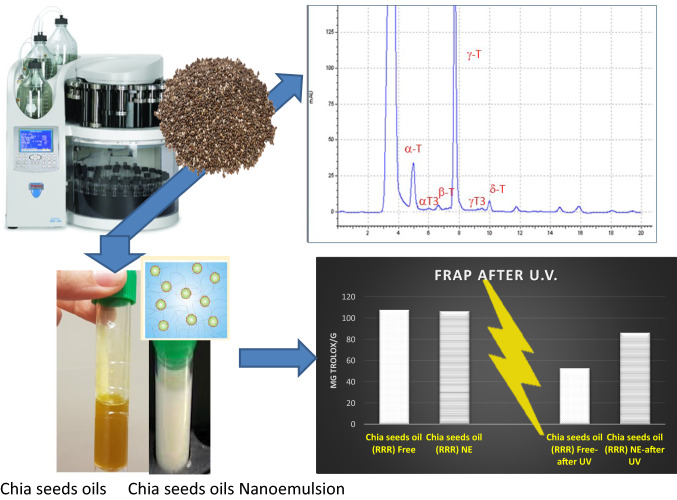
Graphic abstract
The objective of this study was to use accelerated-solvent-extraction to achieve antioxidant extracts from chia seeds oils, enriched in tocopherols and tocotrienols, namely tocochromanols. Nanotechnology applications have been also incorporated to develop an innovative formulation of chia seeds oil nanoemulsion that preserve its antioxidant potential after conditions of oxidative stress. Chia seeds oils proved to be a valuable source of tocochromanols, from 568.84 to 855.98 μg g−1, depending on the geographical provenance. Quantitative data obtained by LC-DAD-ESI-MS/MS showed outstanding levels of γ-Tocopherol, over 83%, followed far behind by Tocopherols-(α, β, δ) and Tocotrienols-(α, β, δ, γ)-tocotrienols. The characteristic tocochromanols fingerprint of chia seeds oils was positively correlated with the FRAP and DPPH antioxidant activity of the extracts (between 18.81 and 138.48 mg Trolox/g). Formulation of the Chia seeds oils as nanoemulsions did not compromised the antioxidant properties of fresh extracts. Interestingly, nanoemulsions retained about the 80% of the initial antioxidant capacity after UV-induced stress, where the non-emulsified oils displayed a remarkable reduction (50–60%) on its antioxidant capacity under the same conditions. These antioxidant chia seeds formulations can constitute a promising strategy to vectorizing vitamin E isomers, in order to be used for food fortification, natural additives and to increase the self-life of food products during packing.
Keywords: Pressurized-liquid-extraction, Chia-seeds, Tocopherols, Tocotrienols, Tocochromanols, Nanoemulsions, Antioxidant activity
Introduction
Nowadays, food industries have started to implement emerging technologies for food processing to achieve food products with high levels of compounds/nutrients for health promotion greatly demanded by consumers (Ixtaina et al. 2015).
In recent years we are witnessing to the development of millenary crops that offer an attractive alternative source of healthy nutrients. In this sense, it is increasing the popularity of chia seeds (Salvia hispanica L.), an endemic Mexican pseudograin used for medicinal and nutritional purposes by Mesoamerican cultures for thousands of years. Currently chia seeds and chia seeds oils are recognized as new foods that can positively contribute to the prevention of obesity, cardiovascular diseases, diabetes and cancer, as well as autoimmune and inflammatory diseases (Ixtaina et al. 2011; Poudyal et al. 2012). These benefits stem mainly from chia seeds levels of omega-6 fatty polyunsaturated fatty acids as α-linolenic (18:3n-3) and linoleic (18:2n-6), dietary-fiber and protein. Indeed, chia seeds are incorporated into foodstuffs like cereal bars, cookies and baking products (Borneo et al. 2010; Aranibar et al. 2019). Chia seeds also possess natural antioxidants that reinforce their nutritional value and health benefits, as well as a renewed interest in this food (Marineli et al. 2014; Pizarro et al. 2015; Da Silva et al. 2017). In this context, it has been outstanding the levels of vitamin E in chia seeds, a powerful antioxidant able to react directly with chain-carrying species decreasing the oxidative reactions (Ayerza et al. 2002; Falk and Munne-Bosh 2010).
Vitamin E from food provenance includes (α-, β-, δ-, γ)-tocopherols and (α-, β-, δ-, γ)-tocotrienols, depending on the number and position of the methyl group attached to the chromanol ring (Kua et al. 2016). The sum of tocopherols (T) and tocotrienols (T3) is collectively known as tocochromanols. It is noteworthy that each isomers of tocopherol (-α, -ß, -γ and -δ) possess a racemic equimolar mixture of eight stereoisomers (four 2R and four 2S) with different chemical and biological properties and characteristics. Among all of them, just 2R-stereoisomeric forms are considered active forms of VitE in human, since they (mainly RRR-α-T) are available for the specific carrier protein called α-tocopherol transfer protein (α-TTP) (Brigelius-Flohe 2006). It is necessary to highlight that synthetic supplements of vitamin E contain all the variability of steroisomers (all-racemic), while vitamin E from foods only possess RRR-forms. This fact is determinant for the bioavailability of vitamin E, since the carrier selectivity transport only RRR-forms (Dersjant-Li and Peisker 2010). Based on the above-mentioned, it is easy to understand the interest in finding natural sources of the RRR-isomers of vitamin E.
In this sense, chia seeds are postulated to be a source of natural vitamin E isomers based on its levels of γ-RRR-tocopherol isomer that represents up to > 80% of total tocopherols content (Ixtaina et al. 2011; Capitani et al. 2012).
Some studies have reported the influence of chia seeds’ geographical origins on γ-RRR-tocopherol content (Ayerza et al. 2002). In fact, the vitamin E levels found in Brazilian chia seeds were around twofold higher than those found in samples from Argentina (Capitani et al. 2012). Likewise, β-tocopherol has been only found in chia seeds oil from Brazil (Ixtaina et al. 2011; Capitani et al. 2012). The same trend has been observed in the tocotrienols (T3) levels of chia seeds from different countries (Castejon et al. 2017; Da Silva et al. 2017) related with the antioxidant potential (Ahsan et al. 2015).
The main stability problem of chia seeds oils (and all fatty foods) is the photochemical oxidation that can be suffers, e.g. the vitamin E isomers. Nowadays, the incorporation of nanotechnology in food industry represents an important option for the development of efficient edible nanoemulsions with good-quality protective features against lipid oxidation by UV light. These nanoemulsions can enhance the viability of some antioxidants (carotenoids, tocopherols, flavonoids, phytosterols, quinones, ect) in oils such as corn, soybean, sunflower and olive oil (McClements and Xiao 2012; Coradini et al. 2014). Recently it has been reported the significant effect of the protein–carbohydrate relation on the droplet size of chia O/W emulsions (Julio Luciana et al. 2016).
The formulation of oily-foods as nanoemulsions could help to reduce the degradation of natural RRR-isomers of vitamin E during manufacturing and storage processes, thus increasing their bioavailability and avoiding the premature degradation of several active molecules (Augustin and Hemar 2009; Chen et al. 2006; Fang and Bhandari 2010; Plaza Oliver et al. 2015; Villaseca-Gonzalez et al. 2018).
