ABSTRACT
The transient receptor potential (TRP) channel superfamily consists of a large group of non-selective cation channels that serve as cellular sensors for a wide spectrum of physical and environmental stimuli. The 28 mammalian TRPs, categorized into six subfamilies, including TRPC (canonical), TRPV (vanilloid), TRPM (melastatin), TRPA (ankyrin), TRPML (mucolipin) and TRPP (polycystin), are widely expressed in different cells and tissues. TRPs exhibit a variety of unique features that not only distinguish them from other superfamilies of ion channels, but also confer diverse physiological functions. Located at the plasma membrane or in the membranes of intracellular organelles, TRPs are the cellular safeguards that sense various cell stresses and environmental stimuli and translate this information into responses at the organismal level. Loss- or gain-of-function mutations of TRPs cause inherited diseases and pathologies in different physiological systems, whereas up- or down-regulation of TRPs is associated with acquired human disorders. In this Cell Science at a Glance article and the accompanying poster, we briefly summarize the history of the discovery of TRPs, their unique features, recent advances in the understanding of TRP activation mechanisms, the structural basis of TRP Ca2+ selectivity and ligand binding, as well as potential roles in mammalian physiology and pathology.
KEY WORDS: TRP channels, TRPC, TRPV, TRPM, TRPA, TRPML, TRPP, Structure–function relationship, Physiological and pathological roles, Inherited and acquired disorders
Summary: TRP channels are molecular sensors for a wide spectrum of cellular stimuli and environmental cues. Here, we describe how they integrate this information into physiological and pathological responses.
Introduction
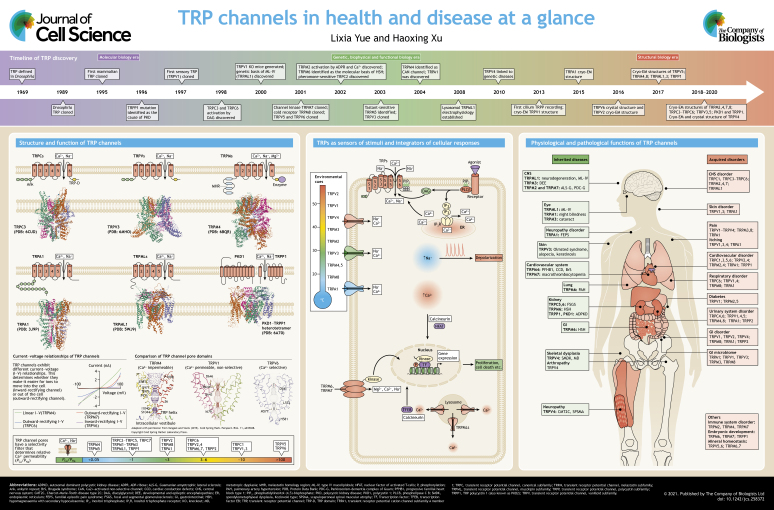
The transient receptor potential (TRP) protein was first designated in the 1960s as the underlying mediator of impaired visual adaptation in Drosophila, where photoreceptors carrying mutations of the trp gene exhibit a transient voltage response to continuous light illumination (Cosens and Manning, 1969; Minke, 1977; Montell et al., 1985). The trp gene in Drosophila was cloned in the 1980s using positional cloning (Montell and Rubin, 1989), and its function as a receptor-operated cation channel in photoreceptors was demonstrated in 1992 (Hardie and Minke, 1992). Since the 1990s, homology cloning has been used to clone TRP genes (Wes et al., 1995; Zhu et al., 1995), leading to the discovery of TRP channels in various organisms, including 17 TRP genes in Drosophila and Caenorhabditis elegans, and 28 TRP genes in mammals (Nilius and Owsianik, 2011).
Since the late 1990s, the molecular cloning of mammalian TRPs, in combination with heterologous overexpression and targeted mouse gene inactivation approaches, has brought significant advances in characterization of the unique features of TRP channels and understanding of their functions (Clapham, 2003; Nilius and Szallasi, 2014; Zhang et al., 2018) (see poster). Unlike other ion channels, the polymodal activation features of TRPs, such as activation by endogenous ligands or various environmental stimuli, confer diverse physiological and pathological roles. TRPs are non-selective cation channels with a wide spectrum of relative permeability to Ca2+ versus Na+ (PCa/PNa), ranging from 0 to 100 (see poster) (Owsianik et al., 2006). Mutagenesis studies have mapped the residues in the pore region responsible for the high selectivity for Ca2+. Further detailed analyses of Ca2+ selectivity, structure–function relations and gating mechanisms have benefitted from a recent revolutionary breakthrough in single-particle cryo-electron microscopy (cryo-EM), highlighting a new era in the study of TRP structural biology (see poster). Since the first report of the TRPV1 structure at 3.4 Å (0.34 nm) resolution using cryo-EM in 2013 (Liao et al., 2013), there has been a rapid increase in the number of TRPs whose atomic structure has been resolved at even higher resolutions (Madej and Ziegler, 2018; Vangeel and Voets, 2019; Zhang et al., 2018). These atomic-resolution structures have provided novel insights into the assembly, gating, permeation and regulatory mechanisms of TRP channels, which will undoubtedly accelerate the design and development of novel therapies for TRP-related diseases.
Here, we briefly discuss the structure–function relations of TRP channels, which include general and unique features, the structural bases of Ca2+ permeation and gating, and the sensing and decoding of environmental cues and cellular stimuli. We also summarize the physiological and pathological functions of TRP channels. Recent advances in the atomic-resolution structures of TRP channels may lay the framework for the development of therapies for various diseases associated with TRP channel dysfunction.
Unique features of TRP channels
TRP channel classification and common features
Based on sequence homology, the 28 mammalian TRP channels are classified into six subfamilies: TRPC, TRPV, TRPM, TRPA1, TRPP and TRPML (Clapham, 2003). These subfamilies can be separated into two groups based on sequence and topological differences: group I comprises the TRPC, TRPV, TRPM and TRPA1 subfamilies, all of which have a large cytosolic domain, whereas group II comprises the TRPP and TRPML subfamilies, both of which have a large exoplasmic domain (Venkatachalam and Montell, 2007).
Although TRP channels share a common overall architecture of six transmembrane (TM) domains (S1–S6) and a central pore loop linking S5 and S6, their intracellular N- and C-termini vary considerably (Montell, 2005). There are variable numbers of ankyrin repeats at the N terminus of TRPCs (which have 3 or 4 repeats), TRPVs (6 repeats) and TRPAs (14 or 15 repeats), which are implicated in protein–protein interactions, trafficking and gating, whereas the N terminus of TRPM proteins is characterized by four stretches of residues designated as the TRPM homology domain (MHD). At the C terminus, TRPCs, TRPVs and TRPMs contain a conserved TRP domain, which has been implicated in channel gating or coupling between different channel domains and is important for allosteric modulation (Vangeel and Voets, 2019). Uniquely, TRPM6 and TRPM7 have an atypical α-kinase domain at the C terminus (Nadler et al., 2001; Runnels et al., 2001), whereas TRPM2 has a NUDT9 nudix hydrolase (NUDT9-H) domain (Perraud et al., 2001), which functions as a putative ADP-ribose (ADPR) pyrophosphatase (Iordanov et al., 2019).
TRPs form homo- or hetero-tetrameric channels with subunits from the same subfamily. TRPP1 (also known as PKD2), however, forms heterotetramers with one subunit comprised of the last six TM domains (S6–S11) of the 11 TM domain-containing PKD1 (Hardy and Tsiokas, 2020) at a stoichiometry of 3:1 (PKD2:PKD1; Su et al., 2018). Different TRP channels have different current–voltage (I–V) relations, including inward-rectifying, outward-rectifying and linear I–V relations, which determine whether they make it easier for ions to move into or out of the cell (see poster). For some TRP channels, the I–V relations may vary depending on the degree of activation. The single-channel conductance of different TRP channels ranges from 10 to 100 pS (Clapham, 2003).
The architecture of TRPs (see poster) contains a pore loop for ion conduction, which is surrounded by the S1–S4 domains, and the voltage-sensing-like domain (VSD-L). The S4–S5 linker connecting the pore domain and VSD-L play an essential role in channel gating (Zhang et al., 2018). Although the VSD-L does not have a canonical voltage sensor, it is involved in the formation of binding sites for TRP agonists such as Ca2+ (in TRPM2, TRPM4 and TRPM8), phosphatidylinositol (4,5)-bisphosphate (PIP2), menthol and icilin (in TRPM8) (Huang et al., 2020). These organized domains assemble into the three or four layers of TRP architecture: a layer consisting of the extracellular domain, a layer consisting of the TM domains and one or two layers consisting of the cytosolic domain (CD) (Huang et al., 2020).
Cationic selectivity of TRP channels
As Ca2+-permeable, non-selective cation channels, TRPs exhibit a wide range of relative permeability to Ca2+ versus monovalent cations (PCa/PNa or PCa/PK, with higher numbers indicating higher Ca2+ selectivity; Owsianik et al., 2006). Two members of the TRPM subfamily, TRPM4 and TRPM5, are virtually Ca2+ impermeable (PCa/PNa<0.01), whereas TRPV5 and TRPV6 are highly Ca2+ selective (PCa/PNa>100). TRPs are permeable not only to abundant physiological cations, but also to trace metal cations such as Zn2+ and Fe2+ (Bouron et al., 2015).
The selectivity filter of TRPs is located at the upper part of the ion-conducting pore (see poster; the representative pore structures of TRPM4, TRPV1 and TRPV6 are adapted with permission from Vangeel and Voets, 2019, copyright Cold Spring Harbor Laboratory Press). Early mutagenesis studies have established that the negatively-charged aspartate and glutamate residues in the pore loop are critical for divalent cation permeation and selectivity (Owsianik et al., 2006). Recent structural analyses have provided the atomic mechanisms for Ca2+ selectivity. The selective filter domain contains the amino acid sequence TIGMG[D/E] in TRPV1–TRPV4 (Deng et al., 2018), FGE in TRPM7 (Duan et al., 2018b), FGQ in TRPM4 (Duan et al., 2018c), LGD in TRPPs (Shen et al., 2016), GLIN in TRPC4 and TRPC5 (Duan et al., 2018a, 2019), FGN in TRPM2 (Iordanov et al., 2019), LGD in TRPA1 (Suo et al., 2020), NGDD in TRPMLs (Chen et al., 2017; Fine et al., 2018; Hirschi et al., 2017) and TIID in TRPV6 (Saotome et al., 2016). Like TRPV5 and TRPV6 (Dang et al., 2019; Hughes et al., 2018; Singh et al., 2018), most non-selective TRPs have negatively-charged amino acid residues in the selective filter domain. However, the key difference between TRPV5 and TRPV6 and the other TRPs is that the side chain of the negatively charged aspartate residue of TRPV5 and TRPV6 is located at the narrowest part of the pore, leading to an extremely strong binding for Ca2+ and a small interatomic distance at the outer channel pore to form a narrow constriction (Saotome et al., 2016). In contrast, the negatively charged residues of other TRPs are not located at the narrowest part, creating a much weaker binding site for Ca2+, and hence low values of PCa/PNa (Huynh et al., 2016; Liao et al., 2013; Paulsen et al., 2015; Zubcevic et al., 2016).
Activation and regulation of TRP channels
Localized at the plasma membrane or intracellular organelles (Zhang et al., 2018), TRPs are activated by a wide variety of extra- and intra-cellular stimuli (Box 1) (Clapham, 2003). Several TRPs are sensitive to thermal transitions. For example, TRPV1–TRPV4, TRPM2 and TRPM3 are sensitive to warmth or noxious heat, whereas TRPM8, TRPA1 and TRPC5 are activated by cooling or by noxious cold temperatures (Bamps et al., 2020; Buijs and McNaughton, 2020; Dhaka et al., 2006; Tan and McNaughton, 2018). The thermal sensing mechanisms of TRPs are yet to be fully understood (Baez et al., 2014; Singh et al., 2019). Activation of TRPC channels is mediated by the phospholipase C (PLC) pathway, either directly through diacylglycerol (DAG) for TRPC2, TRPC3, TRPC6 and TRPC7, or indirectly through cooperative PLCδ activity and Gi/o stimulation for TRPC4 (Hofmann et al., 1999; Thakur et al., 2016; Venkatachalam et al., 2003). Activation of TRPV5 or TRPV6 requires PIP2 binding to a pocket formed by the N-linker, which is located between the pre-S1 and ankyrin repeat domains and features a helix-turn-helix motif (Pumroy et al., 2020), the S4–S5 linker and the S6 helix (Hughes et al., 2018; Singh et al., 2018). Moreover, calmodulin (CaM) is associated with the C terminus of TRPV5 and TRPV6, blocking the channel gate at high intracellular Ca2+ concentrations ([Ca2+]i) (Hughes et al., 2018; Pumroy et al., 2020; Singh et al., 2018). In epithelial cells, TRPV6 is constitutively active in vivo (Xin et al., 2019).
