Abstract
This study aims to examine the effect of the non-Saccharomyces yeast Saccharomycopsis fibuligera on the sensory quality and flavour characteristics of a sweet rice alcoholic beverage. The strain S. fibuligera was isolated from a traditional Chinese hand-made starter with the purpose to improving sweet rice wine fragrance. Here, sweet rice wines were produced by six combinations of three species of fermentation strains, including S. fibuligera, Rhizopus and Saccharomyces cerevisiae, for evaluation. The study results showed significant diversities within these rice wines based on indicators including the score of quantitative descriptive analysis and volatile variety and content as well as odour activity value (OAV). Quantitative results showed that 43 volatile compounds were identified by headspace-solid phase microextraction with gas chromatography-mass spectrometry among samples. Based on the principal component analysis and OAV calculation, the two samples (S-2 and S-3) fermented with S. fibuligera and Rhizopus possessed high scores and were distinguished from the others, and ethyl butanoate, ethyl hexanoate, β-phenylethyl alcohol and 1-octen-3-one with high OAVs were responsible for the key aroma of sweet rice wine fermented with S. fibuligera. Co-inoculating S. fibuligera, Rhizopus or/and S. cerevisiae generated more pleasant aroma compounds in a sweet rice alcoholic beverage than when inoculated individually.
Electronic supplementary material
The online version of this article (10.1007/s13197-020-04833-4) contains supplementary material, which is available to authorized users.
Keywords: Sweet rice alcoholic beverage, Saccharomycopsis fibuligera, Key aroma compounds
Introduction
Rice wine is a traditional alcoholic beverage with approximately 4000 years of history in Southeast Asia, including China, Korea, Japan, India, Vietnam, the Philippines, Thailand and Indonesia. In China, according to the differences in brewing procedures, rice wine can be divided into three classes. Sweet rice alcoholic beverages are made of glutinous rice without filtration and distillation, and Huangjiu, also called rice wine, requires filtration after fermentation (Jiao et al. 2017). Rice-flavoured liquor possesses a higher alcohol content than those of rice wine and sweet rice alcoholic beverages, ranging from 25 to 68%, and has the most complex process with distillation and blending. Sweet rice wine is also called sweet, fermented rice. The typical raw material of sweet rice wine is glutinous rice with a high amylopectin content, and the starter of sweet rice wine, called Jiuqu or Jiuyao, is made of rice powder and microorganisms. After fermentation, the mixture of solid–liquid rice wine contains high levels of glucose with a desirable sweet taste. The alcohol level of sweet rice wine is usually low, between 0.5 and 14% (Cai et al. 2018), due to the short fermentation period of just a few days (approximately 3 days) (Liu et al. 2002). Therefore, sweet rice alcoholic beverages with enjoyable sweetness and low alcohol content are one of the most popular beverages in China and other Asian regions.
As a result of the differences in the fermentation process and raw materials, including non-glutinous rice and glutinous rice, rice wine has slight differences in aroma, but there are still great similarities in aroma among various rice wines so that people can directly distinguish rice wine from other wines; thus, the recognizable aroma represents the typical odour of rice wine. At present, β-phenylethyl alcohol, presenting a rosy and honey-like odour (Cullere et al. 2004), ethyl lactate, possessing a fruity odour and ethyl acetate, with a solvent, fruity and pineapple-like odour (Chen et al. 2019; Ma et al. 2019), are universally recognized as core fragrances of rice wine. Nevertheless, there are still generally approximately 30 to 70 or more than 100 volatile compounds in rice wine. For instance, 61 aroma compounds were detected in dry, semi-dry, semi-sweet, and sweet Huangjiu (Yu et al. 2019a, b), and 79 volatile compounds were identified in Hong Qu glutinous rice wine (Huang et al. 2018). The analytical methods of aroma extract dilution analysis (ADEA) and odour activity value (OAV) were used to identify key aroma compounds, and compounds with OAV ≥ 1 are recognized as important contributors to the overall flavour of rice wine. In several studies, the OAVs of ethyl octanoate, nonanal, and benzeneacetaldehyde as well as isoamyl acetate (Fan and Xu 2012; Yang et al. 2018) were greater than 1, which means that β-phenyl ethanol, ethyl lactate and ethyl acetate were not the only key aroma components.
One of the most important factors of food is definitely flavour. In particular, the overall aroma is a critical component in appraising sweet rice wine quality. Some commercial starters only contain Rhizopus and/or S. cerevisiae, and this resulting wine aroma is insufficient; these wines lack characteristic sweet rice wine aromas of floral and honey. Currently, there is an extensive tendency to isolate pure strains and ferment with multiple pure strains (Xiang et al. 2019). In recent studies, some non-Saccharomyces cerevisiae yeasts were used to improve rice wine flavour quality because non-Saccharomyces yeasts produce a significant amount of volatile compounds during the fermentation period, which can exert a positive effect on wine aroma and flavour. For instance, Candida pulcherrima is a commercially available yeast and is also famous as producing high concentrations of esters such as ethyl octanoate (Jolly et al. 2014). S. fibuligera is also one of the non-Saccharomyces yeasts found in the Makgeolli (a Korean rice wine) brewing process. The level of aroma compounds such as β-phenylethyl alcohol increased continuously with fermentation time, and high contents of most fusel alcohols and acetate esters were achieved by fermentation with S. fibuligera in Makgeolli (Lee et al. 2018; Son et al. 2018). S. fibuligera was also found in the Vietnamese rice wine starter called “Banh Men” (Thanh et al. 2016) to produced amylase. In Chinese spirit making, S. fibuligera can produce approximately thirteen ethyl esters, such as ethyl hexanoate, ethyl octanoate and ethyl acetate (Ma et al. 2019), which all generate pleasant aromas. Therefore, during sweet rice wine fermentation, co-inoculation with S. fibuligera has potentially positive effects on improving the aroma of sweet rice alcoholic beverages.
Consequently, the objectives of the study were (1) to isolate S. fibuligera and then inoculate it in sweet rice wine production, (2) to investigate the varieties and contents of the main aroma compounds of rice wine fermented in the presence and absence of S. fibuligera using headspace solid-phase microextraction (HS-SPME) and gas chromatography-mass spectrometry (GC-MS) analysis; and (3) to identify the key contributors of the sweet rice wine aroma based on the calculation results of OAVs. This study aimed to evaluate the advantages of S. fibuligera YRNN on the production of aroma compounds in a sweet rice alcoholic beverage. Based on these results, our understanding of key aroma compounds and contents of sweet rice wine after fermenting with S. fibuligera can be improved, and the typical aroma of sweet rice wine is a highly popular odour in other Chinese traditional fermented breads, rice cakes, etc. Furthermore, not only sweet rice wine but also other fermented food quality can also be improved because of the pleasant odour produced by S. fibuligera in future production.
Materials and methods
Materials and reagents
Xiaoqu (hand-made starter), made from rice and rice bran powder, was purchased from a workshop in Wuzhou, Guangxi Province, China; Rhizopus Q303 was purchased from Hunan Province Light Industry Scientific Research Institute, Hunan, China; S. cerevisiae was isolated from the sourdough of Mantou by our laboratory and identified based on the 26S rDNA sequence (Huang 2018); YRNN was isolated from Xiaoqu hand-made starter and identified as S. fibuligera. 2-Octanol (internal standard for GC ≥ 99.5% purity) was purchased from Shanghai Macklin Biochemical Co., Ltd. Sodium chloride (NaCl) was purchased from China National Pharmaceutical Group Corp. (Shanghai, China). Ethanol (HPLC grade) was supplied by Tianjin Chemical Reagent Research Institute Co., Ltd.
