Abstract
Films that incorporate antioxidant agents are widely used and improve the stability of food products that are prone to oxidation. This work evaluated the potential antioxidant activity of PVA/gelatine films incorporated with quercetin. The films were prepared by the casting method and characterised by TG-DSC, FTIR spectroscopy, SEM, optical microscopy and swelling index. Antioxidant properties were evaluated with DPPH, ABTS and FRAP assays. According to the thermal characterisation results, the film was stable up to 68 °C and entirely degraded at 632 °C. The FTIR spectroscopic analysis indicated that there was a physical interaction between the quercetin and the polymeric film, and microscopy indicated a homogeneous and uniform film. The film showed DPPH (315.4 ± 8.2) and ABTS radical potential activity (199.4 ± 9.7), as well as potential iron reduction activity—FRAP (740.6 ± 8.9) mainly when analysed in ethanol: water (95:5 v/v) system, all results expressed as milligram of Trolox per gram of film. Hence, PVA/gelatine films incorporated with quercetin have properties that allow a potential application in active packaging systems to delay oxidative processes in food.
Keywords: Active packaging, Natural antioxidants, Quercetin, Environment-friendly copolymers
Introduction
The growing demand for food products with an extended shelf-life has driven the development of active packaging, which is as food packaging systems that act as a carrier for antioxidant (Bitencourt et al. 2014; Lorenzo et al. 2014), antimicrobial (Vásconez et al. 2009; Muriel-Galet et al. 2015) or flavouring compounds (Teixeira et al. 2014). Among active packaging, the active antioxidant packaging is the most developed, and it is based on the incorporation of antioxidant agents into the polymeric package for improving the stability of oxidation-sensitive food products (Gómez-Estaca et al. 2014; Yadav et al. 2020). In this context, the combination of environmentally friendly synthetic polymers with biopolymers, as well as natural antioxidant molecules, is increasing due to their biocompatibility and low toxicity.
Therefore, efforts have focused on the research and development food packaging with antioxidant agents from natural sources, such as tea polyphenols (Shutava et al. 2009; Zhou et al. 2012; Liu et al. 2015), essential oils (Muriel-Galet et al. 2015; Medina-Jaramillo et al. 2017) and quercetin (Han et al. 2015; Souza et al. 2015; Yadav et al. 2020). Quercetin is the most abundant dietary flavonoid in fruits and vegetables (Russo et al. 2012). Besides its high antioxidant property, the addition of quercetin can also protect the polymeric package from degradation (Han et al. 2015). Moreover, quercetin can be combined with polymers such as gelatine and polyvinyl alcohol (PVA) for the development of biocompatible, environmentally friendly and non-toxic active antioxidant food-packaging.
Despite the advantages of biopolymers and natural products, the incorporation of antioxidant molecules in the polymeric film could affect the physical and chemical properties of these films (Valdés et al. 2015), such as microstructure, thermal properties, water vapor transmission rate. A significant decrease of essential mechanical properties such as elongation and tensile strength has been reported (Lorenzo et al. 2014; Nisa et al. 2015). On the other hand, positive effects such as the production of more flexible films from gelatine were reported in the literature (Li et al. 2014). Hence, due to the unique properties of gelatine, such as film-forming properties and easy handling, it is possible to combine gelatine with other biocompatible polymers and natural products to obtain active packages with antioxidant properties and good thermal, mechanical and microscopical properties. In this sense, there are reports in the literature of gelatine-based antioxidant active packages (Giménez et al. 2013; Bitencourt et al. 2014; Liu et al. 2015). It has been reported in the literature that incorporating PVA into gelatine improves the mechanical properties of gelatine (Xiping et al. 2014), mainly elongation at break and tensile strength, which are essential properties for a food packaging material. Thus, gelatine and PVA are fully compatible with each other, and they form stable and resistant films. However, to the best of our knowledge, there are no reports in the literature of gelatine-PVA copolymers as a carrier for natural antioxidants. Hence, to evaluate the potential of gelatine-PVA copolymer as a carrier for natural antioxidants in an active food package, a copolymer derived from gelatine and PVA with incorporated quercetin as a model for an active antioxidant packaging is reported here.