Box 1. Activation and modulation of TRP channels by endogenous and exogenous cues.
TRPs can be activated by a plethora of internal and external stimuli. As cellular sensors in living organisms, TRPs can be directly activated by environmental cues, such as thermal or chemical stimuli, or by natural or synthetic agonists, including capsaicin (TRPV1), menthol (TRPM8), carvacrol (TRPV3), englerin A (TRPC4 and TRPC5), 2-aminoethoxydiphenyl borate (2-APB; TRPV2), 4α-phorbol 12,13-didecanoate (4α-PDD; TRPV4), allyl isothiocyanate (AITC; TRPA1), ML-SA1 (TRPML1) and calmidazolium (TRPP2) (Zhang et al., 2018). TRPs can also be activated via receptor stimulation by endogenous and exogenous agonists, which include environmental cues such as tastants (TRPM5), pheromones (TRPC2) and light (TRPM1), as well as endocrine and paracrine cues, such as local cytokines, neurotransmitters and hormones. Downstream of receptor stimulation, a rise in [Ca2+]i and a change in the PIP2 level are two common signal transduction mechanisms that regulate many TRPs (see poster). Chemical agonists can bind directly to TRPs to activate or cooperate with PIP2 to gate the channels. For example, the binding pocket for menthol in TRPM8 is located in the VSD-L cavity (Yin and Lee, 2020). The vanilloid binding pocket in TRPV1 is located above the S4–S5 linker and is embraced by the S3 and S4 helices in the VSD-L from the same subunit and the S6 helix from the neighboring subunit (Gao et al., 2016). The same binding pocket also accommodates PIP2, providing a competing mechanism between PIP2 and vanilloid compounds for TRPV1 (Gao et al., 2016). Different from other TRPs, TRPCs have a binding pocket for GFB inhibitors located in the pocket surround by S1–S4 (Bai et al., 2020; Vinayagam et al., 2020). TRPs are also modulated by various intracellular and extracellular stimuli (Sakaguchi and Mori, 2020). Oxidants are common regulators that activate TRPM2, TRPA1 (Andersson et al., 2008), TRPC5 and TRPML1, and potentiate TRPV1, TRPC3 and TRPC4 (Yoshida et al., 2006). TRPs expressed in endothelial cells, including TRPC5, TRPC1, TRPC4, TRPV1, TRPV3 and TRPV4, are nitric oxide sensors (Yoshida et al., 2006). Mechanical stretch is another common regulator of TRPs. Unlike the mechanically activated Drosophila TRPN channel (also known as NOMPC), mammalian TRPs, including TRPC1, TRPC3, TRPC5, TRPC6, TRPV2, TRPV4, TRPM3, TRPM4, TRPM7, TRPA1, TRPML2 and TRPP1, are regulated but not activated by mechanical stretch (Chen et al., 2020; Christensen and Corey, 2007; Nikolaev et al., 2019). Moreover, sphingolipids and non-PIP2 phospholipids may also regulate TRPs (Ciardo and Ferrer-Montiel, 2017). For instance, sphingosine activates TRPM3 (Grimm et al., 2005) but inhibits TRPM7 (Qin et al., 2013).
Whereas a rise in [Ca2+]i inactivates TRPV5 and TRPV6, it activates TRPM4, TRPM5, TRPM2 and TRPA1. A Ca2+-binding site coordinated by the amino acid sequence EQND, first described as being close to the S3 helix in TRPM4 (Autzen et al., 2018), is also present in TRPM2 and TRPM8 (Huang et al., 2018, 2019; Yin et al., 2019a). Moreover, an ATP-binding site is present at the interface between the MHR1/2 domain and the adjacent MHR3 domain in TRPM4, but not in TRPM5 (Guo et al., 2017), resulting in ATP-mediated inhibition of Ca2+-induced currents only in TRPM4.
Activation of TRPM2 requires ADPR binding to the C-terminal NUDT9-H domain (Csanady and Torocsik, 2009; Kuhn and Luckhoff, 2004). Interestingly, recent cryo-EM structures of TRPM2 have revealed an additional ADPR-binding site, which is located in the MHR1/2 domain (Huang et al., 2018; Wang et al., 2018). Binding of ADPR causes a series of conformational changes preceding channel opening of TRPM2 (Huang et al., 2018; Yin et al., 2019b).
TRPM6 and TRPM7 activation requires PIP2 and the lowering of internal Mg2+ concentration (Nadler et al., 2001; Runnels et al., 2002; Voets et al., 2004; Xie et al., 2011). The function of these channels is regulated by intracellular concentrations of ATP and other nucleotides (Demeuse et al., 2006; Ferioli et al., 2017; Zhang et al., 2014). PIP2 is also crucial for TRPM8 activation, through binding to an interfacial cavity that is formed by the S4–S5 linker and the top layer of CD, assembled by the pre-S1 domain, the S4–S5 junction, the TRP domain and the MH4 domain from the neighboring subunit (Yin et al., 2019a; Yin and Lee, 2020). Binding of PIP2 allosterically enhances the effects of other cooling agonists, such as menthol, producing synergistic activation of TRPM8.
For group II TRPs, PIP2 is required for TRPML activation (Shen et al., 2012; Zhang et al., 2012), but negatively regulates TRPP2 and TRPP3 (Zheng et al., 2018). The cryo-EM structures of TRPML1 (also known as MCOLN1) reveal that binding of PI(3,5)P2 to the extended helices of S1, S2 and S3 causes the S4–S5 linker to move, thereby allosterically opening the channel (Chen et al., 2017; Fine et al., 2018; Schmiege et al., 2017).
Physiological and pathological roles of TRP channels
TRPs act as multifunctional cellular sensors (Clapham, 2003; Zhou et al., 2020) by integrating a plethora of external and internal signals and converting them to physiological and pathological responses (Wu et al., 2010a). Dysfunction of TRP channels causes various inherited and acquired diseases (see poster; Box 2).
Box 2. TRP channels integrate information from internal and external stimuli to mediate Ca2+-dependent and Ca2+-independent physiological and pathological responses.
TRP channels are widely expressed in various cells and tissues. Located at the plasma membrane or in intracellular organelles, TRPs exhibit the most diversified architectures, activation mechanisms and physiological and pathological functions. As thermal and chemical sensors in living organisms, TRPs turn a range of external stimuli into various physiological and pathological responses. By conducting Ca2+ and other cations, such as Na+, TRP channel activation converts environmental stimuli into action potentials and elevates [Ca2+]i , which triggers various signaling pathways to produce cellular responses, for example Ca2+-dependent regulation of gene expression (see poster). For instance, TRPC and Ca2+-mediated activation of calcineurin causes nuclear translocation of the transcription factor nuclear factor of activated T-cells (NFAT). Aberrant regulation of the TRPC–calcineurin–NFAT pathway has been shown to be a common pathological mechanism underlying hypertrophy and heart failure in mouse models (Eder and Molkentin, 2011; Wu et al., 2010b). Likewise, TRPML and Ca2+-dependent activation of calcineurin activates the transcription factor TFEB to promote autophagy (Medina et al., 2015).
In addition to a Ca2+-initiated signal to the nucleus, the kinase domains of TRPM6 and TRPM7 regulate gene expression via translocation to the nucleus after cleavage (Krapivinsky et al., 2014; Krapivinsky et al., 2017) (see poster). The cleaved kinase domain of TRPM6, upon nuclear translocation, binds to subunits of arginine methyltransferase 5 (PRMT5) and phosphorylates specific histone serine and threonine residues, resulting in reduced methylation of adjacent arginine residues and changes in the transcription of many target genes (Krapivinsky et al., 2017). In contrast, the cleaved kinase domain of TRPM7, after nuclear translocation, binds to multiple components of chromatin-remodeling complexes, such as polycomb group proteins. The TRPM7 kinase then phosphorylates the S10 residue of histone H3 at the promotors of many target genes to induce their expression (Krapivinsky et al., 2014).
TRP channels and inherited diseases
Several TRPs, including TRPC6, TRPV4, TRPV3, TRPM1, TRPM3, TRPM4, TRPM6, TRPM2, TRPM7, TRPA1, TRPML1 and TRPP1, have been linked to genetic diseases (Nilius and Owsianik, 2010). Numerous single-nucleotide polymorphisms (SNPs) have also been identified in TRP channels, although their roles in causing disease are not well established.
TRPC6
Mutations in TRPC6 are linked to a human proteinuric kidney disease called focal and segmental glomerulosclerosis (FSGS) (Reiser et al., 2005; Riehle et al., 2016; Winn et al., 2005). Both gain-of-function (GOF) and loss-of-function (LOF) mutations of TRPC6 may contribute to FSGS (Hofstra et al., 2013; Nilius and Owsianik, 2010; Riehle et al., 2016). Although it is still unclear how TRPC6 contributes to the development of FSGS, defects in the permeability barrier function in the glomeruli of FSGS patients may cause proteinuria and progressive kidney failure. Moreover, a functional SNP that causes upregulation of TRPC6 has been associated with idiopathic pulmonary artery hypertension (PAH) (Yu et al., 2009).
TRPV4
Mutations in TRPV4 have been identified in patients with various neurodegenerative disorders, such as Charcot–Marie–Tooth disease type 2C (CMT2C), scapuloperoneal spinal muscular atrophy (SPSMA), distal spinal motor neuropathy, distal spinal muscular atrophy (SMA) (Auer-Grumbach et al., 2010; Deng et al., 2010; Fiorillo et al., 2012; Lamande et al., 2011; Landoure et al., 2010), and severe intellectual disability and neuropathy (Thibodeau et al., 2017). Mutations in TRPV4 also cause other disorders, including various skeletal dysplasias, ranging from mild autosomal dominant brachyolmia to severe metatropic dysplasia (MD) (Nilius and Voets, 2013; Toft-Bertelsen and MacAulay, 2021), arthropathy (Lamande et al., 2011; McNulty et al., 2015), arthrogryposis (Velilla et al., 2019) and progressive osteoarthropathy (Lamande et al., 2011; Nishimura et al., 2012). The majority of these disease-causing TRPM4 mutations are GOF mutations (Nilius and Voets, 2013), whereas some are LOF mutations (Lamande et al., 2011). Moreover, there are genotype–phenotype overlaps, as some mutations, such as A217S, E278K, V620I and P799R, are involved in both skeletal and neurological disorders (Toft-Bertelsen and MacAulay, 2021).
TRPV3
GOF mutations of TRPV3 cause Olmsted syndrome (OS), a rare keratinization disorder (Duchatelet et al., 2014; Eytan et al., 2014; He et al., 2015; Lin et al., 2012; Ni et al., 2016). Moreover, patients carrying G573A mutations in TRPV3 have multiple immune dysfunctions (Danso-Abeam et al., 2013). TRPV3-associated OS can be effectively treated by the epidermal growth factor receptor (EGFR) inhibitor erlotinib (Greco et al., 2020), due to cross-activation of TRPV3 and EGFR (Cheng et al., 2010).
TRPM1
Mutations of TRPM1 cause congenital stationary night blindness (CSNB) (AlTalbishi et al., 2019; Lee et al., 2020; Nilius and Owsianik, 2010; Zhou et al., 2016) due to a loss of function of the ON bipolar neurons that are postsynaptic to the rod and cone photoreceptors, as evident from electroretinography (ERG). Consistent with this, a loss of ON bipolar cell response as characterized by ERG is also observed in Trpm1-knockout mice (Koike et al., 2010; Morgans et al., 2009).
TRPM3
GOF mutations of TRPM3 have recently been identified as the cause of developmental and epileptic encephalopathies (DEE) (Dyment et al., 2019; Van Hoeymissen et al., 2020). In HEK293T cells heterologously overexpressing TRPM3, DEE-causing mutations, such as V990M and P1090Q, result in increased basal activity, enhanced sensitivity to pregnenolone sulfate and heat, and reduced sensitivity to inhibition and inactivation. Moreover, mutations of TRPM3 (I65M and I8M) have been linked with inherited forms of early-onset cataracts, and knockin mice expressing the I65M mutant form of human TRPM3 recapitulate human cataract development (Bennett et al., 2014; Zhou et al., 2021).
