Introduction
Placebo controlled, randomized, double-blind trials are considered the gold standard for assessing treatment efficacy. Shortly after their development and then widespread adoption, Henry Beecher published a landmark paper in 1955, “The Powerful Placebo,” in which he assumed that patients must be blinded to treatment assignment for placebos to have clinical effects[2]. The assumption that placebos require concealment or deception became imbedded in biomedicine. In 2010, we reported a pilot randomized controlled trial (RCT) of non-concealed, ‘open-label’ placebos (OLP) as treatment for irritable bowel syndrome (IBS) that challenged this conventional belief[17]. The study showed that participants receiving open-label placebo (OLP) reported greater improvement in IBS symptoms with meaningful clinical impact compared to a control group who did not receive placebo (i.e. ‘no-pill control’). This pilot was the first RCT to test OLP for any condition. Supporting the credibility of our finding in IBS, subsequent RCTs - all in patients with subjective symptoms who exhibit high placebo response in RCTS - involving chronic low back pain, knee pain, cancer-related fatigue, migraine headaches, and allergic rhinitis, have also suggested that OLP may be an effective method to elicit placebo effects without deception in these conditions[6,14,16,22,24,27]. This evidence suggests the importance to further investigate OLP in IBS patients. In addition, if the presumption that concealment or deception is necessary for placebos to be effective is false, then many theories about the mechanisms that drive placebo effects may need modification or be inaccurate or incomplete.
IBS is a chronic gastrointestinal disorder characterized by abdominal pain associated with alterations in bowel habits (i.e., diarrhea, constipation, or alternating between diarrhea and constipation). It affects approximately 5%-10% of the adult population and is one of the most common reasons for healthcare consultations and absenteeism from work or school. As with other chronic pain conditions that involve central sensitization and hypersensitivity, effective treatment options for IBS are limited, and placebo response rates in RCTs are high[18]. While high placebo response rates have been an impediment in clinical trials, we hypothesize that it may be possible to ethically harness this placebo effect without deception for clinical benefit[19].
In the current RCT we sought to extend the earlier, counter-intuitive finding from our pilot trial that OLP is more effective than no-pill control (NPC) in IBS by including a larger sample size, longer treatment duration and a concurrent comparison to double-blind placebo (DBP). In addition, we investigated whether the treatment efficacy of OLP differs from DBP. To our knowledge, no such study has ever been performed. Based on our previous trial of open-label placebo in IBS[17] as well as OLP RCT studies in other conditions[6,14,16,20,22,24,27] we hypothesized that OLP would be superior to NPC for improving IBS symptoms.
Methods
Study Design
We conducted a six-week RCT in a single academic medical center from June 2016 to January 2019. Patients were randomly assigned in equal proportions to three groups: (1) Open-Label Placebo (OLP), (2) Double-Blind Placebo (DBP), and (3) No-Pill Control (NPC), which controlled for natural history, regression to the mean and Hawthorne effects. To create ethical conditions for double-blind placebo, half as many patients were randomized to a fourth group, Double-Blind Peppermint Oil (DBM).
As prospectively planned in our published protocol [1] and in our NIH grant application, our study focused only on placebo effects in IBS, and more particularly on two primary questions: (1) Is open label placebo superior to no treatment control? and (2) How does the efficacy of open label placebo compare to the efficacy of double blind placebo? From a purely scientific point of view, neither of these questions would require inclusion of an active treatment arm. However, from an ethical point of view, we needed to include an active treatment arm to establish double blind conditions without deceiving patients and clinicians. The emphasis on placebo effects in our two primary aims is reflected in the fact that we randomized half as many patients to the peppermint oil arm, thus allocating more statistical power to our primary aims. Our a priori data analytic plan and our power analysis were both based on the planned three-arm placebo study [1]. Consequently, outcomes from the double-blind assessment of peppermint oil group will be reported elsewhere.
Originally, the study was designed to include 280 participants in the four groups. However, because of a computer malfunction, primary outcome data on the first 26 patients were not collected. In collaboration with the NIH, our funding agency, we were given permission to restart the study from this point onward; moreover, we were also given approval to include an additional 60 participants to replace the lost data and to improve capacity for secondary analyses. See Figure 1 for details.
Figure 1:
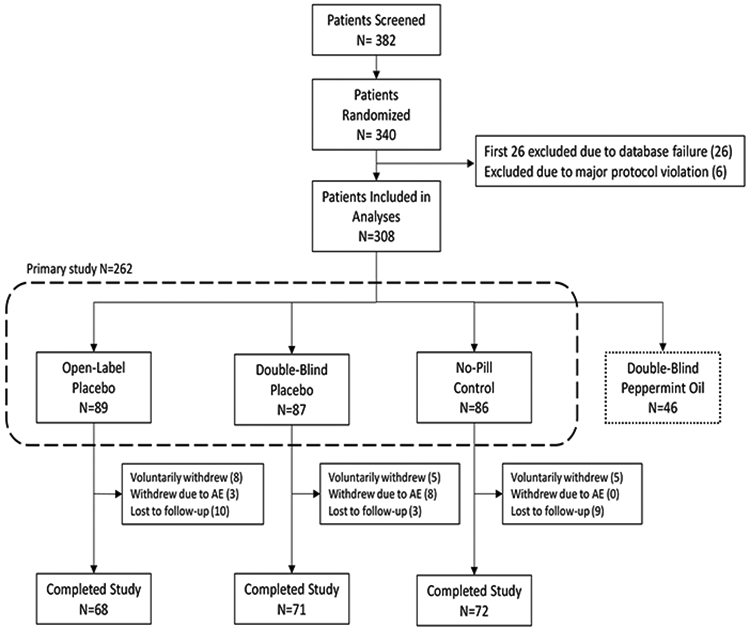
Patient Flow Diagram.
