Abstract
Objective/Background
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection has been associated with thrombotic complications such as deep vein thrombosis or stroke. Recently, numerous cases of acute limb ischemia (ALI) have been reported although pooled data are lacking.
Methods
We systematically searched PubMed, Embase, Scopus, and the Cochrane Library for studies published online up to January 2021 that reported cases with SARS-CoV-2 infection and ALI. Eligible studies should have reported early outcomes including mortality. Primary endpoints included also pooled amputation, clinical improvement, and reoperation rates.
Results
In total, 34 studies (19 case reports and 15 case series/cohort studies) including a total of 540 patients (199 patients were eligible for analysis) were evaluated. All studies were published in 2020. Mean age of patients was 61.6 years (range, 39-84 years; data from 32 studies) and 78.4% of patients were of male gender (data from 32 studies). There was a low incidence of comorbidities: arterial hypertension, 49% (29 studies); diabetes mellitus, 29.6% (29 studies); dyslipidemia, 20.5% (27 studies); chronic obstructive pulmonary disease, 8.5% (26 studies); coronary disease, 8.3% (26 studies); and chronic renal disease, 7.6% (28 studies). Medical treatment was selected as first-line treatment for 41.8% of cases. Pooled mortality rate among 34 studies reached 31.4% (95% confidence interval [CI], 25.4%%–37.7%). Pooled amputation rate among 34 studies reached 23.2% (95% CI, 17.3%–29.7%). Pooled clinical improvement rate among 28 studies reached 66.6% (95% CI, 55.4%%–76.9%). Pooled reoperation rate among 29 studies reached 10.5% (95% CI, 5.7%%–16.7%). Medical treatment was associated with a higher death risk compared with any intervention (odds ratio, 4.04; 95% CI, 1.075–15.197; P = .045) although amputation risk was not different between the two strategies (odds ratio, 0.977; 95% CI, 0.070–13.600; P = .986) (data from 31 studies).
Conclusions
SARS-CoV-2 infection is associated with a high risk for thrombotic complications, including ALI. COVID-associated ALI presents in patients with a low incidence of comorbidities, and it is associated with a high mortality and amputation risk. Conservative treatment seems to have a higher mortality risk compared with any intervention, although amputation risk is similar.
Keywords: Acute limb ischemia, COVID-19, SARS-CoV-2
The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the virus that causes coronavirus disease 2019 (COVID-19), originated in December 2019 and has caused a worldwide pandemic.1 Infection with SARS-CoV-2 has been shown to have a wide range of clinical presentations from asymptomatic in a large percentage of patients to devastating pulmonary failure, sepsis, and death.2 , 3 Additionally, the hypercoagulability associated with this infection has been recognized as a significant cause of morbidity, resulting in thrombotic complications such as pulmonary parenchymal thrombosis, venous thrombosis, myocardial infarction, and stroke.4 Lately, there have been reports of acute limb ischemia (ALI) observed among infected patients as well.
Although pooled data have been published on other thrombotic presentations such as acute mesenteric ischemia or stroke,5 , 6 pooled data on ALI are lacking. Therefore, aim of this review was to evaluate pooled data on patients with COVID-19 infection and ALI.
Methods
Data sources and search
We systematically searched PubMed, Embase, Scopus, and the Cochrane Library (up to January 2021) for clinical studies published online that included patients suffered from ALI while diagnosed with COVID-19 infection. Eligible studies should have reported at least early (30-day) mortality among other outcomes. This review was conducted according to established methods for systematic reviews in cardiovascular medicine (PRISMA criteria).7 The following medical subject terms were used for the online search: “acute,” “limb,” “ischemia,” “COVID-19,” “SARS-CoV-2,” and “infection.” In addition to searching databases, the reference lists of all included studies, meta-analyses, and reviews were evaluated manually, including unpublished data. Only studies published in English were included in this review. References from eligible articles or textbooks were also reviewed to identify further potential sources.
Data extraction: Outcomes and definitions
Three authors independently completed data extraction after following search criteria and quality assessment. Disagreements were resolved by consensus or after review by the senior author of the study, when necessary. Data were obtained from tables, graphs, and text as well. When data were presented in percentages, the absolute values were calculated. For each study, the following data were collected: first author, year of publication, country of origin, total number of patients included in the studies, total number of patients with ALI and COVID-19 infection, patient characteristics (mean age, gender, and comorbidities when reported), localization of ischemia (type of limbs, type of arteries), type of symptoms owing to infection (eg, fever, dyspnea, intubation need), type of medical treatment, type of interventional treatment, early outcomes (eg, mortality, amputation, and cardiac events), improvement of ischemia, late follow-up, and late outcomes when reported.
ALI was defined as acute ischemia presented in the lower or upper extremities. The cause of ischemia should have been arterial thrombosis or embolism and this cause should have been confirmed with some type of imaging within the included studies. Any cases with superficial or cutaneous limb necrosis in patients with COVID-19 infection who had no imaging evidence showing thrombosis or embolism of limb arteries were not included in this review. If the studies under evaluation included patients with acute ischemia of other type (such as mesenteric or cerebral), only patients with ALI were included in the analysis. If a patient happened to present with ALI and another type of ischemic complication, he or she was still included in the analysis.
Primary endpoints included early (30-day) mortality, amputation, clinical improvement, and reoperation rates.
Affected limb arteries included the subclavian, axillary, brachial, ulnar, radial, palmar, and digital arteries for the upper limb and the aorta, iliac, femoral, popliteal, tibial, peroneal, and plantar arteries for the lower limb.
All eligible patients should have tested positive for COVID-19 infection whether they presented with typical symptoms or not.
Amputations reported in this review included both major and minor amputations. Major amputations included transfemoral and transbrachial amputations as well as amputations below the level of the knee or elbow. Minor amputations included amputations below the level of the ankle or wrist. Pooled amputation rate included primary as well as secondary amputations.
Cardiac adverse events included cardiac arrest, myocardial infarction, arrhythmias, or acute cardiac failure.
Improvement of limb ischemia included the improvement of clinical symptoms/signs of ischemia without the need for further intervention or amputation.
Comorbidities are reported in the same way as reported in the included studies owing to lack of specific definitions in the majority of studies.
Quality assessment
Three authors independently reviewed study eligibility and quality. Disagreements were resolved by consensus or after review by the senior author of the study, when necessary. The quality of each study was assessed using well-established criteria for nonrandomized studies, specifically evaluating the collection of data, the aim of the studies, incomplete outcome data, statistical analysis, and other sources of bias.8 The quality of each study was evaluated and reported as high, medium, or low based on the design and methodology of study according to these criteria.
Inclusion and exclusion criteria
Studies included in this meta-analysis met the following criteria: (i) clinical studies or reports presenting cases with COVID-19 infection and ALI and (ii) studies should have reported early (30-day) mortality at least. ALI cases should have been documented with some type of imaging in the included studies. Studies reporting patients with acute ischemia at different body locations were also included but only patients with ALI from these studies were eligible for analysis.
Exclusion criteria included (i) types of publication other than clinical studies or reports, such as reviews, meta-analyses or editorials; (ii) studies not reporting at least early mortality among outcomes; (iii) studies presenting patients with superficial or cutaneous necrosis without evidence of an arterial thrombosis or embolism; (iv) studies reporting cases of ALI among patients without COVID-19 infection only; (v) studies reporting cases with COVID-19 infection and acute ischemia of other body parts such as mesenteric or cerebral ischemia; (vi) studies reporting cases with COVID-19 infection and acute thrombotic events without reporting outcomes for limb ischemia separately; (vii) studies published in a language other than English; (viii) studies not referring to humans; and (ix) studies reported as only abstracts or presented at conferences.
Statistical analysis
A meta-analysis was carried out using the StatsDirect Statistical software (Version 2.8.0, StatsDirect Ltd, Cambridge, UK). Odds ratios (OR) were used to determine effect size, along with 95% confidence intervals (CI). Regarding major outcomes, ORs were pooled with Der Simonian and Laird random effects models being used for sensitivity analysis. P values were calculated for evaluating statistical significance, with a P value of less than .05 indicating a statistically significant difference. Interstudy variations and heterogeneities were estimated using the Q-statistic with a P value of less than .05 also indicating a statistically significant heterogeneity. The present meta-analysis also quantified the effect of heterogeneity by using the I2 index (range, 0%-100%), which represents the proportion of inter-study variability attributed to heterogeneity, rather than to chance.
In circumstances where more than one study reported data from the same cohort (introducing the potential for duplicate inclusion of patients), only the largest cohort was included in the main analysis. A χ 2 test with Yate's correction was used for comparing categorical variables between the two groups of patients. All statistical analyses were conducted using the absolute values and not percentages. The risk of bias was also assessed applying the Habbord-Egger test.
Results
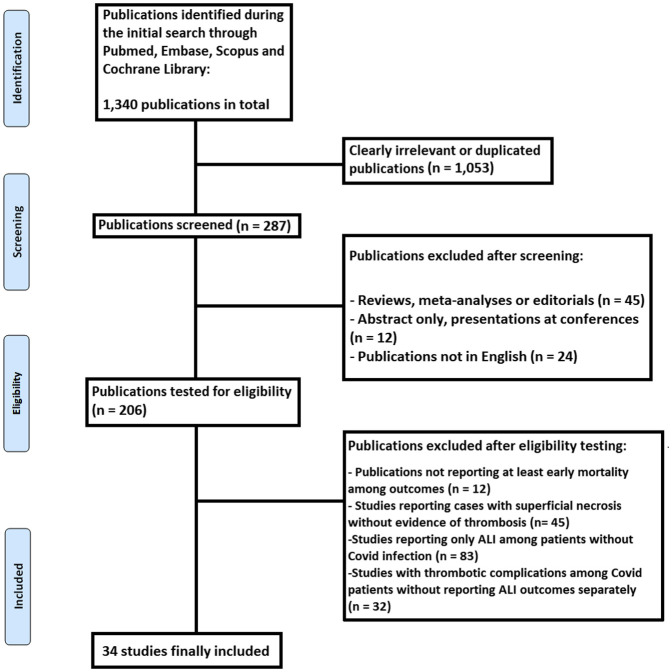
In total, 34 eligible studies (19 case reports and 15 case series/cohort studies) were included9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42 (Fig 1 ). Regarding quality, 5 studies were of high quality, 5 studies of medium quality, and 24 studies (mainly case reports) of lower quality. These studies evaluated a total of 540 patients, out of which 199 were eligible for this analysis. Table I presents basic characteristics of the included studies. The mean age of the patients was 61.6 years (range, 39-84 years; data from 32 studies) and 78.4% of patients were male (data from 32 studies), although data on age or gender were not provided by the two largest studies in size. Among 138 cases, the following limbs were affected: lower limb (n = 102), upper limb (n = 27), and bilateral lower limbs (n = 9). The exact affected arteries are presented in Table I as well.
