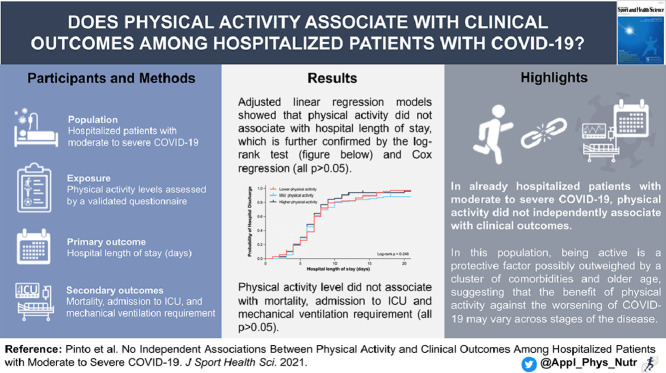
Highlights
-
•
In already hospitalized patients with moderate to severe coronavirus disease 2019 (COVID-19), physical activity did not independently associate with hospital length of stay.
-
•
There were no independent associations between physical activity and admission to the intensive care unit, mechanical ventilation requirement, or mortality.
-
•
In patients with more severe forms of COVID-19, being physically active is likely outweighed by a cluster of comorbidities (e.g., type 2 diabetes, hypertension, weight excess) and older age, suggesting that the benefit of physical activity against the worsening of COVID-19 may vary across stages of the disease.
Keywords: Hospital length of stay, Lifestyle, Physical inactivity, Prognosis, SARS-CoV-2
Abstract
Background
Regular physical activity (PA) has been postulated to improve, or at least maintain, immunity across the life span. However, the link between physical (in)activity and coronavirus disease 2019 (COVID-19) remains to be established. This small-scale prospective cohort study is nested within a randomized controlled trial aimed to investigate the possible associations between PA levels and clinical outcomes among hospitalized patients with moderate to severe COVID-19.
Methods
Hospitalized patients with COVID-19 (mean age: 54.9 years) were recruited from the Clinical Hospital of the School of Medicine of the University of Sao Paulo (a quaternary referral teaching hospital) and from Ibirapuera Field Hospital, both located in Sao Paulo, Brazil. PA level was assessed using the Baecke Questionnaire of Habitual Physical Activity. The primary outcome was hospital length of stay. The secondary outcomes were mortality, admission to the intensive care unit (ICU), and mechanical ventilation requirement.
Results
The median hospital length of stay was 7.0 ± 4.0 days, median ± IQR; 3.3% of patients died, 13.8% were admitted to the ICU, and 8.6% required mechanical ventilation. Adjusted linear regression models showed that PA indices were not associated with hospital length of stay (work index: β = –0.57 (95% confidence interval (95%CI): –1.80 to 0.65), p = 0.355; sport index: β = 0.43 (95%CI: –0.94 to 1.80), p = 0.536; leisure-time index: β = 1.18 (95%CI: –0.22 to 2.59), p = 0.099; and total activity index: β = 0.20 (95%CI: –0.48 to 0.87), p = 0.563). None of the PA indices were associated with mortality, admission to the ICU, or mechanical ventilation requirement (all p > 0.050).
Conclusion
Among hospitalized patients with COVID-19, PA did not independently associate with hospital length of stay or any other clinically relevant outcomes. These findings should be interpreted as meaning that, among already hospitalized patients with more severe forms of COVID-19, being active is a potential protective factor likely outweighed by a cluster of comorbidities (e.g., type 2 diabetes, hypertension, weight excess) and older age, suggesting that the benefit of PA against the worsening of COVID-19 may vary across stages of the disease.
