Abstract
This study was aimed to evaluate the effects of using different levels of olive (Olea europaea) leaf extract on fresh and preserved mutton meatballs. Meatballs were divided into four different groups and treated as T0 (0), T1 (0.1), T2 (0.2) and T3 (0.3%), respectively based on olive leaf extract supplementation. Days of intervals of experiment were 0, 5, 10 days. Samples were preserved at 4˚C for up to 10 days. Different types of analysis such as, sensory (color, flavor, juiciness and overall acceptability), proximate composition (CP %), physicochemical (pH), biochemical (POV, FFA and TBARS) and microbiological (TVC, TCC and TYMC) were determined. Color, flavor and acceptability reduced significantly (p < 0.05) with the increase of the storage periods. Values of the studied quality parameters in all the treatment groups differed significantly (p < 0.05). Based on our findings 0.3% olive leaf extract is found suitable to add in mutton meatballs as a source of natural antioxidant.
Keywords: Antioxidant, Olive leaf extract, Mutton meatball, Refrigerated storage, Qualities
Introduction
Short shelf life caused by chemical or microbial changes is a great concern for the meat industry. The most ordinary form of deterioration in meat caused by chemical change is the oxidation of lipid and heme-containing proteins. Oxidation in meat results in a rancid taste, off flavors, texture and color changes, which adversely affect consumer acceptability and limits the meat shelf life, causing issues in marketing and distribution. Antioxidants can be applied in meat processing to preserve meat quality and extend its storage time. Nutritional and functional properties of antioxidants can be utilized for prevention or reduction of cancer, oxidative stress, arteriosclerosis and aging process. These antioxidants can be extracted from many natural sources through ultrafiltration and nano filtration techniques (Galanakis 2015).
Even low concentrations of antioxidants (ppm) added to meat products can slow down the oxidation of meat lipids and proteins, therefore increasing the storage time of meat by saving them from chemical oxidation (Karre et al. 2013). Both commercial synthetic and natural antioxidants could control or delay the oxidation in meats. With the increasing focus on organic foods, food safety and consumer’s health, more natural sources that show high antioxidant activity and can be applied to meat processing are being explored. Many plant-derived sources that have been suggested to contain a high level of antioxidant ingredients to inhibit oxidative deterioration have been studied, such as bearberry, cranberry, grape seed extract, pine bark extract, plum, pomegranate, rosemary, and oregano (Karre et al. 2013).
Now-a-days the use of novel bioactive components like glucosinolates and isothiocyanates as natural antimicrobials and anti-carcinogenic agents is increasing due to their healthy properties in food industry (Deng et al. 2015). However, only a few can be used in meat processing due to each natural additive needing to gain federal approval first. A manufacturer or other sponsor may be required to petition UDSA with evidence for certain natural additives to document their safety when used in meat products. The FSIS, a department within the UDSA, must then approve additives by inspecting safety, technical function, and conditions of use for each additive that take into consideration of the unique characteristics of meat and poultry products. In addition, some state laws also regulate the usage of antioxidants for meat processing (Mikova 2001).
Different types of preservatives are used to preserve meat and meat products in our country. Salt is the mostly used preservative because of its low price, availability and having different types of properties. It has a preservative and antimicrobial effect as a direct consequence of the capacity of sodium chloride to reduce water activity values. Antioxidants are also added to fresh and processed meat and meat products to prevent lipid oxidation, retard the development of off-flavors, and improve color stability. Two types of anti-oxidants are used in food industry, one is natural and another is synthetic (Matthews and Strong 2005).
Synthetic antioxidants have been confirmed for their toxicological and carcinogenic effects. Awareness about the harmful effects of these chemicals in food is increasing. Meanwhile, natural preservatives offer greater advantages due to their non-toxic nature along with a wide range of health benefits. Olive phenols have the potential to be utilized in meat and meat products due to its ability to provide multidimensional approaches such as color retention, reduction of microbial growth, slowing down of fat oxidation and ultimate extension of shelf life (Galanakis 2018). Due to the antioxidant properties, polyphenols from plant sources are used for food preservation. This natural source of antioxidants will also help to prevent oxidative stress and related diseases like cancer. In the last few years, the identification and development of phenolic compounds or extracts from different plants has become a major area of health- and medical-related research. Different poly-phenolic compounds like oleuropein in olive leaves’ extract have anti-microbial and anti-oxidant properties (Sikora et al. 2008). The most abundant phenolic component is oleuropein which gives the bitter taste to olive and olive oil. Olive leave extracts have been associated with health benefits and preservation of food rich in unsaturated fats (Sikora et al. 2008). Different bio-phenols like verbascoside, tyrosol or hydroxytyrosol, oleuropein, ligostroside etc. are abundant in olive leaves. These compounds have shown several biological activities such as antioxidant and antimicrobial, and consequently can be used in food application (Al-Rimawi et al. 2014). No studies have yet been done on the effect of olive leaf extracts on mutton as a source of antioxidants in Bangladesh. Having the above views in mind the present study will be undertaken to study the incorporation of different levels of olive leaf extract as a natural antioxidant in mutton meatballs.
