Abstract
Nanomaterials (NMs) have revolutionized multiple aspects of medicine by enabling novel sensing, diagnostic, and therapeutic approaches. Advancements in processing and fabrication have also allowed significant expansion in the applications of the major classes of NMs based on polymer, metal/metal oxide, carbon, liposome, or multi-scale macro-nano bulk materials. Concomitantly, concerns regarding the nanotoxicity and overall biocompatibility of NMs have been raised. These involve putative negative effects on both patients and those subjected to occupational exposure during manufacturing. In this review, we describe the current state of testing of NMs including those that are in clinical use, in clinical trials, or under development. We also discuss the cellular and molecular interactions that dictate their toxicity and biocompatibility. Specifically, we focus on the reciprocal interactions between NMs and host proteins, lipids, and sugars and how these induce responses in immune and other cell types leading to topical and/or systemic effects.
Keywords: nanomaterials, nanoparticles, biocompatibility
1. Introduction
Advances in nanotechnology and nanofabrication have allowed the development of a wide range of material classes, geometry, and sizes [1, 2]. As per ISO/TS 80004, the term NM is defined as ‘a material with any external dimension in the nanoscale or having internal structure or surface structure in the nano-scale.’ Many material features are unique at this scale due to size-dependent properties like high surface to volume ratio, high reactivity, pronounced quantum effects, etc [3]. In addition, NMs or materials with nano features can be engineered and optimized for optimal performance due to their ability to be processed at the nanoscale [4, 5]. Biomedical applications are one area where NMs have been utilized and their versatility in creating infinite geometries and hierarchical designs has allowed rapid and remarkable development [6–10]. Naturally, exposure of living systems to NMs, intentional or otherwise, leads to complex interactions including the FBR [11–13]. These interactions can vary significantly and depend on the type of NMs and the tissues and cells involved [14]. For example, some NMs display desirable interactions with the immune system and are tailored for therapeutic purposes [7, 15, 16]. Others elicit undesirable responses that might lead to health complications [17, 18]. Moreover, due to their novel scale, NMs might pose a threat to those involved in their manufacturing [19].
2. Nanomaterial compositions and applications
Despite the major breakthrough in engineering NMs for biological applications, the NM design parameter, and the tissue- and cell-specific responses they can potentially elicit, continue to pose unique challenges [10, 14]. For example, the biocompatibility of a given NM is largely dependent on its surface properties [20]. In addition to the desired properties such as drug release, biointegration, etc, acute or chronic inflammation is almost always present as a result of the FBR [11]. Inflammation and encapsulation by themselves are not necessarily undesirable, in fact, in some cases, it can also be beneficial such as for intrauterine contraceptives [21]. Given its ubiquity, modulation of the inflammatory response is perhaps a more suitable goal for bioengineers. Suggestions for integrating biological regulators such as immunomodulatory compounds with biomaterials have been made in this direction [22].
Here, we discuss five common classes of NMs that have been utilized in or suggested for therapeutic purposes: polymeric, metal, carbon-based, liposomes, and surfaces with nanofeatures (discussed in sections 2.1 to 2.5). The macroscopic surfaces with nanofeatures include macroscale materials like implants whose surfaces have engineered nanofeatures like nanorods or nanopores [23]. Table 1 summarizes the major NM classes and their most common features and applications. It also features diagrams showing the geometry of various NMs.
Table 1.
NM classes, their applications, and current research or development stage.

|
PEG: polyethylene glycol; NM: nanomaterials; CNT: carbon nanotube; CVD: chemical vapor deposition; BMG: bulk metallic glass.
2.1. Polymer-based nanomaterials
Polymers, long chains of repeating monomers, are widely used in nanomedicine due to their biocompatible and biodegradable properties. Polymeric NMs are primarily composed of carbon, hydrogen, oxygen, and nitrogen and are sourced from either synthetic or biological sources. Biodegradable polymeric NPs have been identified as great candidates for controlled drug delivery, as they are capable of selective targeting, controlled drug release, protection of the encapsulated payload, and prolonged circulation time [24]. Polymers can be used to form various types of NMs such as drug/protein conjugates, polymeric micelles, and NPs. Micelles are composed of a hydrophilic outer shell and a hydrophobic core, a design that increases drug stability [24]. Polymer conjugation occurs when a polymer chain is attached to a protein or drug, altering its delivery properties. For example, PEGylation, also known as PEG conjugation, is often used for drug delivery due to its hydrophilic nature, resulting in the body’s inability to recognize it as foreign [68]. Liposomes, metals, drugs, and other proteins have also been modified by PEGylation to avoid immune detection.
Polymeric NM synthesis can occur through various methods. The polymer used and the molecule or drug encapsulated will determine the optimal synthetic method. When beginning with a monomer, the first step is polymerization. The polymer is processed at the nanoscale through an emulsion technique, where a mixed water-surfactant system is used to generate nanodroplets, which are then precipitated to form NPs [24]. Other methods of synthesis include interfacial polymerization and controlled living radical polymerization, which includes nitroxide-mediated polymerization and reversible addition-fragmentation chain transfer [24]. When beginning with a preformed polymer, the first step is to disperse the polymer. The NPs are formed through solvent evaporation, nanoprecipitation, salting-out, dialysis, or supercritical fluid technology [24].
Solvent evaporation, where the polymer is first emulsified into an aqueous phase followed by solvent evaporation resulting in precipitation into NPs, is a common synthesis process. NP size can be altered by adjusting the temperature or the stir rate during evaporation [25]. Additionally, conjugation on the surface of polymeric NMs allows for potential targeted delivery. Many characteristics of polymeric NPs can be modified such as the surface charge, surface receptors for targeting, size, and morphology [24]. These characteristics can alter NP behavior by altering the cellular uptake, biocompatibility, or specificity. Most polymer NMs range from 10 nm to 200 nm as materials at those sizes have a high potential for prolonged circulation [24].
Polymeric NPs can be categorized as natural or synthetic. Synthetic NPs can be further categorized as non-degradable or biodegradable. Historically, synthetic polymers used for NPs were non-biodegradable such as poly(methyl methacrylate), polyacrylamide, and polystyrene [24]. Due to their toxicity and inflammatory effects, the focus shifted to biodegradable polymers such as PLA, PLGA, and PEG [24]. Natural polymers such as starch, cellulose, and chitin have also been investigated as materials for nanomedicine. Starches have been used to produce films with nano features and nanocomposites for use in biomedical applications [69]. CNF, CNC, and bacterial nanocellulose represent NMs derived from cellulose, a highly abundant, and therefore considered a renewable, source of biopolymers [70]. Cellulose can be processed into nanofibers, nanowhiskers, or NPs. Chitosan, a derivative of chitin, is biocompatible but displays varying degrees of toxicity under different chemical modifications, such as different levels of deacetylation [69]. The adhesive nature of chitosan allows adherence with tissues for many applications including dentistry, wound healing, and ophthalmology. Furthermore, chitosan possesses a variety of biological properties such as mucoadhesion and enhanced permeation [71].
When used in cancer treatments, polymeric NPs were found to decrease systemic drug toxicity while providing targeted treatment to the cancerous site [24]. Due to the ease of functionalization, they have also been engineered to penetrate the BBB for the potential treatment of neurodegenerative diseases [24]. A newer innovation in polymeric NPs is the creation of stimuli-responsive NPs. These NPs experience a conformational change due to a stimulus, such as temperature or pH, which triggers controlled release [24]. For example, cancerous tissues exhibit hyperthermia and temperature triggered NPs have been designed as a treatment method [24]. pH-triggered NPs can be used for treatment because many tumors create an acidic extracellular space [24]. Polymeric NPs are also being investigated for nucleic acid delivery since they have been found to protect them from degradation and increase cellular uptake [24]. It should be noted that most of these applications are still in the experimental phase. Polymeric NMs on the market are restricted to PEGylated nanopharmaceuticals, such as Cimzia or Adagen [27]. There are also a number of polymeric NPs and micelles in clinical trials for cancer therapy [24].
2.2. Metals and metal oxide-based nanomaterials
Metal NMs have been used for various applications, mostly in the form of NPs on the scale of 1–200 nm [20]. Due to their optical properties and propensity for scattering light, metals are optimal materials for diagnostics and have been used in imaging as contrast agents, for cancer detection, or disease therapy [72]. Metal NPs are synthesized through various chemical or physical methods. In chemical synthesis, metal salts undergo a redox reaction by either electrochemical synthesis, which involves conduction of current through an electrolytic solution, sonochemical synthesis, where a high energy pulse is used to generate radical species, or chemical reduction, which utilizes metal precursors or reducing agents to form NPs [26]. Physical methods of synthesis include thermal decomposition, which uses high temperatures to create NMs, and laser ablation, where the metal is removed by a laser to create highly controlled NMs [72].
All metal NMs induce an inflammatory response that is dependent on composition, size, and shape. Studies have shown that immune responses can be triggered by inhalation or direct contact, impacting not only patients but those who manufacture them [20]. Generally, as the size of metal NPs decrease, they become more toxic [73]. Other properties that can influence the toxicity of metal NPs include elemental composition, charge, shape, crystallinity, and NP solubility [73].
Gold and silver are two noble metals used often in nanomedicine. Silver NPs contain unique optical, anti-microbial, and electronic properties, while gold NPs have a realtively low toxicity, are easy to prepare and are often used in diagnostics due to their surface plasmon resonance effect [72]. Similarly, surface-enhanced Raman spectroscopy properties of silver NPs have been utilized to develop a blood test for esophageal cancer detection [74]. Reactive metals are also used for NPs and include copper and aluminum. Copper NPs are inexpensive, have good physical properties, and can be used in a wide range of applications [72]. Copper selenide nanocrystals have been created to produce photo-thermal heating, which can destroy human colorectal cancer cells upon irradiation [75]. Aluminum NPs are used to create vaccine adjuvants [29]. Magnetic metals, such as iron, nickel, cobalt, and manganese are useful for MRI imaging [72]. Gold, silver, titanium, and zinc are known to be anti-microbial which, when formed into NPs, can be used for antibacterial therapies [72]. Although many metal NMs are being investigated in an experimental setting, due to their aforementioned relative toxicity, only a few metal NMs have been approved by the FDA. Currently, super-paramagnetic iron oxide is approved as an MRI contrast agent while other iron NPs have been approved for treating iron deficiencies [28]. Many vaccines containing aluminum adjuvants are approved by the FDA [29].
2.3. Carbon-based nanomaterial compositions and applications
Carbon nanoforms are one of the most widely studied categories of NMs, with sizes ranging from several nm to a few 100 nm. Carbon atoms can bond together in various ways depending on their hybridization state, giving rise to a vast array of NMs [76]. These NMs are found in various kinds of geometry and size ranges. For example, graphite is one atomic monolayer thick sheet made of hexagonally arranged carbon atoms. Often, many carbon NMs are referred to as graphitic NMs, because they have carbon atoms arranged primarily in hexagonal rings, like those in graphite. These may be cylindrical CNTs, spherical fullerenes, nanocones, helical tubes, and can even combine to form multiwalled CNTs, nano-onions, and nano-peas (fullerenes in tubes) [37–39, 77–79]. Other structures include nanodiamonds with tetra-hedrally arranged carbon atoms and amorphous carbon black particles. Sometimes, CQDs are referred to as zero-dimensional materials as they are extremely small even amongst NMs. They may be crystalline or amorphous and can be generated by breaking down other NMs like graphite, nanodiamonds, CNTs, etc into sizes less than 10 nm [80].
Some common methods for generating graphitic NMs include graphite vaporization using a discharge arc or laser, CVD using hydrocarbons, HiPco, and hydrocarbon combustion (see table 1 for details) [30]. Size and geometry selectivity for the resultant NMs can be achieved using catalysts. Alternatively, processes like gel chromatography (used for CNTs) may be used for extracting required NM after a nonselective generation process [81]. High pressure is needed in addition to high temperature for the production of nanodiamonds, mimicking the natural process of diamond formation [43].
The physical and chemical properties of many of these nanoforms are dependent on their size, geometry, and surface chemistry, and so are their biological interactions [18, 45, 82]. Functionalization of these NMs by adding certain functional groups/surfactants/polymers on their outer surface is often done to make them more water dispersible, less cytotoxic, and more biocompatible [46]. Specific processes like Hummer’s or Brodie’s method are used for oxidizing graphene sheets [32]. Other materials like CNTs can be functionalized with −COOH through ultrasonic treatment in a mixture of acids [83]. They can also be modified with therapeutic loads for delivery applications.
NMs like nanotubes and fullerenes stand out as therapeutic delivery systems. They can encapsulate therapeutic loads and bind with other molecules covalently on their external surface. In this manner, they can be conjugated with anticancer drugs, bioactive peptides, proteins, nucleic acids, DNA strands, etc [31]. CNTs can be used as photothermal agents owing to significant near-infrared absorption [84]. They have been shown to be promising candidates as diagnostic and imaging tools, tissue engineering scaffolds and are used in neuron prosthesis applications [76]. Fluorescent properties of CQDs are extensively studied and, owing to their extremely small sizes and passivated surface, seem to have good potential for passing the BBB [80].
Similar nanoforms can be produced unintentionally during the burning of fossil fuels, and other incineration processes [85]. They can easily get introduced through pulmonary routes and result in adverse responses. Hence, studies have focused on resulting conditions such as fibrosis caused by exposure to CNTs [86, 87]. A study indicated that SWCNTs may be perceived as pathogens, and the conditioned medium from SWCNT-exposed cells acted as a chemoattractant for DCs [88]. Since these materials are manufactured for various laboratory and industrial applications, there is a growing concern for the safety of personnel working at mass manufacturing sites owing to frequent, long-term exposure. A recent study discussed functional immune response in workers from 12 different U.S. facilities exhibiting generally low levels of exposure to CNTs or nanofibers [19]. However, recent studies with macrophages and fibroblasts exposed to graphene and carbon-based nano-onions, respectively, showed very low ROS generation and cytotoxicity [89, 90]. These observations prompted investigators to suggest that cytotoxicity results may be influenced by additional contaminating factors, such as bacterial endotoxin, introduced during manufacturing [89].
Carbon-based NMs have also been shown to activate the inflammasome complex in macrophages, followed by IL-1β secretion. It has also been suggested that inflammation (in many cases unavoidable for carbon-based NMs) is not always a detrimental response, and strategies should not seek to prevent acute inflammation at every cost, but rather focus on chronic effects [91].
2.4. Liposomes and other self-assembled material compositions and applications
Liposomes are self-assembled lipid bilayer vesicles often but not always composed of phospholipids, one of the most common examples being phosphatidylcholine [48, 92]. Liposomes may be mono- or multi-layered and have sizes ranging from 30 nm to several micrometers [48]. They are suitable candidates for delivering therapeutic payloads owing to their biocompatibility and ability to carry both hydrophilic and hydrophobic loads [48, 92, 93].
Sonication of a suspension containing lipid membranes to disrupt the layers and allow for self-assembly into smaller vesicles is one of the most popular synthesis methods. Multilayered liposomal suspension can also be extruded through a polycarbonate filter to yield particles with a diameter near the pore size of the filter [48]. A heating method developed by Mozafari et al is also popular as it does not involve any toxic components [94]. They are often PEGylated, which reduces surface protein adsorption and uptake by macrophages and results in an increase in their residence time [51, 92]. PEGylation can be done on the lipid bilayer before or after vesicle formation (pre- or post-insertion), resulting in PEG chain(s) on both or only the external surface of the liposome [50]. Water-soluble drugs may be dissolved in an aqueous suspension used to disperse the bilayer and consequently, drug molecules get trapped in the aqueous liposome core. For loading hydrophobic loads, they can be trapped in the liposomes’ nonpolar bilayer compartment [51]. Both the main components of these carriers, lipid bilayer and PEG polymer, are generally considered to be biocompatible, and hence such liposomes are used extensively for drug carrier applications [48, 92]. Consequently, they form the single largest combined category of NMs utilized in FDA approved and investigational drugs [28, 95]. Even so, there have been some unanticipated immune responses against these carriers including accelerated blood clearance, CARPA, and some lipid related allergies, e.g. immediate hyper allergy on the first administration of Doxil® [92].
Non-PEGylated liposomes are preferred for specific cases. For example, conventional liposomes perform better for the delivery of high membrane permeability drugs like vincristine, as PEGylation may make the liposome walls less rigid by hindering hydrogen bonding in the bilayer [53]. Other self-assembled materials, such as nucleic acids, polypeptide nanofibers, etc are being evaluated in preclinical studies for tunable drug delivery and immunological response properties [93]. These may be synthesized using various wet synthesis methods such as emulsification, desolvation, or complex coacervation [54].
2.5. Nanopatterned surface compositions and applications
Apart from surface chemistry, which may be altered through functionalization, topology at micro- and nano-scales can also be utilized to modulate cell response, and consequently immune response in more complex biological systems [96–99]. Patterning may directly affect properties like hydrophobicity and alter more complex cell response pathways. Such patterning may also mechanically force the cells to grow along a certain direction in specific morphology and/or may alter cell response by locally altering the stiffness of the substrate [100, 101]. A surface may be nanopatterned using laser sculpting, ion/electron beam drilling, chemical etching, electrochemical anodization, EBL-assisted RIE/imprinting, or thermo-mechanical nano molding [58, 61, 102].
It has been shown that nanopatterned surfaces may have desirable interactions with certain cells involved in the FBR [57]. Nanopatterning may include creating protrusions, such as nanorods on the surface or pores of certain size distribution. These features can be used to engineer cellular responses such as improved inflammatory and re-endothelialization to increase stent performance [103]. Another example involves improving titanium biocompatibility by surface nanostructuring [104]. Similarly, modulation of macrophage responses has been achieved by BMG nanopatterns [57].
