Abstract
Opioid-based drugs are frequently used for pain management in both males and females despite the known risk of prefrontal cortex dysfunction and cognitive impairments. Although poorly understood, loss of cognitive control following chronic drug use has been linked to decreased activation of frontal cortex regions. Here, we show that self-administration of the potent opioid, remifentanil, causes a long-lasting hypoactive basal state evidenced by a decrease in ex vivo excitability that is paralleled by an increase in firing capacity of layer 5/6 pyramidal neurons in the prelimbic, but not infralimbic region of the medial prefrontal cortex. This phenomenon was observed in females after as few as 5 days and up to 25–30 days of self-administration. In contrast, pyramidal neurons in males showed increased excitability following 10–16 days of self-administration, with hypoactive states arising only following 25–30 days of self-administration. The emergence of a hypoactive, but not hyperactive basal state following remifentanil self-administration aligned with deficits in cognitive flexibility as assessed using an operant-based attentional set-shifting task. In females, the hypoactive basal state is driven by a reduction in excitatory synaptic transmission mediated by AMPA-type glutamate receptors. Alternatively, hyper- and hypoactive states in males align selectively with decreased and increased GABAB signaling, respectively. Chemogenetic compensation for this hypoactive state prior to testing restored cognitive flexibility, basal hypoactive state, and remifentanil-induced plasticity. These data define cellular and synaptic mechanisms by which opioids impair prefrontal function and cognitive control; indicating that interventions aimed at targeting opioid-induced adaptations should be tailored based on biological sex.
Subject terms: Addiction, Cognitive control
Introduction
Even when taken as prescribed, use of opioid-based drugs carry a risk of misuse and dependence that can lead to out of control drug use. Deficits in cognitive control, whether intrinsic or arising from drug use, increase the risk and severity of substance use disorders [1]. Although the range of cognitive problems in substance use disorders is diverse, one of the most consistently documented is cognitive inflexibility [1]. Clinical data indicate that deficits in cognitive flexibility can increase the risk and severity of addiction by strengthening drug-seeking behaviors, increasing relapse vulnerability, and impair an individual’s ability to resist habitual drug use [2–7]. Thus, identifying adaptations responsible for impaired decision-making that precede or parallel out of control drug use provides an opportunity to increase efficacy of treatments aimed at mitigating relapse and susceptibility for developing opioid use disorders.
The prelimbic (PrLC) region of the medial prefrontal cortex (mPFC) in rodents is known to govern various cognitive functions, including encoding of flexible decision-making, inhibitory control, and opioid seeking [8–11, rodents is known to govern various cognitive functions]. Impaired cognition in numerous pathological states has been linked to reduced or increased/irregular spike firing activity in the PrLC cortex [12, 13]. Clinical imaging studies have identified a dichotomous dysfunction of the prefrontal cortex in individuals addicted to heroin, showing reduced basal levels of metabolic activity and craving related increases in activity in response to drug cues [14–16]. Rodent addiction models have separately shown a similar phenomenon whereby PrLC pyramidal neurons exhibit a progressive enhancement in their response to cocaine and cocaine cues, while reduced excitability of PrLC layer 5/6 (L5/6) pyramidal neurons following extended cocaine exposure promotes compulsive drug-seeking [5, 9, 15, 17–23]. Compared to drugs of abuse, such as cocaine, far less is known regarding the impact of opioid exposure on PFC cellular physiology and synaptic regulation, with even less known about how biological sex confers vulnerability to these adaptations. Here, we outline previously unexplored time- and sex-specific effects of opioid self-administration on intrinsic and synaptic regulation of pyramidal neurons in the PrLC and infralimbic (ILC) regions of the mPFC, and the resultant impact on cognitive flexibility.
Materials and methods
Subjects
Adult male and female wild-type C57BL/6 mice (postnatal day 68 ± 0.82 at self-administration onset) were bred in-house or commercially purchased (Jackson Laboratory) and maintained in a temperature and humidity-controlled room. Animal use was approved by the Institutional Animal Care and Use Committee at Marquette University.
Catheter surgery
Mice were implanted with an intravenous standard mouse jugular vein catheter under general isoflurane anesthesia. Mice began self-administration following 5 days of recovery. Catheters were flushed daily with heparinized saline containing either gentamicin sulfate or enrofloxacin. Brevital sodium was used to periodically check catheter patency. Procedures are described in detail in Supplementary information.
Self-administration
For all experiments, mice were initially food restricted to 85–90% of their original weight and habituated to 50% liquid Ensure® in the home cage. In initial experiments (N = 37), training to lever press for a reward involved 1 day of training on a fixed ratio (FR1) schedule for a liquid reward (50% vanilla Ensure®) alone (i.e., not paired) followed by 10–16 days of self-administration for remifentanil or saline on an FR1 schedule of reinforcement (+cue-light, 20-s timeout; 3-h sessions). To control for lever pressing differences between saline and remifentanil exposed mice, expedite acquisition of lever pressing, and reduce the rate of failed acquisition; the majority of mice (N = 233) were involved in a ‘paired’ design where mice were food restricted and trained to press the active lever on an increasing FR schedule where Ensure® was paired with an intravenous infusion of saline or remifentanil. After reaching criterion, mice underwent one self-administration session remifentanil or saline without Ensure® before food was returned ad libitum and self-administration ensued for a total of 5, 10–16, or 25–30 days (2- or 3-h sessions) with a minimum of 10 and maximum of 100 infusions for remifentanil mice. For all self-administration, each infusion was either 0.025 mL saline or 0.025 mL of 5 µg/kg/infusion remifentanil (Ultiva®), a μOR-specific and potent synthetic opioid. Procedures are described in detail in Supplementary information.
In vivo designer receptor exclusively activated by designer drugs (DREADD) activation
Two to three days following self-administration completion, a subset of mice received bilateral intracranial PrLC infusion of AAV8-CamKII-hm3d(Gq)-mcherry (Addgene) or AAV8-CamKII-GFP (UNC Vector Core). Mice recovered for 5 days then were food-deprived and began the attention set-shifting task. Mice received a saline injection prior to the visual cue test and 1.5–2.0 mg/kg clozapine-n-oxide (CNO) prior to the extradimensional (ED) shift test. For ex vivo assessment of in vivo CNO [24], slices were prepared immediately following conclusion of behavioral testing or in some instances, a second CNO injection on a subsequent day and slices prepared at least 45 min following injection. Only mice with virus expressed bilaterally in the PrLC were used for analyses.
Behavioral assessments
The morning following the last self-administration session, a subset of mice were tested during a 5 min elevated plus maze (EPM; San Diego Instruments) test as previously described [25]. Percent time in the open arms was calculated as total time in the open arms divided by total time in the maze. Testing for immobility in the forced swim test (FST) was done ~16 days following self-administration, as described [25]. Cognitive flexibility was measured using an operant-based attention set-shifting task. Following 3–8 days of withdrawal, mice went through food and lever training. Following an average of 14 days after the last self-administration session, visual cue testing was conducted until 150 trials or 10 consecutive correct responses were made. During the visual cue test, the correct response was on the lever below the illuminated cue light. Incorrect responses resulted in only the time out (no reward). Failure to respond (i.e., omission) was tracked but not counted towards a trial to criterion. The day following visual cue testing, ED shift testing was conducted. The reinforced lever was always the lever opposite of the lever bias; however, the cue light was presented similar to that during the visual cue test. The day after criterion of the ED shift was reached (i.e., 10 consecutive correct responses), reversal testing was conducted with the reinforced lever always that of the bias and the cue light presented identical to that during the visual cue test. Increased detail of behavioral assessment methods are available in Supplementary information.
Slice electrophysiology
Whole-cell recordings were performed in L5/6 pyramidal neurons as previously described [25, 26] and described in detail in Supplementary information. Current-clamp recordings and voltage-clamp recordings of baclofen-evoked currents were taken using a potassium gluconate internal solution, with recordings filtered at 2 kHz and sampled at 5 kHz. Postsynaptic current recordings were filtered at 2 kHz and sampled at 20 kHz performed with cesium methylsulfate internal at −72 mV (EPSCs) and 0 mV (IPSCs) and 0.7 mM lidocaine added to the recording solution for miniature excitatory postsynaptic currents (mEPSCs)/miniature inhibitory postsynaptic currents (mIPSCs).
Statistical analysis
ANOVAs were used for comparisons unless only two groups were compared in which case independent-samples t-test were used. When applicable, Student–Newman–Keuls post hoc comparisons were conducted. For more detail and description of software, see Supplementary information.