Isolation of the Saccharomycopsis fibuligera strain
Ground, hand-made starter was added to YPD broth, and then the diluted broth was spread onto rose bengal medium plates with incubation at 30 °C for 24 h. Single colonies with milk-white colour and concentric circle shapes were picked and streaked on YPD agar medium several times for purification to obtain pure isolate strains. The microscopic characteristics of the pure strain (yeast cell type and mycelium shape) were determined under a Motic BA200 optical microscope (Motic China Group Co., Ltd.) at 10 × 40 magnification. Microscopic images were taken by Motic Images Advanced software. The strain, named YRNN, was stored at 4 °C for later study.
Identification of the YRNN strain
One single colony of YRNN was isolated and ground with liquid nitrogen, and the powder was added to a 1.5 mL sterile Eppendorf tube. Then, total genomic DNA was extracted according to the manufacturer’s protocols of the Ezup Column Fungi Genomic DNA Purification Kit (Sangon Biotech Shanghai Co., Ltd, China).
The internally transcribed spacer (ITS) rDNA region of YRNN was amplified by PCR using the primers ITS1 (5′-TCCGTAGGTGAACCTGCGG-3′) and ITS4 (5′-TCCTCCGCTTATTGATATGC-3′). The PCR amplification reaction programme was as follows: 94 °C for 4 min, 94 °C for 30 s, 55 °C for 30 s, and 72 °C for 45 s and 35 cycles, and 72 °C for 2 min. The purity of PCR products was evaluated by electrophoresis in a 1% agarose gel. Amplified PCR products were sequenced at Sangon Biotech (Shanghai) Co., Ltd, China.
The ITS sequence of YRNN was compared to the sequence homology in GenBank (https://www.ncbi.nlm.nih.gov/blast) using the BLAST tool. The phylogenetic tree (Fig. S1) was drawn by MEGA 7 software. The ITS sequence was aligned by ClustalW, and phylogenetic analysis was carried out by MEGA 7 using the neighbour-joining method with a bootstrap value of 1000.
Winemaking
Rhizopus was inoculated onto YPD medium and cultured at 30 °C until the spores matured. Then, Rhizopus moss was washed with 0.05% Tween-80 and shaken for 2 min in a vortex. The spore suspension was filtered and diluted to 107 spores/mL. S. fibuligera YRNN and S. cerevisiae were inoculated into YPD broth and cultured at 30 °C for 48 h and then diluted to 107 cfu/mL. Rice powder solid starters of the three strains were made with sterilized rice powder. Three rice powders were sprayed with a 2% (w/v) suspension of three microorganisms separately and cultured at 30 °C for 3 days and dried to obtain dry Rhizopus, S. fibuligera YRNN and S. cerevisiae starters for subsequent winemaking.
Thirty grams of rice was filled into a 250 mL Erlenmeyer flask, and 42 mL of deionized water was added. The starters of Rhizopus Q303, YRNN and S. cerevisiae were inoculated into sterilized rice. The amounts of the three starters are shown below in Table S1. The difference between S-1 and S-6 is that S-6 was under airtight conditions and sealed with a plastic film. All samples were incubated at 30 °C for 3 days in duplicate and then stored at − 20 °C for later study.
Sensory evaluation of rice wine samples
The linear scale method for quantitative descriptive analysis (QDA) was applied to sensory evaluation. The left end represented “minimum” or “lowest”, and the right end represented “maximum” or “highest”. Fifteen panellists (20–27 years old, 8 females and 7 males) were trained for sensory evaluation. Six equal amounts of sample were coded randomly in three digits for the test, and the temperature of the samples and room was between 20 and 25 °C. After evaluation, the length of the linear odour descriptions was measured using a ruler and transformed to data for statistical analysis. The aroma profiles were obtained based on the mean data of odour descriptions evaluated by the 15 trained panellists.
Analysis of volatile compounds by HS-SPME and GC-MS
Headspace-solid phase microextraction (HS-SPME)
One gram of rice wine sample, 1 mL of double-distilled H2O and 1 g of NaCl were added into a 20 mL screw-capped headspace glass vial with 10 μL of internal standard 2-octanol (483 μg/mL in absolute ethanol). All vials were equilibrated at 50 °C for 5 min, and then the SPME fibre (100 μm PDMS, SUPELCO Co., Bellefonte, PA, USA) was inserted into the vials 1 cm above the liquid level and used to adsorb volatile components for 30 min at 50 °C. After extraction, the fibre was inserted into the GC injection port at 220 °C to desorb volatile compounds for 5 min.
Gas chromatography-mass spectrometry (GC-MS)
Analysis was carried out on a GCMS-QP2010 Ultra gas chromatograph-mass spectrometer (Shimadzu Co., Ltd.). A DB-5 capillary column (30 m × 0.25 mm i.d., 0.25 μm film thickness, Agilent Technologies Inc.) was used to analyse samples. Helium was used as the carrier gas at a flow rate of 1 mL/min. The temperature programme was as follows: The initial oven temperature was 40 °C, held for 5 min, ramped to 180 °C at a rate of 5 °C/min, increased to 220 °C at a rate of 8 °C/min, and held for 5 min at the final temperature.
The temperature of the ion source was 220 °C, and electron impact (EI) ionization was implemented at 70 eV. The m/z range was set from 35 to 350 amu in full-scan mode. The volatile compounds were identified based on the NIST 11 Database. The concentrations of volatile compounds using semi-quantitative analysis were calculated referring to internal standards according to the following equation:
where C represents the concentration of volatile compounds (μg/g), Cis represents the concentration of the internal standard, A represents the peak area of volatile compounds, and Ais represents the peak area of the internal standard.
Determination of odour activity values (OAVs) of major volatile compounds
The major volatile compounds in rice wine samples were determined by using principal component analysis (PCA). Comparing the results of the semi-quantitative analysis of major volatile compounds with the threshold values, OAVs were calculated according to the following equation:
where C represents the concentration of volatile compounds (μg/g) and OT represents the odour threshold of volatile compounds obtained from the literature. The volatile compounds with OAVs ≥ 1 were considered important to the rice wine flavour profile.
Statistics analysis
Statistical analyses of GC-MS data, principal component analysis (PCA) and cluster analysis (CA) were performed using SPSS 22 software. One-way analysis of variance (ANOVA) was applied to the GC-MS data.
Results and discussion
Characterization and identification of YRNN
The colony morphology of the YRNN isolate was a single colony that was round, white, dry and had a compact filamentous surface and edge, as shown in Fig. 1a. The cellular morphology of the YRNN isolate was determined by an optical microscope (Motic BA200, Motic China Group Co., Ltd.). Figure 1b illustrates that YRNN has two cellular morphology types: single-cell yeast and mycelium-shaped yeast. The result was consistent with the study of Farh, who isolated dimorphic yeasts named S. fibuligera from different Nuruk samples, and this species has been regarded as the predominant yeast in the Nuruk used for South Korean rice wine production (Farh et al. 2017).
Fig. 1.