Graphical abstract
1. Introduction
Physical inactivity has been considered a predisposing factor to acquired infections in several cohorts.1 A solid body of literature provides biological plausibility for the negative impact of physical inactivity on the immune system. For instance, chronic inactivity has been linked to increased systemic inflammation, impaired natural killer cell cytolytic activity, and reduced T-cell proliferation and cytokine production, which can ultimately lead to a loss of viral control.2
The immunoregulatory role of physical activity (PA) is also well-known. Regular PA has been postulated to improve, or at least maintain, immunity across the life span.2 There is also evidence that PA reduces the incidence as well as the number and severity of symptoms associated with acute respiratory infections (e.g., upper respiratory tract infection).3
The link between physical (in)activity and coronavirus disease 2019 (COVID-19) remains to be established. A study showed that physical inactivity increases the relative risk for COVID-19 hospital admission in a UK cohort by 32%,4 suggesting that the adoption of an active lifestyle could lower the risk of severe infection. This finding was corroborated by another population-based study showing that consistently meeting PA guidelines was strongly associated with a reduced risk for severe COVID-19 outcomes among infected adults.5 However, studies involving cohorts with more severe forms of the disease are scarce, so it remains unclear whether the benefits of PA will hold for more advanced disease states.
Recently, a retrospective study showed that a sedentary lifestyle was associated with mortality among hospitalized patients with COVID-19 (hazard ratio (HR) = 5.91).6 However, for patients who died, the PA questionnaires were completed by their relatives, which may have led to a reporting bias. Also, respondents had up to 120 days following discharge to complete the questionnaires, which may have increased the chance of memory bias. Therefore, it remains uncertain whether and to what extent physical (in)activity confers a better prognosis for patients already hospitalized with moderate-to-severe COVID-19.
This small-scale, prospective cohort study aimed to investigate the possible associations between PA levels and clinical outcomes (hospital length of stay, mortality, intensive care unit (ICU) admission, and mechanical ventilator requirement) among hospitalized patients with moderate-to-severe COVID-19.
2. Materials and methods
2.1. Study design
This is a prospective cohort study nested within a multicenter randomized controlled trial designed to test the safety and efficacy of vitamin D3 supplementation in hospitalized patients with moderate-to-severe COVID-19 (ClinicalTrials.gov Identifier: NCT04449718), conducted between June 2, 2020 and October 7, 2020 and published elsewhere.7 We assessed patients’ clinical status, coexisting chronic diseases, demographic characteristics, self-reported body weight and height, ethnicity, and PA upon hospital admission. Clinical outcomes were assessed through medical records.
2.2. Patients
Hospitalized patients were recruited from the Clinical Hospital of the School of Medicine of the University of Sao Paulo (a quaternary referral teaching hospital) and from Ibirapuera Field Hospital, both located in Sao Paulo, Brazil. Inclusion criteria were: (1) patients aged 18 years or older; (2) diagnosis of COVID-19 either by polymerase chain reaction for severe acute respiratory syndrome coronavirus 2 from nasopharyngeal swabs or by computed tomography scan findings (bilateral multifocal ground-glass opacities ≥50%) compatible with the disease; (3) diagnosis of flu syndrome with hospitalization criteria upon hospital admission (i.e., presenting with respiratory rate of ≥24 breaths per minute and saturation of ≤93% on room air) or risk factors for complications, such as heart disease, diabetes mellitus, systemic arterial hypertension, neoplasms, immunosuppression, pulmonary tuberculosis, and obesity, followed by COVID-19 confirmation. Patients who met these criteria were considered to have moderate-to-severe COVID-19. Exclusion criteria were: (1) patient unable to read and sign the written informed consent; (2) patient already admitted under invasive mechanical ventilation; (3) previous vitamin D3 supplementation (>1000 IU/day); (4) renal failure requiring dialysis or creatinine of ≥2.0 mg/dL; (5) hypercalcemia defined by total calcium of >10.5 mg/dL; (6) pregnant or lactating women; and (7) patients with expected hospital discharge of less than 24 h from admission.
The study was approved by the Ethics Committee of the Clinical Hospital of the School of Medicine of the University of Sao Paulo and by the Ethics Committee of Ibirapuera Field Hospital. All procedures were conducted in accordance with the Declaration of Helsinki. Participants provided written informed consent before being enrolled in the study (approval number: 30959620.4.0000.0068).