TRPM4
Since the first TRPM4 mutation (E7K) was identified in patients with progressive familial heart block type 1 (PFHB1) (Kruse et al., 2009), many GOF or LOF mutations have been found in patients with various forms of cardiac conduction defects (CCD) (Liu et al., 2010; Stallmeyer et al., 2012) and in patients with Brugada syndrome (BrS) (Liu et al., 2013). Moreover, TRPM4 mutations have been found in childhood cases of CCD and arrhythmia (Bianchi et al., 2018; Syam et al., 2016), as well as in patients with hypoplastic right heart (Reuter et al., 2020).
TRPM6
LOF mutations of TRPM6 cause familial hypomagnesaemia with secondary hypocalcaemia (HSH) (Schlingmann et al., 2007). Both intestinal absorption and renal re-absorption of Mg2+ are defective in HSH patients (Chubanov et al., 2007; Nilius and Owsianik, 2010).
TRPM2 and TRPM7
SNPs of TRPM2 and TRPM7 have been identified in a subset patients with Guamanian amyotrophic lateral sclerosis (ALS-G) and Parkinsonism-dementia complex of Guam (PDC-G) (Hermosura et al., 2005, 2008; Hermosura and Garruto, 2007), although the causality remains to be established (Hara et al., 2010). LOF mutations in TRPM7 may be associated with inherited macrothrombocytopenia (Stritt et al., 2016).
TRPA1
A GOF mutation of TRPA1, N855S, is linked to familial episodic pain syndrome (FEPS) (Kremeyer et al., 2010; Nilius and Szallasi, 2014). Moreover, some SNPs of TRPA1 may be associated with defects in heat or cold sensation (Binder et al., 2011; Kim et al., 2006; Naert et al., 2020).
TRPML1
LOF mutations in TRPML1 result in an autosomal recessive neurodegenerative lysosomal disorder, type IV mucolipidosis (ML-IV) (Wang et al., 2014).
TRPP1
Mutations in TRPP1 (PKD2) and PKD1 account for 15% and 85% of autosomal dominant polycystic kidney disease (ADPKD), respectively (Audrezet et al., 2012, 2016). ADPKD is characterized by the progressive development of large epithelium-lined cysts in the kidney, liver, pancreas, seminal tract and arachnoid membrane (Nilius and Szallasi, 2014). ADPKD also causes cardiovascular abnormalities, such as small artery aneurysms in the heart and brain (Dietrich et al., 2006) and defective septum formation of the heart, which is caused by TRPP1 mutations (Wu et al., 2000).
TRP channels in acquired diseases
TRP channels are ubiquitously expressed and are involved in diverse physiological and pathological functions (see poster). Extensive research using genetically modified animal models and pharmacological agents has provided strong evidence to substantiate TRPs as therapeutic targets for various diseases.
TRPs in central nervous system disorders
Several TRPs are abundantly expressed in the brain. Knockout of Trpc3 in mice causes a deficit in metabotropic glutamate receptor-dependent synaptic transmission (Hartmann et al., 2008), suggesting an important role of TRPC3 in this process. Loss of TRPC1, TRPC4 and TRPC5 has also been shown to cause synaptic depression (Schwarz et al., 2019). Activation of TRPC1, TRPC4 and TRPC5 produces detrimental effects in ischemic stroke (Jeon et al., 2020), whereas activation of TRPC6 produces beneficial effects in ischemic stroke (Shekhar et al., 2021). Deletion of Trpc5 or Trpc4 results in reduced innate fear in mice (Riccio et al., 2009, 2014). Moreover, overactivation of TRPM2 and TRPM7 is involved in ischemic stroke (Alim et al., 2013; Ye et al., 2014), and these proteins are considered therapeutic targets for ischemic brain injury (Belrose and Jackson, 2018; Lin and Xiong, 2017).
Sensory TRPs in pain and itching
Several thermosensitive TRPs, including TRPV1, TRPM2, TRPM3, TRPM8 and TRPA1, are expressed in nociceptive sensory neurons (Julius, 2013; Voets et al., 2019). Knockout of Trpv1 impairs but does not abolish thermal hyperalgesia (Caterina et al., 2000; Davis et al., 2000), whereas knockout of Trpm3 (Alkhatib et al., 2019; Su et al., 2021; Vriens et al., 2011) or triple knockout of Trpv1, Trpm3 and Trpa1 can fully eliminate acute noxious heat sensing in mice (Vandewauw et al., 2018). TRPV1, TRPV3, TRPA1 and TRPM8 have been pursued as analgesic targets to treat cluster headaches, ocular pain, neuropathic pain, diabetic peripheral neuropathic pain, osteoarthritis, overactive bladder disorder, inflammatory pain and cancer pain (Kaneko and Szallasi, 2014). Moreover, TRP channels such as TRPA1, TRPV1, TRPV3 and TRPV4, which are expressed in the nociceptive neurons and skin tissue-resident cells, are involved in itching (Feng et al., 2017; Kittaka and Tominaga, 2017; Wilson et al., 2011; Xie and Hu, 2018).
TRP in cardiovascular diseases
Multiple TRPs are associated with cardiovascular diseases (Hof et al., 2019). TRPC1, TRPC3, TRPC4, TRPC6, TRPC7 and TRPM4 are involved in cardiac hypertrophy and heart failure in mouse models (Freichel et al., 2017). Whereas TRPV2 is essential for maintaining cardiac structure (Katanosaka et al., 2014), the role of TRPM2 in ischemic heart injury has been controversial (Hiroi et al., 2013; Miller et al., 2013). Both TRPM7 and TRPP1 are required for embryonic heart development (Sah et al., 2013; Wu et al., 2000). Several TRPs, including TRPM4, TRPC3, TRPC6 and TRPM7, have been implicated in cardiac arrhythmia. Moreover, TRPs that are expressed in endothelial and smooth muscle cells, such as TRPV1, TRPV4, TRPC6, TRPM4 and TRPP1, contribute to vascular pathologies, including pulmonary hypertension, subarachnoid hemorrhage and aneurism (Yue et al., 2015).
TRPs in respiratory disorders
Because of its stimulation by environmental irritants and inflammatory stimuli, TRPA1 plays a role in chronic asthma and chronic obstructive pulmonary disease (COPD), and TRPV1 also plays a role in asthma as well as in chronic cough (Belvisi and Birrell, 2017). TRPM8 may exacerbate COPD and asthma (Kaneko and Szallasi, 2014), whereas TRPV4 and TRPC6 are associated with lung edema (Weber et al., 2020; Weissmann et al., 2012).
TRPs in the urinary system
TRPC1, TRPC3, TRPC5 and TRPC6 are reportedly involved in kidney disorders (Chubanov et al., 2017), including diabetic nephropathy and kidney fibrosis (Saliba et al., 2015). Whereas TRPC5 and TRPC6 may play roles in acquired and inherited FSGS, TRPC5 has been proposed to be a target for acquired FSGS (Pablo and Greka, 2019; Zhou et al., 2017). Moreover, TRPV1, TRPV2, TRPV4, TRPM4, TRPM8 and TRPA1 are implicated in lower urinary tract disorders, such as urinary incontinence and bladder-associated pain (Kaneko and Szallasi, 2014; Voets et al., 2019).
TRPs in diabetes
TRPM2, TRPM4, TRPM5 and TRPA1 have been implicated in insulin release from pancreatic β-cells (Colsoul et al., 2013). Deletion of Trpm2 or Trpm5 causes impaired glucose tolerance in mice (Kaneko and Szallasi, 2014), whereas activation of TRPM4 and TRPM5 promotes glucagon-like peptide 1-induced insulin release (Shigeto et al., 2015). Moreover, both TRPV1 and TRPA1 may be indirectly involved in diabetes (Derbenev and Zsombok, 2016).
TRPs in gastrointestinal disorders
TRPML1 in parietal cells mediates gastric secretion (Sahoo et al., 2017), and ML-IV patients exhibit constitutive achlorhydria. TRPV1, TRPV4 and TRPA1 are implicated in irritable bowel syndrome, visceral hypersensitivity (VH) and inflammatory bowel disease (IBD). Whereas TRPM8 also plays a role in IBD and VH, TRPC6 and TRPA1 are associated with intestinal fibrosis (Alaimo and Rubert, 2019).
TRPs and the gut microbiome
There is emerging evidence indicating a role of the bacterial endotoxin-sensitive TRP channels, such as TRPA1, TRPV4, TRPV1, TRPM3 and TRPM8, in gut–brain cross talk in several entero-neuronal pathologies (Boonen et al., 2018; Meseguer et al., 2014; Startek and Talavera, 2020; Ye et al., 2021). Moreover, knockout of Trpv1, Trpa1 or both in mice has been shown to influence the gut microbiome and its metabolic pathways (Nagpal et al., 2020).
TRPs in skin disorders
Activation of TRPV3, TRPV4, TRPC6, TRPM8 and TRPA1, and inhibition of TRPV1, contribute to skin barrier formation and wound closure. TRPV1, TRPV3, TRPA1, TRPC1, TRPC4 and TRPM8 are involved in skin inflammation. Moreover, activation of TRPA1, TRPM1 and TRPM7 contributes to hair growth and pigmentation, whereas TRPV1 and TRPV3 are negative regulators of these processes (Caterina and Pang, 2016; Yang et al., 2017).
TRPs in other physiological systems
TRPs are also involved in pathophysiological functions in other systems. For example, TRPM6 and TRPM7 are critical for maintaining Mg2+ homeostasis, and TRPV5 and TRPV6 are important in maintaining Ca2+ homeostasis (Chubanov et al., 2018; Mittermeier et al., 2019; van Abel et al., 2005). A number of TRPs contribute to immunity and the inflammatory response (Spix et al., 2020). Moreover, several TRPs have been proposed as potential targets of anti-cancer therapy (Stewart, 2020; Yang and Kim, 2020).
Concluding remarks and future perspectives
TRP channels play important physiological and pathological roles in various systems, and have been proposed as therapeutic targets for cardiovascular, neurological, renal and metabolic diseases. Although Qutenza (a capsaicin patch) is currently the only FDA-approved drug that targets TRPs, there are multiple ongoing clinical trials assessing other TRP-targeting compounds. The recent breakthroughs in solving atomic-resolution structures of TRP channels in the agonist-bound state will undoubtedly accelerate drug development targeting TRPs for various diseases.
Poster
Poster Panels
Acknowledgements
We apologize to researchers whose work is not cited owing to space limitations.
Footnotes
Competing interests
The authors declare no competing or financial interests.
Funding
Our work in this area is partially supported by the National Institutes of Health (HL143750 to L.Y.) and the American Heart Association (19TPA34890022 to L.Y.). Deposited in PMC for release after 12 months.
