Abstract
The gastrointestinal (GI) tract is a highly dynamic structure essential for digestion, nutrient absorption, and providing an interface to prevent gut bacterial translocation. In order to maintain the barrier function, the gut utilizes many defense mechanisms including proliferation, apoptosis, and apical junctional complexes. Disruption of any of these parameters due to injury or disease could negatively impact the intestinal barrier function and homeostasis resulting in increased intestine inflammation, permeability, bacterial dysbiosis, and tissue damage. MicroRNAs are small noncoding RNA sequences that are master regulators of normal cellular homeostasis. These regulatory molecules affect cellular signaling pathways and potentially serve as candidates for providing a mechanism of impaired gut barrier integrity following GI-related pathologic conditions, ethanol exposure, or trauma such as burn injury. MicroRNAs influence cellular apoptosis, proliferation, apical junction complex expression, inflammation, and the microbiome. Due to their widespread functional affiliations, altered expression of microRNAs are associated with many pathologic conditions. This review explores the role of microRNAs in regulation of intestinal barrier integrity. The studies reviewed demonstrate that microRNAs largely impact intestine barrier function and provide insight behind the observed adverse effects following ethanol and burn injury. Furthermore, these studies suggest that microRNAs are excellent candidates for therapeutic intervention or for biomarkers to manage gut barrier integrity following trauma such as burn injury and other GI-related pathologic conditions.
Keywords: ethanol, inflammation, burn, small RNAs
Summary Sentence:
The studies reviewed in this article focus on microRNAs that largely impact intestine barrier integrity and provide an insight into the mechanism underlying gut barrier disruption in acute injuries.
1. INTRODUCTION
The gastrointestinal (GI) tract performs multiple functions including digestion, nutrient absorption, as well as maintaining a barrier that limits the translocation of bacteria living in the GI tract. Constant exposure to antigens from the diet and commensal bacteria (up to 1012 microorganisms per gram of tissue) requires the gut to employ physical and immunologic defense barriers that limit luminal bacteria translocation.2–7 As shown in Figure 1, the intestine forms a semi-permeable barrier composed of a single layer of columnar epithelial cells that are sealed by tight junction proteins, a semi-permeable protein complex comprised of transmembrane, scaffold, and adaptor proteins.3,8–10 Although the majority of columnar epithelial cells are absorptive enterocytes, there are other cell types (e.g., goblet cells, M cells, and Paneth cells) that participate in intestinal defense.2,3,8 Additionally, the intestine contains a mucus barrier composed of mucins secreted by goblet cells, which prevents luminal bacteria from adhering to the epithelial cells.3 Paneth cells contribute to gut homeostasis by producing a large number of antimicrobial peptides (AMPs). The immune component of the intestine includes a layer of loose connective tissue (lamina propria) beneath the epithelial cells and intestinal lymphoid tissue called Peyer’s patches.2,11 This barrier is tightly regulated by apoptosis, a natural physiologic process resulting in death and removal of unwanted cells,12–14 and proliferation that counterbalances the constant cell turnover.15,16
FIGURE 1.
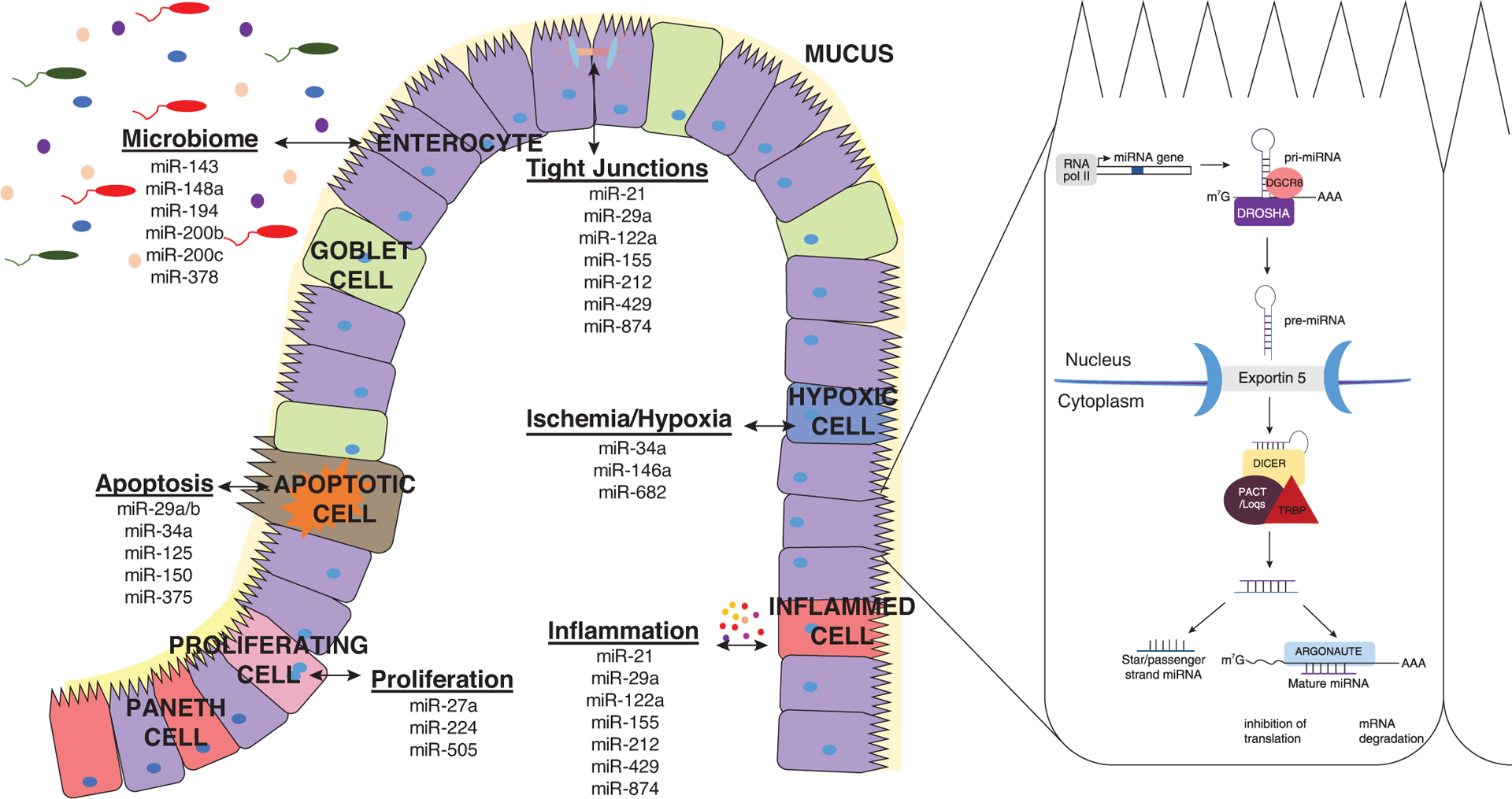
Role of microRNAs in intestinal barrier integrity. Schematic representation of the intestinal barrier. The intestinal barrier can be directly and indirectly affected by cellular regulators, microRNAs. MicroRNAs require a multistep maturation in order to be fully functional. Aberrant expression of microRNAs can in turn affect intestinal barrier integrity by impacting apoptosis, proliferation, tight junction protein expression, ischemia/hypoxia, inflammation, and microbiota composition in the intestine
Together, these components maintain intestinal homeostasis by providing a physical and immunologic barrier. Disruption in any of these barrier functions due to disease, misuse of alcohol, or trauma (e.g., burn injury) may compromise the barrier integrity resulting in increased intestinal permeability.17,18 Understanding mechanisms by which intestinal barrier components are regulated could provide unique therapeutic strategies to treat pathophysiologic conditions that reduce intestinal barrier integrity.