2.3. Outcome measures
PA levels were assessed using the Baecke Questionnaire of Habitual Physical Activity. The questionnaire consists of 3 sections: work, sport, and leisure-time activity. Scores in each section range between 0 and 5, where higher scores indicate a higher PA level. A total activity index is obtained by summing all scores (maximum score = 15).
The primary outcome was hospital length of stay, defined as the total number of days that patients remained hospitalized from the date of hospital admission until the date of hospital discharge or death. The criteria used for patient discharge were: (1) no need for supplemental oxygen in the past 48 h; (2) no fever in the past 72 h; and (3) oxygen saturation of >93% on room air without respiratory distress. The secondary outcomes were: (1) mortality; (2) admission to the ICU; and (3) mechanical ventilation requirement.
2.4. Statistical analysis
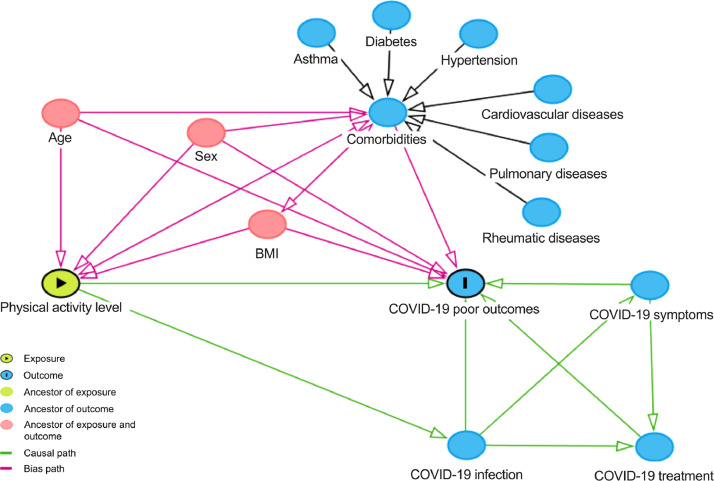
Associations between PA levels (independent variables) and hospital length of stay (primary outcome) were tested using linear regression models, whereas associations between PA levels and mortality, admission to ICU, and mechanical ventilation requirement (secondary outcomes) were tested using logistic regression models. In a sensitivity analysis for the primary outcome, patients were divided into tertiles according to total activity index. The log-rank test was used to compare the Kaplan–Meier estimate curves for length of stay according to tertiles of PA, with deaths being right-censored in the analysis. Cox regression models to estimate HRs, with corresponding 2-sided 95% confidence interval (95%CI), were also performed (the upper tertile was used as reference group). Regression models were performed without adjustments (unadjusted models) and with adjustments for potential confounders (adjusted models). Confounders were selected based on a Direct Acyclic Graph (DAG, www.dagitty.net), which is a causal diagram based on causal relations between the exposure, outcome, and potential confounders.8 The DAG was developed from a priori knowledge to identify a minimum yet sufficient set of covariates to remove confounding factors from the statistical analysis.9 The following covariates were identified to be included in the statistical model: age, sex, body mass index (BMI), and presence of comorbidities (i.e., sum of comorbidities) (Fig. 1). For the primary outcome, models were also adjusted for mortality.
Fig. 1.
Direct acyclic graph of the association between physical activity level and COVID-19 clinical outcomes. BMI = body mass index; COVID-19 = coronavirus disease 2019.
Normality tests (Shapiro–Wilk test) were used to determine whether total activity index and hospital length of stay were normally distributed. Then, potential differences between tertiles were assessed using the Kruskal–Wallis test for independent samples.
Statistical analyses were performed in SAS software (Version 9.4; SAS Institute Inc., Cary, NC, USA). Data are expressed as median ± interquartile range (IQR), β and 95%CI, odds ratio (OR) and 95%CI, or HR and 95%CI, unless stated otherwise. The significance level was set at p ≤ 0.050.
