronic kidney disease: synopsis of the kidney disease: improving global outcomes 2012 clinical practice guideline. Ann Intern Med. 2013;158(11):825‐830. [DOI] [PubMed] [Google Scholar]
- 19.Oh TR, Choi HS, Kim CS, et al. Hyperuricemia has increased the risk of progression of chronic kidney disease: propensity score matching analysis from the KNOW‐CKD study. Sci Rep. 2019;9(1):6681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fu EL, Franko MA, Obergfell A, et al. High‐sensitivity C‐reactive protein and the risk of chronic kidney disease progression or acute kidney injury in post‐myocardial infarction patients. Am Heart J. 2019;216:20‐29. [DOI] [PubMed] [Google Scholar]
- 21.Tsurugano S, Nakao M, Takeuchi T, Nomura K, Yano E. Job stress strengthens the link between metabolic risk factors and renal dysfunction in adult men. Tohoku J Exp Med. 2012;226(2):101‐108. [DOI] [PubMed] [Google Scholar]
- 22.Lambert S. Passing the buck: labor flexibility practices that transfer risk onto hourly workers. Hum Relat. 2008;61:1203‐1227. [Google Scholar]
- 23.Golden L. Irregular work scheduling and its consequences. Economic Policy Institute. https://www.epi.org/publication/irregular‐work‐scheduling‐and‐its‐consequences. Accessed January 18, 2021. [Google Scholar]
- 24.Lee K, Suh C, Kim JE, Park JO. The impact of long working hours on psychosocial stress response among white‐collar workers. Ind Health. 2017;55(1):46‐53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jeon S‐H, Leem J‐H, Park S‐G, et al. Association among working hours, occupational stress, and presenteeism among wage workers: results from the second Korean Working Conditions Survey. Ann Occup Environ Med. 2014;26(1):6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cho SS, Ju YS, Paek D, Kim H, Jung‐Choi K. The combined effect of long working hours and low job control on self‐rated health: an interaction analysis. J Occup Environ Med. 2018;60(5):475‐480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee J, Kim HR. The association between long working hours and high‐sensitivity C‐reactive protein in older aged individuals: the Korea National Health and Nutrition Examination Survey (KNHANES) 2015. J Occup Environ Med. 2018;60(9):775‐780. [DOI] [PubMed] [Google Scholar]
- 28.Lee W, Kang SK, Choi WJ. Effect of long work hours and shift work on high‐sensitivity C‐reactive protein levels among Korean workers. Scand J Work Environ Health. 2020;47(3):200‐207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Uetani M, Suwazono Y, Kobayashi E, Inaba T, Oishi M, Nogawa K. A longitudinal study of the influence of shift work on serum uric acid levels in workers at a telecommunications company. Occup Med (Lond). 2006;56(2):83‐88. [DOI] [PubMed] [Google Scholar]
- 30.Raymond NT, Zehnder D, Smith SC, Stinson JA, Lehnert H, Higgins RM. Elevated relative mortality risk with mild‐to‐moderate chronic kidney disease decreases with age. Nephrol Dial Transplant. 2007;22(11):3214‐3220. [DOI] [PubMed] [Google Scholar]
- 31.Kovesdy CP, Furth SL, Zoccali C. World Kidney Day Steering C . Obesity and kidney disease: hidden consequences of the epidemic. Can J Kidney Health Dis. 2017;4:2054358117698669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Goldberg I, Krause I. The role of gender in chronic kidney disease. EMJ. 2016;1(2):58‐64. [Google Scholar]
- 33.Neugarten J, Golestaneh L. Gender and the prevalence and progression of renal disease. Adv Chronic Kidney Dis. 2013;20(5):390‐395. [DOI] [PubMed] [Google Scholar]
- 34.Sandberg K. Mechanisms underlying sex differences in progressive renal disease. Gend Med. 2008;5(1):10‐23. [DOI] [PubMed] [Google Scholar]
- 35.Artazcoz L, Cortes I, Escriba‐Aguir V, Cascant L, Villegas R. Understanding the relationship of long working hours with health status and health‐related behaviours. J Epidemiol Community Health. 2009;63(7):521‐527. [DOI] [PubMed] [Google Scholar]
- 36.Thorp AA, Healy GN, Winkler E, et al. Prolonged sedentary time and physical activity in workplace and non‐work contexts: a cross‐sectional study of office, customer service and call centre employees. Int J Behav Nutr Phys Act. 2012;9:128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Martens RJH, van der Berg JD, Stehouwer CDA, et al. Amount and pattern of physical activity and sedentary behavior are associated with kidney function and kidney damage: the Maastricht Study. PLoS One. 2018;13(4):e0195306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee S, Lee S, Bae S, et al. Impact of sedentary time on chronic kidney disease and disability incidence in community‐dwelling Japanese older adults: a 4‐year prospective cohort study. J Aging Phys Act. 2019;27(2):184‐190. [DOI] [PubMed] [Google Scholar]
- 39.Virtanen M, Ferrie JE, Gimeno D, et al. Long working hours and sleep disturbances: the Whitehall II prospective cohort study. Sleep. 2009;32(6):737‐745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bo Y, Yeoh E‐K, Guo C, et al. Sleep and the risk of chronic kidney disease: a cohort study. J Clin Sleep Med. 2019;15(3):393‐400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.VanderWeele TJ, Ding P. Sensitivity analysis in observational research: introducing the e‐value. Ann Intern Med. 2017;167(4):268‐274. [DOI] [PubMed] [Google Scholar]
- 42.Mathur MB, Ding P, Riddell CA, VanderWeele TJ. Web site and R package for computing E‐values. Epidemiology. 2018;29(5):e45‐e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1.
Data Availability Statement
The data are not available to be shared publicly, because we do not have permission from the Institutional Review Board to distribute the data. However, data can be available from the Kangbuk Samsung Health Study upon reasonable request, whose authors may be contacted through the corresponding author of this manuscript.