2.6. Nanoceramic composition and applications
Ceramics are a broad class of materials defined as inorganic compounds of metal or metalloid and non-metal with ionic or covalent bonds. They have high mechanical strength and are pH and temperature resistant but have low biodegradability [67]. Synthesis of ceramic NMs can occur through methods such as microemulsion precipitation or hydrothermal synthesis [67]. Ceramic NMs are often used for coatings due to their heat resistance and chemical inertness and their applications range from drug delivery and tissue engineering to diagnostics and biosensors [67].
There are certain materials that stand out in their category for their prevalent use in biomedical applications, e.g. HA. Other materials used for ceramic NMs are based on calcium phosphates, silicon, titanium, and zirconium. Ceramic NMs can be synthesized into a variety of shapes. Ceramic NPs consist of an inorganic core that can encapsulate and protect a drug [67]. Ceramic nanoscaffolds contain pores that allow for high porosity, high surface area, and long degradation times for controlled drug release [67]. Nano-clay is composed of thin nanosized layers of ceramic material known for their high adsorption ability, surface area, and chemical inertness [67]. Some HA based drugs and formulations, such as nanocrystal-line HA-paste based Ostim®, have been approved by the FDA for bone growth stimulation.
3. Nanomaterial toxicity and biocompatibility
NMs designed for biomedical applications must be assessed in terms of cytotoxicity and overall biocompatibility [20, 105]. Cytotoxicity refers to their effects on cell functions and viability. Biocompatibility, on the other hand, involves a comprehensive evaluation of NMs in vitro and in vivo. To ensure their biosafety, they must get tested following standard protocols. For example, under the guidance on NMs by ISO, ISO/TR 10993-22:2017 part 22, it is required that NMs must be tested on (a) cytotoxicity, (b) genotoxicity, carcinogenicity, and reproductive toxicity, (c) immunotoxicity, irritation, and sensitization, (d) hemocompatibility, (e) systemic toxicity, (f) pyrogenicity, and (g) implantation [106]. These tests are crucial to ensure the safety of patients that receive NM-based treatments, as well as the manufacturing workers [107].
Although toxicology tests are crucial to prevent adverse effects, fundamentally understanding the associations between NM physicochemical features and their specific bio-effects is important to broaden their applications. For example, research that focuses on the therapeutic applications of NMs has shown their ability to control the scarring process, attenuate fibrosis, and suppress cancer growth [108]. Wide ranges of material types, biological systems, and analytical metrics that quantify their interactions, however, complicate such assessment. A previous review has listed a number of challenges in understanding the bio-interactions of NPs [109]. Here we expand the list to NMs. It is challenging to understand the association between physicochemical features of NMs and their effects on biological systems because of (a) a broad scale of different types of NMs, varying in chemical composition, size, shape, and surface coating [109, 110] (b) a large number of different model systems that can be used for testing that often interact with different NMs in varying manners (such as changes specific to different cell types) [111], (c) the lack of standardized protocols (e.g. incubation times and NP concentrations used in cell exposure [112, 113], and (d) increased complexity related to in vivo tests [114]. Overall, these challenges limit the number of materials that can be tested and prevents our complete understanding of the bio-effects of NMs.
Here, we opted to cover the most relevant tests and evaluate them based on the specific application requirements. We focused on the effects of NMs on cytotoxicity, immunomodulation, fibrosis, and genotoxicity, which are most commonly described in the literature and also relate to occupational and manufacturing exposures [107]. Figure 1 highlights the most common NM features that influence these processes.
Figure 1.

Examples of NM features that are reported to affect biocompatibility and cytotoxicity.
3.1. Nanomaterial cytotoxicity and genotoxicity
Multiple parameters such as composition, molecular structure, and size can influence NM cytotoxicity, and their effects are mediated by engaging pathways that cause direct necrosis, induced apoptosis, or antibody-triggered immune clearance [115]. Common cytotoxicity tests are based on assessing the following criteria associated with injured/dead cells: (a) damaged cell membrane, (b) production of cell-death markers, and (c) impaired metabolism. Figure 2(a) provides an example of a cytotoxicity assay for ZnO NPs.
Figure 2.
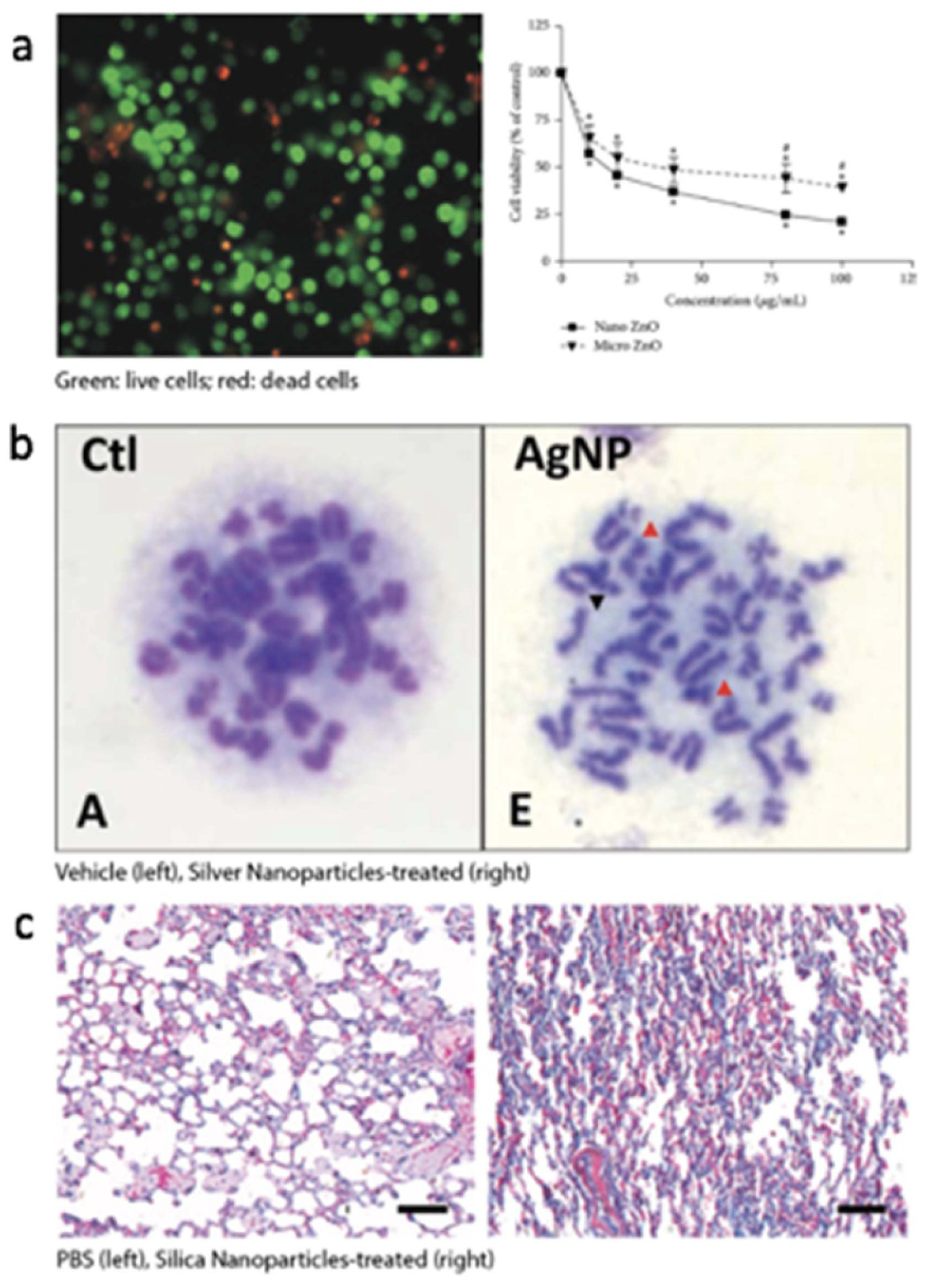
Examples of NP effects such as cytotoxicity, genotoxicity, and fibrosis. (a) Nano ZnO is reported to cause more cell death of THP-1 cells (human monocytes) than micro ZnO. The picture on the left shows THP-1 treated with nano ZnO. Reprinted with permission from Sahu et al. Journal of Nanoscience, 2016, Article ID 4023852 (Hindawi Publications) [116]. (b) AgNP causes chromosome aberration in rat bone marrow cells (red arrow shows breakage, black arrow shows gap). Reprinted with permission from Wen et al. PLOS ONE, 2017, v.12(9) (plos.org) [117]. (c) Mice administered with 10 nm SiNPs show densified ECM in the lung tissue. Representative images of treatment with PBS (left, control) or 10 nm SiNPs (right) are shown. Reprinted with permission from Wang et al. ACS Nano, 2017, 11:1659–1672 (ACS Publications) [118].
Some specific examples include the LDH test that detects the release of LDH, which usually resides inside intact cells. Caspase-3/7 assay assesses the production of caspases, which are mediators of apoptosis. Other tests, such as the MTT assay, measure changes in metabolic activity to evaluate cell viability. Specifically, the MTT assay measures the reduction of tetrazolium salt using redox indicators that correlate with cell viability. Alternatively, the reduction of resazurin can be assayed to evaluate cell viability in a similar manner [119]. Interestingly, other in vitro assays utilize cell-membrane mimic lipid models to study specific damage by the interactions with NMs [120]. In such studies, the mechanism behind those interactions includes membrane disruption, which leads to cell death [120].
NMs can also reduce cell viability by damaging DNA and impairing metabolic activities based on their composition or geometry. For example, CuO, which is a semiconductor and also a popular catalyst used in chemical reactions, has been reported to be cytotoxic [115]. Specifically, it was shown that when compared to Fe2O3, Fe3O4, and TiO2, CuO NPs caused more damage to DNA and mitochondria leading to cell death [89]. In addition, SiO2 NPs and CNTs were reported to be more cytotoxic to bronchiolar epithelial cells than TiO2 [115]. NM geometry was also shown to influence cytotoxicity via ROS generation [121]. For example, cell viability was reported to increase with a decrease in the size of a silver NP (5, 25, 50, and 110 nm silver NPs in diameter were tested) [121]. In comparison on a larger scale, nanometer-sized particles of CuO were shown to be more toxic than the micrometer particles [115].
When a material does not affect cell mortality, it may still induce sub-lethal effects on the genome and epigenome. These changes can happen especially at low doses. The genotoxicity of NMs has been previously reviewed [122, 123]. Standard methods to test for such effects include the Ames test, comet test, micronuclei test, DNA laddering test, and chromosome aberration test [122, 123]. More recently, next-generation sequencing has also been used in chemical mutagenicity evaluation [124]. It should be noted that NMs can not only cause genomic alterations by directly entering the cell through passive diffusion and endocytosis but can also catalyze the intracellular production of −OH radicals, which can lead to elevation of ROS and DNA damage [122]. For example, NMs that have been shown to raise ROS level include Ag [125–130], TiO2 [131, 132], silica [133], quantum dots [134], and CNTs [122, 135, 136]. Besides material composition, studies have also shown that surface charges affect the genotoxicity of liposomes and that positively charged nanocarriers were less genotoxicity [81]. Figure 2(b) shows an example of chromosomal aberrations induced by Ag NPs.
3.2. Nanomaterial immunomodulation
NMs, by design or otherwise, can lead to direct and indirect immunomodulation, which includes immunosuppression and immunostimulation [137]. The role of NMs in immunomodulation is of particular interest in drug delivery applications, in which NPs serve as vehicles for vaccines or drugs. Here, however, we focus on the immune reaction caused by the NMs themselves, which involves the innate and adaptive immune responses [138]. Innate immunity refers to the interactions between NMs with non-specific immune cells such as macrophages and natural killer cells. Whereas adaptive immune response activates T cells and B cells that elicit antigen-specific effects. For example, zinc oxide and silver NMs can significantly increase IL-6 and IL-8 production in kidney cells, implying an increase in both the innate and adaptive responses [139].
Immunomodulation by NMs is a complex process influenced by many parameters, including chemical composition, molecular structure, surface chemistry, and size [20]. Previous reviews have addressed this process but are limited to a subset of parameters. For example, some focus on immune adjuvants and their therapeutic effects [137, 140]. Other reviews have more extensively elaborated on the effects of various NM categories and compositions on systemic immunomodulation [105, 141, 142]. In addition, the role of biophysicochemical features at the nano-bio interface such as protein adsorption and corona formation in immunomodulation has been addressed [20, 143, 144].
NP size, as mentioned above, has also been shown to be relevant in this process. For example, nano-size (193 nm) particles induced a stronger immune response than micro-size ones (1530 nm) [145]. Interestingly, studies have also shown that larger size may elicit stronger serum immunoglobulin response [137]. In addition, bulk material with spiky nanostructures was shown to modulate the immune response. Specifically, in vitro studies showed an increased expression level of CD 40 in DCs after 12 h incubation with spiky particles [146]. Usually, the expression of CD 40 indicates a relevant higher level of the immune response of APCs. The nanostructures in a bulk material such as nanospikes might exert mechanical stress on the cells, which results in potassium efflux and inflammasome activation in DCs [146]. Due to the diverse immunomodulatory responses elicited by different NMs, coupled with the somewhat inconsistent methods used to assess them, it is difficult to associate them with specific features. In addition, multiple parameters have been shown to have synergistic effects on this process, which complicates interpretation [144].
3.3. Nanomaterial-induced fibrosis
Fibrosis involves the excessive deposition and remodeling of dense ECM and it can occur in response to NMs [147–151]. As a process, it is not entirely understood because it can occur in the absence of an acute response and the lack of standardized predictive tests. In vitro tests for fibrotic responses include analyzing cells for the expression of fibrosis-associated proteins such as α-SMA, TGF-β, and ECM components such as fibronectin, laminin, COL I, and hydroxyproline (modified amino acid common in collagens). However, cells are most often cultured on a stiff substrate (2D culture) and this can lead to alteration of gene expression due to the nucleus pore opening caused by mechanical forces, which might require in vivo studies to be performed [152, 153].
NPs, due to their small size, can gain easy access to the lung alveoli. Upon contacting lung cells, NPs can affect them in multiple ways leading to fibrosis. For example, NPs can induce the production of ROS and increase TGF-β production [154]. But such effects may not cause near-term discomfort in the subject, therefore making it difficult to associate NPs with these adverse events. Moreover, NMs engage in complex mechanisms to induce fibrosis. For example, CNTs have been reported to activate type 2 immune mechanisms and induce fibrosis and cancer through the IL-1—IL-17—TGF-β axis [86, 155–157]. In contrast, metal oxides, such as those of copper [158], titanium [159], cerium [160], have been shown to induce fibrosis via ROS elevation, inflammation, and increased TGF-β expression. Figure 2(c) shows an example of silica NPs causing lung fibrosis in mice.
3.4. Therapeutic use of nanomaterials
Because of the exceptional ability of NMs to transverse biological membranes, therapies involving drug delivery and photodynamic therapy have been developed [161]. More interestingly, the cytotoxic attributes of NPs have been co-opted to treat illnesses associated with excessive cell growth [162, 163]. For example, studies have used NPs to treat hypertrophic scarring [163]. Moreover, some NPs could be used to treat solid cancer too. For instance, it was found that a biosynthesized gold NP was cytotoxic to a breast cancer cell [164]. In addition, NMs such as Au, quantum dots, and fullerenes can quench ROS and have been used to alleviate genome damage [122]. However, precise targeting in these therapies has proven to be challenging, and topical and systemic responses of these therapies need to be evaluated.
4. Topical vs systemic interactions of nanomaterials
NMs present both opportunities and challenges because they can overcome size limitations and achieve unique distributions in physiological systems. In this section, we review applications pertaining to tissue regeneration and drug delivery for immunotherapies while considering their intended topical and systemic effects. Systemic refers to those processes affecting the whole body or at least multiple organ systems whereas topical describes a response that affects the immediate area of contact with the NM, whether it be a single organ or tissue type. The term topical in this section, is not used in its common definition of being physically superficial, but rather affecting a localized area of the body. Particularly, topical and systemic effects are observed as NMs have enhanced routes of delivery, including oral, implantable, and injectable administrations. In addition, targeting of specific organs or tissues is attempted through surface modifications, which aim to evade innate immune responses and oxidizing inflammatory mechanisms. For example, CNTs and graphene oxide, while designed for effective drug delivery, face enzymatic degradation, and other immune regulatory challenges [165]. Finally, NMs used for topical reparative and regenerative processes can be subjected to systemic immune responses. For example, biopolymeric fillers, carbon-based NMs, and porous metals cannot achieve full restoration of damaged musculoskeletal tissues due to the innate immune response they elicit [166]. It is therefore important to consider the reciprocal impact between NMs and immunity and examine it in the proper context in terms of intended and unintended topical vs systemic effects. Figure 3 exemplifies possible unintended systemic and topical effects that can be anticipated from NMs depending on their route of administration.
Figure 3.

NMs used to target specific organ systems or specialized tissue often carry unintended effects at levels of production, administration, and delivery. For instance, the synthesis of non-functionalized CNTs, while intended to locally promote neural attachment, foster neuronal growth, and cell membrane incorporation, pose an inhalation risk which could lead to cytotoxic pulmonary inflammation, tumorigenesis, and necrosis. After 2 mg CNT intratracheal administration (E; macroscopic view), hematoxylin/eosin-stained lung tissue displays CNT dispersion. CNT-induced pulmonary lesions are characterized by collagen rich granulomas and are seen in 10× Masson trichrome-stained bronchi (D), alveolar space (I), and in 100× hematoxylin and eosin-stained lung tissue (N). Reprinted with permission from Muller et al. Toxicology Applied Pharmacology, 2005, 3: 221–231 (Elsevier Ltd) [167]. FDA-approved Abraxane® protein-based liposome, intended as an IV administered vehicle for treating pancreatic cancer, is seen to induce peripheral neuropathy, low white and red blood count, and abnormal electrocardiogram. The bottom left set of images show hematoxylin and eosin staining of pancreatic adenocarcinoma in a murine model. The panel on the right represents untreated control tissue which shows a dense tumor stromal network. The panel on the left shows pancreatic adenocarcinoma tissue treated with NP albumin-bound paclitaxel (Nab-PTX) demonstrating a less organized architecture and a reduction in the cancer tissue components. Reprinted with permission from Rossignoli et al. Theranostics, 2019, 9(2): 436–448 [168].