3. Results
3.1. Patients
Two hundred and nine patients had complete PA data and were included in this study. Patients’ age was 54.9 ± 14.5 years (mean ± SD); BMI = 31.0 ± 6.5 kg/m2; 51.7% were men; 48.3% were White; 48.8% had hypertension; 28.7% had diabetes; and 11.5% had cardiovascular diseases (Table 1). Overall, 62% had a positive severe acute respiratory syndrome coronavirus 2 polymerase chain reaction test at enrollment, and 59.6% had computed tomography scan findings suggestive of COVID-19. During the follow-up, all patients had their diagnosis confirmed by serological tests. One hundred and fifty-three patients (73.2%) required supplemental oxygen (134 were on oxygen therapy, and 19 were on non-invasive ventilation), and 62.2% had a computed tomography scan findings suggestive of COVID-19. Median ± IQR hospital length of stay was 7.0 ± 4.0 days, 3.3% of patients died, 13.8% were admitted to ICU, and 8.6% required mechanical ventilation. Mean work index was 2.1 ± 1.4 (range: 0.9–3.8), sport index was 1.8 ± 1.0 (range: 1.0–4.3), leisure-time index was 2.5 ± 1.0 (range: 1.0–4.3), and total activity index was 6.6 ± 2.3 (range: 3.1–11.1) (Table 1).
Table 1.
Demographic and clinical characteristics.
| n = 209 | |
|---|---|
| Age (year) | 54.9 ± 14.5 |
| Sex | |
| Male | 108 (51.7) |
| Female | 101 (48.3) |
| Race | |
| White | 101 (48.3) |
| Brown | 78 (37.3) |
| Black | 30 (14.4) |
| BMI (kg/m²) (n = 192) | 31.0 ± 6.5 |
| Underweight | 2 (1.0) |
| Normal | 28 (14.6) |
| Overweight | 58 (30.2) |
| Obesity | 104 (54.2) |
| Acute COVID-19 symptoms | |
| Fever | 149 (71.3) |
| Cough | 177 (84.7) |
| Fatigue | 184 (88.0) |
| Arthralgia | 71 (34.0) |
| Myalgia | 144 (68.9) |
| Nasal congestion | 78 (37.3) |
| Coryza | 87 (41.6) |
| Sore throat | 69 (33.0) |
| Diarrhea | 95 (45.5) |
| Coexisting diseases | |
| Hypertension | 102 (48.8) |
| Cardiovascular disease | 24 (11.5) |
| Diabetes | 60 (28.7) |
| Chronic obstructive pulmonary disease | 7 (3.3) |
| Asthma | 10 (4.8) |
| Rheumatic disease | 17 (8.1) |
| Concomitant medications | |
| Antibiotic | 207 (99.0) |
| Anticoagulant | 188 (90.0) |
| Analgesic | 139 (66.5) |
| Corticosteroids | 156 (74.6) |
| Antihypertensive | 101 (48.3) |
| Hypoglycemic | 42 (20.1) |
| Hypolipidemic | 28 (13.4) |
| Antiemetic | 117 (56.0) |
| Antiviral | 3 (1.4) |
| Proton pump inhibitor | 112 (53.6) |
| Levothyroxine | 17 (8.1) |
| Physical activity level | |
| Work index | 2.1 (1.4) |
| Sport index | 1.8 (1.0) |
| Leisure-time index | 2.5 (1.0) |
| Total activity index | 6.6 (2.3) |
Note: Data are expressed as mean ± SD (age and BMI), median ± IQR (physical activity level), or absolute and relative frequencies (n (%)).
Abbreviations: BMI = body mass index; COVID-19 = coronavirus disease 2019; IQR = interquartile range.