Fig 1.
PRISMA flowchart of this review. ALI, Acute limb ischemia.
Table I.
Basic characteristics of the included studies
| Study | Year of publication | Country of origin | No. of patients with COVID infection and ALI (total number of patients in the study) | Male/female gender | Mean age, years [SD or range are reported when provided by the studies] | Limb affected | Arteries affected |
|---|---|---|---|---|---|---|---|
| Veerasuri et al9 | 2020 | UK | 1 | 1/0 | 56 | Bilateral lower limbs | Right SFA, right trifurcation, left trifurcation |
| Kaur et al10 | 2020 | USA | 1 | 1/0 | 43 | Right lower limb | Right SFA, POPA, trifurcation |
| Brugliera et al11 | 2020 | Italy | 3 | 3/0 | 68 | Bilateral lower limbs (n = 1) Right lower limb (n = 2) |
SFA, POPA (n = 1) NR for 2 patients |
| Hanif et al12 | 2020 | Pakistan | 1 | 0/1 | 75 | Left upper limb | Left UA and RA |
| Hasan et al13 | 2020 | Pakistan | 1 | 1/0 | 60 | Right lower limb | Right POPA and trifurcation |
| Gubitosa et al14 | 2020 | USA | 1 | 1/0 | 65 | Right lower limb | Right POPA |
| Anwar et al15 | 2020 | USA | 1 | 1/0 | 58 | Left lower limb | Left SFA, trifurcation |
| Singh et al16 | 2020 | USA | 1 | 1/0 | 77 | Left lower limb | Left SFA, trifurcation |
| Wang et al17 | 2020 | USA | 2 | 2/0 | 54 | Right lower limb Right upper limb |
Right dorsalis pedis, toes Right digital arteries, digits |
| Goldman et al18 | 2020 | USA | 16 (48) | 9/7 | 70 ± 14 | Lower limbs (n = 16) | From the POPA and proximally (n = 15) Distally from the POPA (n = 1) |
| Sánchez et al19 | 2020 | Peru | 30 | 23/7 | 60 ± 15 | Lower limb (n = 22) Upper limb (n = 8) |
NR |
| Galanis et al20 | 2020 | Greece | 1 | 1/0 | 80 | Right Upper limb | RA and UA |
| Bozzani et al21 | 2020 | Italy | 6 (38) | 4/2 | 71 (49-83) | Lower limb (n = 6) | Iliac–SFA–POPA (n = 6) |
| Mietto et al22 | 2020 | Italy | 1 | 1/0 | 53 | Left lower limb | Iliac–SFA–POPA–tibial arteries |
| Baccellieri et al23 | 2020 | Italy | 1 | 1/0 | 67 | Right lower limb | Iliac-SFA-POPA |
| Shao et al24 | 2020 | USA | 1 | 1/0 | 67 | Right upper limb | Brachial artery, UA, RA |
| Etkin et al25 | 2020 | USA | 42 (49) | NR | NR | Lower extremities (n = 35) Upper limbs (n = 7) |
Aortoiliac (n = 8) Femoral (n = 12) POPA (n = 15) Above elbow (n = 4) UA and RA (n = 3) |
| Muhammad et al26 | 2020 | UK | 1 | 1/0 | 49 | Left lower limb | Aorti-iliac–POPA–trifurcation |
| Heald et al27 | 2020 | USA | 1 | 1/0 | 65 | Left hand-digital ischemia | Digital arteries (left first and second digits) |
| Bellosta et al28 | 2020 | Italy | 20 | 18/2 | 75 ± 8 | Upper and lower limbs (number NR) | NR |
| Kaur et al29 | 2020 | USA | 1 | 1/0 | 71 | Upper limb (right) | Right brachial artery and RA |
| Kahlberg et al30 | 2020 | Italy | 41 (305) | NR | NR | Upper and lower limbs | NR |
| Schultz et al31 | 2020 | USA | 2 | 1/1 | 57 | 3 fingers (right) 2 fingers (right) |
Digital arteries (right) RA (right) |
| Vacirca et al32 | 2020 | Italy | 1 | 1/0 | 58 | Right lower limb | ATA, PTA, PA (right) |
| Fan et al33 | 2020 | Singapore | 1 | 1/0 | 39 | Right lower limb | ATA Abdominal aorta |
| Perini et al34 | 2020 | Italy | 2 | 2/0 | 45 | Bilateral lower limbs Upper limb (left) |
Aortoiliac Brachial bifurcation |
| Kashi et al35 | 2020 | France | 5 (7) | 4/1 | 69 | Lower limbs (2 bilateral, 2 right, 1 left) | Femoral (n = 3) POPA (n = 1) Iliac (n = 1) Fem-pop bypass (n = 1) NR (n = 1) |
| Baeza et al36 | 2020 | Spain | 3 | 1/2 | 72 | Bilateral lower limbs (n = 3) | Aortic-bilateral iliac (n = 3) |
| Garg et al37 | 2020 | USA | 3 (4) | 2/1 | 63 | Lower limb (2 right, 1 left) | POPA (n = 3) |
| Thompson et al38 | 2020 | USA | 1 | 0/1 | 42 | Right upper limb | Right subclavian + UA |
| Levolger et al39 | 2020 | Netherlands | 2 (4) | 2/0 | 53 | Right lower limb Left upper limb |
Right common iliac artery Left subclavian artery |
| Wengerter et al40 | 2020 | USA | 3 (4) | 3/0 | 53 | Left lower limb (n = 2) Bilateral lower limbs (n = 1) |
Aortoiliac (n = 1) Femoral–POPA (n = 3) |
| Chowdhury et al41 | 2020 | USA | 1 | 1/0 | 75 | Right upper extremity | Brachial artery |
| Liu et al42 | 2021 | China | 1 | 1/0 | 70 | Right lower limb | CFA and SFA |
ATA, Anterior tibial artery; CFA, common femoral artery; NR, not reported; POPA, popliteal artery; RA, radial artery; PA, peroneal artery; PTA, posterior tibial artery; SD, standard deviation; SFA, superficial femoral artery; UA, ulnar artery.
Pooled demographics were the following: arterial hypertension, 49% (29 studies); diabetes mellitus, 29.6% (29 studies); dyslipidemia, 20.5% (27 studies); chronic obstructive pulmonary disease, 8.5% (26 studies); coronary disease, 8.3% (26 studies); arrhythmias, 14.1% (28 studies); chronic heart failure, 8.0% (25 studies); and chronic renal disease, 7.6% (28 studies). All comorbidities are presented in Table II .
Table II.
Demographics of the included patients
| Study | Arterial hypertension | Diabetes mellitus | Dyslipidemia | COPD | CAD | Arrhythmia | CHF | Renal Disease | Other comorbidities |
|---|---|---|---|---|---|---|---|---|---|
| Veerasuri et al9 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | None |
| Kaur et al10 | 1/1 | 1/1 | 0 | 0 | 0 | 0 | 0 | 0 | None |
| Brugliera et al11 | 3/3 | 2/3 | 1/3 | 1/3 | 0 | 0 | 0 | 1/3 | Hypothyroidism (n = 1) |
| Hanif et al12 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | None |
| Hasan et al13 | 1/1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | None |
| Gubitosa et al14 | 1/1 | 1/1 | 1/1 | 0 | 0 | 0 | 0 | 1/1 | Smoking history |
| Anwar et al15 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | None |
| Singh et al16 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | None |
| Wang et al17 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | None |
| Goldman et al18 | 13/16 | 8/16 | 8/16 | NR | NR | NR | 4/16 | 0/16 | Smoking history (n = 8) PAD (n = 8) |
| Sánchez et al19 | 10/30 | 8/30 | 1/30 | NR | 4/30 | 3/30 | NR | 1/30 | Smoking history (n = 3) PAD (n = 4) |
| Galanis et al20 | 1/1 | 1/1 | 0 | 0 | 0 | 0 | 0 | 0 | Dementia |
| Bozzani et al21 | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| Mietto et al22 | 1/1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | Obesity |
| Baccellieri et al23 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | Obesity |
| Shao et al24 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | Lupus anticoagulant positive |
| Etkin et al25 | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| Muhammad et al26 | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| Heald et al27 | 1/1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | History of smoking |
| Bellosta et al28 | 11/20 | 3/20 | NR | 2/20 | 2/20 | 5/20 | NR | 4/20 | Previous VS (n = 4) Obesity (n = 4) |
| Kaur et al29 | 0 | 1/1 | 0 | 0 | 0 | 0 | 0 | 0 | None |
| Kahlberg et al30 | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| Schultz et al31 | 1/2 | 0 | 1/2 | 0 | 0 | 0 | 0 | 0 | Obesity (n = 1) |
| Vacirca et al32 | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| Fan et al33 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | None |
| Perini et al34 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | None |
| Kashi et al35 | 4/5 | 1/5 | NR | 1/5 | NR | 2/5 | NR | 1/5 | Smoking (n = 2) PAD (n = 2) |
| Baeza et al36 | 2/3 | 1/3 | 2/3 | 1/3 | 0 | 2/3 | 0 | 0 | Smoking (n = 2) Obesity (n = 1) PAD (n = 1) |
| Garg et al37 | 1/3 | 1/3 | 1/3 | NR | NR | 1/3 | NR | NR | NR |
| Thompson et al38 | 0 | 1/1 | 0 | 0 | 0 | 0 | 0 | 0 | Rheumatoid arthritis |
| Levolger et al39 | 0 | 1/2 | 0 | 0 | 0 | 0 | 0 | 0 | - |
| Wengerter et al40 | 1/3 | 2/3 | 1/3 | 0 | 0 | 0 | 0 | 0 | Smoking (n = 1) |
| Chowdhury et al41 | 1/1 | 0 | 1/1 | 0 | 1/1 | 0 | 0 | 0 | Dementia |
| Liu et al42 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | Lung cancer |
CAD, Coronary artery disease; CHF, chronic heart failure; COPD, chronic obstructive pulmonary disease; NR, not reported; PAD, peripheral artery disease; VS, vascular surgery.
