Abstract
Anaeromyces robustus is an anaerobic rumen microorganism which can produce plant cell wall degrading enzymes. In this study, a new GH10 xylanase gene xylAr10 from A. robustus was identified, cloned and expressed in Pichia pastoris GS115. The recombinant protein ArXyn10 was characterized after being purified by Ni–NTA. The optimal pH and temperature of ArXyn10 was determined at 5.5 and 40 °C, respectively. ArXyn10 was stable at the pH range of 4.0–8.0, and could maintain high stability from 35 to 45 °C. The hydrolysis products released from beechwood xylan by ArXyn10 showed chromatographic mobility similar to xylobiose and xylotriose according to thin-layer chromatography analysis. It was shown that the addition of 7.5 mg of ArXyn10 in 100 g high-gluten wheat flour during bread making could increase the reducing sugar content by 10.80%, indicating that xylo-oligosaccharides were produced. With the addition of ArXyn10, the hardness and chewiness of the bread decreased and the quality was improved. The new discovered xylanase ArXyn10 have potential application prospect in bread making.
Keywords: Xylanase, Pichia pastoris, Ruminal microorganisms, Anaeromyces robustus, Bread
Introduction
First discovered in 1975, rumen fungi are the only known anaerobic fungal taxa now. More than twenty species have been successfully isolated so far, including six genera: Piromyces, Anaeromyces, Neocallimastix, Cyllamyces, Caecomyes, and Orpinomyces, approximately account for 8% of the total number of rumen microorganisms. Rumen fungi just account for a rather small proportion, but they play an important role in the degradation of xylan in plant cell walls in ruminants (Tiwari et al. 2018). Enzymes such as cellulases and xylanases derived from rumen fungi possess higher enzymatic activity than those of bacterial origin. The abundance of anaerobic fungi is low in the rumens of animals fed with feeds containing high starch while the situation of animals fed with high fiber content is opposite (Griffith et al. 2009). Experiments in animals in vivo have shown that the degradation of crude feed by sheep decreased significantly after removal of anaerobic fungi. However, after reintroduction of Neocallimastix sp., the feed intake of sheep recovered (Gordon and Phillips. 1993). In a word, rumen anaerobic fungi are an excellent source of xylanases. They have been highly adapted to fiber degradation, which can be important for the degradation of difficult-to-process biological resources currently exsiting in industry, such as for the treatment of bark in paper industry (Edwards et al. 2017; Seppala et al. 2016).
Xylan is a complex polysaccharide, usually composed of β-d-xylopyranoses with β-1,4 glycosidic bonds to form the main chain, and arabinose residues, glucose residues, acetyl, feruloyl, glucuronosyl, and 4-O-methyl-d-glucuronosyl to form the side chain (Valenzuela et al. 2010). Most of the natural xylans are difficult to be utilized efficiently, and their complete hydrolysis requires a key enzyme: endo-1,4-β-d-xylanase (EC 3.2.1.8) to cleave xylans into xylo-oligosaccharides (Collins et al. 2005). Based on a comparison of structures at the catalytic domain level, almost all xylanases to date are grouped into the glycoside hydrolase family (GH) 5, 8, 10, 11, 43, 62 (Al-Darkazali et al. 2017), in which most of them are classified into the GH10 and GH11 families. In addition, xylanases belonging to other glycoside hydrolase families are also being discovered. A new GH30 xylanase has been found to be able to release xylobiose from the non-reducing end (Suchova et al. 2020). A GH26 xylanase was found to possess a combination activity of mannanase, xylanase, pectin esterase and endoglucanase (Patel et al. 2016). A GH141 xylanase has also been reported (Heinze et al. 2017). Generally speaking, xylanases of the GH10 family possess a large molecular weight and complex structure, usually containing one or more non-catalytic regions, including repetitive sequences, heat-stable regions, linkage regions, carbohydrate-binding domains, and most of them belong to multi-domain proteins, while xylanases of the GH11 family have a small molecular weight, mostly belong to single-domain proteins (Li et al. 2019).
Xylanase is widely used in the food, pharmaceutical, paper industry and so on. The arabinoxylans in cereals are insoluble and xylanase is able to hydrolyze them to water-soluble xylans, thus altering the fibre fraction during dough formation (Dornez et al. 2009). Water-soluble xylan has a low water affinity and can induce water redistribution in the gluten network, improving the quality of dough and bread. Xylanase was evaluated by Both et al. (2019) for the improvement of whole-meal bread. It was confirmed that xylanase could reduce undesirable effects of fibre in the dough. Guo et al. (2018) revealed that compared with high molecular weight arabinoxylan, hydrolyzed arabinoxylan could improve the rheological properties and processing properties of wheat dough by enhancing the interaction among water molecules, starch and gluten.
The first genome sequencing of anaerobic rumen fungi was completed in 2013 (Youssef et al. 2013), and Anaeromyces robustus was first compiled by Li (2016). However, studies on the enzymatic properties of xylanases originating from A. robustus have not been reported so far. A GH10 xylanase gene xylAr10 was identified from A. robustus genome in this study. The xylAr10 gene was cloned and expressed in Pichia pastoris GS115 to produce the recombinant protein ArXyn10. The purification and characterization of ArXyn10, analysis of the hydrolysis products of beechwood xylan by ArXyn10 was then studied. Further, the application of ArXyn10 in bread making was also reported in this paper. The protein ArXyn10 could decrease the hardness and chewiness of the bread and thus improved the quality. The ability of ArXyn10 to break down xylan into xylo-oligosaccharides can facilitate the utilization of plant materials with high xylan content. In addition, xylo-oligosaccharides cannot be absorbed by human body and thus can be used as sweetener for diabetics or obese people. It is expected that ArXyn10 may have wide application prospect in food industry in future.
Materials and methods
Strains, vectors, and reagents
Escherichia coli DH5α was purchased from TransGen Biotech (Beijing, China), Pichia pastoris GS115 and plasmid pPIC9K were preserved in our laboratory. G418 and biotin were purchased from Macklin (Shanghai, China), used for construction of the recombinant plasmids and P. pastoris strains. SnaBI, SalI, NotI were purchased from Takara (Dalian, China), EnDo H was purchased from New England Biolabs (Beijing, China). Xylose, xylobiose, xylotriose, xylotetraose, and xylopentaose were purchased from Solarbio (Beijing, China) for thin layer chromatography. All other chemicals in this study were of analytical grade unless otherwise stated.