Since, chia seeds can constitute a new source of tocochromanols with interesting applications related with the innovation and valorization of food products; the goals of this paper were to: (I) design an Accelerated-Solvent-Extraction procedure to maximize the isolation of tocochromanols from chia seeds; (II) develop an analytical methodology to identify and quantify the tocochromanols profile in chia seeds oils; (III) incorporate nanotechnology to formulate tocochromanols-enriched oil-in-water nanoemulsions of chia seeds oil and (IV) Estimation of the antioxidant behavior of free and nanoemulsioned chia oils after UV-induced stress.
Materials and methods
Chia seeds samples
Commercial chia seeds used in this study have been certified as organic production. They were purchased from three different cropping areas: Special line from Mexico (set 1), Natur Grainway from Peru (set 2) and Organic chia seeds from Argentina (set 3). They were packed in hermetic plastic vessels and stored at 5 °C until further use. Randomized samples of each independent set of commercial chia seeds (7–8% moisture content) were used to obtain oils by pressing or solvent extraction. Each set of oils obtained by the extraction systems were analyzed in duplicate.
Chemicals and reagents
DL-all-rac-α-Tocopherol, DL-all-rac-β-Tocopherol, DL-all-rac-δ-Tocopherol and DL-all-rac-γ-Tocopherol were supplied by Merk (Darmstadt, Germany). Tocotrienols, Trolox, 2,4,6- tris(2-pyridyl)-S-triazine (TPTZ), (DPPH), albumin from bovine serum (BSA), Foline Ciocalteu’s reagent and gallic acid were all obtained from Sigma Aldrich (Sigma Co., St. Louis, MO, US A). All solvents, analytical grade, were acquired from Panreac S.A. (Madrid, Spain).
Chia seeds oil extraction
The extraction of oil from chia seeds was carried by means of an accelerated solvent extractor ASE 200 (Dionex Corp,Sunnyuale, CA, USA) (Santos Freitas et al. 2008; Castro-Vazquez et al. 2016). Chia seeds samples were grinded using a coffee mill. Four grams of ground powder was placed into ASE-stainless steel extraction cells of 11 mL. Every cell was filled with hexane and raised to 60 °C. Then, two static extraction phases lasting 10 min was carried out under 1500 psi. Between extractions, a rinse of the complete system was performed to avoid any carry-over. Extracts were evaporated using a rotavapor with a vacuum controller (Heidolph, Schwabach, Germany) at 40 °C until remove the hexane and obtain the chia seeds oil. Samples from chia oil were they were filtered through a Whatman No. 1 filter paper. Samples were kept at − 20 °C prior to be used to determine antioxidant activity and tocopherols isomers.
Optimization of pressure solvent extraction (PLE) parameters
Solvent extraction
In order to optimize the PLE extraction solvent, and taking into account the hydrophobic characteristics of vitamin E, methanol, isopropanol-acetonitrile (80:20 v/v) and hexane were tested.
Sample amount
The extraction cell in the PLE system contains the chia seeds samples mixed with a drying agent to avoid a dead volume in the cell and to allow the best contact of sample and solvent. To achieve the best tocopherols extraction conditions, amounts of sample ranging from 0.5 to 5 g were weighed and mixed with the dispersant agent (diatomaceous earth) until the extraction cell was full.
Analysis of tocopherols and tocotrienols from chia seeds oils by HPLC-UV and LC-ESI-MS/MS. Separation and quantification of tocopherols and tocotrienols isomers of vitamin E were performed on a high pressure liquid chromatography (HPLC) using optical detector (UV–vis). The samples, after filtration (0.20 μm, polyester membrane, Chromafil PET 20/25) were injected in duplicate on a column amino phase Supelcosil LC-NH2-NPHPLC column (250 × 4.6 mm IDx 5 µm particles). The chromatography conditions were the following: an isocratic mobile phase consisting of isopropanol: acetonitrile 70:30 v/v, a flow of mobile phase of 1 mLmin−1, injection volume of 20 μL and a wavelength of 295 nm as a compromise for the simultaneous detection of each compound. Quantification of tocochromanols was made by means of external standard calibration lines. The calibration curves, of six points, were obtained covering a concentration range from 0.5 to 100 mg L−1.
Confirmation of tocochromanols were performed on an Agilent 1260 Infinity LC system (Agilent, Waldbronn, Germany), equipped with a DAD with a wavelength of 295 nm and a LC/MSD Trap VL. The injections were carried out by an Agilent autosampler and the samples were analysed using an Agilent Technologies 6460 triple quadrupole mass spectrometer equipped with an electrospray ionization mass spectrometry (ESI/MSn). For the MS characterization of chia seeds oils tocochromanols, the selection of the best precursor ion, the most abundant ion in full scan mode was chosen, providing first-generation product ions in this mode. Specific precursor ion parameters such as transitions, fragmentor (V) and collision energy (CE) were optimized using Optimizer (Agilent, MassHunter).
These conditions for each compounds (m/z) were: (a) 429.1–163.0 with a CE of 25 eV for α-tocopherol, (b) 415.4–149.0 with a CE of 30 eV for β-tocopherol and δ-tocopherol, (c) 429.1–135.0 with a CE of 28 eV for δ-tocopherol, using a fragmentor value of 100 V in all cases; and tocotrienols were (m/z): (a) 423.4–163.0 with a CE of 25 eV for α-tocotrienol, (b) 409.4–149.0 for β-tocotrienol and δ-tocotrienol, with a CE of 30 eV (c) 395.4–135.0 for δ-tocotrienol with a CE of 30 eV. The fragmentor value was 100 V for all tocotrienols.
Zero-grade nitrogen was produced by a nitrogen generator (PEAK Scientific, IL, USA) and high purity was supplied by Linde (Valencia, Spain). The Mass Hunter Workstation B.07.00 software (Agilent Technologies) was used for computerized instrument control, data acquisition and analysis.
The samples, after filtration were injected in duplicate on a reversed-phase narrow-bore column Zorbax Eclipse Plus-C18 (2.1 × 50 mm; 1.8 μm particle), protected by a guard column Zorbax Eclipse XDB-C8 (2.1 × 12.5 mm; 5 μm particle; Agilent), both thermostated at 40 °C. The mobile phase consisted isopropanol: acetonitrile 90:20 v/v. The volume injection for samples was of 5 μL as previously described Villaseca-Gonzalez (Villaseca-Gonzalez et al. 2018).
Chia seeds oil nanoemulsions
Systems formulation
Chia seeds oil-in-water nanoemulsions were formulated by the solvent displacement technique (Lozano et al. 2013). Briefly, the organic phase was composed by 63 µL of chia seeds oil extracted as commented above was emulsified using 20 mg of lecithin (Epikuron 145 V) kindly donated by Cargill (Spain), ethanol and acetone (Sigma, Spain). This organic phase was poured onto 10 ml of ultrapure water under magnetic stirring. After emulsification, the oil-in-water formulations were rotaevaporated to a final volume of 5 mL at 37 °C. Afterwards the formulations were stored at 4 °C and protected from the light to avoid the premature degradation of the oil components.