4.1. Polymer
Polymeric NMs are widely used in drug delivery and drug targeting systems as their particle sizes are conducive to prolonged circulation in the blood, increasing payload half-life [70]. Atrisorb® (Tolmar Inc., Fort Collins, CO, USA), a PDLLA polymer, is a biodegradable material made from the ring-opening polymerization of lactide monomers. Membranes functionalized with antibiotics and growth factor-coated PDLLA nanofibers have been shown to facilitate healing while reducing the risk of bacterial infections [169]. While this nanopolymer offers both great surface area to volume ratio and many possible chemical modifications, its efficacy is hampered by host interactions. For example, PDLLA may be eliminated following application to a focal periodontal defect via phagocytosis. Moreover, if the PDLLA nanofibers gain access into the GI route, they can either be endocytosed by intestinal epithelial cells, remain in the submucosa, or enter the bloodstream and accumulate in the liver and spleen [170] Accumulation of polymer NPs, due to slow biodegradation, could potentially result in hepatocellular injury through protein synthesis inhibition while accumulation in the spleen may affect immunopathology [71].
CNCs, typically on the order of a few hundred nanometers in length and a few nanometers in width, are rod-like acicular structures extracted from ligno-cellulose fiber by hydrolysis with a strong acid [171]. CNCs have been investigated for topical treatments. For example, a nanobiocomposite hydrogel consisting of a mixture of CNCs and silver NPs mixed in a Vaseline® base was used to treat wounds in a diabetic mouse model [172]. Semi-quantitative analysis of H&E images showed reduced inflammation as Ag is thought to decrease the time needed for granulation tissue formation, fibroblast invasion, and possess anti-inflammatory properties. These nanocomposites significantly enhanced the expression of vascular endothelial growth factor and fibroblast growth factor allowing for increased angiogenesis, permeability, and recruitment of inflammatory cells to the injured site, aiding the proliferation and migration of endothelial cells [172]. Although recent studies have not provided evidence of permeation of the dermal barrier by CNCs, it is possible that skin sensitization can occur at high concentrations. In this scenario, nanocellulose could penetrate the stratum corneum and form a stable association with proteins, triggering DCs to migrate to the lymph nodes and consequently activate T lymphocytes [173]. Therefore, it is possible for topically applied NMs to induce a systemic immune response.
Chitosan, another naturally derived polymeric NM, is cationic in nature and its electrostatic interactions with nucleic acids allow it to act as a suitable carrier for strong immune adjuvants for cancer vaccines [71]. In a study evaluating its potential to target bladder cancer, peptide-carrying glycol chitosan NPs demonstrated long circulation times and tumor accumulation for at least 48 h [71]. Despite such enhanced and targeted penetrations, the chitosan NPs also accumulated in the liver and kidneys. In this circumstance, an intended systemic targeted delivery system resulted in non-targeted accumulation in other tissues, which is fairly common with NMs.
4.2. Carbon
Carbon-based NMs can achieve nanofibrous architectures and have tunable physical properties that make them suitable for the fabrication of scaffolds [174]. In nerve regeneration studies, for example, CNTs are often employed as scaffolds since they display morphological features analogous to neurites such as their anisotropic conductivity and elongated structure. Furthermore, bundles of CNTs mimic dimensions similar to that of dendrites, and the tunable physical and chemical properties of CNTs, such as length, diameter, chirality, surface functionalization, and band gaps are compatible with neural activity [175]. While CNTs have demonstrated biocompatibility, there are studies showing accumulation-related side effects. Figure 3 provides an example of this phenomenon where in the majority of non-functionalized CNTs, inhalation can lead to pulmonary inflammation where CNTs engulfed by macrophages can be encapsulated by granulomas. In both acute and chronic inflammatory settings, phagocytic and necrotic tissues contain cytotoxic factors such as ROS, digestive enzymes, and cytokines that can enhance progression to fibrosis and tumorigenesis of the local tissue [176]. Furthermore, distant from the local site of administration, CNTs can induce inflammatory responses to pleural and mesothelial tissues, as well as the liver upon unintended systemic exposures [156].
Carbon NMs are also used in designing innovative and biocompatible targeted drug delivery strategies. For example, fullerenes/buckyballs possess a strong apolar character and their specific geometry, size, and surface characteristics make them appropriate for crossing cell membranes [39]. While intravenous injection and oral delivery are the most common routes of administration, pulmonary, transdermal, transmucosal, and ocular introductions have also been demonstrated [177]. In a study by Misra et al, the FDA-approved anticancer drug DTX was tested for its effectiveness using C60 fullerenes conjugated with APA. C60-OH-APA-DTX fullerenols administered intravenously demonstrated hemocompatibility and pharmacokinetic neutrality, allowing them to bypass the mononuclear phagocytic system and achieve prolonged circulation time [178]. It should be noted that local and systemic biocompatibility of fullerenes may have a dosage threshold. For example, in a study by Prylutska et al, at C60 fullerene concentrations of 300 mg kg−1 or higher, behavior disturbances, hematotoxicity, and spleen, kidney, and liver pathologies were observed [179].
4.3. Metal
4.3.1. Noble metals
Many studies have demonstrated that gold is an efficient nanocarrier for systemic delivery. AuroShell®, for example, is an FDA approved gold nanoshell for treating head and neck cancers using photothermal therapy [180]. After intravenous administration, these nanoshells accumulate in the tumor through EPR effects. A near-infrared laser is then applied to the tumor, allowing the particles to selectively absorb the laser energy and convert the light into heat. As a result, the tumor and its blood vessels are destroyed [180]. However, there are also unintended findings that suggest the toxicity of gold NPs. For example, after intravenous injection of polyelectrolyte multilayer coated gold NPs, which are used for treating prion disease, the particles can accumulate in the hippocampus, thalamus, hypothalamus, and the cerebral cortex [181].
4.3.2. Reactive metals
Although copper (Cu) is essential for normal physiological functions including hemoglobin formation and catecholamine biosynthesis, concentrations in excess of the range of biological tolerance may cause unintended effects such as hemolysis, GI distress, liver, and kidney damage [182]. In a comparative study investigating the effects of single oral administrations of Cu NPs, Cu was reported to accumulate at high concentrations and low solubilities in the liver, kidney, heart, and spleen [183]. Histopathological analysis of these tissues showed unintended local effects such as dilated liver sinusoids, atrophy of glomeruli in the kidneys, and multinucleated cells in the spleen [183].
Aluminum oxide NPs (Al2O3 NPs), despite being commonly employed, have been shown to contribute to pulmonary fibrosis due to occupational exposure in the workplace [184]. Using a nose-only inhalation system in Sprague Dawley rats, researchers found that despite a lack of adverse systemic effects, aluminum accumulation in the kidneys and increases in LDH, IL-6, and TNF-α in the lung were due to Al2O3 NPs inhalation exposure and were dependent on particle size [185]. In studies looking at aluminum oxide NPs after oral administration, mice were observed to have significant Al2O3 NPs accumulation in the liver, lung, and kidney [186].
4.3.3. Magnetic metals
Feraheme®, an FDA-approved iron oxide NP for treating iron deficiency, offers another example of desirable systemic effects. In addition to treating anemia, Feraheme® has been shown to kill acute myeloid leukemia cells, as well as the stem cells that give rise to them, in patient samples and mouse models [187]. It does so by providing a source for induction of ROS, which in turn leads to an increase in oxidative stress, resulting in cell death. However, one major unintended effect is interference with MRI, which could last up to three months after injection [187].
As an example of a locally intended approach, cobalt ions have been frequently investigated in alveolar bone regenerative studies for clinical dentistry applications. Cobalt is of interest in regenerative approaches since it has anoxic-mimicking capabilities that promote tissue angiogenesis and osteogenesis [188]. On the other hand, cobalt chloride-based cobalt-substituted HA (COHAC) NPs, despite demonstrating anti-inflammatory and antibacterial effects and enhanced differentiation of bone cells due to their high degradation rate, caused a decrease cell viability in concentrations higher than 10 ppm [189].
4.4. Self-assembled materials (PEG, non-PEG, protein-based)
4.4.1. PEGylated liposomes
Liposomes possess several characteristics that hold a great promise in drug delivery with relatively low levels of negative effects, including solubility, large payloads, protected carrier, and reduced toxicity [190]. After intravenous infusion, PEGylated liposomes can allow a systemic response with prolonged circulation time for delivering drugs to target sites such as infected tissues and tumor areas [191].
Doxil®, for example, is an FDA approved chemotherapy drug used to treat ovarian cancer, AIDS-related Kaposi’s sarcoma, and multiple myeloma through intravenous infusion. However, there are lipid-related side effects associated with Doxil® via interactions with the complement system. HSRs have been reported immediately after the first intravenous injection in up to 45% of patients [92, 192]. The reactions are mainly due to CARPA in which an adverse immune response is triggered through the activation of the complement system upon infusion. Particularly, EPR effects of Doxil® also contribute to CARPA [92, 193]. The hypothesized process is that anaphylatoxins C5a and C3a are released after complement activation and stimulation of mast cells and basophils, followed by secretion of inflammatory mediators that activate endothelial and smooth muscle cells, resulting in CARPA. Symptoms of CARPA observed in patients include flushing, shortness of breath, and chills, which usually decrease or disappear after subsequent infusion.
4.4.2. Non-PEGylated liposomes
Non-PEGylated liposomes are delivered in the same way as PEGylated ones. Upon intravenous administration, they circulate systemically in the bloodstream for delivering drugs and interact with circulating proteins or target sites, resulting in local responses [191]. AmBisome®, for example, is a liposome that encapsulates fungal pathogen amphotericin B used for treating a wide range of fungal infections, including cryptococcal meningitis in HIV patients, visceral leishmaniasis, and those caused by Aspergillus species. After administering intravenously, AmBisome® circulates in the blood and binds to fungal cell walls at local sites, followed by the disruption of liposome, and the release of amphotericin B [194]. Unintended effects of AmBisome® include HSRs and CARPA due to local cell–cell interactions from the activation of the complement system. Similarly, several other non-PEGylated liposomal drugs such as DaunoXome® and Visudyne® also induce CARPA [92].
4.4.3. Protein-based liposomes
Protein-based liposomes are administered intravenously as well, leading to systemic response and topical effects at target sites. The effects of protein-based liposomes depend largely on the protein themselves due to their specific binding. For example, Abraxane®, an FDA-approved NP formed by albumin with conjugated paclitaxel, dissolves into soluble complexes after intravenous injection [27]. The particles can then accumulate in tumors due to the EPR effect and can be transported across endothelial by binding to membrane protein gp60 [90]. Albumin serves as a delivery vehicle and allows Abraxane® to achieve a high response rate for treating breast and pancreatic cancer. As described in figure 3, Abraxane® also has several unintended side effects such as low blood cell counts, hair loss, peripheral neuropathy, and abnormal electrocardiogram.
Ontak®, consisting of recombinant diphtheria toxin protein conjugated to IL-2, selectively binds to IL-2 receptors upon intravenous injection and delivers diphtheria toxin to target cells [27]. This allows T-cell stimulation for the treatment of leukemia and lymphoma. However, it was discontinued in 2014 due to severe HSRs and loss of vision effects.
5. Nanomaterials and molecular interactions
NM cytotoxicity and biocompatibility are dictated by complex interactions that are not fully elucidated. Physicochemical properties, such as morphology, size, defects, and chemical stability of NMs, trigger biological responses that cause varying levels of toxicity and must be studied intensely prior to the application in humans [195, 196]. Protein corona, the coating created when the NM acquires proteins on its surface upon entering biological environments, plays a critical role in triggering immune responses and giving NMs new functional identities [197]. Other than the characterization of the biological identity of NMs, the physiological response triggered within the body is also an important area of concern in NM design and production. For most applications, NMs must evade innate immune cell activity and the body’s adaptive immune response.
5.1. Protein corona
NMs adsorb proteins that form a layer, also known as the ‘protein corona,’ on their surfaces that then mediate interactions with the cells and tissues [198]. Protein corona, with its efficiency and efficacy, has proven useful in the diagnosis and treatment of many diseases, interacting and interfering with distinct biological microenvironments [199]. In NPs, it also plays a role in the alteration of immune responses, governing cell uptake, accumulation, biodegradation, and clearance [200]. Existing in two forms on the surface of NPs, protein corona can be either ‘soft’ or ‘hard,’ consisting of loosely bound proteins with a short lifespan in the former or of tightly bound proteins with a long lifespan in the latter [199]. Its formation affects the biological identity of NMs and their ability to trigger immune responses and is modified by a variety of factors, such as physicochemical properties of the NM, denaturation of proteins bound to the NM, protein source, environmental factors, and route of administration. A better understanding of protein corona modifications and interactions will allow for improved biocompatibility and further the development of drug delivery and other applications. Figure 4 shows how different kinds of protein corona can be formed on various NMs.
Figure 4.

Upon entering the body, NMs can interact with various proteins to create different kinds of protein coronas, which determine the biological and immunological identity of these NMs.
5.2. Molecular interactions and nanomaterial biological identity
As NMs enter biological environments, biomolecules adhere to their surfaces. As mentioned above, the nature of the protein corona is affected by many factors, including the composition of the NM. Characterization of corona formation on polystyrene NPs showed that equilibrium in the protein corona was reached a few minutes after dilution in plasma and over time continued to change in terms of the amount of protein-bound but not composition [201]. Furthermore, it was also shown that the newly formed corona influenced how NMs interacted with platelets and blood cells to affect hemolysis, thrombocyte activation, NP uptake, and endothelial cell death at an early exposure time. Recently, the protein affinity of three core-crosslinked polymeric NPs with long circulation times (poly(N-(2-hydroxypropyl) methacrylamide, polysarcosine, and PEG) was investigated and it was found that neither of these three polymers formed a substantial protein corona that could affect their in vivo interactions [202]. Another experiment used disulfide-stabilized poly(methacrylic acid) nanoporous polymer NPs to show that their internalization in monocytes and macrophages was differentially affected by the presence of protein corona [203]. It was observed that while a protein corona decreased the internalization efficiency in human monocytic cells, it induced a class A scavenger receptor-mediated phagocytosis in macrophage-like-cells without affecting the overall internalization efficiency. In addition to polymeric NPs, metallic NPs have also been extensively investigated. Recently, it was shown that DNA can be hybridized onto gold NPs with a bovine serum albumin-induced protein corona and that the conjugate had greater colloidal stability in different solutions of salt, acid, and alkali [204]. PEG-grafted gold NPs were also used to investigate the role of size and surface chemistry in mediating serum protein adsorption and their subsequent uptake by macrophages [205]. Specifically, this study found that variations in serum protein adsorption correlated with differences in the mechanism and efficiency of NP uptake by a macrophage cell line. The differential effect of a protein corona was also corroborated in another study where ultra-small gold NPs were used to investigate the effect of the human serum albumin protein corona and it was found that the conformation-transited protein corona-AuNP complex could induce cell apoptosis [206].
Corona formation also affects the dissolution rates of NPs. Cobalt NPs were found to not dissolve in the presence of amino acids but the dissolution rate was higher in the presence of bigger proteins that adsorbed onto the NPs [207]. Aside from metallic NPs, liposomal NPs have also been investigated. A library of ten liposomal formulations was created and these formulations exhibited different targetability and levels of uptake by PANC-1 (human pancreatic cancer cell line) and INS-1 (rat insulinoma cell line) cells when exposed to human plasma [208]. Similarly, dioleoyl-3-trimethylammonium propane based lipoplex formulations were used to show that different lipid compositions resulted in different degrees of total protein adsorption as well as different compositions of individual proteins from fetal calf serum [209]. The same study also showed that PEGylation increases protein adsorption but attempts to identify individual proteins that alter protein adsorption and cellular uptake of NPs was unsuccessful.
In the past 20 years, carbon-based NMs have revealed many chemical and physical properties that are both academically fascinating and commercially appealing. Recently, it has been shown that human serum albumin corona formation on CQDs reduced their cytotoxicity and changed the mode of energy metabolism in mouse cells. In addition, the impact of the size of carbon NMs on protein binding has been confirmed and is consistent with previous studies showing that binding was more efficient with larger CNTs and NPs [210].
One of the major issues with NM-mediated drug delivery is the inevitable denaturation of proteins bound to the NM during interaction with immune cells. As a potential way to evade this phenomenon, it has been shown that corona formation on silica NPs provided a stealth effect on macrophage recognition [211, 212]. Efforts to exploit the properties of the protein corona were made by pre-coating polystyrene NPs stabilized with the PEG-based surfactant lutensol AT50 with immunoglobulin-depleted plasma, which showed a marked reduction in cellular uptake by immune cells [213]. Another example of a stealth effect was shown in a study where the protein corona of liposomes played a major role in their sequestration from peripheral blood mononuclear cells [214]. Capture of NMs by immune cells continues to be one of the most significant barriers to in vivo anti-tumor drug delivery. To overcome that, designer-artificial coronas were fashioned that were able to reduce capture by distinct populations of circulating leukocytes [133]. Another example of a bio-inspired NP surface capable of evading components of the immune system was described recently where CD47 was added to NPs and prevented phagocytic clearance [215].