Construction of the recombinant plasmid pPIC9K-xylAr10
The xylanase gene sequence of the strain A. robustus (GenBank: ORX87577.1) was obtained at the NCBI website (Lu et al. 2020) (https://www.ncbi.nlm.nih.gov/) and the gene was synthesized by GENWIZ with codon optimization and the addition of 6 × His at the C-terminus. The xylAr10 gene was amplified by PCR using the two primers xylAr10F (5′-TACGTATATGAAAGTTTGAAATCTCAC-3′) and xylAr10R (5′-GCGGCCGCTTAATGGTGAT-3′), SnaBI and NotI restriction enzyme sites are underlined. The amplification fragment was then inserted into pPIC9K to obtain the recombinant plasmid pPIC9K-xylAr10.
Transformation and expression of xylAr10 in P. pastoris GS115
The pPIC9K-xylAr10 was linearized by SalI and transformed into P. pastoris GS115 by electroporation. The recombinant cells were cultured on MD plates at 30 °C for 2 days. Transformants were cultured on YPD plates with different G418 concentrations (0.25–2.0 mg/mL) at 30 °C for 4–5 days. The colonies were then inoculated and incubated in 5 mL BMGY medium at 30 °C for 2 days. Cells were harvested by centrifugation at 5000×g, 4 °C for 10 min and incubated in 50 mL BMMY medium for 4–5 days. The xylanase activity of the positive colonies was determined by DNS assay.
Purification and deglycosylation of ArXyn10
The culture was centrifuged at 5000×g, 4 °C for 10 min to remove the yeast cells. The supernatant was applied on Ni–NTA resin. The protein was eluted with gradient elution buffer (0.25 M NaCl, and 20 mM Tris–HCl buffer, 0.2 M imidazole, pH 7.5). Endo H was used to remove N-linked glycan of purified ArXyn10. SDS-PAGE was performed with the deglycosylated enzyme and purified enzyme to detect the degree of ArXyn10 glycosylation.
Sequence analysis and structure prediction
Sequence alignments and database homology searches were performed by BLASTP at NCBI. The protein sequences were analyzed on Expasy to predict isoelectric point, glycosylation sites and hydrophobicity. Functional protein domains of the ORFs were identified on the Pfam website (http://pfam.xfam.org) (Kim et al. 2018b). The sequences with 35–75.4% similarity to ArXyn10 were collected to construct the phylogenetic tree by MEGA5.2 (https://www.megasoftware.net/index.php). Structure model was generated on SWISS-MODEL (https://swissmodel.expasy.org/interactive), and the structural figures were prepared by SPDBV program (https://spdbv.vital-it.ch/) (He et al. 2021).
ArXyn10 enzyme activity assay
The enzyme activity of xylanase was determined by the dinitrosalicylic acid (DNS) method (Souza et al. 2016) to detect the amount of reducing sugar produced. The reaction solution contained 90 μL 1% (W/V) beechwood xylan and 30 μL diluted enzyme. The reaction mixture was incubated at pH 5.5 and 40 °C for 30 min. After 180 μL of DNS was added to the reaction, the mixture was boiled for 5 min and cooled with ice water immediately, and then 1.2 mL deionized water was added to set the volume to 1.5 mL (Qiu et al. 2017). The released reducing sugar was quantified by spectrometric method at 540 nm. All assays of ArXyn10 activity were performed in triplicate. Under the conditions of pH 5.5 and 40 °C, the amount of xylanase required to degrade beechwood xylan to produce 1 μmoL xylose per minute is defined as one unit of enzyme activity.
Effects of pH and temperature on ArXyn10 activity and stability
The optimal pH of purified ArXyn10 was determined at 40 °C in 50 mM citric acid/sodium citrate buffers at the pH range of 3.0–6.5 and in 50 mM Tris–HCl buffers at the pH range of 7.0–9.0. The pH stability of ArXyn10 was determined in different buffers at 40 °C for 5–60 min and diluted with 0.1 M citric acid/sodium citrate buffer at pH 5.5, and then the residual activity was determined by standard assay and relative activity was calculated using untreated samples as control (100%).
The optimal temperature of ArXyn10 was determined at pH 5.5 at the temperature range of 30–80 °C. Thermostability of ArXyn10 was determined at different temperature (50, 60, 70 °C) at pH 5.5 for 5–60 min, and the residual activity was determined by standard assay and relative activity was calculated as described above.
Effect of metal ions and chemicals on the ArXyn10 activity
To determine the effect of metal ions and chemicals on the ArXyn10 activity, metal ions or chemical reagents, Na+, K+, Ca2+, Cu2+, Mn2+, Zn2+, Cu2+, Mg2+, Co2+, SDS, and EDTA were added to the reaction solution at pH 5.5 and 40 °C at the concentration of 1 mM and 5 mM. ArXyn10 incubated without metal ions or chemicals was used as control, and the enzyme activity was set at 100%.
Substrate specificity and kinetic of ArXyn10
Different concentrations of beechwood xylan (1 mg/mL, 2 mg/mL, 2.5 mg/mL, 3.3 mg/mL, 5 mg/mL, 10 mg/mL) were used in the standard reaction system. The initial velocity was measured using 1 mg/mL beechwood xylan as substrate, and the Km, Vmax, and kcat values for ArXyn10 were calculated from Lineweaver–Burk plots, y = Vmax* [S]/(Km + [S]). (Yang et al. 2018).
To test specificity of ArXyn10, beechwood xylan, sodium carboxymethyl cellulose (CMC-Na), microcrystalline cellulose, filter paper (Cut into 2 × 1 cm strips) were used in the standard activity assay.
Thin-layer chromatography (TLC) assay
The purified ArXyn10 was diluted and incubated with 1.0% (w/v) beechwood xylan at pH 5.5 and 40 °C for 30 min. The hydrolysis products were separated on silica gel plate GF254. The solvent system included chloroform, acetic acid, and water (6:7:1. V/V/V). The plate was developed for 2–3 h. After spraying with ethanol: sulfuric acid (95:5, V/V) solvent, the reducing sugars were visualized by heating at 105 °C for 5 min (Kim et al. 2017). A mixture consisting of xylose (X1), xylobiose (X2), xylotriose (X3), xylotetraose (X4) and xylopentaose (X5) was used as standard.