Physicochemical characterization and colloidal stability
Dynamic light scattering (DLS) was used to determine the hydrodynamic mean size and the ζ-potential of the formulations using a Z-Sizer NanoZS from Malvern (UK). Analysis of the formulations by DLS was performed by diluting the samples in phosphate buffer 2 mM.
Samples oxidation process by UV-induced stress
The antioxidant behaviors of free and nanoemulsioned chia seeds oils against light degradation were studied after UV irradiation, as oxidizing agents with an UV-transiluminator (Syngene SYTC2/1067).
The duration of the UV-induced stress was previously optimized at 15 min (data not shown). After this time, ABTS and DPPH assays were again carried out as above described.
Total phenol index (TPI)
The total phenols content of extracts was determined according to the Folin–Ciocalteu procedure described by (Alañon et al. 2011). Deionised water (1.8 mL) was added to 0.2 mL of each extract from the three chia seeds oils. Folin–Ciocalteu reagent (0.2 mL) was then added and tubes were shaken vigorously. After 3 min, 0.4 ml sodium carbonate solution (35% w/v) was added, along with 1.4 ml of deionised water. Samples were well mixed and left in the dark for 1 h. The absorbance was measured at 725 nm using a UV–vis spectrophotometer (Lambda 5, Perkin–Elmer, Seer Green, UK). Results were expressed mg gallic acid equivalents (GAE) per gram of chia oil using a gallic acid standard curve (0–0.2 mg mL−1).
Antioxidant assays of free and nanoemulsioned chia seeds oils
The antioxidant power of free chia seeds oils extracts coming from Mexico (set 1), Peru (set 2) and Argentina (set 3), and their respective nanoemulsions formulations (NE) was evaluated. Their antioxidant behavior after UV degradation were also assessed.
FRAP assay
The FRAP assay was performed as previously described by Benzie and Strain (Benzie and Strain 1996) with some modifications. This spectrophotometric assay measures the ferric reducing ability of antioxidants. The experiment was conducted at 37 °C and pH 3.6. In the FRAP assay, antioxidants present in the extract reduce Fe (III)-tripyridyltriazine complex to the blue ferrous form, with an absorption maximum at 593 nm. The assay was performed by means of an automated microplate reader (Tecan Ltd., Dorset, UK)) with 96-well plates. Reagents included 300 mM acetate buffer pH 3.6; 40 mM hydrochloric acid, 10 mM TPTZ solution and 20 mM ferric chloride solution. The working FRAP reagent was prepared fresh on the day of analysis by mixing acetate buffer, TPTZ solution and ferric chloride solutions in the ratio 10:1:1 and the mixture was incubated at 37 °C. Followed 30 μL of chia seeds oil nanoemulsions, were submitted to the same procedure. The absorbance of the blue colored complex was read against a reagent blank. The absorbance at time zero and after four min was recorded at 593 nm. The calculated difference in absorbance is proportional to the ferric reducing/antioxidant power of the samples. For quantification, a calibration curve of trolox was prepared. The final results were expressed as milligrams of trolox per gram of oil. The analysis was performed in quadruplicate.
DPPH radical scavenging assay
The DPPH assay was carried out according to the method proposed by Alañon (Alañon et al. 2011) where 1,1-diphenyl-2-picrylhydrazyl radical was used as a stable radical. One hundred microliters of the chia oils and chia oils nanoemulsions samples above mentioned as (I), (II) and (III), added to 2.9 mL of a 0.06 mM methanol DPPH radical solution. Methanol was used to adjust the zero and the decrease in absorbance was measured at 515 nm every minute for 25 min in a UV–vis spectrophotometer (Helios, Thermo Spectronic, Cambridge, UK). Only values between 20 and 80% of the initial DPPH values were taken into consideration. Results, calculated from a calibration curve, were expressed as milligrams of trolox per gram of chia seeds oil.
Statistical analysis
Analysis of variance and multivariate analysis were performed using SPSS 19.0 for Windows statistical package. Differences among means were determined for significance at p ≤ 0.05 using the Student-Newman-Keuls test. Principal Component Analysis was performed to classify the samples into groups according to tocopherols, tocotrienols levels and antioxidant activity.
Results and discussion
Optimization of pressure solvent extraction parameters
Solvent extraction
As shown in Fig. 1, there were no significant differences between tocochromanols recovery rates when it was used methanol or isopropanol-acetonitrile (80:20 v/v). Similar results have been reported for other grain extracts (Bustamante-Rangel et al. 2007). The best results were obtained using hexane, mainly in the case of β-tocopherol and γ-tocopherol isolation that displayed recovery percentages ranged between 92.69 and 95.92%, respectively (Fig. 1). Consequently, hexane was selected as the elution solvent.
Fig. 1.
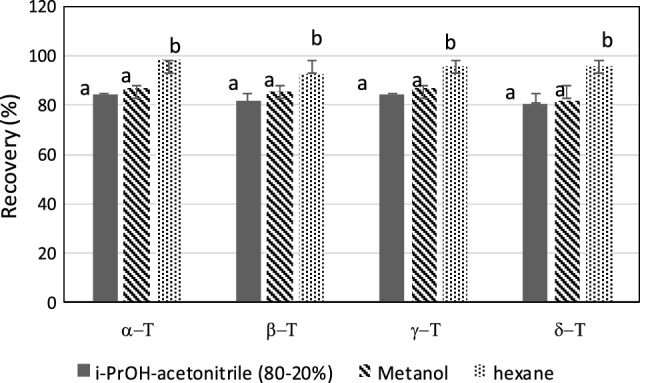
The effect of different solvents on the recovery yield of α, β, γ, δ-tocopherols using Accelerated Solvent Extraction (ASE). a, b: Different letters in the same column denote a significant difference according to the Student–Newman–Keuls test, at p ≤ 0.05
Data confirm the effectiveness of the liquid extraction (PLE) observed in seed in crambe seed oil with methyl acetate that showed a yield of 49.1% and displaying higher concentration of tocopherols, compared to the oil from Soxhlet method (Ferreirade-Mello et al. 2019). It also have been observed that PLE of crambe seed oil, with dimethyl carbonate, was an efficient extraction method, since tocopherols levels were 62% higher than those obtained with soxhlet (Portilho et al. 2020).
Amount of sample
For any sample bigger than 1 g, the signal came close to a constant value. The amount of 5 g was selected after considering the relationship between the levels of tocochromanols and the linear chromatographic signal.