Cell science at a glance
Individual poster panels are available for downloading at https://journals.biologists.com/jcs/article-lookup/doi/10.1242/jcs.258372#supplementary-data
References
- Alaimo, A. and Rubert, J. (2019). The Pivotal Role of TRP Channels in Homeostasis and Diseases throughout the Gastrointestinal Tract. Int. J. Mol. Sci. 20, 5277. 10.3390/ijms20215277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alim, I., Teves, L., Li, R., Mori, Y. and Tymianski, M. (2013). Modulation of NMDAR subunit expression by TRPM2 channels regulates neuronal vulnerability to ischemic cell death. J. Neurosci. 33, 17264-17277. 10.1523/JNEUROSCI.1729-13.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alkhatib, O., da Costa, R., Gentry, C., Quallo, T., Bevan, S. and Andersson, D. A. (2019). Promiscuous G-Protein-Coupled Receptor Inhibition of Transient Receptor Potential Melastatin 3 Ion Channels by Gbetagamma Subunits. J. Neurosci. 39, 7840-7852. 10.1523/JNEUROSCI.0882-19.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- AlTalbishi, A., Zelinger, L., Zeitz, C., Hendler, K., Namburi, P., Audo, I., Sheffer, R., Yahalom, C., Khateb, S., Banin, E.et al. (2019). TRPM1 Mutations are the Most Common Cause of Autosomal Recessive Congenital Stationary Night Blindness (CSNB) in the Palestinian and Israeli Populations. Sci. Rep. 9, 12047. 10.1038/s41598-019-46811-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson, D. A., Gentry, C., Moss, S. and Bevan, S. (2008). Transient receptor potential A1 is a sensory receptor for multiple products of oxidative stress. J. Neurosci. 28, 2485-2494. 10.1523/JNEUROSCI.5369-07.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Audrezet, M. P., Cornec-Le Gall, E., Chen, J. M., Redon, S., Quere, I., Creff, J., Benech, C., Maestri, S., Le Meur, Y. and Ferec, C. (2012). Autosomal dominant polycystic kidney disease: comprehensive mutation analysis of PKD1 and PKD2 in 700 unrelated patients. Hum. Mutat. 33, 1239-1250. 10.1002/humu.22103 [DOI] [PubMed] [Google Scholar]
- Audrezet, M. P., Corbiere, C., Lebbah, S., Moriniere, V., Broux, F., Louillet, F., Fischbach, M., Zaloszyc, A., Cloarec, S., Merieau, E.et al. (2016). Comprehensive PKD1 and PKD2 Mutation Analysis in Prenatal Autosomal Dominant Polycystic Kidney Disease. J. Am. Soc. Nephrol. 27, 722-729. 10.1681/ASN.2014101051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auer-Grumbach, M., Olschewski, A., Papic, L., Kremer, H., McEntagart, M. E., Uhrig, S., Fischer, C., Frohlich, E., Balint, Z., Tang, B.et al. (2010). Alterations in the ankyrin domain of TRPV4 cause congenital distal SMA, scapuloperoneal SMA and HMSN2C. Nat. Genet. 42, 160-164. 10.1038/ng.508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Autzen, H. E., Myasnikov, A. G., Campbell, M. G., Asarnow, D., Julius, D. and Cheng, Y. (2018). Structure of the human TRPM4 ion channel in a lipid nanodisc. Science 359, 228-232. 10.1126/science.aar4510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baez, D., Raddatz, N., Ferreira, G., Gonzalez, C. and Latorre, R. (2014). Gating of thermally activated channels. Curr. Top. Membr. 74, 51-87. 10.1016/B978-0-12-800181-3.00003-8 [DOI] [PubMed] [Google Scholar]
- Bai, Y., Yu, X., Chen, H., Horne, D., White, R., Wu, X., Lee, P., Gu, Y., Ghimire-Rijal, S., Lin, D. C.et al. (2020). Structural basis for pharmacological modulation of the TRPC6 channel. eLife 9, e53311. 10.7554/eLife.53311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bamps, D., Vriens, J., Hoon, J. and Voets, T. (2020). TRP Channel Cooperation for Nociception: Therapeutic Opportunities. Annu. Rev. Pharmacol. Toxicol., 61, 655.-. 10.1146/annurev-pharmtox-010919-023238 [DOI] [PubMed] [Google Scholar]
- Belrose, J. C. and Jackson, M. F. (2018). TRPM2: a candidate therapeutic target for treating neurological diseases. Acta Pharmacol. Sin. 39, 722-732. 10.1038/aps.2018.31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belvisi, M. G. and Birrell, M. A. (2017). The emerging role of transient receptor potential channels in chronic lung disease. Eur. Respir. J. 50, 1601357. 10.1183/13993003.01357-2016 [DOI] [PubMed] [Google Scholar]
- Bennett, T. M., Mackay, D. S., Siegfried, C. J. and Shiels, A. (2014). Mutation of the melastatin-related cation channel, TRPM3, underlies inherited cataract and glaucoma. PLoS One 9, e104000. 10.1371/journal.pone.0104000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianchi, B., Ozhathil, L. C., Medeiros-Domingo, A., Gollob, M. H. and Abriel, H. (2018). Four TRPM4 Cation Channel Mutations Found in Cardiac Conduction Diseases Lead to Altered Protein Stability. Front Physiol 9, 177. 10.3389/fphys.2018.00177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder, A., May, D., Baron, R., Maier, C., Tölle, T. R., Treede, R. D., Berthele, A., Faltraco, F., Flor, H., Gierthmuhlen, J.et al. (2011). Transient receptor potential channel polymorphisms are associated with the somatosensory function in neuropathic pain patients. PLoS One 6, e17387. 10.1371/journal.pone.0017387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boonen, B., Alpizar, Y. A., Meseguer, V. M. and Talavera, K. (2018). TRP Channels as Sensors of Bacterial Endotoxins. Toxins (Basel) 10, 326. 10.3390/toxins10080326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouron, A., Kiselyov, K. and Oberwinkler, J. (2015). Permeation, regulation and control of expression of TRP channels by trace metal ions. Pflugers Arch. 467, 1143-1164. 10.1007/s00424-014-1590-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buijs, T. J. and McNaughton, P. A. (2020). The Role of Cold-Sensitive Ion Channels in Peripheral Thermosensation. Front Cell Neurosci 14, 262. 10.3389/fncel.2020.00262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caterina, M. J. and Pang, Z. (2016). TRP Channels in Skin Biology and Pathophysiology. Pharmaceuticals (Basel) 9, 77. 10.3390/ph9040077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caterina, M. J., Leffler, A., Malmberg, A. B., Martin, W. J., Trafton, J., Petersen-Zeitz, K. R., Koltzenburg, M., Basbaum, A. I. and Julius, D. (2000). Impaired nociception and pain sensation in mice lacking the capsaicin receptor. Science 288, 306-313. 10.1126/science.288.5464.306 [DOI] [PubMed] [Google Scholar]
- Chen, Q., She, J., Zeng, W., Guo, J., Xu, H., Bai, X. C. and Jiang, Y. (2017). Structure of mammalian endolysosomal TRPML1 channel in nanodiscs. Nature 550, 415-418. 10.1038/nature24035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, C. C., Krogsaeter, E., Butz, E. S., Li, Y., Puertollano, R., Wahl-Schott, C., Biel, M. and Grimm, C. (2020). TRPML2 is an osmo/mechanosensitive cation channel in endolysosomal organelles. Sci. Adv. 6, eabb5064. 10.1126/sciadv.abb5064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng, X., Jin, J., Hu, L., Shen, D., Dong, X. P., Samie, M. A., Knoff, J., Eisinger, B., Liu, M. L., Huang, S. M.et al. (2010). TRP channel regulates EGFR signaling in hair morphogenesis and skin barrier formation. Cell 141, 331-343. 10.1016/j.cell.2010.03.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen, A. P. and Corey, D. P. (2007). TRP channels in mechanosensation: direct or indirect activation?. Nat. Rev. Neurosci. 8, 510-521. 10.1038/nrn2149 [DOI] [PubMed] [Google Scholar]
- Chubanov, V., Schlingmann, K. P., Waring, J., Heinzinger, J., Kaske, S., Waldegger, S., Schnitzler, M. M. Y. and Gudermann, T. (2007). Hypomagnesemia with Secondary Hypocalcemia due to a Missense Mutation in the Putative Pore-forming Region of TRPM6. J. Biol. Chem. 282, 7656-7667. 10.1074/jbc.m611117200 [DOI] [PubMed] [Google Scholar]
- Chubanov, V., Kubanek, S., Fiedler, S., Mittermeier, L., Gudermann, T. and Dietrich, A. (2017). Renal Functions of TRP Channels in Health and Disease. In Neurobiology of TRP Channels (eds Emir T. L. R.), pp. 187-212. Boca Raton, FL: CRC Press/Taylor & Francis. [PubMed] [Google Scholar]
- Chubanov, V., Mittermeier, L. and Gudermann, T. (2018). Role of kinase-coupled TRP channels in mineral homeostasis. Pharmacol. Ther. 184, 159-176. 10.1016/j.pharmthera.2017.11.003 [DOI] [PubMed] [Google Scholar]
- Ciardo, M. G. and Ferrer-Montiel, A. (2017). Lipids as central modulators of sensory TRP channels. Biochim Biophys Acta Biomembr 1859, 1615-1628. 10.1016/j.bbamem.2017.04.012 [DOI] [PubMed] [Google Scholar]
- Clapham, D. E. (2003). TRP channels as cellular sensors. Nature 426, 517-524. 10.1038/nature02196 [DOI] [PubMed] [Google Scholar]
- Colsoul, B., Nilius, B. and Vennekens, R. (2013). Transient receptor potential (TRP) cation channels in diabetes. Curr. Top. Med. Chem. 13, 258-269. 10.2174/1568026611313030004 [DOI] [PubMed] [Google Scholar]
- Cosens, D. J. and Manning, A. (1969). Abnormal electroretinogram from a Drosophila mutant. Nature 224, 285-287. 10.1038/224285a0 [DOI] [PubMed] [Google Scholar]
- Csanady, L. and Torocsik, B. (2009). Four Ca2+ Ions Activate TRPM2 Channels by Binding in Deep Crevices near the Pore but Intracellularly of the Gate. J. Gen. Physiol. 133, 189-203. 10.1085/jgp.200810109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dang, S., van Goor, M. K., Asarnow, D., Wang, Y., Julius, D., Cheng, Y. and van der Wijst, J. (2019). Structural insight into TRPV5 channel function and modulation. Proc. Natl. Acad. Sci. U.S.A. 116, 8869-8878. 10.1073/pnas.1820323116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danso-Abeam, D., Zhang, J., Dooley, J., Staats, K. A., Van Eyck, L., Van Brussel, T., Zaman, S., Hauben, E., Van de Velde, M., Morren, M. A.et al. (2013). Olmsted syndrome: exploration of the immunological phenotype. Orphanet J. Rare Dis. 8, 79. 10.1186/1750-1172-8-79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis, J. B., Gray, J., Gunthorpe, M. J., Hatcher, J. P., Davey, P. T., Overend, P., Harries, M. H., Latcham, J., Clapham, C., Atkinson, K.et al. (2000). Vanilloid receptor-1 is essential for inflammatory thermal hyperalgesia. Nature 405, 183-187. 10.1038/35012076 [DOI] [PubMed] [Google Scholar]
- Demeuse, P., Penner, R. and Fleig, A. (2006). TRPM7 Channel Is Regulated by Magnesium Nucleotides via its Kinase Domain. J. Gen. Physiol. 127, 421-434. 10.1085/jgp.200509410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng, H. X., Klein, C. J., Yan, J., Shi, Y., Wu, Y., Fecto, F., Yau, H. J., Yang, Y., Zhai, H., Siddique, N.et al. (2010). Scapuloperoneal spinal muscular atrophy and CMT2C are allelic disorders caused by alterations in TRPV4. Nat. Genet. 42, 165-169. 10.1038/ng.509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng, Z., Paknejad, N., Maksaev, G., Sala-Rabanal, M., Nichols, C. G., Hite, R. K. and Yuan, P. (2018). Cryo-EM and X-ray structures of TRPV4 reveal insight into ion permeation and gating mechanisms. Nat. Struct. Mol. Biol. 25, 252-260. 10.1038/s41594-018-0037-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derbenev, A. V. and Zsombok, A. (2016). Potential therapeutic value of TRPV1 and TRPA1 in diabetes mellitus and obesity. Semin. Immunopathol. 38, 397-406. 10.1007/s00281-015-0529-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhaka, A., Viswanath, V. and Patapoutian, A. (2006). Trp ion channels and temperature sensation. Annu. Rev. Neurosci. 29, 135-161. 10.1146/annurev.neuro.29.051605.112958 [DOI] [PubMed] [Google Scholar]
