Abstract
The aim of this work was to assess the effectiveness of dipping chicken breast in lactic, malic and fumaric acid 3% solutions for 15 s on Salmonella counts, as well as on chicken meat quality and sensory characteristics. All three treatments effectively reduced Salmonella counts. The values of Salmonella log reduction were 2.22, 1.55 and 1.30 log CFU/g for fumaric, malic and lactic treatments, respectively. Although fumaric acid was the most effective for reducing Salmonella counts, chicken meat quality and sensory characteristics were significantly affected, even in cooked samples. Conversely, malic and lactic acids treatments caused minimal changes in chicken meat quality and sensory characteristics compared to control samples. This study shows effective alternatives to reduce Salmonella contamination on chicken breast fillets, although further studies should be considered to improve the effects on quality and sensory attributes.
Keywords: Foodborne pathogen, Organic acids, Poultry meat, Color, Flavor, Texture
Introduction
Salmonella is a zoonotic worldwide-distributed pathogenic bacteria. It is frequently found as a contaminant on chicken carcasses destined for human consumption and is considered one of the most important causes of food-borne disease globally (Adams and Moss 2008). Poultry meat is frequently associated with Salmonella outbreaks (Allerberger 2016; Antunes et al. 2016). Chickens often carry non-typhoidal Salmonella strains in their guts, which are plausible of contaminating meat surface during slaughter even when good processing practices are being followed (Padungtod and Kaneene 2005). Mckee (2011) has reported that only 3–4% broilers entering the slaughterhouses were positive for Salmonella, but 35% of the positive at the end of the plant process, clearly showing the spread of contamination at this stage.
Organic acids are widely used in the food industry as chemical sanitizers. It has been probed their antibacterial activity in several food matrices and they are generally recognized as safe (GRAS) for human consumption (Mani-López et al. 2012). Implementation of organic acids as antimicrobial agents in the food material depends on several characteristic properties of the acids such as chemical formula, physical form, pKa value, molecular weight, minimum inhibitory concentration, nature of the microorganism, buffering properties of the food, and acid-food exposure time (Moore et al. 2017; Dittoe et al. 2018; Coban 2019).
Lactic acid is one of the most widely accepted organic acids used for carcass decontamination by the food industry (Huffman 2002). Over the past ten years, many studies have reported lactic acid effectiveness against foodborne pathogens in meat (Ransom et al. 2003; Killinger et al. 2010). Malic acid has also been studied as a sanitizer in foods. It has been reported that the combination of malic acid and acetic acid was able to significantly reduce Salmonella counts on chicken breasts (Olaimat et al. 2018). As to fumaric acid antimicrobial efficacy in foods, studies are scarce, however, its bactericide effect has been demonstrated in lettuce and apple cider (Comes and Beelman 2002; Kondo et al. 2006).
Few studies have been conducted in order to assess the effects of organic acids on chicken meat quality and sensory characteristics. Hecer and Guldas (2011) studied the effect of dipping treatment with lactic acid (0.5–1%) and fumaric acid (0.5–1%) in broiler wings and reported that the panelists did not perceive negative effects on the sensorial attributes of the treated samples. Zhu et al. (2016) demonstrated that the combined treatment of 0.5% of lactic acid and 1% of citric acid did not affect the physicochemical properties and sensory attributes of the quick-frozen chicken drumsticks during storage. To the best of our knowledge, no studies have been carried out to assess the effects of malic acid on meat quality and sensory characteristics of poultry products. The present study aimed to evaluate the effects of lactic, malic and fumaric acid not only on Salmonella spp. counts but also on chicken meat quality and sensory characteristics.
Materials and methods
Inoculum preparation
Salmonella strains used in this work were identified as Salmonella enterica subsp. enterica serotype Typhimurium, Enteritidis, Thompson, Heidelberg and Schwarzengrund. Strains were originally isolated at different stages of the poultry chain and they were kindly provided by Dr. Pablo Chacana from Pathobiology Institute, INTA Castelar, Argentina. Cells in stationary phase of growth were prepared by individually subculturing one single colony from each serotype in 10 ml of Tryptic Soy Broth (TSB, Oxoid, UK) and incubating the preparation for 24 h at 37 °C. After incubation, 1 ml of each test tube was centrifuged at 3000 × g for 5 min and pellets were twice washed with phosphate-buffered saline (PBS, pH 7.2, Oxoid). Finally, the pool of strains was prepared by mixing equal volumes of each serotype in PBS.