Concerning COVID-19 infection, 49.1% of patients presented with fever (27 studies), 62.3% of patients presented with dyspnea (27 studies), and 36.4% of patients needed to be intubated (30 studies). Basic laboratory findings are also presented in Table III . Increased d-Dimers levels (>5 μg/mL; 26 studies) were not associated with death (OR, 1.169; 95% CI, 0.360-3.756; P = .792) or amputation (OR, 2.0; 95% CI, 0.334-11.969; P = .448) risk. Increased C-reactive protein (CRP) levels (>20 mg/L; 16 studies) were not associated with death (OR, 3.261; 95% CI, 0.164-65.012; P = .438) or amputation (OR, 3.627; 95% CI, 0.183-72.070; P = .398) risk. Increased fibrinogen levels (>400 mg/dL; 13 studies) were not associated with death (OR, 0.667; 95% CI, 0.152-2.925; P = .485) or amputation (OR, 0.639; 95% CI, 0.184-2.222; P = .481) risk.
Table III.
Symptoms and laboratory findings of the included patients
| Study | Fever | Dyspnea | Need for intubation | Mean leucocyte count (× 109/L) | Mean platelet count (× 109/L) | Fibrinogen (mg/dL) | CRP (mg/L) | d-Dimers (μg/mL) | PCT (ng/mL) |
|---|---|---|---|---|---|---|---|---|---|
| Veerasuri et al9 | 1/1 | 1/1 | 0 | 0.8 | NR | NR | NR | 23.138 | NR |
| Kaur et al10 | 1/1 | 1/1 | 1/1 | 16 | 484 | 853 | 289.7 | 20 | 67 |
| Brugliera et al11 | 1/3 | 3/3 | 1/3 | NR | Normal for all | 466.3 | NR | 20, NR, 5.04 | NR |
| Hanif et al12 | 1/1 | 0 | 0 | 5.9 | 733 | NR | NR | 0.867 | NR |
| Hasan et al13 | 1/1 | 1/1 | 0 | 13.9 | 95 | NR | 82.5 | 1.96 | 0.3 |
| Gubitosa et al14 | 1/1 | 1/1 | 1/1 | Lymphopenia | 82 | 247 | NR | 7.955 | NR |
| Anwar et al15 | 1/1 | 1/1 | 0 | NR | NR | 312 | 25.23 | 15.653 | 25.23 |
| Singh et al16 | 0 | 1/1 | 0 | 41 | 534 | NR | 301 | 2.77 | 0.6 |
| Wang et al17 | 2/2 | 2/2 | 2/2 | NR | NR | NR | NR | 8.25 | NR |
| Goldman et al18 | 3/16 | 8/16 | 4/16 | 13.5 ± 4 | NR | NR | NR | NR | NR |
| Sánchez et al19 | NR | NR | NR | 11.6 [9.7-16.1] | 284 [220-371] | 4.8 [4.7-6.3] | 35.5 [24-61] | 3.2 [1.6-4.3] | NR |
| Galanis et al20 | 1/1 | 1/1 | 1/1 | 5.6 | 174 | 360 | 166 | 13.6 | 0.1 |
| Bozzani et al21 | NR | NR | 2/6 | NR | NR | NR | 78.17 [3-240] | NR | NR |
| Mietto et al22 | 0 | 0 | 0 | NR | NR | NR | NR | NR | NR |
| Baccellieri et al23 | 1/1 | 1/1 | 0 | NR | NR | 711 | 114.1 | 20 | NR |
| Shao et al24 | 0 | 1/1 | 1/1 | Increased | NR | NR | NR | >5 | NR |
| Etkin et al25 | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| Muhammad et al26 | 1/1 | 1/1 | 0 | 11.8 | 520 | NR | 12 | NR | NR |
| Heald et al27 | 0 | 1/1 | 1/1 | NR | NR | NR | NR | 0.79 | NR |
| Bellosta et al28 | NR | NR | NR | 14 ± 2 | 239 ± 82 | NR | NR | 2.2 | NR |
| Kaur et al29 | 1/1 | 1/1 | 8.6 | 331 | - | 111.9 | 1.85 | - | |
| Kahlberg et al30 | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| Schultz et al31 | 1/2 | 2/2 | 2/2 | NR | NR | 486, NR | 25, NR | 7.56 | NR |
| Vacirca et al32 | 0 | 1/1 | 1/1 | 6.07 | 322 | 524 | NR | 1.19 | 0.1 |
| Fan et al33 | 1/1 | 1/1 | NR | NR | 770 | 136.2 | 2.55 | NR | |
| Perini et al34 | NR | NR | 1/2 | NR | NR | NR | NR | 9.0 | NR |
| Kashi et al35 | NR | NR | 3/5 | NR | 160 | NR (n = 2) 547 |
NR | NR (n = 3) 20 |
NR |
| Baeza et al36 | 3/3 | 0/3 | 0/3 | 18.2 | 193.7 | 766.3 | 4.1 | 5.07 | NR |
| Garg et al37 | 1/3 | 2/3 | 1/3 | NR | NR | NR | NR | 3.3 | NR |
| Thompson et al38 | 0 | 0 | 0 | NR | NR | NR | NR | NR | NR |
| Levolger et al39 | 2/2 | 1/2 | 0/2 | 10.6 | 360 | NR | 167 | NR | NR |
| Wengerter et al40 | 1/3 | 0 | 1/3 | NR | NR | NR | 144.3 | 15.6 | NR |
| Chowdhury et al41 | 1/1 | 1/1 | 1/1 | 8.6 | 172 | NR | 20 | 1.2 | NR |
| Liu et al42 | 0 | 0 | 0 | 9.83 | 282 | 560 | 79.1 | 6.55 | NR |
CRP, C-reactive protein; NR, not reported; PCT, procalcitonin.
Laboratory values are reported either with standard deviation or value range, whatever was reported.
Regarding treatment, medical treatment was chosen in 41.8% of patients (33 studies) as a first-line treatment. In total (among 33 studies), 92 patients (58.2%) underwent the following procedures: thrombembolectomy (n = 81), fasciotomy (n = 9), angioplasty with or without stenting (n = 7), thrombolysis (n = 7), thrombosuction (n = 2), bypass (n = 3), and endarterectomy (n = 2). All patients were covered with unfractionated or low-molecular-weight heparin. All medical and interventional treatment is presented in Table IV .
Table IV.