Material and methods
Film preparation
Gelatine/PVA copolymer was obtained according to previously published methods (Singh et al. 2010) using the casting technique. The PVA was prepared by dissolving 2 g of polymer powder (Sigma-Aldrich, Art. No. 363146) in 100 mL of distilled water under magnetic stirring at 80 ± 2 °C for 1 h. Then, 1 g of gelatin (type A) was dissolved in the aqueous solution of PVA followed by Fischer esterification with concentrated hydrochloric acid at 37% (1% of total solution volume) at 80 ± 2 °C for 1 h under reflux. After the reaction, 150 mg of quercetin (Sigma-Aldrich, Art. No. 337951) was added and stirred for 1 h. The reaction mixture was poured into a petri-dish (150 mm diameter) and submitted to solvent evaporation in an oven at 35 °C until film formation.
Determination of swelling index (Si) and water vapour permeability (WVP)
The swelling index of films was determined by the method described by Cavalcanti (Cavalcanti et al. 2002), with modifications in the time interval. Initially, film samples were cut into 4.0 cm2 (2 × 2 cm) slices and kept in a desiccator with silica-gel for seven days. After this procedure, samples were weighed and then subjected to immersion in beakers (250 mL) containing distilled water for different time intervals (0.5. 1, 3, 5, 7, 10, 30 and 60 min) at room temperature (25 °C). At each time interval, samples were removed, dried and weighted. The swelling index (Si%) was calculated by Eq. (1):
| 1 |
The water vapour permeability was determined as previously reported (Silva-Pereira et al. 2015).
Spectroscopy and TG-DSC analysis
FTIR spectra were obtained from a PerkinElmer spectrophotometer model Spectrum 100, with a 4 cm−1 resolution in the region between 4000 and 600 cm−1 using crystal diamond/ZnSe and the technique of attenuated reflectance (ATR). The TG-DSC was performed in a Mettler Toledo instrument model TG-DSC-1 and used an α-Al2O3 crucible (70 µL) with a sample mass of approximately 4 mg, a heating rate of 20 °C min−1, a dry air flow of 60 mL min−1 and a temperature range of 30–1000 °C.
Light microscopy (LM) and texture analysis (GLCM)
The light microscopic analysis was performed using a Nikon Eclipse-Ci high-resolution fluorescence microscope at five different magnifications (4×, 10×, 20× 40×and 100×). Images were further submitted to texture analysis performed with the ImageJ software (Abràmoff et al. 2004) using the grey-level co-occurrence matrix (GLCM) algorithms, according to methodology previously purposed (Arzate-Vázquez et al. 2012). In this study, four textural features were calculated (contrast, homogeneity index, energy and entropy). For image texture analysis, all the images were converted from RGB to grey-scale images.
Field emission gun scanning electron microscopy (FEG-SEM)
The film surface morphology was also examined using field emission gun scanning electron microscopy (FEG-SEM) with an accelerating voltage from 10 to 15 kV. Before analysis, all samples were cut with a sharp scalpel and mounted on aluminium stubs using carbon adhesive tape and sputter-coated with gold.
Determination of the antioxidant potential
Antioxidant potential was determined by classical DPPH, ABTS and FRAP tests. The release of quercetin from the films was carried out according to the literature (López-de-Dicastillo et al. 2011) with modifications. Different release media were prepared to simulate different types of food in accordance with European Regulation (UNE-EN. 1186-3 2002) in three release media: (1) ethanol:water (95:5 v/v), (2) ethanol:water (10:90 v/v) and (3) acetic acid:water (3:97 v/v). Films were cut in 2 cm2 slices and added to 10 mL of each release medium in 100 mL beakers, followed by sonication fo 10 min. Samples were collected from the medium every five minutes during a thirty-minute time interval.
The DPPH antioxidant test was performed according to the modified method of Brand-Williams (Brand-Williams et al. 1995). Briefly, a DPPH (2,2-diphenyl-1-picrylhydrazyl) stock solution was prepared by dissolving 24 mg of DPPH in 100 mL methanol and stored it under refrigeration. For analysis, 10 mL of the stock solution was diluted with 45 mL methanol to prepare the working solution. The absorbance of this solution was adjusted to 1.1 ± 0.02. Then, 100 μL of sample was added to 3.9 mL of the DPPH working solution to quantify the antioxidant potential. Sample absorbance at 517 nm was measured after 30 min, 24 h and compared to a standard Trolox ((+)-6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid) curve. Results were expressed as µmol Trolox TE/g dry film equivalent.
