Abstract
This study evaluates and compares the milled fractions of buckwheat (Fagopyrum esculentum) for their physical, functional properties and nutritional composition. Four samples of buckwheat from the Indian Himalayan regions were roller-milled into five fractions: fine-flour, grits, bran, hull-1 and hull-2. The water activity of the grains lies between 0.77 and 0.83%. The L* values were in the range of 22.77 ± 0.36 to 24.46 ± 0.37 for the four buckwheat grains. Water binding capacity and oil binding capacity showed an increasing pattern of fine flour > grits > bran > hull-1 whereas the swelling power exhibited a reverse trend. The maximum (20.03 ± 1.82%) and the minimum (3.19 ± 0.12%) protein content were found in bran and fine-flour fractions respectively. Highest starch content was detected in fine-flour (73.19 ± 2.00–76.32 ± 2.36%) and lowest in hull-1 (11.02 ± 2.00–17.54 ± 2.36%) with no significant (p > 0.05) difference between different samples. Fine-flour exhibited the lowest phytates content (0.59 ± 0.00–0.62 ± 0.01%) whereas bran has highest tannins (0.06 ± 0.03–1.39 ± 0.02). Milled fractions with specific functional properties and nutrients composition have potential for different food applications.
Keywords: Buckwheat, Milling fraction, Functional properties, Minerals, Dietary fiber
Introduction
In spite of the fact that buckwheat is grown in India since time immemorial and have been a traditional food in the regions where it is grown, almost no works have been done to understand the compositional quality of buckwheat or its products. Although buckwheat is an underutilized crop in India, it still plays an important role in the food and nutritional security of the people living in remote settlements of the Indian Himalayan and North-Eastern regions. In South India, it is sporadically grown in Nilgiris and Palani hills. Compared to the amount of work done on nutritional composition, bioactivity, health benefits and product development, very little has been done on the processing aspects of buckwheat grain. Poor understanding of the quality characteristics of ready-to-use buckwheat products is one of the reasons for lack of use in commercial food product formulations. There is no organized market for buckwheat/buckwheat milled products and thus its production has not been encouraged. Limited reports have been published related to milling of buckwheat into different fractions using roller mill.
Most of the earlier works on buckwheat are related to use of buckwheat as functional ingredient to enhance nutritional properties (Yoo et al. 2012; Vogrincic et al. 2010) and development of gluten-free products for people who are gluten intolerant. Buckwheat is well known for its protein quality, since the amino acid composition is well- balanced. Moreover, buckwheat is also a good source of dietary fiber (27.4%) which is a non-starch polysaccharide (Bonafaccia et al. 2003) and a good source of micro-minerals like zinc, copper and manganese (Ikeda and Yamashita 1994). Mariotti et al. (2013) evaluated the positive effect buckwheat flour in bread performance of commercial gluten free mixtures. Recently, many buckwheat gluten-free products including bread, cookies, biscuits, breakfast cereals, pasta and noodles have been developed (Gimenez et al. 2015). Buckwheat has gained much research interests owing to the healing and preventive roles in various non-communicable diseases (Guo et al. 2013). Buckwheat is potential ingredient for low glycemic index food as it has high resistant starch of 33.5–37.8% of its total starch (Skrabanja et al. 1998). He et al. (1995) have reported on positive effect of buckwheat in the preventive and treatment of both hypertension and hypercholesterolemia. Besides, buckwheat is a rich source of bioflavonoids especially rutin (Skrabanja et al. 2004; Guo et al. 2007) which is known to have many health benefits and has been shown to successfully treat seasonal allergy, increase vascular elasticity and prevent internal bleeding (Gawlik-Dziki et al. 2009), reduce blood glucose level and have high antioxidant activity (Zhang et al. 2010). Some works have been done on composition of buckwheat milled fractions (Skrabanja et al. 2004; Steadman et al. 2001), effect of dehulling and puffing of buckwheat grains on the functional properties of protein and starch fraction (Mariotti et al. 2008).
The objective of this study was to (1) obtain different milled fractions; (2) assess functional and nutritional properties of the milled fractions. Having such technical information about specific functional property and composition will be helpful for application of the milled fractions in different end-products. Moreover, it can pave a way for wider use of buckwheat in food industry.
Materials and methods
Materials
Buckwheat (Fagopyrum esculentum) grains were procured from the local market of four different Himalayan regions of India and were named as sample 1, sample 2, sample 3, sample 4. The grains were cleaned to remove all the foreign materials, dust, straws, dirt, etc. using laboratory cleaner called Labofix (Brabender, Germany). The cleaned grains were packed properly and refrigerated at − 4 °C until further use. All chemicals and solvents used for chemical analysis were of analytical grade (AR) and procured from HiMedia and Sigma Aldrich, India. Pancreatin (P7545, activity equivalent to 8× U.S.P specifications), pepsin (P7000, ≥ 250units/mg solid), α- amylase (A3176, 10 units/mg solid) and amyloglucosidase (A7255, 22,500 units/g solid) were all procured from Sigma (St. Louis, MO USA Sigma, St. Louis, MO, USA).
Moisture content and water activity (aw) of buckwheat grains
The moisture content of the grains was evaluated as per the standard method of AACC (AACC 2000). The water activity of buckwheat grains was observed in water activity meter (Rotronic AG, Bassersdorf, Switzerland).
Determination of grain size, thousand grain weight (TGW), hardness and color
The length and width of the grains were measured using a vernier caliper in 20 randomly selected seeds and the average value was taken. Thousand grain weight was determined by weighing 1000 randomly selected seeds while the density was determined by weighing 100 g of the seed and was transferred into a 500 ml measuring cylinder to record the volume. The grain hardness was measured using the instrument Universal texture measuring instrument (LR5K-USA). The measurement was conducted using a cylindrical probe with diameter 35 mm. The texture parameters were determined with a test speed of 50 mm/s. The compression test was a single compression (50%) on five individual grains from each sample. The force in Newton (N) required to break the grain was recorded as the grain hardness values. The color measurements was done for grains as well as the milled fractions of the different samples using Hunter Lab color measuring system (Model Labscan, XE, Reston USA). The L* value was considered for the grains whereas, L*, a*, b* values were determined for the milled fractions. Each sample was individually measured in triplicate.
Processing of buckwheat into milled fractions
Each sample of buckwheat grains weighing 3000 g were milled through the laboratory scale roller mill (MLU 202, Buhler, Switzerland) consisting of three breaks and three reduction rolls followed by sifter for size separation. The milled fractions were divided on basis of sieve size into five fractions: fine flour (< 129 µm) contained only the endosperm; grits (150–183 µm) resembled semolina having more of endosperm and small amount of bran; bran (505–656 µm) contained pure bran portion, hull-1 contained hull as well as bran portion in a coarse form and hull-2 contained only the hull. Physical and chemical analyses were performed for all the milled fractions except for hull-2 as it is normally not used for human consumption. Milling yield of all the five fractions were calculated.