Application of ArXyn10 in bread making
The bread was prepared by adding 2.5 mg, 5.0 mg and 7.5 mg of ArXyn10 to 100 g of high gluten flour, respectively. Bread without ArXyn10 was used as control to determine specific volume, texture, and reducing sugar content. The specific volume1 was tested by the rapeseed replacement method (Tebben et al. 2020). The specific volume was calculated by dividing the bread volume (mL) by bread weight (g) and was expressed in cm3/g. The texture was measured by using a texture analyser (TMS-PRO) equipped with a cylindrical probe 35 mm in diameter (the cross speed: 60 mm/min, trigger point: 0.05 N, and the samples were compressed to 50% of their initial thickness), and the bread was sliced to a thickness of 10 mm. 10 g dough was dissolved in 100 mL water and vortexed for 5 min to mix well, the reducing sugar content was determined using the supernatant of the dough by DNS method.
The bread recipe was as follows: 100 g high-gluten wheat flour, 60 mL water, 10 g sugar, 5 g butter, 0.3 g yeast, 1 g salt. The yeast used for the bread is a kind of commercial yeast (Instant dry yeast, Saccharomyces cerevisiae) from Angel Yeast Co., Ltd. The bread making process was to knead the dough for 15 min and to rest at 40 °C, 80% humidity for 2 h, take out for shaping, then rest again for 1 h, to heat upper tube at 160 °C, lower tube at 180 °C, and bake for 30 min.
Scanning electron microscopy and optical microscope
A small amount of dough was pressed onto a slide, observed and photographed on the microscope (OLYMPUS, BX63) using a 100 × oil objective lens. The rest of the dough was freeze-dried.
The freeze-dried dough was exposed to gold sputtering. The samples were then observed and photographed with a scanning electron micrograph apparatus model TM4000 (Japan) at an accelerating voltage of 5 kV.
Results and discussion
Sequence and structure analysis
According to sequence alignment, ArXyn10 shared 75.4% similarity to the β-xylanase from Neocallimastix californiae (GenBank: ORY42966.1). A phylogenetic tree was constructed using xylanases with 35–75.4% similarity to ArXyn10 (Fig. 1). It showed in the tree that ArXyn10 evolved as an independent branch, and it was most close to the branch of the xylanases from N. californiae. It was the first discovered xylanase being identified from A. robustus, which enriched the source of xylanases and could promote found of new xylanases from other anaerobic origins.
Fig. 1.
Phylogenetic tree of ArXyn10 based on protein sequence homology
Analysis of the protein sequence showed that 18 amino acids at the N-terminus of the protein were signal peptides, amino acid (AA) 61–319 belonged to GH10 domain, and AA 366–398 belonged to CBM1 domain. The predicted molecular weight of the protein encoded by xylAr10 was 44 KDa and the isoelectric point was 7.6. E142 and E253 were the catalytical sites of ArXyn10. ArXyn10 possessed two possible N-glycosylation sites, N149 and N361, and two possible O-glycosylation sites, S147 and S177. The instability index was 33.13, indicating that ArXyn10 was stable. The aliphatic index was 69.92, and the grand average of hydropathicity was − 0.648.
Swiss-Model was used to perform homology modeling of proteins (Fig. 2). Sequence of ArXyn10 shared the highest identity (40.27%) with the characterized endo-1,4-β-d-xylanase (2d1z.1.A) from Streptomyces olivaceoviridis from those with solved 3D structure. The structure of GH10 xylanases usually had (α/β)8TIM-barrel folds, which was consistent with the homology modeling structure of ArXyn10.
Fig. 2.

Homology modeling of ArXyn10 (a) and surface structure of ArXyn10 (b), two catalytic sites (E142 and E253) are identified in red, the catalytic cleft is in the middle of the model
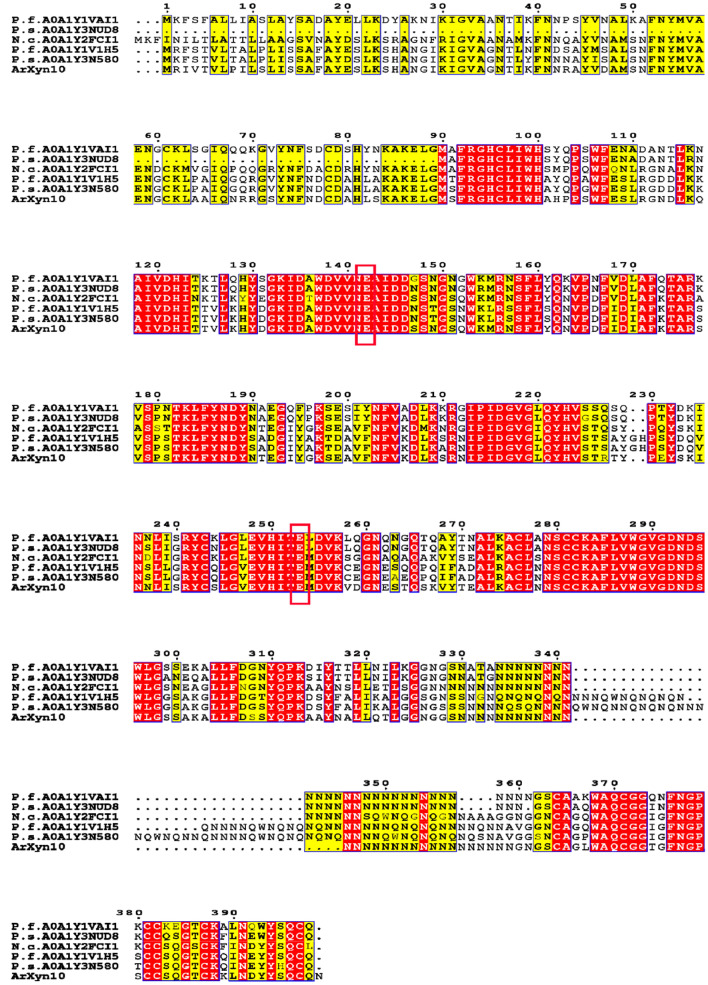
Multiple sequence comparisons of several GH10 xylanases produced by rumen microorganisms revealed that their catalytic sites are highly consistent and their relative positions in the GH10 domain were conserved, as shown in Fig. 3. The catalytic sites of these proteins are in red boxes. In the model of ArXyn10, the catalytic sites located at the inner edge of the depression of the bowl-shaped structure formed by the (α/β)8TIM-barrel structure. E142 and E253 were speculated as catalytic sites and key amino acids in the xylanases derived from anaerobic rumen fungi in this study.
Fig. 3.