2. MicroRNAs AND THE INTESTINAL BARRIER
MicroRNAs (miRs) regulate many cellular processes that maintain the intestinal barrier (e.g., apoptosis/proliferation, tight junction proteins, and inflammation; Fig. 1). They are small noncoding RNAs that control gene expression at the post-transcriptional level by binding to the 3′ untranslated region of their target mRNA resulting in gene silencing.19,20 As shown in the Figure 1, they undergo a maturation process in order to function as gene silencers. MiRs are transcribed by RNA polymerase II forming a primary miR (pri-miR). The pri-miR undergoes a nuclear cleavage by a microprocessor composed of drosha and its cofactor DiGeorge syndrome critical region gene 8. The nuclear cleavage by the microprocessor forms a 60–70 nucleotide precursor miR (pre-miR), which is exported into the cytoplasm by the ran-GTP dependent exportin-5. In the cytoplasm, the pre-miR is cleaved by dicer and its cofactors trans-activation response RNA-binding protein and protein activator of PKR. The second cleavage forms a 21–24 nucleotide duplex miR consisting of a guide and passenger strand. The passenger strand is usually degraded, whereas the guide strand and an argonaute protein form the miRNA-Induced Silencing Complex (miRISC). The formation of the miRISC permits complementary binding of the seed region of the miR to its target resulting in mRNA degradation or translational repression.19–21
MicroRNAs are estimated to target over 60% of all genes and each miR can have multiple target genes.22,23 Aberrant miR expression can impair normal organ function including the intestinal barrier function. Villin-specific dicer knockout mice exhibited altered intestinal morphology, decreased differentiation, increased intestinal inflammation, and apoptosis. Furthermore, ablation of dicer-1 led to diminished expression and mislocalization of tight junction proteins.24,25 These observations coincided with diminished barrier integrity providing evidence of the indispensable role of miRs in the intestine.
MicroRNA levels and biogenesis are affected in a number of clinical conditions including trauma/injury, ethanol, and disease.26–33 Furthermore, as miRs are ubiquitously expressed by cells in both circulation and tissues, their differential expression can be used as diagnostic tools and for possible therapeutic interventions.31,32,34 MiRs can both be affected and in turn affect cellular process/responses: apoptosis,35–40 proliferation,41–47 tight junction protein expression,29,48–54 ischemia/hypoxia,26,40,55 inflammation,26,28,56–64 and the microbiome65 (Fig. 1) all of which can impact the intestinal barrier function.
3. miRs AND PROLIFERATION AND APOPTOSIS
The intestinal epithelium is one of the most rapidly renewing tissues within the body. The intestine is renewed every 2–3 days in mice and 3–5 days in humans.16 Intestinal epithelial stem cells develop into transit amplifying progenitor cells and differentiate into various subtypes of intestinal cells. Proliferation occurs in the crypts, allowing for differentiation and migration up the intestinal villi. Proliferation is counterbalanced with the cellular process, apoptosis. Proliferation and apoptosis are tightly regulated in order to maintain the intestinal barrier function. Apoptosis is a natural physiologic process resulting in death and removal of unwanted cells.12–14 Apoptosis therefore is crucial to enable normal structure and function of the GI tract.14 Increased levels of apoptosis beyond this normal cellular maintenance however results in an impaired intestinal barrier.14 It is widely accepted that if the delicate balance between proliferation and apoptosis is disrupted, it can result in increased intestinal permeability. This was clearly described following injury. Burn injury alone or in combination with ethanol decreases intestinal proliferation. Furthermore, ki67 staining was significantly decreased following ethanol and burn injury compared with sham animals suggesting that the combined insult diminished intestinal epithelial cell proliferation.66 The balance is further disrupted as ethanol and burn injury increased intestinal apoptosis,67 which contributes to impaired intestinal barrier function. Understanding the mechanism by which this balance is disrupted can lead to therapeutic strategies to mitigate barrier impairment following trauma as well as in other pathologic conditions that have similar adverse effects. Several studies have illustrated the role of miRs in proliferation41–47 and intestinal apoptosis.35–37,39,40,55
As alterations of cell proliferation is a hallmark of cancer, the majority of work on miRs and proliferation is performed in cancer models. Overexpression of miR-27a and miR-505 influenced cell proliferation and invasion of colonic cancer cells.46,47 Numerous studies have shown that miR-224 is increased in colorectal cancer patients.41–45 In vitro studies using HCT-116 and SW-480 colon cancer cell lines demonstrated that up-regulation of miR-224 increased proliferation, and cell migration while promoting cell cycle progression.41,42,44,45 Aberrant expression of miRs involved in proliferation such as the ones described here could negatively impact barrier integrity. Similarly, miRs have been described to affect apoptosis, the cellular process that counterbalances proliferation in order to maintain the intestinal barrier.35–39
miR-150 was significantly elevated in the dextran sodium sulfate (DSS) model of colitis in mice and in active ulcerative colitis in human colonic tissue. The miR-150 target gene, c-Myb, was reduced in DSS-treated colon tissue, which led to decreased levels of the antiapoptotic protein Bcl-2 resulting in increased apoptosis.35 This relationship between miR-150 and apoptosis, however, may be cell-type specific. Christensen et al.36 preformed a TaqMan Human MicroRNA Array and profiled expression of 667 miRs including miR-150 in normal colon mucosa and tissue samples from clinical stage II colorectal cancer patients. The group validated these high-throughput analyses in different colon cancer cell lines. The group found that miR-150 only decreased viability in 1 colorectal cancer cell line suggesting that the apoptotic ability of miR-150 may be cell-type dependent. miR-375 however reduced cell viability by inducing apoptosis. miR-375 indirectly affected apoptosis by regulating its target Yes-associated protein 1 (identified using TarBase6.0) whose downstream targets are antiapoptotic genes BIRC5 and BCL2L1 (also known as Bcl-xl).36