- Dietrich, A., Chubanov, V., Kalwa, H., Rost, B. R. and Gudermann, T. (2006). Cation channels of the transient receptor potential superfamily: their role in physiological and pathophysiological processes of smooth muscle cells. Pharmacol. Ther. 112, 744-760. 10.1016/j.pharmthera.2006.05.013 [DOI] [PubMed] [Google Scholar]
- Duan, J., Li, J., Zeng, B., Chen, G. L., Peng, X., Zhang, Y., Wang, J., Clapham, D. E., Li, Z. and Zhang, J. (2018a). Structure of the mouse TRPC4 ion channel. Nat. Commun. 9, 3102. 10.1038/s41467-018-05247-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan, J., Li, Z., Li, J., Hulse, R. E., Santa-Cruz, A., Valinsky, W. C., Abiria, S. A., Krapivinsky, G., Zhang, J. and Clapham, D. E. (2018b). Structure of the mammalian TRPM7, a magnesium channel required during embryonic development. Proc. Natl. Acad. Sci. U.S.A. 115, E8201-E8210. 10.1073/pnas.1810719115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan, J., Li, Z., Li, J., Santa-Cruz, A., Sanchez-Martinez, S., Zhang, J. and Clapham, D. E. (2018c). Structure of full-length human TRPM4. Proc. Natl. Acad. Sci. U.S.A. 115, 2377-2382. 10.1073/pnas.1722038115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan, J., Li, J., Chen, G. L., Ge, Y., Liu, J., Xie, K., Peng, X., Zhou, W., Zhong, J., Zhang, Y.et al. (2019). Cryo-EM structure of TRPC5 at 2.8-A resolution reveals unique and conserved structural elements essential for channel function. Sci. Adv. 5, eaaw7935. 10.1126/sciadv.aaw7935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duchatelet, S., Pruvost, S., de Veer, S., Fraitag, S., Nitschke, P., Bole-Feysot, C., Bodemer, C. and Hovnanian, A. (2014). A new TRPV3 missense mutation in a patient with Olmsted syndrome and erythromelalgia. JAMA Dermatol 150, 303-306. 10.1001/jamadermatol.2013.8709 [DOI] [PubMed] [Google Scholar]
- Dyment, D. A., Terhal, P. A., Rustad, C. F., Tveten, K., Griffith, C., Jayakar, P., Shinawi, M., Ellingwood, S., Smith, R., van Gassen, K.et al. (2019). De novo substitutions of TRPM3 cause intellectual disability and epilepsy. Eur. J. Hum. Genet. 27, 1611-1618. 10.1038/s41431-019-0462-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eder, P. and Molkentin, J. D. (2011). TRPC channels as effectors of cardiac hypertrophy. Circ. Res. 108, 265-272. 10.1161/CIRCRESAHA.110.225888 [DOI] [PubMed] [Google Scholar]
- Eytan, O., Fuchs-Telem, D., Mevorach, B., Indelman, M., Bergman, R., Sarig, O., Goldberg, I., Adir, N. and Sprecher, E. (2014). Olmsted syndrome caused by a homozygous recessive mutation in TRPV3. J. Invest. Dermatol. 134, 1752-1754. 10.1038/jid.2014.37 [DOI] [PubMed] [Google Scholar]
- Feng, J., Yang, P., Mack, M. R., Dryn, D., Luo, J., Gong, X., Liu, S., Oetjen, L. K., Zholos, A. V., Mei, Z.et al. (2017). Sensory TRP channels contribute differentially to skin inflammation and persistent itch. Nat. Commun. 8, 980. 10.1038/s41467-017-01056-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferioli, S., Zierler, S., Zaißerer, J., Schredelseker, J., Gudermann, T. and Chubanov, V. (2017). TRPM6 and TRPM7 differentially contribute to the relief of heteromeric TRPM6/7 channels from inhibition by cytosolic Mg(2+) and Mg.ATP. Sci. Rep. 7, 8806. 10.1038/s41598-017-08144-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fine, M., Schmiege, P. and Li, X. (2018). Structural basis for PtdInsP2-mediated human TRPML1 regulation. Nat. Commun. 9, 4192. 10.1038/s41467-018-06493-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiorillo, C., Moro, F., Brisca, G., Astrea, G., Nesti, C., Balint, Z., Olschewski, A., Meschini, M. C., Guelly, C., Auer-Grumbach, M.et al. (2012). TRPV4 mutations in children with congenital distal spinal muscular atrophy. Neurogenetics 13, 195-203. 10.1007/s10048-012-0328-7 [DOI] [PubMed] [Google Scholar]
- Freichel, M., Berlin, M., Schurger, A., Mathar, I., Bacmeister, L., Medert, R., Frede, W., Marx, A., Segin, S. and Londono, J. E. C. (2017). TRP Channels in the Heart. In Neurobiology of TRP Channels (eds Emir T. L. R.), pp. 149-185. Boca Raton, FL: CRC Press/Taylor & Francis. [PubMed] [Google Scholar]
- Gao, Y., Cao, E., Julius, D. and Cheng, Y. (2016). TRPV1 structures in nanodiscs reveal mechanisms of ligand and lipid action. Nature 534, 347-351. 10.1038/nature17964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greco, C., Leclerc-Mercier, S., Chaumon, S., Doz, F., Hadj-Rabia, S., Molina, T., Boucheix, C. and Bodemer, C. (2020). Use of epidermal growth factor receptor inhibitor Erlotinib to Treat Palmoplantar Keratoderma in patients with Olmsted Syndrome caused by TRPV3 mutations. JAMA Dermatol 156, 191-195. 10.1001/jamadermatol.2019.4126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm, C., Kraft, R., Schultz, G. and Harteneck, C. (2005). Activation of the melastatin-related cation channel TRPM3 [corrected] by D-erythro-sphingosine. Mol. Pharmacol. 67, 798-805. 10.1124/mol.104.006734 [DOI] [PubMed] [Google Scholar]
- Guo, J., She, J., Zeng, W., Chen, Q., Bai, X. C. and Jiang, Y. (2017). Structures of the calcium-activated, non-selective cation channel TRPM4. Nature 552, 205-209. 10.1038/nature24997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara, K., Kokubo, Y., Ishiura, H., Fukuda, Y., Miyashita, A., Kuwano, R., Sasaki, R., Goto, J., Nishizawa, M., Kuzuhara, S.et al. (2010). TRPM7 is not associated with amyotrophic lateral sclerosis-parkinsonism dementia complex in the Kii peninsula of Japan. Am. J. Med. Genet. B Neuropsychiatr. Genet. 153B, 310-313. 10.1002/ajmg.b.30966 [DOI] [PubMed] [Google Scholar]
- Hardie, R. C. and Minke, B. (1992). The trp gene is essential for a light-activated Ca2+ channel in Drosophila photoreceptors. Neuron 8, 643-651. 10.1016/0896-6273(92)90086-S [DOI] [PubMed] [Google Scholar]
- Hardy, E. and Tsiokas, L. (2020). Polycystins as components of large multiprotein complexes of polycystin interactors. Cell. Signal. 72, 109640. 10.1016/j.cellsig.2020.109640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartmann, J., Dragicevic, E., Adelsberger, H., Henning, H. A., Sumser, M., Abramowitz, J., Blum, R., Dietrich, A., Freichel, M., Flockerzi, V.et al. (2008). TRPC3 channels are required for synaptic transmission and motor coordination. Neuron 59, 392-398. 10.1016/j.neuron.2008.06.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He, Y., Zeng, K., Zhang, X., Chen, Q., Wu, J., Li, H., Zhou, Y., Glusman, G., Roach, J., Etheridge, A.et al. (2015). A gain-of-function mutation in TRPV3 causes focal palmoplantar keratoderma in a Chinese family. J. Invest. Dermatol. 135, 907-909. 10.1038/jid.2014.429 [DOI] [PubMed] [Google Scholar]
- Hermosura, M. C. and Garruto, R. M. (2007). TRPM7 and TRPM2-Candidate susceptibility genes for Western Pacific ALS and PD?. Biochim. Biophys. Acta 1772, 822-835. 10.1016/j.bbadis.2007.02.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermosura, M. C., Nayakanti, H., Dorovkov, M. V., Calderon, F. R., Ryazanov, A. G., Haymer, D. S. and Garruto, R. M. (2005). A TRPM7 variant shows altered sensitivity to magnesium that may contribute to the pathogenesis of two Guamanian neurodegenerative disorders. Proc. Natl. Acad. Sci. U.S.A. 102, 11510-11515. 10.1073/pnas.0505149102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermosura, M. C., Cui, A. M., Go, R. C., Davenport, B., Shetler, C. M., Heizer, J. W., Schmitz, C., Mocz, G., Garruto, R. M. and Perraud, A. L. (2008). Altered functional properties of a TRPM2 variant in Guamanian ALS and PD. Proc. Natl. Acad. Sci. U.S.A. 105, 18029-18034. 10.1073/pnas.0808218105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiroi, T., Wajima, T., Negoro, T., Ishii, M., Nakano, Y., Kiuchi, Y., Mori, Y. and Shimizu, S. (2013). Neutrophil TRPM2 channels are implicated in the exacerbation of myocardial ischaemia/reperfusion injury. Cardiovasc. Res. 97, 271-281. 10.1093/cvr/cvs332 [DOI] [PubMed] [Google Scholar]
- Hirschi, M., Herzik, M. A., Jr., Wie, J., Suo, Y., Borschel, W. F., Ren, D., Lander, G. C. and Lee, S. Y. (2017). Cryo-electron microscopy structure of the lysosomal calcium-permeable channel TRPML3. Nature 550, 411-414. 10.1038/nature24055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hof, T., Chaigne, S., Recalde, A., Salle, L., Brette, F. and Guinamard, R. (2019). Transient receptor potential channels in cardiac health and disease. Nat Rev Cardiol 16, 344-360. 10.1038/s41569-018-0145-2 [DOI] [PubMed] [Google Scholar]
- Hofmann, T., Obukhov, A. G., Schaefer, M., Harteneck, C., Gudermann, T. and Schultz, G. (1999). Direct activation of human TRPC6 and TRPC3 channels by diacylglycerol. Nature 397, 259-263. 10.1038/16711 [DOI] [PubMed] [Google Scholar]
- Hofstra, J. M., Lainez, S., van Kuijk, W. H., Schoots, J., Baltissen, M. P., Hoefsloot, L. H., Knoers, N. V., Berden, J. H., Bindels, R. J., van der Vlag, J.et al. (2013). New TRPC6 gain-of-function mutation in a non-consanguineous Dutch family with late-onset focal segmental glomerulosclerosis. Nephrol. Dial. Transplant. 28, 1830-1838. 10.1093/ndt/gfs572 [DOI] [PubMed] [Google Scholar]
- Huang, Y., Winkler, P. A., Sun, W., Lu, W. and Du, J. (2018). Architecture of the TRPM2 channel and its activation mechanism by ADP-ribose and calcium. Nature 562, 145-149. 10.1038/s41586-018-0558-4 [DOI] [PubMed] [Google Scholar]
- Huang, Y., Roth, B., Lu, W. and Du, J. (2019). Ligand recognition and gating mechanism through three ligand-binding sites of human TRPM2 channel. eLife 8, e50175. 10.7554/eLife.50175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang, Y., Fliegert, R., Guse, A. H., Lu, W. and Du, J. (2020). A structural overview of the ion channels of the TRPM family. Cell Calcium 85, 102111. 10.1016/j.ceca.2019.102111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes, T. E. T., Pumroy, R. A., Yazici, A. T., Kasimova, M. A., Fluck, E. C., Huynh, K. W., Samanta, A., Molugu, S. K., Zhou, Z. H., Carnevale, V.et al. (2018). Structural insights on TRPV5 gating by endogenous modulators. Nat. Commun. 9, 4198. 10.1038/s41467-018-06753-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huynh, K. W., Cohen, M. R., Jiang, J., Samanta, A., Lodowski, D. T., Zhou, Z. H. and Moiseenkova-Bell, V. Y. (2016). Structure of the full-length TRPV2 channel by cryo-EM. Nat. Commun. 7, 11130. 10.1038/ncomms11130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iordanov, I., Toth, B., Szollosi, A. and Csanady, L. (2019). Enzyme activity and selectivity filter stability of ancient TRPM2 channels were simultaneously lost in early vertebrates. eLife 8, e44556. 10.7554/eLife.44556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeon, J., Bu, F., Sun, G., Tian, J. B., Ting, S. M., Li, J., Aronowski, J., Birnbaumer, L., Freichel, M. and Zhu, M. X. (2020). Contribution of TRPC Channels in Neuronal Excitotoxicity Associated With Neurodegenerative Disease and Ischemic Stroke. Front Cell Dev Biol 8, 618663. 10.3389/fcell.2020.618663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julius, D. (2013). TRP channels and pain. Annu. Rev. Cell Dev. Biol. 29, 355-384. 10.1146/annurev-cellbio-101011-155833 [DOI] [PubMed] [Google Scholar]