Functional properties
The milled fractions were ground to fine powder of < 60 mesh for the analysis of functional properties. Water binding capacity (WBC) and oil binding capacity (OBC) were determined according to Beuchat (1977). One gram of the sample was vortexed with 10 ml distilled water or refined vegetable oil for 30 s in centrifuge tube. The mixture was allowed to stand at room temperature (28 ± 2 °C) for 30 min, centrifuged (5000 g, 30 min) and the volume of supernatant was measured in a 10 ml graduated cylinder. Swelling power (SP) was estimated according to the method of Leach et al. (1959). The sample (100 mg) was heated in 10 ml distilled water in a water bath at 60 °C for 30 min with constant mixing. The samples were centrifuged at 1600 rpm for 15 min. The precipitated part was weighted and calculated as follows:
| 1 |
Water solubility index (WSI) was determined as described by Anderson et al. (1969). A 2.5 g sample was suspended in 3 mL distilled water at 30 °C in a tarred centrifuged tube for 30 min with gentle intermittent stirring. The content was then centrifuged at 3000g for 10 min (Baird & Tatlock Auto Bench Centrifuge, London, England). The supernatant was taken into a tarred dish and the amount of dried solids recovered by evaporating the water was expressed as percentage of dry solids in the 2.5 g sample. The WSI was calculated as follows:
| 2 |
Macronutrient composition
The moisture, ash, total fat, protein and carbohydrate content of milling fractions of buckwheat were estimated by AACC method (AACC 2000). Protein content was determined using N/Protein Analyzer (FLASH 2000). The calculation: N × 6.25 was used to convert nitrogen content into protein content. The moisture, fat, protein, ash and total dietary fiber (TDF) were subtracted from the total weight and the difference was taken as total carbohydrates.
Determination of starch content, percent digestibility and dietary fiber fractions
Total starch and percent digestibility
Total starch was determined using Batey method (1982) with a slight modification. The absorbance was read at 505 nm using UV–VIS- 1800 spectrophotometer (Shimadzu, Japan). Glucose content was calculated from glucose standard curve. All analyses were performed in triplicate. The total starch content was calculated as follows:
| 3 |
The method of Holm et al. (1986) was adopted to determine the in-vitro starch digestibility which involves sequential incubation with α- amylase, pepsin, pancreatin and amyloglucosidase. The glucose produced from enzymatic digestion was determined by using GOD/POD reagent kit. Percentage of starch digestibility was calculated as percent starch hydrolyzed from the total starch content in the sample. The following equation was used for calculation.
| 4 |
Dietary fibers
The dietary fiber fractions of soluble dietary fiber (SDF) and insoluble dietary fiber (ISDF) were determined by enzymatic–gravimetric method AOAC 991.43 (AOAC 2005). Defatted samples were suspended in MES-TRIS buffer and digested sequentially with heat stable α- amylase, protease and amyloglucosidase enzymes. To obtain the ISDF, the digested samples were filtered through silica crucibles with fritted disk of porosity 46–60 µm. For the SDF, the filtrate was precipitated for 1 h by adding 96% ethanol previously warmed to 60 °C and filtered similarly as for ISDF. Total dietary fiber was obtained by adding ISDF and SDF. Residues were corrected for nitrogen and inorganic matter content.
Determination of minerals content in buckwheat milled fractions
Minerals content was determined using Microwave Plasma Atomic Emission Spectrometry (MPAES). The determined minerals are Potassium (K), Magnesium (Mg), Calcium (Ca), Sodium (Na), Iron (Fe), Copper (Cu), Zinc (Zn) and Manganese (Mn). Amongst these minerals K, Mg, Ca, Na and Fe are the major minerals whereas Cu, Zn, Mn are the minor minerals. Ash solutions were made to carry out the experiment. A standard curve of the respective minerals was used to calibrate the instrument.
Determination of phytates and tannins content in buckwheat milled fractions
Phytate content was determined according to Vaintraub and Lapteva (1988) method with slight modifications using phytic acid as the standard. 1 g each of the milled fractions was extracted with 10 ml of 0.2 N HCl for 1 hr at room temperature then centrifugation was done at 3000 rpm for 30 min. The clear supernatant was used for phytate estimation. 2–3 mL Wade’s reagent and 0.5–3 mL of the supernatant was taken depending on the sample of milling fractions. The solution prepared was again centrifuged. The absorbance of the supernatant was measured at 500 nm in double beam UV–VIS Spectrophotometer (Shimadzu/UV-1800). The standard curve was prepared using absorbance values of working standard solutions of 5–40 mg/ml concentrations. The absorbance of Wade reagent not bound by phytic acid was read as the blank. The amount of phytic acid extracted from sample was calculated by subtracting from the absorbance of the blank. The difference was compared with the absorbance readings of the standard solutions, which corresponds with the various concentrations of phytic acid and expressed as phytic acid in mg/100 g.
Vanillin hydrochloride method was adopted to estimate tannins content (Skrabanja et al. 1998). 1 g each of the milled fractions was extracted with 50 mL of methanol and was kept in shaking incubator for 20–24 h. Then the samples were centrifuged at 3000 rpm for 15 min. 0.5 mL of supernatant was taken and mixed with 2.5 mL of freshly prepared vanillin-HCl reagent. The absorbance was taken at 500 nm in a double beam UV–VIS Spectrophotometer. Catechin was used as the standard. The total tannins content was expressed as percentage. All the tests were done in three replicates and the average is reported.
Results and discussion
Physico-chemical characteristics of buckwheat grains
The physico-chemical parameters namely moisture content, water activity, thousand grain weight (TGW), bulk density, length and width, grain hardness and L color index were determined for the four buckwheat grain samples. Samples 1, 3 and 4 were within the range of critical moisture content of 14% except for sample 2 which had moisture as high as 14.17 ± 0.17%. On the other hand water activity (aw) was in the range of 0.77–0.83% for all the four samples. Grains harvested with high percentage of moisture will tend to have high aw. Determining physical properties of grains help in better understanding of processing quality (Ponce-Garcia et al. 2016, Unal et al. 2017). All the samples were of similar length (6.30 ± 0.55–6.52 ± 0.67 mm) and width (3.79 ± 0.60–4.02 ± 0.64 mm). A slight difference in TGW, bulk density and grain hardness among the samples was observed with sample 2 showing the highest values. Grain hardness was highest for sample 2 (92.39 ± 8.13 N) and lowest for sample 4 (62.32 ± 15.49 N). The L* value which indicates the lightness in color was in the range of 22.77 ± 0.36 to 24.46 ± 0.37 for all the four samples.