Sample preparation
Bags of skinless chicken breast were purchased from a local supermarket. After freezing they were irradiated with 10 kGy in order to eliminate the interference of local microbiota. This procedure was carried out in a semi-industrial irradiation facility (cobalt-60 source) at the Ezeiza Atomic Center, National Commission of Atomic Energy of Argentina (CNEA), with an activity of 820 kCi. Irradiated chicken breasts were aseptically sliced with a sterile punch, to obtain samples of 25 cm2 and 25 g. Samples were individually disposed on sterile petri dishes.
Organic acid solutions
For the 3 organic acids evaluated, the concentration used was of 3% w/v. The solutions of lactic acid (LA; Sigma-Aldrich, Canada), malic acid (MA; Biopack, Argentina) and fumaric acid (FA; Sigma-Aldrich, Canada) were prepared and handled according to manufacturer’s recommendations. Regarding FA, it was kept at a temperature of 50 °C, to avoid its precipitation, over the whole procedure, from acid preparation to sample treatment. The other acids were utilized at room temperature.
Sample inoculation
For the inoculation procedure, 50 µl of Salmonella pool was applied onto the sample surface and evenly spread with a sterile Drigalsky spatula. Inoculated samples were allowed to dry for 40 min at room temperature in a biological safety cabinet (BSL-2) before treatment.
Treatments
Following aseptic procedures, samples were individually immersed for 15 s in 100 ml of the respective antimicrobial solution: AL 3%, AF, 3% and AM 3%. Controls were immersed for 15 s in 100 ml of sterile tap water, while another group of samples was left untreated. After treatment, samples were individually packed in sterile stomacher bags and kept at 4 °C overnight before microbiological analysis was performed.
Microbiological analysis
A total of 225 ml of 0.1% Peptone Water (PW, Biokar diagnostics, France) was added to each stomacher bag containing an individual sample. Immediately after, samples were passed through a Stomacher (easy Mix, AES, France) for 60 s and serially decimal dilutions were prepared. Salmonella counts were performed in Tryptic Soy agar (TSA, Biokar, France) as non-selective media and on XLD, as selective media. The values of log reduction were estimated by subtracting TSA counts of samples treated with the different organic acids from TSA counts of samples treated with tap water. Injured cells were expressed as percentages and calculated as the difference in microbial counts between TSA and XLD, divided by TSA counts and multiplied by 100.
Quality analysis
Cooking procedure
Samples were cooked at 80 °C using an electric convection oven (Oster, CKSTPA488, China) until reached 70 °C at the core. The internal temperature was monitored with T thermocouples and all samples were cooked at the same time.
Chromatic parameters analysis
The chromatic parameters of the raw and cooked chicken samples were determined using a colorimeter (model CR-400, Konica Minolta Sensing, Osaka, Japan) with a D 65 illuminant, 2° observer angle and calibrated using a standard white reflector tile. Five random points were measured on each piece. Results were expressed as lightness (L*), hue angle (h°), saturation index (C*), and color difference (ΔE) and were determined using the software of the colorimeter.
Texture profile analysis
The texture profile was evaluated using the Warner–Bratzler test. Measurements were carried out in a Texture Analyzer Stable MicroSystems (model TA-XT2i, Surrey, U.K.) at 25 °C. The assay parameters used were: test speed 1 mm/s, a cutting distance of 30 mm, a trigger force of 5 g, and a load cell of 50 kg. Cooked samples were cut into sticks of 1.5 cm in diameter and 1.5 cm in thickness, parallel to the muscle fiber orientation. Firmness (maximum cutting force, g) and work of shear (area under the force–deformation curve, g.s.) were determined using the Texture Expert software.
Sensory analysis
The visual color evaluation was carried out with raw samples and by a test of difference against a control using two blind controls (Muñoz et al. 1992). Twenty consumers randomly selected evaluated the color following verbal scale with 7 points: “much clearer than R (control samples)” (− 3), “quite clearer than R” (− 2), “slightly clearer than R” (− 1), “no difference of color concerning R” (0); “Slightly darker than R” (1); “Quite darker than R” (2) and “much darker than R” (3). The test was developed in a cabinet with standardized light (Verivide, CAC 120, UK) and repeated twice with an intermediate time of 15 min. The flavor test was carried out by a triangular similarity test with a panel of semi-trained evaluators (32, without repetition). Each evaluator received three series of cooked chicken samples and identified which was the different sample. Data analysis was carried out by the comparison of the correct responses (identify of the different samples) with the table data based in the binomial distribution (minimum number of responses required to declare significance at the defined α level) (Lawless and Heymann 2010).