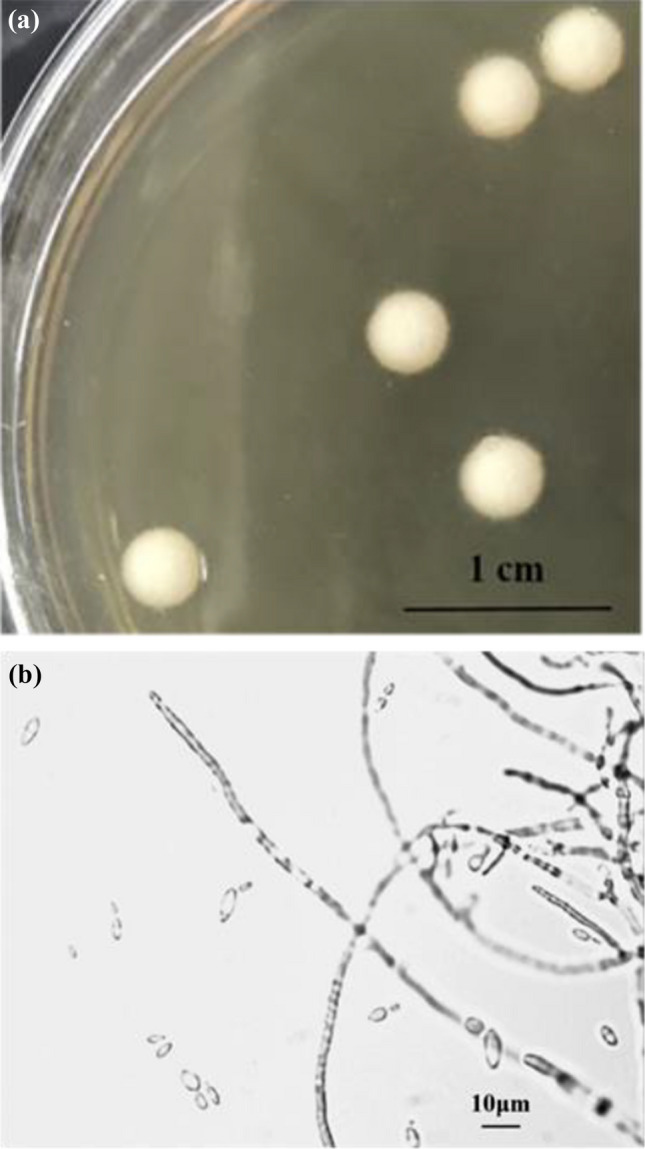
Colony (a) and cellular (b) morphologies of YRNN
ITS sequence analysis and phylogenetic tree
The internal transcribed spacer (ITS) region of the nuclear ribosomal repeat unit is commonly applied in the taxonomy of fungi at and below the genus level. The ITS sequence of YRNN was 612 bp in length. The sequence was compared to that of relevant strains using BLAST analysis. As shown in Table 1, S. fibuligera ADJ4 had the highest score (1098), which was related to its high homology with YRNN. Moreover, the E-value of S. fibuligera ADJ4 was 0.0, which indicates the high reliability of homology. The lower the E-value was, the higher the reliability of the homology of two strains. The percent identity equal to ITS sequence similarity between YRNN and S. fibuligera ADJ4 reached 99.84%. Furthermore, Fig. 2 illustrates that YRNN with S. fibuligera ADJ4 was in a branch of the neighbour-joining (NJ) phylogenetic tree, and the bootstrap value was 100. Therefore, combining the results of BLAST and phylogenetic tree analysis, YRNN was identified as S. fibuligera and named S. fibuligera YRNN.
Table 1.
BLASTN for ITS sequence of YRNN on NCBI
| Strain | Score | E-value | Percent identity (%) | Accession |
|---|---|---|---|---|
| Saccharomycopsis fibuligera ADJ4 | 1098 | 0.0 | 99.84 | KX904348.1 |
| Pichia farinose NWS116 | 1068 | 0.0 | 98.71 | FJ797683.1 |
| Millerozyma farinose DBMY87 | 1067 | 0.0 | 98.71 | KJ706306.1 |
| Pichia jadinii NWS115 | 1057 | 0.0 | 99.83 | FJ797685.1 |
| Cyberlindnera jadinii DBMY412 | 985 | 0.0 | 95.76 | KJ706629.1 |
| Millerozyma farinose DBMY407 | 985 | 0.0 | 94.82 | KJ706624.1 |
Fig. 2.
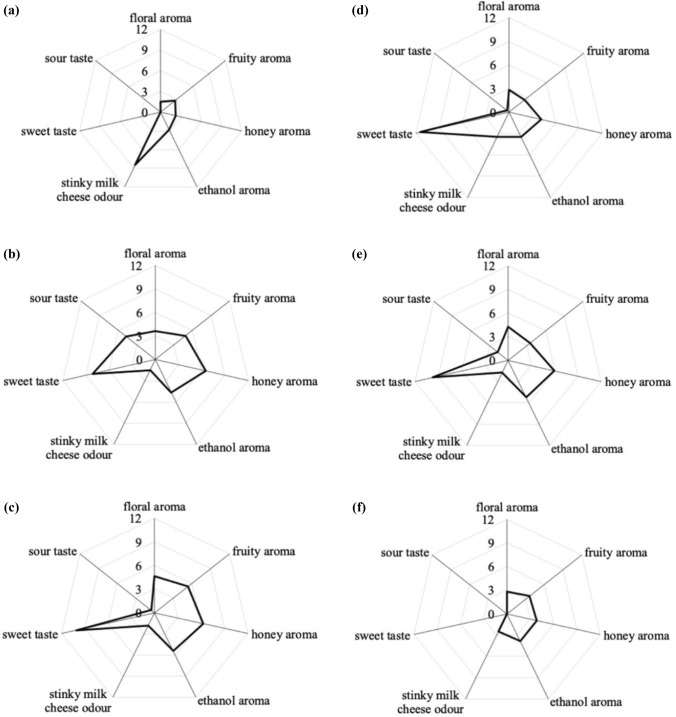
Aroma profiles of six rice wine samples. The scores of descriptors were evaluated by 15 panellists and averaged. S-1, rice wine fermented only by S. fibuligera YRNN (a). S-2, rice wine fermented with Rhizopus and S. fibuligera YRNN (b). S-3, rice wine fermented with Rhizopus, S. fibuligera YRNN and S. cerevisiae (c). S-4, rice wine fermented only by Rhizopus (d). S-5, rice wine fermented with Rhizopus and S. cerevisiae (e). S-6, rice wine fermented only by S. fibuligera and under air-tight conditions, i.e., sealed with plastic film (f)
Application of the quantitative descriptive analysis (QDA) method in the sensory evaluation of sweet rice wines
Figure 2 shows the QDA results of six rice wines in terms of five odour notes as well as sweet and sour taste notes. Among the six samples, the total aroma profiles of S-2 and S-3 fermented with S. fibuligera had relatively high scores for fruity and honey aroma and low scores for stinky milk cheese odour. Floral, fruity and honey aromas were deemed to be key fragrant and pleasant odours in sweet rice wine (Huang et al. 2018; Lee et al. 2018). Regarding the taste, S-2, S-3, S-4 and S-5 all had a strong sweet taste, while the sour taste of S-2 was slightly outstanding among the samples. Although the sweet taste intensity was highest in S-4, the overall aroma profile was not prominent. According to the sensory evaluation results, rice wine fermented with S. fibuligera (S-1 and S-6) or Rhizopus (S-4) did not perform well in overall flavour, while the overall flavour of S-3 fermented with all three fermentation strains was comprehensively better. To further analyse the specific aroma compounds, GC-MS analysis was applied to study the aroma of rice wines.