Percentage yield and color index of the milled fractions
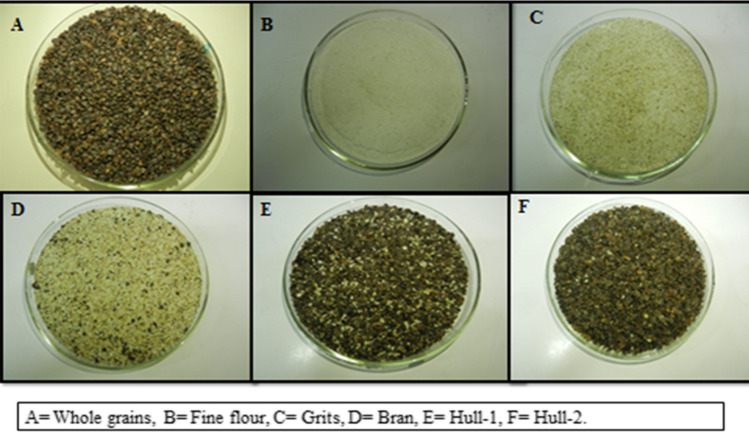
Figure 1 shows the different milled fractions. The milling yield of fine flour was in the range of 21.09 ± 2.0 to 26.57 ± 2.10% (Table 1). The bran fraction was in the range of 24.54 ± 2.40% to 31.54 ± 3.20% with sample 1 exhibiting the highest yield of 31.54 ± 3.20% whereas the lowest was seen in sample 2 (24.54 ± 2.40%). The yield of grits, which contains partly the endosperm and bran was in the range of 18.41 ± 1.20 to 26.47 ± 4.10%. Figure 1 shows the images of the five milled fractions. The yields of fine flour, bran and husk were 23.6%, 14.3% and 27.6% respectively, which is in accordance with that of Skrabanja et al. (2004).
Fig. 1.
Image of buckwheat milled fractions
Table 1.
Milling yield, color and functional properties of milled fractions
| Buckwheat | Milled fractions | Milling yield (%) | Colour index | Functional properties | |||||
|---|---|---|---|---|---|---|---|---|---|
| L* | a* | b* | WBC (%) | OBC (%) | SP (g/g) | WSI (%) | |||
| Sample 1 | Fine flour | 21.09 ± 2.0 | 82.53 ± 0.50 | − 1.12 ± 0.05 | 5.24 ± 0.18 | 188.46 ± 49.0 | 172.17 ± 0.85 | 9.09 ± 0.04 | 8.26 ± 0.14 |
| Grits | 18.41 ± 1.20 | 73.77 ± 1.11 | − 0.39 ± 0.10 | 7.29 ± 0.11 | 192.44 ± 11.00 | 175.53 ± 5.01 | 7.21 ± 0.77 | 12.16 ± 0.80 | |
| Bran | 31.54 ± 3.20 | 65.62 ± 2.07 | 0.73 ± 0.01 | 10.34 ± 0.24 | 192.84 ± 18.80 | 186.82 ± 2.56 | 7.34 ± 0.18 | 16.17 ± 1.53 | |
| Hull-1 | 7.70 ± 0.50 | 39.41 ± 2.29 | 1.16 ± 0.14 | 7.22 ± 0.51 | 233.78 ± 19.28 | 206.31 ± 1.35 | 5.30 ± 0.11 | 16.95 ± 1.95 | |
| Hull-2 | 21.23 ± 1.30 | 24.43 ± 0.46 | 2.00 ± 0.13 | 4.16 ± 0.30 | nd | nd | nd | nd | |
| Sample 2 | Fine flour | 26.57 ± 2.10 | 82.82 ± 0.26 | − 1.14 ± 0.02 | 5.32 ± 0.02 | 190.58 ± 14.16 | 183.70 ± 2.39 | 9.09 ± 0.04 | 8.26 ± 0.14 |
| Grits | 26.47 ± 4.10 | 76.67 ± 0.61 | − 0.71 ± 0.07 | 6.47 ± 0.28 | 205.22 ± 11.54 | 190.01 ± 0.56 | 7.21 ± 0.77 | 12.16 ± 0.80 | |
| Bran | 24.54 ± 2.40 | 65.64 ± 0.51 | 0.62 ± 0.11 | 9.64 ± 0.37 | 214.03 ± 20.52 | 203.10 ± 6.86 | 7.34 ± 0.18 | 16.17 ± 1.53 | |
| Hull-1 | 12.35 ± 0.60 | 46.11 ± 1.58 | 1.34 ± 0.07 | 9.62 ± 0.21 | 246.52 ± 22.48 | 220.26 ± 3.03 | 5.30 ± 0.11 | 16.95 ± 1.95 | |
| Hull-2 | 10.07 ± 1.70 | 24.99 ± 0.90 | 1.86 ± 0.19 | 4.22 ± 0.25 | nd | nd | nd | nd | |
| Sample 3 | Fine flour | 20.75 ± 2.40 | 82.60 ± 0.36 | − 1.13 ± 0.04 | 5.51 ± 0.08 | 180.10 ± 12.05 | 177.61 ± 0.06 | 9.53 ± 0.07 | 9.41 ± 0.21 |
| Grits | 24.70 ± 1.62 | 73.57 ± 1.44 | − 0.3 ± 0.14 | 7.76 ± 0.27 | 202.69 ± 7.23 | 184.91 ± 2.20 | 8.24 ± 0.07 | 13.22 ± 0.32 | |
| Bran | 25.75 ± 1.38 | 65.25 ± 0.52 | 0.52 ± 0.09 | 9.38 ± 0.07 | 241.87 ± 5.59 | 193.39 ± 7.87 | 7.39 ± 0.16 | 18.01 ± 0.07 | |
| Hull-1 | 10.27 ± 0.32 | 45.62 ± 2.18 | 1.00 ± 0.10 | 8.36 ± 0.40 | 299.00 ± 9.06 | 221.17 ± 5.78 | 5.38 ± 0.42 | 19.48 ± 0.01 | |
| Hull-2 | 18.51 ± 0.58 | 31.27 ± 2.14 | 1.43 ± 0.07 | 5.44 ± 0.79 | nd | nd | nd | nd | |
| Sample 4 | Fine flour | 20.16 ± 2.50 | 81.87 ± 0.68 | − 1.12 ± 0.05 | 5.34 ± 0.06 | 187.29 ± 4.30 | 186.12 ± 9.55 | 9.12 ± 0.20 | 7.63 ± 0.61 |
| Grits | 23.28 ± 2.10 | 75.89 ± 0.88 | − 0.7 ± 0.06 | 6.29 ± 0.23 | 213.13 ± 6.88 | 186.30 ± 1.03 | 8.36 ± 0.15 | 13.95 ± 2.08 | |
| Bran | 25.32 ± 2.65 | 63.19 ± 1.05 | 0.64 ± 0.13 | 10.02 ± 0.74 | 244.71 ± 10.19 | 183.76 ± 3.77 | 7.29 ± 0.20 | 18.43 ± 0.52 | |
| Hull-1 | 12.03 ± 0.51 | 40.63 ± 2.60 | 1.00 ± 0.30 | 7.00 ± 0.59 | 308.59 ± 12.74 | 216.17 ± 4.83 | 5.14 ± 0.05 | 19.81 ± 0.52 | |
| Hull-2 | 19.28 ± 2.20 | 27.31 ± 3.59 | 1.44 ± 0.20 | 4.33 ± 0.89 | nd | nd | nd | nd | |
Values are means ± standard deviation, N = 4. nd- not determined. WBC- Water binding capacity, OBC- Oil binding capacity, SP- Swelling power, WSI- Water solubility index
Fine flour was lightest in color with L* value in the range of 81.87 ± 0.68 to 82.82 ± 0.26 across the four samples. The fractions became darker as it moves towards the hull which is indicated by the decreasing L* values. The lightness value was in the range of 73.57 ± 1.44 to 75.89 ± 0.88 for grits, 63.19 ± 1.05 to 65.64 ± 0.51 for bran, 39.41 ± 2.29 to 46.11 ± 1.58 for hull-1 and 24.43 ± 0.46 to 31.27 ± 2.14 for hull-2. Redness (a*) was not consistently different, and yellowness (b*) was highest in bran fraction. No significant difference was noted in the color values of the samples within the same fractions.