Materials and methods
Sample preparation for different treatments
About 2 kg of fresh mutton was taken for the preparation of the mutton meatballs. First the mutton was cleaned with tap water and then the excess fat was removed by knife. After that the mutton meat was ground and then the ground meat was mixed with different types of native spices, salt, oil, ice flakes, 5% corn flower, and sauce according to the experimental design. There were four treatment groups. These were treated by 0%, 0.1%, 0.2% and 0.3% olive leaves extract as T0, T1, T2 and T3 respectively. Then mutton meatballs of proper shape were prepared separately. After that the meatballs were boiled in water at 100ºC for 2–3 min. Then the water was removed from the meatball and then they were fried in vegetable oil until they become reddish brown in color. All the meatballs were weighing 25 g. These samples of different treatments were used for sensory, physicochemical, biochemical and microbiological analyses.
Experimental procedure
To characterize the quality of the different treatment groups at the different storage periods, sensory, physicochemical, biochemical and microbiological analyses were conducted for each treatment group at 0, 5th, 10th days interval. Each treatment had three replications. The meat ball preparation and all the analyses were performed at the Animal Science Laboratory, Bangladesh Agricultural University, Mymensingh.
Sensory evaluation
Sensory qualities of the meatballs were judged by a trained 6-member panel at 0 day and repeated at 5th days and 10th days of storage. The different state of flavor, color, tenderness, juiciness and acceptability was measured in a 5-point scale (weak to strong). The judges evaluated the samples based on the above criterions. Panelists were selected among department staff and students and trained according to the American Meat Science Association guidelines. Sensory evaluation was carried out in individual booths under controlled conditions of light, temperature and humidity. Panelists had to be present in an orientation program to become familiarized with the sensory quality scale. Sensory scores were 5 for excellent, 4 for very good, 3 for good, 2 for fair and 1 for poor (AMSA 1995). All samples were served in Petri dishes.
Physicochemical analysis
Proximate composition
Crude protein (CP) was carried out according to the micro kjeldahl method, following the protocol described by Kratchanova et al. (2004). For each sample CP was determined for three times and the mean value was shown.
pH measurement
The pH values of cooked meatballs were measured using a digital pH meter (CON60; Trans-Wiggens) from a homogenate. A mixture of 5 g blended meatball and 10 ml distilled water was used for homogenate preparation (AACC 1983).
Biochemical analysis
Free fatty acid (FFA), peroxide value (POV), thiobarbituric acid value (TBARS) were analyzed to know the biochemical properties of the meatball. The FFA value and the peroxide value (POV) were determined according to Sallam et al. (2004). Thiobarbituric acid value (TBARS) was assessed by the method described by Schmedes and Holmer (1989).
Microbial assessment
Total viable count (TVC), total coliform count (TCC) and total yeast-mould count (TYMC) were measured for microbial quality assessment. Ten g of the mutton meatball sample were aseptically excised from the stored stock sample. Each of the stored mutton meatball samples was thoroughly and uniformly macerated in a mechanical blender using a sterile diluent (0.1% peptone water) as per recommendation of the International Organization for Standardization (ISO 1995). 10 g minced meatball with 90 ml of 1% peptone water were taken in a sterile container. A homogenized suspension was made in a sterile blender. Thus a 1:10, w/v dilution of the samples was obtained. Later on, using a whirly mixture machine serial dilutions ranging from 10–2 to 10–6 were prepared according to the instruction of the standard method (ISO 1995). The media employed for these bacteriological analysis included plate count agar (PCA) for total viable count, Macconkey agar (MA) for total coliform count and potato dextrose agar (PDA) for total yeast mold count. The media were prepared according to the guidelines of the manufacturer. After inoculation, the PCA and MA media were incubated at 35 °C for 24–48 h and the PDA agar at 25 °C for 48–72 h. The visual as well as colony counter was used for counting the incubated cultured media. The microbiological count was expressed as log10 CFU/g.
Statistical model and analysis
For statistical analyses, a factorial experiment was conducted with two factors—A (treatments) and B (days of intervals). The model is shown below
where yijk = observation k in level i of factor A and level j of factor B, μ = the overall mean, Ai = the effect of level i of factor A, Bj = the effect of level j of factor B.
Data were statistically analyzed using SAS Statistical Discovery software, NC, USA. DMRT test was used to determine the significance of differences among treatments’ means.
Results and discussion
Sensory evaluation
Olive leaves’ extracts were used on mutton meatballs as a source of natural antioxidant. The color, flavor, juiciness and overall acceptability score of different treatments with days’ intervals were shown in Table 1. The score for color at different treatments ranged from 4.18 to 4.88. Among the four treatments the most preferable color was observed from T3 whereas the control group showed the less preferred color. The most preferable color was observed from 0 day and the less preferable color at the 10th day. The lowest color score was found to be 4.45 at 10 days of storage irrespective of the treatment groups. A similar observation was reported by Singh et al. (2011). There were significant differences (p > 0.05) for the flavor of all the treatments. The most preferable flavor was observed in the T3 group and the lowest flavor from the T0 group. With the increasing of storage time, the flavor from treatments decreased gradually. Raghavan et al. (2007) found similar results of reduction of flavor quality with the increase of storage time because storage time negatively affect sensory attributes such as color, texture, and flavor as well as the nutritional quality of the product. For the antioxidant and antimicrobial properties of Ocimum leaf, it is incorporated in different food items. A decline in flavor scores during a long storage period was observed by Zargar et al. (2014). From the experiment, a significant difference (p > 0.05) was found for the juiciness of all treatments. Among these four treatments, the most preferable juiciness score was observed for the T3 group. A significant (p < 0.05) variation for juiciness was also found among these days of observation. The most preferable tenderness was observed from 0 day and the less preferable tenderness from the 10th day. There were significant differences (p > 0.05) of overall acceptability for all the treatments. Among these four treatments the most preferable overall acceptability was observed for the T3 group and the less preferable overall acceptability was observed for the control and the T0 group. The different superscripts were observed from the 0, 5th and 10th days of observation, which indicates that there were significant differences (p < 0.05) between those days of observation. The data show that the quality of the meatball deteriorated with time. Decrease in overall acceptability scores was for the reason of decrease in sensory qualities of the meatballs. Chacko and Patterson (2011) reported a similar type of findings for octopus meatballs which was also reported for goat meat by Agnihotri et al. (2006). Olive leave extract is found to increase the sensory value of meat products which was undervalued previously. These kinds of extracts can be used in the food production line and have potential to be used for future research as mentioned by Galanakis (2013).