The ABTS antioxidant assay was performed as described by Re et al. (1999). After samples incubation for 25 min at 30 °C, the absorbance at 737 nm was measured and compared to a standard Trolox curve. Results were expressed as µmol Trolox equivalents TE/g dry film.
The reduction of ferric tripyridyl triazine (Fe+3-TPTZ-2,4,6-tris (2-pyridyl)-s-triazine) (FRAP test) was conducted with the method of Benzie and Strain (Benzie and Strain 1996), in which the absorbance at 593 nm was measured. Results were expressed as µmol Trolox equivalents TE/g dry film.
Statistical analysis
Data analysis was carried using computational program statistics. The data were submitted to an analysis of variance (ANOVA) and the study of differences between averages, detected by the Tukey test (p < 0.05). Where applicable, analyses were made in triplicate and the results were given as means.
Results and discussion
The film reached its maximum water absorption in 7 min (125.8% ± 3.2%) and then remained constant after 20 min (108.6% ± 1.7%). Thus, the results show that despite the recorded changes, there was an equilibrium hydration period between 5 and 7 min (123.2% ± 4.4% to 125.8% ± 3.2%). The final value for the film swelling index was 108.3% ± 1.7%. Films kept their integrity during the swelling test, showing good water absorbency properties and low water solubility. This property is desirable to absorb extra water from the outer surface of high moisture food. This higher water absorption is attributed to the nature of hydrophilic compounds present in the film, making hydrogen bonds with the water.
Water vapor permeability (WVP) is the standard measure by which films are ranked by their ability to achieve moisture transmission under specific conditions. The smallest values of WVP indicate a better moisture barrier property. These values relate this behaviour with the increase of the water solubility coefficient in the polymer matrix, which makes the films more hygroscopic. Gelatine/PVA/Quercetin films presented maximum absorption rate of 0.20 ± 0.02 g h−1 mm m−2 kPa−1. After approximately 31 h, the film presented humidity uniformity (Fig. 1), keeping a constant rate of humidity absorption. After this constant period, the samples reached saturation. That is, they absorbed all the possible humidity and remained at a fixed humidity of 75% and temperature of 24.5 °C. It is clear that samples absorbed moisture, but there was no visual, chemical or physical alteration, and after 108 h of analysis, the film returned to its original state. Thus, water was not intramolecularly trapped into the polymeric matrix; this result is similar to those previously reported (Silva-Pereira et al. 2015). The results also indicate that the films remain stable and keep integrity under moisture, having low moisture permeability, and therefore could be indicated for moisture control for moisture sensitive foods.
Fig. 1.

WVP of the copolymeric film with incorporated quercetin
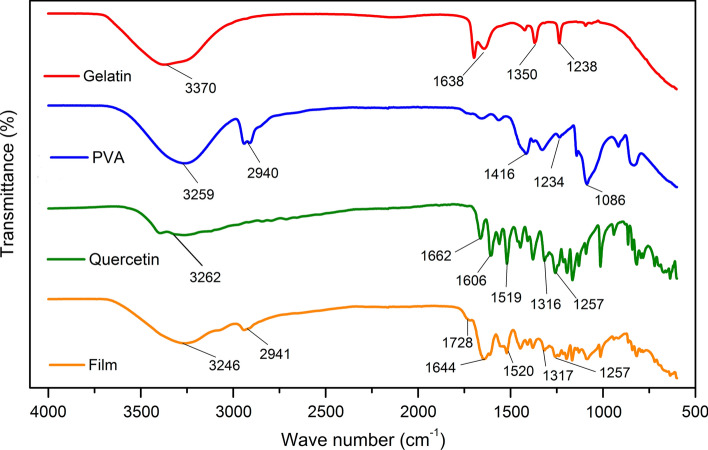
Figure 2 shows FTIR spectra for the pure gelatine, PVA, quercetin and gelatine/PVA copolymer films. The spectrum for the gelatine film shows absorption peaks at about 3370 cm−1, corresponding to the -OH and -NH groups due to the presence of water and amino acids typical of gelatine structure (Li et al. 2014); 1638 cm−1, (stretching vibration C=O) corresponding to the presence of primary amides; and 1350 and 1238 cm−1 (C–N stretching band), which are typical of tertiary amines and assigned to the gelatin polymer chain. The results for the gelatine are also in accordance with previously reported data (Zhang et al. 2013), which showed amide I absorption was primarily due to the stretching vibration of the C=O bond and the amide II band was due to the coupling of the bending of the N–H bond and the stretching of the C–N bond. In the PVA spectrum (Fig. 2), the O–H stretching band is present at 3259 cm−1 (Table 2) due to the presence of hydroxyl groups characteristic of the material, as well as C–H stretching bands at 2940 cm−1, 1416 cm−1 (CH2 deformation), 1234 and 1086 cm−1, corresponding to the stretching C–O and (C–O)–C–OH, respectively.