Engineering artificially designed protein coats and developing strategies to avoid capture by the immune system requires a thorough understanding of the protein interactions at play. Investigations of the biological identity of different liposomal formulations after incubation in human and mouse plasma revealed that in the latter, liposomes were more negatively charged, less enriched in opsonins, and appreciably more enriched in apolipoproteins [211]. In addition to protein sources, there are other environmental factors that affect the biological identity of NMs such as protein concentration, temperature, incubation time, shear stress, etc. Researchers were able to show that increasing serum protein concentrations affected corona formation and led to desorption of abundant serum proteins (such as albumin, immunoglobulins, transferrin, etc) and adsorption of lower abundance proteins with higher affinity (apolipoproteins, lipoproteins, and complement factors) [216]. Another study with silver NPs showed that by varying the pH and temperature, the different conformational spaces and charge localization of the plasma proteins could be assessed, and this was correlated with the binding affinities of the proteome [217].
Corona formation is also affected by the flow. Specifically, protein-NPs complexes formed from fetal bovine serum after flow had decreased cellular binding, as measured with flow cytometry [218]. Surface charge is another important factor for corona formation. It has been shown that compared to polyanionic NPs, distinctly lower amounts of proteins were adsorbed onto polyzwitterionic hybrid NPs and the corona composition showed elevated relative ratios of medium molecular weight proteins [219]. Finally, the route of administration: systemic or topical also plays an important role in determining the biological identity of a NM. Orally delivered NMs need to cross: (a) the harsh GI environment (i.e. low pH and digestive enzymes); (b) the mucus barrier, and then (c) the intestinal enterocyte lining before reaching the bloodstream. In protein-rich GI fluids, a protein corona is likely to form and affect the surface characteristics [220]. NMs may also be inhaled, and research has revealed that when they come into contact with porcine lung surfactant, the protein coronas that formed were different from the ones formed in plasma [221]. Specifically, there was a striking prevalence of molecules with high lipid and surface binding in the protein corona that forms with the lung surfactant, which is markedly different from coronas formed with plasma proteins.
With regards to protein corona formation, an additional consideration is that it leads to loss of targetability and undesired off-target effects. For example, it was previously shown that silicon NPs functionalized with bicyclononyne and exposed to serum proteins exhibited a significant loss in targeting efficiency as compared to NPs with pristine surfaces [222]. Such observations prompted the development of strategies to exploit protein corona as a targeting ligand by synthesizing NMs that are coated by plasma proteins that are naturally shuttled to cells. Two proteins that have been correlated with the cellular association are apolipoproteins and vitronectin. One research group has developed predictive-validation modeling that provides a means of assessing the relative significance of the identified corona proteins [223]. They found that just eight proteins including vitronectin and some apolipoproteins were the main promoters of liposome-HeLa cell association. More recently, the targeting ability of the protein corona was studied using four temozolomide-loaded liposomes and a few typical proteins (vitronectin, apoA1, apoA2, apoB, apoC2) were identified, which bind to receptors overexpressed at the BBB (e.g. scavenger receptor class B, type I and LDLR) [224].
5.3. Molecular interactions and nanomaterial immunological identity
Physicochemical characterization of NM biological identity can also provide information about their equally important immunological identity. Understanding the interactions of NMs with the immune system, which is designed for the efficient detection and elimination of foreign substances, is critical to either ‘avoid immune recognition or to specifically interact with the immune cells’ [225]. Therefore, it is important to consider both the material-intrinsic properties of the NM and the context-dependent identity formed through pattern recognition of the immune system. Through the introduction of non-stealth NMs, the initial response is dominated by components of innate immunity [226]. This response triggers the release of pro-inflammatory cytokines, activating key pathways in the immune and other cells [142]. Because of such effects, cytotoxicity assays are routinely employed to assess the safety of NMs. However, NM features including high adsorption capacity, hydrophobicity, surface charge, optical, and magnetic properties, or catalytic activity have been shown to interfere with such assays [227]. In addition, protein corona has also been shown to affect the nutrition balance of cell culture media, which interfered further with the accuracy of cytotoxicity assays [228].
It is appreciated that different NM types trigger the release of unique cytokine profiles, causing distinct symptoms and responses. A variety of assays are employed to characterize these responses including bead-based immunoassays and other cytotoxicity assays to measure the levels of released cytokines such as IL-6, IL-1, IL-8, IFN-γ, TNF-α, and CCL2 [139]. During inflammation and observation of high cytotoxicity induced by zinc oxide and silver NMs, IL-6, and IL-8 production increased significantly [139]. Increased NF-kB activation, a hallmark of inflammatory responses, was also observed when a conformational change in serum albumin bound to NPs switched cellular uptake from the albumin receptor to scavenger receptor-mediated internalization [229].
Many different types of toxicological assays exist, yet the focus has shifted to in vitro testing due to ethical issues and high cost of in vivo testing. Cell culture assays test for cytotoxicity (altered metabolism, decreased growth, lytic, or apoptotic cell death), proliferation, genotoxicity, and altered gene expression [230]. In addition to increased cytokine production, interactions between NM corona and macrophage receptors led to a generation of ROS and permeability of lysosomes [142]. The release of proinflammatory cytokines also can stimulate the activation of phagocytes. Compounding these effects, another study has shown that the NM-specific release of cytokines could also stimulate the activation of phagocytes [231]. Specifically, it was shown that cobalt and nickel NPs and HA crystals have inflammogenic effects, stimulating macrophage release of TNF-α, and activating phagocytes [231]. The presence of corona also influenced the internalization of poly(methacrylic acid) nanoporous polymer NPs by monocytes and [203]. Consistent with this observation, the uptake efficiency of NM-corona complexes by macrophages was higher than that of pristine material, resulting in a remarkable increase of TNF-α and nitric oxide production [232]. It was also discovered that protein-coated systems enhanced the expression of the FcγRIIB inhibitory receptor, a key negative regulator of B cell activation, leading to the impairment of cellular uptake and activation of cell apoptosis via the AKT/Caspase 3 signaling pathway [233].
While the innate immune system relies on the complement system and phagocytic cells, the adaptive immune system functions through T and B lymphocytes [234]. With cells and tissues that have already been compromised with ongoing innate immune reactions, the adaptive immune response occurs and takes over, enhancing the existing inflammatory process [200]. The response begins with the macrophage cytokine release and then progresses to the involvement of T and B lymphocytes, further attacking the NMs. For example, in a study of multiwalled CNTs, the mRNA expression of proinflammatory macrophage-produced cytokines significantly increased upon injection, leading to the stimulation of T lymphocytes [142]. It is also appreciated that NMs are recognized by the adaptive immune system due to their shared biochemical properties with viruses [200]. In addition, if only a soft corona is formed on the NP, or if the NP is not sterile, the adaptive immune response treats the NP as a foreign ‘danger’ signal [200]. Secondary and tertiary conformational changes of the absorbed proteins can also lead to adaptive immune response, with the tertiary structure change from the NP-protein corona formation leading to protein aggregation and the induction of immunogenic potential [200].
6. Cellular interactions
Before delving into specific cellular interactions with NMs, we briefly discuss the general mechanism of NM uptake by cells. It should be noted that this is only a short overview and that more in-depth coverage of these topics can be found in recent reviews [235, 236]. Cell–NM interactions can be broadly divided into endocytic uptake and receptor-mediated recognition. Endocytic uptake can then be divided into clathrin-dependent endocytosis, caveolae-dependent endocytosis, macropinocytosis, and phagocytosis. For larger NMs (>200 nm), phagocytosis and micropinocytosis are the dominant uptake methods. Phagocytosis is carried out by professional phagocytes (neutrophils and macrophages) and is initiated by NMs being recognized by specific receptors [235]. These receptors then mediate actin cytoskeletal activity that allows the plasma membrane to enclose the NM in a cup-shaped protrusion and subsequently pinch off inwardly to become a phagosome. In contrast, macropinocytosis does not rely on receptor-mediated NM recognition, and it non-specifically engulfs NMs into the cell. For smaller NMs (50–80 nm), cells will rely on either clathrin- or caveolae-dependent endocytosis. Both clathrin and caveolin are proteins that facilitate plasma membrane invaginations of NMs into the cell [236]. These vesicles are then trafficked to lysosomes for subsequent degradation. However, some NMs may take advantage of the acidic environment of lysosomes to escape degradation and deliver drugs or proteins to the cell [237].
While cellular uptake of these NMs can lead to therapeutic effects via internalization, NMs may also interact with cells via specific membrane receptor-mediated pathways. NMs can interact with cells by activating certain signaling cascades via specific receptors and may not require uptake into the cell. Some NMs can inherently activate certain signaling pathways while other NMs can be coated with antibodies or other ligands for specific receptors to stimulate pathways. In the context of the immune response, specific receptors include but are not limited to TLRs, Fc receptors, scavenger receptors, and integrins [238]. Table 2 outlines the most common cellular interactions of different NMs and the subsequent cell-specific responses they elicit.
Table 2.
Polymer based: fluorescent microscopy of PLGA NPs (green) in macrophages. Reprinted with permission from Kalluru et al. Journal of Cell Science, 2013, Vol. 126, Pg. 3046 [239]. Carbon based: SEM image of multi-walled CNT and alveolar macrophage. Reprinted with permission from Kasai et al. Particle and Fibre Toxicology, 2016,13:53 (Springer Nature) [240]. Metal/metal oxide based: TEM image of magnetic NPs in alveolar macrophages. Boris A. Katsnelson et al. ISRN Nanotechnology,2012, Vol.2012, Article ID 143 613 (Hindawi) [241]. Nanoliposomes: U-118 MG cells treated with nanoliposome encapsulated doxorubicin. DAPI stain shown in blue and nanoliposomes shown in red. Reprinted with permission from Lin. et al. PLoS ONE, 2014, Vol.9, Issue 7 (PLOS) [242]. Nanopatterned BMG: cell on micro/nano patterned Pt-BMG. Reprinted with permission from Wang et al., ACS Applied Bio Materials, 2018, Vol. 1, Issue 1, Pg. 51–58 (American Chemical Society) [243].

|
6.1. Cellular interactions of polymer-based nanomaterials
Polymer-based NMs represent a popular method for immune modulation due to their loading capability, shape, and size tunability, and biodegradability, which allow for precise NM optimization specific to individual cell types. Polymer NMs are usually loaded with cytokines to stimulate the immune response or are coated with antibodies specific to immune response receptors [26]. Polymer NPs have been tested on a wide variety of immune cells with their cellular effects being well-documented. Common polymer NM bases are PLGA, PBAE, and polystyrene with special attention given to PLGA NPs. PBAE NPs have mainly been tested on immune cells in cancer mouse models. For example, PBAE NPs loaded with IL-12 were delivered into a melanoma mouse model, causing M2 macrophages to repolarize into M1 macrophages and thus limiting subsequent tumor progression [246]. More recently, PBAE NPs loaded with cyclic dinucleotides, potential inhibitors of tumor growth, were used to target monocytes and improve outcomes in the same mouse model [260]. Numerous studies have used PLGA based NPs conjugated to antibodies specific APCs for immune tolerance induction. For example, PLGA NPs coated with the DC specific receptor DEC205 was shown to increase the levels of IL-10 produced by DCs and T cells in vitro [261]. In more recent studies, PLGA NPs were combined with the delivery of nucleic acid-based approaches to induce changes in the immune system. Specifically, PLGA NPs carrying siRNA against PD-1 and PDL1 were used to target tumor lymphocytes and suppress growth in a colon cancer mouse model [262]. In addition, PLGA NPs were loaded with an eCRISPR-Cas9 system to target and genetically edit murine-derived macrophages [263]. Collectively, these studies highlight the potential of NPs to be used for in vivo gene modulation.
6.2. Cellular interactions of carbon nanomaterials
The carbon NM family consists of a number of formations such as carbon black NPs, fullerene, carbon nanohorns, nanographite, and CNTs. The cellular interactions with each of these materials have been reviewed recently [142]. Here, we emphasize the cellular interactions with CNT’s due to their prevalence in the field. We also paid special attention to their interactions with immune cells and more specifically with macrophages. It has been shown that stimulation of macrophages via CNT uptake is modulated by molecular recognition receptors such as TLRs, scavenger receptors, complement receptors, integrins, and lectin-like receptors [264]. The method by which CNT’s are sensed ultimately affects the downstream signals that are activated. It has been suggested that complement opsonization on CNT’s leads to reduced expression of proinflammatory cytokines and upregulation of anti-inflammatory cytokines [248]. While PEG-coating is commonly used to bypass these interactions and reduce cytotoxicity, it has been shown that this may also reduce the level of uptake by macrophages. Furthermore, the shape of CNT’s affects macrophage activity. Specifically, Palomaki et al showed that while most CNT’s can induce IL-1β expression, only long needle-like CNT’s can induce IL-1α expression in LPS-primed macrophages [265]. Many recent studies have focused on CNT’s as potential vaccines against viruses, though these studies have been performed in nonmammalian organisms and lower order mammals, such as in fish and in hamsters [266–268].
A common concern with CNT’s is their stability and susceptibility to degradation by the immune system during therapeutic use. It is thought that macrophages can uptake CNT’s and activate NADPH oxidase which stimulates ROS that contributes to their degradation. However, the rate at which this happens varies in different organ systems. In vivo studies on mice found multi-walled CNTs injected into the cortex of the brain can be degraded as early as 2 d post injection while those injected into lungs could last for as long as 6 months [269]. In addition, a recent study showed that the cytotoxicity effect of CNT’s on lung cells increased susceptibility to H1N1 infection [19].
6.3. Cellular interactions of metal/metal oxide nanoparticle
The interactions between macrophages and metal oxide NPs as well as the downstream responses have been reviewed previously and therefore will not be addressed in detail here [249]. In brief, besides being directly endocytosed, binding of metal oxide NPs to integrins, carbohydrate receptors, TLRs, nucleotide oligomerization domain-like receptors, Fc receptors, or scavenger receptors can induce macrophage activation [249]. The downstream effects are complex, including pro-inflammatory/anti-inflammatory cytokine production, toxic compound production, or pre-apoptosis membrane component translocation. These downstream processes are dependent on the intrinsic properties of NPs and the activation states of macrophages [249]. Commercial silver NPs have been reported to induce inflammation biomarker expression in murine RAW 264.7 macrophages and anti-inflammation biomarker expression in human whole blood cell cultures [251]. Gold NP-loaded mesoporous silica has been demonstrated to induce M2 polarization and promoted the secretion of anti-inflammatory factors of macrophages [252]. Cobalt NPs, mimicking the debris coming from failed CoCr alloy hip prostheses, have been shown to alter the migration, microtubule acetylation, spreading, and podosome formation of U937 macrophages owning to ROS production and downregulation of RhoA [253]. Neutrophils have been reported to interact with gold and silver NPs with the induction of neutrophil extracellular traps. Detailed mechanisms and downstream effects can be found in a review by Yang et al [270].
A recent study described the development of an antibody targeted amphiphilic organic ligand-protected gold NP vehicle capable of delivering small molecular weight drugs to lymphocytes [254]. The untargeted NP vehicle’s gold core was surrounded by a mixed monolayer of alkanethiols terminated by hydrophobic methyl and water-solubilizing sulfate groups. This NP vehicle was capable of entering cells through lipid membrane penetration [235]. When conjugated with anti-CD8 antibody and intravenously delivered in mice in vivo, compared to the untargeted group, the uptake of anti-CD8 NP vehicles by CD8+ T cells increased 35-fold [254]. In addition, radionuclide-embedded gold NPs were demonstrated to be able to induce DC maturation and antitumor immunity towards cervical cancer [255].
6.4. Cellular interactions of nanoliposome
Nanoliposomes are widely used as antigen delivery vehicles and immune adjuvants in anti-cancer drugs, anti-infection drugs, and vaccines. The liposomal membrane can be prepared with natural or synthetic phospholipids and cholesterols similar to the lipids that are naturally present in cell membranes [271]. Nanoliposomes can be taken up by cells through phagocytosis or fusion. Immunological effects and factors influencing the outcome of the process of nanoliposome administration as well as specific examples of nanoliposome delivery systems used in cancer immunotherapy were reviewed in detail by Zamani et al [271].
To summarize, when employed as adjuvants, the physicochemical properties of nanoliposomes, such as membrane fluidity, surface charge, and size all influence the degree of immune responses [271]. Nanoliposomes encapsulating antigens designed to be phagocytosed by APCs are preferred to possess higher membrane fluidity in order to elicit humoral immune responses whereas rigid membranes are preferred for nanoliposomes designed to present antigens on the membrane [256, 271]. In general, liposomes with antigens encapsulated by positively charged lipids exhibit higher APC uptake efficiency than that of those composed of negatively charged or neutral lipids [271, 272]. Larger nanoliposomes induce more efficient immune responses owing to their better retention at the site of injection and more effective phagocytic uptake in the lymph nodes [271]. A previous study reported Ag formulated in liposomes with vesicle size ⩾225 nm preferentially induced Th1 immune response in BAL-B/c mice whereas vesicles of the same type with the size ⩽155 nm preferentially induced Th2 immune response [273].