Table 1.
Effect of olive on sensory quality in mutton meatballs
| Parameters | DI | Treatments | Mean | Level of significance | |||||
|---|---|---|---|---|---|---|---|---|---|
| T0 | T1 | T2 | T3 | Treat | DI | T × DI | |||
| Color | 0 | 4.94 ± 0.0088 | 4.90 ± 0.0057 | 4.91 ± 0.0057 | 4.92 ± 0.0033 | 4.91a ± 0.01 | < 0.05 | < 0.05 | < 0.05 |
| 5 | 4.31 ± 0.0088 | 4.87 ± 0.003 | 4.88 ± 0.0057 | 4.90 ± 0.0057 | 4.74b ± 0.31 | ||||
| 10 | 3.31 ± 0.0115 | 4.84 ± 0.0033 | 4.84 ± 0.0057 | 4.87 ± 0.0057 | 4.46c ± 0.80 | ||||
| Mean | 4.19c ± 0.83 | 4.87b ± 0.03 | 4.87b ± 0.02 | 4.89a ± 0.02 | |||||
| Odor | 0 | 4.50 ± 0.0057 | 4.47 ± 0.0057 | 4.42 ± 0.0033 | 4.44 ± 0.0057 | 4.45a ± 0.03 | < 0.05 | < 0.05 | < 0.05 |
| 5 | 4.33 ± 0.0000 | 4.35 ± 0.0057 | 4.38 ± 0.0057 | 4.35 ± 0.0057 | 4.35b ± 0.03 | ||||
| 10 | 3.50 ± 0.0057 | 4.09 ± 0.0057 | 4.06 ± 0.0033 | 4.03 ± 0.0057 | 3.92c ± 0.27 | ||||
| Mean | 4.11d ± 0.54 | 4.30c ± 0.22 | 4.28b ± 0.22 | 4.27a ± 0.22 | |||||
| Juiciness | 0 | 4.57 ± 0.0057 | 4.50 ± 0.0057 | 4.50 ± 0.0033 | 4.52 ± 0.0057 | 4.52a ± 0.01 | < 0.05 | < 0.05 | < 0.05 |
| 5 | 4.51 ± 0.0057 | 4.39 ± 0.0057 | 4.30 ± 0.0057 | 4.41 ± 0.0057 | 4.40b ± 0.07 | ||||
| 10 | 3.71 ± 0.0057 | 4.29 ± 0.0057 | 4.27 ± 0.0057 | 4.30 ± 0.0057 | 4.14c ± 0.29 | ||||
| Mean | 4.26d ± 0.48 | 4.39b ± 0.12 | 4.35c ± 0.14 | 4.41a ± 0.12 | |||||
| Overall acceptability | 0 | 4.67 ± 0.0057 | 4.45 ± 0.0057 | 4.45 ± 0.0033 | 4.47 ± 0.0057 | 4.51a ± 0.09 | < 0.05 | < 0.05 | < 0.05 |
| 5 | 4.50 ± 0.0057 | 4.30 ± 0.0057 | 4.30 ± 0.0057 | 4.35 ± 0.0057 | 4.36b ± 0.07 | ||||
| 10 | 3.33 ± 0.0057 | 4.25 ± 0.0057 | 4.27 ± 0.0057 | 4.29 ± 0.0057 | 4.03c ± 0.49 | ||||
| Mean | 4.17c ± 0.73 | 4.33b ± 0.11 | 4.34b ± 0.10 | 4.40a ± 0.08 | |||||
Sensory scores were 5 for excellent, 4 for very good, 3 for good, 2 for fair, and 1 for poor. Mean in each row having different superscript varies significantly at values *P < 0.05. Again, mean values having same superscript in each row did not differ significantly at P > 0.05. T0 = 0% olive leaves extract, T1 = 0.1% olive leaves extract, T2 = 0.2% olive leaves extract, T3 = 0.3% olive leaves extract
DI days of intervals, Treat treatment, T*DI interaction of treatment and day intervals
Change in physiochemical properties
Crude protein (CP)
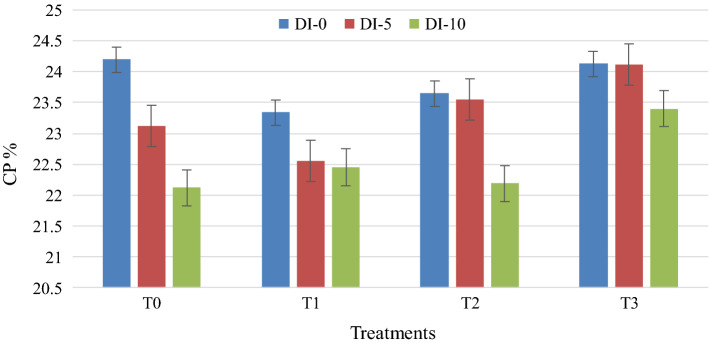
The CP content of the different treatments with days intervals are shown in Fig. 1. CP content of different treatments ranged from 23.34 to 24.13%. There were significant differences (p < 0.05) of CP content among these treatments. The CP content at different days of storage ranged from 24.01 to 22.88%. The different superscript was observed at 0, 5th, and 10th days, which indicates that there were significant differences (p < 0.05) of CP content among these days. Consumers always prefer the highest CP content of products. The most preferable CP content was observed at 0 days. The less preferable CP content was observed at 10 days. The protein result was higher compared to the protein content of Indonesian Mutton Meatballs, where the range was 13.38 to 14.44% (Purnomo and Rahardiyan 2008). The reason for retaining a higher CP content may be the utilization of the pressurized hot water extraction method which is similar to Kovačević et al. (2018). Traditional koefte meatballs showed higher protein content (25.51%). Koefte meatballs prepared with different levels of fat and flour also showed a higher protein content, ranged from 16.1 to 19.58% (Serdaroglu et al. 2005). Pork meatballs were also reported to have higher a protein content, ranged from 17.30 to 19.26% mentioned by Huang et al. (2005). Traditional Taiwan meatballs, called Kung-Wang showed a broad range of protein content, ranging from 12 to 22%, as reported by Hsu and Yu (1999). The antioxidant property of the olive leave extract was similar to the property of white cabbage outer leaves which was used on sponge cake quality by Prokopov et al. (2015) to retain the percentage of protein.