In silico analyses (TargetScan and MicroCosm programs) were used to identify sequences matches between the miR and the antiapoptotic protein Mcl-1. miR-29a was increased in a mouse model of DSS-induced ulcerative colitis, which resulted in decreased levels of Mcl-1. In vitro analysis in colonic epithelial cells HT29 showed that miR-29a caused apoptosis, by down-regulation of its target molecule Mcl-1, which activated caspase-3.37 miR-mediated regulation of Mcl-1 have also been investigated in other studies.38,39 As mRNAs can have numerous binding sites allowing for binding of different miRs, it is not surprising that multiple miRs (miR-125 and miR-29b) have been shown to reduce Mcl-1 levels.38,39 Together, these studies suggest that aberrant miR expression in the intestines can directly and indirectly disrupt the balance between proliferation and apoptosis and result in barrier disruption. Thus, miRs provide novel therapeutic targets to mitigate intestinal barrier impairment as well as they can be used as markers for prognosis and predictions for barrier disruption.
4. miRs AND TIGHT JUNCTION PROTEINS
Tight junction proteins play an indispensable role in maintenance of intestinal barrier integrity in epithelial and endothelial cells.3,10 The claudin family and occludin proteins make up the essential tetra-span transmembrane proteins to function in regulation of paracellular flux between intestinal epithelial cells.68,69 The adaptor proteins zonula occludens (ZO) anchor occludin and claudin proteins to actin cytoskeleton and adherens junction.8,10,68,70 This protein complex plays an integral role in maintaining the intestinal barrier. Trauma such as burn injury with or without ethanol exposure negatively impact intestinal tight junction proteins.67,71–73 Better understanding of cellular regulators such as miRs and how they impact tight junction proteins can help prevent intestinal barrier dysfunction observed following major injury and other disease conditions.
Numerous studies show that altered expression of miRs (miR-21, miR-122a, miR-212, miR-429, and miR-874) can directly and indirectly modulate tight junction proteins and apical junctional complexes resulting in increased intestinal permeability.29,49–51,54,74 miR-122a has been shown to directly target occludin resulting in degradation of its mRNA that negatively impacted intestinal barrier function.49,54 Administration of 109 CFU/day of probiotics L. rhamnosus Gorbach-Goldin per day for 4 weeks reduced miR-122a expression resulting in increased occludin protein levels ablating the detrimental effects of chronic ethanol exposure on intestinal barrier integrity.54 A similar study investigating the role of ethanol exposure and miRs showed that exposure of Caco-2 cells to 0.1 %–1% ethanol for 3 h resulted in increased expression of miR-212 in a time- and dose-dependent manner, which correlated with decreased ZO-1 expression.29 Reduced ZO-1 levels are associated with impaired intestinal barrier function.29,74
MicroRNAs can also target molecules that indirectly result in reduced tight junction expression. miR-21 was up-regulated in both the colon and serum of patients with ulcerative colitis, which is associated with decreased expression of occludin, ZO-1, and Ras homolog gene family, member B (RhoB).50 Overexpression of miR-21 in Caco-2 cells resulted in a loss of both occludin, ZO-1, and RhoB protein levels while increasing epithelial permeability. The group used Targetscan and miRanda to predict miR-21 targets and identified that miR-21 targeted RhoB expression. They further found that siRNA-mediated ablation of RhoB resulted in decreased occludin and increased permeability.50 Similarly, occludin can be indirectly targeted by miR-874. In vitro overexpression of miR-874 resulted in increased paracellular permeability, bacterial translocation, and diminished occludin and claudin-1 levels.52 These studies establish that miRs could influence intestinal barrier integrity by direct or indirect regulation of tight junction protein expression.
5. miRs AND THE MICROBIOME
The gut microbiota is a relatively constant microbe population comprising over 100 trillion organisms consisting of 1,000 bacterial species.75,76 Alterations in the gut microbiome can lead to pathologic conditions (inflammatory bowel disease, obesity, and diabetes). In particular, trauma (e.g., burn injury or ethanol intoxication combined with burn injury) has the ability to alter the intestinal microbiome and increase gut bacterial load.71,77,78 Studies profiling miR expression utilizing either germ-free or antibiotic-treated mice show that the microbiota composition can influence expression of miRs.65,79–81 Germ-free mice infected with the foodborne pathogen Listeria monocytogenes had reduced ability to clear bacteria in multiple tissues compared to conventional (C57BL6/J) mice suggesting that the microbiota is protective against infection. Furthermore, miRs (miR-143, miR-148a, miR-194, miR-200b, miR-200c, and miR-378) were significantly decreased in conventional mice infected with Listeria, which coincided with increased expression in several of their predicted targets (identified by Targetscan).65 These changes were not observed in germ-free mice infected with Listeria, with the exception of miR-378, which was significantly elevated 72 h post infection.65 Other studies have reported similar findings that host miR expression is altered in response to infections with pathogenic bacteria such as Helicobacter pylori.82,83 As the microbiota/host relationship is so interconnected, it is not surprising that host miRs can also influence the microbiota composition.25
The host can influence microbiota composition and growth through intestinal epithelial cell and intestinal epithelial +4 niche stem cells expressing cell derived miRs. These miRs are released via extracellular vesicles into the feces where they can enter bacteria and control bacterial growth and gene expression. It is unclear how the miRs enter bacteria and how the miRs are processed by the bacteria; however, the fecal miRs could be binding to complementary binding sites on bacterial genes. Furthermore, dysregulation of host-derived miRs using mice with an intestinal epithelial cell deficiency in dicer expression resulted in gut microbiota dysbiosis exacerbating DSS-induced colitis.25 These studies display the essential symbiotic relationship between the host and the microbiota, which in part is shaped by miRs. These studies reveal that the interplay between the host and microbiota is mediated through miRs shaping the intestinal microbiota and the microbiota in turn modifies miR expression. This suggests that the microbiota/miR crosstalk plays an important role in cultivating the gut microbiota composition.