Experimental design and statistical analysis
Experiments were conducted independently three times, with three samples per test in each replicate. The effects of organics acids on Salmonella counts and quality parameters (assays 2.7.1 and 2.7.2) were analyzed using a one-way ANOVA analysis (Adhikari et al. 2020; Mohan and Purohit 2020). Treatments that showed a P < 0.05 indicated significant effects. Based on the data of the Levene test, the mean values were compared through a comparison test (Tukey or Thamane) to determine significant differences (P < 0.05). Regarding, the visual color evaluation data were analyzed using a two-factor ANOVA (samples—evaluator) followed by a Dunnet test to determine significant differences (P < 0.05) between samples (Rogers 2017). The software used for the analyses was SPSS version 21 (SPSS Inc., Chicago, Ill, USA).
Results
Microbiological analysis
The mean Salmonella counts in TSA and XLD, the log reductions and the percentages of injured cells after treatments are shown in Table 1. No significant differences (P > 0.05) were observed between samples treated with tap water and untreated samples nor in TSA neither in XLD. Salmonella counts of samples treated with FA were significantly different (P < 0.05) from samples treated with tap water and from samples treated with MA and LA in both, TSA and XLD. The log reduction achieved for these samples was 2.22 log CFU/g and the percentage of injured cells was 17%. Salmonella counts of samples treated with MA were significantly different (P < 0.05) from samples treated with tap water but similar to samples treated with LA in both, TSA and XLD. The log reduction achieved for these samples was 1.55 log CFU/g and the percentage of injured cells was of 11%. Finally, the log reduction achieved for samples treated with LA was 1.30 log CFU/g and the percentage of injured cells was 11% (P < 0.05).
Table 1.
Salmonella counts in TSA and XLD in chicken breast of untreated samples and samples treated with tap water, fumaric, malic and lactic acid
| Treatment | TSA (log CFU/g) | XLD (log CFU/g) | Log reductions (log CFU/g) | Injured cells (%) |
|---|---|---|---|---|
| Untreated | 8.09 ± 0.38 a | 7.90 ± 0.43 a | N/A | N/A |
| Tap water | 8.09 ± 0.45 a | 7.70 ± 0.21 b | 0 | 5 |
| Fumaric acid 3% | 5.87 ± 0.63 c | 4.86 ± 0.72 d | 2.22 | 17 |
| Malic acid 3% | 6.54 ± 0.20 bc | 5.83 ± 0.19 c | 1.55 | 11 |
| Lactic acid 3% | 6.79 ± 0.16 b | 6.03 ± 0.17 c | 1.30 | 11 |
Results are expressed as mean (SD); n = 9 per treatment
a, b, c Interventions with no common letter differed significantly (P < 0.05; one-way ANOVA)
Quality analysis
Chromatic parameters and the texture profile analysis
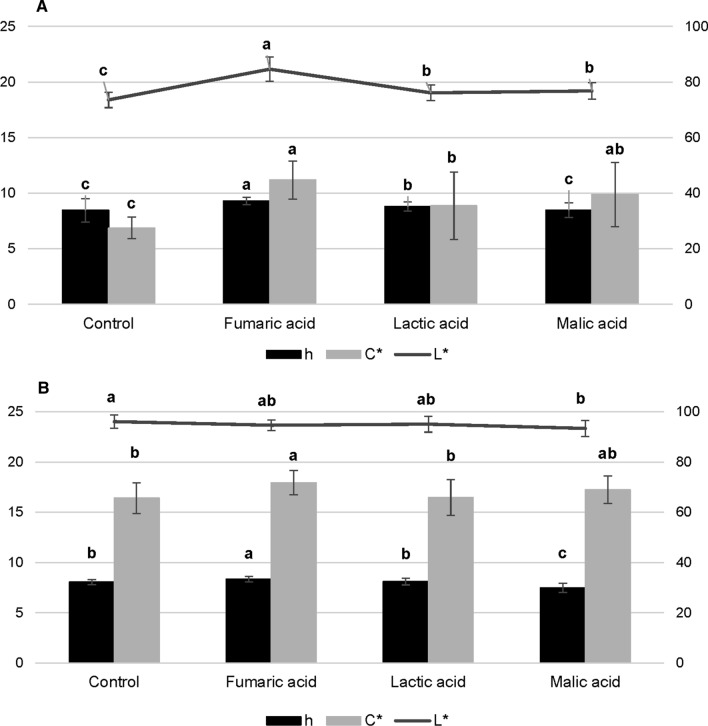
Chromatic parameters and the texture profile are shown in Figs. 1 and 2, respectively. Overall, FA, MA, and LA caused a significant increase in L*, h, and C* values (P < 0.05) in raw samples. Only samples treated with MA presented a similar h than samples treated with tap water. The color difference (ΔE) found between samples treated with tap water and samples treated with FA was 11.9, with MA was 4.47 and with LA was 3.35. As to cooked samples, small differences (P ≤ 0.05) in L* values were found among samples treated with tap water, FA, and LA. Only cooked samples treated with MA (< L* values) were significantly different (P < 0.05) from samples treated with tap water. As to C* and h, the FA treatment caused the highest effect on these parameters, while significant differences (P > 0.05) were not found between samples treated with LA and tap water. The color difference (ΔE) found between control samples treated with tap water and samples treated with FA was 2.5, with MA was 3.65 and with LA was 1.03.