Fig. 1.
Effect of olive extract on CP in mutton meatballs (DI days interval; T treatments)
pH of cooked meat
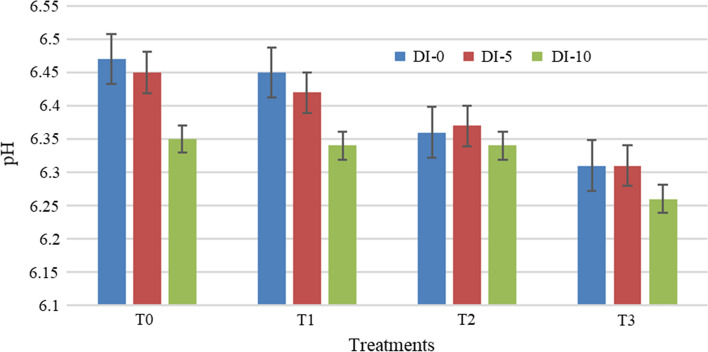
The pH of the cooked meat from the different treatments with day’s intervals is shown in Fig. 2. The pH range found for the different treatments was from 6.29 to 6.42. There were significant differences (p < 0.05) of pH among these treatments. An increase in the pH value as well as a decrease in the acidity value was found for the different samples. For different days’ intervals, the pH ranged from 6.32 to 6.40. The different superscript was observed from 0, 5th and 10th days of observation which indicates significant (p < 0.05) differences among these observations. The most preferable pH value was found at 0 day and the less preferred pH was found at the 10th day of storage. This may be due to the properties of olive extract at initial stage which is dependent on concentration and temperature. Similar properties were also found in pink guava extraction studies by Nagarajan et al. (2019).
Fig. 2.
Effect of olive leaves extract on pH in mutton meatballs (DI days interval; T treatments)
Biochemical properties
Free fatty acid value (FFA %)
The free fatty acid value (FFA %) of different treatment levels with day intervals is shown in Table 2. The FFA value for the different treatment levels ranged from 0.33 to 0.40%. The different superscripts indicated the significant variation (p < 0.05) among the different treatment groups. The most preferable value was observed from T3. The range of FFA at different days of intervals was from 0.33 to 0.37%. The different superscripts observed at 0, 5th and 10th days indicates the significant variation (p < 0.05). With prolonged storage time, the FFA value increased. The most preferable FFA was observed for the 0 day and the less preferable FFA was observed for the 10th day of observation. The significant (p < 0.05) increase in FFA content of the products during storage might be due to the growth of lipolytic microorganisms and an increased FFA value in fermented sausage for different storage periods after adding baechu-kimchi and kimchi-powder as an antioxidant source (Das et al. 2012). Overall, the free fatty acids level was not very high even in the 10th day of storage. The reason may be the use of non-conventional extraction technologies like the use of hot water extraction that retained the antioxidant properties. A similar result was found by Barba et al. (2015).
Table 2.