Functional properties of milling fractions
The water binding capacity and oil binding capacity of the milled fractions were in the ranges of 172.17 ± 0.85–308.59 ± 12.74% across the different buckwheat samples (Table 1). The WBC and OBC showed an increasing pattern of fine flour > grits > bran > hull-1. This may be attributed to the higher dietary fiber content (as observed in Table 3) as the fraction moves from endosperm towards the hull layer of the seed (Dural NH & Hines AL, 1993; Holloway, WD & Greig, RI, 1984). The results are in agreement with that of Devisetti et al. (2014) where lower water absorption capacity in polished millet flours was reported. Retention of liquid in seed flours is an index of the ability of protein and fiber to absorb and retain water and/or oil, which in turn influences the texture and mouthfeel of food products. In particular, the WBC, SP and WSI are the three indices linked to the prediction of the behaviour of the material if further processed for use as a binder, a stabilizer, or a source of nutrients in final food systems. The SP and WSI were in the ranges of 5.14 ± 0.05–9.53 ± 0.07 g/g and 7.63 ± 0.61–19.81 ± 0.52% respectively. The SP was highest in the fine flour fraction in all the four buckwheat samples followed by grits, bran and hull-1, whereas, the WSI was highest in hull-1 and least in fine flour fraction. The differences in the functional properties between the milled fractions are influenced by the compositional difference of carbohydrate and proteins. Polysaccharides, which are hydrophilic have good water holding capacity. Usually, the protein and polysaccharide contents are higher in the outer layers than in the endosperm part of the grains. Moreover, polar amino acid residues of proteins have an affinity for water molecules and thus imparting water binding property. On the other hand, the mechanism of oil binding is mainly due to physical entrapment of oil by capillary attraction (Beuchat 1977). Protein and carbohydrate in the form of starch have good affinity for oil. The fat and oil binding property of protein and starch has extensive application in processing of ground meats, gravy or saucy type products to reduce cooking loss and maintaining of dimensional stability in the cooked product.
Table 3.
Starch content, percent digestibility and dietary fibers of buckwheat milling fractions
| Milled fractions | Buckwheat | Total starch (%) | IVSD (%) | IDF (%) | SDF (%) | TDF (%) |
|---|---|---|---|---|---|---|
| Fine flour | Sample1 | 75.01 ± 3.38a | 91.56 ± 2.44ab | 5.20 ± 0.40b | 3.39 ± 0.96c | 8.59 ± 0.56b |
| Sample2 | 76.32 ± 2.36a | 88.94 ± 3.60ab | 4.65 ± 0.30c | 1.54 ± 0.32a | 6.19 ± 0.40a | |
| Sample3 | 73.19 ± 2.00a | 92.94 ± 0.65b | 6.00 ± 0.40d | 3.40 ± 0.16b | 9.40 ± 0.56b | |
| Sample4 | 75.20 ± 1.00a | 85.24 ± 2.47a | 6.13 ± 0.09a | 2.30 ± 0.28c | 8.43 ± 0.19b | |
| Grits | Sample1 | 63.00 ± 5.03a | 80.33 ± 3.33a | 10.77 ± 0.63b | 5.82 ± 3.23a | 16.59 ± 3.63a |
| Sample2 | 64.66 ± 5.28a | 81.64 ± 2.11a | 10.94 ± 0.58b | 3.74 ± 1.46a | 14.68 ± 2.02a | |
| Sample3 | 67.17 ± 2.00a | 84.29 ± 1.60a | 12.05 ± 0.45b | 5.57 ± 0.39a | 17.62 ± 0.06a | |
| Sample4 | 63.16 ± 3.00a | 81.95 ± 3.62a | 7.33 ± 1.61a | 6.83 ± 0.29a | 14.16 ± 1.32a | |
| Bran | Sample1 | 52.12 ± 3.59a | 84.13 ± 1.30ab | 19.01 ± 1.40a | 3.70 ± 0.70a | 23.41 ± 2.10a |
| Sample2 | 49.81 ± 0.52a | 83.17 ± 2.23a | 18.35 ± 2.45a | 7.34 ± 0.50b | 25.69 ± 1.95a | |
| Sample3 | 49.63 ± 4.51a | 84.22 ± 2.09ab | 24.60 ± 0.02b | 6.57 ± 0.37b | 31.17 ± 0.35b | |
| Sample4 | 46.62 ± 0.50a | 88.06 ± 0.95b | 16.27 ± 1.13a | 9.26 ± 0.38c | 25.53 ± 1.51a | |
| Hull-1 | Sample1 | 15.54 ± 2.50ab | 71.45 ± 0.83b | 54.56 ± 0.64c | 4.21 ± 0.57a | 58.41 ± 1.19b |
| Sample2 | 17.54 ± 2.36b | 54.56 ± 2.13a | 55.28 ± 0.84ab | 1.15 ± 0.01a | 56.43 ± 0.83a | |
| Sample3 | 11.02 ± 2.00a | 71.45 ± 0.83a | 50.13 ± 3.64bc | 7.65 ± 2.37b | 57.78 ± 1.42c | |
| Sample4 | 12.03 ± 1.00a | 55.22 ± 2.57a | 50.90 ± 3.05a | 6.68 ± 1.56b | 57.58 ± 1.48b |
Values are means ± standard deviation, N = 3, Values in the same column with different letters are significantly different at p < 0.05
IVSD- In-vitro starch digestibility; IDF- Insoluble dietary fiber; SDF- Soluble dietary fiber; TDF- Total dietary fiber
Macronutrient composition
Table 2 indicates a considerable variation in the macronutrient composition among different fractions for the same sample. However, the moisture content remained consistent in the range 13% to 15% irrespective of different fractions or different samples. The milling fractions had ash and fat content of 0.23 ± 0.01–4.17 ± 0.17% and 1.84 ± 0.06–4.48 ± 0.47% respectively. High amount of fat in the grits (2.27 ± 0.02–2.97 ± 0.95%) and bran (3.19 ± 0.45–3.70 ± 0.24%) is due to the proximity of embryo (which is rich in oil) near the bran region of the seed. The distribution of protein and carbohydrate is different from the distribution of ash and fat. The maximum protein value (20.03 ± 1.82%) was seen in bran fraction of sample 3 and minimum value (3.19 ± 0.12%) was observed in fine flour of sample 1. Overall, the richest