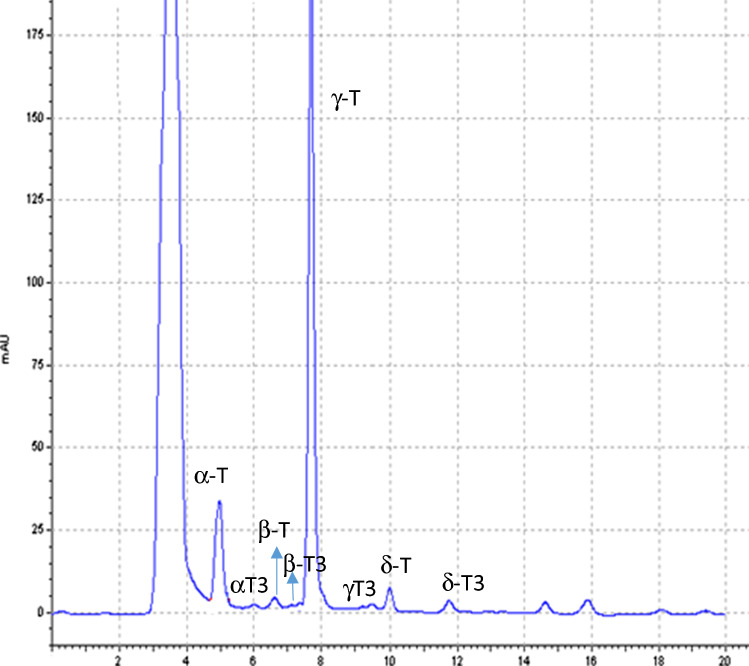
Determination of tocopherols and tocotrienols from chia seeds oils
Four tocopherols (α-T, β-T, γ-T and δ-T) and four tocotrienols (α-T3, β-T3, γ-T3 and δT3) were isolated from chia seeds oils. The proposed methodology by HPLC-UV is useful to achieve the simultaneous separation of eight vitamin E isomers from chia seeds oils. As far as the authors know, this is the first time that tocopherols and tocotrienols have been successfully separated from chia seeds oils by HPLC-UV instead of HPLC-fluorescence detection (Pilarova et al 2016). The advantage of the proposed methodology is the speed, accuracy, sensitivity, specificity and simplicity, which make it highly suitable for the quantification of all vitamin E isoforms even in quality control analyses.
The total levels of tocochromanols in the chia seeds oils, from three provenances, fell within the range of 568.84–855.98 μg g−1 (see Table 1). The tocochromanols identification was confirmed by using an LC-DAD online ESI-MS/MS with a triple quadrupole as a detector in the negative mode [M-H]−. The mass spectrometry data are shown in Table 1.
Table 1.
Identification of tocochromanols by ASE and LC–ESI–MS. Quantification of vitamin E isomers (μg/g) in three natural chia seeds oils
| μg/g | Identification | chia seeds oil-1 (RRR) | chia seeds oil-2 (RRR) | chia seeds oil-3 (RRR) | ||||
|---|---|---|---|---|---|---|---|---|
| MS [M-H]− (m/z) | Products ions (m/z) | Mean | SD | Mean | SD | Mean | SD | |
| α-Tocopherol (α-T) | 429.1 | 163 | 130.40a | (3.72) | 6.93b | (0.36) | 10.34c | (0.96) |
| α-Tocotrienol (α-T3) | 415.4 | 149 | 2.88a | (0.16) | 4.67b | (0.21) | 2.70a | (0.26) |
| β-Tocopherol (β-T) | 415.4 | 149 | 10.98a | (0.41) | 5.27b | (0.43) | 4.64b | (0.58) |
| β-Tocopherol (β-T) | 429.1 | 135 | traces | traces | traces | |||
| γ-Tocopherol (γ-T) | 423.4 | 163 | 699.23a | (24.58) | 545.82b | (27.90) | 557.20b | (20.40) |
| γ-Tocotrienol (γ-T3) | 409.4 | 148.8 | 2.34a | (0.14) | 2.37a | (0.16) | 2.16a | (0.20) |
| δ-Tocopherol (δ-T) | 409.4 | 148.8 | 6.48a | (0.18) | 0.46b | (0.02) | 14.51c | (1.37) |
| δ-Tocotrienol (δ-T3) | 395.4 | 134.8 | 3.67a | (0.27) | 2.32a | (0.35) | 2.84a | (0.29) |
| TOTAL | 855.98 | 568.84 | 594.39 | |||||
Samples provenance: Chia seeds oil-1 (RRR) from Mexico; chia seeds oil-2 (RRR) from Peru chia seeds oil-3 (RRR); from Brazil
a, b, cDifferent letters in the same column denote a significant difference according to the Student–Newman–Keuls test, at p ≤ 0.05
The amounts of vitamin E quantified in the ASE extracts from chia seeds were clearly higher than those obtained by classic techniques, such as liquid extraction, pressing extraction and ultrasonic system, which hardly reach 238–545 μg g−1 (Ixtaina et al. 2011; Capitani et al. 2012). The levels of tocochromanols here found are 1.6–3.5-times higher than those reported using the mentioned isolation techniques. The reason for the better performance of PLE was the use of hexane above its atmospheric pressure boiling (Delgado-Zamarreno et al. 2004; Castro-Vazquez et al. 2016). These conditions improved the extraction efficiency of tocochromanols from chia seeds also reducing their degradation and avoiding underestimating their values, as it happens in classic isolation techniques.
All the obtained chia seeds oils displayed the same vitamin E-isomers fingerprint, although significant differences were noted in relation to their levels that depending on the chia geographical origin. The Student’s t test for independent samples displayed significant differences (p < 0.05) between the content of tocochromanols in the chia seeds grown in Mexico (855.98 µg g−1), compared to those coming from Peru and Brazil (568.84 and 594.39 µg g−1, respectively; see Table 1). A typical chromatogram, that includes the listed peaks, is shown in Fig. 2.
Fig. 2.
HPLC–UV–vis Chromatographic profile of tocopherols and tocotrienols of chia seeds oil under conditions described within the text
It is important to highlight the γ-tocopherol levels (> 83% of vitamin E) in all the extracts from chia seeds oils regardless of its provenance. This compound was confirmed by their precursor-ion at m/z 415.4 [M-H]− and their fragment ion at m/z 149 (Table 1). The amounts of γ-tocopherol varied from 548.96 to 713.92 μg g−1. Data agree with values reported by other studies (Da Silva et al. 2017); although they were 28–62% higher than those obtained using soxhlet extraction (Ixtaina et al. 2011; Capitani et al. 2012).
From a quantitative point of view, the Mexican chia seeds oil extracts displayed statistically larger amounts of γ-tocopherol (p ≤ 0.05) than those coming from Brazil and Peru. Literature has reported the higher capacity of γ -tocopherol vs to α- tocopherol to trap nitrogen oxide species and to inhibit the cyclooxygenase activity (Chen and Bergman 2005). For this reason, one of the most outstanding findings of this study was the remarkably γ-tocopherol levels found in the three series of ASE-chia oil extracts, ranged between 557.20 and 699.23 μg g−1.
The levels of α-tocopherol (m/z [M-H]− 429.1 to 163.0) found in the three provenances from chia seeds were between 6.93 and 130.40 µg/g. This fluctuating behavior has also been previously described by (Ixtaina et al. 2011). The α-tocopherol amounts observed in chia seeds oils from Peru and Brazil (Table 1) agreed with the results reported for South American chia oil samples (Da Silva et al. 2013). Remarkably, the Mexican chia seeds oil extracts showed 13–18 folds higher α-tocopherol levels (p ≤ 0.05) than the other geographical origin.