Results
Effects of short- and long-term remifentanil self-administration on PrLC L5/6 pyramidal neurons
To determine how contingent administration of a clinically relevant opioid impacts PFC function, male and female mice originally underwent 10–16 days of saline or remifentanil (Ultiva®) self-administration followed by 14–21 days of forced abstinence (Fig. 1a). During initial studies, mice were lever trained using a liquid Ensure® reward on an FR1 schedule (not paired), followed by 10–16 days of intravenous (i.v.) saline or remifentanil self-administration (3-h sessions). Subsequent studies utilized a training approach whereby i.v. infusions were initially paired with Ensure®, followed by i.v. administration without Ensure®. When Ensure® was not paired, remifentanil mice had significantly more active lever presses compared to saline mice (Supplementary Fig. 1a), whereas pairing with Ensure® prompted a sustained increase in active lever responding in saline mice (Supplementary Fig. 1b), negating a difference compared to remifentanil mice (Supplementary Fig. 1c) and allowing us to control for any differences lever pressing may have on later electrophysiology measures. Importantly, active lever responding and cumulative infusions remained similar in paired and not paired remifentanil mice (Supplementary Fig. 1d, e) and paired male remifentanil mice exhibited higher break points for remifentanil compared to saline mice for a saline infusion following self-administration (Supplementary Fig. 1f), indicating that the selected dose is reinforcing and mice receiving remifentanil are more motivated to lever press compared to mice receiving a saline infusion. Comparison of 2- and 3-h sessions showed no difference in total infusions earned in male and female remifentanil mice (Supplementary Fig. 2a, c). Further, neither active nor inactive lever responding was different in males (Supplementary Fig. 2b) while females in 2-h sessions showed significantly greater active lever responding only on day 7 of self-administration (Supplementary Fig. 2d); thus, data from initial cohorts were combined. For 10–16-day self-administration studies, active lever responding during maintenance in mice that were trained using the ‘paired’ procedure and were allowed to self-administer for 3-h a day (see Supplementary Fig. 3 for responding across all days) did not vary in males (N = 18) vs. females (N = 21; sex: F(1,37) = 0.21, p = 0.65; day: F(3,111) = 1.89, p = 0.14; interaction: F(3,111) = 0.29, p = 0.83). In contrast, a main effect of sex was observed for inactive lever responding, with females exhibiting greater responding compared to males (sex: F(1,37) = 4.57, p = 0.04; day: F(3, 111) = 1.12, p = 0.34; interaction: F(3,111) = 0.30, p = 0.83; Fig. 1b). Cumulative remifentanil infusions across all days of self-administration did not differ in remifentanil males vs. females (t(37) = −1.69, p = 0.12; Fig. 1c).
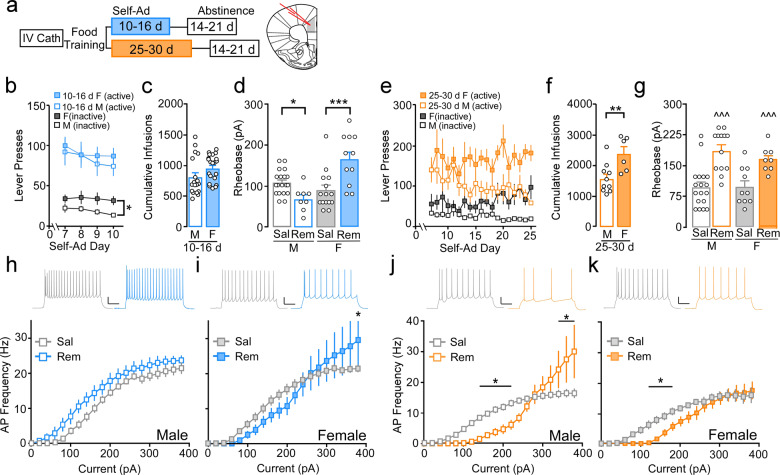
Fig. 1. Bidirectional effect of remifentanil self-administration on PrLC L5/6 pyramidal neurons in males and females.
a Self-administration, abstinence timeline, and location of whole-cell recordings in the PrLC. b For mice that received 10–16 days of self-administration, no difference was observed for active presses during maintenance between remifentanil males (blue, unfilled) and females (blue, filled) whereas females had greater inactive responses (gray, filled) compared to males (gray, unfilled). c Cumulative remifentanil infusions during the entire 10–16-day self-administration procedure did not differ in males (M) vs. females (F). d Comparison of mean action potential (AP) threshold (rheobase) in PrLC L5/6 pyramidal neurons following 14–21-day abstinence from 10- to 16-day self-administration in males and females showed that rheobase was similar in females compared to males under saline conditions (SAL). Alternatively, remifentanil (REM) increased rheobase in females but reduced it in males compared to respective controls. e For 25–30-day self-administration groups, active lever responding did not differ between remifentanil males (orange, unfilled) and females (orange, filled) or across days during maintenance. Alternatively, males exhibited significantly lower inactive lever responding compared to females on days 9, 13, and 19–25. f Cumulative remifentanil infusions were elevated in females (F) compared to males (M). g Comparison of rheobase following 14–21-day abstinence from 25–30 days of self-administration showed a significantly higher firing threshold in remifentanil males and females compared to respective saline controls. h–i Current-spike analysis in 10–16-day self-administering mice showed a trend toward increased firing frequency in remifentanil vs. saline males (h) whereas i remifentanil females exhibited similar firing at lower currents and increased firing at higher potentials (380 pA) compared to controls. j Following 25–30 days of self-administration, current-spike analysis showed firing frequency was reduced at lower currents (140–220 pA) but increased at more depolarized potentials (340–380 pA) in remifentanil vs. saline males. k Remifentanil females showed reduced firing frequency at lower currents (100–180 pA) but similar firing frequency at more depolarized potentials vs. saline females. Representative scale bars: 20 pA/200 ms. Representative spike firing following 200 pA current injection. All data are presented as the mean ± SEM. *p < 0.05, **p < 0.01, ***p < 0.001; ^^^p < 0.001 main effect of treatment.
Functional integrity of mPFC information processing is dependent on extrasynaptic factors that dictate pyramidal neuron firing threshold and responsivity to synaptic input (i.e., excitability) [27–30]. Ex vivo electrophysiology was used to examine these properties in layer 5/6 (L5/6) PrLC pyramidal neurons following 10–16 days of self-administration and a 14–21-day period of abstinence by measuring the threshold to evoke an initial action potential (AP; rheobase) in response to depolarizing current steps (1 s, 0–380 pA, 20 pA steps). Comparison of rheobases revealed a significant sex by treatment interaction (F(1,49) = 21.50, p < 0.001) with post hoc comparisons showing rheobase was similar in females compared to males under saline conditions (male, N/n = 13/19; female, N/n = 9/15; p = 0.23). Alternatively, remifentanil increased rheobase in females (N/n = 5/11; p < 0.001) but reduced it in males (N/n = 6/8; p = 0.039) compared to saline controls (Fig. 1d). As cell excitability reflects firing threshold and spike activity states and rheobase is often defined as a measure of basal excitation, we will refer to reductions and increases in threshold to fire an action potential as hyperexcitable and hypoexcitable basal states, respectively. Notably, rheobase did not differ in paired vs. not paired saline mice (data not shown; males: t(21) = 0.85, p = 0.41; females: t(26)= −0.56, p = 0.58), indicating that neither the training or sustained instrumental responding impacted observed changes in physiology. Further, extinction from initial Ensure® training did not influence threshold to fire, as additional cohorts undergoing Ensure® self-administration for 10–16 days showed similar rheobase compared to those undergoing subsequent saline administration and rheobase remained different from remifentanil mice (Supplementary Fig. 4).
To determine whether this hypoexcitable basal state is unique to females or merely arises on a more rapid time scale compared to males, rheobase was next measured following abstinence from 25 to 30 days of self-administration. Similar to 10–16 days, active lever pressing during maintenance following ‘paired’ training in mice that were allowed to self-administer for 3-h a day did not vary across sex (sex: F(1,14) = 4.24, p = 0.06; day: F(18,252) = 0.80, p = 0.71; interaction: F(18,252) = 1.34, p = 0.16; males: N = 10, females: N = 6) or across all days of self-administration (Supplementary Fig. 5). For inactive lever pressing, a sex by day interaction was observed (F(28,252) = 1.91, p = 0.02), with males having significantly lower inactive presses on days 9 and 13 (p < 0.05), 19 (p < 0.01), 20 (p < 0.001), 21 (p < 0.05), 22 (p < 0.01), 23 (p < 0.001), 24 (p < 0.05), and 25 (p < 0.001). In contrast to 10–16 days, cumulative remifentanil infusions were elevated in females compared to males (t(14) = −3.12, p = 0.008; Fig. 1f). Ex vivo assessments showed a main effect of treatment with remifentanil males (N/n = 6/13) and females (N/n = 4/8) exhibiting an increase in rheobase compared to controls (male, N/n = 9/18; female, N/n = 5/10; sex: F(1,45) = 0.59, p = 0.45; treatment: F(1,45) = 30.48, p = 0.001; interaction: F(1,45) = 0.64, p = 0.43; Fig. 1g). Thus, opioid self-administration promotes a hypoactive basal state in PrLC pyramidal neurons in males and females, however, this dysfunction arises more rapidly in females.