Fig. 2.
FTIR spectra of gelatine, PVA, quercetin and copolymeric film with incorporated quercetin
Table 2.
Film antioxidant potential
| Simulant | ABTS | FRAP | DPPH 30’ | DPPH 24 h |
|---|---|---|---|---|
| Acetic acid: water (3:97; v/v) | 22.8 ± 0.5bC | 21.3 ± 0.9bC | 49.2 ± 0.9bB | 78.5 ± 2.4cA |
| Ethanol: water (10:90; v/v) | 27.7 ± 2.3bD | 40.1 ± 3.6bC | 57.9 ± 3.7bB | 118.4 ± 4.1bA |
| Ethanol: water (95:5; v/v) | 199.4 ± 9.7aC | 740.6 ± 8.9aA | 315.4 ± 8.2aB | 308.8 ± 5.4a |
Values are expressed in (µmol TE/g). Means followed by the same capital letters in the same line and non-capital letters in different columns, do not differ significantly (p < 0.05)
The FTIR spectrum for the quercetin was characteristic of the main functional groups typical of flavonoid compounds, with an absorption band at 3262 cm−1. This result suggests that there were a few hydroxyl groups in the quercetin molecule, which might form the intra- and intermolecular hydrogen bonds30. A carbon–oxygen double-bound stretching vibration peak for the carbonyl group was found at 1662 cm−1. Also, stretching vibration peaks of the benzene skeleton in the quercetin molecule were found at 1606 and 1519 cm−1, these measurements are corroborated by the literature (Lin et al. 2006).
The gelatine/PVA copolymer has main absorption bands at 3246, 2941, 1728, 1644, 1520, 1317 and 1257 cm−1, corresponding to the stretching vibration of O–H, C–H, C=O, C=C, C–O and C–O–C. The band observed at 1644 cm−1 was the first shifted relative to that observed in the gelatine spectrum (1638 cm−1). The band at and 1728 cm−1 which was not observed in the spectrum of gelatine, indicates the formation of an ester grouping. Thus, there was a chemical Fischer-type esterification reaction between the PVA and gelatine, producing a copolymer with different chemical properties than the starting polymer (Singh et al. 2010). Therefore, considering the copolymer spectrum in comparison to the others, it is clear that there was a physical interaction between the gelatine/PVA copolymer and quercetin due to the significant decrease in band corresponding to the grouping –OH (3246 cm−1). The decrease was due the formation of intramolecular bonds between hydroxyl groups present in the copolymer and quercetin and the influence of conjugated π bonds with the phenolic groups (Li et al. 2014). The absorption bands between 1520 and 1606 cm−1, overlapped by the band at 1644 cm−1, showed the presence of aromatic groups belonging to quercetin. This indicated that the quercetin was incorporated into the copolymeric film in an adsorption process and may be subsequently released in a controlled manner.
The TG-DSC curve (Fig. 3) shows the thermal degradation of the copolymer film. The degradation occured in five stages of mass loss. The first stage occurred between approximately 41 and 68 °C in the TG curve due to loss of adsorbed water[0.55% (m/m)] with an endothermic peak in the DSC curve. The second stage was observed in the TG curve between 68 and 173 °C with mass loss of 9.01% (m/m) and endothermic peak in the DSC curve, which initiated the thermal degradation of organic material. In other words, the film lost its stability from this temperature. The third stage observed in TG curve occurred between 173 and 400 °C with mass loss of 37.57% (m/m) and accompanied by a discrete endothermic peak in the curve DSC. The fourth stage occurred between 400 and 505 °C with mass loss of 21.38% (m/m) and endothermic peak in the DSC curve. The fifth and last stage occurred between 505 and 632 °C with mass loss of 24.42% (m/m). This stage is characteristic of oxidative degradation, as evidenced by the exothermic peak in the DSC curve, and indicating a significant release of energy from the release of gas. Therefore, the total thermal decomposition of the film occurred at 632 °C. The data TG-DSC corroborates the observations of the FTIR data on the formation of a copolymer.