Eligibility and Recruitment
Participants who were 18-80 years old, met Rome IV criteria for IBS, and had at least moderately severe IBS symptoms (defined as a score of ≥ 175 on the IBS-Symptom Severity Scale (IBS-SSS)) were eligible for an initial visit. Participants were eligible to participate if their IBS medication regimen (e.g., fiber, tricyclic antidepressants, anti-spasmodics, etc.) had been stable for at least 30 days and agreed not to change their IBS treatment for the duration of the study.
Participants were excluded from the study if they reported: (1) unexplained or uninvestigated alarm features (e.g. rectal bleeding, unintentional weight loss, iron deficiency anemia, family history of colon cancer, etc.), (2) severe acid reflux (defined as an average of three or more episodes of heartburn or regurgitation per day over a week), (3) use of peppermint oil in the past 30 days, (4) if the investigators judged that the patient had a diagnosis that would interfere with the assessment of efficacy, or the safety of the participant, or (5) allergy to soybean oil (since the placebo contained soybean oil to match the appearance of the peppermint oil pills). See below and our published protocol paper for further details[1].
Participants were recruited from advertisements on public transportation, newspapers, direct mailings to patients, and referrals from healthcare professionals. When potential participants contacted the study staff, the study staff explained the entire trial transparently.
Study visits
Visit 1 (Baseline).
After informed consent was obtained and baseline questionnaires were completed, participants were seen by a board-certified gastroenterologist (AL, JN, JI, or VR), who performed a routine supportive GI-focused interview and physical examination, as would be done in clinical practice, to verify eligibility. Physician assignment was quasi-randomized and based on availability. All participants received the same brief rationale describing the overall study. This rationale was semi-scripted and emphasized three main points: (1) we know that placebos can produce clinically meaningful improvement in double-blind trials, (2) we don’t know if placebos work when honestly given (i.e. un-blinded or open-label), and (3) it is not necessary to believe that placebos will work in order to experience benefit. For further details, see our previously published protocol paper[1]. While the bullet points were standardized, we allowed physicians to follow their usual therapeutic style. We did not train or direct the physicians to use any communication enhancements different from the regular practice. After this brief rationale, the physician opened a sealed, opaque envelope and informed the participant of their allocation to either: OLP, NPC, or double-blind (placebo or peppermint oil). Given the complexity of the design, physicians then briefly reviewed the semi-scripted information for the assigned arm (see Supplement for additional details). For participants receiving pills (OLP, DBP or DBM), it was emphasized that taking the pills as prescribed was critical, and that any improvements could happen either rapidly or gradually. The scientific importance of the no-pill control was emphasized. Study physicians were trained in delivering the script transparently and with equipoise. Honesty was emphasized. If participants spontaneously expressed skepticism about OLP, physicians validated their doubts by discussing their own puzzlement and reflected on the unique design of this trial. Participants were encouraged participants to keep an open mind and “see what happens.” For patients on NTC, the scientific importance of this control group was emphasized and it was repeated that they would receive advice on their IBS at the end of the study.
Visits 2 (Midpoint) and 3 (Endpoint).
During Visit 2 (week 3) and Visit 3 (week 6), all participants completed questionnaires, were verbally asked about adverse events, and briefly met with a study physician (AL, JN, JI, or VR based on availability).
Placebo pills
Placebo pills contained 0.2mL of soybean oil in enteric-coated softgels (~ 14mm x 8mm; manufactured by SoftGel Technologies Inc.; Los Angeles, CA, USA) and were designed to match the peppermint oil pills (Pepogest™; Greenbay, WI, USA). All participants in the treatment arms received the same instructions to take one softgel, three times per day, 30 minutes before meals. All pills were undisguisable. The bottles were labeled as “Open-Label Placebo” in the OLP arm and as “Double-Blind Placebo or Peppermint Oil” in the double-blind arm.
Randomization, stratification, and blinding
Treatment assignments were randomly generated by a program written by one of our biostatisticians (RD) employing SAS statistical software (SAS Institute, Inc.; Cary, NC, USA), and using permuted block randomization with randomly varying block sizes. Randomization for the full study was done in a 2:2:2:1 ratio (OLP, NPC, DBP, and DBM). Given that our placebo questions were primary and that peppermint oil was our foil, we randomized half as many participants to peppermint oil. We also stratified randomization based on IBS-SSS severity (<300 and ≥300) and sex, resulting in four strata. Furthermore, 34 participants were randomly assigned and completed a 30-minute qualitative interview after completing the study. (These results will be published elsewhere.)