6.5. Nanotopology
Nanopatterning and other techniques allow the processing of bulk biomaterials at the nanoscale and the creation of nanofeatures. Several studies have reported the ability of nanopatterned implants to modulate the behavior of cells involved in FBR. For example, Padmanabhan et al reported cell-type-specific remodeling of the actin cytoskeleton based on nanorod size [23]. Specifically, fibroblasts exhibited increasingly smaller size and circularity in response to the increasing size of nanorods (50–200 nm) on Pt-BMG. In contrast, macrophages were only able to detect nanofeatures greater than 200 nm. Finally, endothelial cells were able to detect BMG nano-features greater than 55 nm and exhibited greater area, perimeter, and elongation. Importantly, intracellular levels of Rho-A, which regulates actin remodeling and type I collagen production, were reduced in fibroblasts cultured on the nanopatterned BMG. These results suggested that fibroblast behavior in FBR could be modulated by specific nanopatterns. In a separate study with the same material, it was reported that nanotopography could modulate macrophage polarization [57]. Specifically, IFN/lipopolysaccharide-induced polarization was reduced in macrophages cultured on Pt-BMG with 55 nm diameter nanorods. In addition, implantation of the nanopatterned Pt-BMG in vivo induced reduced foreign body giant cell formation and fibrous capsule thickness and increased blood vessel formation when compared to that of the flat BMG group. Therefore, processing of bulk materials at nanoscale has the potential to modulate complex biological processes.
7. Conclusions and future perspectives
The relationship between NM biological identity and physiological responses is central to both their safe application and successful targeting. For example, lipid-based NPs have enabled the successful development of COVID-19 mRNA vaccines [274]. However, in order to improve the efficiency and effectiveness of NM-based treatments, undesired off-target effects must be minimized, the shuttling of NMs to desired cells must be optimized, and further studies regarding protein corona composition and cellular association should be pursued. A recent review identified key remaining challenges including the difficulty in quantifying and characterizing the vast number of proteins on NPs, the lack of reliable theoretical models, the difficulty in correlating theoretical and experimental models, the variation in serum proteins from species to species, discrepancies between in vitro and in vivo studies, and the subsequent issues in translating novel NMs into clinically relevant therapies [275]. It is expected that once the molecular mechanisms of these interactions are clarified, more effective NMs can be produced to fully benefit the field of nanomedicine. Moreover, issues involving scale-up, correlation between preclinical data and clinical outcomes, and difficulties of incorporating NMs in a final compatible form have been raised [276]. Despite these many challenges, the field is poised to continue improving existing NMs and discovering new ones that can interface with biological systems with greater efficacy. For example, the emergence of green NMs represents a new avenue to explore alternatives to chemical and physical methods for nanosynthesis [277]. In terms of new applications, exciting opportunities include the delivery of machinery for genome editing (CRISPR/Cas9), engineering of chimeric antigen receptor T cells for immunotherapy, and biofunctionalization of bulk materials via nanoprocessing. However, potential health hazards still exist both for those exposed during manufacturing as well as patients receiving treatments. Therefore, attempts to develop NM-based therapeutic and diagnostic strategies should also include more extensive and targeted studies to evaluate their potential cytotoxicity and overall biocompatibility. It is our belief that better understanding of these processes will contribute to enhanced designs with greater therapeutic potential.
Acknowledgments
This work was supported by NIH grant DK115969 (T R K) and T T was supported by NIH T32 GM007324 (T T). Figures 1, 3, and 4, and the icons in table 1 are created with the help of BioRender software.
Abbreviations
- APA
aspartic acid
- APC(s)
antigen-presenting cell(s)
- BBB
blood brain barrier
- BMG
bulk metallic glass
- CARPA
complement activation-related pseudoallergy
- CCL2
chemokine ligand 2, also known as monocyte chemoattractant protein 1
- CD
cluster of differentiation
- CNC
cellulose nanocrystal
- CNF
cellulose nanofiber
- CNT
carbon nanotube
- COL I
collagen type I
- CQD(s)
carbon quantum dot(s)
- CRISPR-Cas9
clustered regularly interspaced short palindromic repeats and CRISPR-associated protein 9
- CVD
chemical vapor deposition
- DC(s)
dendritic cell(s)
- DTX
docetaxel
- EBL
electron-beam lithography
- ECM
extracellular matrix
- EPR
enhanced permeability and retention
- FBR
foreign body response
- FDA
Food and Drug Administration
- GI
gastrointestinal
- H&E
haemotoxylin and eosin
- HA
hydroxyapatite
- HiPco
high pressure conversion (reduction) of carbon monoxide
- HIV
human immunodeficiency virus
- HSR
hypersensitivity reaction
- IFN
interferon
- IL
interleukin
- ISO
International Organization for Standardization
- LDH
lactate dehydrogenase
- MRI
magnetic resonance imaging
- MTT
3-(4,5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide
- NF-kB
nuclear factor kappa-light-chain-enhancer of activated B cells
- NM
nanomaterials
- NP(s)
nanoparticle(s)
- PBAE
poly(B-amino ester)
- PDA
pancreatic ductal adenocarcinoma
- PDLLA
poly-DL-lactic acid
- PEG
polyethylene glycol
- PLA
poly(lactide)
- PLGA
poly(lactide-co-glycolide)
- Pt-BMG
platinum-based BMG
- RIE
reactive ion etching
- ROS
reactive oxygen species
- SMA
smooth muscle actin
- SWCNT
single-walled CNTs
- TGF-β
transforming growth factor beta
- TLR
toll-like receptor
- TNF
tumor necrosis factor
References
- [1].Jeevanandam J, Barhoum A, Chan YS, Dufresne A and Danquah MK 2018. Review on nanoparticles and nanostructured materials: history, sources, toxicity and regulations Beilstein J. Nanotechnol 9 1050–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Sharma VP, Sharma U, Chattopadhyay M and Shukla VN 2018. Advance applications of nanomaterials: a review Mater. Today Proc 5 6376–80 [Google Scholar]
- [3].Guisbiers G 2010. Size-dependent materials properties toward a universal equation Nanoscale Res. Lett 5 1132–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Caruso F 2001. Nanoengineering of particle surfaces Adv. Mater 13 11–22 [Google Scholar]
- [5].Dobrzanski LA. et al. Advanced nanoengineering materials. J. Nanomater. 2018;2018 8/3847348. [Google Scholar]
- [6].Alivisatos AP 2001. Less is more in medicine—sophisticated forms of nanotechnology will find some of their first real-world applications in biomedical research, disease diagnosis and, possibly, therapy Sci. Am 285 66–73 [Google Scholar]
- [7].Davis SS 1997. Biomedical applications of nanotechnology—implications for drug targeting and gene therapy Trends Biotechnol. 15 217–24 [DOI] [PubMed] [Google Scholar]
- [8].Lehner R, Wang X, Marsch S and Hunziker P 2013. Intelligent nanomaterials for medicine: carrier platforms and targeting strategies in the context of clinical application Nanomedicine 9 742–57 [DOI] [PubMed] [Google Scholar]
- [9].Soares S, Sousa J, Pais A and Vitorino C 2018. Nanomedicine: principles, properties, and regulatory issues Front. Chem 6 360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Kim J, Kim HN, Lang Y and Pandit A 2014. Biologically inspired micro- and nanoengineering systems for functional and complex tissues Tissue Eng. A 20 2127–30 [DOI] [PubMed] [Google Scholar]
- [11].Padmanabhan J and Kyriakides TR 2015. Nanomaterials, inflammation, and tissue engineering Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol 7 355–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Syed S, Zubair A and Frieri M 2013. Immune response to nanomaterials: implications for medicine and literature review Curr. Allergy Asthma Rep 13 50–7 [DOI] [PubMed] [Google Scholar]
- [13].Dobrovolskaia MA and Mcneil SE 2007. Immunological properties of engineered nanomaterials Nat. Nanotechnol 2 469–78 [DOI] [PubMed] [Google Scholar]
- [14].Szeto GL and Lavik EB 2016. Materials design at the interface of nanoparticles and innate immunity J. Mater. Chem. B 4 1610–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Bhardwaj V and Kaushik A 2017. Biomedical applications of nanotechnology and nanomaterials Micromachines 8 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Grasso G, Zane D and Dragone R 2020. Microbial nanotechnology: challenges and prospects for green biocatalytic synthesis of nanoscale materials for sensoristic and biomedical applications Nanomaterials 10 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Whitman AG et al. 2008. Applications of nanotechnology in the biomedical sciences: small materials, big impacts, and unknown consequences Emerg. Conceptual Ethical Policy Issues Bionanotechnol 101 117–30 [Google Scholar]
- [18].Ganguly P, Breen A and Pillai SC 2018. Toxicity of nanomaterials: exposure, pathways, assessment, and recent advances ACS Biomater. Sci. Eng 4 2237–75 [DOI] [PubMed] [Google Scholar]
- [19].Schubauer-Berigan MK, Dahm MM, Toennis CA, Sammons DL, Eye T, Kodali V, Zeidler-Erdely PC and Erdely A 2020. Association of occupational exposures with ex vivo functional immune response in workers handling carbon nanotubes and nanofibers Nanotoxicology 14 404–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Najafi-Hajivar S, Zakeri-Milani P, Mohammadi H, Niazi M, Soleymani-Goloujeh M, Baradaran B and Valizadeh H 2016. Overview on experimental models of interactions between nanoparticles and the immune system Biomed. Pharmacother 83 1365–78 [DOI] [PubMed] [Google Scholar]
- [21].Boehler RM, Graham JG and Shea LD 2011. Tissue engineering tools for modulation of the immune response Biotechniques 51 239–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Marrazzo P and O’Leary C 2020. Repositioning natural antioxidants for therapeutic applications in tissue engineering Bioengineering 7 104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Padmanabhan J, Kinser ER, Stalter MA, Duncan-Lewis C, Balestrini JL, Sawyer AJ, Schroers J and Kyriakides TR 2014. Engineering cellular response using nanopatterned bulk metallic glass ACS Nano 8 4366–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Banik BL, Fattahi P and Brown JL 2016. Polymeric nanoparticles: the future of nanomedicine Wiley Interdiscip. Rev. Nanomed Nanobiotechnol 8 271–99 [DOI] [PubMed] [Google Scholar]
- [25].Reis CP, Neufeld RJ, Ribeiro AJ and Veiga F 2006. Nanoencapsulation I. Methods for preparation of drug-loaded polymeric nanoparticles Nanomedicine 2 8–21 [DOI] [PubMed] [Google Scholar]
- [26].Ben-Akiva E, Est Witte S, Meyer RA, Rhodes KR and Green JJ 2018. Polymeric micro- and nanoparticles for immune modulation Biomater. Sci 7 14–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Farjadian F, Ghasemi A, Gohari O, Roointan A, Karimi M and Hamblin MR 2019. Nanopharmaceuticals and nanomedicines currently on the market: challenges and opportunities Nanomedicine 14 93–126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Bobo D, Robinson KJ, Islam J, Thurecht KJ and Corrie SR 2016. Nanoparticle-based medicines: a review of FDA-approved materials and clinical trials to date Pharm. Res 33 2373–87 [DOI] [PubMed] [Google Scholar]
- [29].HogenEsch H, O’Hagan DT and Fox CB 2018. Optimizing the utilization of aluminum adjuvants in vaccines: you might just get what you want NPJ Vaccines 3 51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Erol O et al. 2018. Recent advances in bioactive 1D and 2D carbon nanomaterials for biomedical applications Nanomed. Nanotechnol. Biol. Med 14 2433–54 [DOI] [PubMed] [Google Scholar]
- [31].Maiti D, Tong X, Mou X and Yang K 2019. Carbon-based nanomaterials for biomedical applications: a recent study Front Pharmacol. 9 1–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Alegret N, Dominguez-Alfaro A, Gonźalez-Domínguez JM, Arnaiz B, Cossío U, Bosi S, Vázquez E, Ramos-Cabrer P, Mecerreyes D and Prato M 2018. Three-dimensional conductive scaffolds as neural prostheses based on carbon nanotubes and polypyrrole ACS Appl. Mater. Interfaces 10 43904–14 [DOI] [PubMed] [Google Scholar]
- [33].Marchesan S, Kostarelos K, Bianco A and Prato M 2015. The winding road for carbon nanotubes in nanomedicine Mater. Today 18 12–9 [Google Scholar]
- [34].Yu HT. et al. High-efficient synthesis of graphene oxide based on improved hummers method. Sci. Rep. 2016;6:36413. doi: 10.1038/srep36143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Park J et al. 2015. Graphene oxide flakes as a cellular adhesive: prevention of reactive oxygen species mediated death of implanted cells for cardiac repair ACS Nano 9 4987–99 [DOI] [PubMed] [Google Scholar]
- [36].Kairi MI, Dayou S, Kairi NI, Bakar SA, Vigolo B and Mohamed AR 2018. Toward high production of graphene flakes—a review on recent developments in their synthesis methods and scalability J. Mater. Chem. A 6 15010–26 [Google Scholar]
- [37].Yang YQ, Asiri AM, Tang Z, Du D and Lin Y 2013. Graphene based materials for biomedical applications Mater. Today 16 365–73 [Google Scholar]
- [38].Mojica M, Alonso JA and Mendez F 2013. Synthesis of fullerenes J. Phys. Org. Chem 26 526–39 [Google Scholar]
- [39].Goodarzi S et al. 2017. Fullerene: biomedical engineers get to revisit an old friend Mater. Today 20 460–80 [Google Scholar]
- [40].Rondags A, Yuen WY, Jonkman MF and Horváth B 2017. Fullerene C-60 with cytoprotective and cytotoxic potential: prospects as a novel treatment agent in dermatology? Exp. Dermatol 26 220–4 [DOI] [PubMed] [Google Scholar]
- [41].Zhang CG, Li J, Shi C, Liu E, Du X, Feng W and Zhao N 2011. The efficient synthesis of carbon nano-onions using chemical vapor deposition on an unsupported Ni–Fe alloy catalyst Carbon 49 1151–8 [Google Scholar]
- [42].Gottlieb S, Wöhrl N, Schulz S and Buck V 2016. Simultaneous synthesis of nanodiamonds and graphene via plasma enhanced chemical vapor deposition (MW PE-CVD) on copper Springerplus 5 568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Mochalin VN, Shenderova O, Ho D and Gogotsi Y 2011. The properties and applications of nanodiamonds Nat. Nanotechnol 7 11–23 [DOI] [PubMed] [Google Scholar]
- [44].Camisasca A and Giordani S 2017. Carbon nano-onions in biomedical applications: promising theranostic agents Inorganica Chim. Acta 468 67–76 [Google Scholar]
- [45].d’Amora M, Rodio M, Bartelmess J, Sancataldo G, Brescia R, Cella Zanacchi F, Diaspro A and Giordani S 2016. Biocompatibility and biodistribution of functionalized carbon nano-onions (f-CNOs) in a vertebrate model Sci. Rep 6 33923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Chauhan S, Jain N and Nagaich U 2020. Nanodiamonds with powerful ability for drug delivery and biomedical applications: recent updates on in vivo study and patents J. Pharm. Anal 10 1–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Turcheniuk K and Mochalin VN 2017. Biomedical applications of nanodiamond Nanotechnology 28 2001. [DOI] [PubMed] [Google Scholar]
- [48].Akbarzadeh A, Rezaei-Sadabady R, Davaran S, Joo SW, Zarghami N, Hanifehpour Y, Samiei M, Kouhi M and Nejati-Koshki K 2013. Liposome: classification, preparation, and applications Nanoscale Res. Lett 8 102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Panahi Y, Farshbaf M, Mohammadhosseini M, Mirahadi M, Khalilov R, Saghfi S and Akbarzadeh A 2017. Recent advances on liposomal nanoparticles: synthesis, characterization and biomedical applications Artif. Cells, Nanomed. Biotechnol 45 788–99 [DOI] [PubMed] [Google Scholar]
- [50].Nosova AS, Koloskova OO, Nikonova AA, Simonova VA, Smirnov VV, Kudlay D and Khaitov MR 2019. Diversity of PEGylation methods of liposomes and their influence on RNA delivery Medchemcomm 10 369–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Beltran-Gracia E, López-Camacho A, Higuera-Ciapara I, Velázquez-Fernández JB and Vallejo-Cardona AA 2019. Nanomedicine review: clinical developments in liposomal applications Cancer Nanotechnol. 10 11 [Google Scholar]
- [52].Ventola CL 2017. Progress in nanomedicine: approved and investigational nanodrugs Pharm. Ther 42 742–55 [PMC free article] [PubMed] [Google Scholar]
- [53].Wang XL, Song Y, Su Y, Tian Q, Li B, Quan J and Deng Y 2016. Are PEGylated liposomes better than conventional liposomes? A special case for vincristine Drug Deliv. 23 1092–100 [DOI] [PubMed] [Google Scholar]