6. miRs ROLE IN ISCHEMIA/HYPOXIA
Ischemia is a major consequence in the intestine following trauma and burn injury, where blood flow is redistributed to more vital organs resulting in hypoxia (diminished oxygen delivery) in the gut.84–87 Elevated levels of hypoxia-inducible factor (HIF) −1α, a marker of hypoxia, in the gut has been associated with diminished intestinal epithelial and endothelial barrier function.85,88 HIF-1 (heterodimer composed of HIF-1α and HIF-1β) also plays a role in site repair and healing after hypoxic conditions. As described by Ahluwalia and Tarnawski,89 elevated HIF-1α stabilization in endothelial cells induces expression of vascular endothelial growth factor leading to reestablishment of the microvascular network allowing for mobilization of blood flow.
Hypoxia/ischemia influences both expression of miRs and miR biogenesis components.40,52,55,90–94 Intestinal ischemia/reperfusion (I/R) injury leads to intestinal injury through increased inflammation, overproduction of reactive oxygen species, and apoptosis. Using the miRNA target prediction tools miRanda, TargetScan, and PicTar, the group identified miRs that target Sirtuin-1. In particular, miR-34a was significantly up-regulated following I/R. Knockdown of miR-34a was shown to increase Sirtuin-1, which reduced I/R-related oxidative damage and apoptosis.40 In a similar study, miR-682 was identified by TargetScan to target phosphatase and tensin homology (PTEN). miR-682 mediated intestinal I/R injury by targeting PTEN. In vivo overexpression of miR-682 prior to I/R injury significantly reduced apoptosis and other effects of I/R injury.55 Chassin et al.26 utilized a mouse model of I/R to show the connection between miR-146a and IRAK1 expression. The group demonstrated that increased IRAK1 due to I/R injury resulted in increased expression of the proinflammatory chemokine CXCL2, apoptosis, and intestinal permeability.26 In vivo induction of miR-146a via diindolylmethase (DIM), a miR-146a-inducing agent, or administration of miR-146a reduced I/R-mediated inflammation and IRAK1 elevation. Furthermore, exposure of human intestinal tissues ex vivo to hypoxic conditions elevated IRAK1, which was attenuated with DIM treatment and reduced CXCL8 mRNA expression following LPS stimulation.26 These studies demonstrate that the relationship between hypoxia and inflammation could be influenced by miRs.
7. miRs ROLE IN INFLAMMATION
Intestinal inflammation is a hallmark of intestinal pathology. Numerous disease conditions/injuries (e.g., ulcerative colitis, Crohn’s disease, ethanol, and burn injury67,72,95–98) are associated with excess inflammation and exhibit modulation of miR biogenesis and/or miR expression. Inflammation in the gut contributes to increased apoptosis, tissue damage, and dismantling tight junction complexes making it a major contributor of increased intestinal permeability.
MicroRNAs can disrupt inflammatory pathways contributing to increased inflammation following disease and trauma. KEGG pathways developed using Gene Set Enrichment Analysis from the dicer mutants identified that ablation of miRs differentially activated pathways with immune pathways making up a third of the differentially expressed genes.24 Similarly, the expression of miR biogenesis components (drosha and argonaute-2) were diminished following alcohol and burn injury, which correlated with reduced expression of miR-150. Overexpression of miR-150 in young adult mouse colonocytes reduced LPS-mediated inflammation (IL-6 and KC) compared with empty vector controls.28 Other models have also demonstrated a similar relationship between miR-150 and inflammatory mediators.34,99–101
Furthermore, immunomodulatory miRs (miR-21, miR-146a and miR-155) have been shown to be overexpressed in patients with inflammatory bowel disease, vibrio cholera infection, and acute intestinal obstruction.56,58,59,63,64 Knockout of immunomodulatory miR-21 resulted in a reduction in intestinal inflammation (TNF-α and MIP-2) in a model of DSS-induced colitis while improving survival.58 Several studies have implicated miR-155 and miR-146a as negative feedback regulators of the inflammatory response modulating signal molecules in the NFκB pathway.
miR-155 influenced splenic T cell release of IFN-γ in a murine model following ethanol exposure prior to burn injury. miR-155 was reduced in T cells following ethanol and burn injury compared with sham-injured animals. There was however no difference in ex vivo T cell release of IFN-γ between miR-155 knockout mice and wild-type mice 1 day following ethanol and burn injury.102 These studies suggest that the immunomodulatory role of miR-155 in the gut may be cell-type specific. Interestingly, miR-146a and miR-155 are up-regulated in inflammatory bowel disease and vibrio cholera infection, suggesting that these miRs are not involved in the hyperinflammatory response associated with the diseases, but may have a role in the resolution of the disease state. Chronic ethanol exposure (Lieber-DeCarli diet for 5 weeks) significantly elevated miR-155 while significantly reducing the AMP, Reg3β. Knockout of miR-155 in the presence of chronic ethanol intoxication, reduced ethanol-mediated increases in serum endotoxins levels, NFκB activation and inflammation (TNF-α) in the small intestine. In contrast, acute ethanol exposure (5 g/kg ethanol in water for 3 days) resulted in elevated AMPs but did not alter miR-155 expression or TNF-α protein levels; however, it significantly increased NFκB activation.61
These studies demonstrate that miRs are particularly important in regulating the inflammatory response in the gut. Interestingly, atypical miR expression may be both protective to the gut or contribute to the pathology of the disease. As inflammation is paramount for intestinal barrier dysfunction, more investigation is required to elucidate the relationship between miRs and inflammation.