Type of treatment for the included patients
| Study | Medical to interventional treatment as first line treatment | HCQ | Antibiotics | Antiviral treatment | Heparin or LMWH | Other medication | Thrombectomy or Embolectomy | Other intervention | Reoperation |
|---|---|---|---|---|---|---|---|---|---|
| Veerasuri et al9 | 1/0 | NR | NR | NR | 1/1 | Rivaroxaban | 0 | None | 0 |
| Kaur et al10 | 1/0 | 1/1 | 1/1 | NR | 1/1 | NR | 0 | None | 0 |
| Brugliera et al11 | 2/1 | 3/3 | 2/3 | 1/3 | 3/3 | Iloprost (n = 1) ASA (n = 2) |
1 | None | 0 |
| Hanif et al12 | 0/1 | 0 | 1/1 | 0 | 1/1 | NR | 1 | None | 0 |
| Hasan et al13 | 1/0 | 0 | 1/1 | 0 | 1/1 | Rivaroxaban post discharge | 0 | None | 0 |
| Gubitosa et al14 | 0/1 | 0 | 0 | 0 | 1/1 | FDPX | 1 | Fasciotomy | 0 |
| Anwar et al15 | 0/1 | 1/1 | 1/1 | 0 | 1/1 | CTDS, ASA | 0 | Angioplasty | 0 |
| Singh et al16 | 0/1 | 1/1 | 1/1 | 0 | 1/1 | NR | 1 | None | 0 |
| Wang et al17 | 2/0 | NR | NR | NR | 1/1 | Argatroban | 0 | None | 0 |
| Goldman et al18 | 9/7 | NR | NR | NR | NR | NR | 6 | None | 0 |
| Sánchez et al19 | 2/28 | NR | NR | NR | NR | NR | 23 | Fasciotomy (n = 6) | 2 |
| Galanis et al20 | 1/0 | 1/1 | 1/1 | 0 | 1/1 | FDPX | 1 | None | 0 |
| Bozzani et al21 | 0/6 | NR | NR | NR | 6/6 | ASA (3/6) ASA + clopidogrel (3/6) |
6 | PTA (n = 2) PTA + stenting (n = 1) |
1 |
| Mietto et al22 | 0/1 | NR | NR | NR | 1/1 | Prostacyclin | 1 | Thrombolysis, fasciotomy | 1 |
| Baccellieri et al23 | 0/1 | 1/1 | 1/1 | NR | 1/1 | NR | 1 | Thrombectomy at right upper limb | 0 |
| Shao et al24 | 0/1 | NR | NR | NR | 1/1 | NR | 1 | Thrombolysis, fasciotomy | 0 |
| Etkin et al25 | 31/11 | NR | NR | NR | NR | NR | 9 | Endovascular thrombosuction (n = 2) | NR |
| Muhammad et al26 | 0/1 | NR | NR | NR | 1/1 | ASA 75 mg Dabigatran 150 mg bid |
0 | Thrombolysis | 0 |
| Heald et al27 | 1/0 | NR | NR | NR | 1/1 | NR | 0 | None | 0 |
| Bellosta et al28 | 3/17 | NR | NR | NR | 20/20 | NR | 15 | Below the knee fem-pop bypass (n = 2) Additional thrombolysis (n = 2) Kissing stents (n = 2) Femoral endarterectomy (n = 1) Below the knee angioplasty (n = 1) |
2 |
| Kaur et al29 | 0/1 | 1/1 | 1/1 | NR | 1/1 | NR | 1 | Endarterectomy of the right arm | 0 |
| Kahlberg et al30 | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| Schultz et al31 | 2/0 | 2/2 | 2/2 | 2/2 | 2/2 | Nitroglycerin (n = 2) Apixaban (n = 1) |
0 | None | 0 |
| Vacirca et al32 | 0/1 | NR | NR | NR | 1/1 | NR | 1 | Thrombolysis | 0 |
| Fan et al33 | 0/1 | NR | NR | 1/1 | 1/1 | ASA | 1 | Aortic stent graft placement | 0 |
| Perini et al34 | 1/1 | NR | NR | NR | 1/1 | NR | 1 | None | 1 |
| Kashi et al35 | 4/1 | NR | NR | NR | 2/5 | Apixaban (n = 1) ASA (n = 2) |
1 | None | NR |
| Baeza et al36 | 0/3 | 3/3 | 3/3 | 2/3 | 3/3 | Acenocoumarol (n = 1) | 2 | Aortobifemoral bypass (n = 1) | 0 |
| Garg et al37 | 2/1 | 1/3 | 1/3 | 1/3 | 3/3 | – | 1 | NR | NR |
| Thompson et al38 | 0/1 | 0 | 0 | 0 | 1/1 | – | 1 | None | 0 |
| Levolger et al39 | 2/0 | 1/2 | 0 | 0 | 2/2 | Rivaroxaban (n = 1) Apixaban (n = 1) |
1 | Thrombolysis | 0 |
| Wengerter et al40 | 0/3 | NR | NR | NR | 3/3 | NR | 3 | None | NR |
| Chowdhury et al41 | 0/1 | 1/1 | 1/1 | 0 | 1/1 | Prednisolone | 1 | None | 0 |
| Liu et al42 | 1/0 | NR | 1/1 | 1/1 | 1/1 | NR | 0 | None | 0 |
| Total (n/total n) | 66/158 (1 study NR) | 17/24 (18 studies NR) | 18/24 (18 studies NR) | 8/23 (19 studies NR) | 65/65 (4 studies NR) | ASA (n = 13) Clopidogrel (n = 3) Apixaban (n = 3) Dabigatran (n = 1) Argatroban (n = 1) Rivaroxaban (n = 3) CTDS (n = 2) FDPX (n = 1) Acenocoumarol (n = 1) Prostaglandins (n = 2) Nitroglycerine (n = 2) (15 studies NR) |
N = 81 (1 study NR) | Thrombolysis (n = 7) PTA/stenting (n = 5) Endarterectomy (n = 2) Fasciotomy (n = 9) Bypass (n = 3) Thrombectomy at other site (n = 1) Thrombosuction Stent graft placement (n = 1) |
n = 7 (5 studies NR) |
ASA, Acetylsalicylic acid; bid, 2 times per day; CTDS, corticosteroids; FDPX, fondaparinux; HCQ, hydroxychloroquine; LMWH, low molecular weight heparin; NR, not reported; PTA, percutaneous transluminal angioplasty.
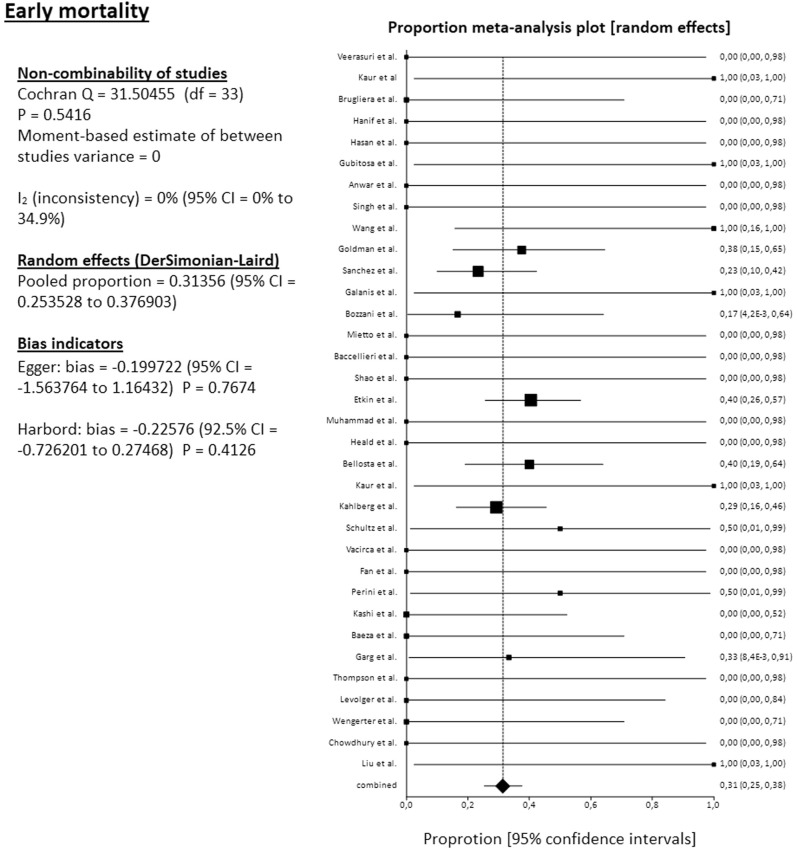
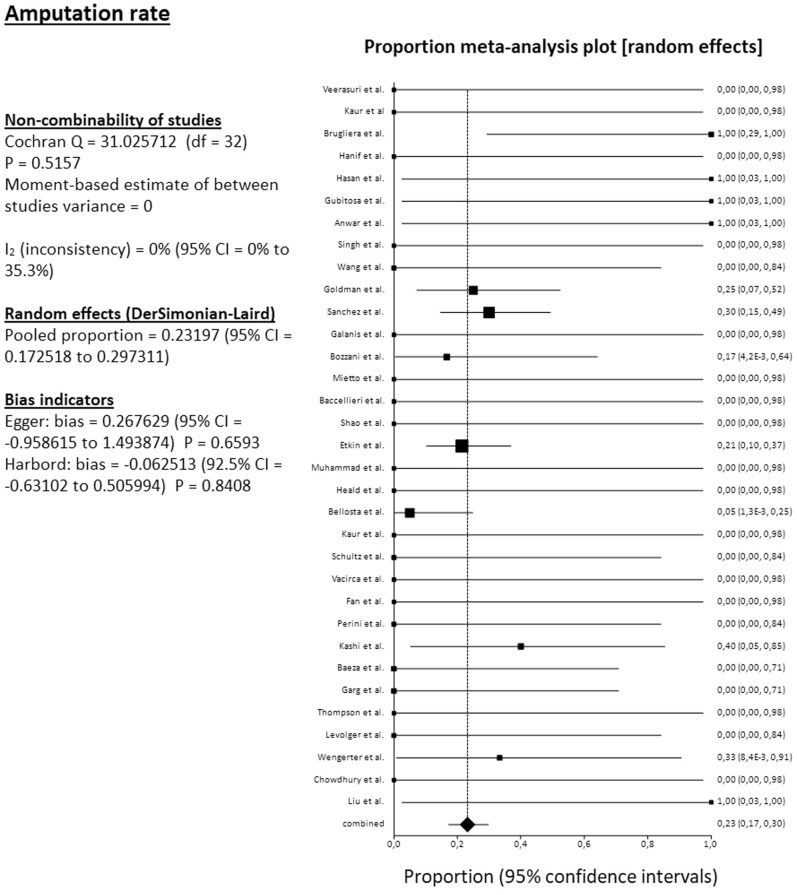
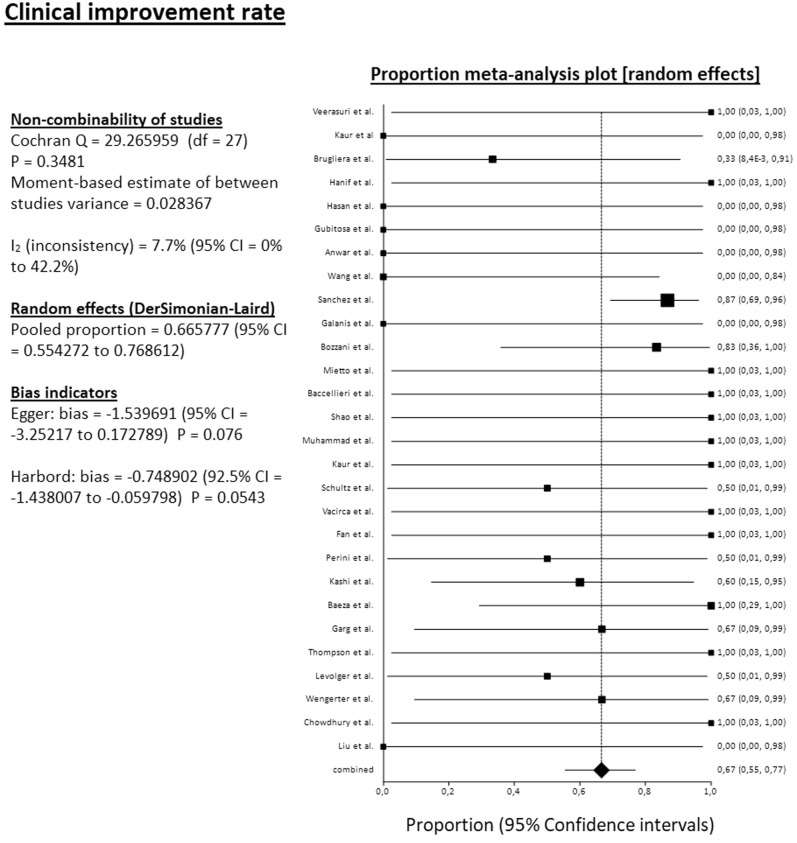
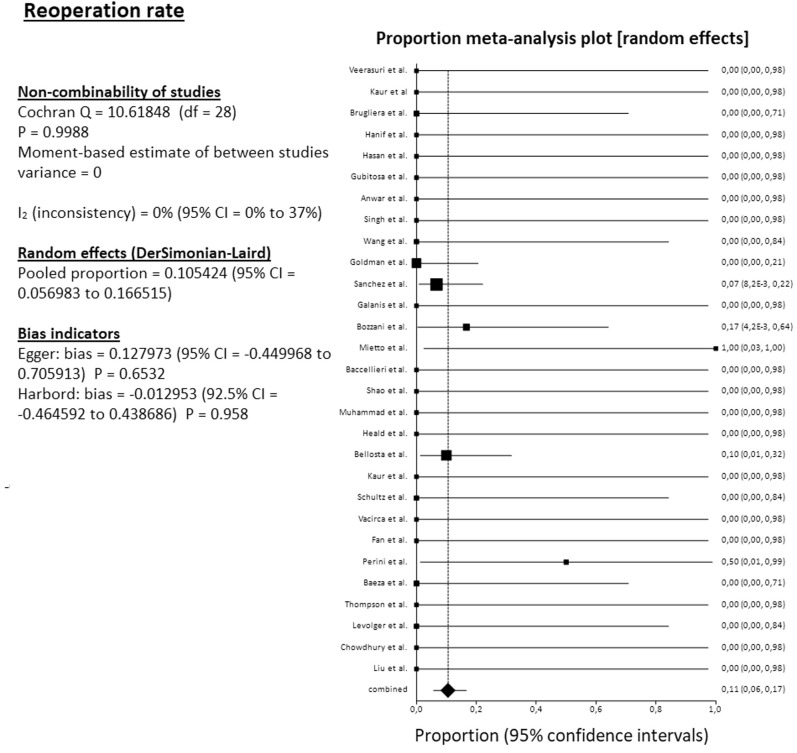