- [54].Verma D. et al. Protein based nanostructures for drug delivery. J. Pharm. 2018;2018:9285854. doi: 10.1155/2018/9285854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Jain A, Singh SK, Arya SK, Kundu SC and Kapoor S 2018. Protein nanoparticles: promising platforms for drug delivery applications ACS Biomater. Sci. Eng 4 3939–61 [DOI] [PubMed] [Google Scholar]
- [56].Bhaskar S and Lim S 2017. Engineering protein nanocages as carriers for biomedical applications NPG Asia Mater. 9 e371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Shayan M, Padmanabhan J, Morris AH, Cheung B, Smith R, Schroers J and Kyriakides TR 2018. Nanopatterned bulk metallic glass-based biomaterials modulate macrophage polarization Acta Biomater. 75 427–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Liu Z, Han G, Sohn S, Liu N and Schroers J 2019. Nanomolding of crystalline metals: the smaller the easier Phys. Rev. Lett 122 036101. [DOI] [PubMed] [Google Scholar]
- [59].Loye AM, Kinser ER, Bensouda S, Shayan M, Davis R, Wang R, Chen Z, Schwarz UD, Schroers J and Kyriakides TR 2018. Regulation of mesenchymal stem cell differentiation by nanopatterning of bulk metallic glass Sci. Rep. 8 8758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].FDA 2019. FDA takes action to protect patients from risk of certain textured breast implants; requests Allergan voluntarily recall certain breast implants and tissue expanders from market. FDA press anouncement (available at: www.fda.gov/news-events/press-announcements/fda-takes-action-protect-patients-risk-certain-textured-breast-implants-requests-allergan) (Accessed 15 July 2020) [Google Scholar]
- [61].Chen Q and Liu ZW 2019. Fabrication and applications of solid-state nanopores Sensors 19 1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Jani AMM, Losic D and Voelcker NH 2013. Nanoporous anodic aluminium oxide: advances in surface engineering and emerging applications Prog. Mater. Sci 58 636–704 [Google Scholar]
- [63].Chen ZT, Ni S, Han S, Crawford R, Lu S, Wei F, Chang J, Wu C and Xiao Y 2017. Nanoporous microstructures mediate osteogenesis by modulating the osteo-immune response of macrophages Nanoscale 9 706–18 [DOI] [PubMed] [Google Scholar]
- [64].Dumas V. et al. Femtosecond laser nano/micro patterning of titanium influences mesenchymal stem cell adhesion and commitment. Biomed. Mater. 2015;10:055002. doi: 10.1088/1748-6041/10/5/055002. [DOI] [PubMed] [Google Scholar]
- [65].Luu TU, Gott SC, Woo BWK, Rao MP and Liu WF 2015. Micro- and nanopatterned topographical cues for regulating macrophage cell shape and phenotype ACS Appl. Mater. Interfaces 7 28665–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Sforza M, Zaccheddu R, Alleruzzo A, Seno A, Mileto D, Paganelli A, Sulaiman H, Payne M and Maurovich-Horvat L 2018. Preliminary 3-year evaluation of experience with silksurface and velvetsurface motiva silicone breast implants: a single-center experience with 5813 consecutive breast augmentation cases Aesthet. Surg. J 38 S62–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Singh D, Singh S, Sahu J, Srivastava S and Singh MR 2016. Ceramic nanoparticles: recompense, cellular uptake and toxicity concerns Artif Cells, Nanomed. Biotechnol 44 401–9 [DOI] [PubMed] [Google Scholar]
- [68].Peres C et al. 2017. Poly(lactic acid)-based particulate systems are promising tools for immune modulation Acta Biomater 48 41–57 [DOI] [PubMed] [Google Scholar]
- [69].Torres FG, Troncoso OP, Pisani A, Gatto F and Bardi G 2019. Natural polysaccharide nanomaterials: an overview of their immunological properties Int. J. Mol. Sci 20 5092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Dufresne A. Cellulose nanomaterials as green nanoreinforcements for polymer nanocomposites. Phil. Trans. R. Soc. A. 2018;376:20170040. doi: 10.1098/rsta.2017.0040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Key J, Dhawan D, Cooper CL, Knapp DW, Kim K, Kwon IC, Choi K, Park K, Decuzzi P and Leary JF 2016. Multicomponent, peptide-targeted glycol chitosan nanoparticles containing ferrimagnetic iron oxide nanocubes for bladder cancer multimodal imaging Int. J. Nanomedicine 11 4141–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Singla R et al. 2016. Metallic nanoparticles, toxicity issues and applications in medicine Nanoscale Materials in Targeted Drug Delivery, Theragnosis and Tissue Regeneration ed Yadav SK (Berlin: Springer; ) pp 41–80 [Google Scholar]
- [73].Luo YH, Chang LW and Lin P 2015. Metal-based nanoparticles and the immune system: activation, inflammation, and potential applications Biomed. Res. Int 2015 143720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Li D, Feng S, Huang H, Chen W, Shi H, Liu N, Chen L, Chen W, Yu Y and Chen R 2014. Label-free detection of blood plasma using silver nanoparticle based surface-enhanced Raman spectroscopy for esophageal cancer screening J. Biomed. Nanotechnol 10 478–84 [DOI] [PubMed] [Google Scholar]
- [75].Hessel CM, Pattani VP, Rasch M, Panthani MG, Koo B, Tunnell JW and Korgel BA 2011. Copper selenide nanocrystals for photothermal therapy Nano Lett. 11 2560–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Cha CY, Shin SR, Annabi N, Dokmeci MR and Khademhosseini A 2013. Carbon-based nanomaterials: multifunctional materials for biomedical engineering ACS Nano 7 2891–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Iijima S 1991. Helical microtubules of graphitic carbon Nature 354 56–8 [Google Scholar]
- [78].Iijima S 2002. Carbon nanotubes: past, present, and future Physica B 323 1–5 [Google Scholar]
- [79].Rao CNR, Sood A, Subrahmanyam K and Govindaraj A 2009. Graphene: the new two-dimensional nanomaterial Angew. Chem., Int. Ed 48 7752–77 [DOI] [PubMed] [Google Scholar]
- [80].Molaei MJ 2019. Carbon quantum dots and their biomedical and therapeutic applications: a review RSC Adv. 9 6460–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Wei XJ, Tanaka T, Hirakawa T, Wang G and Kataura H 2017. High-efficiency separation of (6,5) carbon nanotubes by stepwise elution gel chromatography Phys. Status Solidi A 254 1700279 [Google Scholar]
- [82].Zhang MF, Yang M, Morimoto T, Tajima N, Ichiraku K, Fujita K, Iijima S, Yudasaka M and Okazaki T 2018. Size-dependent cell uptake of carbon nanotubes by macrophages: a comparative and quantitative study Carbon 127 93–101 [Google Scholar]
- [83].Hoa LTM 2018. Characterization of multi-walled carbon nanotubes functionalized by a mixture of HNO3/H2SO4 Diam. Relat. Mater 89 43–51 [Google Scholar]
- [84].Sobhani Z, Behnam MA, Emami F, Dehghanian A and Jamhiri I 2017. Photothermal therapy of melanoma tumor using multiwalled carbon nanotubes Int. J. Nanomedicine 12 4509–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Nowack B, David RM, Fissan H, Morris H, Shatkin JA, Stintz M, Zepp R and Brouwer D 2013. Potential release scenarios for carbon nanotubes used in composites Environ. Int 59 1–11 [DOI] [PubMed] [Google Scholar]
- [86].Dong J and Ma Q 2018. Type 2 immune mechanisms in carbon nanotube-induced lung fibrosis Front Immunol. 9 1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Duke KS and Bonner JC 2018. Mechanisms of carbon nanotube-induced pulmonary fibrosis: a physicochemical characteristic perspective Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol 10 e1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Mukherjee SP. et al. Macrophage sensing of single-walled carbon nanotubes via Toll-like receptors. Sci. Rep. 2018;8:1115. doi: 10.1038/s41598-018-19521-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Lahiani MH, Gokulan K, Williams K, Khodakovskaya MV and Khare S 2017. Graphene and carbon nanotubes activate different cell surface receptors on macrophages before and after deactivation of endotoxins J. Appl. Toxicol 37 1305–16 [DOI] [PubMed] [Google Scholar]
- [90].Jang J, Kim Y, Hwang J, Choi Y, Tanaka M, Kang E and Choi J 2019. Biological responses of onion-shaped carbon nanoparticles Nanomaterials 9 1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Keshavan S, Calligari P, Stella L, Fusco L, Delogu LG and Fadeel B 2019. Nano-bio interactions: a neutrophil-centric view Cell Death Dis. 10 1–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Mohamed M, Abu Lila AS, Shimizu T, Alaaeldin E, Hussein A, Sarhan HA, Szebeni J and Ishida T 2019. PEGylated liposomes: immunological responses Sci. Technol. Adv. Mater 20 710–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Tostanoski LH and Jewell CM 2017. Engineering self-assembled materials to study and direct immune function Adv. Drug Deliv. Rev 114 60–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Mozafari MR 2010. Nanoliposomes: preparation and analysis Liposomes: Methods and Protocols vol 605 (New York: Humana Press; ) pp 29–50 [DOI] [PubMed] [Google Scholar]
- [95].Caster JM, Patel AN, Zhang T and Wang A 2017. Investigational nanomedicines in 2016: a review of nanotherapeutics currently undergoing clinical trials Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol 9 1416. [DOI] [PubMed] [Google Scholar]
- [96].Bello DG, Fouillen A, Badia A and Nanci A 2020. Nanoporosity stimulates cell spreading and focal adhesion formation in cells with mutated paxillin ACS Appl. Mater. Interfaces 12 14924–32 [DOI] [PubMed] [Google Scholar]
- [97].Karazisis D, Petronis S, Agheli H, Emanuelsson L, Norlindh B, Johansson A, Rasmusson L, Thomsen P and Omar O 2017. The influence of controlled surface nanotopography on the early biological events of osseointegration Acta Biomater. 53 559–71 [DOI] [PubMed] [Google Scholar]
- [98].Kim DH, Provenzano PP, Smith CL and Levchenko A 2012. Matrix nanotopography as a regulator of cell function J. Cell Biol 197 351–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Qian WY, Gong L, Cui X, Zhang Z, Bajpai A, Liu C, Castillo AB, Teo JCM and Chen W 2017. Nanotopographic regulation of human mesenchymal stem cell osteogenesis ACS Appl. Mater. Interfaces 9 41794–806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Mullen CA, Vaughan T, Billiar K and McNamara L 2015. The effect of substrate stiffness, thickness, and cross-linking density on osteogenic cell behavior Biophys. J 108 1604–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Yang Y et al. 2017. Biophysical regulation of cell behavior-cross talk between substrate stiffness and nanotopography Engineering 3 36–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Kumar G, Tang HX and Schroers J 2009. Nanomoulding with amorphous metals Nature 457 868–72 [DOI] [PubMed] [Google Scholar]
- [103].Bae IH, Jeong MH, Lim KS, Park DS, Shim JW, Park J-K, Oh KH, Jin MR and Sim DS 2018. Novel polymer-free everolimus-eluting stent fabricated using femtosecond laser improves re-endothelialization and anti-inflammation Sci. Rep 8 7383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Barthes J et al. 2018. Review: the potential impact of surface crystalline states of titanium for biomedical applications Crit. Rev. Biotechnol 38 423–37 [DOI] [PubMed] [Google Scholar]
- [105].Zolnik BS, González-Fernández A, Sadrieh N and Dobrovolskaia MA 2010. Minireview: nanoparticles and the immune system Endocrinology 151 458–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Platform IOB 2013. ISO/TR 10993–22:2017(en) Biological Evaluation of Medical devices—Part 22: Guidance on Nanomaterials (Geneva: International Organization for Standardization; ) (www.iso.org/obp/ui#iso:std:iso:tr:10993:-22:ed-1:v1:en) [Google Scholar]
- [107].Rodríguez-Ibarra C, Déciga-Alcaraz A, Ispanixtlahuatl-Meráz O, Medina-Reyes EI, Delgado-Buenrostro NL and Chirino YI 2020. International landscape of limits and recommendations for occupational exposure to engineered nanomaterials Toxicol. Lett 322 111–9 [DOI] [PubMed] [Google Scholar]
- [108].Soenen SJ et al. 2015. (Intra)cellular stability of inorganic nanoparticles: effects on cytotoxicity, particle functionality, and biomedical applications Chem. Rev 115 2109–35 [DOI] [PubMed] [Google Scholar]
- [109].Hu X, Zheng Y, Zhou J, Fang D, Jiang H and Wang X 2018. Silver-assisted thiolate ligand exchange induced photoluminescent boost of gold nanoclusters for selective imaging of intracellular glutathione Chem. Mater 30 1947–55 [Google Scholar]
- [110].Guimarães PP, Gaglione S, Sewastianik T, Carrasco RD, Langer R and Mitchell MJ 2018. Nanoparticles for immune cytokine TRAIL-based cancer therapy ACS Nano 12 912–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Ahmed S, Miyawaki O and Matsumura K 2018. Enhanced adsorption of a protein–nanocarrier complex onto cell membranes through a high freeze concentration by a polyampholyte cryoprotectant Langmuir 34 2352–62 [DOI] [PubMed] [Google Scholar]
- [112].Duong HV, Chau TTL, Dang NTT, Vanterpool F, Salmerón-Sánchez M, Lizundia E, Tran HT, Nguyen LV and Nguyen T-D 2018. Biocompatible chitosan-functionalized upconverting nanocomposites ACS Omega 3 86–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Li M, Luo Z and Zhao Y 2018. Self-assembled hybrid nanostructures: versatile multifunctional nanoplatforms for cancer diagnosis and therapy Chem. Mater 30 25–53 [Google Scholar]
- [114].Council NR 2007. Toxicity Testing in the 21st Century: A Vision and a Strategy (Washington: National Academies Press; ) ( 10.17226/11970) [DOI] [Google Scholar]
- [115].Karlsson HL, Gustafsson J, Cronholm P and Möller L 2009. Size-dependent toxicity of metal oxide particles—a comparison between nano- and micrometer size Toxicol. Lett 188 112–8 [DOI] [PubMed] [Google Scholar]
- [116].Sahu D, Kannan GM, Tailang M and Vijayaraghavan R 2016. In vitro cytotoxicity of nanoparticles: a comparison between particle size and cell type J. Nanosci 2016 4023852 [Google Scholar]
- [117].Wen H, Dan M, Yang Y, Lyu J, Shao A, Cheng X, Chen L and Xu L 2017. Acute toxicity and genotoxicity of silver nanoparticle in rats PLoS One 12 e0185554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Wang Z et al. 2017. Specifically formed corona on silica nanoparticles enhances transforming growth factor β1 activity in triggering lung fibrosis ACS Nano 11 1659–72 [DOI] [PubMed] [Google Scholar]
- [119].Riss TL and Moravec RA 2004. Use of multiple assay endpoints to investigate the effects of incubation time, dose of toxin, and plating density in cell-based cytotoxicity assays Assay. Drug Dev. Technol 2 51–62 [DOI] [PubMed] [Google Scholar]
- [120].Farnoud AM and Nazemidashtarjandi S 2019. Emerging investigator series: interactions of engineered nanomaterials with the cell plasma membrane; what have we learned from membrane models? Environ. Sci.: Nano 6 13–40 [Google Scholar]
- [121].Perde-Schrepler M, Florea A, Brie I, Virag P, Fischer-Fodor E, Vâlcan A, Gurzău E, Lisencu C and Maniu A 2019. Size-dependent cytotoxicity and genotoxicity of silver nanoparticles in cochlear cells in vitro J. Nanomater 2019 6090259 [Google Scholar]
- [122].Singh N, Manshian B, Jenkins GJS, Griffiths SM, Williams PM, Maffeis TGG, Wright CJ and Doak SH 2009. NanoGenotoxicology: the DNA damaging potential of engineered nanomaterials Biomaterials 30 3891–914 [DOI] [PubMed] [Google Scholar]
- [123].Mahaye N et al. 2017. Genotoxicity of metal based engineered nanoparticles in aquatic organisms: a review Mutat. Res. Rev. Mutat. Res 773 134–60 [DOI] [PubMed] [Google Scholar]
- [124].Du H, Pan B and Chen T 2017. Evaluation of chemical mutagenicity using next generation sequencing: a review J. Environ. Sci. Health C 35 140–58 [DOI] [PubMed] [Google Scholar]
- [125].Tran QH and Le A-T 2013. Silver nanoparticles: synthesis, properties, toxicology, applications and perspectives Adv. Nat. Sci.: Nanosci. Nanotechnol 4 033001 [Google Scholar]
- [126].Kim S and Ryu DY 2013. Silver nanoparticle-induced oxidative stress, genotoxicity and apoptosis in cultured cells and animal tissues J. Appl. Toxicol 33 78–89 [DOI] [PubMed] [Google Scholar]
- [127].de Lima R, Seabra AB and Duŕan N 2012. Silver nanoparticles: a brief review of cytotoxicity and genotoxicity of chemically and biogenically synthesized nanoparticles J. Appl. Toxicol 32 867–79 [DOI] [PubMed] [Google Scholar]
- [128].Foldbjerg R, Dang DA and Autrup H 2011. Cytotoxicity and genotoxicity of silver nanoparticles in the human lung cancer cell line, A549 Arch. Toxicol 85 743–50 [DOI] [PubMed] [Google Scholar]
- [129].Park MV, Neigh AM, Vermeulen JP, de la Fonteyne LJJ, Verharen HW, Briedé JJ, van Loveren H and de Jong WH 2011. The effect of particle size on the cytotoxicity, inflammation, developmental toxicity and genotoxicity of silver nanoparticles Biomaterials 32 9810–7 [DOI] [PubMed] [Google Scholar]
- [130].Gliga AR, Skoglund S, Odnevall Wallinder I, Fadeel B and Karlsson HL 2014. Size-dependent cytotoxicity of silver nanoparticles in human lung cells: the role of cellular uptake, agglomeration and Ag release Part Fibre Toxicol 11 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Shukla RK, Sharma V, Pandey AK, Singh S, Sultana S and Dhawan A 2011. ROS-mediated genotoxicity induced by titanium dioxide nanoparticles in human epidermal cells Toxicol. In Vitro 25 231–41 [DOI] [PubMed] [Google Scholar]