Fig. 1.
Effect of organic acid treatments on chromatic parameters of chicken meat: A raw samples and B cooked samples. (a–c) Interventions with no common letter differed significantly (P < 0.05) using one-way ANOVA
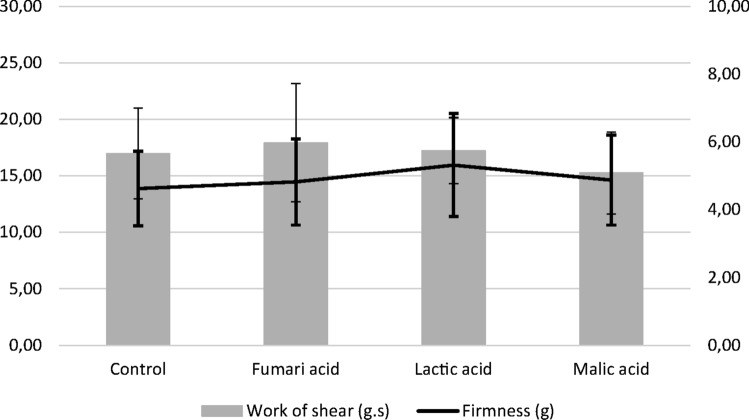
Fig. 2.
Effect of organic acid treatments on the texture profile of chicken meat
Regarding the texture profile, FA and LA treatments caused a slight increase in firmness and work of shear, while a decrease was observed in samples treated with MA. However, significant differences (P > 0.05) were not found with the samples treated with tap water.
Sensory analysis
As to the visual color evaluation, panelists perceived significant differences (P < 0.05) among samples treated with FA, MA, and samples treated with tap water. As to samples treated with LA no differences were perceived with samples treated with tap water (P < 0.05). Regarding flavor, the panelists indicated that samples treated with FA were significant different (P < 0.05) from samples treated with water, while samples treated with MA and LA were perceived as similar to samples treated with tap water.
Discussion
In the present study, Salmonella log reduction after dipping chicken breast in LA 3% solution for 15 s was 1.30 log CFU/g. Similar results were reported by Ilhak et al. (2018) who evaluated the reduction of Salmonella Typhimurium after dipping skinless chicken breast in LA 4% solution for 1 min and informed a reduction of 1.4 log CFU/g. This finding was very interesting as Ilhak et al. (2018) evaluated a longer exposure time (1 min vs 15 s) and a higher LA concentration (4 vs 3%) than those evaluated in the present study. The fact of achieving similar reductions with shorter exposure time and lower LA concentration represents an advantage since, under these conditions; it is less likely that the decontamination treatment adversely affects the general appearance and nutritional content of the poultry products. Likewise, shorter decontamination treatment times are more feasible with regard to integration of decontamination as an in-line operation during processing (Riedel et al. 2009). The application method also appears to have a significant impact on the decontamination efficacy of organic acids solutions. Ramirez-Hernandez et al. (2018) evaluated the effectiveness of a 15 s LA 2.84% treatment applied by spray to reduce Salmonella in chicken breasts and reported that no significant reduction was observed. In this case, LA concentrations were similar to our study and the exposure time was the same as ours (15 s). The main difference, however, was that Ramirez-Hernandez et al. (2018) used a spray method while we used a dip one. Based on these findings, dipping treatment appears to be more effective than spraying. This has been previously reported by Kim and Slavik (1995) and Wolf et al. (2012).