8. CONCLUSIONS AND FUTURE DIRECTIONS
As demonstrated by the studies reviewed in this article, miRs expression can be altered in pathologic conditions and that they have a significant role in pathology and resolution of disease. These findings suggest that miRs can be used as diagnostic tools. Furthermore, they can also serve as targets for potential therapeutic interventions. Collectively, the findings in this review exemplify the role of miRs in the regulation of intestinal barrier function. These studies demonstrate that miRs can directly and indirectly affect intestinal apoptosis, proliferation, tight junction protein expression, inflammation, and microbiota composition. There are studies that have investigated the effect of ethanol or trauma including burn injury on miR expression in other organ systems. There is however a big gap in research examining how miRs can be influenced or influence ethanol’s effects on trauma particularly in the context of burn injury in the gut. Understanding regulation strategies to maintain the epithelial barrier could lead to therapeutic interventions. Furthermore, aberrant miRs expression can be further exploited and used as biomarkers or for therapeutic design.
ACKNOWLEDGMENTS
The authors acknowledge the support from the National Institutes of Health R01 AA015731, R01 GM128242, and T32 AA013527.
Funding information
National Institutes of Health, Grant/Award Numbers: R01 AA015731, R01 GM128242, T32 AA013527
Abbreviations:
- AMPs
antimicrobial peptides
- DIM
diindolylmethase
- DSS
dextran sodium sulfate
- GI
gastrointestinal
- HIF
hypoxia inducible factor
- I/R
ischemia/reperfusion
- miRISC
miRNA-Induced Silencing Complex
- miRs
microRNAs
- pre-miR
precursor microRNA
- pri-miR
primary microRNA
- PTEN
phosphatase and tensin homology
- RhoB
Ras homolog gene family member B
- ZO
Zonula occludens
Footnotes
This manuscript originated from the “Review of Related Literature: Role of microRNAs on the Intestinal Barrier and Microbiome” chapter of the author’s (NLM) PhD dissertation.1
DISCLOSURES
The authors declare no conflicts of interest.
REFERENCES
- 1.Morris NL. Role of MicroRNAs in Impaired Gut Permeability Following Ethanol and Burn Injury. PhD Thesis. 2018;2831. [Google Scholar]
- 2.Mowat AM, Agace WW. Regional specialization within the intestinal immune system. Nat Rev Immunol. 2014;14(10):667–685. [DOI] [PubMed] [Google Scholar]
- 3.Peterson LW, Artis D. Intestinal epithelial cells: regulators of barrier function and immune homeostasis. Nat Rev Immunol. 2014;14(3):141–153. [DOI] [PubMed] [Google Scholar]
- 4.Garrett WS, Gordon JI, Glimcher LH. Homeostasis and inflammation in the intestine. Cell. 2010;140(6):859–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wittkopf N, Neurath MF, Becker C. Immune-epithelial crosstalk at the intestinal surface. J Gastroenterol. 2014;49(3):375–387. [DOI] [PubMed] [Google Scholar]
- 6.Stadnyk AW. Intestinal epithelial cells as a source of inflammatory cytokines and chemokines. Can J Gastroenterol. 2002;16(4):241–246. [DOI] [PubMed] [Google Scholar]
- 7.Kurashima Y, Goto Y, Kiyono H. Mucosal innate immune cells regulate both gut homeostasis and intestinal inflammation. Eur J Immunol. 2013;43(12):3108–3115. [DOI] [PubMed] [Google Scholar]
- 8.Hammer AM, Morris NL, Earley ZM, Choudhry MA. The first line of defense: The effects of alcohol on post-burn intestinal barrier, immune cells, and microbiome. Alcohol Res. 2015;37(2):209–222. [PMC free article] [PubMed] [Google Scholar]
- 9.Laukoetter MG, Bruewer M, Nusrat A. Regulation of the intestinal epithelial barrier by the apical junctional complex. Curr Opin Gastroenterol. 2006;22(2):85–89. [DOI] [PubMed] [Google Scholar]
- 10.Steed E, Balda MS, Matter K. Dynamics and functions of tight junctions. Trends Cell Biol. 2010;20(3):142–149. [DOI] [PubMed] [Google Scholar]
- 11.Shen L Functional morphology of the gastrointestinal tract. Curr Top Microbiol Immunol. 2009;337:1–35. [DOI] [PubMed] [Google Scholar]
- 12.Majno G, Joris I. Apoptosis, oncosis, and necrosis. An overview of cell death. Am J Pathol. 1995;146(1):3–15. [PMC free article] [PubMed] [Google Scholar]
- 13.Renehan AG, Booth C, Potten CS. What is apoptosis, and why is it important?. BMJ. 2001;322(7301):1536–1538.Clinical research ed.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ramachandran A, Madesh M, Balasubramanian KA. Apoptosis in the intestinal epithelium: its relevance in normal and pathophysiological conditions. J Gastroenterol Hepatol. 2000;15(2):109–120. [DOI] [PubMed] [Google Scholar]
- 15.Hall PA, Coates PJ, Ansari B, Hopwood D. Regulation of cell number in the mammalian gastrointestinal tract: the importance of apoptosis J Cell Sci. 1994. December;107 (Pt 12):3569–77. [DOI] [PubMed] [Google Scholar]
- 16.Peck BCE, Shanahan MT, Singh AP, Sethupathy P. Gut microbial influences on the mammalian intestinal stem cell niche. Stem Cells Int. 2017:5604727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rendon JL, Li X, Akhtar S, Choudhry MA. Interleukin-22 modulates gut epithelial and immune barrier functions following acute alcohol exposure and burn injury. Shock: 2013;39(1):11–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pathmakanthan S, Hawkey CJ. A lay doctor’s guide to the inflammatory process in the gastrointestinal tract. Postgrad Med J. 2000;76(900):611–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Finnegan EF, Pasquinelli AE. MicroRNA biogenesis: regulating the regulators. Crit Rev Biochem Mol Biol. 2013;48(1):51–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.He L, Hannon GJ. MicroRNAs: small RNAs with a big role in gene regulation. Nat Rev Genet. 2004;5(7):522–531. [DOI] [PubMed] [Google Scholar]
- 21.Treiber T, Treiber N, Meister G. Regulation of microRNA biogenesis and function. Thromb Haemost. 2012;107(4):605–610. [DOI] [PubMed] [Google Scholar]
- 22.Friedman RC, Farh KK, Burge CB, Bartel DP. Most mammalian mRNAs are conserved targets of microRNAs. Genome Res. 2009;19(1):92–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Du T, Zamore PD. Beginning to understand microRNA function. Cell Res. 2007;17(8):661–663. [DOI] [PubMed] [Google Scholar]