The average δ-T concentration in the analyzed chia seeds oils extracts fluctuated from 2.16 to 2.37 μg g−1, and it was similar to the values previously reported for South American chia seeds oils (Ixtaina et al. 2011). The δ-T identification, using its molecular weight and isotopic or fragmentation patterns by mass spectrometry, allowed the unmistakable identification of δ-T in the current chia seeds oils.
Concerning to β-tocopherol, identified by its precursor and product ions, the analyzed samples showed amounts within a range from 4.67 to 10.98 μg g-1 (Table 1). These are relevant data because, to the best of our knowledge, very few authors have reported the presence of this isomer in chia oils (Da Silva et al. 2013; Castejon et al. 2017). It is necessary to underline that β and γ-tocopherol displayed the same electrospray ionization mass spectrometry (ESI-MS) fragmentation patterns m/z. This characteristic hindered their separate identification. Bustamante-Rangel et al. 2007, referred that the peak assigned as γ-isomer corresponded, on several occasions, to the sum of the (β + γ)-isomers. This aspect reveals the importance of the proposed analytical methodology to achieve a successful isolation of both isomers.
Four tocotrienols, α-T3, β-T3, γ-T3 and δ-T3, were also identified and quantified in the analyzed chia seeds oils (Fig. 2). Their presence was confirmed by their [H–H] − precursors and product ions. The mean tocotrienols levels in the three sets of chia seeds oils were ranged between 7.70 and 9.36 µg g−1 oil (Table 1). These values are ninefold higher than those reported fusing the classical extraction techniques (Ixtaina et al. 2011), and they are in line with those found in Bolivian chia oils also by PLE technique (Castejon et al. 2017).
α-Tocotrienol (α-T3) was detected at concentrations of 2.70–4.67 μg g−1 in the chia seeds oils. Its presence constitutes a significant advantage since it have been documented that α-T3, but not α-T, has neuroprotective effects against glutamate-induced toxicity (Khanna et al. 2010).
Discreet amounts of γ-T3 and δ-T3, ranging between 2.16 and 3.67 μg g−1 were found in all the series of analyzed chia seeds oils (Table 1). As far as the authors know γ-T3 is especially significant since it has hardly found in any research works. Despite the low γ-T3 concentration, its presence in chia seeds oils can play a key antioxidant role due to its proven almost threefold higher free radical scavenging activities than α-T isomer (Ahsan et al. 2015).
Formulation of chia seeds oil nanoemulsions
Formulation methods were properly designed to avoid premature degradation of vitamin E isomers as a consequence of the temperature and light stress. By bearing this in mind, we adapted the well-known solvent displacement technique to keep the antioxidant capacity of the chia seeds oil extracts intact. The mild conditions of this formulation method, where ethanol and acetone were quickly eliminated after the chia oil nanoemulsion formation, led to the formation of oily droplets in the aqueous medium, whose hydrodynamic mean size were 200.76 nm (SD = 0.06), and a low polydispersity index (PDI) of 0.12 that did not depend of chia seeds’ origin. The three analyzed chia seeds oils systems presented a negative surface charge (ζ-potential) of − 50.02 ± 2.52 mv that confers to chia oil nanoemulsion a suitable electrostatic stability to avoid vesicle coalescence. The numerical value of ζ-potential is related to the stability of emulsions. Emulsions with high absolute f-potential (higher than + 30 mV or lower than − 30 mV) are electrostatically stabilized while emulsions with low absolute ζ–potential tend to coagulate or flocculate. In this sense the current chia seeds NE improves the rheological characteristics of previously described emulsions enriched with chia mucilage (Julio Luciana et al. 2016).
These physicochemical characteristics ensure the potential use of these nanoemulsions to improve the bioavailability of chia seeds tocochromanols after oral intake as previously reported (Plaza-Oliver et al. 2015) with similar physicochemical data in other matrices. From an industrial point of view, the use of GRAS materials (generally recognized as safe by its English acronym) facilitates that chia oil nanoemulsion be approved by regulatory agencies in order to be used in food formulation purposes.
Total phenolic content
The total phenolic content (TPC) of the fresh chia seeds oils were ranged from 1.00 to 1.89 mg GAE/g (Table2), which agrees with the results reported by Martínez-Cruz (Martinez-Cruz and Paredes-Lopez 2014), although they are also at least 1.3-fold higher than those reported using classic extraction techniques (Reyes-Caudillo et al. 2008; Porras-Loaiza et al. 2014). Our higher TPC values were surely supported by a greater co-extraction of several compounds such as antioxidant polyphenols, e.g., myricetin, quercetin, kaempferol and chlorogenic acid, that can be efficiently isolated by the PLE technique (Marineli et al. 2014).
Table 2.
Total Polyphenol Content (TPC), FRAP and DPPH assays mean values and standard deviation (SD) of free chia seeds oils, nanoemulsioned chia seeds oils, and samples after oxidation (A.O) process
| Samples | TPC | FRAP | DPPH |
|---|---|---|---|
| Mean ± SD | Mean ± SD | Mean ± SD | |
| Chia oil extract-1 (RRR) Free | 1.89a ± 0.19 | 107.53 ± 6.56 | 135.64 ± 12.65 |
| Chia oil extract-1 (RRR) NE | 1.89a ± 0.27 | 106.36 ± 9.74 | 138.48 ± 10.44 |
| Chia oil extract-1 (RRR) Free AO | 0.78b ± 0.19 | 52.58 ± 3.75 | 71.97 ± 4.95 |
| chia oil extract-1 (RRR) NE. AO | 1.5c ± 0.22 | 86.15 ± 7.93 | 111.22 ± 11.03 |
| Chia oil extract-2 (RRR) Free | 1.00a ± 0.21 | 63.83 ± 6.3 | 69.32 ± 8.05 |
| Chia oil extract-2 (RRR) NE | 1.02a ± 0.11 | 59.62 ± 7.09 | 64.97 ± 9.22 |
| Chia oil extract-2 (RRR) Free. AO | 0.61b ± 0.10 | 34.03 ± 2.28 | 39.43 ± 3.04 |
| Chia oil extract-2 (RRR) NE. AO | 0.84c ± 0.16 | 46.50 ± 6.97 | 49.056 ± 6.67 |
| Chia oil extract-3 (RRR) Free | 1.20a ± 0.31 | 81.76 ± 9.51 | 99.24 ± 7.21 |
| Chia oil extract-3 (RRR) NE | 1.26a ± 0.22 | 89.60 ± 12.3 | 99.13 ± 6.44 |
| Chia oil extract-3 (RRR) Free. AO | 0.69b ± 0.18 | 18.81 ± 3.84 | 41.81 ± 4.01 |
| Chia oil extract-3 (RRR) NE. AO | 0.97c ± 0.19 | 71.22 ± 5.07 | 77.41 ± 7.66 |
TPC are expressed as mg gallic acid equivalents per gram of oil
FRAP and DPPH assays are expressed as mg Trolox per gram of chia seeds oil
Chia oil extract-1 (RRR) free, natural oil coming from Mexican chia seeds; Chia oil extract-1 (RRR) NE, Nanoemulsion from Mexican chia seeds oil; Chia oil extract-1 (RRR) Free. AO, natural oil coming from Mexican chia seeds after oxidation; Chia oil extract-1 (RRR) NE. AO, Nanoemulsion from Mexican chia seeds oil after oxidation processes.