- [132].Yang H, Liu C, Yang D, Zhang H and Xi Z 2009. Comparative study of cytotoxicity, oxidative stress and genotoxicity induced by four typical nanomaterials: the role of particle size, shape and composition J. Appl. Toxicol 29 69–78 [DOI] [PubMed] [Google Scholar]
- [133].Betker JL, Gomez J and Anchordoquy TJ 2013. The effects of lipoplex formulation variables on the protein corona and comparisons with in vitro transfection efficiency J. Control Release 171 261–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [134].Brunetti V, Chibli H, Fiammengo R, Galeone A, Malvindi MA, Vecchio G, Cingolani R, Nadeau JL and Pompa PP 2013. InP/ZnS as a safer alternative to CdSe/ZnS core/shell quantum dots: in vitro and in vivo toxicity assessment Nanoscale 5 307–17 [DOI] [PubMed] [Google Scholar]
- [135].Seabra AB, Paula AJ, de Lima R, Alves OL and Durán N 2014. Nanotoxicity of graphene and graphene oxide Chem. Res. Toxicol 27 159–68 [DOI] [PubMed] [Google Scholar]
- [136].Ema M, Gamo M and Honda K 2017. A review of toxicity studies on graphene-based nanomaterials in laboratory animals Regul. Toxicol. Pharmacol 85 7–24 [DOI] [PubMed] [Google Scholar]
- [137].Gutierro I, Herńandez RM, Igartua M, Gascón AR and Pedraz JL 2002. Size dependent immune response after subcutaneous, oral and intranasal administration of BSA loaded nanospheres Vaccine 21 67–77 [DOI] [PubMed] [Google Scholar]
- [138].Ngobili TA and Daniele MA 2016. Nanoparticles and direct immunosuppression Exp. Biol. Med 241 1064–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Kermanizadeh A, Vranic S, Boland S, Moreau K, Baeza-Squiban A, Gaiser BK, Andrzejczuk LA and Stone V 2013. An in vitro assessment of panel of engineered nanomaterials using a human renal cell line: cytotoxicity, pro-inflammatory response, oxidative stress and genotoxicity BMC Nephrol. 14 96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].Feng X, Xu W, Li Z, Song W, Ding J and Chen X 2019. Immunomodulatory nanosystems Adv. Sci 6 1900101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [141].Čolić M, Tomić S and Bekić M 2020. Immunological aspects of nanocellulose Immunol. Lett 222 80–9 [DOI] [PubMed] [Google Scholar]
- [142].Yuan X, Zhang X, Sun L, Wei Y and Wei X 2019. Cellular toxicity and immunological effects of carbon-based nanomaterials Part Fibre Toxicol. 16 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [143].Nel AE, Mädler L, Velegol D, Xia T, Hoek EMV, Somasundaran P, Klaessig F, Castranova V and Thompson M 2009. Understanding biophysicochemical interactions at the nano-bio interface Nat. Mater 8 543–57 [DOI] [PubMed] [Google Scholar]
- [144].Rahmati M and Mozafari M 2019. Nano-immunoengineering: opportunities and challenges Curr. Opin. Biomed. Eng 10 51–9 [Google Scholar]
- [145].Kumar S, Anselmo AC, Banerjee A, Zakrewsky M and Mitragotri S 2015. Shape and size-dependent immune response to antigen-carrying nanoparticles J. Control. Release 220 141–8 [DOI] [PubMed] [Google Scholar]
- [146].Wang J et al. 2018. Physical activation of innate immunity by spiky particles Nat. Nanotechnol 13 1078–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [147].Oberdürster G 2000. Toxicology of ultrafine particles: in vivo studies Phil. Trans. R. Soc. A 358 2719–40 [Google Scholar]
- [148].Byrne JD and Baugh JA 2008. The significance of nanoparticles in particle-induced pulmonary fibrosis McGill J. Med 11 43. [PMC free article] [PubMed] [Google Scholar]
- [149].Sanchez VC et al. 2009. Biopersistence and potential adverse health impacts of fibrous nanomaterials: what have we learned from asbestos? Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol 1 511–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [150].Song Y, Li X and Du X 2009. Exposure to nanoparticles is related to pleural effusion, pulmonary fibrosis and granuloma Eur. Respir. J 34 559–67 [DOI] [PubMed] [Google Scholar]
- [151].Cho W-S, Duffin R, Poland CA, Howie SEM, MacNee W, Bradley M, Megson IL and Donaldson K 2010. Metal oxide nanoparticles induce unique inflammatory footprints in the lung: important implications for nanoparticle testing Environ. Health Perspect 118 1699–706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [152].Porras AM, Hutson HN, Berger AJ and Masters KS 2016. Engineering approaches to study fibrosis in 3D in vitro systems Curr. Opin. Biotechnol 40 24–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [153].Mabry KM, Payne SZ and Anseth KS 2016. Microarray analyses to quantify advantages of 2D and 3D hydrogel culture systems in maintaining the native valvular interstitial cell phenotype Biomaterials 74 31–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [154].Gonzalez-Gonzalez FJ, Chandel NS, Jain M and Budinger GRS 2017. Reactive oxygen species as signaling molecules in the development of lung fibrosis Trans. Res 190 61–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [155].Dong J and Ma Q 2016. Myofibroblasts and lung fibrosis induced by carbon nanotube exposure Part Fibre Toxicol 13 60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [156].Dong J and Ma Q 2019. Integration of inflammation, fibrosis, and cancer induced by carbon nanotubes Nanotoxicology 13 1244–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [157].Vietti G, Ibouraadaten S, Palmai-Pallag M, Yakoub Y, Bailly C, Fenoglio I, Marbaix E, Lison D and van den Brule S 2013. Towards predicting the lung fibrogenic activity of nanomaterials: experimental validation of an in vitro fibroblast proliferation assay Part Fibre Toxicol 10 52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [158].Lai X, Zhao H, Zhang Y, Guo K, Xu Y, Chen S and Zhang J 2018. Intranasal delivery of copper oxide nanoparticles induces pulmonary toxicity and fibrosis in C57BL/6 mice Sci. Rep 8 1–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [159].Huang K-T, Wu C-T, Huang K-H, Lin W-C, Chen C-M, Guan S-S, Chiang C-K and Liu S-H 2015. Titanium nanoparticle inhalation induces renal fibrosis in mice via an oxidative stress upregulated transforming growth factor-β pathway Chem. Res. Toxicol 28 354–64 [DOI] [PubMed] [Google Scholar]
- [160].Ma JY, Zhao H, Mercer RR, Barger M, Rao M, Meighan T, Schwegler-Berry D, Castranova V and Ma JK 2011. Cerium oxide nanoparticle-induced pulmonary inflammation and alveolar macrophage functional change in rats Nanotoxicology 5 312–25 [DOI] [PubMed] [Google Scholar]
- [161].Harmatys KM, Overchuk M and Zheng G 2019. Rational design of photosynthesis-inspired nanomedicines Acc. Chem. Res 52 1265–74 [DOI] [PubMed] [Google Scholar]
- [162].Denoyer D, Masaldan S, La Fontaine S and Cater MA 2015. Targeting copper in cancer therapy: ‘copper that cancer’ Metallomics 7 1459–76 [DOI] [PubMed] [Google Scholar]
- [163].Xiao Y et al. 2019. Cuprous oxide nanoparticles reduces hypertrophic scarring by inducing fibroblast apoptosis Int. J. Nanomedicine 14 5989–6000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [164].Jeyarani S, Vinita NM, Puja P, Senthamilselvi S, Devan U, Velangani AJ, Biruntha M, Pugazhendhi A and Kumar P 2020. Biomimetic gold nanoparticles for its cytotoxicity and biocompatibility evidenced by fluorescence-based assays in cancer (MDA-MB-231) and non-cancerous (HEK-293) cells J. Photochem. Photobiol. B 202 111715. [DOI] [PubMed] [Google Scholar]
- [165].Bhattacharya K, Mukherjee SP, Gallud A, Burkert SC, Bistarelli S, Bellucci S, Bottini M, Star A and Fadeel B 2016. Biological interactions of carbon-based nanomaterials: from coronation to degradation Nanomed.: Nanotechnol. Biol. Med 12 333–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [166].Egli RJ and Luginbuehl R 2012. Tissue engineering—nanomaterials in the musculoskeletal system Swiss Med. Wkly 142 w13647. [DOI] [PubMed] [Google Scholar]
- [167].Muller J, Huaux F, Moreau N, Misson P, Heilier J-F, Delos M, Arras M, Fonseca A, Nagy JB and Lison D 2005. Respiratory toxicity of multi-wall carbon nanotubes Toxicol. Appl. Pharmacol 207 221–31 [DOI] [PubMed] [Google Scholar]
- [168].Rossignoli F et al. 2019. MSC-delivered soluble TRAIL and paclitaxel as novel combinatory treatment for pancreatic adenocarcinoma Theranostics 9 436–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [169].Iviglia G, Kargozar S and Baino F 2019. Biomaterials, current strategies, and novel nano-technological approaches for periodontal regeneration J. Funct. Biomater 10 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [170].Chen X, Wu G, Feng Z, Dong Y, Zhou W, Li B, Bai S and Zhao Y 2016. Advanced biomaterials and their potential applications in the treatment of periodontal disease Crit. Rev. Biotechnol 36 760–75 [DOI] [PubMed] [Google Scholar]
- [171].Seabra AB, Bernardes JS, Fávaro WJ, Paula AJ and Durán N 2018. Cellulose nanocrystals as carriers in medicine and their toxicities: a review Carbohydr. Polym 181 514–27 [DOI] [PubMed] [Google Scholar]
- [172].Singla R, Soni S, Patial V, Kulurkar PM, Kumari A, S. M, Padwad YS and Yadav SK 2017. In vivo diabetic wound healing potential of nanobiocomposites containing bamboo cellulose nanocrystals impregnated with silver nanoparticles Int. J. Biol. Macromol 105 45–55 [DOI] [PubMed] [Google Scholar]
- [173].Roman M 2015. Toxicity of cellulose nanocrystals: a review Ind. Biotechnol 11 25–33 [Google Scholar]
- [174].Jodat YA and Shin SR 2020. Functional carbon-based nanomaterials for engineered tissues toward organ regeneration Biomaterials for Organ and Tissue Regeneration (Amsterdam: Elsevier; ) pp 529–50 [Google Scholar]
- [175].Redondo-Gómez C et al. 2019. Recent Advances in Carbon Nanotubes for Nervous Tissue Regeneration vol 2020 (Hoboken, NJ and London: Wiley and Hindawi; ) p 6861205 [Google Scholar]
- [176].Palmer BC, Phelan-Dickenson SJ and DeLouise LA 2019. Multi-walled carbon nanotube oxidation dependent keratinocyte cytotoxicity and skin inflammation Part Fibre Toxicol 16 1–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [177].Hendrickson OD, Morozova OV, Zherdev AV, Yaropolov AI, Klochkov SG, Bachurin SO and Dzantiev BB 2015. Study of distribution and biological effects of fullerene C60 after single and multiple intragastrical administrations to rats Fulleren. Nanotub. Carbon Nanostruct 23 658–68 [Google Scholar]
- [178].Misra C, Thotakura N, Kumar R, Singh B, Sharma G, Katare OP and Raza K 2017. Improved cellular uptake, enhanced efficacy and promising pharmacokinetic profile of docetaxel employing glycine-tethered C60-fullerenes Mater. Sci. Eng. C 76 501–8 [DOI] [PubMed] [Google Scholar]
- [179].Prylutska SV et al. 2019. In vitro and in vivo toxicity of pristine C60 fullerene aqueous colloid solution Fulleren. Nanotub. Carbon Nanostruct 27 715–28 [Google Scholar]
- [180].Kim EM and Jeong HJ 2017. Current status and future direction of nanomedicine: focus on advanced biological and medical applications Nucl. Med. Mol. Imaging 51 106–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [181].Sousa F et al. 2010. Functionalized gold nanoparticles: a detailed in vivo multimodal microscopic brain distribution study Nanoscale 2 2826–34 [DOI] [PubMed] [Google Scholar]
- [182].Uauy R, Olivares M and Gonzalez M 1998. Essentiality of copper in humans Am. J. Clin. Nutr 67 952S–9S [DOI] [PubMed] [Google Scholar]
- [183].Lee IC et al. 2016. Comparative toxicity and biodistribution of copper nanoparticles and cupric ions in rats Int. J. Nanomedicine 11 2883–900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [184].Jederlinic PJ, Abraham JL, Churg A, Himmelstein JS, Epler GR and Gaensler EA 1990. Pulmonary fibrosis in aluminum oxide workers. Investigation of nine workers, with pathologic examination and microanalysis in three of them Am. Rev. Respir. Dis 142 1179–84 [DOI] [PubMed] [Google Scholar]
- [185].Kim YS, Chung Y-H, Seo D-S, Choi H-S and Lim C-H 2018. Twenty-eight-day repeated inhalation toxicity study of aluminum oxide nanoparticles in male Sprague-Dawley rats Toxicol. Res 34 343–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [186].Park EJ, Lee G-H, Yoon C, Jeong U, Kim Y, Cho M-H and Kim D-W 2016. Biodistribution and toxicity of spherical aluminum oxide nanoparticles J. Appl. Toxicol 36 424–33 [DOI] [PubMed] [Google Scholar]
- [187].Trujillo-Alonso V et al. 2019. FDA-approved ferumoxytol displays anti-leukaemia efficacy against cells with low ferroportin levels Nat. Nanotechnol 14 616–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [188].Ignjatović N et al. 2013. Nanoparticles of cobalt-substituted hydroxyapatite in regeneration of mandibular osteoporotic bones J. Mater. Sci. Mater. Med 24 343–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [189].Lin W et al. 2020. Effect of cobalt precursors on cobalt-hydroxyapatite used in bone regeneration and MRI J. Dent. Res 99 277–84 [DOI] [PubMed] [Google Scholar]
- [190].Zununi Vahed S et al. 2017. Liposome-based drug co-delivery systems in cancer cells Mater. Sci. Eng. C: Mater. Biol. Appl 71 1327–41 [DOI] [PubMed] [Google Scholar]
- [191].Inglut CT, Sorrin AJ, Kuruppu T, Vig S, Cicalo J, Ahmad H and Huang H-C 2020. Immunological and toxicological considerations for the design of liposomes Nanomaterials 10 190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [192].Szebeni J et al. 2012. Liposome-induced complement activation and related cardiopulmonary distress in pigs: factors promoting reactogenicity of Doxil and AmBisome Nanomedicine 8 176–84 [DOI] [PubMed] [Google Scholar]
- [193].Weissig V, Pettinger TK and Murdock N 2014. Nanopharmaceuticals (part 1): products on the market Int. J. Nanomedicine 9 4357–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [194].Stone NR, Bicanic T, Salim R and Hope W 2016. Liposomal amphotericin B (AmBisome®): a review of the pharmacokinetics, pharmacodynamics, clinical experience and future directions Drugs 76 485–500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [195].Podila R and Brown JM 2013. Toxicity of engineered nanomaterials: a physicochemical perspective J. Biochem. Mol. Toxicol 27 50–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [196].Lanone S and Boczkowski J 2006. Biomedical applications and potential health risks of nanomaterials: molecular mechanisms Curr. Mol. Med 6 651–63 [DOI] [PubMed] [Google Scholar]
- [197].Digiacomo L. et al. Impact of the protein corona on nanomaterial immune response and targeting ability. Wiley Interdiscip. Rev. Nanomed Nanobiotechnol. 2020;12:e1615. doi: 10.1002/wnan.1615. [DOI] [PubMed] [Google Scholar]
- [198].Pino PD, Pelaz B, Zhang Q, Maffre P, Nienhaus GU and Parak WJ 2014. Protein corona formation around nanoparticles—from the past to the future Mater. Horiz 1 301–13 [Google Scholar]
- [199].Foroozandeh P and Aziz AA 2015. Merging worlds of nanomaterials and biological environment: factors governing protein corona formation on nanoparticles and its biological consequences Nanoscale Res. Lett 10 221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [200].Corbo C, Molinaro R, Parodi A, Toledano Furman NE, Salvatore F and Tasciotti E 2016. The impact of nanoparticle protein corona on cytotoxicity, immunotoxicity and target drug delivery Nanomedicine 11 81–100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [201].Tenzer S et al. 2013. Rapid formation of plasma protein corona critically affects nanoparticle pathophysiology Nat. Nanotechnol 8 772–81 [DOI] [PubMed] [Google Scholar]
- [202].Alberg I. et al. Polymeric nanoparticles with neglectable protein corona. Small. 2020;16:e1907574. doi: 10.1002/smll.201907574. [DOI] [PubMed] [Google Scholar]
- [203].Yan Y, Gause KT, Kamphuis MMJ, Ang C-S, O’Brien-Simpson NM, Lenzo JC, Reynolds EC, Nice EC and Caruso F 2013. Differential roles of the protein corona in the cellular uptake of nanoporous polymer particles by monocyte and macrophage cell lines ACS Nano 7 10960–70 [DOI] [PubMed] [Google Scholar]
- [204].Wu R. et al. Attaching DNA to gold nanoparticles with a protein corona. Front. Chem. 2020;8:121. doi: 10.3389/fchem.2020.00121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [205].Walkey CD, Olsen JB, Guo H, Emili A and Chan WCW 2012. Nanoparticle size and surface chemistry determine serum protein adsorption and macrophage uptake J. Am. Chem. Soc 134 2139–47 [DOI] [PubMed] [Google Scholar]