PFC dysfunction and impaired cognitive control has been linked to irregularities in prefrontal pyramidal neuron firing patterns across numerous disorders [12, 13]. Thus, we next examined how opioid self-administration impacted patterned spike firing. Following 10–16 days of self-administration, pyramidal neurons in remifentanil males (N/n = 5/6) exhibited a nonsignificant leftward shift in the current-spike relationship reflecting a trend towards increased firing frequency compared to saline males (N/n = 9/18; treatment: F(1,19) = 3.78, p = 0.07; interaction: F(19,361) = 0.62, p = 0.90; Fig. 1h). Alternatively, pyramidal neurons in remifentanil females (N/n = 4/10) showed a nonsignificant rightward shift at lower currents, but increased firing at more depolarized potentials (p = 0.03 for 380 pA; interaction: F(19,418) = 1.61, p = 0.05; Fig. 1i). Following 25–30 days of self-administration in males, current-spike analysis showed firing frequency was reduced at lower currents but increased at more depolarized potentials in remifentanil (N/n = 6/13) vs. saline (N/n = 9/18) males (interaction: F(19,551) = 5.43, p < 0.001; p < 0.05 at 140–220 pA and 340–380 pA). The divergence following 10–16 days of self-administration in females was no longer present (saline, N/n = 5/10; remifentanil, N/n = 4/8), with firing frequency reduced at lower currents vs. controls but similar firing at more depolarized potentials (interaction: F(19,304) = 1.87, p = 0.016; p < 0.05 at 100–180 pA; Fig. 1k). Notably, no significant effects on excitability or firing were observed in ILC pyramidal neurons following short- or long-term self-administration (Supplementary Fig. 6).
To thoroughly characterize the temporal nature of these adaptations, we next asked whether hyperexcitable states observed in males also occurs in females, but on a shorter time scale. Following 14–21 days abstinence from 5 days of self-administration, despite males and females having similar responding and intake (data not shown), mean rheobase was reduced in remifentanil male mice but increased in females (Supplementary Fig. 7a), correlating with a left- and rightward shift in firing, respectively (Supplementary Fig. 7b, c). To determine how plasticity varied with more prolonged abstinence, excitability was measured 40–45 days following 10–16 days of self-administration. Following prolonged abstinence, only hypoexcitable basal states and elevated firing capacity in females remained (Supplementary Fig. 7d–f), with increased excitability previously observed in males no longer present. These data highlight the enduring nature of hypoexcitable basal states in females. Examination of additional intrinsic properties related to neuronal firing including action potential threshold, amplitude, duration, and after hyperpolarization amplitude were also examined for all self-administration exposure and withdrawal lengths. Although there were some significant measures, no noticeable patterns of significance were apparent (Supplementary Tables 1–4).
Impact of remifentanil on PrLC inhibitory and excitatory synaptic regulation
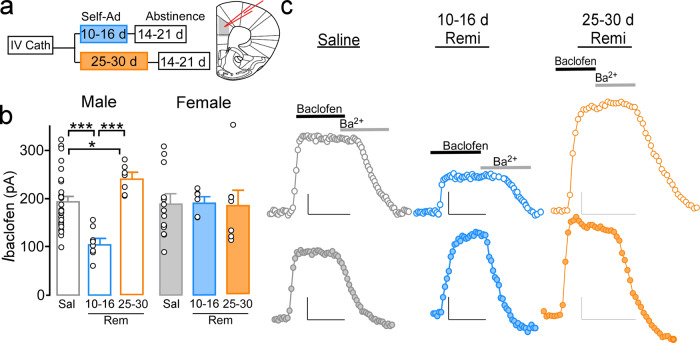
Activation and firing patterns of pyramidal neurons are heavily influenced by synaptic and perisynaptic excitatory and inhibitory signaling at the soma and dendrites [28, 29]. We have previously shown that cocaine-induced hyperexcitablity of PrLC pyramidal neurons is linked to reductions in GABABR-dependent activation of G protein inwardly-rectifying K+ channels (GABABR-GIRK)[26]. Thus, we examined if changes in excitability also aligned with altered GABABR-GIRK signaling (Fig. 2a). Somatodendritic currents evoked by the GABABR agonist, baclofen (IBaclofen; 200 µM), were similar in 10–16- (N/n = 11/13) and 25–30-day (N/n = 8/12) saline males (t(22) = 0.442, p = 0.663) and females (10–16 days, N/n = 5/5; 25–30 days, N/n = 7/9; t(12) = −0.842, p = 0.416) and thus each sex was combined into a single saline group. Subsequent analysis identified a significant sex by treatment interaction (F(2,60) = 5.79, p = 0.005). IBaclofen was reduced in 10–16-day remifentanil males (N/n = 6/8) compared to saline (p < 0.001) and 25–30-day remifentanil males (N/n = 6/7; p < 0.001), but increased in 25–30-day remifentanil males compared to saline (p = 0.037; Fig. 2b, c), paralleling the decrease and increase in rheobase previously observed in males, respectively. Conversely, IBaclofen was not altered in 10–16-day (N/n = 4/6; p = 0.913) or 25–30-day (N/n = 5/7; p = 0.868) females compared to saline. However, IBaclofen was greater in 10–16-day remifentanil females vs. 10–16-day remifentanil males (p = 0.006). Similar to changes in rheobase and spike firing in 10–16-day males, alterations in IBaclofen were no longer present in males at 40–45 days after abstinence (Supplementary Fig. 8).
Fig. 2. Remifentanil effects on PrLC L5/6 pyramidal neuron GABAB-GIRK signaling.
a Self-administration and abstinence timepoints. b Baclofen-evoked currents (IBaclofen) from male (unfilled) and female (filled) PrLC L5/6 pyramidal neurons 14–21 days after saline (gray), 10–16-day remifentanil self-administration (blue), or 25–30-day remifentanil self-administration (orange). IBaclofen was no different in 10–16-day vs. 25–30-day saline males or females and thus were combined. IBaclofen was reduced in 10–16-day remifentanil males vs. saline males and 25–30-day remifentanil males, whereas 25–30-day remifentanil males showed an increase in IBaclofen vs. saline males. IBaclofen did not differ when comparing saline females with 10–16- or 25–30-day remifentanil females. A difference was observed between 10- and 16-day remifentanil males and females. c Representative IBaclofen traces in males (top) and females (bottom) across treatments. Representative scale bars: 50 pA/200 s. *p < 0.05, ***p < 0.001.
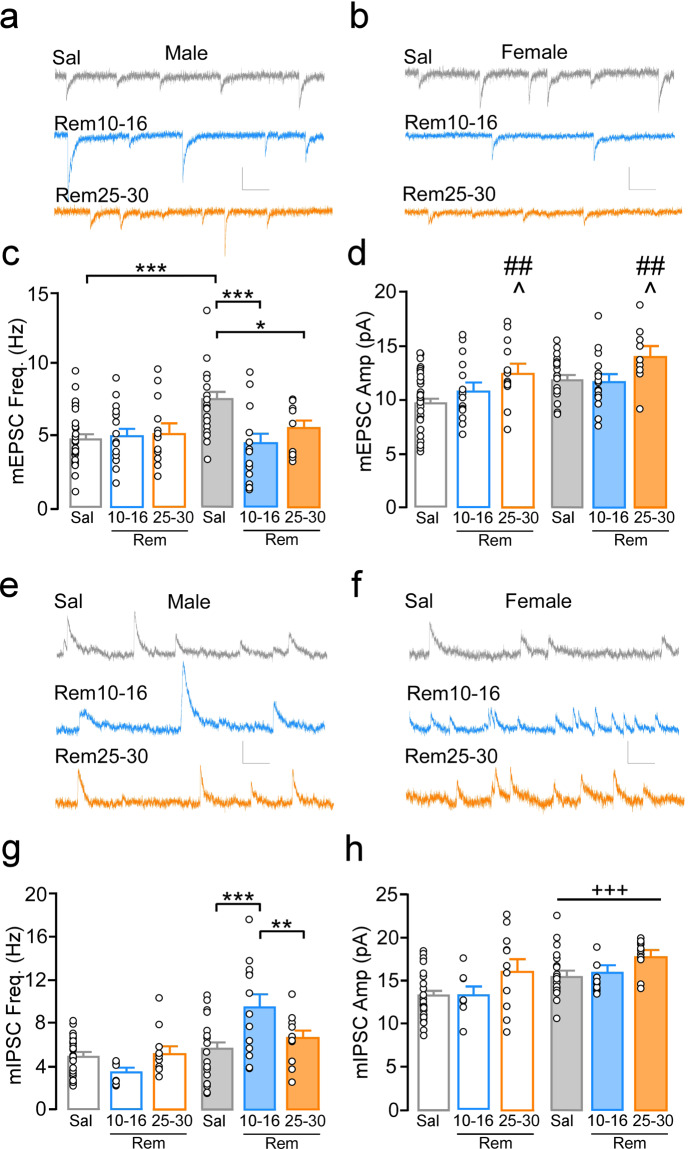
Given the lack of change in GABABR-GIRK signaling in females, we next examined modifications in ionotropic excitatory and inhibitory synaptic transmission by measuring AMPA receptor-specific mEPSCs and GABAAR-specific mIPSCs. Saline groups were again not different between 10–16 and 25–30-day groups and were therefore combined (Supplementary Fig. 9). Comparison of mEPSC frequency identified an interaction of sex by treatment (F(1,94) = 4.66, p = 0.01). Post hoc comparisons indicate that mEPSC frequency was greater in saline females (N/n = 11/21) compared to saline males (N/n = 13/27; p < 0.001). mEPSC frequency was reduced in females following 10–16 (N/n = 8/15; p = 0.001) and 25–30 days (N/n = 4/9; p = 0.038) of remifentanil self-administration compared to saline controls (Fig. 3a–c). mEPSC frequency did not differ when comparing saline males with 10–16-day (N/n = 8/15, p = 0.73) or 25–30-day (N/n = 5/13, p = 0.79) remifentanil males. Only a main effect of treatment was identified for mEPSC amplitudes, with frequency elevated in 25–30-day remifentanil mice compared to 10–16-day remifentanil (p = 0.019) and saline groups (p = 0.006; treatment: F(2,94) = 4.77, p = 0.01; sex: F(1,94) = 3.63, p = 0.06; interaction: F(2,94) = 10.27, p = 0.76; Fig. 3d).