3.2. Primary outcome
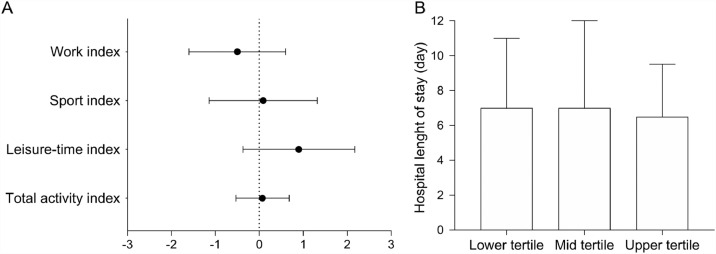
Unadjusted linear regression models showed that PA indices were not associated with hospital length of stay, except by the work index (β = –1.2 (95%CI: –2.4 to –0.1), p = 0.038) (Table 2). None of the PA indices were associated with hospital length of stay when adjusted for confounders (work index: β = –0.5 (95%CI: –1.6 to 0.6), p = 0.368; sport index: β = 0.1 (95%CI: –1.1 to 1.3), p = 0.882; and leisure-time index: β = 0.9 (95%CI: –0.4 to 2.2), p = 0.162; total activity index: β = 0.1 (95%CI: –0.5 to 0.7), p = 0.810) (Fig. 2A).
Table 2.
Unadjusted linear and logistic regression models between physical activity level and COVID-19 clinical outcomes.
| Variable | β or OR (95%CI) | p |
|---|---|---|
| Work index | ||
| Hospital length of stay | –1.2 (–2.4 to –0.1) | 0.038 |
| Mortality | 2.4 (0.9 to 6.5) | 0.073 |
| Admission to ICU | 1.5 (0.9 to 2.4) | 0.097 |
| Mechanical ventilation requirement | 1.1 (0.6 to 1.9) | 0.810 |
| Sport index | ||
| Hospital length of stay | 0.4 (–0.9 to 1.8) | 0.549 |
| Mortality | 0.9 (0.3 to 2.3) | 0.810 |
| Admission to ICU | 1.1 (0.7 to 2.0) | 0.635 |
| Mechanical ventilation requirement | 1.1 (0.5 to 2.1) | 0.852 |
| Leisure-time index | ||
| Hospital length of stay | 1.1 (–0.3 to 2.5) | 0.132 |
| Mortality | 1.2 (0.4 to 3.3) | 0.757 |
| Admission to ICU | 0.8 (0.4 to 1.3) | 0.336 |
| Mechanical ventilation requirement | 0.9 (0.4 to 1.7) | 0.677 |
| Total activity index | ||
| Hospital length of stay | –0.1 (–0.7 to 0.6) | 0.855 |
| Mortality | 1.3 (0.8 to 2.1) | 0.274 |
| Admission to ICU | 1.1 (0.8 to 1.4) | 0.470 |
| Mechanical ventilation requirement | 1.0 (0.7 to 1.4) | 0.973 |
Notes: Hospital length of stay is presented as β (95%CI) and mortality, admission to ICU, and mechanical ventilation requirement are presented as OR (95%CI). Bolded values indicate p < 0.050.
Abbreviations: 95%CI = 95% confidence interval; COVID-19 = coronavirus disease 2019; ICU = intensive care unit; OR = odds ratio.
Fig. 2.
Physical activity and hospital length of stay in hospitalized patients with severe coronavirus disease 2019 (COVID-19). (A) Adjusted linear regression models between physical activity level and hospital length of stay, data are presented as β and 95%CI. (B) Sensitivity analysis comparing hospital length of stay among tertiles of total physical activity, data are presented as median and IQR. 95%CI = 95% confidence interval; IQR = interquartile range.