- 24.McKenna LB, et al. MicroRNAs control intestinal epithelial differentiation, architecture, and barrier function. Gastroenterology. 2010;139(5):1654–1664.1664.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu S, et al. The host shapes the gut microbiota via fecal microRNA. Cell Host Microbe. 2016;19(1):32–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chassin C, et al. MicroRNA-146a-mediated downregulation of IRAK1 protects mouse and human small intestine against ischemia/reperfusion injury. EMBO Mol Med. 2012;4(12):1308–1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gaulke CA, et al. Intestinal epithelial barrier disruption through altered mucosal microRNA expression in human immunodeficiency virus and simian immunodeficiency virus infections. J Virol. 2014;88(11):6268–6280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Morris NL, Hammer AM, Cannon AR, Gagnon RC, Li X, Choudhry MA. Dysregulation of microRNA biogenesis in the small intestine after ethanol and burn injury. Biochimica et Biophysica Acta (BBA) - Molecular Basis of Disease. 2017;1863 (10):2645–2653. 10.1016/j.bbadis.2017.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tang Y, et al. Effect of alcohol on miR-212 expression in intestinal epithelial cells and its potential role in alcoholic liver disease. Alcoholism. Clin Exp Res. 2008;32(2):355–364. [DOI] [PubMed] [Google Scholar]
- 30.Nata T, et al. MicroRNA-146b improves intestinal injury in mouse colitis by activating nuclear factor-kappaB and improving epithelial barrier function. J Gene Med. 2013;15(6–7):249–260. [DOI] [PubMed] [Google Scholar]
- 31.Wu F, et al. MicroRNAs are differentially expressed in ulcerative colitis and alter expression of macrophage inflammatory peptide-2 alpha. Gastroenterology. 2008;135(5):1624–1635.e24. [DOI] [PubMed] [Google Scholar]
- 32.Wu F, et al. Identification of microRNAs associated with ileal and colonic Crohn’s disease. Inflamm Bowel Dis. 2010;16(10): 1729–1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhou Q, Souba WW, Croce CM, Verne GN. MicroRNA-29a regulates intestinal membrane permeability in patients with irritable bowel syndrome. Gut. 2010;59 (6):775–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vasilescu C, et al. MicroRNA fingerprints identify miR-150 as a plasma prognostic marker in patients with sepsis. PLoS One. 2009;4(10):e7405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bian Z, et al. Role of miR-150-targeting c-Myb in colonic epithelial disruption during dextran sulphate sodium-induced murine experimental colitis and human ulcerative colitis. J Pathol. 2011;225(4):544–553. [DOI] [PubMed] [Google Scholar]
- 36.Christensen LL, et al. Functional screening identifies miRNAs influencing apoptosis and proliferation in colorectal cancer. PLoS One. 2014;9(6):e96767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lv B, et al. MiR-29a promotes intestinal epithelial apoptosis in ulcerative colitis by down-regulating Mcl-1. Int J Clin exp Pathol. 2014;7(12):8542–8552. [PMC free article] [PubMed] [Google Scholar]
- 38.Nijhuis A, et al. MCL-1 is modulated in Crohn’s disease fibrosis by miR-29b via IL-6 and IL-8. Cell Tissue Rese. 2017.368(2):325–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Balakrishnan A, et al. Upregulation of proapoptotic microRNA mir-125a after massive small bowel resection in rats. Ann Surg. 2012;255(4):747–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang G, et al. miR-34a-5p inhibition alleviates intestinal ischemia/reperfusion-induced reactive oxygen species accumulation and apoptosis via activation of SIRT1 signaling. Antiox Redox Signal. 2016;24(17):961–973. [DOI] [PubMed] [Google Scholar]
- 41.Zhang X, Zhang X, Liu C, Jia N, Li X, Xiao J. miR-224 promotes colorectal cancer cells proliferation via downregulation of P21WAF1/CIP1. Molecular Medicine Reports. 2014;9(3):941–946. [DOI] [PubMed] [Google Scholar]
- 42.Zhang GJ, Zhou H, Xiao HX, Li Y, Zhou T. Up-regulation of miR-224 promotes cancer cell proliferation and invasion and predicts relapse of colorectal cancer. Cancer Cell International. 2013;13(1):104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liao WT, et al. microRNA-224 promotes cell proliferation and tumor growth in human colorectal cancer by repressing PHLPP1 and PHLPP2. Clin Cancer Res. 2013;19(17):4662–4672. [DOI] [PubMed] [Google Scholar]
- 44.Wang Y, et al. MicroRNA-224 targets SMAD family member 4 to promote cell proliferation and negatively influence patient survival. PLoS One. 2013;8(7):e68744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li T, et al. MicroRNA-224 sustains Wnt/beta-catenin signaling and promotes aggressive phenotype of colorectal cancer. J Exp Clin Cancer Res. 2016;35:21–016.–0287–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gao Y, Li BD, Liu YG. Effect of miR27a on proliferation and invasion in colonic cancer cells. Asian Pacific J Cancer Prevent. 2013;14(8):4675–4678. [DOI] [PubMed] [Google Scholar]
- 47.Wang Y, Zhu X, Xuan C. MicroRNA-505 inhibits proliferation and promotes apoptosis of colorectal cancer cells. Int J Clin Exp Med. 2016;9(6):10488–10496. [Google Scholar]
- 48.Tian R, Wang R, Xie H, Jin W, Yu K. Overexpressed miRNA-155 dysregulates intestinal epithelial apical junctional complex in severe acute pancreatitis. World J Gastroenterol. 2013;19(45):8282–8291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ye D, Guo S, Al-Sadi R, Ma TY. MicroRNA regulation of intestinal epithelial tight junction permeability. Gastroenterology. 2011;141(4):1323–1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yang Y, et al. Overexpression of miR-21 in patients with ulcerative colitis impairs intestinal epithelial barrier function through targeting the Rho GTPase RhoB. Biochem Biophys Res Commun. 2013;434(4):746–752. [DOI] [PubMed] [Google Scholar]
- 51.Yu T, et al. Overexpression of miR-429 impairs intestinal barrier function in diabetic mice by down-regulating occludin expression. Cell Tissue Res. 2016.366(2):341–352. [DOI] [PubMed] [Google Scholar]