The pooled mortality rate among 34 studies reached 31.4% (95% CI, 25.4%-37.7%). The pooled amputation rate (both primary and secondary) among 34 studies reached 23.2% (95% CI, 17.3%-29.7%). From the 34 reported amputations, 22 were major (transfemoral or below the knee), 1 minor (below the ankle), and 11 were at an unknown level. The pooled clinical improvement rate among 28 studies reached 66.6% (95% CI, 55.4%-76.9%). The pooled reoperation rate among 29 studies reached 10.5% (95% CI, 5.7%-16.7%) (Fig 2, Fig 3, Fig 4, Fig 5 ). For all these pooled outcomes, heterogeneity was very low (I2 = 0%-7.7%). All outcomes are presented in Table V . Finally, there was no difference regarding death risk (OR, 1.10; 95% CI, 0.284%-4.272; P = .884) and amputation risk (OR, 1.16; 95% CI, .288-4.649; P = .834) between upper limb and lower limb location (data available from 31 studies). Regarding first-line strategy, medical treatment was associated with a higher risk of death compared with any intervention (OR, 4.04; 95% CI, 1.075-15.197; P = .045), although the risk of amputation was not different between the two strategies (OR, 0.977; 95% CI, 0.070-13.600; P = .986) (data from 31 studies).
Fig 2.
Forest plot on pooled early mortality. CI, Confidence interval; df, degrees of freedom.
Fig 3.
Forest plot on pooled amputation rate. CI, Confidence interval; df, degrees of freedom.
Fig 4.
Forest plot on pooled clinical improvement rate. CI, Confidence interval; df, degrees of freedom.
Fig 5.
Forest plot on pooled reoperation rate. CI, Confidence interval; df, degrees of freedom.
Table V.
Main outcomes for all included patients
| Study | Death | Amputations | Cardiac | Other complications | Improvement of the ischemia | Mean follow-up | Late outcomes |
|---|---|---|---|---|---|---|---|
| Veerasuri et al9 | 0 | 0 | 0 | 0 | 1 | 2 months | Numbness in right foot |
| Kaur et al10 | 1 | 0 | 1 | Hemodialysis | 0 | NR | NR |
| Brugliera et al11 | 0 | 3 (all TF) | 0 | DVT (n = 1) | 1 | NR | NR |
| Hanif et al12 | 0 | 0 | 0 | 0 | 1 | NR | NR |
| Hasan et al13 | 0 | 1 (TF) | 0 | 0 | 0 | NR | NR |
| Gubitosa et al14 | 1 | 1 (TF) | 1 | HIT | 0 | NR | NR |
| Anwar et al15 | 0 | 1 (below ankle) | 0 | 0 | 0 | NR | NR |
| Singh et al16 | 0 | 0 | 0 | 0 | NR | NR | NR |
| Wang et al17 | 2 | 0 | 0 | Encephalopathy (n = 1) | 0 | NR | NR |
| Goldman et al18 | 6 | 4 (all TF) | NR | NR | NR | NR | NR |
| Sánchez et al19 | 7 | 9 (all TF) | NR | NR | 26 | NR | NR |
| Galanis et al20 | 1 | 0 | 0 | DVT | 0 | NR | NR |
| Bozzani et al21 | 1 | 1 (TF) | 0 | Rethrombosis (n = 2) | 5 | NR | NR |
| Mietto et al22 | 0 | 0 | 0 | Rhabdomyolysis, acute renal failure | 1 | 40 days | Superficial peroneal nerve impairment |
| Baccellieri et al23 | 0 | 0 | 0 | Nephrotic syndrome, bilateral acroischemia, thrombosis of upper limb (intraoperative) | 1 | 2 months | No new event |
| Shao et al24 | 0 | 0 | 0 | PE, GI bleeding | 1 | 2 months | Dry gangrene of digits |
| Etkin et al25 | 17 | 9 (NR) | NR | NR | NR | NR | NR |
| Muhammad et al26 | 0 | 0 | 0 | NR | 1 | 6 weeks | Full recovery |
| Heald et al27 | 0 | 0 | 0 | 0 | NR | NR | NR |
| Bellosta et al28 | 8 | 1 (major) | 1 | NR | NR | NR | NR |
| Kaur et al29 | 1 | 0 | 1 | NR | 1 | NR | NR |
| Kahlberg et al30 | 12 | NR | NR | NR | NR | NR | NR |
| Schultz et al31 | 1 | 0 | 0 | Septic shock, ARDS, AKI (n = 2) DVT (n = 2) Hemorrhagic shock (n = 1) |
1 | NR | NR |
| Vacirca et al32 | 0 | 0 | 0 | 0 | 1 | NR | NR |
| Fan et al33 | 0 | 0 | 0 | NR | 1 | NR | NR |
| Perini et al34 | 1 | 0 | 0 | 0 | 1 | NR | NR |
| Kashi et al35 | 0 | 2 (NR) | NR | DVT (n = 1) | 3 | NR | NR |
| Baeza et al36 | 0 | 0 | 0/3 | 0 | 3 | NR | NR |
| Garg et al37 | 1 | 0 | 0 | DVT (n = 1) Stroke (n = 1) |
2 | NR | NR |
| Thompson et al38 | 0 | 0 | 0 | – | 1 | Several days | Digit tip gangrene |
| Levolger et al39 | 0 | 0 | 0 | Contralateral limb ischemia (n = 1) | 1 | NR | NR |
| Wengerter et al40 | 0 | 1 (BTK) | 0 | Stroke (n =1) | 2 | NR | NR |
| Chowdhury et al41 | 0 | 0 | 0 | 0 | 1 | NR | NR |
| Liu et al42 | 1 | 1 (TF) | 0 | 0 | 0 | NR | NR |
| Total (n) | 61 | 34 (1 study NR) | 4 (5 studies NR) | DVT/PE (n = 7) Rethrombosis (n = 2) Stroke (n = 1) AKI/hemodialysis (n = 4) Encephalopathy (n = 1) Shock (n = 2) Other limb ischemia (n = 2) Rhabdomyolysis (n = 1) HIT (n = 1) Other (n = 3) (8 studies NR) |
56 (6 studies NR) | 0-2 months (28 studies NR) | No new event (n = 2) Digit gangrene (n = 2) Numbness/nerve impairment (n = 2) (28 studies NR) |
ARDS, Acute respiratory distress syndrome; AKI, acute kidney injury; BTK, below the knee; DVT, deep vein thrombosis; GI, gastrointestinal; HIT, heparin-induced thrombopenia; NR, not reported; PE, pulmonary embolism; TF, transfemoral.