Effect of olive leaves extract on bio-chemical parameters in mutton meatballs
| Parameters | DI | Treatments | Mean | Level of significance | |||||
|---|---|---|---|---|---|---|---|---|---|
| T0 | T1 | T2 | T3 | Treat | DI | T × DI | |||
| TBARS (mg-MA/kg) | 0 | 0.45 ± 0.02 | 0.39 ± 0.03 | 0.37 ± 0.02 | 0.36 ± 0.02 | 0.39c ± 0.03 | < 0.05 | < 0.05 | < 0.05 |
| 5 | 0.48 ± 0.05 | 0.41 ± 0.05 | 0.39 ± 0.05 | 0.38 ± 0.05 | 0.42b ± 0.03 | ||||
| 10 | 0.57 ± 0.03 | 0.48 ± 0.02 | 0.46 ± 0.01 | 0.43 ± 0.03 | 0.49a ± 0.05 | ||||
| Mean | 0.50a ± 0.06 | 0.43b ± 0.05 | 0.41c ± 0.04 | 0.39c ± 0.04 | |||||
| POV (meq/kg) | 0 | 4.02 ± 0.03 | 3.95 ± 0.01 | 3.94 ± 0.04 | 3.88 ± 0.03 | 3.95c ± 0.05 | < 0.05 | < 0.05 | < 0.05 |
| 5 | 4.33 ± 0.04 | 4.04 ± 0.01 | 3.98 ± 0.05 | 3.96 ± 0.06 | 4.08b ± 0.16 | ||||
| 10 | 4.56 ± 0.03 | 4.14 ± 0.02 | 4.08 ± 0.02 | 4.01 ± 0.03 | 4.22a ± 0.25 | ||||
| Mean | 4.30a ± 0.21 | 4.04b ± 0.04 | 4.00c ± 0.03 | 3.98d ± 0.04 | |||||
| FFA (%) | 0 | 0.37 ± 0.01 | 0.33 ± 0.01 | 0.31 ± 0.03 | 0.3 ± 0.01 | 0.33c ± 0.01 | < 0.05 | < 0.05 | < 0.05 |
| 5 | 0.40 ± 0.03 | 0.35 ± 0.02 | 0.34 ± 0.01 | 0.33 ± 0.03 | 0.36b ± 0.01 | ||||
| 10 | 0.42 ± 0.04 | 0.36 ± 0.04 | 0.36 ± 0.02 | 0.35 ± 0.05 | 0.37a ± 0.02 | ||||
| Mean | 0.40a ± 0.01 | 0.35b ± 0.01 | 0.34c ± 0.03 | 0.33c ± 0.02 | |||||
Mean in each row having different superscript varies significantly at values P < 0.05. T0 = 0% olive leaves extract, T1 = 0.1% olive leaves extract, T2 = 0.2% olive leaves extract, T3 = 0.3% olive leaves extract
DI day intervals, Treat treatment, T*DI interaction of treatment and day intervals, FFA free fatty acid, POV per oxide value, TBARS thiobarbituric acid reactive substances
Peroxide value (POV-meq/kg)
The peroxide value (POV) of the different treatment groups ranged from 4.30 to 3.98. For the different storage periods, the control groups had a higher POV than the others. As shown in Table 2, the higher anti-oxidative effect on POV came from the T0 group. The different superscripts that were observed for the treatment groups indicate that there were significant differences (p < 0.05) of peroxide value among these treatments. Among these four treatment group most preferable POV was observed for the T3 group. For the consumer, it is always desired to have low POV for food items. The range of POV at different days of interval was from 3.95 to 4.22. The different superscripts observed at 0, 5th and 10th days showed the significant variations (p < 0.05). For prolonged storage periods, all treatments showed high POV. Some studies showed increased POV in food products for storage period with the absence or presence of antioxidants. Generally, antioxidants reduce POV in food items for different storage conditions. High POV in sausage (salame) with a long storage period was reported by Novelli et al. (1998). They found 4.20, 4.02 and 1.67 meq O2/kg fat for refrigerated storage without antioxidant at 3, 1 and 0 month respectively. At 0, 30, 60, 90, 120, 150 and 180 days of frozen storage for rosemary treated sheep meat burger the POV was 0.24, 0.45, 0.66, 1.05, 1.27, 1.46 and 1.59 meq O2/kg fat respectively. The lower POV may be due to the polyphenols present in the olive leave extract, which was also stated by Galanakis et al. (2018a).
Thiobarbituric acid value (TBARS)
Table 2 shows the TBARS values for the different treatment groups. The TBARS value for different treatment groups ranged from 0.50 to 0.39. Different superscripts were observed for the treatment groups, which indicates that there were significant differences (p < 0.05) of peroxide value among these treatments. Among these four treatments, the most preferable TBARS value was found for T3 group. For the health of consumers, a low TRARS value should be present in food items. For different days intervals, TBARS value ranged from 0.39 to 0.49. The different superscript was observed for the 0, 5th and 10th days of observation, which indicates that there were significant differences (p < 0.05) among these observations. Significantly higher (p < 0.05) TBARS value was found in all the treatments for the long storage period. Similar findings were reported by Nassu et al. (2003) in goat meat sausage during refrigerated storage.
Microbiological assessment
Total viable count (TVC)
The TVC value of the different treatment levels with different days of intervals is shown in Table 3. TVC for mutton meatballs ranged from 4.40 to 5.01 (log10 CFU/g), at different treatment levels. For the TVC values, no significant differences were found among the treatments. The plate count in the T0 group (5.01 log CFU/g) was significantly higher for the treated samples. A low TVC value is always desired for consumer’s health. At different days’ intervals, the TVC value ranged from 4.53 to 4.66. A different superscript was observed from 0, 5th, and 10th days intervals, which indicates there were significant differences (p < 0.05) of TVC values. The amount of TVC was increased with the storage periods. At different storage period, significantly lower TVC and pathogenic microorganisms were observed when diallyl disulfide and diallyl sulfide treatments were used. However, it is also possible that the effectiveness of the antimicrobial extracts could be reduced due to physical interactions with the food matrix (Fernandez et al. 2005). Galanakis et al. (2018b) found a reduction of the microbial growth in bakery products due to the presence of polyphenols recovered from olive mill wastewater. The lower TVC levels were found in different stages of meatball, which may be due to the presence of polyphenols in the olive leaf extract.
Table 3.