Salmonella log reduction after dipping chicken breast in a MA 3% solution for 15 s was 1.55 log CFU/g. Tamblyn and Conner (1997) evaluated the effectiveness of a 15 s and a 2 min dip treatments in 4% MA solution but on chicken skin instead of chicken breast. Cited authors reported no reduction in Salmonella counts after the 15 s treatment and a 1.46 log CFU/g reduction after the 2 min treatment. Conversely, Mohan and Pohlman (2016) reported a reduction of 2.23 log CFU/g of coliform populations after dipping beef trimmings in a 3% MA solution for 15 s. As the antimicrobial activity of organic acids depends on the pH and the buffering capacity of the food matrix, the discrepancies between results may be attributed to the type of treated tissue (Lianou et al. 2012). Olaimat et al. (2018) evaluated a dip treatment in a 0.5% MA solution for 5 min to decontaminate chicken breasts and reported a reduction lower than 0.5 log CFU/g in Salmonella population after 1 day of storage and a reduction of 2.7 log CFU/g after 10 days of storage. The lower reductions reported by Olaimat et al. (2018) at day 1 may be due to the lower concentration in MA solution (3 vs 0.5%) that could not be compensated by the higher exposure time (5 min vs 15 s). However, after 10 days of storage, the reductions were higher than those reported in the present study. These results suggest that an increase in Salmonella´s reduction may be expected after several days of storage. This is due to the fact that organic acids not only exhibit an immediate bactericidal effect but also a residual effect (Lianou et al. 2012). Further studies should be done to demonstrate this hypothesis.
Salmonella log reduction after dipping chicken breast in a FA 3% solution for 15 s was 2.22 log CFU/g. To the best of our knowledge, no previous studies have been conducted to assess the effectiveness of FA in reducing Salmonella population in poultry products. However, some studies demonstrated its effectiveness as an antimicrobial treatment. Tango et al. (2015) reported that a concentration of 0.25% of FA was capable of reducing, to an undetectable level, an in vitro Salmonella Typhimurium culture of 8–9 log CFU/ml. Other studies demonstrated its effectiveness against Salmonella Typhimurium on fresh cut lettuce and against Escherichia coli O157:H7 in apple cider (Comes and Beelman 2002; Kondo et al. 2006). Based on these results, FA appears as an interesting alternative to conventional interventions frequently used for poultry product decontamination.
The percentages of injured Salmonella cells after treatment were 17% for FA and 11% for MA and LA. Regarding LA, similar results were reported by Dickson and Siragusa (1994) who informed 15% of injured Salmonella cells after washing beef tissue with 1% LA solution. The presence of injured cells in a food can pose major public health concerns, since many bacterial pathogens can become more resistant to cooking and other commonly used microbial reduction strategies as a result of sublethal injury (Wesche et al. 2009).
LA was the most effective treatment at preserving chicken meat quality and sensory characteristics, followed by MA and FA treatments. Regarding the chromatic parameters of raw samples, all 3 organic acids evaluated caused significant differences compared to the samples treated with tap water. Samples treated with FA were the most affected, presenting a pinker color with greater intensity and clarity than the samples treated with tap water. These results were, in general, consistent with those observed in the sensory analysis. The panelists perceived color differences among samples treated with tap water and samples treated with FA and MA while no differences were perceived between samples treated with tap water and samples treated with LA. Several authors had reported that after the use of organic acids, the pH value of the samples decreases (Kim and Slavik 1995; Lim and Mustapha 2004; Harris et al. 2012). That could explain the changes in the chromatic parameters of raw samples observed in our experiment. The decrease in pH value causes protein denaturation, giving a paler appearance to treated meat. Given the fact that we did not include measurements of pH values as part of our methodology, we can only speculate about the possible existence of a causal relationship between those two observed results. After cooking, these color differences decreased to the point that LA treated samples were similar to samples treated with tap water. Regarding flavor, the panelists indicated that samples treated with FA were significantly different from samples treated with water, while samples treated with MA and LA were perceived as similar to samples treated with tap water.