- 52.Zhi X, et al. MiR-874 promotes intestinal barrier dysfunction through targeting AQP3 following intestinal ischemic injury. FEBS Lett. 2014;588(5):757–763. [DOI] [PubMed] [Google Scholar]
- 53.Zhang L, Shen J, Cheng J, Fan X. MicroRNA-21 regulates intestinal epithelial tight junction permeability. Cell Biochemistry and Function. 2015;33(4):235–240. 10.1002/cbf.3109. [DOI] [PubMed] [Google Scholar]
- 54.Zhao H, et al. Inhibition of miR122a by Lactobacillus rhamnosus GG culture supernatant increases intestinal occludin expression and protects mice from alcoholic liver disease. Toxicol Lett. 2015;234(3):194–200. [DOI] [PubMed] [Google Scholar]
- 55.Liu Z, et al. MicroRNA-682-mediated downregulation of PTEN in intestinal epithelial cells ameliorates intestinal ischemia-reperfusion injury. Cell Death Dis. 2016;7(4):e2210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Min M, Peng L, Yang Y, Guo M, Wang W, Sun G. MicroRNA-155 Is Involved in the Pathogenesis of Ulcerative Colitis by Targeting FOXO3a. Inflammatory Bowel Diseases. 2014;20(4):652–659. 10.1097/mib.0000000000000009. [DOI] [PubMed] [Google Scholar]
- 57.Chassin C, et al. miR-146a mediates protective innate immune tolerance in the neonate intestine. Cell Host Microbe. 2010;8(4): 358–368. [DOI] [PubMed] [Google Scholar]
- 58.Shi C, et al. MicroRNA-21 knockout improve the survival rate in DSS induced fatal colitis through protecting against inflammation and tissue injury. PLoS One. 2013;8(6):e66814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Schaefer JS, et al. MicroRNA signatures differentiate Crohn’s disease from ulcerative colitis. BMC Immunol. 2015;16:5–015.–0069–0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bitar A, et al. Induction of immunomodulatory miR-146a and miR-155 in small intestinal epithelium of Vibrio cholerae infected patients at acute stage of cholera. PLoS One. 2017;12(3): e0173817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lippai D, Bala S, Catalano D, Kodys K, Szabo G. Micro-RNA-155 Deficiency Prevents Alcohol-Induced Serum Endotoxin Increase and Small Bowel Inflammation in Mice. Alcoholism: Clinical and Experimental Research. 2014;38(8):2217–2224. 10.1111/acer.12483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bitar A, Aung KM, Wai SN, Hammarström ML. Vibrio cholerae derived outer membrane vesicles modulate the inflammatory response of human intestinal epithelial cells by inducing microRNA-146a. Scientific Reports. 2019;9(1). 10.1038/s41598-019-43691-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Takagi T, et al. Increased expression of microRNA in the inflamed colonic mucosa of patients with active ulcerative colitis. J Gastroenterol Hepatol. 2010;25:S129–S133. Suppl 1. [DOI] [PubMed] [Google Scholar]
- 64.Lin J, et al. Novel specific microRNA biomarkers in idiopathic inflammatory bowel disease unrelated to disease activity. Modern Pathol. 2014;27(4):602–608. [DOI] [PubMed] [Google Scholar]
- 65.Archambaud C, et al. The intestinal microbiota interferes with the microRNA response upon oral Listeria infection. mBio 2013;4 (6):e00707–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hammer AM, et al. Interleukin-22 prevents microbial dysbiosis and promotes intestinal barrier regeneration following acute injury. Shock (Augusta, Ga). 2017;48(6):657–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Li X, Akhtar S, Choudhry MA. Alteration in intestine tight junction protein phosphorylation and apoptosis is associated with increase in IL-18 levels following alcohol intoxication and burn injury. Biochim Biophys Acta. 2012;1822(2):196–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hartsock A, Nelson WJ. Adherens and tight junctions: structure, function and connections to the actin cytoskeleton. Biochim Biophys Acta. 2008;1778(3):660–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Al-Sadi R, Khatib K, Guo S, Ye D, Youssef M, Ma T. Occludin regulates macromolecule flux across the intestinal epithelial tight junction barrier. American Journal of Physiology-Gastrointestinal and Liver Physiology. 2011;300(6):G1054–G1064. 10.1152/ajpgi.00055.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cichon C, Sabharwal H, Rüter C, Schmidt MA. MicroRNAs regulate tight junction proteins and modulate epithelial/endothelial barrier functions. Tissue Barriers. 2014;2(4):e944446. 10.4161/21688362.2014.944446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hammer AM, et al. The effects of alcohol intoxication and burn injury on the expression of claudins and mucins in the small and large intestines. Shock (Augusta, Ga). 2015.45(1):73–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zahs A, Bird MD, Ramirez L, Choudhry MA, Kovacs EJ. Anti–IL-6 antibody treatment but not IL-6 knockout improves intestinal barrier function and reduces inflammation after binge ethanol exposure and burn injury. Shock. 2013;39 (4):373–379. 10.1097/shk.0b013e318289d6c6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zahs A, Bird MD, Ramirez L, Turner JR, Choudhry MA, Kovacs EJ. Inhibition of long myosin light-chain kinase activation alleviates intestinal damage after binge ethanol exposure and burn injury. American Journal of Physiology-Gastrointestinal and Liver Physiology. 2012;303(6):G705–G712. 10.1152/ajpgi.00157.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tang Y, Zhang L, Forsyth CB, Shaikh M, Song S, Keshavarzian A. The role of miR-212 and iNOS in alcohol-induced intestinal barrier dysfunction and steatohepatitis. Alcoholism: Clinical and Experimental Research. 2015;39(9):1632–1641. 10.1111/acer.12813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Faith JJ, et al. The long-term stability of the human gut microbiota. Science (New York, NY). 2013;341(6141):1237439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Guinane CM, Cotter PD. Role of the gut microbiota in health and chronic gastrointestinal disease: understanding a hidden metabolic organ. Therap Adv Gastroenterol. 