Volatile compound analysis of rice wine fermented with and without Saccharomycopsis fibuligera YRNN
The identified and semi-quantitative statistics are listed in Table 2. There were significant differences (p < 0.05) among the six samples, mainly between the varieties and contents of esters, alcohols and carbonyl compounds. A total of 43 aroma compounds, including 16 esters, 13 alcohols, 4 aldehydes, 3 acids, 4 ketones and 3 other compounds, were identified in different rice wine groups. The rice wine sample S-3 produced with S. fibuligera, Rhizopus and S. cerevisiae had the largest number of volatile compounds (36), followed by S-2 fermented with S. fibuligera and Rhizopus at 31. Next, S-4 produced with only Rhizopus and S-5 fermented with Rhizopus and S. cerevisiae were set as similar traditional Chinese sweet rice wine groups, and they contained 22 and 26 volatile compounds, respectively. The difference between S-1 and S-6 was fermentation under aerobic conditions or not, and the number of aroma compounds in these two samples was 18 and 17, respectively; thus, the existence of oxygen influenced the production of volatiles by S. fibuligera.
Table 2.
Volatile compound profiles and content analysis of six rice wine samples by GC–MS
| Compounds* | RI | RIL | Concentration (μg/g) | |||||
|---|---|---|---|---|---|---|---|---|
| S-1 | S-2 | S-3 | S-4 | S-5 | S-6 | |||
| Esters | ||||||||
| Ethyl acetate | 586 | 584 | 0.71 ± 0.28b | 8.58 ± 2.40a | 9.21 ± 0.16a | 1.67 ± 0.28b | 3.10 ± 0.29b | 2.72 ± 1.30b |
| Butyl acetate | 785 | 812 | ND | 0.31 ± 0.19 | 0.30 ± 0.08 | ND | ND | ND |
| Ethyl butanoate | 785 | 800 | ND | ND | 0.38 ± 0.01 | ND | ND | 0.17 ± 0.02 |
| Ethyl hexanoate | 984 | 980 | ND | 0.45 ± 0.03a | 0.49 ± 0.08a | ND | 0.11 ± 0.02b | ND |
| Hexyl formate | 981 | – | ND | ND | 0.12 ± 0.01 | 0.08 ± 0.05 | ND | 0.11 ± 0.02 |
| Ethyl octanoate | 1183 | 1189 | ND | 0.98 ± 0.20a | 0.85 ± 0.09a | ND | 0.29 ± 0.05b | ND |
| Ethyl 2-octenoate | 1191 | – | ND | 0.24 ± 0.16 | 0.11 ± 0.03 | ND | ND | ND |
| Ethyl caprate | 1381 | 1394 | ND | 0.28 ± 0.01b | 0.56 ± 0.10a | 0.14 ± 0.03c | 0.52 ± 0.01a | ND |
| Diethyl succinate | 1151 | 1162 | ND | 1.78 ± 0.16 | 1.88 ± 044 | 1.07 ± 0.35 | 2.36 ± 0.83 | ND |
| β-Phenethyl acetate | 1259 | 1260 | ND | 3.74 ± 0.56 | 4.15 ± 0.95 | ND | ND | ND |
| Ethyl dodecanoate | 1580 | 1563 | ND | 0.46 ± 0.06b | 0.80 ± 0.03a | 0.29 ± 0.05c | 0.43 ± 0.07b | ND |
| Ethyl-9,12-octadecadienoate | 2193 | – | ND | ND | 6.61 ± 0.36 | 4.46 ± 2.57 | 3.39 ± 1.15 | ND |
| Ethyl pentadecanoate | 1878 | 1863 | 1.29 ± 0.20d | 11.35 ± 1.80a | 9.07 ± 0.57ab | 5.32 ± 0.25c | 7.48 ± 1.44bc | ND |
| Ethyl hexadecanoate | 1978 | 1974 | 5.00 ± 1.81c | 23.23 ± 1.71a | 22.72 ± 3.27a | 11.33 ± 0.19b | 13.84 ± 3.97b | 4.07 ± 1.10c |
| Ethyl (E)-9-octadecenoate | 2185 | 2174 | ND | ND | 3.13 ± 0.15 | ND | ND | ND |
| Ethyl 3-methylbutanoate | 820 | 852 | 0.10 ± 0.01 | ND | ND | ND | ND | ND |
| Alcohols | ||||||||
| Ethanol | 463 | – | 38.51 ± 0.23c | 123.55 ± 23.14a | 113.09 ± 1.37a | 84.18 ± 18.16ab | 94.15 ± 23.09ab | 54.14 ± 14.19bc |
| 2-Methyl-1-propanol | 597 | 606 | 0.12 ± 0.04b | 6.19 ± 1.03a | 6.77 ± 1.09a | 4.65 ± 0.01a | 5.54 ± 0.85a | 0.61 ± 0.15b |
| 1-Butanol | 662 | 656 | ND | 0.16 ± 0.03 | 0.14 ± 0.09 | ND | 0.08 ± 0.00 | 0.09 ± 0.04 |
| 3-Methyl-3-buten-1-ol | 852 | – | ND | 0.33 ± 0.04 | 0.38 ± 0.08 | 0.27 ± 0.13 | 0.55 ± 0.20 | ND |
| 3-Methyl-1-butanol | 697 | 736 | ND | 44.30 ± 13.28ab | 50.54 ± 7.79a | 27.45 ± 3.71b | 29.79 ± 4.71b | 8.72 ± 2.63c |
| 1-Octen-3-ol | 969 | 972 | 1.08 ± 0.48a | 0.40 ± 0.04b | 0.43 ± 0.01ab | ND | ND | 0.21 ± 0.01b |
| 2,3-Butanediol | 743 | 776 | ND | 1.02 ± 0.17 | 1.64 ± 0.08 | 2.13 ± 1.82 | 1.48 ± 0.27 | ND |
| 1-Octanol | 1059 | – | 0.07 ± 0.00 | 0.17 ± 0.13 | 0.12 ± 0.01 | ND | ND | ND |
| 2-Octen-1-ol | 1067 | – | 0.53 ± 0.14a | 0.23 ± 0.09b | 0.21 ± 0.01b | ND | ND | 0.07 ± 0.02b |
| 1-Nonanol | 1159 | – | 0.16 ± 0.03 | ND | ND | ND | ND | ND |
| 1-Hexanol | 860 | 863 | 0.13 ± 0.00 | ND | ND | ND | 0.06 ± 0.01 | ND |
| Benzyl alcohol | 1036 | 1039 | 0.14 ± 0.02b | 0.28 ± 0.03a | 0.30 ± 0.04a | 0.22 ± 0.02ab | 0.32 ± 0.09a | ND |
| β-Phenylethyl alcohol | 1136 | 1118 | 7.59 ± 0.05 cd | 117.9 ± 85.87b | 171.35 ± 10.37a | 8.62 ± 1.62 cd | 19.62 ± 0.37c | 5.31 ± 0.29d |
| Aldehydes | ||||||||
| Acetaldehyde | 408 | < 500 | 0.44 ± 0.01 | 0.59 ± 0.48 | 1.06 ± 0.67 | 1.36 ± 0.44 | 0.98 ± 0.24 | 1.05 ± 0.16 |
| Nonanal | 1104 | 1104 | ND | 0.13 ± 0.01ab | 0.10 ± 0.02b | 0.12 ± 0.05ab | 0.19 ± 0.01a | 0.07 ± 0.01b |
| Benzaldehyde | 982 | 993 | 0.17 ± 0.09 | 0.21 ± 0.05 | 0.22 ± 0.04 | ND | ND | 0.08 ± 0.02 |
| Benzeneacetaldehyde | 1081 | 1053 | 0.39 ± 0.15c | 1.49 ± 0.05ab | 1.59 ± 0.10a | 0.94 ± 0.26bc | 1.24 ± 0.43ab | 0.49 ± 0.07c |
| Acids | ||||||||
| Acetic acid | 576 | 582 | ND | 0.67 ± 0.32 | 1.32 ± 0.30 | ND | ND | 0.70 ± 0.51 |
| 3-Methyl-butanoic acid | 811 | 877 | 8.34 ± 2.88a | 6.29 ± 0.64a | 2.52 ± 0.13b | 1.07 ± 0.27b | 0.86 ± 0.16b | 1.43 ± 0.74b |
| 2-Methyl-propanoic acid | 711 | 789 | 1.97 ± 0.45 | ND | ND | ND | ND | ND |
| Ketones | ||||||||
| 1-Octen-3-one | 943 | 980 | ND | 0.32 ± 0.26 | 0.14 ± 0.06 | ND | ND | ND |
| 2-Nonadecanone | 2046 | – | ND | ND | 0.42 ± 0.40 | 0.34 ± 0.03 | ND | ND |
| Acetone | 455 | – | ND | ND | ND | 0.50 ± 0.12 | 0.29 ± 0.05 | ND |
| 2-Pentadecanone | 1648 | – | ND | 0.10 ± 0.01 | ND | ND | 0.23 ± 0.25 | ND |
| Others | ||||||||