- Kaneko, Y. and Szallasi, A. (2014). Transient receptor potential (TRP) channels: a clinical perspective. Br. J. Pharmacol. 171, 2474-2507. 10.1111/bph.12414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katanosaka, Y., Iwasaki, K., Ujihara, Y., Takatsu, S., Nishitsuji, K., Kanagawa, M., Sudo, A., Toda, T., Katanosaka, K., Mohri, S.et al. (2014). TRPV2 is critical for the maintenance of cardiac structure and function in mice. Nat. Commun. 5, 3932. 10.1038/ncomms4932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, H., Mittal, D. P., Iadarola, M. J. and Dionne, R. A. (2006). Genetic predictors for acute experimental cold and heat pain sensitivity in humans. J. Med. Genet. 43, e40. 10.1136/jmg.2005.036079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kittaka, H. and Tominaga, M. (2017). The molecular and cellular mechanisms of itch and the involvement of TRP channels in the peripheral sensory nervous system and skin. Allergol. Int. 66, 22-30. 10.1016/j.alit.2016.10.003 [DOI] [PubMed] [Google Scholar]
- Koike, C., Obara, T., Uriu, Y., Numata, T., Sanuki, R., Miyata, K., Koyasu, T., Ueno, S., Funabiki, K., Tani, A.et al. (2010). TRPM1 is a component of the retinal ON bipolar cell transduction channel in the mGluR6 cascade. Proc. Natl. Acad. Sci. U.S.A. 107, 332-337. 10.1073/pnas.0912730107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krapivinsky, G., Krapivinsky, L., Manasian, Y. and Clapham, D. E. (2014). The TRPM7 Chanzyme Is Cleaved to Release a Chromatin-Modifying Kinase. Cell 157, 1061-1072. 10.1016/j.cell.2014.03.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krapivinsky, G., Krapivinsky, L., Renthal, N. E., Santa-Cruz, A., Manasian, Y. and Clapham, D. E. (2017). Histone phosphorylation by TRPM6's cleaved kinase attenuates adjacent arginine methylation to regulate gene expression. Proc. Natl. Acad. Sci. U.S.A. 114, E7092-E7100. 10.1073/pnas.1708427114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kremeyer, B., Lopera, F., Cox, J. J., Momin, A., Rugiero, F., Marsh, S., Woods, C. G., Jones, N. G., Paterson, K. J., Fricker, F. R.et al. (2010). A gain-of-function mutation in TRPA1 causes familial episodic pain syndrome. Neuron 66, 671-680. 10.1016/j.neuron.2010.04.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruse, M., Schulze-Bahr, E., Corfield, V., Beckmann, A., Stallmeyer, B., Kurtbay, G., Ohmert, I., Brink, P. and Pongs, O. (2009). Impaired endocytosis of the ion channel TRPM4 is associated with human progressive familial heart block type I. J. Clin. Invest. 119, 2737-2744. 10.1172/JCI38292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn, F. J. and Luckhoff, A. (2004). Sites of the NUDT9-H domain critical for ADP-ribose activation of the cation channel TRPM2. J. Biol. Chem. 279, 46431-46437. 10.1074/jbc.M407263200 [DOI] [PubMed] [Google Scholar]
- Lamande, S. R., Yuan, Y., Gresshoff, I. L., Rowley, L., Belluoccio, D., Kaluarachchi, K., Little, C. B., Botzenhart, E., Zerres, K., Amor, D. J.et al. (2011). Mutations in TRPV4 cause an inherited arthropathy of hands and feet. Nat. Genet. 43, 1142-1146. 10.1038/ng.945 [DOI] [PubMed] [Google Scholar]
- Landoure, G., Zdebik, A. A., Martinez, T. L., Burnett, B. G., Stanescu, H. C., Inada, H., Shi, Y., Taye, A. A., Kong, L., Munns, C. H.et al. (2010). Mutations in TRPV4 cause Charcot-Marie-Tooth disease type 2C. Nat. Genet. 42, 170-174. 10.1038/ng.512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, Y. J., Joo, K., Seong, M. W., Park, K. H., Park, S. S. and Woo, S. J. (2020). Congenital Stationary Night Blindness due to Novel TRPM1 Gene Mutations in a Korean Patient. Korean J. Ophthalmol. 34, 170-172. 10.3341/kjo.2019.0080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao, M., Cao, E., Julius, D. and Cheng, Y. (2013). Structure of the TRPV1 ion channel determined by electron cryo-microscopy. Nature 504, 107-112. 10.1038/nature12822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin, J. and Xiong, Z. G. (2017). TRPM7 is a unique target for therapeutic intervention of stroke. Int. J. Physiol. Pathophysiol. Pharmacol. 9, 211-216. [PMC free article] [PubMed] [Google Scholar]
- Lin, Z., Chen, Q., Lee, M., Cao, X., Zhang, J., Ma, D., Chen, L., Hu, X., Wang, H., Wang, X.et al. (2012). Exome sequencing reveals mutations in TRPV3 as a cause of Olmsted syndrome. Am. J. Hum. Genet. 90, 558-564. 10.1016/j.ajhg.2012.02.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, H., El Zein, L., Kruse, M., Guinamard, R., Beckmann, A., Bozio, A., Kurtbay, G., Megarbane, A., Ohmert, I., Blaysat, G.et al. (2010). Gain-of-function mutations in TRPM4 cause autosomal dominant isolated cardiac conduction disease. Circ Cardiovasc Genet 3, 374-385. 10.1161/CIRCGENETICS.109.930867 [DOI] [PubMed] [Google Scholar]
- Liu, H., Chatel, S., Simard, C., Syam, N., Salle, L., Probst, V., Morel, J., Millat, G., Lopez, M., Abriel, H.et al. (2013). Molecular genetics and functional anomalies in a series of 248 Brugada cases with 11 mutations in the TRPM4 channel. PLoS One 8, e54131. 10.1371/journal.pone.0054131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madej, M. G. and Ziegler, C. M. (2018). Dawning of a new era in TRP channel structural biology by cryo-electron microscopy. Pflugers Arch. 470, 213-225. 10.1007/s00424-018-2107-2 [DOI] [PubMed] [Google Scholar]
- McNulty, A. L., Leddy, H. A., Liedtke, W. and Guilak, F. (2015). TRPV4 as a therapeutic target for joint diseases. Naunyn Schmiedebergs Arch. Pharmacol. 388, 437-450. 10.1007/s00210-014-1078-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina, D. L., Di Paola, S., Peluso, I., Armani, A., De Stefani, D., Venditti, R., Montefusco, S., Scotto-Rosato, A., Prezioso, C., Forrester, A.et al. (2015). Lysosomal calcium signalling regulates autophagy through calcineurin and TFEB. Nat. Cell Biol. 17, 288-299. 10.1038/ncb3114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meseguer, V., Alpizar, Y. A., Luis, E., Tajada, S., Denlinger, B., Fajardo, O., Manenschijn, J. A., Fernandez-Pena, C., Talavera, A., Kichko, T.et al. (2014). TRPA1 channels mediate acute neurogenic inflammation and pain produced by bacterial endotoxins. Nat. Commun. 5, 3125. 10.1038/ncomms4125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller, B. A., Wang, J., Hirschler-Laszkiewicz, I., Gao, E., Song, J., Zhang, X. Q., Koch, W. J., Madesh, M., Mallilankaraman, K., Gu, T.et al. (2013). The second member of transient receptor potential-melastatin channel family protects hearts from ischemia-reperfusion injury. Am. J. Physiol. Heart Circ. Physiol. 304, H1010-H1022. 10.1152/ajpheart.00906.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minke, B. (1977). Drosophila mutant with a transducer defect. Biophys. Struct. Mech. 3, 59-64. 10.1007/BF00536455 [DOI] [PubMed] [Google Scholar]
- Mittermeier, L., Demirkhanyan, L., Stadlbauer, B., Breit, A., Recordati, C., Hilgendorff, A., Matsushita, M., Braun, A., Simmons, D. G., Zakharian, E.et al. (2019). TRPM7 is the central gatekeeper of intestinal mineral absorption essential for postnatal survival. Proc. Natl. Acad. Sci. U.S.A. 116, 4706-4715. 10.1073/pnas.1810633116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montell, C. (2005). The TRP superfamily of cation channels. Sci. STKE 2005, 1-24. 10.1126/stke.2722005re3 [DOI] [PubMed] [Google Scholar]
- Montell, C. and Rubin, G. M. (1989). Molecular characterization of the Drosophila trp locus: a putative integral membrane protein required for phototransduction. Neuron 2, 1313-1323. 10.1016/0896-6273(89)90069-X [DOI] [PubMed] [Google Scholar]
- Montell, C., Jones, K., Hafen, E. and Rubin, G. (1985). Rescue of the Drosophila phototransduction mutation trp by germline transformation. Science 230, 1040-1043. 10.1126/science.3933112 [DOI] [PubMed] [Google Scholar]
- Morgans, C. W., Zhang, J., Jeffrey, B. G., Nelson, S. M., Burke, N. S., Duvoisin, R. M. and Brown, R. L. (2009). TRPM1 is required for the depolarizing light response in retinal ON-bipolar cells. Proc. Natl. Acad. Sci. U.S.A. 106, 19174-19178. 10.1073/pnas.0908711106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadler, M. J., Hermosura, M. C., Inabe, K., Perraud, A. L., Zhu, Q., Stokes, A. J., Kurosaki, T., Kinet, J. P., Penner, R., Scharenberg, A. M.et al. (2001). LTRPC7 is a Mg.ATP-regulated divalent cation channel required for cell viability. Nature 411, 590-595. 10.1038/35079092 [DOI] [PubMed] [Google Scholar]
- Naert, R., Talavera, A., Startek, J. B. and Talavera, K. (2020). TRPA1 gene variants hurting our feelings. Pflugers Arch. 472, 953-960. 10.1007/s00424-020-02397-y [DOI] [PubMed] [Google Scholar]
- Nagpal, R., Mishra, S. K., Deep, G. and Yadav, H. (2020). Role of TRP Channels in Shaping the Gut Microbiome. Pathogens 9, 753. 10.3390/pathogens9090753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni, C., Yan, M., Zhang, J., Cheng, R., Liang, J., Deng, D., Wang, Z., Li, M. and Yao, Z. (2016). A novel mutation in TRPV3 gene causes atypical familial Olmsted syndrome. Sci. Rep. 6, 21815. 10.1038/srep21815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikolaev, Y. A., Cox, C. D., Ridone, P., Rohde, P. R., Cordero-Morales, J. F., Vasquez, V., Laver, D. R. and Martinac, B. (2019). Mammalian TRP ion channels are insensitive to membrane stretch. J. Cell Sci. 132, jcs238360. 10.1242/jcs.238360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilius, B. and Owsianik, G. (2010). Transient receptor potential channelopathies. Pflugers Arch. 460, 437-450. 10.1007/s00424-010-0788-2 [DOI] [PubMed] [Google Scholar]
- Nilius, B. and Owsianik, G. (2011). The transient receptor potential family of ion channels. Genome Biol. 12, 218. 10.1186/gb-2011-12-3-218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilius, B. and Szallasi, A. (2014). Transient Receptor Potential Channels as Drug Targets: From the Science of Basic Research to the Art of Medicine. Pharmacol. Rev. 66, 676-814. 10.1124/pr.113.008268 [DOI] [PubMed] [Google Scholar]
- Nilius, B. and Voets, T. (2013). The puzzle of TRPV4 channelopathies. EMBO Rep. 14, 152-163. 10.1038/embor.2012.219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura, G., Lausch, E., Savarirayan, R., Shiba, M., Spranger, J., Zabel, B., Ikegawa, S., Superti-Furga, A. and Unger, S. (2012). TRPV4-associated skeletal dysplasias. Am. J. Med. Genet. C Semin. Med. Genet. 160C, 190-204. 10.1002/ajmg.c.31335 [DOI] [PubMed] [Google Scholar]
- Owsianik, G., Talavera, K., Voets, T. and Nilius, B. (2006). Permeation and Selectivity of TRP Channels. Annu. Rev. Physiol. 68, 685-717. 10.1146/annurev.physiol.68.040204.101406 [DOI] [PubMed] [Google Scholar]
- Pablo, J. L. and Greka, A. (2019). Charting a TRP to Novel Therapeutic Destinations for Kidney Diseases. Trends Pharmacol. Sci. 40, 911-918. 10.1016/j.tips.2019.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulsen, C. E., Armache, J. P., Gao, Y., Cheng, Y. and Julius, D. (2015). Structure of the TRPA1 ion channel suggests regulatory mechanisms. Nature 520, 511-517. 10.1038/nature14367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perraud, A. L., Fleig, A., Dunn, C. A., Bagley, L. A., Launay, P., Schmitz, C., Stokes, A. J., Zhu, Q., Bessman, M. J., Penner, R.et al. (2001). ADP-ribose gating of the calcium-permeable LTRPC2 channel revealed by Nudix motif homology. Nature 411, 595-599. 10.1038/35079100 [DOI] [PubMed] [Google Scholar]