Chia oil extract-2 (RRR) Free, natural oil coming from Peruvian chia seeds; Chia oil extract-2 (RRR) NE, Nanoemulsion from Peruvian chia seeds oil; Chia oil extract-2 (RRR) free. AO, natural oil coming from Peruvian chia seeds after oxidation; Chia oil extract-2 (RRR) NE. AO, Nanoemulsion from Peruvian chia seeds oil after oxidation processes
Chia oil extract-3 (RRR) Free, oil coming from Brazilian chia seeds; Chia oil extract-3 (RRR) NE, Nanoemulsion from Brazilian chia seeds oil; Chia oil extract-3 (RRR. AO) free, oil coming from Brazilian chia seeds after oxidation; Chia oil extract-3 (RRR) NE. AO, Nanoemulsion from Brazilian chia seeds oil after oxidation processes.
a, b, cDifferent letters in the same column denote a significant difference according to the Student–Newman–Keuls test, at p ≤ 0.0
The total polyphenol content, for fresh chia seeds oils (both free and NE) was quite similar. Nevertheless, TPC values of fresh free chia seeds oils significantly decreased after oxidation while their oxidized nanoemulsions maintained practically stable their values.
Hence, the promising formulations of chia seeds oil nanoemulsions seem to be quite appropriate to keep its phenolic potential in order to be used for packing purposes and to increase the self-life of food products.
Antioxidant activity of fresh chia seeds oils (free and nanoemulsioned)
Free Mexican chia seeds oil [chia oil 1-(RRR)-free] showed the highest levels of FRAP and DPPH activities, 107.53 and 135.64 mg trolox g-1 respectively, which are according with levels reported by da Silva et al. 2017. Free Peruvian and Brazilian chia seeds oils [chia oil 2-(RRR)-free and chia oil 3-(RRR)-free] displayed FRAP and DPPH values between 63.83 and 99.24 mg trolox g-1 respectively (Table 2), revealing the influence of the crop area on the antioxidant activity of chia seeds oils.
On the other hand, there were not significant differences between the FRAP and DPPH values reached by the Mexican, Peruvian and Brazilian fresh chia seeds oils-NE in comparison with their respective values of fresh free chia oils. This fact reinforces the suitability of the current formulation which does not compromise the antioxidant activity.
To obtain more detailed information about the relation between the individual isomers of vitamin E in chia oils from different origins in relation with their antioxidant activities, a principal component analysis (PCA) was applied to the data matrix with two-dimensional projection of the variables (data not shown). The first principal component axis explained 58.40% of total variation and clearly isolated the Mexican chia seeds oils which were correlated with higher levels of FRAP, DPPH•+, and α-T, β-T and γ-T. The PC-2 explained 30.15% of total variation and showed positive loadings with tocotrienols γ-T3 and α-T3, which is consistent with the slightly higher contents found in Peruvian chia oil seeds oil plotted at the top of the axis. The Brazilian chia seeds oil extracts were correlated with δ-tocopherol and they were grouped at the bottom of PC-2.
Antioxidant behavior of free and nanoemulsioned chia seeds oils after UV-induced stress
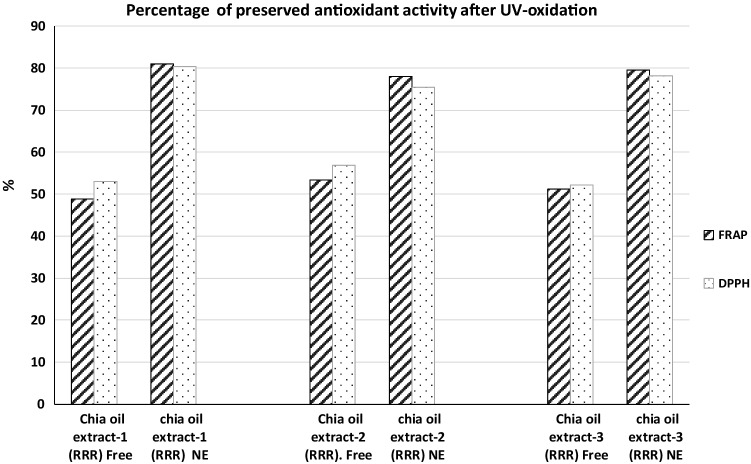
Decreases of FRAP and DPPH values were observed both for free and nanoemulsioned chia seed oils after the induced stress by exposition to UV light. However, the differences between samples were statistically significant, being particularly favourable for nanoemulsions. The percentage of antioxidant activity maintenanced by chia seeds oils-NE after UV oxidation treatment was between 78.08 and 80.99%. Data seems to be very promising and proved the techno-functional and antioxidant properties of the current nanoemulsions, compared with the just only 49–57% that preserved the Free-oxidised chia seeds oils (Fig. 3).
Fig. 3.
Percentage of preserved antioxidant activity observed in Free and NE chia seeds oils after UV-induced stress
Obviously, the global antioxidant activity of chia oils extracts is not only caused by the profile of vitamin E isomers, but it also depends of the synergical effect of several not light-sensitive polyphenolics that does suffer less deterioration than photosensible antioxidants. However, it seems clear that DPPH and FRAP values after UV radiation were significantly higher in nanoemulsioned samples than those found in free chia seeds extracts. This behaviour was observed in the three matrixes of chia seeds oils, but specially in Nanoemulsion from Mexican chia seeds oil that displayed the highest values of FRAP and DPPH assays. Data are in a good agreement with those reported in previous works in which similar photo-stabilization effect has been described for other types of antioxidant nanostructures (Coradini et al. 2014).
These results emphasize the importance of the nanotechnology incorporation to development chia seeds oils-NE that protect against UV-induced oxidative stress. It's encouraging to display food formulations alternatives and these chia seeds oils nanoemulsions could be taken into account as an important source of antioxidants with food applications as valorising innovative products with antioxidant health benefit.
Conclusion
Chia seeds have proved to be an important source of natural tocochromanols. Accelerated solvent extraction (ASE) is a suitable technique to obtain antioxidant oily extracts from chia seeds enriched in vitamin E isomers reducing the T and T3 degradations, as happens with classic isolation techniques.
LC-DAD-ESI-MS/MS methodology is suitable to separately identify and quantify eight tocochromanols from chia seeds oils, which are especially rich in γ-T followed far behind by α-T and even presenting α-T3, β-T3 δ-T3 and γ-T3.
We have demonstrated that the geographical origin of chia seeds influences the levels of their tocochromanols, the total polyphenols and therefore the antioxidant activity; being Mexican provenance which offers the oily extracts richest in tocochromanols with the higher antioxidant power.
The successful design of chia seeds oil-in-water NE, using lecithin as GRAS emulsifier, provides effective lipid nanocarriers enriched on tocochromanols. Interestingly, the mild conditions of the formulation methods did not compromise the antioxidant capacity of the Chia seed oils.
Additionally, The NE helped to maintain around the 80% of the initial antioxidant capacity after UV-induced stress. This proves the potential of NE-chia seeds oils in food formulations to improve the shelf-life and the nutritional properties of other food products.