source of protein was the bran fraction whereas, the hull-1 and grits also showed good amount. The difference in the protein content within the same fractions of different samples was negligible. The good amount of protein present in some of the milling fractions has potential application in special dietary products. The carbohydrate present in the milled fractions followed an order of Fine flour > Grit > Bran > Hull-1. Hull-1 is the outer pericarp of the grain which has high ash and fat but low carbohydrate content. Fine flour which is the starchy endosperm has the highest carbohydrate. The variation in chemical composition for different fractions is in agreement with previous reports (Bonafaccia et al. 2003; Skrabanja et al. 2004) even though the streams selection from different studies varies slightly, the trend in changing composition is common.
Table 2.
Macronutrient composition of buckwheat milled fractions
| Buckwheat | Milled fractions | Moisture content (%) | Ash (% db) | Fat (% db) | Protein (% db) | Carbohydrate (%) |
|---|---|---|---|---|---|---|
| Sample 1 | Fine flour | 14.01 ± 0.12 | 0.97 ± 0.02 | 2.09 ± 0.04 | 3.19 ± 0.12 | 79.74 ± 2.5 |
| Grits | 15.23 ± 0.00 | 1.02 ± 0.05 | 2.97 ± 0.95 | 9.71 ± 0.09 | 71.07 ± 4.21 | |
| Bran | 14.77 ± 00.07 | 2.16 ± 0.04 | 3.70 ± 0.24 | 16.46 ± 0.75 | 62.91 ± 1.36 | |
| Hull-1 | 14.90 ± 0.03 | 2.72 ± 0.00 | 4.48 ± 0.47 | 14.27 ± 1.13 | 58.77 ± 0.15 | |
| Sample 2 | Fine flour | 14.46 ± 0.37 | 0.26 ± 0.00 | 1.84 ± 0.06 | 4.54 ± 0.27 | 78.90 ± 5.62 |
| Grits | 14.32 ± 0.07 | 1.36 ± 0.22 | 2.55 ± 0.04 | 7.51 ± 0.63 | 74.26 ± 3.81 | |
| Bran | 14.72 ± 0.00 | 2.58 ± 0.26 | 3.27 ± 0.01 | 19.55 ± 0.74 | 59.88 ± 2.5 | |
| Hull-1 | 14.46 ± 0.05 | 3.68 ± 0.06 | 3.55 ± 0.13 | 15.18 ± 3.96 | 63.13 ± 0.05 | |
| Sample 3 | Fine flour | 15.53 ± 0.25 | 0.44 ± 0.01 | 1.94 ± 0.09 | 5.06 ± 1.96 | 77.03 ± 6.21 |
| Grits | 15.48 ± 0.11 | 1.34 ± 0.00 | 2.27 ± 0.02 | 10.09 ± 1.01 | 70.82 ± 3.84 | |
| Bran | 15.03 ± 0.18 | 2.59 ± 0.01 | 3.19 ± 0.45 | 20.03 ± 1.82 | 59.16 ± 1.39 | |
| Hull-1 | 15.00 ± 0.01 | 4.00 ± 0.01 | 3.50 ± 0.04 | 18.27 ± 0.22 | 59.23 ± 0.08 | |
| Sample 4 | Fine flour | 15.44 ± 0.19 | 0.23 ± 0.01 | 1.87 ± 0.22 | 4.24 ± 0.60 | 78.22 ± 8.14 |
| Grits | 14.84 ± 0.12 | 0.93 ± 0.01 | 2.59 ± 0.01 | 9.72 ± 0.13 | 71.92 ± 2.25 | |
| Bran | 14.39 ± 0.08 | 2.18 ± 0.39 | 3.52 ± 0.09 | 16.92 ± 1.27 | 62.99 ± 2.65 | |
| Hull-1 | 13.68 ± 0.37 | 4.17 ± 0.17 | 4.47 ± 0.12 | 14.93 ± 2.12 | 62.75 ± 0.09 |
Values are means ± standard deviation, N = 3
Carbohydrate profile of buckwheat milling fractions
Table 3 indicates that there is a wide range of differences in the total starch, percentage digestibility of starch and dietary fibers. A non-significant (p > 0.05) starch content decrease was detected in fine flour (73.19 ± 2.00–76.32 ± 2.36%), grits (63.00 ± 5.03–67.17 ± 2.00%), bran (46.62 ± 0.5–52.12 ± 3.59%) and hull-1 (11.02 ± 2.00–17.54 ± 2.36%). This variation may be attributed to the extraction of flour during milling to obtain various milled streams. Our values are slightly lower than that of Skrabanja et al. (2004) for flours (70.4 – 91.7%) and more for the bran fraction (20.3–42.6%). The percentage digestibility of starch in all the fractions is > 50%. Table 3 indicates that buckwheat roller milled fractions are good source of dietary fiber and the variation is significant (p < 0.05) among different samples for same fractions. The range of total dietary fiber fine flour, grits and bran were 6.19 ± 0.40–9.40 ± 0.56%, 14.16 ± 1.32–17.62 ± 0.06%, 23.41 ± 2.10–31.17 ± 0.35% respectively. As expected, in hull-1, which is composed mainly of the cell wall material like cellulose, hemicellulose, lignin, pectin etc., the total fiber content was highest with all the samples showing > 55%. Similarly, the insoluble dietary fiber (ISDF), Soluble dietary fiber (SDF) also showed an increasing order from fine flour, grits, bran to hull-1. This is due to pure endosperm part present in fine flour which is devoid of bran and outer pericarp. The ISDF values range from 2.30 ± 0.28–6.00 ± 0.40%, 7.33 ± 1.61–12.05 ± 0.45%, 16.27 ± 1.13–24.60 ± 0.02% and 53.90 ± 3.05–62.86 ± 0.64%; whereas for the SDF was 1.54 ± 0.32–6.13 ± 0.09%, 3.74 ± 1.46–6.83 ± 0.29%, 3.70 ± 0.70–9.26 ± 0.38% and 1.15 ± 0.01–10.68 ± 1.56% for fine flour, grits, bran and hull-1 respectively. Though fine flour contains lower TDF (6.19 ± 0.40–9.40 ± 0.56%) than other milled fractions, it contains a good quantity of SDF (1.54 ± 0.32—3.40 ± 0.16%). These results are in accordance with the reports by Steadman et al. (2001) where the observed readings for flour, semolina, bran and hull fractions were between 1.4–5.5, 2–4.5, 2.4–6.6 and 2.7% for SDF and 0.7–4.4, 3.9–9.3, 2.4–6.6 and 89.1% for IDF respectively. Though high dietary fiber content is a desirable aspect considering its innumerable health benefits, milled fraction like hull or husk can have high amount of other impurities like silica which are hazardous to health. Thus, their application in food products should be considered only after safety check for any unwanted associating material.