All outcomes measures were administered by blinded research assistants. Participants in the DBP group were blinded to their treatment assignment (i.e., they did not know whether their pills contained peppermint oil or not); however, participants in the OLP and NPC groups were not blinded.
Outcome Assessments
The validated IBS Severity Scoring System (IBS-SSS) was the primary outcome measure. The IBS-SSS measures 5 items (severity of abdominal pain, number days with abdominal pain, severity of abdominal distension, dissatisfaction with bowel habits, and interference with quality of life), each on a 0-100 scale. IBS-SSS scores can range from 0-500, with higher scores indicating greater symptom severity. IBS symptoms can be categorized as mild (75-174), moderate (175-300), or severe (>300). A decrease of 50 points is considered a clinically meaningful improvement in symptoms[11].
We used two additional instruments as secondary IBS outcomes: (1) the IBS global improvement scale (IBS-GIS)[12], which measures participants’ global improvement in the last seven days on a scale that ranges from 1(substantially worse), to 7(substantially improved), and (2) the IBS adequate relief scale (IBS-AR)[25] which is a single dichotomous question: “Have you had adequate relief of your IBS symptoms over the past week?” Unlike the IBS-SSS, the secondary outcomes are not measured at baseline.
To evaluate participants’ attitudes towards the treatments, participants were asked to rate their expectancy for improvement (0-100 VAS) if they received: (a) placebo, or (b) peppermint oil. These questions were asked at baseline prior to learning randomization assignments.
Statistical analysis
To calculate power for our primary analysis, we used our previous pilot trial of OLP in IBS[17], in which the effect size for the standardized mean difference (Cohen’s d) between OLP and NPC on IBS-SSS improvement was d = 0.53. We calculated that a total of 240 participants, with 80 participants in each of the three groups was sufficient to achieve 90% power to detect such an effect size.
We conducted a modified intent-to-treat analysis that included all randomized patients who provided at least one post-baseline, primary outcome assessment, without any exclusions other than major protocol violations and database error (see Figure 1 for details). To address any potential bias due to missing data, we also planned to conduct a sensitivity analysis using multiple imputation by chained equations (MICE) to replace missing data and allow for a full intent-to-treat analysis that would include all randomized participants with no exclusions.
To test whether six weeks of OLP, DBP, or NPC treatment resulted in different clinical outcomes in IBS, we conducted a one-way analysis of covariance (ANCOVA) on IBS-SSS scores, with gender and baseline IBS-SSS scores as a covariates, and treatment condition (OLP vs. DBP vs. NPC) as the independent variable. If the omnibus ANCOVA was significant, we planned to conduct Fisher’s Least Significant Difference (LSD) tests to make pairwise comparisons between the three groups. In the special case of three groups, it has been shown that this two-step, Fisher’s LSD procedure controls Type I family-wise error rate (FWER) at the nominal alpha level, in this case 5%[13]. For each contrast, we also planned to compute effect sizes in the form of Cohen’s d, the standardized mean difference between groups. By convention, a small effect size is d=.20, medium is d=.50, and large is d=.80[7].
We also conducted a one-way analysis of covariance (ANCOVA) on IBS-GIS scores, with gender, and initial severity (moderate vs. severe), the factors used for stratifying the randomization, as covariates, and treatment condition (OLP vs. DBP vs. NPC) as the independent variable. If the ANCOVA was significant, we planned to use Fisher’s LSD tests to make pairwise comparisons between the three groups. For each contrast, we also computed effect sizes in the form of Cohen’s d.
For IBS-AR, we conducted logistic regression analyses, with gender and initial severity (moderate vs. severe) as covariates, and treatment condition (OLP vs. DBP vs. NPC) as the independent variable. If the overall test for the three groups was significant, we planned to follow-up with pairwise post hoc tests.
Missing data minimization strategies included patient retention efforts and a modified intent-to-treat analysis. Patient-reported assessments were captured electronically at each visit, and the system prohibited participants from omitting items.
Results
Participants
A total of 340 participants were randomized to the four arms of the study. However, data from the first 26 participants were excluded due to a database failure. Six additional participants were excluded due to major protocol violations (e.g., a patient assigned to double-blind placebo began taking over-the-counter peppermint oil). Only participants who were randomized to open-label placebo (n=89), double-blind placebo (n=87), or no-pill control (n=86) are included in the current analyses (n=262). For additional details, see the Methods section and Figure 1.
The demographic characteristics of the three groups are shown in Table 1. The mean age was 42.0 years (SD=18.1). The majority were women (72.9%), and most reported their race as white (83.6%). Based on IBS-SSS (0-500), symptom severity at baseline was moderate (175-299) for 63.4% and severe (≥300) for 36.6% of participants. Overall, the mean baseline IBS-SSS severity was 282.1 (SD=67.4). Participants reported having consulted with a median of 2 physicians and 1 gastroenterologist for their IBS. Nearly half of participants (47.7%) reported having had IBS for more than 10 years. There were no significant differences between the groups on any of the baseline characteristics reported in Table 1.