Little information is available regarding the effects of organic acids treatments on quality parameters of chicken breasts treated under conditions similar to the ones defined in the present study. Therefore, comparison between assays should be interpreted cautiously. As to the use of LA, our results were in agreement with those reported by González-Fandos and Dominguez (2006) and, Hecer and Guldas (2011). González-Fandos and Dominguez (2006) reported that the sensory properties (odor, color, texture, and overall appearance) of poultry legs were not affected by the LA treatments at 0.22 M (~ 2% w/v) and 0.11 M (~ 1% w/v) for 5 min. Hecer and Guldas (2011) reported that treatments using concentrations of 0.5–1% for 10 min, did not affect sensory properties of chicken wings. Hecer and Guldas (2011) also evaluated the effects of FA treatment and reported that, after dipping broiler wings in a 0.5–1% FA solution, panelists did not detect undesirable effects on the sensory attributes. Regarding MA, González-Fandos and Naiara (2015) reported that after MA treatments (1% and 2% for 5 min) on chicken legs, non-undesirable effects on quality characteristics (color and odor) were detected. Likewise, Skřivanová et al. (2011) reported that the sensory analysis revealed no changes in the appearance or odor of the raw chicken skin treated with MA (0.5% for 60 s). These results differed from our results as in our case, panelists perceived color differences among samples treated with tap water and samples treated with FA and MA. This discrepancy may be due to the lower concentration of FA and MA solution used by González-Fandos and Dominguez (2006), Hecer and Guldas (2011) and Skřivanová et al. (2011) compared to the concentration used in the present study (0.5–1–2% vs 3%).
Conclusion
Dipping skinless chicken breast in a 3% lactic, malic or fumaric acid solutions for 15 s significantly reduced Salmonella counts. Lactic and malic acid treatments caused minimal changes while fumaric acid treatment caused significantly changes in chicken meat quality and sensory characteristics.
Acknowledgements
This work was funded with grants from a PICT of Universidad de Moron and from the Instituto Nacional de Tecnología Agropecuaria (INTA) of Argentina coded named PNAIyVA 1130042. We thank Celina Horak and Juan Ignacio Garrido from the Centro Nacional de Energía Atómica (CNEA) for their assistance.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Marina Mozgovoj and Mariana Cap have contributed equally to this work
References
- Adams R, Moss MO. Food microbiology. 3. Cambridge: RSC Publishing; 2008. [Google Scholar]
- Adhikari P, Yadav S, Cosby DE, et al. Research note: effect of organic acid mixture on growth performance and Salmonella Typhimurium colonization in broiler chickens. Poult Sci. 2020;99:2645–2649. doi: 10.1016/j.psj.2019.12.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allerberger F. Poultry and human infections. Clin Microbiol Infect. 2016;22:101–102. doi: 10.1016/j.cmi.2015.10.016. [DOI] [PubMed] [Google Scholar]
- Antunes P, Mourão J, Campos J, Peixe L. Salmonellosis: the role of poultry meat. Clin Microbiol Infect. 2016;22:110–121. doi: 10.1016/j.cmi.2015.12.004. [DOI] [PubMed] [Google Scholar]
- Coban HB. Organic acids as antimicrobial food agents: applications and microbial productions. Bioprocess Biosyst Eng. 2019 doi: 10.1007/s00449-019-02256-w. [DOI] [PubMed] [Google Scholar]
- Comes JE, Beelman RB. Addition of fumaric acid and sodium benzoate as an alternative method to achieve a 5-log reduction of Escherichia coli O157:H7 populations in apple cider. J Food Prot. 2002;65:476–483. doi: 10.4315/0362-028X-65.3.476. [DOI] [PubMed] [Google Scholar]