Effect of olive leaves extract on microbial quality in mutton meatballs
| Parameters | DI | Treatments | Level of significance | ||||||
|---|---|---|---|---|---|---|---|---|---|
| T0 | T1 | T2 | T3 | Mean | Treat | DI | T × DI | ||
| TCC (log CFU/g) | 0 | 1.23 ± 0.02 | 1.16 ± 0.01 | 1.14 ± 0.03 | 1.13 ± 0.03 | 1.17a ± 0.04 | < 0.05 | < 0.05 | < 0.05 |
| 5 | 1.3 ± 0.03 | 1.14 ± 0.04 | 1.01 ± 0.05 | 1.08 ± 0.02 | 1.16b ± 0.06 | ||||
| 10 | 1.34 ± 0.00 | 1.01 ± 0.04 | 1.9 ± 0.04 | 1.04 ± 0.02 | 1.14c ± 0.09 | ||||
| Mean | 1.29a ± 0.04 | 1.13b ± 0.04 | 1.11c ± 0.02 | 1.08d ± 0.05 | |||||
| TYMC (log CFU/g) | 0 | 1.97 ± 0.04 | 1.82 ± 0.01 | 1.77 ± 0.06 | 1.77 ± 0.03 | 1.83a ± 0.05 | < 0.05 | < 0.05 | < 0.05 |
| 5 | 2.02 ± 0.02 | 1.73 ± 0.04 | 1.74 ± 0.04 | 1.73 ± 0.05 | 1.81b ± 0.10 | ||||
| 10 | 2.13 ± 0.02 | 1.74 ± 0.04 | 1.72 ± 0.02 | 1.71 ± 0.02 | 1.83c ± 0.20 | ||||
| Mean | 2.04a ± 0.06 | 1.76b ± 0.02 | 1.74c ± 0.05 | 1.74c ± 0.04 | |||||
| TVC (log CFU/g) | 0 | 4.64 ± 0.03 | 4.51 ± 0.02 | 4.5 ± 0.02 | 4.46 ± 0.08 | 4.53a ± 0.04 | < 0.05 | < 0.05 | < 0.05 |
| 5 | 4.94 ± 0.02 | 4.45 ± 0.04 | 4.43 ± 0.03 | 4.40 ± 0.04 | 4.56b ± 0.31 | ||||
| 10 | 5.44 ± 0.02 | 4.44 ± 0.03 | 4.42 ± 0.03 | 4.35 ± 0.06 | 4.67c ± 0.52 | ||||
| Mean | 5.01a ± 0.09 | 4.47b ± 0.03 | 4.45c ± 0.04 | 4.41d ± 0.03 | |||||
Mean in each row having different superscript varies significantly at values P < 0.05. T0 = 0% olive leaves extract, T1 = 0.1% olive leaves extract, T2 = 0.2% olive leaves extract, T3 = 0.3% olive leaves extract
DI day intervals, Treat treatment, T*DI interaction of treatment and day intervals, TVC total viable count, TCC total coliform count, TYMC total yeast-mold count
Total coliform count (TCC)
The TCC value of different treatment levels with different days of intervals is shown in Table 3. The total coliform count for different treatment groups ranged from 1.08 to 1.29 (log CFU/g). A significant variation (p < 0.05) of TCC values is indicated by dissimilar superscripts for the treatment groups. The highest TCC was found for the control group (1.29 log CFU/g). The most preferable TCC content was observed for the T3 group. The less amount of TCC is expected for food safety of consumers. For different days of storage, the TCC value ranged from 1.14 to 1.17. During storage, the TCC value decreased. The different superscripts observed from 0, 5th and 10th days of observation indicated a significant variation (p < 0.05) among the observations. Reducing the auto-oxidation and fat metabolism, antioxidants prevent the fat from deterioration. Therefore, a low bacterial growth was found in mutton meatballs treated with antioxidants. For lamb meat preservation, antioxidant active packages were used in an experiment (Camo et al. 2008). In this experiment, oregano active film, rosemary extract and rosemary active film were used at a higher level of oxygen and the meat was preserved under illumination for 0, 5, 8, 11, and 13 days at 11 °C. They reported a gradual reduction of TCC with the storage period.
Total yeast-mold count (TYMC)
Table 3 shows the TYMC value for the treatment groups as well as for the day intervals of storage period. TYMC for different treatments ranged from 1.74 to 2.04 (log CFU/g). A significant variation (p < 0.05) for TYMC in treatment groups were indicated by dissimilar superscripts. The highest TYMC was found for the control group (2.04 log CFU/g). For the significance of food borne diseases, always low TYMC is desired in food items. The TYMC ranged from 1.81 to 1.83 for the interval of storage. During storage the TYMC value decreased. A significant variation (p < 0.05) in days of storage was indicated by the different superscripts. A report on the antimicrobial effect of dichloromethane root extract of C. caudatus showed that it inhibits the growth of Candida albicans and Cladosporium cucumerinum on thin layer chromatography. No yeast or mold was found in cooked mutton meatballs when an antimicrobial component was used. The antimicrobial activity of essential oils was investigated and found to prevent the spoilage and pathogenic organisms in meat (Fernandez et al. 2005).
The results obtained showed that the quality of mutton meatballs could be preserved for extended days with the use of an olive leaf extract. Other than meatball, olive leaf extract can also be used for the preservation of other food products like meat, milk, and milk products due to the presence of phenols in it. Also, it may have some diversified use as Ultra-violet booster in cosmetics (Galanakis et al. 2018c). The freshness, sensory, physicochemical, biochemical and microbial properties of these meatballs are highly linked with the anti-oxidant properties of the olive leaf.