Multiple sequence alignment of ArXyn10 with the other xylanases from anaerobic rumen fungus. Abbreviation of the xylanases and Uniprot accession numbers are as follows: piromyces finnis A0A1Y1V1H5 (P.F.A0A1Y1V1H5), Piromyces sp. A0A1Y3N580 (P.S.A0A1Y3N580), Neocallimastix californiae A0A1Y2FCI1 (N.C.A0A1Y2FCI1), Piromyces finnis A0A1Y1VAI1 (P.F.A0A1Y1VAI1), Piromyces sp. A0A1Y3NUD8 (P.S.A0A1Y3NUD8), two predicted catalytic sites are shown in red box
Expression and purification of ArXyn10
The gene fragment of xylAr10 was obtained and cloned into pPIC9K, resulting in the recombinant plasmid pPIC9K-xylAr10. The open reading frame of xylAr10 was 1200 bp, encoding a protein of 399 AA. After expression in P. pastoris GS115 and subsequent purification, the recombinant protein ArXyn10 was subjected to SDS-PAGE. A single band of ArXyn10 was observed near 44 kDa in SDS-PAGE (Fig. 4), which was approximately the calculated size of the protein. After digestion by Endo H, the molecular weight of ArXyn10 did not change significantly, indicating that ArXyn10 was not glycosylated.
Fig. 4.
SDS-PAGE analysis of the purified recombinant ArXyn10. Lanes: M-Marker, 1: Endo H, 2. ArXyn10 digested with Endo H, 3. ArXyn10 purified by Ni–NTA, 4. Cultured supernatant
Characterization of the purified ArXyn10
As shown in Fig. 5, the optimal pH of the purified ArXyn10 was 5.5, and ArXyn10 maintained more than 80% of the enzyme activity between pH 5.0–6.5, showing it was an acidic xylanase. ArXyn10 had a wide range of pH stability at 40℃. After treatment at pH 4.0–8.0 for 1 h, the residual enzyme activity was above 90%, indicating that ArXyn10 could maintain high enzyme activity in the pH range of 4.0–8.0 (Fig. 5B).
Fig. 5.
Effect of pH and temperature on the catalytic activity and stability of the ArXyn10. a Optimum pH. b pH stability. c Optimum temperature. d Thermostability
The optimum temperature of ArXyn10 was 40 °C. ArXyn10 could maintain high enzyme activity at 35–45 °C, indicating it belongs to mesophilic enzyme. After treatment of ArXyn10 at 50, 60 and 70 °C for 30 min, 39.29%, 18.40% and 2.24% of the enzymatic activity remained, respectively, indicating that ArXyn10 was thermolabile. (Fig. 5D).
Collins et al. (2005) found that most fungal xylanases are mesophilic, with an optimum temperature usually at 40–50 °C, and ArXyn10 is consistent with this finding. However, the lack of heat resistance of ArXyn10 may result in application restriction in industry. For example, in the treatment of plant tissues such as straw and bark, where hemicelluloses such as xylan are difficult to degrade, thus steam blasting is used and it requires the aid of heat resistant enzymes, which is unacceptable for ArXyn10. For this purpose, it may need further modification for heat resistance.
The effects of different metal ions and chemical reagents on the activity of ArXyn10 were shown in Table 1. K+ and Na+ had little or no effect on ArXyn10 activity (> 90% activity remained). Under the condition of low concentration metal ions and reagents (1 mM), ArXyn10 was slightly enhanced by Ba2+ (114.43%), moderately inhibited by Fe2+ (47.70%), Mn2+ (63.25%), EDTA (75.62%), Co2+ (85.40%), strongly inhibited by Zn2+ (21.69%), SDS(23.75%), Cu2+ (24.14%). At high concentration (5 mM), ArXyn10 was significantly activated by Ba2+ (138.25%), moderately inhibited by Fe2+ (35.64%), EDTA (43.05%), Mg2+ (68.60%), Ca2+ (81.86), but strongly inhibited by Cu2+ (17.55%), Zn2+ (20.74%), Mn2+ (25.65%), SDS (25.65%), Co2+ (27.15%).
Table 1.
Effect of metal ions and chemical reagents at 1 mM and 5 mM concentrations on the activity of ArXyn10
| Ions or chemicals | Relative activity (%) | |
|---|---|---|
| 1 mM | 5 mM | |
| Control | 100 ± 1.02 | 100 ± 1.01 |
| K+ | 100.39 ± 3.70 | 96.42 ± 2.99 |
| Ca2+ | 96.55 ± 1.54 | 81.86 ± 3.33 |
| Mn2+ | 63.25 ± 2.32 | 25.65 ± 0.95 |
| Cu2+ | 24.14 ± 0.34 | 17.55 ± 0.13 |
| Mg2+ | 94.70 ± 0.72 | 68.60 ± 2.59 |
| Ba2+ | 114.43 ± 1.17 | 138.25 ± 4.03 |
| Fe2+ | 47.70 ± 4.92 | 35.64 ± 5.96 |
| Zn2+ | 21.69 ± 1.74 | 20.74 ± 0.93 |
| Co2+ | 85.40 ± 1.01 | 27.15 ± 0.65 |
| SDS | 23.75 ± 0.59 | 25.65 ± 0.88 |
| EDTA | 75.62 ± 2.85 | 43.05 ± 1.13 |
| Na+ | 95.74 ± 3.43 | 101.85 ± 2.07 |
The enzyme activity of ArXyn10 for beechwood xylan was 134.49 U/mL (100%), the enzyme activities on microcrystalline cellulose, filter paper and CMC-Na were 2.44%, 1.49% and 0.85%, respectively, and it showed no activity on soluble starch.
The kinetic parameters of ArXyn10 were obtained by Lineweaver–Burk plot using 5 min reaction time. Vmax and Km were 65.36 U/mg and 4.61 mg/mL, respectively. Kcat was determined as 48.01 s−1, and Kcat/Km was 10.45 s−1 (mg/mL)−1.
As shown in Table 2, among several different GH10 family xylanases, ArXyn10 showed high catalytic efficiency and substrate specificity, and therefore has potential application prospect. Xylanases could show differences in catalytic efficiency and substrate specificity, indicating that xylanase activity can be affected by multiple factors, including CBM domains (Arantes and Saddler 2011; Li et al. 2018), GH domains, N-terminus or C-terminus, non-domain amino acids. In addition, changes in the substrate binding site, salt bridges and disulfide bonds can also affect the catalytic efficiency and function mechanism of xylanase (Wang et al. 2016, 2012; Voutilainen et al. 2010).
Table 2.