Fig. 3. Remifentanil effects on PrLC L5/6 pyramidal neuron excitatory and inhibitory transmission.
Representative miniature excitatory postsynaptic current (mEPSC) traces from PrLC L5/6 pyramidal neurons in a males and b females following 14–21 days abstinence from 10–16 and 25–30 days of self-administration. c mEPSC frequency was significantly greater in saline females compared to 10–16- and 25–30-day remifentanil females as well as saline males. mEPSC frequency did not differ when comparing saline to 10–16-day or 25–30-day remifentanil males. d mEPSC amplitude was elevated in 25–30-day remifentanil mice compared to 10–16-day and saline mice. Representative miniature inhibitory postsynaptic current (mIPSC) traces in males (e) and females (f) following 14–21 days abstinence from 10–16 and 25–30 days of self-administration. g mIPSC frequency was greater in 10–16-day remifentanil females vs. saline and 25–30-day remifentanil females, with no differences between 25- and 30-day remifentanil and saline groups. No differences were observed when comparing saline males to 10–16- or 25–30-day remifentanil males. h Mean mIPSC amplitude was elevated in females compared to males regardless of treatment. Scale bars: 10 pA/100 ms. *p < 0.05, **p < 0.01, ***p < 0.001; +++p < 0.001 main effect of sex, ^p < 0.05 vs. Rem10-16, ##p < 0.01 vs Rem25-30.
mIPSC frequency was significantly different between groups (interaction: F(2,88) = 6.21, p = 0.003). mIPSC frequency was elevated in 10–16-day remifentanil females (N/n = 10/13) compared to saline (N/n = 10/20; p = 0.001) and 25–30-day remifentanil females (N/n = 4/11; p = 0.011), while no differences were observed between 25- and 30-day remifentanil vs. saline (N/n = 10/20; p = 0.53) females, indicating that these effects were no longer present following more prolonged self-administration (Fig. 3e–g). Similar to mEPSC frequency, no differences were observed when comparing saline (N = 13/30) to 10–16-day (N/n = 5/8; p = 0.19) or 25–30-day (N/n = 5/11; p = 0.67) remifentanil males. A main effect of sex was observed for mIPSC amplitude, with mean amplitudes greater in females compared to males, regardless of treatment (sex: F(1,88) = 11.82, p < 0.001; treatment: F(2,88) = 1.83, p = 0.17; interaction: F(2,88) = 0.22, p = 0.81; Fig. 3h). These findings indicate that hypoexcitable states are driven via distinctly different mechanisms in males and females.
Time-dependent effects of remifentanil on affect and cognitive flexibility
PrLC dysfunction has been linked to impairments in flexible behavior [8, 31] and affect dysregulation that are known to increased risk for relapse and heightened drug use[5]. We first examined whether 10–16 days of self-administration, the exposure period where sex differences in plasticity were identified, produced changes in performance in the EPM and a FST. Percent open arm time in the EPM was not altered in male or females following acute abstinence; however, females showed an increase in time immobile in the FST following more prolonged abstinence (Supplementary Fig. 10).
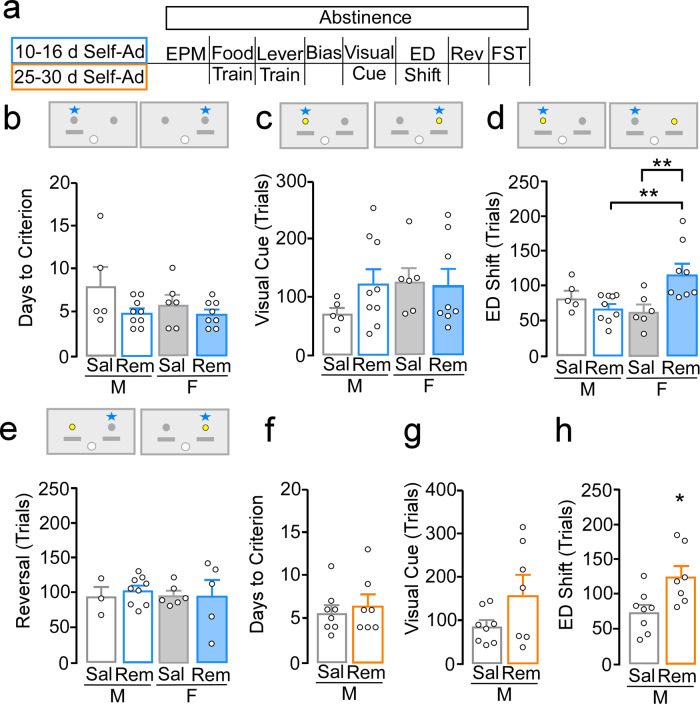
To examine effects on cognitive flexibility, we used an operant-based attentional set-shifting model. This task resembles the Wisconsin Card Sorting Task in the sense that the stimuli are easy to detect, but rules are implicit and learned while the task is performed (Fig. 4a). Remifentanil exposure for 10–16 days did not alter the number of days to reach lever training criterion in males (saline, N = 5; remifentanil, N = 9) or females (saline, N = 6; remifentanil, N = 8; sex: F(1,24) = 1.24, p = 0.28; treatment: F(1,24) = 4.09, p = 0.054; interaction: F(1,24) = 0.96, p = 0.34; Fig. 4b). It also did not alter number of trials (sex: F(1,24) = 1.07, p = 0.31; treatment: F(1,24) = 0.80, p = 0.38; interaction: F(1,24) = 1.21, p = 0.28; Fig. 4c) or errors to reach criterion (Supplementary Fig. 11a) during the visual cue discrimination task; indicating that associative learning processes remain intact. Alternatively, comparison of trials to criterion during the ED shift test showed that remifentanil females required more trials vs. saline females (p = 0.002) and remifentanil males (p = 0.002), whereas performance in remifentanil males did not differ compared to saline males (p = 0.33); interaction: (F(1,24) = 9.62, p = 0.005). Remifentanil females, but not males, made a greater number of errors prior to reaching criterion during an extradimensional shift test (Supplementary Fig. 11b). Performance during a subsequent reversal learning test was unaffected in both males and females (sex: F(1,19) = 0.05, p = 0.82; treatment: F(1,19) = 0.12, p = 0.73; interaction: F(1,19) = 0.24, p = 0.63; Fig. 4e; Supplementary Fig. 11c for errors to criterion).
Fig. 4. Remifentanil-induced deficits in cognitive flexibility.
a Timeline of behavioral assessments following 10–16 or 25–30-day self-administration and schematic of operant set-shifting procedure depicting levers (gray), dipper (white), cue location (yellow), and correct response (star). b, e Testing following 10–16 days of self-administration in males and females. b Number of days to pass lever training criterion (2 consecutive days ≤ 5 omissions) was similar acrosss treatment and sex. c Number of trials to criterion during a visual cue test also showed no significant effect of sex, treatment, or interaction. d Number of trials to criterion during the extradimensional (ED) shift test showed remifentanil females required more trials vs. saline females and remifentanil males, whereas performance in remifentanil vs. saline males was similar. e Number of trials to criterion during a reversal test showed no significant effect of sex, treatment, or interaction. f–h Testing following 25–30 days of self-administration in males. f, g No difference was observed in number of days to pass lever training criterion or trials to reach criterion during a visual cue test. h During the ED test, remifentanil males required more trials to reach criterion compared to saline. *p < 0.05, **p < 0.01.
We next measured cognitive flexibility in males (saline, N = 8; remifentanil, N = 7) following 25–30 days of self-administration, a timepoint where males exhibit a similar hypoactive basal state. There were no differences in days to reach lever training criterion (t(13) = −0.54, p = 0.60; Fig. 4f). There were also no differences in trials (t(13) = −1.89, p = 0.08; Fig. 4g) or errors (Supplementary Fig. 11d) to reach criterion during the visual cue test. However, remifentanil males required significantly more trials (t(13) = −2.64, p = 0.02; Fig. 4h) and errors (Supplementary Fig. 11e) to reach criterion during the extradimensional shift test These findings indicate that opioid self-administration produces cognitive flexibility deficits in both males and females, and that this deficit arises in females with less drug exposure and aligns with emergence of a hypoexcitable basal state.