Table 1:
Demographics and Baseline Characteristics.
| Demographics and Baseline Characteristics |
Open-Label Placebo n = 89 |
Double-Blind Placebo n = 87 |
No-Pill Control n = 86 |
|---|---|---|---|
| Age | 42.2 (17.8) | 43.8 (19.2) | 40.0 (17.0) |
| % Female | 71.9 | 73.6 | 73.3 |
| % African American | 4.5 | 3.4 | 3.5 |
| % Asian | 3.4 | 4.6 | 10.5 |
| % Caucasian | 84.3 | 86.2 | 80.2 |
| No. Doctors Seen for IBS | 2.5 (1.8) | 2.3 (1.5) | 2.9 (2.1) |
| Baseline Severity (IBS-SSS) | 286.0 (62.0) | 285.8 (69.0) | 274.4 (71.1) |
| % Moderate (IBS-SSS 175-299) | 60.7 | 62.1 | 67.4 |
| % Severe (IBS-SSS ≥ 300) | 39.3 | 37.9 | 32.6 |
| % IBS-Constipation | 20.2 | 20.7 | 27.9 |
| % IBS-Diarrhea | 41.6 | 44.8 | 39.5 |
| % IBS-Mixed | 34.8 | 31.0 | 30.2 |
| % IBS-Undefined | 3.4 | 3.4 | 2.3 |
| % IBS Duration > 10 years | 44.4 | 44.7 | 54.0 |
| PHQ-8 Depression | 4.7 (4.2) | 5.3 (4.9) | 5.7 (5.3) |
| GAD-7 Generalized Anxiety | 4.2 (4.2) | 4.8 (4.5) | 5.2 (5.6) |
Note: Values are means (standard deviations) unless otherwise specified. IBS-SSS = IBS-Severity Scoring System (range 0-500). PHQ-8 = Patient Health Questionnaire depression scale (range 0-24). GAD-7 = Generalized Anxiety Disorder scale (range 0-21).
Since the study physicians may have differed in their communication styles, we examined whether the four gastroenterologists each saw roughly an equal number of patients in each of the three treatment arms. By chance, one would expect that 33.3% of each physician’s clinical encounters should be in each of the three treatment groups. In fact, the lowest percentage was 27.8% and the highest was 37.5%, and the distribution of visits across treatment arms did not differ significantly from chance.
Primary Outcome
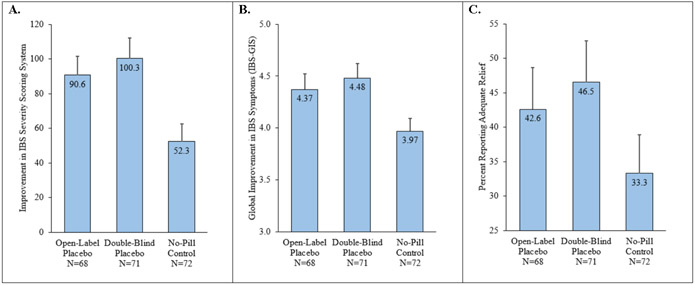
The omnibus ANCOVA comparing OLP, DBP, and NPC on mean IBS-SSS improvement from baseline to 6-week endpoint was statistically significant (p=.011). The mean improvement in IBS-SSS from baseline to the 6-week endpoint, our primary outcome, was significantly greater in OLP compared to NPC (90.6 vs. 52.3, p=0.031). OLP and DBP did not differ significantly on IBS-SSS improvement (p=.485). The effect sizes were moderate for OLP vs. NPC (d=.43) and small for OLP vs DBP (d=.10). These results are illustrated in Figure 2 and Table 2. Additionally, DBP was superior to NPC (100.3 vs.52.3, p=0.004.
Figure 2:
Outcomes at 6-week Endpoint. (A) Primary Outcome: Improvement on the IBS Severity Scoring System (IBS-SSS). (B) Secondary Outcome: Global Improvement in IBS Symptoms (IBS-GIS). (C) Secondary Outcome: Percent of Participants Reporting Adequate Relief of Symptoms (IBS-AR).