| Acetoin | 717 | 723 | ND | ND | 0.20 ± 0.03 | ND | 0.24 ± 0.04 | ND |
| 3-(Methylthio)-1-propanol | 912 | 978 | ND | 0.29 ± 0.03 | 0.28 ± 0.05 | ND | ND | ND |
| 2-Furanmethanol | 885 | 854 | ND | ND | ND | 0.19 ± 0.02 | 0.19 ± 0.03 | ND |
*Compounds were identified by MS spectra and by comparison with the retention index from the literature (RIL) (Chen et al. 2019; Fan and Qian 2005, 2006; Fan and Xu 2012; Su et al. 2015; Yu et al. 2019a, b; Zhang et al. 2013; Zhao et al. 2018). ND: not detected. Identification based on mass spectra and similarity index (SI) ≥ 80
Values with different lowercase letters in a row are significantly different using Duncan’s multiple comparison tests (p < 0.05)
Alcohols were the major aroma compounds in all six samples. The six rice wines showed quantitative and qualitative differences in terms of alcohol composition. β-Phenylethyl alcohol, which produces honey and rose aromas (Lin et al. 2018), was the most abundant alcohol in S-3 at a concentration 171.35 μg/g. It is well known that β-phenylethyl alcohol is one of the key aroma components of the typical rice wine flavour. Furthermore, the comparison of the level of β-phenylethyl alcohol among S-2, S-4 and S-5 showed that the content in S-2 was approximately 13.7 times higher than that in S-4, while that in S-5 was only 2.3 times higher than that in S-4, which indicated that S. fibuligera in rice wine fermentation produced more β-phenylethyl alcohol than the other strains, improving the positive floral aroma of rice wine; additionally, S. cerevisiae had a slight impact on the increase in β-phenylethyl alcohol. Moreover, the rice wines produced with S. fibuligera, such as S-2 and S-3, showed higher contents of 3-methyl-1-butanol, which generated an alcohol-like aroma, than those in the other samples (Yu et al. 2019a, b). Although eleven alcohols were detected in the six rice wines, 1-octen-3-ol and 2-octen-1-ol were especially abundant in S. fibuligera-fermented groups, and these alcohols can impart mushroom flavour to rice wine (Xu et al. 2015).
Esters were the second-most abundant substances in the aroma components of rice wine. Due to the desirable odour of esters, they play an important role in the overall fragrance of rice wine. Ethyl pentadecanoate, ethyl hexadecanoate and ethyl acetate had high concentrations in all rice wines and were common and fundamental aroma compounds in rice wine. This result was consistent with that of Yang’s study, in which ethyl pentadecanoate, ethyl hexadecanoate and ethyl acetate were detected in different brewing stages of Chinese rice wine samples (Yang et al. 2017). Particularly, in the fermentations with S. fibuligera (S-2 and S-3), ethyl acetate, ethyl hexanoate and ethyl octanoate were significantly higher than in other samples (Table 2), and butyl acetate, ethyl 2-octenoate and β-phenethyl acetate, which provide fruity and floral odours, were unique to S-2 and S-3. Moreover, ethyl (E)-9-octadecenoate, a long-chain fatty acid ethyl esters with floral odours, was uniquely found in S-3 at 3.13 μg/g. Based on the above analysis, medium- (C6–C12) and long-chain fatty acid ethyl esters were the main esters, accounting for 56.25% of esters, and they exerted positive effects on the sweet rice wine flavour. In another study, middle-chain and long-chain FAEEs were also considered representative and significant flavours in rice wine (Yang et al. 2018).
Carbonyl compounds included aldehydes and ketones. Among these compounds, the highest level of benzeneacetaldehyde (1.59 μg/g) was observed for S-3, followed by that in S-2 (1.49 μg/g). Benzeneacetaldehyde is considered an important aroma in rice wine compound because of its floral and rose aroma (Xu et al. 2015). Similarly, in Korean rice wine (Makgeolli), benzeneacetaldehyde was also detected with higher content in fermentation with S. fibuligera compared with fermentation by A. oryzae (Son et al. 2018). Moreover, 1-octen-3-one only existed in S-2 and S-3 expressing mushroom-like flavours, and it was reported to be the major contributor to the characteristic aroma of Chinese rice wine (Chen et al. 2019).
Other aroma compounds in wines were acetoin, 3-(methylthio)-1-propanol and 2-furanmethanol. 3-(Methylthio)-1-propanol only existed in S-2 and S-3, and in grape wine, 3-(methylthio)-1-propanol was considered to be one of the most important sulfur compounds and interesting aromatic substances (Garde-Cerdán and Ancín-Azpilicueta 2006). The 2-Furanmethanol, with a burnt sugar aroma, was only detected in S-4 and S-5, which might be related to the presence of Rhizopus. Furthermore, a study reported that enzymatic extrusion-processed rice wine did not contain 3-(methylthio)-1-propanol and 2-furanmethanol (Xu et al. 2015), which concluded that volatile compounds might be related to microorganism metabolism.
The similarities and differences of aroma compounds in rice wine were revealed from the analysis above, and it was concluded that sweet rice wine fermented with S. fibuligera and Rhizopus had pleasant volatile components, including ethyl acetate, β-phenethyl acetate, medium- and long-chain fatty acid ethyl esters (ethyl hexanoate, ethyl dodecanoate, ethyl pentadecanoate, ethyl hexadecanoate, ethyl (E)-9-octadecenoate), 3-methyl-1-butanol, β-phenylethyl alcohol, benzeneacetaldehyde and 3-(methylthio)-1-propanol. These substances contributed to improving the overall aroma of rice wine.