- Pumroy, R. A., Fluck, E. C., 3rd, Ahmed, T. and Moiseenkova-Bell, V. Y. (2020). Structural insights into the gating mechanisms of TRPV channels. Cell Calcium 87, 102168. 10.1016/j.ceca.2020.102168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin, X., Yue, Z., Sun, B., Yang, W., Xie, J., Ni, E., Feng, Y., Mahmood, R., Zhang, Y. and Yue, L. (2013). Sphingosine and FTY720 are potent inhibitors of the transient receptor potential melastatin 7 (TRPM7) channels. Br. J. Pharmacol. 168, 1294-1312. 10.1111/bph.12012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiser, J., Polu, K. R., Moller, C. C., Kenlan, P., Altintas, M. M., Wei, C., Faul, C., Herbert, S., Villegas, I., Avila-Casado, C.et al. (2005). TRPC6 is a glomerular slit diaphragm-associated channel required for normal renal function. Nat. Genet. 37, 739-744. 10.1038/ng1592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuter, M. S., Chaturvedi, R. R., Liston, E., Manshaei, R., Aul, R. B., Bowdin, S., Cohn, I., Curtis, M., Dhir, P., Hayeems, R. Z.et al. (2020). The Cardiac Genome Clinic: implementing genome sequencing in pediatric heart disease. Genet. Med. 22, 1015-1024. 10.1038/s41436-020-0757-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riccio, A., Li, Y., Moon, J., Kim, K. S., Smith, K. S., Rudolph, U., Gapon, S., Yao, G. L., Tsvetkov, E., Rodig, S. J.et al. (2009). Essential role for TRPC5 in amygdala function and fear-related behavior. Cell 137, 761-772. 10.1016/j.cell.2009.03.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riccio, A., Li, Y., Tsvetkov, E., Gapon, S., Yao, G. L., Smith, K. S., Engin, E., Rudolph, U., Bolshakov, V. Y. and Clapham, D. E. (2014). Decreased anxiety-like behavior and Gαq/11-dependent responses in the amygdala of mice lacking TRPC4 channels. J. Neurosci. 34, 3653-3667. 10.1523/JNEUROSCI.2274-13.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riehle, M., Büscher, A. K., Gohlke, B. O., Kaßmann, M., Kolatsi-Joannou, M., Bräsen, J. H., Nagel, M., Becker, J. U., Winyard, P., Hoyer, P. F.et al. (2016). TRPC6 G757D Loss-of-Function Mutation Associates with FSGS. J. Am. Soc. Nephrol. 27, 2771-2783. 10.1681/ASN.2015030318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Runnels, L. W., Yue, L. and Clapham, D. E. (2001). TRP-PLIK, a bifunctional protein with kinase and ion channel activities. Science 291, 1043-1047. 10.1126/science.1058519 [DOI] [PubMed] [Google Scholar]
- Runnels, L. W., Yue, L. and Clapham, D. E. (2002). The TRPM7 channel is inactivated by PIP(2) hydrolysis. Nat. Cell Biol. 4, 329-336. 10.1038/ncb781 [DOI] [PubMed] [Google Scholar]
- Sah, R., Mesirca, P., Mason, X., Gibson, W., Bates-Withers, C., Van den Boogert, M., Chaudhuri, D., Pu, W. T., Mangoni, M. E. and Clapham, D. E. (2013). Timing of myocardial Trpm7 deletion during cardiogenesis variably disrupts adult ventricular function, conduction, and repolarization. Circulation 128, 101-114. 10.1161/CIRCULATIONAHA.112.000768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahoo, N., Gu, M., Zhang, X., Raval, N., Yang, J., Bekier, M., Calvo, R., Patnaik, S., Wang, W., King, G.et al. (2017). Gastric Acid Secretion from Parietal Cells Is Mediated by a Ca(2+) Efflux Channel in the Tubulovesicle. Dev. Cell 41, 262-273e6. 10.1016/j.devcel.2017.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakaguchi, R. and Mori, Y. (2020). Transient receptor potential (TRP) channels: Biosensors for redox environmental stimuli and cellular status. Free Radic. Biol. Med. 146, 36-44. 10.1016/j.freeradbiomed.2019.10.415 [DOI] [PubMed] [Google Scholar]
- Saliba, Y., Karam, R., Smayra, V., Aftimos, G., Abramowitz, J., Birnbaumer, L. and Farès, N. (2015). Evidence of a Role for Fibroblast Transient Receptor Potential Canonical 3 Ca2+ Channel in Renal Fibrosis. J. Am. Soc. Nephrol. 26, 1855-1876. 10.1681/ASN.2014010065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saotome, K., Singh, A. K., Yelshanskaya, M. V. and Sobolevsky, A. I. (2016). Crystal structure of the epithelial calcium channel TRPV6. Nature 534, 506-511. 10.1038/nature17975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlingmann, K. P., Waldegger, S., Konrad, M., Chubanov, V. and Gudermann, T. (2007). TRPM6 and TRPM7--Gatekeepers of human magnesium metabolism. Biochim. Biophys. Acta 1772, 813-821. 10.1016/j.bbadis.2007.03.009 [DOI] [PubMed] [Google Scholar]
- Schmiege, P., Fine, M., Blobel, G. and Li, X. (2017). Human TRPML1 channel structures in open and closed conformations. Nature 550, 366-370. 10.1038/nature24036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz, Y., Oleinikov, K., Schindeldecker, B., Wyatt, A., Weißgerber, P., Flockerzi, V., Boehm, U., Freichel, M. and Bruns, D. (2019). TRPC channels regulate Ca2+-signaling and short-term plasticity of fast glutamatergic synapses. PLoS Biol. 17, e3000445. 10.1371/journal.pbio.3000445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shekhar, S., Liu, Y., Wang, S., Zhang, H., Fang, X., Zhang, J., Fan, L., Zheng, B., Roman, R. J., Wang, Z.et al. (2021). Novel Mechanistic Insights and Potential Therapeutic Impact of TRPC6 in Neurovascular Coupling and Ischemic Stroke. Int. J. Mol. Sci. 22, 2074. 10.3390/ijms22042074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen, D., Wang, X., Li, X., Zhang, X., Yao, Z., Dibble, S., Dong, X. P., Yu, T., Lieberman, A. P., Showalter, H. D.et al. (2012). Lipid storage disorders block lysosomal trafficking by inhibiting a TRP channel and lysosomal calcium release. Nat. Commun. 3, 731. 10.1038/ncomms1735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen, P. S., Yang, X., DeCaen, P. G., Liu, X., Bulkley, D., Clapham, D. E. and Cao, E. (2016). The Structure of the Polycystic Kidney Disease Channel PKD2 in Lipid Nanodiscs. Cell 167, 763-773e11. 10.1016/j.cell.2016.09.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shigeto, M., Ramracheya, R., Tarasov, A. I., Cha, C. Y., Chibalina, M. V., Hastoy, B., Philippaert, K., Reinbothe, T., Rorsman, N., Salehi, A.et al. (2015). GLP-1 stimulates insulin secretion by PKC-dependent TRPM4 and TRPM5 activation. J. Clin. Invest. 125, 4714-4728. 10.1172/JCI81975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh, A. K., McGoldrick, L. L., Twomey, E. C. and Sobolevsky, A. I. (2018). Mechanism of calmodulin inactivation of the calcium-selective TRP channel TRPV6. Sci. Adv. 4, eaau6088. 10.1126/sciadv.aau6088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh, A. K., McGoldrick, L. L., Demirkhanyan, L., Leslie, M., Zakharian, E. and Sobolevsky, A. I. (2019). Structural basis of temperature sensation by the TRP channel TRPV3. Nat. Struct. Mol. Biol. 26, 994-998. 10.1038/s41594-019-0318-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spix, B., Chao, Y. K., Abrahamian, C., Chen, C. C. and Grimm, C. (2020). TRPML Cation Channels in Inflammation and Immunity. Front Immunol 11, 225. 10.3389/fimmu.2020.00225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stallmeyer, B., Zumhagen, S., Denjoy, I., Duthoit, G., Hebert, J. L., Ferrer, X., Maugenre, S., Schmitz, W., Kirchhefer, U., Schulze-Bahr, E.et al. (2012). Mutational spectrum in the Ca(2+)-activated cation channel gene TRPM4 in patients with cardiac conductance disturbances. Hum. Mutat. 33, 109-117. 10.1002/humu.21599 [DOI] [PubMed] [Google Scholar]
- Startek, J. B. and Talavera, K. (2020). Lipid Raft Destabilization Impairs Mouse TRPA1 Responses to Cold and Bacterial Lipopolysaccharides. Int. J. Mol. Sci. 21, 3826. 10.3390/ijms21113826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart, J. M. (2020). TRPV6 as A Target for Cancer Therapy. J Cancer 11, 374-387, 10.7150/jca.31640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stritt, S., Nurden, P., Favier, R., Favier, M., Ferioli, S., Gotru, S. K., van Eeuwijk, J. M., Schulze, H., Nurden, A. T., Lambert, M. P.et al. (2016). Defects in TRPM7 channel function deregulate thrombopoiesis through altered cellular Mg(2+) homeostasis and cytoskeletal architecture. Nat. Commun. 7, 11097. 10.1038/ncomms11097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su, Q., Hu, F., Ge, X., Lei, J., Yu, S., Wang, T., Zhou, Q., Mei, C. and Shi, Y. (2018). Structure of the human PKD1-PKD2 complex. Science 361, eaat9819. 10.1126/science.aat9819 [DOI] [PubMed] [Google Scholar]
- Su, S., Yudin, Y., Kim, N., Tao, Y. X. and Rohacs, T. (2021). TRPM3 Channels Play Roles in Heat Hypersensitivity and Spontaneous Pain after Nerve Injury. J. Neurosci. 41, 2457-2474. 10.1523/JNEUROSCI.1551-20.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suo, Y., Wang, Z., Zubcevic, L., Hsu, A. L., He, Q., Borgnia, M. J., Ji, R. R. and Lee, S. Y. (2020). Structural Insights into Electrophile Irritant Sensing by the Human TRPA1 Channel. Neuron 105, 882-894e5. 10.1016/j.neuron.2019.11.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syam, N., Chatel, S., Ozhathil, L. C., Sottas, V., Rougier, J. S., Baruteau, A., Baron, E., Amarouch, M. Y., Daumy, X., Probst, V.et al. (2016). Variants of Transient Receptor Potential Melastatin Member 4 in Childhood Atrioventricular Block. J Am Heart Assoc 5, e001625. 10.1161/JAHA.114.001625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan, C. H. and McNaughton, P. A. (2018). TRPM2 and warmth sensation. Pflugers Arch. 470, 787-798. 10.1007/s00424-018-2139-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thakur, D. P., Tian, J. B., Jeon, J., Xiong, J., Huang, Y., Flockerzi, V. and Zhu, M. X. (2016). Critical roles of Gi/o proteins and phospholipase C-δ1 in the activation of receptor-operated TRPC4 channels. Proc. Natl. Acad. Sci. U.S.A. 113, 1092-1097. 10.1073/pnas.1522294113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thibodeau, M. L., Peters, C. H., Townsend, K. N., Shen, Y., Hendson, G., Adam, S., Selby, K., Macleod, P. M., Gershome, C., Ruben, P.et al. (2017). Compound heterozygous TRPV4 mutations in two siblings with a complex phenotype including severe intellectual disability and neuropathy. Am. J. Med. Genet. A 173, 3087-3092. 10.1002/ajmg.a.38400 [DOI] [PubMed] [Google Scholar]
- Toft-Bertelsen, T. L. and MacAulay, N. (2021). TRPing to the Point of Clarity: Understanding the Function of the Complex TRPV4 Ion Channel. Cells 10, 165. 10.3390/cells10010165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Abel, M., Hoenderop, J. G. and Bindels, R. J. (2005). The epithelial calcium channels TRPV5 and TRPV6: regulation and implications for disease. Naunyn Schmiedebergs Arch. Pharmacol. 371, 295-306. 10.1007/s00210-005-1021-2 [DOI] [PubMed] [Google Scholar]
- Van Hoeymissen, E., Held, K., Nogueira Freitas, A. C., Janssens, A., Voets, T. and Vriens, J. (2020). Gain of channel function and modified gating properties in TRPM3 mutants causing intellectual disability and epilepsy. eLife 9, e57190. 10.7554/eLife.57190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandewauw, I., De Clercq, K., Mulier, M., Held, K., Pinto, S., Van Ranst, N., Segal, A., Voet, T., Vennekens, R., Zimmermann, K.et al. (2018). A TRP channel trio mediates acute noxious heat sensing. Nature 555, 662-666. 10.1038/nature26137 [DOI] [PubMed] [Google Scholar]