Chemogenetic manipulation of PrLC function and cognitive flexibility
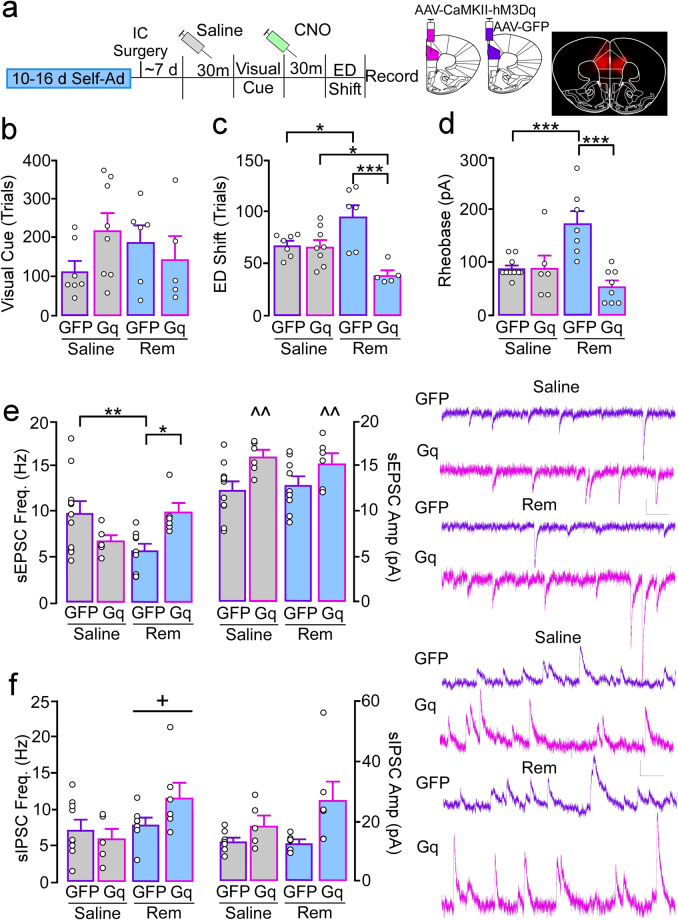
Previous studies have shown that pharmacological inhibition of the PrLC impairs flexible decision-making in a nearly identical model of attentional set-shifting [32]. To determine if the hypoexcitable basal state underlies reductions in cognitive flexibility, we used an in vivo chemogenetic approach to express the excitatory hm3d-Gq DREADD [33] in CaMKII-expressing cells (i.e., pyramidal neurons) in females following 10–16 days of self-administration (GFP-saline, N = 7; Gq-saline, N = 8; GFP-remifentanil, N = 6; Gq-remifentanil, N = 5; Fig. 5a). Following a habituating injection of saline, no differences in trials to criterion during a visual cue test were observed (treatment: F(1,22) = 0.00, p = 0.97; virus: F(1,22) = 0.55, p = 0.46; interaction: F(1,22) = 2.86, p = 0.11; Fig. 5b). Prior to a subsequent extradimensional shift test, all mice received systemic injection of the DREADD agonist, CNO (1.5–2.0 mg/kg), to ensure any effects of CNO were distributed across groups. GFP-remifentanil females took significantly more trials to reach criterion compared to GFP-saline (p = 0.016; interaction: F(1,22) = 13.52, p = 0.001), replicating initial findings. Gq-remifentanil females performed significantly better than GFP-remifentanil (p < 0.001) and Gq-saline females (p = 0.017), while performance in Gq-saline mice was similar to their GFP counterparts (p = 0.86; Fig. 5c). Notably, initial studies using a higher dose of CNO (5 mg/kg) produced a similar rescue of behavior in Gq-remifentanil females; however, it also partially rescued performance in GFP-remifentanil females (Supplementary Fig. 12a) indicating CNO at higher concentrations may have off-target effects due to back metabolism of CNO to clozapine [34], which has been shown to improve set-shifting performance in rats [35].
Fig. 5. Increased excitation of PrLC pyramidal neurons in remifentanil females reverses deficits in cognitive flexibility.
a Timeline of DREADD experiments involving 10–16-day saline and remifentanil female mice expressing an excitatory CaMKII-hM3Dq(Gq)-DREADD or control GFP AAV in the PrLC. All mice received a saline or CNO (1.5–2.0 mg/kg, i.p.) injection 30 min prior to the visual cue and ED shift test, respectively. b Comparison of trials required to reach criterion during visual cue testing showed no effect of treatment, virus, or treatment by virus interaction. c For trials to criterion during the ED shift, female GFP-remifentanil mice required more trials vs. GFP-saline and Gq-remifentanil mice. Gq-remifentanil mice performed significantly better than Gq-saline mice. There was no significant difference between GFP-saline and Gq-saline. d–f Ex vivo comparison of rheobase and synaptic transmission following in vivo systemic CNO injection. d Comparison of rheobase in PrLC pyramidal neurons showed a treatment by virus interaction, with more current required to evoke an action potential in female GFP-remifentanil mice vs. GFP-saline and Gq-remifentanil mice. Rheobase in Gq-saline did not differ compared to GFP-saline or Gq-remifentanil. e Spontaneous EPSC (sEPSC) frequency (left) and amplitude (right) in GFP- and Gq-expressing PrLC pyramidal neurons. Pyramidal neurons in GFP-remifentanil mice showed a reduction in frequency compared to GFP-saline and Gq-remifentanil mice. sEPSC frequency in Gq-saline was not significantly different compared to GFP-saline or Gq-remifentanil. For sEPSC amplitude, Gq+ cells exhibited an overall greater amplitude compared to GFP. f Spontaneous IPSC (sIPSC) frequency and amplitude in GFP and Gq-expressing PrLC pyramidal neurons. sIPSC frequency was increased in remifentanil mice compared to saline mice regardless of virus. For sIPSC amplitude, no pairwise comparisons of interest were significant. Scale bars: 10 pA/100 ms. *p < 0.05, **p < 0.01, ***p < 0.001, ^^p < 0.01 main effect of virus, +p < 0.05 main effect of treatment.
Ex vivo analysis of CNO effects on pyramidal neuron rheobase showed that CNO bath application (5 µM for 10 min) lead to spontaneous firing in four out of five cells and that CNO significantly reduced rheobase in non-virus and GFP-expressing cells, albeit to a lesser extent (Supplementary Fig. 12b). Ex vivo physiological assessments following in vivo systemic injection of CNO showed that, similar to findings in non-virus-expressing animals, rheobase in GFP-remifentanil females (N/n = 3/7) was significantly greater than GFP-saline mice (N/n = 5/10; p < 0.001; interaction: F(1,27) = 14.98, p < 0.001). Alternatively, rheobase in Gq-remifentanil (N/n = 4/8) mice was significantly reduced compared to cells from GFP-remifentanil (p < 0.001) and similar to Gq-saline (N/n = 4/6; p = 0.12; Fig. 5d). No differences were observed between GFP- and Gq-saline mice (p = 0.93).
We next examined if DREADD-mediated deficits in cognitive flexibility and cell excitability also align with restoration of synaptic regulation following in vivo CNO. Rheobase in PrLC pyramidal neurons showed a treatment by virus interaction (F(1,27) = 14.98, p < 0.001), with more current required to evoke an action potential in female GFP-remifentanil mice (N/n = 3/7) vs. GFP-saline (N/n = 5/10; p < 0.001) and Gq-remifentanil mice (N/n = 4/8; p < 0.001). Rheobase in Gq-saline did not differ compared to GFP-saline (N/n = 4/6; p = 0.93) or Gq-remifentanil (p = 0.12; Fig. 5e). sEPSC frequency was significantly reduced in GFP-remifentanil mice (N/n = 3/9) compared to GFP-saline (N/n = 4/11; p = 0.006) and Gq-remifentanil mice (N/n = 3/6; p = 0.013; interaction: F(1,28) = 10.73, p = 0.003), replicating initial findings. Similar to rheobase, sEPSC frequency did not differ between Gq-saline (N/n = 3/6) and GFP-saline (p = 0.058) or Gq-remifentanil (p = 0.082). For sEPSC amplitude, Gq+ cells exhibited an overall greater amplitude compared to GFP-cells regardless of treatment (virus: F(1,28) = 9.81, p = 0.004; treatment: F(1,28) = 0.00, p = 0.94; interaction: F(1,28) = 0.36, p = 0.56). Alternatively, sIPSCs frequency was greater in remifentanil mice (GFP: N/n = 2/7; Gq: N/n = 3/6) compared to saline mice (GFP: N/n = 5/9; Gq: N/n = 3/5), regardless of virus (virus: F(1,28) = 0.55, p = 0.47; treatment: F(1,23) = 4.34, p = 0.049; interaction: F(1,23) = 2.77, p = 0.11; Fig. 5f). For sIPSC amplitude, a Kruskal–Wallis indicated a significant difference between groups (H(3) = 9.73, p = 0.02) however no pairwise comparisons of interest were significant. Taken together, these data demonstrate that the opioid-induced hypoactive basal state underlies impairments in cognitive flexibility, and that this hypoexcitable state is ostensibly driven by a reduction in excitatory drive.
Discussion
The current series of experiments demonstrates that low dose [36–40] exposure of a clinically used opioid is sufficient to promote an enduring hypoexcitable basal state in PrLC pyramidal neurons in male and female mice. This dysfunction occurs on a faster timeline in females and is linked to the emergence of deficits in cognitive flexibility commonly observed in individuals with substance use disorders [4, 5]. Unexpectedly, this hypoexcitable state is preceded by an opposite hyperexcitable state in males that is not apparent in females. Further, while present in both males and females, hypoexcitable states are ostensibly driven by distinct cellular mechanisms, reflecting increased inhibition mediated by GABABR in males and reduced AMPAR-mediated excitatory drive in females.