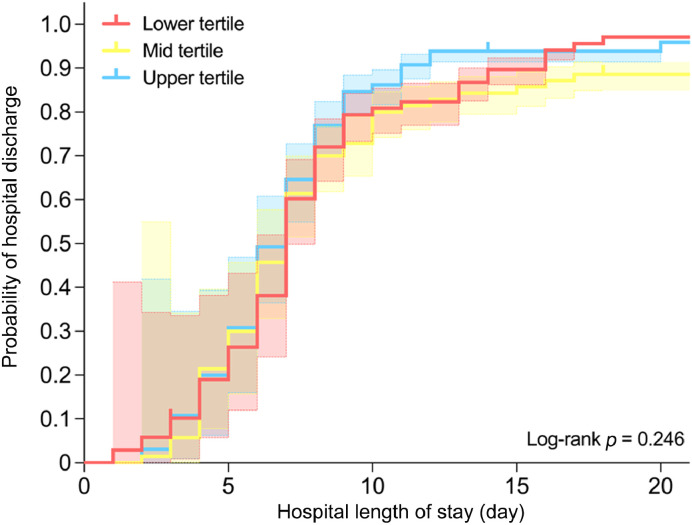
Lower (5.1 ± 1.0), mid (6.6 ± 0.5), and upper (8.1 ± 1.1) tertiles significantly differed in respect to total activity index (p < 0.001). Sensitivity analysis showed that hospital length of stay did not differ between lower (7.0 ± 4.0 days), mid (7.0 ± 5.0 days), and upper (6.5 ± 3.0 days) tertiles of total activity index (p = 0.733) (Fig. 2B), which is further confirmed by the log-rank test (p = 0.246) (Fig. 3) and Cox regression (lower tertile HR: 1.00 (95%CI: 0.69 – 1.45), p = 0.990; mid tertile HR: 0.90 (95%CI: 0.62 – 1.30), p = 0.576).
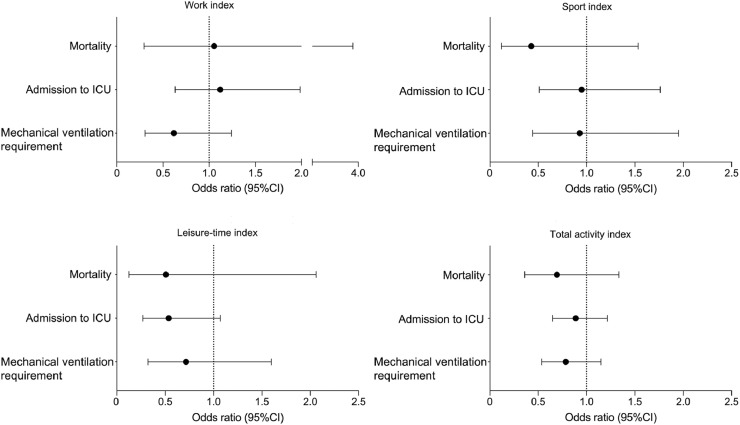
Fig. 4.
Adjusted logistic regression models between physical activity level and mortality, admission to ICU, and mechanical ventilation requirement. Data are presented as odds ratio and 95% confidence interval. ICU = intensive care unit.
Fig. 3.
Kaplan–Meier plot of time from hospital admission to hospital discharge according to tertiles of total activity index.
3.3. Secondary outcomes
Unadjusted logistic regression models showed that PA indices were not associated with any of the secondary outcomes (Table 2). Similarly, PA indices were not associated with mortality following adjustment for confounders (work index: OR = 1.1 (95%CI: 0.3–3.8), p = 0.936; sport index: OR = 0.4 (95%CI: 0.1–1.5), p = 0.192; leisure-time index: OR = 0.5 (95%CI: 0.1–2.1), p = 0.342; total activity index: OR = 0.7 (95%CI: 0.4–1.3), p = 0.272), admission to ICU (work index: OR = 1.1 (95%CI: 0.6–2.0), p = 0.697; sport index: OR = 0.9 (95%CI: 0.5–1.8), p = 0.867; leisure-time index: OR = 0.5 (95%CI: 0.3–1.1), p = 0.077; total activity index: OR = 0.9 (95%CI: 0.7–1.2), p = 0.459), and mechanical ventilation requirement (work index: OR = 0.6 (95%CI: 0.3–1.2), p = 0.176; sport index: OR = 0.9 (95%CI: 0.4–2.0), p = 0.844; leisure-time index: OR = 0.7 (95%CI: 0.3–1.6), p = 0.413; total activity index: OR = 0.8 (95%CI: 0.5–1.2), p = 0.214) (Fig. 4).