Acknowledgements
This study was funded by Ministry of Science and Technology, Bangladesh (Project no.: 2018/111/MoST) and Bangladesh Agricultural University Research System (Project no.: 2017/48/BAU)
Compliance with ethical standards
Conflict of interest
No potential conflict of interest was reported by the authors.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Saju Ahmed Rubel and Z. N. Yu share co-first author.
Contributor Information
Saju Ahmed Rubel, Email: rubel.13bau@gmail.com.
Z. N. Yu, Email: ahnuo123@163.com
H. M. Murshed, Email: hasan.murshed@bau.edu.bd
S. M. Ariful Islam, Email: arifbau95@gmail.com.
Dalia Sultana, Email: daliasultana2019@yahoo.com.
S. M. E. Rahman, Email: syedrahman@bau.edu.bd, Email: ehsan_bau@yahoo.com
Jun Wang, Email: faithmate@gmail.com.
References
- AACC (1983) Approved method of the AACC. Method 02-52, Method 10-90, Method 44-15A, Method 44-40. American Association of Cereal Chemists. The Association, St. Paul, MN, USA
- Agnihotri MK, Rajkumar V, Dutta TK. Effect of feeding complete rations with variable protein and energy levels prepared using by-products of pulses and oilseeds on carcass characteristics, quality of goat meat and meat balls. Asian Aust J Anim Sci. 2006;19:1437–1449. doi: 10.5713/ajas.2006.1437. [DOI] [Google Scholar]
- Al-Rimawi F, Odeh I, Bisher A, Abbadi J, Qabbajeh M. Effect of geographical region and harvesting date on antioxidant activity, phenolic and flavonoid content of olive leaves. J Food Nutr Res. 2014;2:925–930. doi: 10.12691/jfnr-2-12-11. [DOI] [Google Scholar]
- AMSA . Research guidelines for cookery, sensory evaluation, and instrumental tenderness measurements of fresh meat. Chicago III: American Meat Science Association and Nutritional Live Stock and Meat Board; 1995. [Google Scholar]
- Barba FJ, Galanakis CM, Esteve MJ, Frigola A, Vorobie E. Potential use of pulsed electric technologies and ultrasounds to improve the recovery of high-added value compounds from blackberries. J Food Eng. 2015;167:38–44. doi: 10.1016/j.jfoodeng.2015.02.001. [DOI] [Google Scholar]
- Camo J, Beltrán JA, Roncalés P. Extension of the display life of lamb with an antioxidant active packaging. Meat Sci. 2008;80:1086–1091. doi: 10.1016/j.meatsci.2008.04.031. [DOI] [PubMed] [Google Scholar]
- Chacko D, Patterson J. Qualities of octopus meat balls developed using smashed potato and Bengal gram starches. World J Dairy Food Sci. 2011;6:130–135. [Google Scholar]
- Das AK, Rajkumar V, Verma AK, Swarup D. Moringa oleiferia leaves extract a natural antioxidant for retarding lipid peroxidation in cooked goat meat patties. Int J Food Sci Technol. 2012;47:585–591. doi: 10.1111/j.1365-2621.2011.02881.x. [DOI] [Google Scholar]
- Deng Q, Zinoviadou KG, Galanakis CM, Orlien V, Grimi N, Vorobiev E, Lebovka N, Barba FJ. The effects of conventional and non-conventional processing on glucosinolates and its derived forms, isothiocyanates: extraction, degradation, and applications. Food Eng Rev. 2015;7:357–381. doi: 10.1007/s12393-014-9104-9. [DOI] [Google Scholar]
- Fernandez LA, Zhi JN, Carbonell LA, Alvarez JAP, Kuri V. Antioxidant and antibacterial activities of natural extracts: application in beef meatballs. Meat Sci. 2005;69:371–380. doi: 10.1016/j.meatsci.2004.08.004. [DOI] [PubMed] [Google Scholar]
- Galanakis CM. Emerging technologies for the production of nutraceuticals from agricultural by-products: a viewpoint of opportunities and challenges. Food Bioprod Process. 2013;91:575–579. doi: 10.1016/j.fbp.2013.01.004. [DOI] [Google Scholar]
- Galanakis CM. Separation of functional macromolecules and micro molecules: from ultra filtration to the border of nanofiltration. Trends Food Sci Technol. 2015;42(1):44–63. doi: 10.1016/j.tifs.2014.11.005. [DOI] [Google Scholar]
- Galanakis CM. Phenols recovered from olive mill wastewater as additives in meat products. Trends Food Sci Technol. 2018;79:98–105. doi: 10.1016/j.tifs.2018.07.010. [DOI] [Google Scholar]
- Galanakis CM, Tsatalasa P, Charalambous Z, Galanakis IM. Polyphenols recovered from olive mill wastewater as natural preservatives in extra virgin olive oils and refined olive kernel oils. Environ Technol Innov. 2018;10:62–70. doi: 10.1016/j.eti.2018.01.012. [DOI] [Google Scholar]
- Galanakis CM, Tsatalasa P, Charalambous Z, Galanakis IM. Control of microbial growth in bakery products fortified with polyphenols recovered from olive mill wastewater. Environ Technol Innov. 2018;10:1–15. doi: 10.1016/j.eti.2018.01.006. [DOI] [Google Scholar]