Acknowledgements
Authors thank the financial support given by the PEII-2014-040-P research project from the Junta de Comunidades de Castilla-La Mancha as well as the 01110AB026 research project given by the Diputación de Albacete. And also, to the financial support given by University of Castilla-La Mancha.
Compliance with ethical standards
Conflicts of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Lucía Castro-Vázquez, Email: luciaisabel.castro@uclm.es.
Virginia Rodríguez-Robledo, Email: Virginia.RRobledo@uclm.es.
María Plaza-Oliver, Email: plazolii@gmail.com.
Manuel J. Santander-Ortega, Email: Manuel.Santander@uclm.es
M. Victoria Lozano, Email: MVictoria.Lozano@uclm.es
Joaquín González, Email: Joaquin.GFuentes@uclm.es.
Noemí Villaseca, Email: Noemi.Villaseca@alu.uclm.es.
Pilar Marcos, Email: Pilar.Marcos@uclm.es.
M. Mar Arroyo-Jiménez, Email: Mariamar.Arroyo@uclm.es
References
- Ahsan H, Aha A, Siddiqui WA. A review of characterization of tocotrienols from plant oils and foods. J Chem Biol. 2015;8(2):45–59. doi: 10.1007/s12154-014-0127-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alanon ME, Castro-Vazquez L, Diaz-Maroto MC, Hermosin-Gutierrez I, Gordon MH, Perez-Coello MS. Antioxidant capacity and phenolic composition of different woods used in cooperage. Food Chem. 2011;129:1584–1590. doi: 10.1016/j.foodchem.2011.06.013. [DOI] [Google Scholar]
- Aranibar C, Aguirre A, Bo R. Utilization of a by-product of chia oil extraction as a potential source for value addition in wheat muffins. J Food Sci Technol. 2019;56:4189–4197. doi: 10.1007/s13197-019-03889-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Augustin MA, Hemar Y. Nano- and micro-structured assemblies for encapsulation of food ingredients. Chem Soc Rev. 2009;38:902–912. doi: 10.1039/B801739P. [DOI] [PubMed] [Google Scholar]
- Ayerza R, Coates W, Lauria M. Chia seed (Salvia hispanica L.) as an omega-3 fatty acid source for broilers: influence on fatty acid composition, cholesterol and fat content of white and dark meats, growth performance, and sensory characteristics. Poult Sci. 2002;81:826–837. doi: 10.1093/ps/81.6.826. [DOI] [PubMed] [Google Scholar]
- Benzie IFF, Strain JJ. The ferric reducing ability of plasma (FRAP) as a measure of "antioxidant power": The FRAP assay. Anal Biochem. 1996;239(1):70–76. doi: 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]
- Brigelius-Flohé R. Bioactivity of vitamin E. Nutr Res Rev. 2006;19(2):174–186. doi: 10.1017/S0954422407202938. [DOI] [PubMed] [Google Scholar]
- Bustamante-Rangel M, Delgado-Zamarreno MM, Sanchez-Perez A, Carabias-Martinez R. Determination of tocopherols and tocotrienols in cereals by pressurized liquid extraction-liquid chromatography-mass spectrometry. Anal Chim Acta. 2007;587(2):216–221. doi: 10.1016/j.aca.2007.01.049. [DOI] [PubMed] [Google Scholar]
- Borneo R, Aguirre A, Leon AE. Chia (Salvia hispanica L) gel can be used as egg or oil replacer in cake formulations. J Am Diet Assoc. 2010;110:946–9. doi: 10.1016/j.jada.2010.03.011. [DOI] [PubMed] [Google Scholar]
- Capitani MI, Spotorno V, Nolasco SM, Tomas MC. Physicochemical and functional characterization of by-products from chia (Salvia hispanica L.) seeds of Argentina. LWT-Food Sci Technol. 2012;45(1):94–102. doi: 10.1016/j.lwt.2011.07.012. [DOI] [Google Scholar]
- Castejon N, Luna P, Senorans FJ. Ultrasonic removal of mucilage for pressurized liquid extraction of omega-3 rich oil from chia seeds (Salvia hispanica L.) J Agric Food Chem. 2017;65(12):2572–2579. doi: 10.1021/acs.jafc.6b05726. [DOI] [PubMed] [Google Scholar]
- Castro-Vazquez L, Alanon ME, Rodriguez-Robledo V, Perez-Coello MS, Hermosin-Gutierrez I, Diaz-Maroto MC, Jordan J, Galindo MF, Arroyo-Jimenez MD. Bioactive flavonoids, antioxidant behaviour, and cytoprotective effects of dried grapefruit peels (Citrus paradisi Macf.) Oxid Med Cell Longev. 2016;3:1–12. doi: 10.1155/2016/8915729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen MH, Bergman C. A rapid procedure for analysing rice bran tocopherol, tocotrienol and y-oryzanol contents. J Food Compos Anal. 2005;18(4):139–151. doi: 10.1016/j.jfca.2003.09.004. [DOI] [Google Scholar]
- Chen LY, Remondetto GE, Subirade M. Food protein-based materials as nutraceutical delivery systems. Trends Food Sci Technol. 2006;17:272–283. doi: 10.1016/j.tifs.2005.12.011. [DOI] [Google Scholar]
- Coradini K, Lima FO, Oliveira CM, Chaves PS, Athayde ML, Carvalho LM, Beck RC. Co-encapsulation of resveratrol and curcumin in lipid-core nanocapsules improves their in vitro antioxidant effects. Eur J Pharm Biopharm. 2014;88(1):178–185. doi: 10.1016/j.ejpb.2014.04.009. [DOI] [PubMed] [Google Scholar]
- da Silva JK, Cazarin CB, Colomeu TC, Batista AG, Meletti LM, Paschoal JAR, Zollner RD. Antioxidant activity of aqueous extract of passion fruit (Passiflora edulis) leaves: in vitro and in vivo study. Food Res Int. 2013;53(2):882–890. doi: 10.1016/j.foodres.2012.12.043. [DOI] [Google Scholar]
- da Silva BP, Anunciacao PC, Matyelka JC, Della Lucia CM, Martino HS, Pinheiro-Sant'Ana HM. Chemical composition of Brazilian chia seeds grown in different places. Food Chem. 2017;221:1709–1716. doi: 10.1016/j.foodchem.2016.10.115. [DOI] [PubMed] [Google Scholar]
- Delgado-Zamarreno MM, Bustamante-Rangel M, Sanchez-Perez A, Carabias-Martinez R. Pressurized liquid extraction prior to liquid chromatography with electrochemical detection for the analysis of vitamin E isomers in seeds and nuts. Journal of Chromatogr A. 2004;1056(1–2):249–252. doi: 10.1016/j.chroma.2004.09.033. [DOI] [PubMed] [Google Scholar]
- Dersjant-Li Y, Peisker M. A critical review of methodologies used in determination of relative bio-availability ratio of RRR-alpha-tocopheryl acetate and all-rac-alpha-tocopheryl acetate. J Sci Food Agric. 2010;90(10):1571–1577. doi: 10.1002/jsfa.3994. [DOI] [PubMed] [Google Scholar]