Time-dependent and region-specific alterations in pyramidal neuron function
The dichotomy of abstinent heroin users showing reductions in basal metabolic activity in the prefrontal cortex and craving related increases in neural activity in response to drug cues [41, 42], aligns with our current findings where PrLC pyramidal neurons exhibit increased rheobase but also increased firing at more depolarized potentials. This phenomenon is observed following 10–16 days of self-administration in females but takes 25–30 days in males. Increased action potential firing, along with increased variability, appears to be driven by a small portion of cells. What defines this subpopulation as well as the in vivo relevance of these dynamic shifts in ex vivo firing remains unclear, however it may reflect changes in cells based on efferent target or molecular phenotype. For example, dopamine type 1 vs. type 2 receptor expressing PrLC pyramidal neurons have recently been shown to undergo divalent alterations in excitability and firing following prolonged stress exposure [25]. PrLC pyramidal neurons that project to the nucleus accumbens exhibit a high-level of excitation and phase-locking to nucleus accumbens oscillatory dynamics that may be driven by reward cues [43, 44] and reward consumption [45]. Alternatively, increased firing could reflect time-specific plasticity, as this was not observed following 25–30 days of remifentanil self-administration in females. Thus, this may reflect a continuing shift towards greater PrLC dysfunction that underlies emergence of other substance use related maladaptive behaviors beyond cognitive inflexibility [5, 17, 46–49] that are not yet evident in males after only 25–30 days of remifentanil exposure. Regardless, as increased rheobase in females but not the decreased rheobase in males following 10–16 day self-administration was shown to last up to at least 45 days, this adaptation may contribute to enduring deficits in behavioral control.
Activation of both the PrLC and ILC subregions is necessary for heroin seeking [9, 21, 50–54]. It was therefore unexpected that ILC basal state excitation or spike firing was unchanged following remifentanil self-administration in males and females. While it has been previously shown that short-term cocaine exposure does not significantly impact ILC pyramidal neuron excitability [26], the variability in rheobase and spike firing across cells more likely suggests that evident changes are occurring in a cell-type specific (i.e., neurons expressing D1- vs. D2-type receptors) [25, 52, 55, 56] or pathway-specific manner, as past work has identified opioid-induced plasticity at ILC inputs to the nucleus accumbens shell [57].
Sex-specific effects on cognitive deficits and plasticity
Previous studies have implicated mPFC excitatory and inhibitory synaptic modifications in opioid sensitivity and reinstatement [58]; however, these studies did not distinguish subregions of the mPFC and assessed plasticity following extinction [27, 29]. Although reduced excitability of PrLC pyramidal neurons has been previously observed in males following cocaine self-administration [17], female data are lacking and an underlying mechanism remained elusive. Here we find that changes in basal excitability states in males aligns with divergent changes in GABABR-dependent currents. The observed reduction in GABABR signaling is similar to our past findings following repeated cocaine [26]. In females, alterations in GABABR currents were not detected following short- or long-term exposure. Rather, pyramidal neurons in females showed a bidirectional increase and decrease in GABAAR- and AMPAR-mediated currents, respectively. Only reductions in excitatory signaling remained after more prolonged exposure where hypoexcitable and cognitive impairments persist, highlighting this as the primary mechanism driving reduced excitability and cognitive dysfunction. In support, restoration of cognitive performance and hypoexcitable states in Gq-remifentanil female mice aligned with restoration of excitatory transmission to control levels, whereas inhibitory transmission was elevated to levels observed in our initial remifentanil cohorts, suggesting that elevations in GABAAR signaling are insufficient to drive cognitive decline. These findings are in agreement with past work demonstrating that saturation of excitatory signaling is more disruptive to cortical information flow and mPFC-dependent behaviors than increased cortical inhibition [27]. Importantly, the Gq-DREADD rescue of both the hypoactive basal state and changes in ex vivo excitatory drive suggests that DREADDs promote a transient plasticity [24] and that these two phenomenon are related, as direct effects of DREADDs are presumably confined to virus-expressing cells. However, without elimination of synaptic signaling in our slice preparations following in vivo DREADD exposure or during our initial experiments, the contribution of altered synaptic regulation to intrinsic properties and vice versa remains unclear.
It is possible that augmentation in inhibitory signaling contributes more to drug-related behaviors, as increased responsivity of mPFC GABAergic interneurons to heroin-associated stimuli has been previously implicated in relapse vulnerability [59]. Notably, the lack of effect on sIPSCs in GFP-remifentanil females does not likely reflect a lack of replication but rather a distinction in effects on activity-dependent vs. quantal transmission or that back metabolism of CNO alters GABAAR signaling. Interestingly, no impact of Gq-DREADD on rheobase in saline controls was observed, however this lack of effect aligns with a lack of behavioral change. Further, it likely reflects complexity of cortical microcircuits and functionality of mechanisms normally in place to counter acute shifts in cortical activation.
There are several limitations to this study that should be acknowledged. Multiple approaches, including food-based lever training and a fading procedure were used to initiate acquisition of self-administration in a majority of mice. Although the fading approach was done to control for lever pressing differences between saline and remifentanil exposed mice, expedite acquisition of lever pressing, and reduce the rate of failed acquisition; it also resulted in an atypical acquisition curve whereby active lever responding decreased throughout initial self-administration days. This decreased responding suggests that drug administration is driven by prior contingencies, may reflect extinguishing active lever pressing from the previous Ensure® reward, or may reflect a titration of preferred intake as mice reach a steady maintenance period after ~7 days that is held for up to 30 days. While we do not show within animal dose dependent titration to refute the former, we feel this not likely a factor for a number of reasons. First, while active lever responses decreased, inactive lever presses do not, indicating that this change does not reflect a decrease in lever discrimination. Second, we demonstrate increased motivation for remifentanil in a subset of mice indicating the saline group never extinguished from the Ensure® reward (Supplementary Fig. 1f). Moreover, while the paired training saline group has increased responding compared to those where Ensure® was not paired with the infusion during training (Supplementary Fig. 1b), the training only influenced the first day of active lever pressing in remifentanil mice (Supplementary Fig. 1d) and did not change the number of cumulative infusions earned (Supplementary Fig. 1e). Importantly, we would posit that the ability to replicate neural adaptations across varied parameters strengthens the rigor and perhaps validity of our findings compared to those observed only under a finite set of experimental parameters. Finally, our experiments do not address alterations in gonadal hormones. As recent work has demonstrated a role of estrous stage on cue/drug associations [60], heroin self-administration [61], and unconstrained remifentanil demand [62], a critical step in future studies will be determining what role, if any, these hormones have on the observed deficits in cognitive flexibility and how these deficits confer susceptibility to PrLC dysfunction.
Conclusion
Our capacity to prevent and treat substance use disorders is hindered by variability within diagnosed populations. Biological sex is known to dictate drug-related behavioral and neurobiological outcomes [63], as females are at heightened risk for developing substance use disorders and exposure to illicit substances leads females to transition to addiction more rapidly than men [63, 64]. Women report more lifetime use of prescription opioids [65], experience greater self-reported cue-related craving [66], and are at greater risk of overdose related deaths [67]. Opioid use disorders are prevalent among men and women, however clinical data regarding sex differences specifically in opioid use disorders remains lacking. Past rodent models exhibited similar trends with females showing more rapid acquisition of heroin-taking behavior and greater overall intake [68, 69]. Importantly, several recent studies have shown no evidence for increased acquisition and maintenance of opioid self-administration in female rats [70, 71] and that males exhibit higher fentanyl choice while effectiveness is higher in females [72]. Here, we have identified sex-specific and time-dependent plasticity within a key substrate for cognitive control following opioid exposure. While opioid-induced adaptations in male and female mice observed here cannot definitively be mapped on to alterations in humans, our data highlight the possibility that females may be at greater risk for cortical dysfunction and impaired behavioral control on a shorter timescale. Further, data indicate that interventions aimed at targeting opioid-induced neural adaptations may need to be tailored based on biological sex.
Funding and disclosure
These studies were supported by funding from the National Institute on Drug Abuse grant K99 DA038706 (MCH), and R00DA038706 (MCH), as well as funding from the Brain and Behavior Research Foundation (#26299). The authors have no biomedical financial interests or potential conflicts of interest.
Supplementary information
Author contributions
EMA and MCH designed, discussed, and planned all experiments. EMA, AE, SD, EP, and MCH performed experiments and analyzed the data. EMA and MCH wrote the manuscript.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s41386-021-01028-z.