- Dickson JS, Siragusa GR. Survival of Salmonella Typhimurium, Escherichia coli O157:H7 and Listeria monocytogenes during storage on beef sanitized with organic acids. J Food Saf. 1994;14:313–327. doi: 10.1111/j.1745-4565.1994.tb00603.x. [DOI] [Google Scholar]
- Dittoe DK, Ricke SC, Kiess AS. Organic acids and potential for modifying the avian gastrointestinal tract and reducing pathogens and disease. Front Vet Sci. 2018;5:1–12. doi: 10.3389/fvets.2018.00216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González-Fandos E, Dominguez JL. Efficacy of lactic acid against Listeria monocytogenes attached to poultry skin during refrigerated storage. J Appl Microbiol. 2006;101:1331–1339. doi: 10.1111/j.1365-2672.2006.03022.x. [DOI] [PubMed] [Google Scholar]
- González-Fandos E, Naiara M. Efficacy of malic acid against Campylobacter jejuni attached to chicken skin during refrigerated storage. J Food Process Preserv. 2015 doi: 10.1111/jfpp.12637. [DOI] [PubMed] [Google Scholar]
- Harris D, Brashears MM, Garmyn AJ, et al. Microbiological and organoleptic characteristics of beef trim and ground beef treated with acetic acid, lactic acid, acidified sodium chlorite, or sterile water in a simulated commercial processing environment to reduce Escherichia coli O157:H7 and Salmon. Meat Sci. 2012;90:783–788. doi: 10.1016/j.meatsci.2011.11.014. [DOI] [PubMed] [Google Scholar]
- Hecer C, Guldas M. Effects of lactic acid, fumaric acid and chlorine dioxide on shelf-life of broiler wings during storage. J Microbiol Res. 2011;5:3880–3883. [Google Scholar]
- Huffman RD. Current and future technologies for the decontamination of carcasses and fresh meat. Meat Sci. 2002;62:285–294. doi: 10.1016/S0309-1740(02)00120-1. [DOI] [PubMed] [Google Scholar]
- Ilhak OI, Incili GK, Durmusoglu H. Effect of some chemical decontaminants on the survival of Listeria monocytogenes and Salmonella Typhimurium with different attachment times on chicken drumstick and breast meat. J Food Sci Technol Sci Technol. 2018;55:3093–3097. doi: 10.1007/s13197-018-3234-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killinger KM, Kannan A, Bary AI, Cogger CG. Validation of a 2 percent lactic acid antimicrobial rinse for mobile poultry slaughter operations. J Food Prot. 2010;73:2079–2083. doi: 10.4315/0362-028X-73.11.2079. [DOI] [PubMed] [Google Scholar]
- Kim J, Slavik MF. Cetylpyridinium chloride (CPC) treatment on poultry skin to reduce attached Salmonella. J Food Prot. 1995;59:322–326. doi: 10.4315/0362-028X-59.3.322. [DOI] [PubMed] [Google Scholar]
- Kondo N, Murata M, Isshiki K. Efficiency of sodium hypochlorite, fumaric acid, and mild heat in killing native microflora and Escherichia coli O157:H7, Salmonella typhimurium DT104, and Staphylococcus aureus attached to fresh-cut lettuce. J Food Prot. 2006;69:323–329. doi: 10.4315/0362-028X-69.2.323. [DOI] [PubMed] [Google Scholar]
- Lawless HT, Heymann H (2010) Sensory evaluation of food science principles and practices. Chapter 1, 2nd edn. Ithaca, New York. 10.1007/978-1-4419-6488-5
- Lianou A, Koutsoumanis KP, Sofos JN. Organic acids and other chemical treatments for microbial decontamination of food. In: Demirci A, Ngadi MO, editors. Microbial decontamination in the food industry. Amsterdam: Elsevier; 2012. pp. 592–664. [Google Scholar]
- Lim K, Mustapha A. Effects of cetylpyridinium chloride, acidi ed sodium chlorite, and potassium sorbate on populations of Escherichia coli O157:H7, Listeria monocytogenes, and Staphylococcus aureus on fresh beef. J Food Prot. 2004;67:310–315. doi: 10.4315/0362-028X-67.2.310. [DOI] [PubMed] [Google Scholar]
- Mani-López E, García HS, López-Malo A. Organic acids as antimicrobials to control Salmonella in meat and poultry products. Food Res Int. 2012;45:713–721. doi: 10.1016/j.foodres.2011.04.043. [DOI] [Google Scholar]
- Mckee S (2011) Salmonella control in poultry processing. In: 65th Annual Reciprocal Meat Conference, pp 1–4