Table 2:
Outcomes at 6-Week Endpoint.
| Primary Outcome | |||||||
|---|---|---|---|---|---|---|---|
| IBS-SSS Improvement from Baseline to 6-week Endpoint | p-values | ||||||
| Open-Label Placebo (n=68) |
Double-Blind Placebo (n=71) |
No-Pill Control (n=72) |
Global test |
OLP vs. NPC |
DBP vs. NPC |
OLP vs. DBP |
|
| Mean (SD) | 90.6 (89.5) | 100.3 (99.6) | 52.3 (87.0) | 0.015 | 0.038 | 0.005 | 0.485 |
| 95% CI | 68.6 – 112.6 | 78.7 – 121.8 | 30.8 – 73.7 | ||||
| Secondary Outcomes | |||||||
| IBS-SSS reduction from Baseline to 6 week Endpoint | p-values | ||||||
| Open-Label Placebo |
Double-Blind Placebo |
No-Pill Control |
Global test |
OLP vs. NPC |
DBP vs. NPC |
OLP vs. DBP |
|
| 50 point reduction * | 69.1% | 70.4% | 54.2% | 0.083 | -- | -- | -- |
| 150 point reduction * | 29.4% | 29.6% | 12.5% | 0.018 | 0.021 | .014 | .999 |
| Global Improvement (IBS-GIS) at 6-Week Endpoint | p-values | ||||||
| Open-Label Placebo |
Double-Blind Placebo |
No-Pill Control |
Global test |
OLP vs. NPC |
DBP vs. NPC |
OLP vs. DBP |
|
| Mean (SD) | 4.37 | 4.48 | 3.97 | 0.021 | 0.041 | 0.008 | 0.562 |
| Slight, Moderate or Substantial * | 41.7% | 44.6% | 24.3% | 0.019 | 0.047 | 0.008 | 0.607 |
| Moderate or Substantial * | 18.1% | 20.3% | 5.4% | 0.012 | 0.019 | 0.007 | 0.834 |
| Adequate Relief (IBS-AR) at 6-Week Endpoint | p-values | ||||||
| Open-Label Placebo |
Double-Blind Placebo |
No-Pill Control |
Global test |
OLP vs. NPC |
DBP vs. NPC |
OLP vs. DBP |
|
| Percent | 42.6% | 46.5% | 33.3% | 0.258 | -- | -- | -- |
post-hoc analyses
Note: IBS-SSS = IBS Severity Scoring System. IBS-GIS = IBS Global Improvement Scale. IBS-AR = IBS Adequate Relief.
To provide some additional clinical data on response rates, we also performed a post hoc analysis of the percent of participants who improved by 50 points on the IBS-SSS (considered a clinically significant response) and by 150 points (considered a very strong clinical response). As can be seen in Table 2, approximately 70% of OLP and DBP participants reported a 50 point reduction in IBS-SSS, as compared to only 54% of NPC participants. Similarly, approximately 30% of OLP and DBP participants reported a 150 point reduction, as compared to only 12% of NPC participants.
Secondary Outcomes
The omnibus ANCOVA comparing OLP, DBP, and NPC on mean global improvement scores (IBS-GIS) at the 6-week endpoint was statistically significant (p=.021). At the 6 week endpoint, OLP reported significantly higher mean IBS-GIS scores compared to NPC (4.37 vs. 3.97, p=0.041, as did DBP compared to NPC (4.48 vs. 3.97, p=0.008). OLP and DBP did not differ significantly from each other in mean IBS-GIS scores (p=.562). The observed effect sizes were small to medium for OLP vs. NPC (d=.35), medium for DBP vs. NPC (d=.46).and small for OLP vs DBP (d=.09). In addition, the percentage of participants who reported moderate or substantial global improvement was significantly higher for OLP compared to NPC (18.1% vs. 5.4%, p=.019, see Table 2 and Figure 2).
To provide some additional clinical data on response rates, we also performed a post hoc analysis of percent of participants who reported any global improvement (i.e., slight, moderate, or substantial) as well as the percent who reported moderate or substantial global improvement. These values are displayed in Table 2.
Although the rates of adequate relief reported at the 6-week endpoint by OLP (42.6%) and DBP (46.5%) were numerically higher than NPC (33.3%), the logistic regression testing for differences between the three groups was not statistically significant (p=.258); and therefore, no follow-up tests were conducted (see Table 2 and Figure 2).
To address any potential bias due to missing data, we also conducted a sensitivity analysis using multiple imputation by chained equations (MICE) to replace missing data, thus producing a full intent-to-treat analysis that included all randomized participants without any exclusions. As detailed in the Supplement, these multiple imputation analyses produced a similar pattern of effects for all three outcome measures. Finally, outcomes at the 3-week midpoint were not statistically significant, but showed a similar pattern (see Supplement).
Expectancy
Prior to learning their randomization assignment, patients were asked to rate their expectancy for improvement (0-100 VAS) if they received: (a) placebo, or (b) peppermint oil. OLP and DBP participants reported nearly identical mean baseline expectancies for both questions (55.9 vs. 55.3, respectively, for peppermint oil; and 40.1 vs. 41.9 for placebo). These small differences between OLP and DBP were not significant (p>.65 for both tests). Combining OLP and DBP together, a paired t-test showed that expectancies were significantly higher for peppermint oil as compared to placebo (55.6 vs. 41.0, respectively, p<.001). Interestingly, expectancy for the DBP group was significantly correlated with improvement in IBS-SSS scores from baseline to the 6-week endpoint (p=.01) with a medium effect size (r=.30). In contrast, expectancy for the placebo treatment was not significantly correlated with outcome in the OLP group (p=.25), and the observed effect size was negative and of small magnitude (r= −.14). The difference between these two correlations (i.e., r=.25 vs. r= −.14) was statistically significant (p=.01).