- Falk J, Munne-Bosch S. Tocochromanol functions in plants: antioxidation and beyond. J Exp Bot. 2010;61(6):1549–1566. doi: 10.1093/jxb/erq030. [DOI] [PubMed] [Google Scholar]
- Fang ZX, Bhandari B. Encapsulation of polyphenols - a review. Trends Food Sci Technol. 2010;21:510–523. doi: 10.1016/j.tifs.2010.08.003. [DOI] [Google Scholar]
- Ferreirade Mello BT, Iwassa I, Santos Garcia VA, Silva C. Methyl acetate as solvent in pressurized liquid extraction of crambe seed oil. J Supercrit Fluid. 2019;145:66–73. doi: 10.1016/j.supflu.2018.11.024. [DOI] [Google Scholar]
- Ixtaina VY, Martinez ML, Spotorno V, Mateo CM, Maestri DM, Diehl BW, Nolasc SM, Tomas MC. Characterization of chia seed oils obtained by pressing and solvent extraction. J Food Compos Anal. 2011;24:166–174. doi: 10.1016/j.jfca.2010.08.006. [DOI] [Google Scholar]
- Julio LM, Ixtaina VY, Fernández MA, Sánchez RMT, Wagner JR. Chia seed oil-in-water emulsions as potential delivery systems of ω-3 fatty acids. J Food Eng. 2015;162:48–55. doi: 10.1016/j.jfoodeng.2015.04.005. [DOI] [Google Scholar]
- Julio Luciana M, Ixtaina VY, Fernandez M, Torres Sanchez R, Nolasco S, Tomas MC. Development and characterization of functional O/W emulsions with chia seed (Salvia hispanica L.) by-products. J Food Sci Technol. 2016;53(8):3206–3214. doi: 10.1007/s13197-016-2295-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khanna S, Parinandi NL, Kotha SR, Roy S, Rink C, Bibus D, Sen CK. Nanomolar vitamin E alpha-tocotrienol inhibits glutamate-induced activation of phospholipase A2 and causes neuroprotection. J Neurochem. 2010;112(5):1249–1260. doi: 10.1111/j.1471-4159.2009.06550.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kua YL, Gan SY, Morris A. A validated, rapid, simple and economical high-performance liquid-chromatography method to quantify palm tocopherol and tocotrienols. J Food Compos Anal. 2016;53:22–29. doi: 10.1016/j.jfca.2016.09.003. [DOI] [Google Scholar]
- Lozano MV, Esteban H, Brea J, Loza MI, Torres D, Alonso MJ. Intracellular delivery of docetaxel using freeze-dried polysaccharide nanocapsules. J Microencapsul. 2013;30:181–8. doi: 10.3109/02652048.2012.714411. [DOI] [PubMed] [Google Scholar]
- Marineli RD, Moraes E, Lenquiste SA, Godoy AT, Eberlin MN, Marostica MR. Chemical characterization and antioxidant potential of Chilean chia seeds and oil (Salvia hispanica L.) LWT-Food Sci Technol. 2014;59:1304–1310. doi: 10.1016/j.lwt.2014.04.014. [DOI] [Google Scholar]
- Martinez-Cruz O, Paredes-Lopez O. Phytochemical profile and nutraceutical potential of chia seeds (Salvia hispanica L.) by ultra high performance liquid chromatography. J Chromatogr A. 2014;1346:43–48. doi: 10.1016/j.chroma.2014.04.007. [DOI] [PubMed] [Google Scholar]
- McClements DJ, Xiao H. Potential biological fate of ingested nanoemulsions: influence of particle characteristics. Food Funct. 2012;3:202–220. doi: 10.1039/C1FO10193E. [DOI] [PubMed] [Google Scholar]
- Pilarova V, Gottvald T, Svoboda P, Novak O, Benesova K, Belakova S, Novakova L. Development and optimization of ultra-high performance supercritical fluid chromatography mass spectrometry method for high-throughput determination of tocopherols and tocotrienols in human serum. Anal Chim Acta. 2016;934:252–265. doi: 10.1016/j.aca.2016.06.008. [DOI] [PubMed] [Google Scholar]
- Plaza-Oliver M, Baranda JF, Rodriguez Robledo V, Castro-Vazquez L, Gonzalez-Fuentes J, Marcos P, Arroyo-Jimenez MM. Design of the interface of edible nanoemulsions to modulate the bioaccessibility of neuroprotective antioxidants. Int J Pharm Pract. 2015;490(1–2):209–218. doi: 10.1016/j.ijpharm.2015.05.031. [DOI] [PubMed] [Google Scholar]
- Pizarro PL, Lopes AE, Silva CA, Sammán NC, Dupas M, Kil CY. Functional bread with n-3 alpha linolenic acid from whole chia (Salvia hispanica L.) flour. J Food Sci Technol. 2015;52(7):4475–4482. doi: 10.1007/s13197-014-1477-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porras-Loaiza P, Jimenez-Munguia MT, Sosa-Morales ME, Palou E, Lopez-Malo A. Physical properties, chemical characterization and fatty acid composition of Mexican chia (Salvia hispanica L.) seeds. Int J Food Sci Technol. 2014;49(2):571–577. doi: 10.1111/ijfs.12339. [DOI] [Google Scholar]
- Portilho C, Ferreira de Mello BT, Ferreira Cabral V, Camila da Silva C. Crambe seed oil: extraction and reaction with dimethyl carbonate under pressurized conditions. J Supercrit Fluid. 2020;159:104780. doi: 10.1016/j.supflu.2020.104780. [DOI] [Google Scholar]
- Poudyal H, Panchal SK, Waanders J, Ward L, Brown L. Lipid redistribution by alpha-linolenic acid-rich chia seed inhibits stearoyl-CoA desaturase-1 and induces cardiac and hepatic protection in diet-induced obese rats. J Nutr Biochem. 2012;23:153–162. doi: 10.1016/j.jnutbio.2010.11.011. [DOI] [PubMed] [Google Scholar]
- Reyes-Caudillo E, Tecante A, Valdivia-Lopez MA. Dietary fibre content and antioxidant activity of phenolic compounds present in Mexican chia (Salvia hispanica L.) seeds. Food Chem. 2008;107(2):656–663. doi: 10.1016/j.foodchem.2007.08.062. [DOI] [Google Scholar]
- Santos Freitas DL, Jacques RA, Richter MF, Silva AL, Caramao EB. Pressurized liquid extraction of vitamin E from Brazilian grape seed oil. JChromatogr A. 2008;18:80–3. doi: 10.1016/j.chroma.2008.02.067. [DOI] [PubMed] [Google Scholar]
- Villaseca-Gonzalez N, Robledo RV, Castro-Vazquez L, Lozano MV, Santander-Ortega MJ, Gonzalez-Fuentes J, Marcos P, Arroyo-Jimenez Mari MD. Ultrafast determination of vitamin E using LC-ESI-MS/MS for preclinical development of new nutraceutical formulations. Bioanalysis. 2018;10:215–227. doi: 10.4155/bio-2017-0095. [DOI] [PubMed] [Google Scholar]