- [206].Yang H, Wang M, Zhang Y, Li F, Yu S, Zhu L, Guo Y, Yang L and Yang S 2019. Conformational-transited protein corona regulated cell-membrane penetration and induced cytotoxicity of ultrasmall Au nanoparticles RSC Adv. 9 4435–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [207].Mei N, Hedberg J, Odnevall Wallinder I and Blomberg E 2019. Influence of biocorona formation on the transformation and dissolution of cobalt nanoparticles under physiological conditions ACS Omega 4 21778–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [208].Palchetti S, Caputo D, Digiacomo L, Capriotti A, Coppola R, Pozzi D and Caracciolo G 2019. Protein corona fingerprints of liposomes: new opportunities for targeted drug delivery and early detection in pancreatic cancer Pharmaceutics 11 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [209].Betker JL, Jones D, Childs CR, Helm KM, Terrell K, Nagel MA and Anchordoquy TJ 2018. Nanoparticle uptake by circulating leukocytes: a major barrier to tumor delivery J. Control Release 286 85–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [210].Cai X, Ramalingam R, Wong HS, Cheng J, Ajuh P, Cheng SH and Lam YW 2013. Characterization of carbon nanotube protein corona by using quantitative proteomics Nanomedicine 9 583–93 [DOI] [PubMed] [Google Scholar]
- [211].Caracciolo G, Palchetti S, Colapicchioni V, Digiacomo L, Pozzi D, Capriotti AL, La Barbera G and Laganà A 2015. Stealth effect of biomolecular corona on nanoparticle uptake by immune cells Langmuir 31 10764–73 [DOI] [PubMed] [Google Scholar]
- [212].Caracciolo G, Pozzi D, Capriotti AL, Cavaliere C, Piovesana S, La Barbera G, Amici A and Laganà A 2014. The liposome-protein corona in mice and humans and its implications for in vivo delivery J. Mater. Chem. B 2 7419–28 [DOI] [PubMed] [Google Scholar]
- [213].Simon J, Müller LK, Kokkinopoulou M, Lieberwirth I, Morsbach S, Landfester K and Mailänder V 2018. Exploiting the biomolecular corona: pre-coating of nanoparticles enables controlled cellular interactions Nanoscale 10 10731–9 [DOI] [PubMed] [Google Scholar]
- [214].Giulimondi F. et al. Interplay of protein corona and immune cells controls blood residency of liposomes. Nat. Commun. 2019;10:3686. doi: 10.1038/s41467-019-11642-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [215].Qie Y. et al. Surface modification of nanoparticles enables selective evasion of phagocytic clearance by distinct macrophage phenotypes. Sci. Rep. 2016;6:26269. doi: 10.1038/srep26269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [216].Partikel K, Korte R, Mulac D, Humpf H-U and Langer K 2019. Serum type and concentration both affect the protein-corona composition of PLGA nanoparticles Beilstein J. Nanotechnol 10 1002–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [217].Gorshkov V, Bubis JA, Solovyeva EM, Gorshkov MV and Kjeldsen F 2019. Protein corona formed on silver nanoparticles in blood plasma is highly selective and resistant to physicochemical changes of the solution Environ. Sci. Nano 6 1089–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [218].Jayaram DT, Pustulka SM, Mannino RG, Lam WA and Payne CK 2018. Protein corona in response to flow: effect on protein concentration and structure Biophys. J 115 209–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [219].Grafe C, von der Lühe M, Weidner A, Globig P, Clement JH, Dutz S and Schacher FH 2019. Protein corona formation and its constitutional changes on magnetic nanoparticles in serum featuring a polydehydroalanine coating: effects of charge and incubation conditions Nanotechnology 30 265707. [DOI] [PubMed] [Google Scholar]
- [220].Berardi A and Baldelli Bombelli F 2019. Oral delivery of nanoparticles—let’s not forget about the protein corona Expert Opin. Drug Deliv 16 563–6 [DOI] [PubMed] [Google Scholar]
- [221].Raesch SS, Tenzer S, Storck W, Rurainski A, Selzer D, Ruge CA, Perez-Gil J, Schaefer UF and Lehr C-M 2015. Proteomic and lipidomic analysis of nanoparticle corona upon contact with lung surfactant reveals differences in protein, but not lipid composition ACS Nano 9 11872–85 [DOI] [PubMed] [Google Scholar]
- [222].Mirshafiee V, Mahmoudi M, Lou K, Cheng J and Kraft ML 2013. Protein corona significantly reduces active targeting yield Chem. Commun 49 2557–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [223].Palchetti S, Digiacomo L, Pozzi D, Peruzzi G, Micarelli E, Mahmoudi M and Caracciolo G 2016. Nanoparticles-cell association predicted by protein corona fingerprints Nanoscale 8 12755–63 [DOI] [PubMed] [Google Scholar]
- [224].Arcella A et al. 2018. Brain targeting by liposome-biomolecular corona boosts anticancer efficacy of temozolomide in glioblastoma cells ACS Chem. Neurosci 9 3166–74 [DOI] [PubMed] [Google Scholar]
- [225].Dobrovolskaia MA and McNeil SE 2013. Handbook of Immunological Properties of Engineered Nanomaterials (Singapore: World Scientific; ) ( 10.1142/8390) [DOI] [PubMed] [Google Scholar]
- [226].Farrera C and Fadeel B 2015. It takes two to tango: understanding the interactions between engineered nanomaterials and the immune system Eur. J. Pharm. Biopharm 95 3–12 [DOI] [PubMed] [Google Scholar]
- [227].Kroll A, Pillukat MH, Hahn D and Schnekenburger J 2009. Current in vitro methods in nanoparticle risk assessment: limitations and challenges Eur. J. Pharm. Biopharm 72 370–7 [DOI] [PubMed] [Google Scholar]
- [228].Mahmoudi M, Simchi A, Imani M, Shokrgozar MA, Milani AS, Häfeli UO and Stroeve P 2010. A new approach for the in vitro identification of the cytotoxicity of superparamagnetic iron oxide nanoparticles Colloids Surf. B 75 300–9 [DOI] [PubMed] [Google Scholar]
- [229].Fleischer CC and Payne CK 2014. Secondary structure of corona proteins determines the cell surface receptors used by nanoparticles J. Phys. Chem. B 118 14017–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [230].Hillegass JM, Shukla A, Lathrop SA, MacPherson MB, Fukagawa NK and Mossman BT 2010. Assessing nanotoxicity in cells in vitro Wiley Interdiscip. Rev. Nanomed Nanobiotechnol 2 219–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [231].Dwivedi PD, Misra A, Shanker R and Das M 2009. Are nanomaterials a threat to the immune system? Nanotoxicology 3 19–26 [Google Scholar]
- [232].Mo J, Xie Q, Wei W and Zhao J 2018. Revealing the immune perturbation of black phosphorus nanomaterials to macrophages by understanding the protein corona Nat. Commun 9 2480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [233].Wu G, Jiang C and Zhang T 2018. FcgammaRIIB receptor-mediated apoptosis in macrophages through interplay of cadmium sulfide nanomaterials and protein corona Ecotoxicol. Environ. Saf 164 140–8 [DOI] [PubMed] [Google Scholar]
- [234].Dumortier H 2013. When carbon nanotubes encounter the immune system: desirable and undesirable effects Adv. Drug Deliv. Rev 65 2120–6 [DOI] [PubMed] [Google Scholar]
- [235].Behzadi S, Serpooshan V, Tao W, Hamaly MA, Alkawareek MY, Dreaden EC, Brown D, Alkilany AM, Farokhzad OC and Mahmoudi M 2017. Cellular uptake of nanoparticles: journey inside the cell Chem. Soc. Rev 46 4218–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [236].Zhao J and Stenzel MH 2018. Entry of nanoparticles into cells: the importance of nanoparticle properties Polym. Chem 9 259–72 [Google Scholar]
- [237].Paillard A, Hindré F, Vignes-Colombeix C, Benoit J-P and Garcion E 2010. The importance of endo-lysosomal escape with lipid nanocapsules for drug subcellular bioavailability Biomaterials 31 7542–54 [DOI] [PubMed] [Google Scholar]
- [238].Liu Y, Hardie J, Zhang X and Rotello VM 2017. Effects of engineered nanoparticles on the innate immune system Semin. Immunol 34 25–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [239].Kalluru R et al. 2013. Poly(lactide-co-glycolide)-rifampicin nanoparticles efficiently clear Mycobacterium bovis BCG infection in macrophages and remain membrane-bound in phago-lysosomes J. Cell. Sci 126 3043–54 [DOI] [PubMed] [Google Scholar]
- [240].Kasai T, Umeda Y, Ohnishi M, Mine T, Kondo H, Takeuchi T, Matsumoto M and Fukushima S 2016. Lung carcinogenicity of inhaled multi-walled carbon nanotube in rats Part Fibre Toxicol. 13 53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [241].Katsnelson BA, Privalova LI, Kuzmin SV, Gurvich VB, Sutunkova MP, Kireyeva EP and Minigalieva IA 2012. An approach to tentative reference levels setting for nanoparticles in the workroom air based on comparing their toxicity with that of their micrometric counterparts: a case study of iron oxide Fe3O4 ISRN Nanotechnol. 2012 143613 [Google Scholar]
- [242].Lin J, Shigdar S, Fang DZ, Xiang D, Wei MQ, Danks A, Kong L, Li L, Qiao L and Duan W 2014. Improved efficacy and reduced toxicity of doxorubicin encapsulated in sulfatide-containing nanoliposome in a glioma model PLoS One 9 e103736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [243].Wang J, Loye AM, Ketkaew J, Schroers J and Kyriakides TR 2018. Hierarchical micro- and nanopatterning of metallic glass to engineer cellular responses ACS Appl. Bio Mater 1 51–8 [Google Scholar]
- [244].Saluja SS et al. 2014. Targeting human dendritic cells via DEC-205 using PLGA nanoparticles leads to enhanced cross-presentation of a melanoma-associated antigen Int. J. Nanomedicine 9 5231–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [245].Joffre OP, Sancho D, Zelenay S, Keller AM and Reis E Sousa C 2010. Efficient and versatile manipulation of the peripheral CD4+ T-cell compartment by antigen targeting to DNGR-1/CLEC9A Eur. J. Immunol 40 1255–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [246].Wang Y, Lin Y-X, Qiao S-L, An H-W, Ma Y, Qiao Z-Y, Rajapaksha RPYJ and Wang H 2017. Polymeric nanoparticles promote macrophage reversal from M2 to M1 phenotypes in the tumor microenvironment Biomaterials 112 153–63 [DOI] [PubMed] [Google Scholar]
- [247].Sicard A et al. 2016. B cells loaded with synthetic particulate antigens: a versatile platform to generate antigen-specific helper T cells for cell therapy Nano Lett. 16 297–308 [DOI] [PubMed] [Google Scholar]
- [248].Pondman KM, Sobik M, Nayak A, Tsolaki AG, Jäkel A, Flahaut E, Hampel S, Ten Haken B, Sim RB and Kishore U 2014. Complement activation by carbon nanotubes and its influence on the phagocytosis and cytokine response by macrophages Nanomedicine 10 1287–99 [DOI] [PubMed] [Google Scholar]
- [249].Figueiredo Borgognoni C, Kim JH, Zucolotto V, Fuchs H and Riehemann K 2018. Human macrophage responses to metal-oxide nanoparticles: a review Artif Cells, Nanomed. Biotechnol 46 694–703 [DOI] [PubMed] [Google Scholar]
- [250].Dukhinova MS, Prilepskii AY, Shtil AA and Vinogradov VV 2019. Metal oxide nanoparticles in therapeutic regulation of macrophage functions Nanomaterials 9 1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [251].Lategan KL, Walters CR and Pool EJ 2019. The effects of silver nanoparticles on RAW 264.7. Macrophages and human whole blood cell cultures Front. Biosci 24 347–65 [DOI] [PubMed] [Google Scholar]
- [252].Liang H, Jin C, Ma L, Feng X, Deng X, Wu S, Liu X and Yang C 2019. Accelerated bone regeneration by gold-nanoparticle-loaded mesoporous silica through stimulating immunomodulation ACS Appl. Mater. Interfaces 11 41758–69 [DOI] [PubMed] [Google Scholar]
- [253].Xu J, Yang J, Nyga A, Ehteramyan M, Moraga A, Wu Y, Zeng L, Knight MM and Shelton JC 2018. Cobalt (II) ions and nanoparticles induce macrophage retention by ROS-mediated down-regulation of RhoA expression Acta Biomater. 72 434–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [254].Yang YS et al. 2018. Targeting small molecule drugs to T cells with antibody-directed cell-penetrating gold nanoparticles Biomater. Sci 7 113–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [255].Lee SB, Lee YJ, Cho SJ, Kim SK, Lee S-W, Lee J, Lim D-K and Jeon YH 2018. Antigen-free radionuclide-embedded gold nanoparticles for dendritic cell maturation, tracking, and strong antitumor immunity Adv. Healthc. Mater 7 e1701369. [DOI] [PubMed] [Google Scholar]
- [256].Yoshio Nakano MM, Nishinohara S, Takita Y, Naito S, Kato H, Taneichi M, Komuro K and Uchida T 2001. Surface-linked liposomal antigen induces IgE-selective unresponsiveness regardless of the lipid components of liposomes Bioconjug. Chem 12 391–5 [DOI] [PubMed] [Google Scholar]
- [257].Yan W, Chen W and Huang L 2007. Mechanism of adjuvant activity of cationic liposome: phosphorylation of a MAP kinase, ERK and induction of chemokines Mol. Immunol 44 3672–81 [DOI] [PubMed] [Google Scholar]
- [258].Connor J, Yatvin MB and Huang L 1984. pH-sensitive liposomes: acid-induced liposome fusion Proc. Natl Acad. Sci. USA 81 1715–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [259].Siegel DP and Epand RM 1997. The mechanism of lamellar-to-inverted hexagonal phase transitions in phosphatidylethanolamine: implications for membrane fusion mechanisms Biophys. J 73 3089–111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [260].Wilson DR, Sen R, Sunshine JC, Pardoll DM, Green JJ and Kim YJ 2018. Biodegradable STING agonist nanoparticles for enhanced cancer immunotherapy Nanomedicine 14 237–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [261].Bandyopadhyay A, Fine RL, Demento S, Bockenstedt LK and Fahmy TM 2011. The impact of nanoparticle ligand density on dendritic-cell targeted vaccines Biomaterials 32 3094–105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [262].Kwak SY, Hee Dong Han SL, Chang, Kim K-P and Ahn HJ 2019. PLGA nanoparticles codelivering siRNAs against programmed cell death protein-1 and its ligand gene for suppression of colon tumor growth Mol. Pharm 16 4940–53 [DOI] [PubMed] [Google Scholar]
- [263].Jo A, Ringel-Scaia VM, McDaniel DK, Thomas CA, Zhang R, Riffle JS, Allen IC and Davis RM 2020. Fabrication and characterization of PLGA nanoparticles encapsulating large CRISPR-Cas9 plasmid J. Nanobiotechnol 18 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [264].Pondman KM, Salvador-Morales C, Paudyal B, Sim RB and Kishore U 2017. Interactions of the innate immune system with carbon nanotubes Nanoscale Horiz. 2 174–86 [DOI] [PubMed] [Google Scholar]
- [265].Jaana Palomaki EV, Sund J, Vippola M, Clausen PA, Jensen KA, Kai Savolainen SM and Alenius H 2011. Long, needle-like carbon nanotubes and asbestos activate the NLRP inflammasome through a similar mechanism ACS Nano 5 6861–70 [DOI] [PubMed] [Google Scholar]
- [266].Jia YJ, Guo Z-R, Ma R, Qiu D-K, Zhao Z, Wang G-X and Zhu B 2020. Immune efficacy of carbon nanotubes recombinant subunit vaccine against largemouth bass ulcerative syndrome virus Fish Shellfish Immunol. 100 317–23 [DOI] [PubMed] [Google Scholar]
- [267].Oliveira TL, Bacelo KL, Forster KM, Ilha V, Rodrigues OE and Hartwig DD 2020. DNA nanovaccines prepared using LemA antigen protect Golden Syrian hamsters against Leptospira lethal infection Mem. Inst. Oswaldo Cruz 115 e190396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [268].Zhang C, Wang GX and Zhu B 2020. Application of antigen presenting cell-targeted nanovaccine delivery system in rhabdovirus disease prophylactics using fish as a model organism J. Nanobiotechnol 18 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [269].Yang M and Zhang M 2019. Biodegradation of carbon nanotubes by macrophages Front. Mater 6 255 [Google Scholar]
- [270].Yang H, Marion TN, Liu Y, Zhang L, Cao X, Hu H, Zhao Y and Herrmann M 2019. Nanomaterial exposure induced neutrophil extracellular traps: a new target in inflammation and innate immunity J. Immunol. Res 2019 3560180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [271].Zamani P, Momtazi-Borojeni AA, Nik ME, Oskuee RK and Sahebkar A 2018. Nanoliposomes as the adjuvant delivery systems in cancer immunotherapy J. Cell. Physiol 233 5189–99 [DOI] [PubMed] [Google Scholar]
- [272].Foged C, Arigita C, Sundblad A, Jiskoot W, Storm G and Frokjaer S 2004. Interaction of dendritic cells with antigen-containing liposomes: effect of bilayer composition Vaccine 22 1903–13 [DOI] [PubMed] [Google Scholar]
- [273].Brewer JM, Tetley L, Richmond J, Liew FY and Alexander J 1998. Lipid vesicle size determines the Th1 or Th2 response to entrapped antigen J. Immunol 161 4000–7 [PubMed] [Google Scholar]
- [274].Shin MD et al. 2020. COVID-19 vaccine development and a potential nanomaterial path forward Nat. Nanotechnol 15 646–55 [DOI] [PubMed] [Google Scholar]
- [275].Elechalawar CK, Hossen MN, McNally L, Bhattacharya R and Mukherjee P 2020. Analysing the nanoparticle-protein corona for potential molecular target identification J. Control Release 322 122–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [276].Longo JPF et al. 2020. Issues affecting nanomedicines on the way from the bench to the market J. Mater. Chem. B ( 10.1039/D0TB02180F) [DOI] [PubMed] [Google Scholar]
- [277].Saratale RG, Karuppusamy I, Saratale GD, Pugazhendhi A, Kumar G, Park Y, Ghodake GS, Bharagava RN, Banu JR and Shin HS 2018. A comprehensive review on green nanomaterials using biological systems: recent perception and their future applications Colloids Surf. B 170 20–35 [DOI] [PubMed] [Google Scholar]