- Vangeel, L. and Voets, T. (2019). Transient Receptor Potential Channels and Calcium Signaling. Cold Spring Harb Perspect Biol 11, a035048. 10.1101/cshperspect.a035048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velilla, J., Marchetti, M. M., Toth-Petroczy, A., Grosgogeat, C., Bennett, A. H., Carmichael, N., Estrella, E., Darras, B. T., Frank, N. Y., Krier, J.et al. (2019). Homozygous TRPV4 mutation causes congenital distal spinal muscular atrophy and arthrogryposis. Neurol Genet 5, e312. 10.1212/NXG.0000000000000312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkatachalam, K. and Montell, C. (2007). TRP Channels. Annu. Rev. Biochem. 76, 387-417. 10.1146/annurev.biochem.75.103004.142819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkatachalam, K., Zheng, F. and Gill, D. L. (2003). Regulation of canonical transient receptor potential (TRPC) channel function by diacylglycerol and protein kinase C. J. Biol. Chem. 278, 29031-29040. 10.1074/jbc.M302751200 [DOI] [PubMed] [Google Scholar]
- Vinayagam, D., Quentin, D., Yu-Strzelczyk, J., Sitsel, O., Merino, F., Stabrin, M., Hofnagel, O., Yu, M., Ledeboer, M. W., Nagel, G.et al. (2020). Structural basis of TRPC4 regulation by calmodulin and pharmacological agents. eLife 9, e60603. 10.7554/eLife.60603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voets, T., Nilius, B., Hoefs, S., van der Kemp, A. W. C. M., Droogmans, G., Bindels, R. J. M. and Hoenderop, J. G. J. (2004). TRPM6 Forms the Mg2+ Influx Channel Involved in Intestinal and Renal Mg2+ Absorption. J. Biol. Chem. 279, 19-25. 10.1074/jbc.M311201200 [DOI] [PubMed] [Google Scholar]
- Voets, T., Vriens, J. and Vennekens, R. (2019). Targeting TRP Channels – Valuable Alternatives to Combat Pain, Lower Urinary Tract Disorders, and Type 2 Diabetes?. Trends Pharmacol. Sci. 40, 669-683. 10.1016/j.tips.2019.07.004 [DOI] [PubMed] [Google Scholar]
- Vriens, J., Owsianik, G., Hofmann, T., Philipp, S. E., Stab, J., Chen, X., Benoit, M., Xue, F., Janssens, A., Kerselaers, S.et al. (2011). TRPM3 is a nociceptor channel involved in the detection of noxious heat. Neuron 70, 482-494. 10.1016/j.neuron.2011.02.051 [DOI] [PubMed] [Google Scholar]
- Wang, W., Zhang, X., Gao, Q. and Xu, H. (2014). TRPML1: an ion channel in the lysosome. Handb. Exp. Pharmacol. 222, 631-645. 10.1007/978-3-642-54215-2_24 [DOI] [PubMed] [Google Scholar]
- Wang, L., Fu, T. M., Zhou, Y., Xia, S., Greka, A. and Wu, H. (2018). Structures and gating mechanism of human TRPM2. Science 362, eaav4809. 10.1126/science.aav4809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber, J., Rajan, S., Schremmer, C., Chao, Y. K., Krasteva-Christ, G., Kannler, M., Yildirim, A. O., Brosien, M., Schredelseker, J., Weissmann, N.et al. (2020). TRPV4 channels are essential for alveolar epithelial barrier function as protection from lung edema. JCI Insight 5, e134464. 10.1172/jci.insight.134464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissmann, N., Sydykov, A., Kalwa, H., Storch, U., Fuchs, B., Mederos y Schnitzler, M., Brandes, R. P., Grimminger, F., Meissner, M., Freichel, M.et al. (2012). Activation of TRPC6 channels is essential for lung ischaemia-reperfusion induced oedema in mice. Nat. Commun. 3, 649. 10.1038/ncomms1660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wes, P. D., Chevesich, J., Jeromin, A., Rosenberg, C., Stetten, G. and Montell, C. (1995). TRPC1, a human homolog of a Drosophila store-operated channel. Proc. Natl. Acad. Sci. U.S.A. 92, 9652-9656. 10.1073/pnas.92.21.9652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson, S. R., Gerhold, K. A., Bifolck-Fisher, A., Liu, Q., Patel, K. N., Dong, X. and Bautista, D. M. (2011). TRPA1 is required for histamine-independent, Mas-related G protein-coupled receptor-mediated itch. Nat. Neurosci. 14, 595-602. 10.1038/nn.2789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winn, M. P., Conlon, P. J., Lynn, K. L., Farrington, M. K., Creazzo, T., Hawkins, A. F., Daskalakis, N., Kwan, S. Y., Ebersviller, S., Burchette, J. L.et al. (2005). A mutation in the TRPC6 cation channel causes familial focal segmental glomerulosclerosis. Science 308, 1801-1804. 10.1126/science.1106215 [DOI] [PubMed] [Google Scholar]
- Wu, G., Markowitz, G. S., Li, L., D'Agati, V. D., Factor, S. M., Geng, L., Tibara, S., Tuchman, J., Cai, Y., Park, J. H.et al. (2000). Cardiac defects and renal failure in mice with targeted mutations in Pkd2. Nat. Genet. 24, 75-78. 10.1038/71724 [DOI] [PubMed] [Google Scholar]
- Wu, L.-J., Sweet, T.-B. and Clapham, D. E. (2010a). International Union of Basic and Clinical Pharmacology. LXXVI. Current progress in the mammalian TRP ion channel family. Pharmacol. Rev. 62, 381-404. 10.1124/pr.110.002725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu, X., Eder, P., Chang, B. and Molkentin, J. D. (2010b). TRPC channels are necessary mediators of pathologic cardiac hypertrophy. Proc. Natl Acad. Sci. USA 107, 7000-7005. 10.1073/pnas.1001825107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie, Z. and Hu, H. (2018). TRP Channels as Drug Targets to Relieve Itch. Pharmaceuticals (Basel) 11, 100. 10.3390/ph11040100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie, J., Sun, B., Du, J., Yang, W., Chen, H.-C., Overton, J. D., Runnels, L. W. and Yue, L. (2011). Phosphatidylinositol 4,5-bisphosphate (PIP2) controls magnesium gatekeeper TRPM6 activity. Sci. Rep. 1, 12. 10.1038/srep00012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xin, Y., Malick, A., Hu, M., Liu, C., Batah, H., Xu, H. and Duan, C. (2019). Cell-autonomous regulation of epithelial cell quiescence by calcium channel Trpv6. eLife 8, e48003. 10.7554/eLife.48003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, D. and Kim, J. (2020). Emerging role of transient receptor potential (TRP) channels in cancer progression. BMB Rep 53, 125-132. 10.5483/BMBRep.2020.53.3.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, P., Feng, J., Luo, J., Madison, M. and Hu, H. (2017). A Critical Role for TRP Channels in the Skin. In Neurobiology of TRP Channels (eds Emir T. L. R.), pp. 95-111. Boca Raton, FL: CRC Press/Taylor & Francis. [PubMed] [Google Scholar]
- Ye, M., Yang, W., Ainscough, J. F., Hu, X.-P., Li, X., Sedo, A., Zhang, X.-H., Zhang, X., Chen, Z., Li, X.-M.et al. (2014). TRPM2 channel deficiency prevents delayed cytosolic Zn2+ accumulation and CA1 pyramidal neuronal death after transient global ischemia. Cell Death Dis 5, e1541. 10.1038/cddis.2014.494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye, L., Bae, M., Cassilly, C. D., Jabba, S. V., Thorpe, D. W., Martin, A. M., Lu, H. Y., Wang, J., Thompson, J. D., Lickwar, C. R.et al. (2021). Enteroendocrine cells sense bacterial tryptophan catabolites to activate enteric and vagal neuronal pathways. Cell Host Microbe 29, 179-196e9. 10.1016/j.chom.2020.11.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin, Y. and Lee, S. Y. (2020). Current View of Ligand and Lipid Recognition by the Menthol Receptor TRPM8. Trends Biochem. Sci.. 45, 806-819. 10.1016/j.tibs.2020.05.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin, Y., Le, S. C., Hsu, A. L., Borgnia, M. J., Yang, H. and Lee, S. Y. (2019a). Structural basis of cooling agent and lipid sensing by the cold-activated TRPM8 channel. Science 363, eaav9334. 10.1126/science.aav9334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin, Y., Wu, M., Hsu, A. L., Borschel, W. F., Borgnia, M. J., Lander, G. C. and Lee, S. Y. (2019b). Visualizing structural transitions of ligand-dependent gating of the TRPM2 channel. Nat. Commun. 10, 3740. 10.1038/s41467-019-11733-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida, T., Inoue, R., Morii, T., Takahashi, N., Yamamoto, S., Hara, Y., Tominaga, M., Shimizu, S., Sato, Y. and Mori, Y. (2006). Nitric oxide activates TRP channels by cysteine S-nitrosylation. Nat. Chem. Biol. 2, 596-607. 10.1038/nchembio821 [DOI] [PubMed] [Google Scholar]
- Yu, Y., Keller, S. H., Remillard, C. V., Safrina, O., Nicholson, A., Zhang, S. L., Jiang, W., Vangala, N., Landsberg, J. W., Wang, J.-Y.et al. (2009). A functional single-nucleotide polymorphism in the TRPC6 gene promoter associated with idiopathic pulmonary arterial hypertension. Circulation 119, 2313-2322. 10.1161/CIRCULATIONAHA.108.782458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue, Z., Xie, J., Yu, A. S., Stock, J., Du, J. and Yue, L. (2015). Role of TRP channels in the cardiovascular system. Am. J. Physiol. Heart Circ. Physiol. 308, H157-H182. 10.1152/ajpheart.00457.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, X., Li, X. and Xu, H. (2012). Phosphoinositide isoforms determine compartment-specific ion channel activity. Proc. Natl. Acad. Sci. U.S.A. 109, 11384-11389. 10.1073/pnas.1202194109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, Z., Yu, H., Huang, J., Faouzi, M., Schmitz, C., Penner, R. and Fleig, A. (2014). The TRPM6 kinase domain determines the Mg.ATP sensitivity of TRPM7/M6 heteromeric ion channels. J. Biol. Chem. 289, 5217-5227. 10.1074/jbc.M113.512285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, X., Hu, M., Yang, Y. and Xu, H. (2018). Organellar TRP channels. Nat. Struct. Mol. Biol. 25, 1009-1018. 10.1038/s41594-018-0148-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng, W., Cai, R., Hofmann, L., Nesin, V., Hu, Q., Long, W., Fatehi, M., Liu, X., Hussein, S., Kong, T.et al. (2018). Direct Binding between Pre-S1 and TRP-like Domains in TRPP Channels Mediates Gating and Functional Regulation by PIP2. Cell Rep 22, 1560-1573. 10.1016/j.celrep.2018.01.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou, L., Li, T., Xing, Y. Q., Li, Y., Wu, Q. S. and Zhang, M. J. (2016). Novel TRPM1 mutations in two Chinese families with early-onset high myopia, with or without complete congenital stationary night blindness. Int J Ophthalmol 9, 1396-1402. 10.18240/ijo.2016.10.05 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou, Y., Castonguay, P., Sidhom, E. H., Clark, A. R., Dvela-Levitt, M., Kim, S., Sieber, J., Wieder, N., Jung, J. Y., Andreeva, S.et al. (2017). A small-molecule inhibitor of TRPC5 ion channels suppresses progressive kidney disease in animal models. Science 358, 1332-1336. 10.1126/science.aal4178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou, W., Yang, S., Li, B., Nie, Y., Luo, A., Huang, G., Liu, X., Lai, R. and Wei, F. (2020). Why wild giant pandas frequently roll in horse manure. Proc. Natl Acad. Sci. USA 117, 32493-32498. 10.1073/pnas.2004640117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou, Y., Bennett, T. M. and Shiels, A. (2021). Mutation of the TRPM3 cation channel underlies progressive cataract development and lens calcification associated with pro-fibrotic and immune cell responses. FASEB J. 35, e21288. 10.1096/fj.202002037R [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu, X., Chu, P. B., Peyton, M. and Birnbaumer, L. (1995). Molecular cloning of a widely expressed human homologue for the Drosophila trp gene. FEBS Lett. 373, 193-198. 10.1016/0014-5793(95)01038-G [DOI] [PubMed] [Google Scholar]
- Zubcevic, L., Herzik, M. A., Jr., Chung, B. C., Liu, Z., Lander, G. C. and Lee, S. Y. (2016). Cryo-electron microscopy structure of the TRPV2 ion channel. Nat. Struct. Mol. Biol. 23, 180-186. 10.1038/nsmb.3159 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.