Kinetic parameters of different GH10 xylanases
| Enzymes | Km (mg mL−1) | Kcat (s−1) | Kcat/Km (s−1 mL mg−1) | Organism | References |
|---|---|---|---|---|---|
| ArXyn10 | 4.61 | 48.01 | 10.45 | Anaeromyces robustus | Present work |
| McXyn10 | 3.0 | 4.18 | 1.39 | Malbranchea cinnamomea | Fan et al. (2014) |
| Srxyn10 | 8.79 | 64.76 | 7.37 | Streptomyce rochei | Li et al. (2018) |
| rXyn10E | 10.4 | 129.2 | 12.3 | Paenibacillus curdlanolyticus | Sermsathanaswadi et al. (2017) |
| rXylM△RICIN | 3.9 | 22.3 | 5.2 | Luteimicrobium xylanilyticum | Kim et al. (2018a) |
Thin-layer chromatography (TLC) assay
TLC analysis of the hydrolysate of beechwood xylan showed that after incubation, the main hydrolysates exhibited a TLC mobility similar to xylobiose (X2) and xylotriose (X3), and no other xylo-oligosaccharides were produced (Fig. 6). Therefore, ArXyn10 exhibits a typical endo fashion of action. Beechwood xylan is a glucuronoxylan which means that some xylopyranosyl units in the main chain are substituted with glucuronic or 4-O-methylglucuronic acid. It is possible that the hydrolysates produced by ArXyn10 are also substituted.
Fig. 6.

Thin-layer chromatography analysis of the hydrolytic products released by ArXyn10 from beechwood xylan. Lanes: M: the standards of xylose (X1) and xylo-oligosaccharides (X2-X5), 1: ArXyn10 without beechwood xylan, 2: the hydrolytic products released by ArXyn10 at pH 5.5 and 40 °C for 30 min, 3: beechwood xylan
Application of ArXyn10 in bread making
Compared with the control group, the specific volume of the bread was 2.20 without the addition of ArXyn10 and increased by 3.63%, 5.91% and 8.18% with the addition of 2.5 mg, 5.0 mg and 7.5 mg of ArXyn10, respectively. the bread was more fluffy and tasted better after added with ArXyn10.
The bread reducing sugar content increased by 3.75%, 7.11% and 10.80% with the addition of 2.5 mg, 5.0 mg and 7.5 mg of ArXyn10, respectively. The bread was more fluffy and tasted better after addition of ArXyn10. ArXyn10 can degrade hemicellulose in bread into xylo-oligosaccharides. Xylo-oligosaccharides are available to Bifidobacterium (Zheng et al. 2020), which possesses a variety of important physiological functions for human health.
The addition of 2.5 mg ArXyn10 reduced the hardness of bread by 7.76% and chewiness by 13.44%, while the addition of 5.0 mg and 7.5 mg ArXyn10 reduced the hardness by 21.55% and 31.03% and chewiness by 29.72% and 34.76%, respectively (Table 3), which significantly reduced the hardness and chewiness of bread and improved the taste. Among the xylanases in Table 4, ArXyn10 was effective in reducing the hardness and chewiness of the bread at a low addition level. Other properties of the bread also influence the taste and quality of the bread. Elasticity is inextricably linked to the formation of gluten, as the carbon dioxide produced during the fermentation step forms large and small bubbles in the dough. ArXyn10 could improve elasticity, indicating that more gluten networks had been created in the bread. The addition of ArXyn10 decreased the cohesiveness of the bread due to the breakdown of some of the non-starch polysaccharides by xylanase, generating a large number of dietary fibers interspersed in the gluten network structure. The shear dilution of the fibers and competitive water absorption could destroy the gluten structure of the dough, resulting in a reduction in cohesiveness.
Table 3.
Effect of addition of the recombinant xylanase ArXyn10 on the texture of bread
| ArXyn10 (mg) | Hardness | Adhesiveness | Cohesiveness | Springiness | Gumminess | Chewiness |
|---|---|---|---|---|---|---|
| Control(0) | 11.6 | 0.29 | 0.645 | 4.06 | 7.5 | 30.51 |
| 2.5 | 10.7 | 0.27 | 0.590 | 3.93 | 6.0 | 26.41 |
| 5.0 | 9.1 | 0.28 | 0.620 | 4.29 | 5.6 | 23.92 |
| 7.5 | 8.0 | 0.29 | 0.645 | 4.30 | 5.5 | 19.90 |
Table 4.
Effect of xylanases on hardness and chewiness of bread
| Xylanase | Dosage | Hardness (%) | Chewiness (%) | References |
|---|---|---|---|---|
| ArXyn10 | 25 mg/kg | − 7.76 | − 13.44 | Present work |
|
ArXyn10 ArXyn10 |
50 mg/kg | − 21.55% | − 29.72 | Present work |