Minerals content
Buckwheat milled fractions were analyzed for eight minerals namely K, Mg, Ca, Na, Fe, Cu, Zn and Mn. Table 4 shows that the milled fractions exhibit a wide range of variations for minerals content. The mineral content followed an uneven distribution in the order of Hull-1 > Bran > Grits > Fine flour. The macro-minerals in particular, K, Mg and Ca were the highest in all the milled fractions ranging from 136.5 ± 10.18–1106.37 ± 17.17 g/100 g, 36.18 ± 2.02–395.00 ± 6.88 g/100 g and 11.12 ± 1.63–221.31 ± 10.58 g/100 g respectively. There was no significant difference (p < 0.05) in the Ca content in grits and bran fractions across the four samples. Moreover, huge variations for a particular mineral element were observed among the milling fractions. This variation, as explained by Steadman et al. (2001) is due to the diverse chemical composition of central endosperm, aleurone layer, embryo and cell wall tissues in grains and seeds. For fine flour, the distribution of micro-minerals namely Fe, Cu, Zn and Mn were in the range of (5.31 ± 0.65–7.68 ± 0.65 g/100 g), (0.50 ± 0.00–0.56 ± 0.12 g/100 g), (0.87 ± 0.14–2.81 ± 1.96 g/100 g) and (0.25 ± 0.00–0.31 ± 0.12 g/100 g) respectively with not much difference in all the four buckwheat samples. It is interesting to note that the fine flour fraction which consists mainly of endosperm part of the seed contains good amount of the above nutritionally important micro-elements. A much lower values were reported by Steadman et al. (2001) in a similar work on milling fractions of buckwheat. Fe content (16.75 ± 0.64 g/100 g in grits, 11.37 ± 3.35 mg/100 g in bran, 26.06 ± 4.43 g/100 g in hull-1) is the highest in sample 2 whereas, sample 1 showed the highest Zn content (4.37 ± 2.31 g/100 g in grits, 7.25 ± 2.63 g/100 g in bran, 5.00 ± 0.28 g/100 g in hull-1). The difference (p < 0.05) in the content of some of the minerals within same milled fractions across samples may due to the varied growing region and agro-climatic practices. Though hull-1 fraction is also a condensed part for dietary minerals, its application in food products may be limited as compared to the other fractions because of its fibrous nature and other functional properties. In this case, bran fraction which is also equally high in most of the minerals may be a better option for use a minerals rich ingredient.
Table 4.
Minerals content of buckwheat milled fractions
| Minerals (mg/100 g) | Sample 1 | Sample 2 | Sample 3 | Sample 4 | Sample 1 | Sample 2 | Sample 3 | Sample 4 |
|---|---|---|---|---|---|---|---|---|
| Fine flour | Grits | |||||||
| K | 136.5 ± 10.18a | 157.75 ± 5.33bc | 156.43 ± 2.39b | 170.62 ± 6.60c | 407.5 ± 33.90b | 406.62 ± 12.24b | 350.43 ± 30.47a | 369.62 ± 15.99ab |
| Mg | 36.18 ± 2.02a | 38.12 ± 4.33a | 36.62 ± 4.19a | 55.62 ± 4.93b | 193.81 ± 12.34c | 183.18 ± 5.22bc | 157.56 ± 8.33a | 174.31 ± 7.63ab |
| Ca | 11.12 ± 1.63a | 14.12 ± 0.59a | 31.93 ± 5.87b | 26.75 ± 7.60b | 19.62 ± 0.77a | 13.00 ± 0.61a | 76.37 ± 35.37b | 42.62 ± 5.87ab |
| Na | 4.00 ± 2.66a | 3.81 ± 2.68a | 19.12 ± 18.05b | *nd | 6.13 ± 2.00a | 5.81 ± 3.41a | 26.00 ± 28.28a | *nd |
| Fe | 6.75 ± 1.06ab | 7.68 ± 0.65b | 5.31 ± 0.65a | 6.43 ± 0.47ab | 6.25 ± 1.02a | 16.75 ± 0.64b | 6.18 ± 1.24a | 5.43 ± 0.12a |
| Cu | 0.56 ± 0.12a | 0.50 ± 0.00a | 0.50 ± 0.00a | 0.50 ± 0.00a | 0.75 ± 0.00a | 0.75 ± 0.00a | 0.62 ± 0.14a | 0.75 ± 0.00a |
| Zn | 2.81 ± 1.96a | 1.87 ± 1.58a | 2.93 ± 0.51a | 0.87 ± 0.14a | 4.37 ± 2.31a | 2.62 ± 0.32a | 2.43 ± 0.87a | 1.81 ± 0.37a |
| Mn | 0.31 ± 0.12a | 0.25 ± 0.00a | 0.25 ± 0.00a | 0.25 ± 0.00a | 1.56 ± 2.46a | 1.75 ± 0.57a | 1.00 ± 0.00a | 1.12 ± 0.14a |
| Bran | Hull-1 | |||||||
| K | 700.06 ± 72.3b | 711.06 ± 9.76b | 687.00 ± 8.27ab | 607.18 ± 21.31a | 859.68 ± 15.14a | 1106.37 ± 17.17c | 986.93 ± 38.16b | 962.62 ± 30.64b |
| Mg | 320.93 ± 25.57c | 314.18 ± 3.28bc | 287.18 ± 4.92ab | 273.37 ± 4.78a | 327.25 ± 7.85ab | 395.00 ± 6.88c | 321.68 ± 5.87a | 341.88 ± 12.20b |