Fig. 3.

TG-DSC curve of copolymeric film with incorporated quercetin
Figure 4 shows the LM results for the film at all magnifications. It was observed that the film showed uniformity, confirming that there was the formation of the polymer network and the interaction thereof with quercetin. Irregularities in all film surfaces were also observed (Fig. 4a), characterised by the darker parts of the film, with the largest showing a diameter of approximately 70 μm. Furthermore, the images indicate the presence of a granule of a lighter colour are with a diameter of approximately 200 μm. These irregularities can be assigned to the non-solubilization of some components of the film (quercetin). Moreover, air bubbles, cracks and holes in the films were not observed, indicating film uniformity. Figure 4b–f are higher magnification images of Fig. 4a, with a focus on a granule that does not have a defined contour, but has the same aspect when observed inside and outside (Fig. 4e, f)). These images have magnifications of 100× and show structures of different sizes and shapes. The granules have most circular shapes with well-defined contours and sizes ranging from 2–10 µm. The presence of granules are assigned to undissolved Quercetin molecules.
Fig. 4.
Light microscopy images of copolymeric film with incorporated quercetin
To corroborate the description of film structure observed with LM, the images were mathematically treated with GLCM algorithms to obtain their textural features (Table 1). Contrast can be used to measure the variance of the image is a local variation of the grayscale values of a group of pixels (Silva-Pereira et al. 2015). The homogeneity index represents the local homogeneity of the image. Entropy is an indicator of the complexity of the image, because the higher the entropy, the more complex it is.
Table 1.
Textural features extracted from film at different magnifications
| Magnification | Contrast | Homogeneity | Entropy | Energy |
|---|---|---|---|---|
| 4× | 755.737 | 0.113 | 8.688 | 2.667 × 10–4 |
| 10× | 489.442 | 0.138 | 8.146 | 5.437 × 10–4 |
| 20× | 731.649 | 0.132 | 8.341 | 4.780 × 10–4 |
| 40× | 670.948 | 0.116 | 8.517 | 2.993 × 10–4 |
| 100× | 393.360 | 0.154 | 7.636 | 6.791 × 10–4 |
Contrast analysis mathematically represents the presence of impurities, such as granules or fibers on the film. A high difference of contrast between magnifications means a higher presence of impurities. The 4× magnification images showed the largest contrast value (755.734) and magnifications of 100× had lower values, namely 393.360. These differences in the values of the contrast can be explained by the presence of structures in the form of granules on the film, since the presence of fibres or granules leads to higher contrast, a measure that ultimately represents local differences in the image (Ke et al. 2010), that is, the film has some impurties in the form of granules. For the homogeneity index, the images at magnifications of 100× showed higher values, indicating that the film was more homogeneous at this magnification. The entropy index at a magnification of 4× showed the highest value (8.688), followed by magnifications of 40×, 20× and 10×. The lowest entropy was found at magnification 100×, indicating a lower disorder of the system for this case. Overall, the results for entropy and homogeneity indicate that the film is highly uniform with some granules in its surface, representing local variations, mainly due to the presence of quercetin crystals and aggregates.
The energy measures the textural uniformity of the image and is the opposite of entropy (Arzate-Vázquez et al. 2012). Thus, the higher the energy, the higher the uniformity of the image. Thus, the 100× magnification represented the structure of the film inside the granule (6.791 × 10–4) and had a higher uniformity. As expected, the GLCM data corroborate the observations made by optical microscopy, which are the film shows good homogeneity and has large local variations due to the presence of microscopic granules.
Figure 5 shows the SEM micrographs at two magnifications. The surface appears to be uniform, suggesting the formation of an ordered matrix. This observation agrees with LM analysis. Furthermore, Sang-Ho and co-workers (Bae et al. 2014) have also found uniform and smooth surfaces in gelatine/PVA blend films. In SEM, some quercetin crystals and aggregates were observed due to low solubility in water. All features observed in the SEM corroborate with the observations made with the LM.