| 75 mg/kg | − 31.03 | − 34.76 | Present work | |
| Commercial xylanase | 500 mg/kg | − 13.65 | + 15.06 | Sarabhai et al. (2021) |
| 1000 mg/kg | − 36.50 | − 60.24 | Sarabhai et al. (2021) | |
| Extracted xylanase | 0.1 mL/kg | − 15.00 | + 2.42 | Ghoshal et al. (2013) |
| SWT | 1000 U/kg | − 54.24 | − 58.91 | Passarinho et al. (2019) |
| SM2 | 1000 U/kg | − 59.57 | − 61.34 | Passarinho et al. (2019) |
|
XynA XynA XynA |
0.3 U/kg | − 4.91 | + 5.23 | Zheng et al. (2011) |
| 0.9 U/kg | − 7.82 | + 12.98 | Zheng et al. (2011) | |
| 1.5 U/kg | − 1.40 | + 2.13 | Zheng et al. (2011) | |
| Extracted xylanase | 12,000 U/kg | − 77.27 | − 35.23 | Shah et al. (2005) |
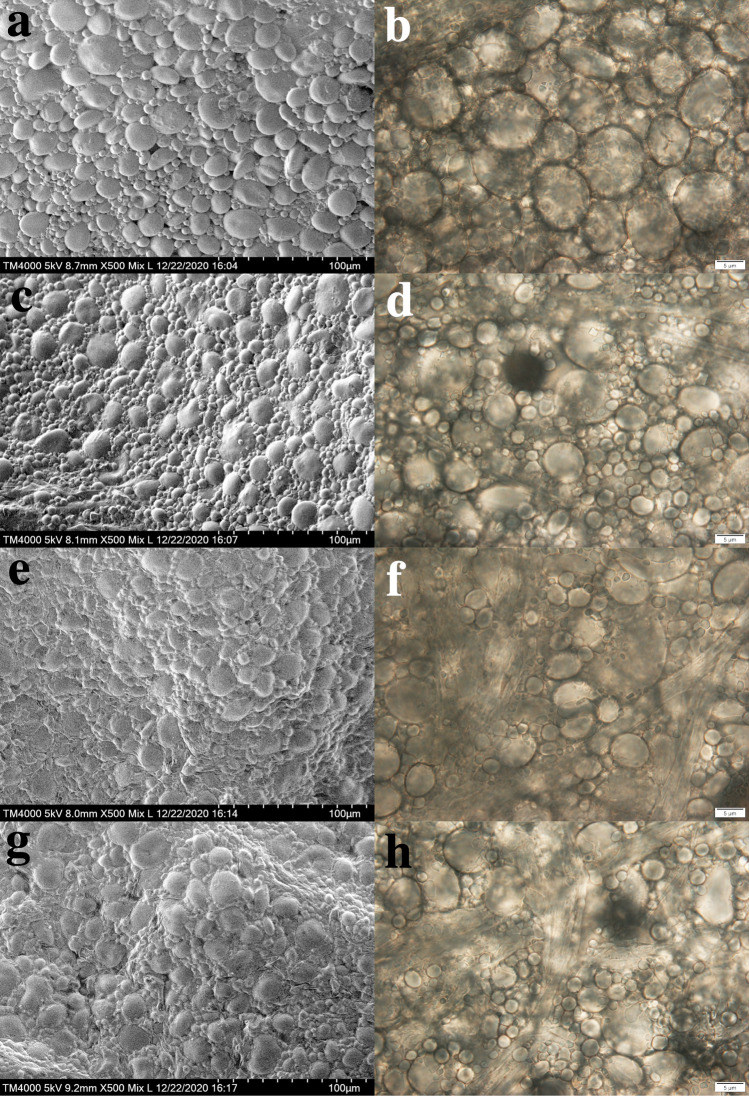
Water-unextractable arabinoxylan (WUAX) is the main component of non-amylose polysaccharides in flour. Although the average content of WUAX in flour is only 1.7% (Saulnier et al. 2007), it possesses excellent water absorption ability and can therefore absorb large amount of water. The water can be released again after the hydrolysis of WUAX. Without the addition of ArXyn10, there was no degradation of arabinoxylan (AX) in the dough (Fig. 7a, b). However, after the addition of ArXyn10, WUAX was hydrolyzed to water-unextractable arabinoxylan (WEAX) (Fig. 7d, f, h) and water was released, resulting in a softer dough bread (Sheikholeslami et al. 2021). As the amount of ArXyn10 increased, more WUAX was hydrolyzed to xylo-oligosaccharides, which is consistent with the above experimental results of increased reducing sugar content. The WEAX and xylo-oligosaccharides combine with starch and gluten protein through hydrogen bonding to form and enhance a composite gluten network structure (Fig. 7c, e, g) (Guo et al. 2018), resulting in a more ductile dough and bread.
Fig. 7.
Effect of ArXyn10 on microstructure of dough, a, c, e, g were taken by SEM, b, d, f, h were taken by optical microscope. a, b Dough without ArXyn10; c, d Dough with 2.5 mg ArXyn10; e, f Dough with 5.0 mg ArXyn10; g, h Dough with 7.5 mg ArXyn10
During the baking of bread, air production and holding capacity of the dough is an important factor in determining the quality of the bread. The increase of free water can lead to softer dough with enhanced ductility and thus quality and taste of the baked bread was improved. The gluten network can enhance the gas holding capacity of the dough, improve the ductility and enable the dough to be fully extended during the fermentation process. Attributed to softer dough, the volume of the bread is increased, and as a result, the taste of the bread is better. Through this study, it can be expected that ArXyn10 has a high potential application prospect in bread making.
Conclusion
In this paper, the ArXyn10 encoding gene was synthesized, cloned into yeast pPIC9K vector, and was successfully expressed in P. pastoris GS115. SDS-PAGE revealed a monomer form of the recombinant protein, whose size conformed to the predicted molecular weight of 44 kDa. The xylanase ArXyn10 was characterized by determination of optimal temperature and pH, thermostability and pH stability. The optimum temperature of ArXyn10 is consistent with the fermentation temperature of yeast, and therefore ArXyn10 can be used as a bread improver. In addition, the xylo-oligosaccharides are beneficial to human intestinal health. ArXyn10 is capable of producing xylo-oligosaccharides and has great potential for application.
Acknowledgements
We would like to thank Yangtze University for laboratory and equipment.
Authors contributions
SW: Methodology, Investigation, Formal analysis, Visualization, Writing—original draft. GW: Investigation, Formal analysis, Writing—Review and Editing. HW: Methodology, Formal analysis, Resources, Writing—Review and Editing.
Funding
This study was supported by Open Research Fund of the Jiangsu Provincial Key Construction Laboratory of Probiotics Preparation (JSYSZJ2017001).
Data availability
Sequence data is available in the NCBI database via accession numbers MW692171.1.
Declarations
Conflict of interests
The authors declare that they have no conflict of interest in the publication.