| Ca | 20.37 ± 4.28a | 13.81 ± 1.46a | 104.87 ± 5.03c | 57.56 ± 2.65b | 64.93 ± 3.34b | 32.87 ± 0.47a | 221.31 ± 10.58d | 168.06 ± 11.78c |
| Na | 5.31 ± 1.34a | 3.37 ± 1.31a | 64.06 ± 9.90b | 4.50 ± 0.20a | 8.62 ± 5.51a | 9.93 ± 6.74a | 54.75 ± 10.99b | 19.25 ± 1.42a |
| Fe | 8.18 ± 2.41a | 11.37 ± 3.35a | 8.12 ± 0.14a | 9.06 ± 1.67a | 13.68 ± 1.23a | 26.06 ± 4.43ab | 17.87 ± 1.19ab | 20.75 ± 8.66b |
| Cu | 0.75 ± 3.66a | 1.00 ± 0.00a | 0.75 ± 0.00a | 0.75 ± 0.00a | 0.87 ± 0.14a | 1.00 ± 0.00a | 0.87 ± 0.14a | 0.87 ± 0.14a |
| Zn | 7.25 ± 2.63b | 3.43 ± 0.37a | 3.62 ± 0.52a | 3.18 ± 0.37a | 5.00 ± 0.28a | 4.31 ± 0.37ab | 4.93 ± 0.12b | 3.50 ± 1.74b |
| Mn | 2.06 ± 0.23a | 2.18 ± 0.12a | 2.00 ± 0.00a | 1.87 ± 0.14a | 4.06 ± 0.23a | 4.12 ± 0.14a | 4.50 ± 0.00b | 4.50 ± 0.00b |
Values are means ± standard deviation, N = 3. Values in the same column with different letters are significantly different at p < 0.05. *nd.- not detected
Phytates and tannins content
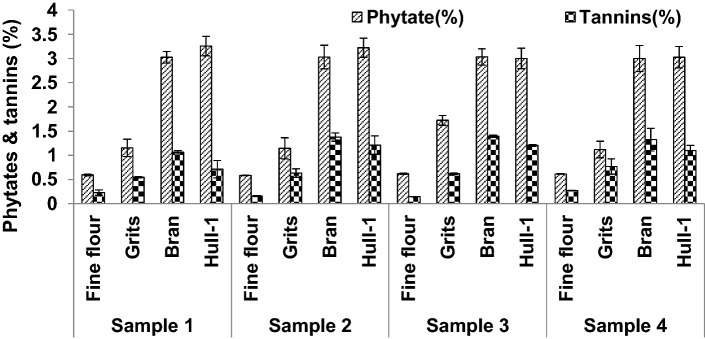
Figure 2 illustrates the content of the two common anti-nutrients namely, phytates and tannins, usually present in cereals and grains. The concentration of phytates was highest in hull-1 (3.00 ± 0.31–3.25 ± 0.20%). Bran fraction also showed a good amount of phytates as comparable to hull-1 in all the samples. On the other hand, the lowest amount of phytates was seen in fine flour and grits in the range 0.59 ± 0.00 to 0.62 ± 0.01% and 1.12 ± 0.97 to 1.72 ± 0.10% respectively. Skrabanja et al. (1998) reported phytates content at 0.21–0.72% in fine flour. Steadman et al. (2001) observed highest content of phytates in bran without hull (35–38 g/kg). They further reported that 60–90% of phosphorus was bound to phytic acid. It is a known fact that phytic acid and associated minerals are concentrated in protein bodies of the embryo and aleurone layer of seeds. The content of tannins showed a trend of bran > hull-1 > grits > fine flour. The detected tannins were the highest in bran fraction (1.06 ± 0.03–1.39 ± 0.02%) in the four buckwheat samples. Our results are in accordance with that of Steadman et al. (2001) where the amount of condensed tannins present in fine flour, grits and bran were 1.18, 1.63 and 4.10 g/Kg dry weight respectively. Phytic acid being a strong chelator of various metal ions forming metal-phytate complex, reduces the bioavailability of dietary minerals. On the other hand, tannins form complexes with protein, thereby reducing availability of protein for absorption. The content of phytates and tannins in plant foods have to be evaluated especially when they are present in high amount or the foods containing these ‘anti-nutrients’ are being consumed in large quantities.
Fig. 2.
Phytates and tannins content of buckwheat milled fractions
Conclusion
No significant differences were found in various physico-chemical properties of buckwheat grains, although milling yield for different fractions varied slightly among the buckwheat samples. In general, there is a positive correlation between the milling yield and physical parameters namely TGW, grain hardness and density. Buckwheat bran is the most nutrient dense having high protein, lipid, dietary fiber and minerals. The macro-minerals in particular, K, Mg and Ca were found highest in all the milled fractions whereas Fe, Zn and Mn are the micro-elements present in large quantities. We can conclude that by milling buckwheat grains into various fractions it is possible to obtain certain milled fractions rich in a particular group of nutrients. Appropriate fractions can be chosen in developing target-specific health products. Fractions with specific functional properties of technological significance can be further exploited to obtain a desired end-use product. Demonstration of the feasibility of the milled fractions in various food products would be a good support for the present study.