Discussion
Although the overall incidence of ALI has decreased worldwide and the hypercoagulable state remains an uncommon cause for limb ischemia,43 the incidence of thromboembolic complications among patients with COVID-19 infection is as high as 35% to 45%.44 In critically ill patients, there is an even higher risk for both venous and arterial thromboembolism associated with high mortality.45 , 46 As we found in this review, ALI was associated with a mortality rate of 31.4%, although the reported mortality in non-COVID populations with ALI ranges from 5% to 9% in literature.47 , 48 Comparative studies have also shown a higher incidence of thrombotic events such as strokes among COVID-infected patients compared with the general wards.49 However, this relative increase of arterial thrombotic events during the pandemic may be attributed to several factors, such as delays in emergency room presentation owing to the lockdown, older patient age, or fear in approaching hospitals because of a high contamination risk.50 Additionally, there are several reports that these thrombotic events occur at a later time point during the course of the infection.36 Some authors have advocated that the virus starts a second attack between 7 and 14 days from the onset of symptoms that perhaps initiates some type of hypercoagulability.2
Additionally, we found a mean age of 61 years with a very wide age range starting from just 39 years. Other authors have also reported that infected patients with thrombotic complications are of relatively young age, and available computed tomography scans and angiography reveal no prior major atherosclerosis in these cases.51 This finding suggests that a significant proportion of arterial thromboses in patients with COVID-19 might occur over nondiseased or mildly diseased vessels. Although male gender, advanced age, hypertension, and diabetes have been found to be independent risk factors of death among patients with COVID-19,52 this review revealed that infected patients with ALI show a low incidence of major comorbidities such as diabetes, dyslipidemia, coronary disease, and renal disease. This finding indicates that even patients without risk factors are at risk of presenting thrombotic complications when infected.
The causative mechanism for ALI seems to be a systematic inflammatory process triggered by a massive activation of macrophages that generate a cytokine storm.53 COVID-19 causes elevated cytokine levels, including but not limited to tumor necrosis factor-α, IL-1β, IL-6, procalcitonin, and interferon γ.54, 55, 56 The coupling of inflammation and coagulation has also been described in the literature, with these procedures sharing common molecular pathways.57 It has been reported that infected patients are prone to thrombotic dysfunction, and especially those with severe symptoms had higher CRP levels and a higher thrombotic risk.58 However, high levels of CRP in our review were not associated with adverse events in patients with ALI. These patients exhibit several risk factors of thrombosis such as blood concentration, vascular endothelial injury, extended bed rest, and blood hypercoagulation. Additionally, recent data indicate that COVID-19 infection is associated with profound and generalized activation of both alternative and lectin-based complement pathways.59
There is growing evidence that this virus promotes a procoagulant state producing both microthrombi and macrothrombi.60 Cutaneous ischemic lesions are frequent in such patients, even in absence of major vessel thrombosis.61 In particular, severe endothelial injury, widespread thrombosis with microangiopathy, alveolar-capillary microthrombi and new vessel growth have been detected in infected cases.62 Vascular pathological changes in such patients include partial vascular endothelial shedding, vascular intimal inflammation, and thrombosis.63 Varga et al63 have even observed viral inclusion bodies under light microscopy in the endothelium of the specimens. The angiotensin-converting enzyme 2, the receptor for SARS-CoV-2, is also expressed on the membrane of vascular muscle and endothelial cells, and therefore, infection of these cells could induce an inflammatory response in the blood vessel walls, predisposing to clot formation.33 The viral infection itself leads to decreased platelet function secondary to decreased production, platelet consumption, and production of autoantibodies such as antiphospholipid antibodies.64 , 65 It is also known that other viral infections including hepatitis or human immune deficiency viral infections have been associated with thrombotic complications such as venous thromboembolism.66 , 67
Regarding thrombotic markers, high d-dimers, fibrinogen degradation products, and a prolonged thromboplastin time have been associated with greater in-hospital mortality, need for mechanical ventilation, and thrombotic complications in infected patients.12 , 68 , 69 Recent pooled data indicate that prothrombin time and d-dimer levels are significantly higher in patients with severe infection than in those with mild disease.70 Some authors advocate that increased levels of d-dimers could serve as an indicator of the time-point at which an intervention with recombinant tissue plasminogen activator or tocilizumab should be considered.71 However, an optimal cut-off level and prognostic value are still not known.72 In our review, all patients had a thrombotic complication, indicating a population at higher mortality risk. This factor could justify that we could not establish a cutoff level for d-dimer as well. The mean fibrinogen concentrations in patients with COVID-19 are in general at the upper limits of normal, presumably as an acute phase response.44 However, we could not establish an association of high fibrinogen levels with worse outcomes either.
Given the increased thrombotic risk, the World Health Organization recommends at least prophylactic doses of low-molecular-weight heparin daily or subcutaneous unfractionated heparin twice daily for venous thromboembolism prophylaxis in critically ill patients with COVID-19.73 However, ALI can even occur among patients already receiving thromboprophylaxis,74 and this outcome has been observed in some cases included in the present review. Despite thromboprophylaxis, the risk of venous thromboembolism remains high in hospitalized patients with COVID-19.75 One study of 94 patients with confirmed COVID-19 demonstrated a statistically significant relative deficiency of antithrombin III compared with control.76 This acquired deficiency would promote further coagulation and could decrease the efficacy of anticoagulant treatment in such patients. Therefore, certain concerns arise whether full therapeutic dosage of anticoagulants would be more appropriate for severely ill patients.
For patients with COVID-19 presenting with ALI, the choice of intervention is guided by the need to limit interventions that would expose these patients to stressful procedures, the desire to limit exposure of medical personnel, and the need to conserve resources. Additionally, in critically ill infected patients, thrombosis may be a terminal event, sometimes being referred to as agonal thrombosis.30 This point probably explains the high number of patients treated conservatively as a first-line treatment in this review as well. However, we found that medical treatment showed a much higher mortality risk compared with intervention, although the amputation risk was similar. In another systematic review by Putko et al,77 a similar mortality rate was found, although the number of included cases and studies was much lower and no further meta-analysis was conducted. However, Tang et al78 have shown that anticoagulant treatment mainly with low-molecular-weight heparin is associated with a lower mortality risk in patients with COVID-19 who have an increased coagulopathy score or high d-dimer levels. Therefore, coverage with anticoagulants is imperative in high-risk patients.
If an intervention is needed, several methods have been used for treating ALI, including thrombembolectomy, thrombolysis, thrombosuction, and others, yielding comparable results regarding limb salvage.79 The choice of surgical intervention is influenced by both the clinical status of the patient and the etiology of the ALI. Data from non-COVID cases show that thrombotic rather than embolic events lead to worse outcomes.80 In our review, we found an almost 10.5% reoperation rate and a 23.5% amputation rate, with the majority being major amputations. Other authors have also found that successful revascularization is disappointingly low in patients with COVID-19 when compared with previously reported series.81 Data indicate that continuation of anticoagulants at admission in patients already receiving such agents for other causes did not affect outcomes, even in patients undergoing operative procedures.12 This finding underlines the strong hypercoagulant and inflammatory storm that this infection releases.
There are certain limitations to this review. First, the total number of patients included is low, and most of studies consist of case reports or case series. Second, many studies do not provide data considering the precise medical treatment or laboratory profile of patients to extract adequately powered pooled data. There was also a lack of specific a definition for the majority of comorbidities in the included studies and, therefore, these events were reported in this review as reported in the studies. Additionally, no follow-up is provided by the majority of studies. This factor is mainly due to the fact that all of studies have been published within the last year. Furthermore, reoperation rates are reported without any detail on the type of procedure in the majority of studies. Finally, data were too limited to conduct any multiregression analysis.
Conclusions
SARS-CoV-2 infection is associated with a high thrombotic risk probably by promoting a systematic inflammatory response and a hypercoagulable state. COVID-associated ALI usually presents in patients with low number of comorbidities, and it is associated with a high mortality and amputation risk. Mortality risk seems to be greater with conservative treatment compared with any intervention, although the amputation risk is similar. Future studies should focus on identifying optimal medical treatment for these patients as well as potential prognostic factors for mortality and amputation risks.
Author contributions
Conception and design: GG, AS, KF
Analysis and interpretation: GG, KS, FS
Data collection: GG, MF, KV, DV, CT, VM
Writing the article: GG, AS, MF, KV, DV, CT, VM
Critical revision of the article: GG, FS, KF
Final approval of the article: GG, AS, MF, KV, DV, CT, VM, FS, KF
Statistical analysis: GG
Obtained funding: Not applicable
Overall responsibility: KF
Audra A. Duncan, MD, SECTION EDITOR
Footnotes
Author conflict of interest: none.
The editors and reviewers of this article have no relevant financial relationships to disclose per the JVS policy that requires reviewers to decline review of any manuscript for which they may have a conflict of interest.