References
- Al-Darkazali H, Meevootisom V, Isarangkul D, Wiyakrutta S. Gene expression and molecular characterization of a xylanase from chicken cecum metagenome. Int J Microbiol. 2017;2017:4018398. doi: 10.1155/2017/4018398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arantes V, Saddler JN. Cellulose accessibility limits the effectiveness of minimum cellulase loading on the efficient hydrolysis of pretreated lignocellulosic substrates. Biotechnol Biofuels. 2011;4:3. doi: 10.1186/1754-6834-4-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Both J, Esteres VP, Santetti GS, Bressiani J, Oro T, Gomez MP, Friedrich MT, Gutkoski LC. Phenolic compounds and free sulfhydryl groups in whole grain wheat flour modified by xylanase. J Sci Food Agr. 2019;99(12):5392–5400. doi: 10.1002/jsfa.9799. [DOI] [PubMed] [Google Scholar]
- Collins T, Gerday C, Feller G. Xylanases, xylanase families and extremophilic xylanases. Fems Microbiol Rev. 2005;29(1):3–23. doi: 10.1016/j.femsre.2004.06.005. [DOI] [PubMed] [Google Scholar]
- Dornez E, Gebruers K, Delcour JA, Courtin CM. Grain-associated xylanases: occurrence, variability, and implications for cereal processing. Trends Food Sci Technol. 2009;20:495–510. doi: 10.1016/j.tifs.2009.05.004. [DOI] [Google Scholar]
- Edwards JE, Forster RJ, Callaghan TM, Dollhofer V, Dagar SS, Cheng Y, Chang J, Kittelmann S, Fliegerova K, Puniya AK, Henske JK, Gilmore SP, O'Malley MA, Griffith GW, Smidt H. PCR and omics based techniques to study the diversity, ecology and biology of anaerobic fungi: insights, challenges opportunities. Front Microbiol. 2017;8:1657. doi: 10.3389/fmicb.2017.01657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan GS, Yang SQ, Yan QJ, Guo Y, Li YX, Jiang ZQ. Characterization of a highly thermostable glycoside hydrolase family 10 xylanase from Malbranchea cinnamomea. Int J Biol Macromol. 2014;70:482–489. doi: 10.1016/j.ijbiomac.2014.07.025. [DOI] [PubMed] [Google Scholar]
- Ghoshal G, Shivhare US, Banerjee UC. Effect of xylanase on quality attributes of whole-wheat bread. J Food Qual. 2013;36(3):172–180. doi: 10.1111/jfq.12034. [DOI] [Google Scholar]
- Gordon GL, Phillips MW. Removal of anaerobic fungi from the rumen of sheep by chemical treatment and the effect on feed consumption and in vivo fibre digestion. Lett Appl Microbiol. 1993;17:220–223. doi: 10.1111/j.1472-765X.1993.tb01451.x. [DOI] [Google Scholar]
- Griffith GW, Ozkose E, Theodorou MK, Davies DR. Diversity of anaerobic fungal populations in cattle revealed by selective enrichment culture using different carbon sources. Fungal Ecol. 2009;2(2):87–97. doi: 10.1016/j.funeco.2009.01.005. [DOI] [Google Scholar]
- Guo XN, Yang S, Zhu KX. Impact of arabinoxylan with different molecular weight on the thermo-mechanical, rheological, water mobility and microstructural characteristics of wheat dough. Int J Food Sci Tech. 2018;53(9):2150–2158. doi: 10.1111/ijfs.13802. [DOI] [Google Scholar]
- He XL, Zhang J, Wang S, Yang Z, Zhang H, Zhou X. Cloning, expression, purification and biochemical characterization of CpxR protein from pectobacterium carotovorum. Biotechnol Appl Biochem. 2021 doi: 10.1002/bab.2161. [DOI] [PubMed] [Google Scholar]
- Heinze S, Mechelke M, Kornberger P, Liebl W, Schwarz WH, Zverlov VV. Identification of endoxylanase XynE from Clostridium thermocellum as the first xylanase of glycoside hydrolase family GH141. Sci Rep-UK. 2017;7(1):73–92. doi: 10.1038/s41598-017-11598-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JY, Nong G, Rice JD, Gallo M, Preston JF, Altpeter F. In planta production and characterization of a hyperthermostable GH10 xylanase in transgenic sugarcane. Plant Mol Biol. 2017;93(4–5):465–478. doi: 10.1007/s11103-016-0573-5. [DOI] [PubMed] [Google Scholar]
- Kim DY, Lee SH, Lee MJ, Cho HY, Lee JS, Rhee YH, Shin DH, Son KH, Park HY. Genetic and functional characterization of a novel GH10 endo-β-1,4-xylanase with a ricin-type β-trefoil domain-like domain from Luteimicrobium xylanilyticum HY-24. Int J Biol Macromol. 2018;106:620–628. doi: 10.1016/j.ijbiomac.2017.08.063. [DOI] [PubMed] [Google Scholar]
- Kim HB, Lee KT, Kim MJ, Lee JS, Kim KS. Identification and characterization of a novel KG42 xylanase (GH10 family) isolated from the black goat rumen-derived metagenomic library. Carbohyd Res. 2018;468:1–9. doi: 10.1016/j.carres.2018.08.010. [DOI] [PubMed] [Google Scholar]
- Li GJ, Hyde KD, Zhao RL, Hongsanan S, Abdel-Aziz FA, et al. Fungal diversity notes 253–366: taxonomic and phylogenetic contributions to fungal taxa. Fungal Divers. 2016;78:1–237. doi: 10.1007/s13225-016-0366-9. [DOI] [Google Scholar]
- Li Q, Sun BG, Li XT, Xiong K, Xu YQ, Yang R, Hou J, Hou J. Improvement of the catalytic characteristics of a salt-tolerant GH10 xylanase from Streptomyce rochei L10904. Int J Biol Macromol. 2018;107:1447–1455. doi: 10.1016/j.ijbiomac.2017.10.013. [DOI] [PubMed] [Google Scholar]
- Li GQ, Chen XJ, Zhou X, Huang R, Li LB, Miao YZ, Liu DY, Zhang RF. Improvement of GH10 family xylanase thermostability by introducing of an extra alpha-helix at the C-terminal. Biochem Bioph Res Co. 2019;515(3):417–422. doi: 10.1016/j.bbrc.2019.05.163. [DOI] [PubMed] [Google Scholar]
- Lu L, Liu YW, Zhang ZY. Global characterization of GH10 family xylanase genes in rhizoctonia cerealis and functional analysis of xylanase rcxyn1 during fungus infection in wheat. Int J Mol Sci. 2020;21(5):1812. doi: 10.3390/ijms21051812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passarinho ATP, Ventorim RZ, Maitan-Alfenas GP, de Oliveira EB, Guimaraes VM. Engineered GH11 xylanases from Orpinomyces sp. PC-2 improve techno-functional properties of bread dough. J Sci Food Agr. 2019;99(2):741–747. doi: 10.1002/jsfa.9242. [DOI] [PubMed] [Google Scholar]