References
- 1.Gould TJ. Addiction and cognition. Addict Sci Clin Pr. 2010;5:4–14. [PMC free article] [PubMed] [Google Scholar]
- 2.Zeng H, Lee TM, Waters JH, So KF, Sham PC, Schottenfeld RS, et al. Impulsivity, cognitive function, and their relationship in heroin-dependent individuals. J Clin Exp Neuropsychol. 2013;35:897–905. doi: 10.1080/13803395.2013.828022. [DOI] [PubMed] [Google Scholar]
- 3.Lee TM, Zhou WH, Luo XJ, Yuen KS, Ruan XZ, Weng XC. Neural activity associated with cognitive regulation in heroin users: A fMRI study. Neurosci Lett. 2005;382:211–6. doi: 10.1016/j.neulet.2005.03.053. [DOI] [PubMed] [Google Scholar]
- 4.Everitt BJ, Belin D, Economidou D, Pelloux Y, Dalley JW, Robbins TW. Review. Neural mechanisms underlying the vulnerability to develop compulsive drug-seeking habits and addiction. Philos Trans R Soc Lond B Biol Sci. 2008;363:3125–35. doi: 10.1098/rstb.2008.0089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goldstein RZ, Volkow ND. Dysfunction of the prefrontal cortex in addiction: neuroimaging findings and clinical implications. Nat Rev Neurosci. 2011;12:652–69. doi: 10.1038/nrn3119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li CS, Sinha R. Inhibitory control and emotional stress regulation: neuroimaging evidence for frontal-limbic dysfunction in psycho-stimulant addiction. Neurosci Biobehav Rev. 2008;32:581–97. doi: 10.1016/j.neubiorev.2007.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Porrino LJ, Smith HR, Nader MA, Beveridge TJ. The effects of cocaine: a shifting target over the course of addiction. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31:1593–600. doi: 10.1016/j.pnpbp.2007.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bissonette GB, Roesch MR. Neurophysiology of rule switching in the corticostriatal circuit. Neuroscience. 2017;345:64–76. doi: 10.1016/j.neuroscience.2016.01.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rogers JL, Ghee S, See RE. The neural circuitry underlying reinstatement of heroin-seeking behavior in an animal model of relapse. Neuroscience. 2008;151:579–88. doi: 10.1016/j.neuroscience.2007.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rich EL, Shapiro M. Rat prefrontal cortical neurons selectively code strategy switches. J Neurosci. 2009;29:7208–19. doi: 10.1523/JNEUROSCI.6068-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Spellman T, Svei M, Kaminsky J, Manzano-Nieves G, Liston C. Prefrontal deep projection neurons enable cognitive flexibility via persistent feedback monitoring. Cell. 2021;184:2750–66.e17. doi: 10.1016/j.cell.2021.03.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Matsuo K, Glahn DC, Peluso MA, Hatch JP, Monkul ES, Najt P, et al. Prefrontal hyperactivation during working memory task in untreated individuals with major depressive disorder. Mol Psychiatry. 2007;12:158–66. doi: 10.1038/sj.mp.4001894. [DOI] [PubMed] [Google Scholar]
- 13.Kehrer C, Maziashvili N, Dugladze T, Gloveli T. Altered excitatory-inhibitory balance in the NMDA-hypofunction model of schizophrenia. Front Mol Neurosci. 2008;1:6. doi: 10.3389/neuro.02.006.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goldstein RZ, Volkow ND. Drug addiction and its underlying neurobiological basis: neuroimaging evidence for the involvement of the frontal cortex. Am J Psychiatry. 2002;159:1642–52. doi: 10.1176/appi.ajp.159.10.1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Botelho MF, Relvas JS, Abrantes M, Cunha MJ, Marques TR, Rovira E, et al. Brain blood flow SPET imaging in heroin abusers. Ann N. Y Acad Sci. 2006;1074:466–77. doi: 10.1196/annals.1369.047. [DOI] [PubMed] [Google Scholar]
- 16.Langleben DD, Ruparel K, Elman I, Busch-Winokur S, Pratiwadi R, Loughead J, et al. Acute effect of methadone maintenance dose on brain FMRI response to heroin-related cues. Am J Psychiatry. 2008;165:390–4. doi: 10.1176/appi.ajp.2007.07010070. [DOI] [PubMed] [Google Scholar]
- 17.Chen BT, Yau HJ, Hatch C, Kusumoto-Yoshida I, Cho SL, Hopf FW, et al. Rescuing cocaine-induced prefrontal cortex hypoactivity prevents compulsive cocaine seeking. Nature. 2013;496:359–62. doi: 10.1038/nature12024. [DOI] [PubMed] [Google Scholar]
- 18.Couey JJ, Meredith RM, Spijker S, Poorthuis RB, Smit AB, Brussaard AB, et al. Distributed network actions by nicotine increase the threshold for spike-timing-dependent plasticity in prefrontal cortex. Neuron. 2007;54:73–87. doi: 10.1016/j.neuron.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 19.Sun W, Rebec GV. Repeated cocaine self-administration alters processing of cocaine-related information in rat prefrontal cortex. J Neurosci. 2006;26:8004–8. doi: 10.1523/JNEUROSCI.1413-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Peters J, De, Vries TJ. Glutamate mechanisms underlying opiate memories. Cold Spring Harb Perspect Med. 2012;2:a012088. doi: 10.1101/cshperspect.a012088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Peters J, Pattij T, De Vries TJ. Targeting cocaine versus heroin memories: divergent roles within ventromedial prefrontal cortex. Trends Pharm Sci. 2013;34:689–95. doi: 10.1016/j.tips.2013.10.004. [DOI] [PubMed] [Google Scholar]
- 22.Shen H, Moussawi K, Zhou W, Toda S, Kalivas PW. Heroin relapse requires long-term potentiation-like plasticity mediated by NMDA2b-containing receptors. Proc Natl Acad Sci USA. 2011;108:19407–12. doi: 10.1073/pnas.1112052108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Childress AR, Mozley PD, McElgin W, Fitzgerald J, Reivich M, O’Brien CP. Limbic activation during cue-induced cocaine craving. Am J Psychiatry. 1999;156:11–8. doi: 10.1176/ajp.156.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Qin L, Ma K, Yan Z. Chemogenetic activation of prefrontal cortex in Shank3-deficient mice ameliorates social deficits, NMDAR hypofunction, and Sgk2 downregulation. iScience. 2019;17:24–35. doi: 10.1016/j.isci.2019.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Anderson EM, Gomez D, Caccamise A, McPhail D, Hearing M. Chronic unpredictable stress promotes cell-specific plasticity in prefrontal cortex D1 and D2 pyramidal neurons. Neurobiol Stress. 2019;10:100152. doi: 10.1016/j.ynstr.2019.100152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hearing M, Kotecki L, Marron Fernandez de Velasco E, Fajardo-Serrano A, Chung HJ, Lujan R, et al. Repeated cocaine weakens GABA(B)-Girk signaling in layer 5/6 pyramidal neurons in the prelimbic cortex. Neuron. 2013;80:159–70. doi: 10.1016/j.neuron.2013.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yizhar O, Fenno LE, Prigge M, Schneider F, Davidson TJ, O’Shea DJ, et al. Neocortical excitation/inhibition balance in information processing and social dysfunction. Nature. 2011;477:171–8. doi: 10.1038/nature10360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Isaacson JS, Scanziani M. How inhibition shapes cortical activity. Neuron. 2011;72:231–43. doi: 10.1016/j.neuron.2011.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sohal VS, Zhang F, Yizhar O, Deisseroth K. Parvalbumin neurons and gamma rhythms enhance cortical circuit performance. Nature. 2009;459:698–702. doi: 10.1038/nature07991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim H, Ahrlund-Richter S, Wang X, Deisseroth K, Carlen M. Prefrontal Parvalbumin neurons in control of attention. Cell. 2016;164:208–18.. doi: 10.1016/j.cell.2015.11.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dalley JW, Cardinal RN, Robbins TW. Prefrontal executive and cognitive functions in rodents: neural and neurochemical substrates. Neurosci Biobehav Rev. 2004;28:771–84. doi: 10.1016/j.neubiorev.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 32.Birrell JM, Brown VJ. Medial frontal cortex mediates perceptual attentional set shifting in the rat. J Neurosci. 2000;20:4320–4. doi: 10.1523/JNEUROSCI.20-11-04320.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Roth BL. DREADDs for Neuroscientists. Neuron. 2016;89:683–94. doi: 10.1016/j.neuron.2016.01.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lin G, McKay G, Midha KK. Characterization of metabolites of clozapine N-oxide in the rat by micro-column high performance liquid chromatography/mass spectrometry with electrospray interface. J Pharm Biomed Anal. 1996;14:1561–77. doi: 10.1016/0731-7085(96)01738-4. [DOI] [PubMed] [Google Scholar]
- 35.Ilg AK, Enkel T, Bartsch D, Bahner F. Behavioral effects of acute systemic low-dose clozapine in wild-type rats: implications for the use of DREADDs in behavioral neuroscience. Front Behav Neurosci. 2018;12:173. doi: 10.3389/fnbeh.2018.00173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.James AS, Chen JY, Cepeda C, Mittal N, Jentsch JD, Levine MS, et al. Opioid self-administration results in cell-type specific adaptations of striatal medium spiny neurons. Behav Brain Res. 2013;256:279–83. doi: 10.1016/j.bbr.2013.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Panlilio LV, Schindler CW. Self-administration of remifentanil, an ultra-short acting opioid, under continuous and progressive-ratio schedules of reinforcement in rats. Psychopharmacology. 2000;150:61–6. doi: 10.1007/s002130000415. [DOI] [PubMed] [Google Scholar]