The principal component analysis of the volatile contents based on GC–MS data of six samples, and the score plot as well as loading plot are illustrated in Fig. 3. The first two PCs explained 75.261% of the variance, maintaining most of the volatile compound information. PC1 and PC2 accounted for 49.419% and 25.842% of the variance, respectively (Fig. 3b).
Fig. 3.
PCA score plot from six rice wine samples (a), loading plot based on the concentration of 43 compounds derived from GC–MS analysis (b), cluster analysis of six rice wine samples (c)
The score plot (Fig. 3a) showed that the six samples had significant differences in the coordinate system. Every sample score was calculated based on PC1 and PC2 and obtained t1 and t2 scores, which corresponded to the x and y axes of the score plot, respectively. Every sample position was specific in the score plot. The closer the sample points are, the closer the compositions of the samples are. Conversely, the farther away the sample points are, the greater the difference. As Fig. 3a shows, samples could be separated into three portions: S-2 and S-3 (fermented with S. fibuligera and Rhizopus) were located in the first quadrant, S-4 and S-5 (traditional sweet rice wine similar groups) were located on the negative PC2 axis, and S-1 and S-6 were located on the negative PC1 axis. Moreover, cluster analysis (CA) was performed using the K-means clustering algorithm. At the scale of 15 (Fig. 3c), the six wines were also separated into 3 classes by Euclidean distance. S-2 and S-3 belonged to the first cluster, the second cluster contained is S-1 and S-6, and S-4 and S-5 were the last cluster, which coincided with the PCA results. The scores of the six samples were − 2.93, 7.31, 7.43, − 5.00, − 3.00 and − 3.80.
As Fig. 3 illustrates, S-2 and S-3 are located in the first quadrant with higher scores than the other samples. Both S-2 and S-3 were fermented with S. fibuligera, Rhizopus or/and S. cerevisiae. Therefore, S. fibuligera had a positive effect on the main body aroma of sweet rice wine because similar groups of traditional sweet rice wine (S-4 and S-5) fermented with Rhizopus and/or S. cerevisiae were located in the fourth quadrant with low scores.
Sixteen aroma compounds, including ethyl acetate, butyl acetate, ethyl butanoate, ethyl hexanoate, ethyl octanoate, β-phenethyl acetate, ethyl hexadecanoate, ethyl (E)-9-octadecenoate, 1-butanol, 1-octanol, β-phenylethyl alcohol, benzaldehyde, acetic acid, 1-octen-3-one and 3-(methylthio)-1-propanol, were clustered in the first quadrant of the loading plot (Fig. 3b). As S-2 and S-3 possessing high contents of ethyl octanoate, β-phenethyl acetate, β-phenylethyl alcohol and so on, they were clustered in the first quadrant of the score plot (Fig. 3a). However, S-1 had higher levels of 3-methyl-butanoic acid, 2-octen-1-ol, and 1-octen-3-ol than those in S-2 and S-3; these compounds were in the second quadrant of the loading plot; hence, correlatively, S-1 was located along the negative x-axis. For the above-mentioned analyses, the presence of different compounds indeed caused the difference among samples. Sixteen aroma compounds possibly in the samples fermented with S. fibuligera possessed a higher score and better aroma than those of other compounds. In the following section, the calculated OAVs of these components are discussed.
OAVs of major volatile compounds
The volatiles with OAVs ≥ 1 can be regarded as contributors to rice wine's main body aroma. According to the calculation results of OAVs, 10 and 11 of the 16 odour-active components in S-2 and S-3, respectively, were recognized as the key aroma compounds in rice wine listed in Table 3. Among them, OAVs greater than 100 were observed for some components, including ethyl butanoate (384 in S-3), β-phenylethyl alcohol (107 and 156) and 1-octen-3-one (632 and 289), with higher OAVs than those of other volatiles; hence, they play a dominant role in rice wine flavour with fruity, rosy, honey and vegetative aromas. Analogously, ethyl butanoate and β-phenylethyl alcohol were also identified by Fan and Xu (Fan and Xu 2012) as contributors to the characteristics rice wine aroma. The OAV of 1-octen-3-one in S-3 was lower than that in S-2, and 1-octen-3-one generates a creamy and mushroom-like odour (Yin et al. 2019), which might not be a very enjoyable flavour in rice wine, but it is very common in Chinese rice wine. In addition, ethyl butanoate in S-3 had a high OAV and has a desirable fruity aroma; therefore, the aroma of S-3 might be slightly better than that of S-2.
Table 3.
Odour thresholds and OAVs of major compounds in S-2 and S-3
| Compounds | Odour threshold (mg/kg) | OAVs | Odour descriptorsa | |
|---|---|---|---|---|
| S-2 | S-3 | |||
| Ethyl acetate | 5 | 2 | 2 | Fruity, pineapple (Li et al. 2019) |
| Butyl acetate | 0.066 | 5 | 5 | Ethereal solvent, fruity, banana (Whitener et al. 2017) |
| Ethyl butanoate | 0.001 | ND | 384 | Sour fruit, strawberry, fruity (Shu et al. 2014) |
| Ethyl hexanoate | 0.005 | 91 | 98 | Floral, fruity, apple peel, pear (Shu et al. 2014) |
| Ethyl octanoate | 0.0193 | 51 | 44 | Fruity, wine, waxy, sweet apricot, banana, brandy, pear (Whitener et al. 2017) |
| β-Phenethyl acetate | 0.24959 | 15 | 17 | Sweet, honey, floral rosy, with a slight yeasty honey note with a cocoa and balsamic nuance (Whitener et al. 2017) |
| Ethyl hexadecanoate | 2 | 12 | 11 | Fatty, rancid, fruity, sweet (Shu et al. 2014) |
| Ethyl (E)-9-Octadecenoate | 0.87 | ND | 4 | Fresh, floral (Shu et al. 2014) |
| 1-Butanol | 0.5 | < 1 | < 1 | Alcoholic (Li et al. 2019) |
| 1-Octanol | 0.19 | < 1 | < 1 | Citrus-like, green (Czerny et al. 2008) |
| β-Phenylethyl alcohol | 1.1 | 107 | 156 | Rosy, honey (Li et al. 2019) |
| Benzaldehyde | 0.3 | < 1 | < 1 | Almond (Chen et al. 2019) |
| Acetic acid | 22 | < 1 | < 1 | Vinegar-like (Li et al. 2019) |
| 1-Octen-3-one | 0.0005 | 632 | 289 | Mushroom-like (Czerny et al. 2008) |
| 3-(Methylthio)-1-propanol | 0.036 | 8 | 8 | Cooked potato-like (Czerny et al. 2008) |
| Ethyl 2-octenoate | NF | – | – | Sweet, fruity (El Hadi et al. 2013) |
aOdour threshold referred to treatise (Gemert 2003); the matrix was a water solution
NF not found
The OAV was greater than 10 for ethyl hexanoate, ethyl octanoate, ethyl hexadecanoate and β-phenethyl acetate. Ethyl esters have been reported in many alcoholic beverages, such as rice wine, sake, wine, and beer. In our study, the concentrations of ethyl hexanoate and ethyl octanoate were not high, while their odour thresholds were very low, yielding high OAVs, which was in accordance with Luo (Luo et al. 2008), who reported that ethyl hexanoate and ethyl octanoate played an important role in the flavour of most Chinese rice wine with a fruity aroma. Particularly, β-phenethyl acetate is unusual in rice wine and is a typical floral compound, which was a desirable odour in rice wine. The β-phenethyl acetate can be formed from β-phenylethyl alcohol by alcohol acetyltransferase, possibly because the relatively large OAV of β-phenethyl acetate was related to the high content of β-phenylethyl alcohol in S-2 and S-3.