- Patel AB, Patel AK, Shah MP, Parikh IK, Joshi CG. Isolation and characterization of novel multifunctional recombinant family 26 glycoside hydrolase from Mehsani buffalo rumen metagenome. Biotechnol Appl Biochem. 2016;63(2):257–265. doi: 10.1002/bab.1358. [DOI] [PubMed] [Google Scholar]
- Qiu HY, Li ZY, Wang H, Zhang HY, Li S, Luo XG, Song YJ, Wang N, He HP, Zhou H, Ma WJ, Zhang TC. Molecular and biochemical characterization of a novel cold-active and metal ion-tolerant GH10 xylanase from frozen soil. Biotechnol Biotechnolog Equip. 2017;31(5):955–963. doi: 10.1080/13102818.2017.1359667. [DOI] [Google Scholar]
- Sarabhai S, Tamilselvan T, Prabhasankar P. Role of enzymes for improvement in gluten-free foxtail millet bread: IT’S effect on quality, textural, rheological and pasting properties. Lwt-Food Sci Technol. 2021;137:110365. doi: 10.1016/j.lwt.2020.110365. [DOI] [Google Scholar]
- Saulnier J, Sado PE, Branlard G, Charmet G, Guillon F. Wheat arabinoxylans: exploiting variation in amount and composition to develop enhanced varieties. J Cereal Sci. 2007;46(3):261–281. doi: 10.1016/j.jcs.2007.06.014. [DOI] [Google Scholar]
- Seppala S, Solomon KV, Gilmore SP, Henske JK, O'Malley MA. Mapping the membrane proteome of anaerobic gut fungi identifies a wealth of carbohydrate binding proteins and transporters. Microb Cell Fact. 2016;15(1):212. doi: 10.1186/s12934-016-0611-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sermsathanaswadi J, Baramee S, Tachaapaikoon C, Pason P, Ratanakhanokchai K, Kosugi A. The family 22 carbohydrate-binding module of bifunctional xylanase/β-glucanase Xyn10E from Paenibacillus curdlanolyticus B-6 has an important role in lignocellulose degradation. Enzyme Microb Tech. 2017;96:75–84. doi: 10.1016/j.enzmictec.2016.09.015. [DOI] [PubMed] [Google Scholar]
- Shah AR, Shah RK, Datta M. Improvement of the quality of whole wheat bread by supplementation of xylanase from Aspergillus foetidus. Bioresource Technol. 2005;97(16):2047–2053. doi: 10.1016/j.biortech.2005.10.006. [DOI] [PubMed] [Google Scholar]
- Sheikholeslami Z, Mahfouzi M, Karimi M, Ghiafehdavoodi M. Modification of dough characteristics and baking quality based on whole wheat flour by enzymes and emulsifiers supplementation. Lwt-Food Sci Technol. 2021;139:110794. doi: 10.1016/j.lwt.2020.110794. [DOI] [Google Scholar]
- Souza AR, Araujo GC, Zanphorlin LM, Ruller R, Franco FC, Torres FAG, Mertens J, Bowman MJ, Gomes E, Silva R. Engineering increased thermostability in the GH-10 endo -1,4-β-xylanase from Thermoascus aurantiacus CBMAI 756. Int J Biol Macromol. 2016;93:20–26. doi: 10.1016/j.ijbiomac.2016.08.056. [DOI] [PubMed] [Google Scholar]
- Suchova K, Puchart V, Spodsberg N, Krogh KBRM, Biely P. A novel GH30 xylobiohydrolase from Acremonium alcalophilum releasing xylobiose from the non-reducing end. Enzyme Microb Tech. 2020;134:109484. doi: 10.1016/j.enzmictec.2019.109484. [DOI] [PubMed] [Google Scholar]
- Tebben L, Chen GJ, Tilley M, Li YH. Individual effects of enzymes and vital wheat gluten on whole wheat dough and bread properties. J Food Sci. 2020;85(12):4201–4208. doi: 10.1111/1750-3841.15517. [DOI] [PubMed] [Google Scholar]
- Tiwari UP, Chen HY, Kim SW, Jha R. Supplemental effect of xylanase and mannanase on nutrient digestibility and gut health of nursery pigs studied using both in vivo and in vitro models. Anim Feed Sci Tech. 2018;245:77–90. doi: 10.1016/j.anifeedsci.2018.07.002. [DOI] [Google Scholar]
- Valenzuela SV, Díaz P, Javier PF. Recombinant expression of an alkali stable GH10 xylanase from Paenibacillus barcinonensis. J Agr Food Chem. 2010;58(8):4814–4818. doi: 10.1021/jf9045792. [DOI] [PubMed] [Google Scholar]
- Voutilainen SP, Murray PG, Tuohy MG, Koivula A. Expression of Talaromyces emersonii cellobiohydrolase Cel7A in Saccharomyces cerevisiae and rational mutagenesis to improve its thermostability and activity. Protein Eng Des Sel. 2010;23(2):69–79. doi: 10.1093/protein/gzp072. [DOI] [PubMed] [Google Scholar]
- Wang YW, Fu Z, Huang HQ, Zhang HS, Yao B, Xiong HR, Turunen O. Improved thermal performance of Thermomyces lanuginosus GH11 xylanase by engineering of an N-terminal disulfide bridge. Bioresour Technol. 2012;112:275–279. doi: 10.1016/j.biortech.2012.02.092. [DOI] [PubMed] [Google Scholar]
- Wang XY, Huang HQ, Xie XM, Ma R, Bai YG, Zheng F, You S, Zhang BY, Xie HF, Yao B, Luo H. Improvement of the catalytic performance of a hyperthermostable GH10 xylanase from Talaromyces leycettanus JCM12802. Bioresour Technol. 2016;222:277–284. doi: 10.1016/j.biortech.2016.10.003. [DOI] [PubMed] [Google Scholar]
- Yang A, Cheng JS, Liu M, Shangguan YJ, Liu LW. Sandwich fusion of CBM9_2 to enhance xylanase thermostability and activity. Int J Biol Macromol. 2018;117:586–591. doi: 10.1016/j.ijbiomac.2018.05.199. [DOI] [PubMed] [Google Scholar]
- Youssef NH, Couger MB, Struchtemeyer CG, Liggenstoffer AS, Prade RA, Najar FZ, Atiyeh HK, Wilkins MR, Elshahed MS. The genome of the anaerobic fungus Orpinomyces sp. strain C1A reveals the unique evolutionary history of a remarkable plant biomass degrader. Appl Microbiol Biot. 2013 doi: 10.1128/AEM.00821-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng H, Guo B, Chen XL, Fan SJ, Zhang YZ. Improvement of the quality of wheat bread by addition of glycoside hydrolase family 10 xylanases. Appl Microbiol Biot. 2011;90(2):509–515. doi: 10.1007/s00253-011-3088-7. [DOI] [PubMed] [Google Scholar]
- Zheng FZ, Song LN, Basit A, Liu JQ, Miao T, Wen JQ, Cao YH, Jiang W. An endoxylanase rapidly hydrolyzes xylan into major product xylobiose via transglycosylation of xylose to xylotriose or xylotetraose. Carbohyd Polym. 2020;237:116121. doi: 10.1016/j.carbpol.2020.116121. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Sequence data is available in the NCBI database via accession numbers MW692171.1.