- 38.Panlilio LV, Secci ME, Schindler CW, Bradberry CW. Choice between delayed food and immediate opioids in rats: treatment effects and individual differences. Psychopharmacology. 2017;234:3361–73.. doi: 10.1007/s00213-017-4726-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Blair G, Wells C, Ko A, Modarres J, Pace C, Davis JM, et al. Dextromethorphan and bupropion reduces high level remifentanil self-administration in rats. Pharm Biochem Behav. 2020;193:172919. doi: 10.1016/j.pbb.2020.172919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Levin ED, Wells C, Hawkey A, Holloway Z, Blair G, Vierling A, et al. Amitifadine, a triple reuptake inhibitor, reduces self-administration of the opiate remifentanil in rats. Psychopharmacology. 2020;237:1681–89. doi: 10.1007/s00213-020-05489-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Daglish MR, Weinstein A, Malizia AL, Wilson S, Melichar JK, Britten S, et al. Changes in regional cerebral blood flow elicited by craving memories in abstinent opiate-dependent subjects. Am J Psychiatry. 2001;158:1680–6. doi: 10.1176/appi.ajp.158.10.1680. [DOI] [PubMed] [Google Scholar]
- 42.Suh JJ, Langleben DD, Ehrman RN, Hakun JG, Wang Z, Li Y, et al. Low prefrontal perfusion linked to depression symptoms in methadone-maintained opiate-dependent patients. Drug Alcohol Depend. 2009;99:11–7. doi: 10.1016/j.drugalcdep.2008.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Otis JM, Namboodiri VM, Matan AM, Voets ES, Mohorn EP, Kosyk O, et al. Prefrontal cortex output circuits guide reward seeking through divergent cue encoding. Nature. 2017;543:103–07. doi: 10.1038/nature21376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.West EA, Niedringhaus M, Ortega HK, Haake RM, Frohlich F, Carelli RM. Noninvasive brain stimulation rescues cocaine-induced prefrontal hypoactivity and restores flexible behavior. Biol Psychiatry. 2021;89:1001–11. [DOI] [PMC free article] [PubMed]
- 45.Donnelly NA, Holtzman T, Rich PD, Nevado-Holgado AJ, Fernando AB, Van Dijck G, et al. Oscillatory activity in the medial prefrontal cortex and nucleus accumbens correlates with impulsivity and reward outcome. PloS One. 2014;9:e111300. doi: 10.1371/journal.pone.0111300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Feil J, Sheppard D, Fitzgerald PB, Yucel M, Lubman DI, Bradshaw JL. Addiction, compulsive drug seeking, and the role of frontostriatal mechanisms in regulating inhibitory control. Neurosci Biobehav Rev. 2010;35:248–75. doi: 10.1016/j.neubiorev.2010.03.001. [DOI] [PubMed] [Google Scholar]
- 47.Smith RJ, Laiks LS. Behavioral and neural mechanisms underlying habitual and compulsive drug seeking. Prog Neuropsychopharmacol Biol Psychiatry. 2018;87:11–21. doi: 10.1016/j.pnpbp.2017.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jentsch JD, Taylor JR. Impulsivity resulting from frontostriatal dysfunction in drug abuse: implications for the control of behavior by reward-related stimuli. Psychopharmacology. 1999;146:373–90. doi: 10.1007/PL00005483. [DOI] [PubMed] [Google Scholar]
- 49.Everitt BJ, Robbins TW. Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nat Neurosci. 2005;8:1481–9. doi: 10.1038/nn1579. [DOI] [PubMed] [Google Scholar]
- 50.Reiner DJ, Fredriksson I, Lofaro OM, Bossert JM, Shaham Y. Relapse to opioid seeking in rat models: behavior, pharmacology and circuits. Neuropsychopharmacology. 2019;44:465–77. doi: 10.1038/s41386-018-0234-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bossert JM, Stern AL, Theberge FR, Cifani C, Koya E, Hope BT, et al. Ventral medial prefrontal cortex neuronal ensembles mediate context-induced relapse to heroin. Nat Neurosci. 2011;14:420–2. doi: 10.1038/nn.2758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bossert JM, Stern AL, Theberge FR, Marchant NJ, Wang HL, Morales M, et al. Role of projections from ventral medial prefrontal cortex to nucleus accumbens shell in context-induced reinstatement of heroin seeking. J Neurosci. 2012;32:4982–91. doi: 10.1523/JNEUROSCI.0005-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Peters J, LaLumiere RT, Kalivas PW. Infralimbic prefrontal cortex is responsible for inhibiting cocaine seeking in extinguished rats. J Neurosci. 2008;28:6046–53. doi: 10.1523/JNEUROSCI.1045-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schmidt ED, Voorn P, Binnekade R, Schoffelmeer AN, De Vries TJ. Differential involvement of the prelimbic cortex and striatum in conditioned heroin and sucrose seeking following long-term extinction. Eur J Neurosci. 2005;22:2347–56. doi: 10.1111/j.1460-9568.2005.04435.x. [DOI] [PubMed] [Google Scholar]
- 55.Bossert JM, Poles GC, Wihbey KA, Koya E, Shaham Y. Differential effects of blockade of dopamine D1-family receptors in nucleus accumbens core or shell on reinstatement of heroin seeking induced by contextual and discrete cues. J Neurosci. 2007;27:12655–63. doi: 10.1523/JNEUROSCI.3926-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bossert JM, Wihbey KA, Pickens CL, Nair SG, Shaham Y. Role of dopamine D(1)-family receptors in dorsolateral striatum in context-induced reinstatement of heroin seeking in rats. Psychopharmacology. 2009;206:51–60. doi: 10.1007/s00213-009-1580-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hearing MC, Jedynak J, Ebner SR, Ingebretson A, Asp AJ, Fischer RA, et al. Reversal of morphine-induced cell-type-specific synaptic plasticity in the nucleus accumbens shell blocks reinstatement. Proc Natl Acad Sci USA. 2016;113:757–62. doi: 10.1073/pnas.1519248113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Van den Oever MC, Spijker S, Smit AB, De Vries TJ. Prefrontal cortex plasticity mechanisms in drug seeking and relapse. Neurosci Biobehav Rev. 2010;35:276–84. doi: 10.1016/j.neubiorev.2009.11.016. [DOI] [PubMed] [Google Scholar]
- 59.Van den Oever MC, Lubbers BR, Goriounova NA, Li KW, Van der Schors RC, Loos M, et al. Extracellular matrix plasticity and GABAergic inhibition of prefrontal cortex pyramidal cells facilitates relapse to heroin seeking. Neuropsychopharmacology. 2010;35:2120–33. doi: 10.1038/npp.2010.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Johnson AR, Thibeault KC, Lopez AJ, Peck EG, Sands LP, Sanders CM, et al. Cues play a critical role in estrous cycle-dependent enhancement of cocaine reinforcement. Neuropsychopharmacology. 2019;44:1189–97. [DOI] [PMC free article] [PubMed]
- 61.Lacy RT, Strickland JC, Feinstein MA, Robinson AM, Smith MA. The effects of sex, estrous cycle, and social contact on cocaine and heroin self-administration in rats. Psychopharmacol (Berl) 2016;233:3201–10. doi: 10.1007/s00213-016-4368-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lacy RT, Austin BP, Strickland JC. The influence of sex and estrous cyclicity on cocaine and remifentanil demand in rats. Addict Biol. 2019;25:12717–26. [DOI] [PMC free article] [PubMed]
- 63.Becker JB, Perry AN, Westenbroek C. Sex differences in the neural mechanisms mediating addiction: a new synthesis and hypothesis. Biol Sex Differ. 2012;3:14. doi: 10.1186/2042-6410-3-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Becker JB, Hu M. Sex differences in drug abuse. Front Neuroendocrinol. 2008;29:36–47. doi: 10.1016/j.yfrne.2007.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Serdarevic M, Striley CW, Cottler LB. Sex differences in prescription opioid use. Curr Opin psychiatry. 2017;30:238–46. doi: 10.1097/YCO.0000000000000337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kennedy AP, Epstein DH, Phillips KA, Preston KL. Sex differences in cocaine/heroin users: drug-use triggers and craving in daily life. Drug Alcohol Depend. 2013;132:29–37. doi: 10.1016/j.drugalcdep.2012.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Unick GJ, Rosenblum D, Mars S, Ciccarone D. Intertwined epidemics: national demographic trends in hospitalizations for heroin- and opioid-related overdoses, 1993-2009. PloS One. 2013;8:e54496. doi: 10.1371/journal.pone.0054496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Cicero TJ, Aylward SC, Meyer ER. Gender differences in the intravenous self-administration of mu opiate agonists. Pharm Biochem Behav. 2003;74:541–9. doi: 10.1016/S0091-3057(02)01039-0. [DOI] [PubMed] [Google Scholar]
- 69.Lynch WJ, Carroll ME. Sex differences in the acquisition of intravenously self-administered cocaine and heroin in rats. Psychopharmacology. 1999;144:77–82. doi: 10.1007/s002130050979. [DOI] [PubMed] [Google Scholar]
- 70.Reiner DJ, Lofaro OM, Applebey SV, Korah H, Venniro M, Cifani C, et al. Role of projections between piriform cortex and orbitofrontal cortex in relapse to fentanyl seeking after palatable food choice-induced voluntary abstinence. J Neurosci. 2020;40:2485–97. doi: 10.1523/JNEUROSCI.2693-19.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bossert JM, Kiyatkin EA, Korah H, Hoots JK, Afzal A, Perekopskiy D, et al. In a rat model of opioid maintenance, the G protein-biased Mu opioid receptor agonist TRV130 decreases relapse to oxycodone seeking and taking and prevents oxycodone-induced brain hypoxia. Biol Psychiatry. 2020;88:935–44. doi: 10.1016/j.biopsych.2020.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Townsend EA, Negus SS, Caine SB, Thomsen M, Banks ML. Sex differences in opioid reinforcement under a fentanyl vs. food choice procedure in rats. Neuropsychopharmacology. 2019;44:2022–29. doi: 10.1038/s41386-019-0356-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.