Adverse Events
Significantly more participants in the DBP group reported adverse events (31.0%) as compared to participants in both OLP (15.7%, p=.008) and NPC (9.3%, p<.001). The proportion of participants in the OLP and DBP groups reporting adverse events did not differ significantly (p=.27). There was a total of 22 adverse events reported in OLP compared with 44 in DBP, and only 11 in NPC. Adverse events reported by two or more participants overall are shown in Table 3, the majority of which were gastrointestinal.
Table 3:
Adverse Events.
| Adverse Events (AEs) | Open-Label Placebo n = 89 |
Double-Blind Placebo n = 87 |
No-Pill Control n = 86 |
|---|---|---|---|
| Total number of AEs | 22 | 44 | 11 |
| Reflux/heartburn | 1 (1.1%) | 10 (11.5%) | 0 (0.0%) |
| Diarrhea | 5 (5.6%) | 5 (5.7%) | 0 (0.0%) |
| Belching | 1(1.1%) | 2 (2.3%) | 0 (0.0%) |
| Nausea | 0 (0.0%) | 3 (3.4%) | 2 (2.3%) |
| Abdominal pain | 2 (2.2%) | 2 (2.2%) | 1 (1.2%) |
| Gas | 1(1.1%) | 4 (4.6%) | 0 (0.0%) |
| Constipation | 0 (0.0%) | 2 (2.3%) | 1 (1.2%) |
| Bloating | 2 (2.2%) | 1 (1.1%) | 0 (0.0%) |
| Epigastric Pain | 0 (0.0%) | 1 (1.1%) | 1 (1.2%) |
| Fatigue | 0 (0.0%) | 2 (2.3%) | 0 (0.0%) |
| Bronchitis | 0 (0.0%) | 1 (1.1%) | 1 (1.2%) |
| Cold-like symptoms | 0 (0.0%) | 1 (1.1%) | 1 (1.2%) |
Note: Only adverse events that occurred in 2 or more participants are listed. Therefore, the total number of adverse events is greater than the sum of the individual events listed.
Discussion
This is the first study to directly compare the effects of open-label placebo and double-blind placebo in any medical condition. We found that open-label placebo was significantly better than no-pill control in improving IBS symptoms as measured by our primary outcome (IBS-SSS), as well as by one of our secondary outcomes, global improvement (IBS-GIS). We also found that improvement in IBS symptoms (IBS-SSS) and global improvement (IBS-GIS) in participants receiving open-label placebo was similar to those receiving double-blind placebo. This study confirms our previous finding in IBS that open-label placebo is superior to usual care (i.e., no-pill controls)2 and challenges the widely held assumption that blinding is necessary in order for participants to improve with placebo.
We found that open-label placebo was significantly better than no-pill control in improving IBS symptoms as measured by our primary outcome (IBS-SSS), as well as by one of our secondary outcomes, global improvement (IBS-GIS). Although the test for the other secondary outcome, adequate relief (IBS-AR), was not statistically significant, a numerically higher percentage of participants in the OLP group reported adequate relief as compared to NPC (42.6% vs. 33.3%). Our study is consistent with previous studies showing that open-label placebo is superior to usual care (i.e., no-pill controls)[6,14,16,17,20,22,24,27]. To our knowledge, the present study had the largest sample size and longest duration of any OLP trial to date.
It is notable that there were twice as many adverse events (AEs) reported in double-blind placebo as compared to open-label placebo. This is likely due to the “nocebo” effect, in which participants receiving double-blind placebo sometimes report side effects due to their knowledge that they might be receiving an active medication. Indeed, in clinical trials, reported side effects to placebo often match the typical side effects associated with the investigational treatment (presumably because participants are given information about possible side effects of the investigational treatment)[31]. We accurately informed participants that side effects were rarely reported in published RCTs of peppermint oil and that those side effects were typically mild and related to reflux/heartburn[10]. Nonetheless, we observed higher reports of reflux in the DBP group compared to OLP and NPC.
The clinical response to OLP in this trial was high with a 69.1% of participants receiving OLP reporting a clinically meaningful improvement in symptoms (i.e. improvement in IBS-SSS ≥ 50 points)[11]. The finding that openly prescribed placebo may be as effective as blinded placebo has implications for clinical practice and for future OLP research, especially in chronic visceral and somatic pain conditions[19]. It has been well-documented that many physicians admit to prescribing medicines that they believe will not have any pharmacological effects in the hope of inducing a placebo effect (sometimes referred to as “impure” placebos)[9,21,23]. For example, in a national survey of 1,200 randomly selected U.S. physicians, approximately 50% reported having regularly prescribed impure placebos[28]. This practice is most often observed in the treatment of patients with chronic functional conditions[5]. The results of the current study suggest, however, that deception may not be necessary, and that at least in some conditions, patients may still show improvement even when prescribed open-label placebos.