Fig. 5.
SEM images of copolymeric film with incorporated quercetin. a (100 uM scale) and b (50 uM) scale
The antioxidant potentials of the simulant media after exposure to the active film are expressed as milligram of Trolox per gram of film (Table 2).
According to the results, it is clear that regardless of the analysis method, the antioxidant potential depends directly on the presence of ethanol in the simulant medium, where higher values were present for ethanol:water (95:5, v/v). This result demonstrates that ethanolic media was the system with greater quercetin extraction capacity when compared to other simulants. The antioxidant potential of 95% ethanol was 3 (DPPH, Acetic Acid:Water 3:97) to 35 (FRAP, Acetic Acid:Water 3:97) times higher than for other simulants. On the other hand, the antioxidant potential in an acidic simulant was the lowest. Acid media are very polar and it is known that quercetin is more active in an least polar medium (Cuvelier et al. 2000). This explains the results for the 95% ethanol when compared to the others.
After 24 h in the DPPH assay, the antioxidant power of the 95% ethanol medium increased (from 315.4 ± 8.2 to 308.8 ± 5.4 µmol TE/g) while the same power in another medium decreased (see Table 1). This can be explained by the differences in the kinetics of quercetin extraction in different systems. As the kinetics is highly dependent on the polarity of the system, on the chemical structure of the released compound and on the properties of polymeric matrix, the combination of lest polar system and weak chemical bonds between polymeric matrix and Quercetin can lead to faster quercetin release in such media. Similar results were found in other works (López-de-Dicastillo et al. 2012). In this work, the polymeric matrix was the same for all systems (gelatine-PVA copolymer), thus the increase of the antioxidant potential of the acid and alcoholic media was related to the lower quercetin extraction power of these media. In other words, the concentration of quercetin in those mediums increased slowly, while in the fatty simulant (ethanol:water 95:5), the extraction was rapid. This can be explained by the better affinity of quercetin to ethanol and structurally related organic solvents. Furthermore, the differences for the quercetin release rate as a function of the medium allows fine-tuning of it according to the pH or polarity of the media or food.
Conclusion
It can be concluded that gelatine/PVA copolymeric films incorporated with quercetin have good thermal, morphological and antioxidant properties. This makes potential applications possible in active packaging systems. Moreover, quercetin release ratio depends on the on the polarity and pH of the medium. This feature enables the development of antioxidant smart packaging that releases quercetin at a rate that depends on pH and polarity of food.
Acknowledgements
Dr. A. B. Siqueira and Dr. J. A Teixeira are thanked for the TG-DSC measurements. G.L.R.R. B. Vinhal acknowledges a grant from CAPES (Comissão de Aperfeiçoamento de Pessoal de Nível Superior), Finance Code #001.
Compliance with ethical standards
Conflict of interest
The authors declare no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Abràmoff MD, Magalhães PJ, Ram SJ. Image processing with imageJ. Biophotonics Int. 2004;11:36–42. [Google Scholar]
- Arzate-Vázquez I, Chanona-Pérez JJ, Calderón-Domínguez G. Microstructural characterisation of chitosan and alginate films by microscopy techniques and texture image analysis. CarbohydrPolym. 2012;87:289–299. doi: 10.1016/j.carbpol.2011.07.044. [DOI] [PubMed] [Google Scholar]