- Mohan A, Pohlman FW. Role of organic acids and peroxyacetic acid as antimicrobial intervention for controlling Escherichia coli O157:H7 on beef trimmings. LWT-Food Sci Technol. 2016;65:868–873. doi: 10.1016/j.lwt.2015.08.077. [DOI] [Google Scholar]
- Mohan A, Purohit AS. Anti-Salmonella activity of pyruvic and succinic acid in combination with oregano essential oil. Food Control. 2020 doi: 10.1016/j.foodcont.2019.106960. [DOI] [Google Scholar]
- Moore A, Nannapaneni R, Kiess A, Sharma CS. Evaluation of USDA approved antimicrobials on the reduction of Salmonella and Campylobacter in ground chicken frames and their effect on meat quality. Poult Sci. 2017;96:2385–2392. doi: 10.3382/ps/pew497. [DOI] [PubMed] [Google Scholar]
- Muñoz AM, Civille GV, Carr BT. Sensory evaluation in quality control. 1. New York: Springer; 1992. [Google Scholar]
- Olaimat AN, Al-Holy MA, Abu Ghoush MH, et al. The use of malic and acetic acids in washing solution to control Salmonella spp. on chicken breast. J Food Sci. 2018;83:2197–2203. doi: 10.1111/1750-3841.14286. [DOI] [PubMed] [Google Scholar]
- Padungtod P, Kaneene JB. Campylobacter in food animals and humans in Northern Thailand. J Food Prot. 2005;68:2519–2526. doi: 10.4315/0362-028X-68.12.2519. [DOI] [PubMed] [Google Scholar]
- Ramirez-Hernandez A, Brashears MM, Sanchez-Plata MX. Efficacy of lactic acid, lactic acid-acetic acid blends, and peracetic acid to reduce Salmonella on chicken parts under simulated commercial processing conditions. J Food Prot. 2018;81:17–24. doi: 10.4315/0362-028X.JFP-17-087. [DOI] [PubMed] [Google Scholar]
- Ransom JR, Belk KE, Sofos JN, et al. Comparison of intervention technologies for reducing Escherichia coli O157:H7 on beef cuts. Food Prot Trends. 2003;23:24–34. [Google Scholar]
- Riedel CT, Brøndsted L, Rosenquist H, et al. Chemical decontamination of Campylobacter jejuni on chicken skin and meat. J Food Prot. 2009;72:1173–1180. doi: 10.4315/0362-028X-72.6.1173. [DOI] [PubMed] [Google Scholar]
- Rogers L. Sensory panel management: a practical handbook for recruitment, training and performance. Sawston: Woodhead Publishing; 2017. [Google Scholar]
- Skřivanová E, Molatová Z, Matěnová M, et al. Inhibitory effect of organic acids on arcobacters in culture and their use for control of Arcobacter butzleri on chicken skin. Int J Food Microbiol. 2011;144:367–371. doi: 10.1016/j.ijfoodmicro.2010.10.021. [DOI] [PubMed] [Google Scholar]
- Tamblyn KC, Conner DE. Bactericidal activity of organic acids in combination with transdermal compounds against Salmonella typhimurium attached to broiler skin. Food Microbiol. 1997;14:477–484. doi: 10.1006/fmic.1997.0112. [DOI] [PubMed] [Google Scholar]
- Tango C, Mansur A, Oh D-H. Fumaric acid and slightly acidic electrolyzed water inactivate gram positive and gram negative foodborne pathogens. Microorganisms. 2015;3:34–46. doi: 10.3390/microorganisms3010034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wesche AM, Gurtler JB, Marks BP, Ryser ET. Stress, sublethal injury, resuscitation, and virulence of bacterial foodborne pathogens. J Food Prot. 2009;72:1121–1138. doi: 10.4315/0362-028X-72.5.1121. [DOI] [PubMed] [Google Scholar]
- Wolf MJ, Miller MF, Parks AR, et al. Validation comparing the effectiveness of a lactic acid dip with a lactic acid spray for reducing Escherichia coli O157:H7, and non-O157 Shiga toxigenic escherichia coli on beef trim and ground beef. J Food Prot. 2012;75:1968–1973. doi: 10.4315/0362-028X.JFP-12-038. [DOI] [PubMed] [Google Scholar]
- Zhu Y, Xia X, Liu A, et al. Effects of combined organic acid treatments during the cutting process on the natural micro fl ora and quality of chicken drumsticks. Food Control. 2016;67:1–8. doi: 10.1016/j.foodcont.2016.02.031. [DOI] [Google Scholar]