We would argue that treatment with open-label placebo fulfills the American Medical Association’s ethical standards of informed consent, transparency and respect for person[4,30].Survey and focus group evidence suggests that patients are willing to try OLP. For example, a survey of 853 US patients indicated that 62% would “probably” or “definitely” take OLP if recommended by a doctor[15]. This finding was replicated in a focus group in the UK (n=58)[3]. In the current study, we only assessed participants’ expectancies for placebo, in general. In future studies, it would be helpful to also assess participants’ expectancies for open-label placebo specifically. There are no data on physicians’ attitudes, but we speculate that OLP may not as be as acceptable to physicians because their professional identify is tied to “medications that are not placebos.” More confirmatory data, engaged discussion, and critical self-examination may be required before physicians would be willing to prescribe open-label placebos.
This study has several strengths, including its relatively large sample size, rigorous endpoints, and innovative design. However, there are also some limitations to consider. Participants in this study may not be representative of the general population of IBS patients since they were individuals who were willing to try open-label placebo and/or herbal medicine (i.e., peppermint oil) as a treatment for IBS. However, we would note that the same limitation applies to all RCTs. For example, an RCT testing a new medication would only be generalizable to patients who are willing try a pharmaceutical for their disorder, thus excluding those who are skeptical about drug treatments. In addition, since no objective markers have been definitively associated with IBS, our results necessarily relied upon the standard measures of self-reported symptoms used by IBS researchers and clinicians. That said, we deliberately chose a functional illness defined by patient-self appraisal because previous research and theoretical models suggest these conditions reliably have robust placebo responses [18]. Finally, despite the positive results for OLP from our two initial RCTs in IBS, we believe that these findings should be independently replicated by a large multi-centered trial of longer duration.
It is important to emphasize that the findings presented here should not be interpreted as meaning that open-label placebos should be considered as a substitute for double-blind placebos in pharmaceutical RCTs. Double-blind placebos not only control for placebo effects, but they also reduce potential biases involving allocation, attention, detection, performance, and attrition[8,29].
Conclusion:
It appears that in some conditions, concealment or deception is not necessary for patients to benefit from placebo treatment. Moreover, our data suggest that open-label placebo has comparable efficacy to double-blind placebo in IBS. Despite these conclusions, more research is required in order to harness open-label placebo as an ethical and effective treatment for IBS, and perhaps, other chronic functional disorders.
Supplementary Material
Acknowledgements:
This study was supported by NIH grant R01AT008573. TJK is partially supported by a grant from the Foundation for the Science of the Therapeutic Encounter. There are no conflicts of interests for the authors.
Footnotes
Ethics approval: This study was approved by the Committee on Clinical Investigations at Beth Israel Deaconess Medical Center.
Data sharing: De-identified data will be made available with formal request with Ethics approval.
References
- [1].Ballou S, Kaptchuk TJ, Hirsch W, Nee J, Iturrino J, Hall KT, Kelley JM, Cheng V, Kirsch I, Jacobson E, Conboy L, Lembo A, Davis RB. Open-label versus double-blind placebo treatment in irritable bowel syndrome: study protocol for a randomized controlled trial. Trials 2017;18:234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Beecher HK. The powerful placebo. J Am Med Assoc 1955;159:1602–1606. [DOI] [PubMed] [Google Scholar]
- [3].Bishop FL, Aizlewood L, Adams AEM. When and why placebo-prescribing is acceptable and unacceptable: a focus group study of patients’ views. PLoS ONE 2014;9:e101822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Blease C, Colloca L, Kaptchuk TJ. Are open-Label Placebos Ethical? Informed Consent and Ethical Equivocations. Bioethics 2016;30:407–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Burke MJ. “It’s All in Your Head”-Medicine’s Silent Epidemic. JAMA Neurol 2019. [DOI] [PubMed] [Google Scholar]
- [6].Carvalho C, Caetano JM, Cunha L, Rebouta P, Kaptchuk TJ, Kirsch I. Open-label placebo treatment in chronic low back pain: a randomized controlled trial. Pain 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Cohen J A power primer. Psychol Bull 1992;112:155–159. [DOI] [PubMed] [Google Scholar]
- [8].Enderlein G Pocock SJ: Clinical Trials — a practical approach. John Wiley & Sons, Chichester — New York — Brisbane — Toronto — Singapore 1983, 265 S., £ 16.95. Biometrical Journal 1985;27:634–634. [Google Scholar]