Acknowledgement
This work was supported by the Department of Science and Technology- Science and Engineering Board (DST-SERB), Government of India (EEQ/2017/000365). The authors are thankful to Mr. Anbalagan K. and Mr. Lokesha C., Central Instruments Facility and Services, Central Food Technological Research Institute (CFTRI), Mysore, for their help in carrying out grain hardness and minerals content analyses.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- AACC (2000) Approved methods of the American Association of Cereal Chemists. 10th ed Vols I and II
- Anderson RA. Gelatinization of corn grits by roll-and extrusion-cooking. Cereal Sci Today. 1969;14:4–12. [Google Scholar]
- AOAC (2005) Official methods of analysis (19th ed) Association of Official Analytical Chemists, Washington
- Batey IL. Starch analysis using thermostable alpha-amylases. Starch-Stärke. 1982;34(4):125–128. doi: 10.1002/star.19820340407. [DOI] [Google Scholar]
- Beuchat LR. Functional and electrophoretic characteristics of succinylated peanut flour protein. J Agric Food Chem. 1977;25(2):258–261. doi: 10.1021/jf60210a044. [DOI] [Google Scholar]
- Bonafaccia G, Marocchini M, Kreft I. Composition and technological properties of the flour and bran from common and tartary buckwheat. Food Chem. 2003;80(1):9–15. doi: 10.1016/S0308-8146(02)00228-5. [DOI] [Google Scholar]
- Devisetti R, Yadahally SN, Bhattacharya S. Nutrients and antinutrients in foxtail and proso millet milled fractions: Evaluation of their flour functionality. LWT-Food Sci Technol. 2014;59(2):889–895. doi: 10.1016/j.lwt.2014.07.003. [DOI] [Google Scholar]
- Dural NH, Hines AL. Adsorption of water on cereal-bread type dietary fibers. J Food Eng. 1993;20(1):17–43. doi: 10.1016/0260-8774(93)90017-E. [DOI] [Google Scholar]
- Gawlik-Dziki U, Dziki D, Baraniak B, Lin R. The effect of simulated digestion in vitro on bioactivity of wheat bread with Tartary buckwheat flavones addition. LWT-Food Sci Tech. 2009;42(1):137–143. doi: 10.1016/j.lwt.2008.06.009. [DOI] [Google Scholar]
- Gimenez-Bastida JA, Piskuła M, Zieliński H. Recent advances in development of gluten free buckwheat products. Trends Food Sci Technol. 2015;44(1):58–65. doi: 10.1016/j.tifs.2015.02.013. [DOI] [Google Scholar]
- Guo X, Yao H, Chen Z. Effect of heat, rutin and disulfide bond reduction on in-vitro pepsin digestibility of Chinese tartary buckwheat protein fractions. Food Chem. 2007;102(1):118–212. doi: 10.1016/j.foodchem.2006.04.039. [DOI] [Google Scholar]
- Guo XD, Zhang DY, Gao XJ, Parry J, Liu K, Liu BL, Wang M. Quercetin and quercetin-3-glucuronide are equally effective in ameliorating endothelial insulin resistance through inhibition of reactive oxygen species-associated inflammation. Mol Nutr Food Res. 2013;57(6):1037–1045. doi: 10.1002/mnfr.201200569. [DOI] [PubMed] [Google Scholar]
- He J, Klag MJ, Whelton PK, Mo JP, Chen JY, Qian MC, Mo PS, He GQ. Oats and buckwheat intakes and cardiovascular disease risk factors in an ethnic minority of China. Am J Clin Nutr. 1995;61(2):366–372. doi: 10.1093/ajcn/61.2.366. [DOI] [PubMed] [Google Scholar]
- Holm J, Björck I, Drews A, Asp NG. A rapid method for the analysis of starch. Starch Stärke. 1986;38(7):224–226. doi: 10.1002/star.19860380704. [DOI] [Google Scholar]
- Holloway WD, Greig RI. Water holding capacity of hemicelluloses from fruits, vegetables and wheat bran. J Food Sci. 1984;49:1632–1633. doi: 10.1111/j.1365-2621.1984.tb12867.x. [DOI] [Google Scholar]
- Ikeda S, Yamashita Y. Buckwheat as a dietary source of zinc copper and manganese. Fagopyrum. 1994;14:29–34. [Google Scholar]
- Leach HW. Structure of starch granules. I. Swelling and solubility patterns of various starches. Cereal Chem. 1959;36:534–544. [Google Scholar]
- Mariotti M, Lucisano M, Pagani MA, Iametti S. Macromolecular interactions and rheological properties of buckwheat-based dough obtained from differently processed grains. J Agric Food Chem. 2008;56(11):4258–4267. doi: 10.1021/jf800009e. [DOI] [PubMed] [Google Scholar]
- Mariotti M, Pagani MA, Lucisano M. The role of buckwheat and HPMC on the breadmaking properties of some commercial gluten-free bread mixtures. Food Hydrocolloids. 2013;30(1):393–400. doi: 10.1016/j.foodhyd.2012.07.005. [DOI] [Google Scholar]
- Ponce-Garcia N, Ramirez-Wong B, Escalante-Aburto A, Torres-Chavez PI, Figueroa J. Mechanical properties in wheat (Triticum aestivum) kernels evaluated by compression tests: a review. In: El-Amin MF, editor. Viscoelastic and viscoplastic materials. 1. London: In Tech; 2016. pp. 21–33. [Google Scholar]
- Skrabanja V, Kreft I, Golob T, Modic M, Ikeda S, Ikeda K, Kreft S, Bonafaccia G, Knapp M, Kosmelj K. Nutrient content in buckwheat milling fractions. Cereal Chem. 2004;81(2):172–176. doi: 10.1094/CCHEM.2004.81.2.172. [DOI] [Google Scholar]
- Skrabanja V, Laerke HN, Kreft I. Effects of hydrothermal processing of buckwheat (Fagopyrum esculentumMoench) groats on starch enzymatic availability in-vitro and in vivo in Rats. J Cereal Sci. 1998;28(2):209–214. doi: 10.1006/jcrs.1998.0200. [DOI] [Google Scholar]
- Steadman KJ, Burgoon MS, Lewis BA, Edwardson SE, Obendorf RL. Minerals phytic acid tannin and rutin in buckwheat seed milling fractions. J Sci Food Agri. 2001;81(11):1094–1100. doi: 10.1002/jsfa.914. [DOI] [PubMed] [Google Scholar]
- Unal H, Izli G, Izli N, Asik BB. Comparison of some physical and chemical characteristics of buckwheat (Fagopyrum esculentum Moench) grains. Cyta-J Food. 2017;52(2):257–265. doi: 10.1080/19476337.2016.1245678. [DOI] [Google Scholar]
- Vaintraub IA, Lapteva NA. Colorimetric determination of phytate in unpurified extracts of seeds and the products of their processing. Anal Biochem. 1988;175(1):227–230. doi: 10.1016/0003-2697(88)90382-X. [DOI] [PubMed] [Google Scholar]
- Vogrincic M, Timoracka M, Melichacova S, Vollmannova A, Kreft I. Degradation of rutin and polyphenols during the preparation of tartary buckwheat bread. J Agric Food Chem. 2010;58(8):4883–4887. doi: 10.1021/jf9045733. [DOI] [PubMed] [Google Scholar]
- Yoo J, Kim Y, Yoo SH, Inglett GE, Lee S. Reduction of rutin loss in buckwheat noodles and their physicochemical characterisation. Food Chem. 2012;132(4):2107–2111. doi: 10.1016/j.foodchem.2011.12.065. [DOI] [Google Scholar]
- Zhang M, Chen H, Li J, Pei Y, Liang Y. Antioxidant properties of tartary buckwheat extracts as affected by different thermal processing methods. LWT-Food Sci Tech. 2010;43(1):81–185. [Google Scholar]