- Bae SH, Son SR, Kumar Sakar S. Evaluation of the potential anti-adhesion effect of the PVA/Gelatin membrane. J Biomed Mater Res Part B ApplBiomater. 2014;102:840–849. doi: 10.1002/jbm.b.33066. [DOI] [PubMed] [Google Scholar]
- Benzie IFF, Strain JJ. The ferric reducing ability of plasma (FRAP) as a measure of ‘“antioxidant power”’: The FRAP assay. Anal Biochem. 1996;239:70–76. doi: 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]
- Bitencourt CM, Fávaro-Trindade CS, Sobral PJA, Carvalho RA. Gelatin-based films additivated with curcuma ethanol extract: antioxidant activity and physical properties of films. Food Hydrocoll. 2014;40:145–152. doi: 10.1016/j.foodhyd.2014.02.014. [DOI] [Google Scholar]
- Brand-Williams W, Cuvelier ME, Berset C. Use of a free radical method to evaluate antioxidant activity. LWT Food SciTechnol. 1995;28:25–30. doi: 10.1016/S0023-6438(95)80008-5. [DOI] [Google Scholar]
- Cavalcanti OA, Van Den MG, Caramico-soares I, Kinget R. Polysaccharides as excipients for colon-specific coatings. permeability and swelling properties of casted films. Drug Dev Ind Pharm. 2002;28:157–164. doi: 10.1081/DDC-120002449. [DOI] [PubMed] [Google Scholar]
- Cuvelier M-E, Bondet V, Berset C. Behavior of phenolic antioxidants in a partitioned medium: structure—activity relationship. J Am Oil ChemSoc. 2000;77:819–824. doi: 10.1007/s11746-000-0131-4. [DOI] [Google Scholar]
- Giménez B, De LAL, Montero P. Release of active compounds from agar and agar-gelatin films with green tea extract. Food Hydrocoll. 2013;30:264–271. doi: 10.1016/j.foodhyd.2012.05.014. [DOI] [Google Scholar]
- Gómez-Estaca J, López-de-Dicastillo C, Hernández-Muñoz P. Advances in antioxidant active food packaging. Trends Food SciTechnol. 2014;35:42–51. doi: 10.1016/j.tifs.2013.10.008. [DOI] [Google Scholar]
- Han T, Lu L, Ge C. Development and properties of high density polyethylene (HDPE) and ethylene-vinyl acetate copolymer (EVA) blend antioxidant active packaging films containing quercetin. PackagTechnolSci. 2015;28:415–423. doi: 10.1002/pts.2114. [DOI] [Google Scholar]
- Ke G, Xu W, Yu W. Preparation and properties of drug-loaded chitosan–sodium alginate complex membrane. Int J Polym Mater. 2010;59:184–191. doi: 10.1080/00914030903231332. [DOI] [Google Scholar]
- Li J-H, Miao J, Wu J-L. Preparation and characterisation of active gelatin-based films incorporated with natural antioxidants. Food Hydrocoll. 2014;37:166–173. doi: 10.1016/j.foodhyd.2013.10.015. [DOI] [Google Scholar]
- Lin W-Y, Fang X-Z, Huang X-C, Dian L, Liu XF. A fast block mode selection approach for H.264 visual coding. Front Electr Electron Eng China. 2006;1:431–436. doi: 10.1007/s11460-006-0082-4. [DOI] [Google Scholar]
- Liu F, Antoniou J, Li Y, et al. Preparation of gelatin films incorporated with tea polyphenol nanoparticles for enhancing controlled-release antioxidant properties. J Agric Food Chem. 2015;63:3987–3995. doi: 10.1021/acs.jafc.5b00003. [DOI] [PubMed] [Google Scholar]
- López-de-Dicastillo C, Nerín C, Alfaro P, et al. Development of new antioxidant active packaging films based on ethylene vinyl alcohol copolymer (EVOH) and green tea extract. J Agric Food Chem. 2011;59:7832–7840. doi: 10.1021/jf201246g. [DOI] [PubMed] [Google Scholar]
- López-de-Dicastillo C, Gómez-Estaca J, Catalá R, et al. Active antioxidant packaging films: development and effect on lipid stability of brined sardines. Food Chem. 2012;131:1376–1384. doi: 10.1016/j.foodchem.2011.10.002. [DOI] [Google Scholar]
- Lorenzo JM, Batlle R, Gómez M. Extension of the shelf-life of foal meat with two antioxidant active packaging systems. LWT Food SciTechnol. 2014;59:181–188. doi: 10.1016/j.lwt.2014.04.061. [DOI] [Google Scholar]
- Medina-Jaramillo C, Ochoa-Yepes O, Bernal C, Famá L. Active and smart biodegradable packaging based on starch and natural extracts. CarbohydrPolym. 2017;176:187–194. doi: 10.1016/j.carbpol.2017.08.079. [DOI] [PubMed] [Google Scholar]