References
- 1.Liu Y.C., Kuo R.L., Shih S.R. COVID-19: the first documented coronavirus pandemic in history. Biomed J. 2020;43:328–333. doi: 10.1016/j.bj.2020.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Li T., Lu H., Zhang W. Clinical observation and management of COVID-19 patients. Emerg Microbes Infect. 2020;9:687–690. doi: 10.1080/22221751.2020.1741327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ejaz H., Alsrhani A., Zafar A., Javed H., Junaid K., Abdalla A.E., et al. COVID-19 and comorbidities: deleterious impact on infected patients. J Infect Public Health. 2020;13:1833–1839. doi: 10.1016/j.jiph.2020.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Castro R.A., Frishman W.H. Thrombotic complications of COVID-19 infection: a review. Cardiol Rev. 2021;29:43–47. doi: 10.1097/CRD.0000000000000347. [DOI] [PubMed] [Google Scholar]
- 5.Keshavarz P., Rafiee F., Kavandi H., Goudarzi S., Heidari F., Gholamrezanezhad A. Ischemic gastrointestinal complications of COVID-19: a systematic review on imaging presentation. Clin Imaging. 2020;73:86–95. doi: 10.1016/j.clinimag.2020.11.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nannoni S., de Groot R., Bell S., Markus H.S. Stroke in COVID-19: a systematic review and meta-analysis. Int J Stroke. 2020;11 doi: 10.1177/1747493020972922. 1747493020972922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liberati A., Altman D.G., Tetzlaff J., Mulrow C., Gøtzsche P.C., Ioannidis J.P., et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339:b2700. doi: 10.1136/bmj.b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25:603–605. doi: 10.1007/s10654-010-9491-z. [DOI] [PubMed] [Google Scholar]
- 9.Veerasuri S., Kulkarni S.R., Wilson W.R., Paravastu S.C.V. Bilateral acute lower limb ischemia secondary to COVID-19. Vasc Endovascular Surg. 2021;55:196–199. doi: 10.1177/1538574420954301. [DOI] [PubMed] [Google Scholar]
- 10.Kaur P., Posimreddy S., Singh B., Qaqa F., Habib H.A., Maroules M., et al. COVID-19 Presenting as acute limb ischaemia. Eur J Case Rep Intern Med. 2020;7:001724. doi: 10.12890/2020_001724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brugliera L., Spina A., Castellazzi P., Cimino P., Arcuri P., Deriu M.G., et al. Rehabilitative of COVID-19 patients with acute lower extremity Ischemia and amputation. J Rehabil Med. 2020;52:jrm00094. doi: 10.2340/16501977-2714. [DOI] [PubMed] [Google Scholar]
- 12.Hanif M., Ali M.J., Haider M.A., Naz S., Ahmad Z. Acute upper limb ischemia due to arterial thrombosis in a mild COVID-19 patient: a case report. Cureus. 2020;12:e10349. doi: 10.7759/cureus.10349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hasan S.A., Haque A., Nazir F. Acute limb ischemia: a rare complication of COVID-19. Cureus. 2020;12:e11488. doi: 10.7759/cureus.11488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gubitosa J.C., Xu P., Ahmed A., Pergament K. COVID-19-associated acute limb ischemia in a patient on therapeutic anticoagulation. Cureus. 2020;12:e10655. doi: 10.7759/cureus.10655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Anwar S., Acharya S., Shabih S., Khabut A. Acute limb ischemia in COVID-19 disease: a mysterious coagulopathy. Cureus. 2020;12:e9167. doi: 10.7759/cureus.9167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Singh B., Kaur P., Ajdir N., Gupta S., Maroules M. Covid-19 presenting as acute limb ischemia. Cureus. 2020;12:e9344. doi: 10.7759/cureus.9344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang J.S., Pasieka H.B., Petronic-Rosic V., Sharif-Askary B., Evans K.K. Digital gangrene as a sign of catastrophic coronavirus disease 2019-related microangiopathy. Plast Reconstr Surg Glob Open. 2020;8:e3025. doi: 10.1097/GOX.0000000000003025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goldman I.A., Ye K., Scheinfeld M.H. Lower-extremity arterial thrombosis associated with COVID-19 is characterized by greater thrombus burden and increased rate of amputation and death. Radiology. 2020;297:263–269. doi: 10.1148/radiol.2020202348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sánchez J.B., Cuipal Alcalde J.D., Ramos Isidro R., Luna C.Z., Cubas W.S., Coaguila Charres A., et al. Acute limb ischemia in a Peruvian cohort infected by COVID-19. Ann Vasc Surg. 2020;72:196–2004. doi: 10.1016/j.avsg.2020.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Galanis N., Stavraka C., Agathangelidis F., Petsatodis E., Giankoulof C., Givissis P. Coagulopathy in COVID-19 infection: a case of acute upper limb ischemia. J Surg Case Rep. 2020;2020:rjaa204. doi: 10.1093/jscr/rjaa204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bozzani A., Arici V., Tavazzi G., Franciscone M.M., Danesino V., Rota M., et al. Acute arterial and deep venous thromboembolism in COVID-19 patients: risk factors and personalized therapy. Surgery. 2020;168:987–992. doi: 10.1016/j.surg.2020.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mietto C., Salice V., Ferraris M., Zuccon G., Valdambrini F., Piazzalunga G., et al. Acute lower limb ischemia as clinical presentation of COVID-19 infection. Ann Vasc Surg. 2020;69:80–84. doi: 10.1016/j.avsg.2020.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Baccellieri D., Bilman V., Apruzzi L., Monaco F., D'Angelo A., Loschi D., et al. A case of Covid-19 patient with acute limb ischemia and heparin resistance. Ann Vasc Surg. 2020;68:88–92. doi: 10.1016/j.avsg.2020.06.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shao T., In-Bok Lee C., Jabori S., Rey J., Duran E.R., Kang N. Acute upper limb ischemia as the first manifestation in a patient with COVID-19. J Vasc Surg Cases Innov Tech. 2020;6:674–677. doi: 10.1016/j.jvscit.2020.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Etkin Y., Conway A.M., Silpe J., Qato K., Carroccio A., Manvar-Singh P., et al. Acute arterial thromboembolism in patients with COVID-19 in the New York City area. Ann Vasc Surg. 2021;70:290–294. doi: 10.1016/j.avsg.2020.08.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Muhammad K., Tantawy T.G., Makar R.R., Olojugba O. Successful catheter-directed thrombolysis for acute lower limb ischemia secondary to COVID-19 infection. Ann Vasc Surg. 2020;71:103–111. doi: 10.1016/j.avsg.2020.09.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Heald M., Fish J., Lurie F. Skin manifestations of COVID-19 resembling acute limb ischemia. J Vasc Surg Cases Innov Tech. 2020;6:514–515. doi: 10.1016/j.jvscit.2020.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bellosta R., Luzzani L., Natalini G., Pegorer M.A., Attisani L., Cossu L.G., et al. Acute limb ischemia in patients with COVID-19 pneumonia. J Vasc Surg. 2020;72:1864–1872. doi: 10.1016/j.jvs.2020.04.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kaur P., Qaqa F., Ramahi A., Shamoon Y., Singhal M., Shamoon F., et al. Acute upper limb ischemia in a patient with COVID-19. Hematol Oncol Stem Cell Ther. 2020 doi: 10.1016/j.hemonc.2020.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kahlberg A., Mascia D., Bellosta R., Attisani L., Pegorer M., Socrate A.M., et al. Vascular surgery during COVID-19 emergency in hub hospitals of Lombardy: experience on 305 Patients. Eur J Vasc Endovasc Surg. 2020;61:306–315. doi: 10.1016/j.ejvs.2020.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schultz K., Wolf J.M. Digital ischemia in COVID-19 patients: case report. J Hand Surg Am. 2020;45:518–522. doi: 10.1016/j.jhsa.2020.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vacirca A., Faggioli G., Pini R., Teutonico P., Pilato A., Gargiulo M. Unheralded lower limb threatening ischemia in a COVID-19 patient. Int J Infect Dis. 2020;96:590–592. doi: 10.1016/j.ijid.2020.05.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fan B.E., Chia Y.W., Sum C.L.L., Kuperan P., Chan S.S.W., Ling L.M., et al. Global haemostatic tests in rapid diagnosis and management of COVID-19 associated coagulopathy in acute limb ischaemia. J Thromb Thrombolysis. 2020;50:292–297. doi: 10.1007/s11239-020-02165-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Perini P., Nabulsi B., Massoni C.B., Azzarone M., Freyrie A. Acute limb ischaemia in two young, non-atherosclerotic patients with COVID-19. Lancet. 2020;395:1546. doi: 10.1016/S0140-6736(20)31051-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kashi M., Jacquin A., Dakhil B., Zaimi R., Mahé E., Tella E., et al. Severe arterial thrombosis associated with Covid-19 infection. Thromb Res. 2020;192:75–77. doi: 10.1016/j.thromres.2020.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Baeza C., González A., Torres P., Pizzamiglio M., Arribas A., Aparicio C. Acute aortic thrombosis in COVID-19. J Vasc Surg Cases Innov Tech. 2020;6:483–486. doi: 10.1016/j.jvscit.2020.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Garg K., Barfield M.E., Pezold M.L., Sadek M., Cayne N.S., Lugo J., et al. Arterial thromboembolism associated with COVID-19 and elevated D-dimer levels. J Vasc Surg Cases Innov Tech. 2020;6:348–351. doi: 10.1016/j.jvscit.2020.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Thompson O., Pierce D., Whang D., O'Malley M., Geise B., Malhotra U. Acute limb ischemia as sole initial manifestation of SARS-CoV-2 infection. J Vasc Surg Cases Innov Tech. 2020;6:511–513. doi: 10.1016/j.jvscit.2020.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Levolger S., Bokkers R.P.H., Wille J., Kropman R.H.J., de Vries J.P.M. Arterial thrombotic complications in COVID-19 patients. J Vasc Surg Cases Innov Tech. 2020;6:454–459. doi: 10.1016/j.jvscit.2020.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wengerter S.P., Wengerter K.R., Masoudpoor H., Sagarwala A., Karim O., Rao N., et al. Acute aortoiliac and infrainguinal arterial thrombotic events in four patients diagnosed with the novel coronavirus 2019. J Vasc Surg Cases Innov Tech. 2020;6:698–702. doi: 10.1016/j.jvscit.2020.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chowdhury Y.S., Mitre C.A., Rotella V.E., Garg K., Lee D.K., Belligund P., et al. Extensive peripheral arterial thrombosis in a patient with SARS-CoV-2 infection. Am J Med Case Rep. 2020;8:486–490. [Google Scholar]