2013;6(4):295–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Earley ZM, et al. Burn injury alters the intestinal microbiome and increases gut permeability and bacterial translocation. PLoS One. 2015;10(7):e0129996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Beckmann N, Pugh AM, Caldwell CC. Burn injury alters the intestinal microbiome’s taxonomic composition and functional gene expression. PLoS One. 2018;13(10):e0205307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Dalmasso G, et al. Microbiota modulate host gene expression via microRNAs. PLoS One. 2011;6(4):e19293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Xue X, et al. Downregulation of microRNA-107 in intestinal CD11c(+) myeloid cells in response to microbiota and proinflammatory cytokines increases IL-23p19 expression. Eur J Immunol. 2014;44(3):673–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Singh N, Shirdel EA, Waldron L, Zhang RH, Jurisica I, Comelli EM. The murine caecal microRNA signature depends on the presence of the endogenous microbiota. International Journal of Biological Sciences. 2012;8(2):171–186. 10.7150/ijbs.8.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Liu Z, et al. Up-regulated microRNA-146a negatively modulate Helicobacter pylori-induced inflammatory response in human gastric epithelial cells. Microbes Infect. 2010;12(11):854–863. [DOI] [PubMed] [Google Scholar]
- 83.Wu K, Zhu Ch, Yao Y, Wang X, Song J, Zhai J. MicroRNA-155-enhanced autophagy in human gastric epithelial cell in response to Helicobacter Pylori. Saudi Journal of Gastroenterology. 2016;22(1). 10.4103/1319-3767.173756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Choudhry MA, Ba ZF, Rana SN, Bland KI, Chaudry IH. Alcohol ingestion before burn injury decreases splanchnic blood flow and oxygen delivery. American Journal of Physiology-Heart and Circulatory Physiology. 2005;288(2):H716–H721. 10.1152/ajpheart.00797.2004. [DOI] [PubMed] [Google Scholar]
- 85.Luo HM, et al. Valproic acid treatment inhibits hypoxia-inducible factor 1alpha accumulation and protects against burn-induced gut barrier dysfunction in a rodent model. PLoS One. 2013;8(10):e77523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Horton JW. Bacterial translocation after burn injury: the contribution of ischemia and permeability changes. Shock (Augusta, Ga). 1994;1(4):286–290. [PubMed] [Google Scholar]
- 87.Magnotti LJ, Deitch EA. Burns, bacterial translocation, gut barrier function, and failure. J Burn Care Rehab. 2005;26(5):383–391. [DOI] [PubMed] [Google Scholar]
- 88.Hu S, et al. Pyruvate is superior to citrate in oral rehydration solution in the protection of intestine via hypoxia-inducible factor-1 activation in rats with burn injury. JPEN J Parent Enteral Nutr. 2015;40(7):924–933. [DOI] [PubMed] [Google Scholar]
- 89.Ahluwalia A, Tarnawski AS. Critical role of hypoxia sensor–HIF-1alpha in VEGF gene activation. Implications for angiogenesis and tissue injury healing. Curr Med Chem. 2012;19(1):90–97. [DOI] [PubMed] [Google Scholar]
- 90.Morris NL, Yeligar SM. Role of HIF-1alpha in alcohol-mediated multiple organ dysfunction. Biomolecules. 2018;8(4).170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Rupaimoole R, et al. Hypoxia-mediated downregulation of miRNA biogenesis promotes tumour progression. Nat Commun. 2014;5:5202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Nallamshetty S, Chan SY, Loscalzo J. Hypoxia: a master regulator of microRNA biogenesis and activity. Free Radic Biol Med. 2013;64:20–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Bruning U, et al. MicroRNA-155 promotes resolution of hypoxia-inducible factor 1alpha activity during prolonged hypoxia. Mol Cell Biol. 2011;31(19):4087–4096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Morris NL, Cannon AR, Li X, Choudhry MA. Protective effects of PX478 on gut barrier in a mouse model of ethanol and burn injury. Journal of Leukocyte Biology. 2020. 10.1002/jlb.3a0820-323rr. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Al-Sadi R, et al. Interleukin-6 modulation of intestinal epithelial tight junction permeability is mediated by JNK pathway activation of claudin-2 gene. PLoS One. 2014;9(3):e85345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Morris NL, Li X, Earley ZM, Choudhry MA. Regional variation in expression of pro-inflammatory mediators in the intestine following a combined insult of alcohol and burn injury. Alcohol. 2015;49(5):507–511. 10.1016/j.alcohol.2015.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Akhtar S, Li X, Chaudry IH, Choudhry MA. Neutrophil chemokines and their role in IL-18-mediated increase in neutrophil O2- production and intestinal edema following alcohol intoxication and burn injury. American Journal of Physiology-Gastrointestinal and Liver Physiology. 2009;297(2):G340–G347. 10.1152/ajpgi.00044.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Akhtar S, Li X, Kovacs EJ, Gamelli RL, Choudhry MA. Interleukin-18 delays neutrophil apoptosis following alcohol intoxication and burn injury. Molecular Medicine. 2011;17(1–2):88–94. 10.2119/molmed.2010.00080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Manoharan P, Basford JE, Pilcher-Roberts R, Neumann J, Hui DY, Lingrel JB. Reduced levels of microRNAs miR-124a and miR-150 are associated with increased proinflammatory mediator expression in Krüppel-like factor 2 (KLF2)-deficient macrophages. Journal of Biological Chemistry. 2014;289(45):31638–31646. 10.1074/jbc.m114.579763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Roderburg C, et al. Circulating microRNA-150 serum levels predict survival in patients with critical illness and sepsis. PLoS One. 2013;8(1):e54612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.How CK, et al. Expression profile of MicroRNAs in gram-negative bacterial sepsis. Shock (Augusta, Ga). 2015;43(2):121–127. [DOI] [PubMed] [Google Scholar]
- 102.Li X, Rendon JL, Choudhry MA. T cell IFN-gamma suppression following alcohol and burn injury is independent of miRNA155. PLoS One. 2014;9(8):e105314. [DOI] [PMC free article] [PubMed] [Google Scholar]