- [9].Fässler M, Meissner K, Schneider A, Linde K. Frequency and circumstances of placebo use in clinical practice--a systematic review of empirical studies. BMC Med 2010;8:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Ford AC, Talley NJ, Spiegel BMR, Foxx-Orenstein AE, Schiller L, Quigley EMM, Moayyedi P. Effect of fibre, antispasmodics, and peppermint oil in the treatment of irritable bowel syndrome: systematic review and meta-analysis. BMJ 2008;337:a2313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Francis CY, Morris J, Whorwell PJ. The irritable bowel severity scoring system: a simple method of monitoring irritable bowel syndrome and its progress. Aliment Pharmacol Ther 1997;11:395–402. [DOI] [PubMed] [Google Scholar]
- [12].Gordon S, Ameen V, Bagby B, Shahan B, Jhingran P, Carter E. Validation of irritable bowel syndrome Global Improvement Scale: an integrated symptom end point for assessing treatment efficacy. Dig Dis Sci 2003;48:1317–1323. [DOI] [PubMed] [Google Scholar]
- [13].Hayter AJ. The Maximum Familywise Error Rate of Fisher’s Least Significant Difference Test. Journal of the American Statistical Association 1986;81:1000–1004. [Google Scholar]
- [14].Hoenemeyer TW, Kaptchuk TJ, Mehta TS, Fontaine KR. Open-Label Placebo Treatment for Cancer-Related Fatigue: A Randomized-Controlled Clinical Trial. Sci Rep 2018;8:2784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Hull SC, Colloca L, Avins A, Gordon NP, Somkin CP, Kaptchuk TJ, Miller FG. Patients’ attitudes about the use of placebo treatments: telephone survey. BMJ 2013;347:f3757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Kam-Hansen S, Jakubowski M, Kelley JM, Kirsch I, Hoaglin DC, Kaptchuk TJ, Burstein R. Altered placebo and drug labeling changes the outcome of episodic migraine attacks. Sci Transl Med 2014;6:218ra5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Kaptchuk TJ, Friedlander E, Kelley JM, Sanchez MN, Kokkotou E, Singer JP, Kowalczykowski M, Miller FG, Kirsch I, Lembo AJ. Placebos without deception: a randomized controlled trial in irritable bowel syndrome. PLoS ONE 2010;5:e15591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Kaptchuk TJ, Hemond CC, Miller FG. Placebos in chronic pain: evidence, theory, ethics, and use in clinical practice. BMJ (Clinical research ed) 2020;370:m1668. [DOI] [PubMed] [Google Scholar]
- [19].Kaptchuk TJ, Miller FG. Open label placebo: can honestly prescribed placebos evoke meaningful therapeutic benefits? BMJ 2018;363:k3889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Kelley JM, Kaptchuk TJ, Cusin C, Lipkin S, Fava M. Open-label placebo for major depressive disorder: a pilot randomized controlled trial. Psychother Psychosom 2012;81:312–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Kermen R, Hickner J, Brody H, Hasham I. Family physicians believe the placebo effect is therapeutic but often use real drugs as placebos. Fam Med 2010;42:636–642. [PubMed] [Google Scholar]
- [22].Kleine-Borgmann J, Schmidt K, Hellmann A, Bingel U. Effects of open-label placebo on pain, functional disability, and spine mobility in patients with chronic back pain: a randomized controlled trial. Pain 2019;160:2891–2897. [DOI] [PubMed] [Google Scholar]
- [23].Meissner K, Höfner L, Fässler M, Linde K. Widespread use of pure and impure placebo interventions by GPs in Germany. Fam Pract 2012;29:79–85. [DOI] [PubMed] [Google Scholar]
- [24].Olliges E, Stroppe S, Haile A, Malhis M, Funke S, Meissner K. Open-label placebo for elderly patients with chronic knee pain: effects of pain, functionality and quality of life. Abstract #P201 2nd Official SIPS Conference2019. [Google Scholar]
- [25].Passos MCF, Lembo AJ, Conboy LA, Kaptchuk TJ, Kelly JM, Quilty MT, Kerr CE, Jacobson EE, Hu R, Friedlander E, Drossman DA. Adequate relief in a treatment trial with IBS patients: a prospective assessment. Am J Gastroenterol 2009;104:912–919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Sandler AD, Bodfish JW. Open-label use of placebos in the treatment of ADHD: a pilot study. Child Care Health Dev 2008;34:104–110. [DOI] [PubMed] [Google Scholar]
- [27].Schaefer M, Harke R, Denke C. Open-Label Placebos Improve Symptoms in Allergic Rhinitis: A Randomized Controlled Trial. Psychother Psychosom 2016;85:373–374. [DOI] [PubMed] [Google Scholar]
- [28].Tilburt JC, Emanuel EJ, Kaptchuk TJ, Curlin FA, Miller FG. Prescribing “placebo treatments”: results of national survey of US internists and rheumatologists. BMJ 2008;337:a1938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Tyson J, Kennedy K. Guide to clinical trials. Bert Spilker, Raven Press. New York, 1991. No. of pages: xxv + 1156. Price: $162.50. ISBN: 0-88167-767-1. Statistics in Medicine 1993;12:1648–1648. [Google Scholar]
- [30].Use of Placebo in Clinical Practice. American Medical Association; n.d. Available: https://www.ama-assn.org/delivering-care/ethics/use-placebo-clinical-practice. Accessed 27 Mar 2020. [Google Scholar]
- [31].Wells RE. To Tell the Truth, the Whole Truth, May Do Patients Harm: The Problem of the Nocebo Effect for Informed Consent. Am J Bioeth 2012;12:22–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.