- 42.Liu Y., Chen P., Mutar M., Hung M., Shao Z., Han Y., et al. Ischemic necrosis of lower extremity in COVID-19: a case report. J Atheroscler Thromb. 2021;28:90–95. doi: 10.5551/jat.57950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Baril D.T., Ghosh K., Rosen A.B. Trends in the incidence, treatment, and outcomes of acute lower extremity ischemia in the United States Medicare population. J Vasc Surg. 2014;60:669–677. doi: 10.1016/j.jvs.2014.03.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Levi M., Thachil J., Iba T., Levy J.H. Coagulation abnormalities and thrombosis in patients with COVID-19. Lancet Haematol. 2020;7:438–440. doi: 10.1016/S2352-3026(20)30145-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Klok F.A., Kruip M.J.H.A., van der Meer N.J.M., Arbous M.S., Gommers D.A.M.P.J., Kant K.M., et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res. 2020;191:145–147. doi: 10.1016/j.thromres.2020.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shah A., Donovan K., McHugh A., Pandey M., Aaron L., Bradbury C.A., et al. Thrombotic and haemorrhagic complications in critically ill patients with COVID-19: a multicentre observational study. Crit Care. 2020;24:561. doi: 10.1186/s13054-020-03260-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Eliason J.L., Wainess R.M., Proctor M.C., Dimick J.B., Cowan J.A., Jr., Upchurch G.R., Jr., et al. A national and single institutional experience in the contemporary treatment of acute lower extremity ischemia. Ann Surg. 2003;238:382–389. doi: 10.1097/01.sla.0000086663.49670.d1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hemingway J., Emanuels D., Aarabi S., Quiroga E., Tran N., Starnes B., et al. Safety of transfer, type of procedure, and factors predictive of limb salvage in a modern series of acute limb ischemia. J Vasc Surg. 2019;69:1174–1179. doi: 10.1016/j.jvs.2018.08.174. [DOI] [PubMed] [Google Scholar]
- 49.Lodigiani C., Iapichino G., Carenzo L., Cecconi M., Ferrazzi P., Sebastian T., et al. Venous and arterial thromboembolic complications in COVID-19 patients admitted to an academic hospital in Milan, Italy. Thromb Res. 2020;191:9–14. doi: 10.1016/j.thromres.2020.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bissacco D., Grassi V., Lomazzi C., Domanin M., Bellosta R., Piffaretti G., et al. Is there a vascular side of the story? Vascular consequences during COVID-19 outbreak in Lombardy, Italy. J Card Surg. 2020;36:1677–1682. doi: 10.1111/jocs.15069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.de Roquetaillade C., Chousterman B.G., Tomasoni D., Zeitouni M., Houdart E., Guedon A., et al. Unusual arterial thrombotic events in Covid-19 patients. Int J Cardiol. 2021;323:281–284. doi: 10.1016/j.ijcard.2020.08.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Albitar O., Ballouze R., Ooi J.P., Sheikh Ghadzi S.M. Risk factors for mortality among COVID-19 patients. Diabetes Res Clin Pract. 2020;166:108293. doi: 10.1016/j.diabres.2020.108293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.McGonagle D., Sharif K., O’Regan A., Bridgewood C. The role of cytokines including interleukin-6 in COVID-19 induced pneumonia and macrophage activation syndrome-like disease. Autoimmun Rev. 2020;19:10253. doi: 10.1016/j.autrev.2020.102537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zeng F., Huang Y., Guo Y., Yin M., Chen X., Xiao L., et al. Association of inflammatory markers with the severity of COVID-19: a meta-analysis. Int J Infect Dis. 2020;96:467–474. doi: 10.1016/j.ijid.2020.05.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Liu B., Li M., Zhou Z., Guan X., Xiang Y. Can we use interleukin-6 (IL-6) blockade for coronavirus disease 2019 (COVID-19)-induced cytokine release syndrome (CRS)? J Autoimmun. 2020;111:102452. doi: 10.1016/j.jaut.2020.102452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mehta P., McAuley D.F., Brown M., Sanchez E., Tattersall R.S., Manson J.J. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395:1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Terpos E., Ntanasis-Stathopoulos I., Elalamy I., Kastritis E., Sergentanis T.N., Politou M., et al. Hematological findings and complications of COVID-19. Am J Hematol. 2020;95:834–847. doi: 10.1002/ajh.25829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tang N., Li D., Wang X., Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 2020;18:844–847. doi: 10.1111/jth.14768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Magro C., Mulvey J.J., Berlin D., Nuovo G., Salvatore S., Harp J., et al. Complement associated microvascular injury and thrombosis in the pathogenesis of severe COVID-19 infection: a report of five cases. Transl Res. 2020;220:1–13. doi: 10.1016/j.trsl.2020.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wichmann D. Autopsy findings and venous thromboembolism in patients with COVID-19. Ann Intern Med. 2020;173:1030. doi: 10.7326/L20-1206. [DOI] [PubMed] [Google Scholar]
- 61.Alonso M.N., Mata-Forte T., García-León N., Vullo P.A., Ramirez-Olivencia G., Estébanez M., et al. Incidence, characteristics, laboratory findings and outcomes in acro-ischemia in COVID-19 patients. Vasc Health Risk Manag. 2020;16:467–478. doi: 10.2147/VHRM.S276530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ackermann M., Verleden S.E., Kuehnel M., Haverich A., Welte T., Laenger F., et al. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in Covid-19. N Engl J Med. 2020;383:120–128. doi: 10.1056/NEJMoa2015432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Varga Z., Flammer A.J., Steiger P., Haberecker M., Andermatt R., Zinkernagel A.S., et al. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020;395:1417–1418. doi: 10.1016/S0140-6736(20)30937-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Goeijenbier M., van Wissen M., van de Weg C., Jong E., Gerdes V.E., Meijers J.C., et al. Review: viral infections and mechanisms of thrombosis and bleeding. J Med Virol. 2012;84:1680–1696. doi: 10.1002/jmv.23354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhang Y., Xiao M., Zhang S., Xia P., Cao W., Jiang W., et al. Coagulopathy and antiphospholipid antibodies in patients with Covid-19. N Engl J Med. 2020;382:38. doi: 10.1056/NEJMc2007575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Galli L., Gerdes V.E., Guasti L., Squizzato A. Thrombosis associated with viral hepatitis. J Clin Transl Hepatol. 2014;2:234–239. doi: 10.14218/JCTH.2014.00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jackson B.S., Pretorius E. Pathological clotting and deep vein thrombosis in patients with HIV. Semin Thromb Hemost. 2019;45:132–140. doi: 10.1055/s-0038-1676374. [DOI] [PubMed] [Google Scholar]
- 68.Zhang L., Yan X., Fan Q., Liu H., Liu X., Liu Z., et al. D-dimer levels on admission to predict in-hospital mortality in patients with Covid-19. J Thromb Haemost. 2020;18:1324–1329. doi: 10.1111/jth.14859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yin S., Huang M., Li D., Tang N. Difference of coagulation features between severe pneumonia induced by SARS-CoV2 and non-SARS-CoV2. J Thromb Thrombolysis. 2020;3:1–4. doi: 10.1007/s11239-020-02105-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Xiong M., Liang X., Wei Y.D. Changes in blood coagulation in patients with severe coronavirus disease 2019 (COVID-19): a meta-analysis. Br J Haematol. 2020;189:1050–1052. doi: 10.1111/bjh.16725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lippi G., Favaloro E.J. D-dimer is associated with severity of coronavirus disease 2019: a pooled analysis. Thromb Haemost. 2020;120:876–878. doi: 10.1055/s-0040-1709650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Al-Ani F., Chehade S., Lazo-Langner A. Thrombosis risk associated with COVID-19 infection. A scoping review. Thromb Res. 2020;192:152–160. doi: 10.1016/j.thromres.2020.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bikdeli B., Madhavan M., Jiminez D., Chuich T., Dreyfus I., Driggin E., et al. COVID-19 and thrombotic or thromboembolic disease: implications for prevention, antithrombotic therapy, and follow-up: JACC state-of-the-art review. J Am Coll Cardiol. 2020;75:2950–2973. doi: 10.1016/j.jacc.2020.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Avila J., Long B., Holladay D., Gottlieb M. Thrombotic complications of COVID-19. Am J Emerg Med. 2021;39:213–218. doi: 10.1016/j.ajem.2020.09.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Artifoni M., Danic G., Gautier G., Gicquel P., Boutoille D., Raffi F., et al. Systematic assessment of venous thromboembolism in COVID-19 patients receiving thromboprophylaxis: incidence and role of D-dimer as predictive factors. J Thromb Thrombolysis. 2020;50:211–216. doi: 10.1007/s11239-020-02146-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Han H., Yang L., Liu R., Liu F., Wu K.L., Li J., et al. Prominent changes in blood coagulation of patients with SARS-CoV-2 infection. Clin Chem Lab Med. 2020;58:1116–1120. doi: 10.1515/cclm-2020-0188. [DOI] [PubMed] [Google Scholar]
- 77.Putko R.M., Bedrin M.D., Clark D.M., Piscoya A.S., Dunn J.C., Nesti L.J. SARS-CoV-2 and limb ischemia: a systematic review. J Clin Orthop Trauma. 2021;12:194–199. doi: 10.1016/j.jcot.2020.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Tang N., Bai H., Chen X., Gong J., Li D., Sun Z. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J Thromb Haemost. 2020;18:1094–1099. doi: 10.1111/jth.14817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Veenstra E.B., van der Laan M.J., Zeebregts C.J., de Heide E.J., Kater M., Bokkers R.P.H. A systematic review and meta-analysis of endovascular and surgical revascularization techniques in acute limb ischemia. J Vasc Surg. 2020;71:654–668.e3. doi: 10.1016/j.jvs.2019.05.031. [DOI] [PubMed] [Google Scholar]
- 80.Torrealba J.I., Osman M., Kelso R. Hypercoagulability predicts worse outcomes in young patients undergoing lower extremity revascularization. J Vasc Surg. 2019;70:175–180. doi: 10.1016/j.jvs.2018.09.062. [DOI] [PubMed] [Google Scholar]
- 81.Piffaretti G., Angrisano A., Franchin M., Ferrario M., Rivolta N., Bacuzzi A., et al. Risk factors analysis of thromboembolectomy for acute thromboembolic lower extremity ischemia in native arteries. J Cardiovasc Surg. 2018;59:810–816. doi: 10.23736/S0021-9509.16.09673-7. [DOI] [PubMed] [Google Scholar]