Ethyl acetate (OAV 2), butyl acetate (OAV 5), ethyl (E)-9-octadecenoate (OAV 4) and 3-(methylthio)-1-propanol (OAV 8) had OAVs greater than 1. Ethyl acetate and butyl acetate are common esters in many kinds of alcoholic beverages. Among these compounds, ethyl (E)-9-octadecenoate, having a floral aroma, was notably abundant in S-3. In addition, 3-(methylthio)-1-propanol was reported to bring a mellow flavour to rice wine, which was determined in dry-type rice wine to be a powerful odourant (Fan and Xu 2012).
Conclusion
Based on these analyses, our research highlighted that co-inoculating S. fibuligera and Rhizopus intensified the characteristics and aroma profiles of sweet rice wines. Furthermore, in sweet rice alcoholic beverage fermentation, co-inoculation of S. fibuligera, Rhizopus and S. cerevisiae produced the greatest amounts and contents of volatile compounds, specifically esters and alcohols such as β-phenethyl acetate and β-phenylethyl alcohol. The largest contributors (OAVs > 50) to the typical aroma of sweet rice wine fermented with S. fibuligera were acetate esters, including ethyl butanoate, ethyl hexanoate and ethyl octanoate, as well as β-phenylethyl alcohol and 1-octen-3-one. In addition, these aromatic substances gave sweet rice wine a comprehensive aroma of floral, fruity and honey, which are typical aromatic characteristics. This study provided a novel aroma-producing yeast, S. fibuligera, for the improvement of sweet rice alcoholic beverage aroma and established a basis for the controlled production of sweet rice wine with a desirable aroma profile.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The authors gratefully acknowledge the project supported by the Research Project of Teaching Reform in Colleges and University of Hunan Province (Grant No. 2019-344).
Data availability
Nucleotide sequence accession number: The sequence data of YRNN in this paper have been deposited in the NCBI GenBank database (Accession Number: MN809231.1).
Compliance with ethical standards
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Yurong Yang, Email: okyong93@outlook.com.
Haiyan Zhong, Email: zhonghaiyan631210@126.com.
Tao Yang, Email: yangtao@csuft.edu.cn, Email: yangtao807@163.com.
Caihong Lan, Email: 1832172746@qq.com.
He Zhu, Email: 251763119@qq.com.
References
- Cai H, Zhang T, Zhang Q, Lou J, Cai C, Mao J. Microbial diversity and chemical analysis of the starters used in traditional Chinese sweet rice wine. Food Microbiol. 2018;73:319–326. doi: 10.1016/j.fm.2018.02.002. [DOI] [PubMed] [Google Scholar]
- Chen S, Wang CC, Qian M, Li Z, Xu Y. Characterization of the key aroma compounds in aged Chinese rice wine by comparative aroma extract dilution analysis, quantitative measurements, aroma recombination, and omission studies. J Agric Food Chem. 2019;67(17):4876–4884. doi: 10.1021/acs.jafc.9b01420. [DOI] [PubMed] [Google Scholar]
- Cullere L, Escudero A, Cacho J, Ferreira V. Gas chromatography-olfactometry and chemical quantitative study of the aroma of six premium quality Spanish aged red wines. J Agric Food Chem. 2004;52:1653–1660. doi: 10.1021/jf0350820. [DOI] [PubMed] [Google Scholar]
- Czerny M, Christlbauer M, Christlbauer M, Fischer A, Granvogl M, Hammer M, Hartl C, Hernandez NM, Schieberle P. Re-investigation on odour thresholds of key food aroma compounds and development of an aroma language based on odour qualities of defined aqueous odorant solutions. Eur Food Res Technol. 2008;228(2):265–273. doi: 10.1007/s00217-008-0931-x. [DOI] [Google Scholar]
- El Hadi MA, Zhang FJ, Wu FF, ZhouCH TJ. Advances in fruit aroma volatile research. Molecules. 2013;18(7):8200–8229. doi: 10.3390/molecules18078200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan WL, Qian MC. Headspace solid phase microextraction and gas chromatography-olfactometry dilution analysis of young and aged Chinese ‘Yanghe daqu’ liquors. J Agric Food Chem. 2005;53(20):7931–7938. doi: 10.1021/jf051011k. [DOI] [PubMed] [Google Scholar]
- Fan WL, Qian MC. Characterization of aroma compounds of Chinese ‘Wuliangye’ and "Jiannanchun" liquors by aroma extract dilution analysis. J Agric Food Chem. 2006;54(7):2695–2704. doi: 10.1021/jf052635t. [DOI] [PubMed] [Google Scholar]
- Fan WL, Xu Y. Characteristic aroma compounds of Chinese dry rice wine by gas chromatography-olfactometry and gas chromatograph-mass spectrometry. Acs Sym Ser. 2012;1104:277–301. doi: 10.1021/bk-2012-1104.ch016. [DOI] [Google Scholar]
- Farh MEA, Cho YJ, Lim JY, Seo JA. A diversity study of Saccharomycopsis fibuligera in rice wine starter nuruk, reveals the evolutionary process associated with its interspecies hybrid. J Microbiol. 2017;55(5):337–343. doi: 10.1007/s12275-017-7115-y. [DOI] [PubMed] [Google Scholar]
- Garde-Cerdán T, Ancín-Azpilicueta C. Contribution of wild yeasts to the formation of volatile compounds in inoculated wine fermentations. Eur Food Res Technol. 2006;222(1–2):15–25. doi: 10.1007/s00217-005-0029-7. [DOI] [Google Scholar]
- Gemert LJV (2003) Compilations of odour threshold values in air, water and other media. (Second Enlarged and Revised ed.). Beijing, China
- Huang H. Aroma producing yeast strain screening, identification for rice aromatic Chinese spirit and research on ester production law of it. Food science and technology (vol master) China: Central South University of Forestry and Technology; 2018. [Google Scholar]
- Huang ZR, Hong JL, Xu JX, Li L, Guo WL, Pan YY, Chen SJ, Bai WD, Rao PF, Ni L, Zhao LN, Liu B, Lv XC. Exploring core functional microbiota responsible for the production of volatile flavour during the traditional brewing of Wuyi Hong Qu glutinous rice wine. Food Microbiol. 2018;76:487–496. doi: 10.1016/j.fm.2018.07.014. [DOI] [PubMed] [Google Scholar]
- Jiao AQ, Xu XM, Jin ZY. Research progress on the brewing techniques of new-type rice wine. Food Chem. 2017;215:508–515. doi: 10.1016/j.foodchem.2016.08.014. [DOI] [PubMed] [Google Scholar]
- Jolly NP, Varela C, Pretorius IS. Not your ordinary yeast: non-Saccharomyces yeasts in wine production uncovered. FEMS Yeast Res. 2014;14(2):215–237. doi: 10.1111/1567-1364.12111. [DOI] [PubMed] [Google Scholar]