- Muriel-Galet V, Cran MJ, Bigger SW. Antioxidant and antimicrobial properties of ethylene vinyl alcohol copolymer films based on the release of oregano essential oil and green tea extract components. J Food Eng. 2015;149:9–16. doi: 10.1016/j.jfoodeng.2014.10.007. [DOI] [Google Scholar]
- Nisa I, Ashwar BA, Shah A. Development of potato starch based active packaging films loaded with antioxidants and its effect on shelf life of beef. J Food SciTechnol. 2015;52:7245–7253. doi: 10.1007/s13197-015-1859-3. [DOI] [Google Scholar]
- Re R, Pellegrini N, Proteggente A. Antioxidant activity applying an improved ABTS radical cationdecolorization assay. Free RadicBiol Med. 1999;26:1231–1237. doi: 10.1016/S0891-5849(98)00315-3. [DOI] [PubMed] [Google Scholar]
- Russo M, Spagnuolo C, Tedesco I. The flavonoid quercetin in disease prevention and therapy: facts and fancies. BiochemPharmacol. 2012;83:6–15. doi: 10.1016/j.bcp.2011.08.010. [DOI] [PubMed] [Google Scholar]
- Shutava TG, Balkundi SS, Vangala P. Layer-by-layer-coated gelatin nanoparticles as a vehicle for delivery of natural polyphenols. ACS Nano. 2009;3:1877–1885. doi: 10.1021/nn900451a. [DOI] [PubMed] [Google Scholar]
- Silva-Pereira MC, Teixeira JA, Pereira-Júnior VA, Stefani R. Chitosan/corn starch blend films with extract from Brassica oleraceae (red cabbage) as a visual indicator of fish deterioration. LWT Food SciTechnol. 2015 doi: 10.1016/j.lwt.2014.11.041. [DOI] [Google Scholar]
- Singh D, Wahl C, Heinhorst S, Morgan SE. Polyvinyl alcohol-gelatin antimicrobial hydrogels. PolymPrepr. 2010;51:663–664. [Google Scholar]
- Souza MP, Vaz AFM, Silva HD. Development and characterization of an active chitosan-based film containing quercetin. Food Bioprocess Technol. 2015;8:2183–2191. doi: 10.1007/s11947-015-1580-2. [DOI] [Google Scholar]
- Teixeira B, Marques A, Pires C. Characterisation of fish protein films incorporated with essential oils of clove, garlic and origanum: physical, antioxidant and antibacterial properties. LWT Food SciTechnol. 2014;59:533–539. doi: 10.1016/j.lwt.2014.04.024. [DOI] [Google Scholar]
- UNE-EN. 1186-3 (2002) Materials and articles in contact with food-stuffs. Plastics. Part 3: Test methods for overall migration into aqueous food simulants by total immersion. 10.3403/02570334U
- Valdés A, Mellinas AC, Ramos M. Use of herbs, spices and their bioactive compounds in active food packaging. RSC Adv. 2015;5:40324–40335. doi: 10.1039/C4RA17286H. [DOI] [Google Scholar]
- Vásconez MB, Flores SK, Campos CA. Antimicrobial activity and physical properties of chitosan–tapioca starch based edible films and coatings. Food Res Int. 2009;42:762–769. doi: 10.1016/j.foodres.2009.02.026. [DOI] [Google Scholar]
- Xiping G, Keyong T, Jie L. Compatibility and properties of biodegradable blend films with gelatin and poly (vinyl alcohol) J Wuhan UnivTechnolSci Ed. 2014;29:351–356. doi: 10.1007/s11595-014-0920-9. [DOI] [Google Scholar]
- Yadav S, Mehrotra GK, Bhartiya P. Preparation, physicochemical and biological evaluation of quercetin based chitosan-gelatin film for food packaging. CarbohydrPolym. 2020;227:115348. doi: 10.1016/J.CARBPOL.2019.115348. [DOI] [PubMed] [Google Scholar]
- Zhang N, Liu X, Yu L. Phase composition and interface of starch–gelatin blends studied by synchrotron FTIR micro-spectroscopy. CarbohydrPolym. 2013;95:649–653. doi: 10.1016/j.carbpol.2013.03.045. [DOI] [PubMed] [Google Scholar]
- Zhou H, Sun X, Zhang L. Fabrication of biopolymeric complex coacervation core micelles for efficient tea polyphenol delivery via a green process. Langmuir. 2012;28:14553–14561. doi: 10.1021/la303062j. [DOI] [PubMed] [Google Scholar]