- Galanakis CM, Tsatalas P, Galanakis IM. Implementation of phenols recovered from olive mill wastewater as UV booster in cosmetics. Ind Crops Prod. 2018;111:30–37. doi: 10.1016/j.indcrop.2017.09.058. [DOI] [Google Scholar]
- Hsu SY, Yu SH. Effects of phosphate, water, fat, and salt on qualities of low-fat emulsified meatball. J Food Eng. 1999;39:123–130. doi: 10.1016/S0260-8774(98)00134-4. [DOI] [Google Scholar]
- Huang SC, Shiau CY, Liu TE, Chu CL, Hwang DF. Effects of rice bran on sensory and physico-chemical properties of emulsified pork meatballs. Meat Sci. 2005;70(4):613–619. doi: 10.1016/j.meatsci.2005.02.009. [DOI] [PubMed] [Google Scholar]
- ISO (1995) Recommendation of the meeting of the subcommittee, International Organization for Standardization, on meat and meat products. ISO/TC- 36/SC-6, pp 10–18
- Karre L, Lopez K, Getty KJ. Natural antioxidants in meat and poultry products. Meat Sci. 2013;94(2):220–227. doi: 10.1016/j.meatsci.2013.01.007. [DOI] [PubMed] [Google Scholar]
- Kovačević DB, Barba FJ, Granato D, Galanakis CM, Herceg Z, Dragović-Uzelac V, Putnik P. Pressurized hot water extraction (PHWE) for the green recovery of bioactive compounds and steviol glycosides from Stevia rebaudiana Bertoni leaves. Food Chem. 2018;254:150–157. doi: 10.1016/j.foodchem.2018.01.192. [DOI] [PubMed] [Google Scholar]
- Kratchanova M, Slavov A, Kratchanov C. Interaction of pectin with amino acids and other amino compounds in aqueous solution. Food Hydrocoll. 2004;18:677–683. doi: 10.1016/j.foodhyd.2003.11.004. [DOI] [Google Scholar]
- Matthews K, Strong M. Salt—its role in meat products and the industry’s action to reduce it. Nutr Bull. 2005;30:55–61. doi: 10.1111/j.1467-3010.2005.00469.x. [DOI] [Google Scholar]
- Mikova K. The regulation of antioxidant in food. In: Pokorny J, Yanishlieva N, Gordon M, editors. Antioxidants in foods. Cambridge: Woodhead Publishing Ltd; 2001. [Google Scholar]
- Nagarajan J, Krishnamurthy NP, Ramanan RN, Raghunandan ME, Galanakis CM, Ooi CW. A facile water-induced complexation of lycopene and pectin from pink guava byproduct: extraction, characterization and kinetic studies. Food Chem. 2019;296:47–55. doi: 10.1016/j.foodchem.2019.05.135. [DOI] [PubMed] [Google Scholar]
- Nassu RT, Goncalves LAG, Silva BFJ. Oxidative stability of fermented goat meat sausage with different levels of natural antioxidant. Meat Sci. 2003;63:43–49. doi: 10.1016/S0309-1740(02)00051-7. [DOI] [PubMed] [Google Scholar]
- Novelli E, Zanardi E, Ghiretti G, Campanini G, Dazzi G, Madarena G, Chizzolini R. Lipid and cholesterol oxidation in frozen stored pork, salame Milano and mortadella. Meat Sci. 1998;48(1–2):29–40. doi: 10.1016/S0309-1740(97)00072-7. [DOI] [PubMed] [Google Scholar]
- Prokopov T, Goranova Z, Baeva M, Slavov A, Galanakis CM. Effects of powder from white cabbage outer leaves on sponge cake quality. Int Agrophys. 2015;29:493–500. doi: 10.1515/intag-2015-0055. [DOI] [Google Scholar]
- Purnomo H, Rahardiyan D. Indonesian traditional meatball: review article. Int Food Res J. 2008;15:101–108. [Google Scholar]
- Raghavan SY, Richards SS, Eu JB, Lee HC, Kim YJ, Chin KB. Evaluation of lipid oxidation and oxidative products as affected by pork meat cut, packaging, method and storage time during frozen storage. J Food Sci. 2007;72(2):114–119. doi: 10.1111/j.1750-3841.2006.00265.x. [DOI] [PubMed] [Google Scholar]
- Sallam KI, Ishioroshi M, Samejima K. Antioxidants and antimicrobial effects of garlic in chicken sausage. Lebensm Wiss Technol. 2004;37:849–855. doi: 10.1016/j.lwt.2004.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmedes A, Holmer G. A new thiobarbituric acid (TBA) method for determining free malondialdehyde (MDA) and hydroperoxides selectively as a measure of lipid peroxidation. J Am Oil Chem Soc. 1989;66:813–817. doi: 10.1007/BF02653674. [DOI] [Google Scholar]
- Serdaroglu M, Yldz-Turp G, Abrodimov K. Quality of low-fat meatballs containing legume flours as extenders. Meat Sci. 2005;70(1):99–105. doi: 10.1016/j.meatsci.2004.12.015. [DOI] [PubMed] [Google Scholar]
- Sikora E, Cieślik E, Topolska K. The sources of natural antioxidants. Acta Sci Pol Technol Aliment. 2008;7:5–17. [Google Scholar]
- Singh VP, Sanyal MK, Dubey PC, Mendirttan SK. Quality assessment of vacuum packaged chicken snacks stored at room temperature. J Stored Prod Res. 2011;2(1):120–126. [Google Scholar]
- Zargar FA, Kumar S, Bhat ZF, Kumar P. Effect of pumpkin on the quality characteristics and storage quality of aerobically packaged chicken sausages. Springer Plus. 2014;3(1):39. doi: 10.1186/2193-1801-3-39. [DOI] [PMC free article] [PubMed] [Google Scholar]