- Lee SM, Jung JH, Seo JA, Kim YS. Bioformation of Volatile and nonvolatile metabolites by Saccharomycopsis fibuligera KJJ81 cultivated under different conditions-carbon sources and cultivation times. Molecules. 2018;23(11):2762. doi: 10.3390/molecules23112762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li HH, Qin D, Wu ZY, Sun BG, Sun XT, Huang MQ, Sun JY, Zheng FP. Characterization of key aroma compounds in Chinese Guojing sesame-flavor Baijiu by means of molecular sensory science. Food Chem. 2019;284:100–107. doi: 10.1016/j.foodchem.2019.01.102. [DOI] [PubMed] [Google Scholar]
- Lin X, Wang QK, Hu XP, Wu WY, Zhang YX, Liu SX, Li CF. Evaluation of different Saccharomyces cerevisiae strains on the profile of volatile compounds in pineapple wine. J Food Sci Technol. 2018;55(10):4119–4130. doi: 10.1007/s13197-018-3338-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu YC, Chen MJ, Lin CW. Studies on La-Chao culture filtrate for a flavoring agent in a yogurt-like product. Asian Aust J Anim. 2002;15(4):602–609. doi: 10.5713/ajas.2002.602. [DOI] [Google Scholar]
- Luo T, Fan WL, Xu Y. Characterization of volatile and semi-volatile compounds in Chinese rice wines by headspace solid phase microextraction followed by gas chromatography-mass spectrometry. J Inst Brewing. 2008;114(2):172–179. doi: 10.1002/j.2050-0416.2008.tb00323.x. [DOI] [Google Scholar]
- Ma RF, Lu S, Zhang JS, Hu JR, Liu P. Polyphasic characterization of yeasts and lactic acid bacteria metabolic contribution in semi-solid fermentation of Chinese baijiu (traditional fermented alcoholic drink): towards the design of a tailored starter culture. Microorganisms. 2019;7(5):147. doi: 10.3390/microorganisms7050147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shu Y, Zhang ZS, Wang ZQ, Ren H, Wang H (2014) Research on characteristic aromatic compounds in jujube brandy. In: Proceedings of the 2012 international conference on applied biotechnology (ICAB 2012), vol. 249, pp 499–506. Tianjin, China: Springer Berlin Heidelberg. 10.1007/978-3-642-37916-1_51
- Son EY, Lee SM, Kim MJ, Seo JA, Kim YS. Comparison of volatile and non-volatile metabolites in rice wine fermented by Koji inoculated with Saccharomycopsis fibuligera and Aspergillus oryzae. Food Res Int. 2018;109:596–605. doi: 10.1016/j.foodres.2018.05.008. [DOI] [PubMed] [Google Scholar]
- Su KR, Liu Y, He CC, Su H, Song HL. Comparative analysis of volatile aroma components of Xiangzaolu via the combination of three extraction methods and gas chromatography-olfactometry-mass spectrometry (GC-O-MS) Mod Food Sci Technol. 2015;31(8):340–347. doi: 10.13982/j.mfst.1673-9078.2015.8.053. [DOI] [Google Scholar]
- Thanh VN, Thuy NT, Chi NT, Hien DD, Ha BT, LuongDT NPD, Ty PV. New insight into microbial diversity and functions in traditional Vietnamese alcoholic fermentation. Int J Food Microbiol. 2016;232:15–21. doi: 10.1016/j.ijfoodmicro.2016.05.024. [DOI] [PubMed] [Google Scholar]
- Wang DS, Tian XJ, Huang JL, Huang H, Zhang GH, Ding JN. Effects of MIG1 gene and glucose on cell morphology of Saccharomycopsis fibuligera and mechanism exploration. Microbiol China. 2014;41(9):1757–1763. doi: 10.13344/j.microbiol.china.130767. [DOI] [Google Scholar]
- Whitener MEB, Stanstrup J, Carlin S, Divol B, Du Toit M, Vrhovsek U. Effect of non-Saccharomyces yeasts on the volatile chemical profile of Shiraz wine. Aust J Grape Wine R. 2017;23(2):179–192. doi: 10.1111/ajgw.12269. [DOI] [Google Scholar]
- Xiang WL, Xu Q, Zhang ND, Rao Y, Zhu L, Zhang Q. Mucor indicus and Rhizopus oryzaeco-culture to improve the flavor of Chinese turbid rice wine. J Sci Food Agr. 2019;99(12):5577–5585. doi: 10.1002/jsfa.9831. [DOI] [PubMed] [Google Scholar]
- Xu EB, Long J, Wu ZZ, Li HY, Wang F, Xu XM, Jin ZY, Jiao AQ. Characterization of volatile flavor compounds in Chinese rice wine fermented from enzymatic extruded rice. J Food Sci. 2015;80(7):C1476–C1489. doi: 10.1111/1750-3841.12935. [DOI] [PubMed] [Google Scholar]
- Yang YJ, Xia YJ, Wang GQ, Yu JS, Ai LZ. Effect of mixed yeast starter on volatile flavor compounds in Chinese rice wine during different brewing stages. LWT Food Sci Technol. 2017;78:373–381. doi: 10.1016/j.lwt.2017.01.007. [DOI] [Google Scholar]
- Yang YJ, Xia YJ, Wang GQ, Zhang H, Xiong ZQ, Yu JS, Yu HY, Ai LZ. Comparison of oenological property, volatile profile, and sensory characteristic of Chinese rice wine fermented by different starters during brewing. Int J Food Prop. 2018;20(sup3):S3195–S3211. doi: 10.1080/10942912.2017.1325900. [DOI] [Google Scholar]
- Yin CM, Fan XZ, Fan Z, Shi DF, Yao F, Gao H. Comparison of non-volatile and volatile flavor compounds in six Pleurotus mushrooms. J Sci Food Agr. 2019;99(4):1691–1699. doi: 10.1002/jsfa.9358. [DOI] [PubMed] [Google Scholar]
- Yu HY, Xie T, Qian XH, Ai LZ, Chen C, Tian HX. Characterization of the volatile profile of Chinese rice wine by comprehensive two-dimensional gas chromatography coupled to quadrupole mass spectrometry. J Sci Food Agr. 2019;99(12):5444–5456. doi: 10.1002/jsfa.9806. [DOI] [PubMed] [Google Scholar]
- Yu HY, Xie T, Xie JR, Ai LZ, Tian HX. Characterization of key aroma compounds in Chinese rice wine using gas chromatography-mass spectrometry and gas chromatography-olfactometry. Food Chem. 2019;293:8–14. doi: 10.1016/j.foodchem.2019.03.071. [DOI] [PubMed] [Google Scholar]
- Zhang YY, Huang MQ, Tian HY, Sun BG, Wang J, Li QH. Preparation and aroma analysis of Chinese traditional fermented flour paste. Food Sci Biotechnol. 2013;23(1):49–58. doi: 10.1007/s10068-014-0007-6. [DOI] [Google Scholar]
- Zhao J, Wang M, Xie JC, WangTZ XQF, Zhao MY, Fan MD. Characterization of key aroma compounds in pork from black pig. Food Sci. 2018;39(02):203–209. doi: 10.7506/spkx1002-6630-20802032. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Nucleotide sequence accession number: The sequence data of YRNN in this paper have been deposited in the NCBI GenBank database (Accession Number: MN809231.1).