4. Discussion
This small-scale prospective cohort study provided novel evidence that, among patients with moderate-to-severe COVID-19, several comorbidities, and older age, PA levels do not independently associate with hospital length of stay or any other relevant clinical outcomes when adjusted for confounders.
The negative impact of physical inactivity on the immune system has been widely reported.2 Inactivity has been associated with higher rates of viral and bacterial infections, immunosenescence, and poor vaccine responses.2 In addition, inactivity is closely related to obesity, a condition that predisposes a person to poor antibody responses to vaccination and impaired lymphocyte proliferation following mitogenic stimulus.2 These immunological disturbances experienced by obese people are implicated in a greater risk of viral and bacterial infections as well as longer hospital length of stay due to more frequent and prolonged complications following surgery.10 This body of literature has supported the speculation that physical inactivity could be a risk factor for COVID-19 as well.
A few recent studies have supported this hypothesis. A large-scale observational study involving individuals residing in England showed that physical inactivity and obesity account for up to 8.6% and 29.5%, respectively, of the population attributable fraction of severe COVID-19,4 defined as a case requiring hospital admission. Another population-based study showed that patients with COVID-19 who were consistently inactive had a greater risk of hospitalization (OR = 2.26), admission to the ICU (OR = 1.73), and death (OR = 2.49) than those who were consistently meeting PA guidelines.5 These findings are corroborated by another study showing that being physically active is associated with a 35% reduction in hospitalization due to COVID-19 in Brazil.11 While these studies suggest that PA may prevent hospital admission and poor outcomes in infected patients, the influence of lifestyle on the prognosis of already hospitalized patients, for whom the presence of risk factors for a poor prognosis could outweigh the benefits of being physically active, remains to be determined. It may be possible that, in the present study, patients with the highest levels of PA were those who did not require hospitalization or who were discharged within 24 h. This could partially explain the absence of associations between PA and outcomes. In fact, none of the patients included in the study scored higher than 11 on the PA questionnaire.
A recent study retrospectively assessed PA levels in a cohort of hospitalized patients with COVID-19 (aged 18–70 years) and estimated that a sedentary lifestyle was associated with an increased in the risk of in-hospital mortality by approximately 6 times.6 However, this remarkable finding may have been influenced by reporting and memory biases and by limited control for confounders associated with poor prognosis for this disease. In the present study, by prospectively following a cohort of hospitalized patients with moderate-to-severe COVID-19, most of them presenting with several comorbidities and older age, we showed that PA levels did not associate with hospital length of stay or any other secondary outcome, such as death. Length of stay was similar in this and the previous study,6 with more active patients staying in hospital for 8 days (vs. a mean of 8.5 days in less active peers; p = 0.875) and 7 days (vs. a median of 7 days in less active peers; p = 0.024), respectively. It is not possible to do a direct comparison between the studies for deaths because the mortality rates were much lower in our cohort than in the other6 (3.3% vs. 8.7%). Therefore, we cannot rule out the possibility that the present study was underpowered for this secondary outcome.
It is difficult to contrast these findings with others since there is a paucity of analogous studies involving hospitalized COVID-19 patients. However, among patients with cardiovascular disease, higher PA levels were related to a slightly shorter hospital stay (0.9 days).12 A similar conclusion was reached by another study which observed that physical inactivity was a predictor of longer hospital lengths of stay among community-dwelling older adults (3.18 days vs. 0.82 days in active individuals).13 One may speculate that the main factor underlying the contrasting outcomes found in these cohorts and in ours are the patients’ characteristics. In fact, the longer length of stay in the current study (8.5 days vs. 2.6 days13 and 2.1 days12) appears to indicate that the disease in our patients was more severe than in those of the other cohorts.
The main strength of this study involves its novelty in assessing the influence of PA levels by using a validated questionnaire in a hospitalized cohort mainly comprised of older adults with numerous comorbidities and moderate-to-severe COVID-19. The main limitations of this study involve: (1) its observational nature, which hampers causative relationships; (2) a potential selection bias (collider), possibly leading to distortion of the associations among the present sample, which could differ from the population of those not selected or those who were unable/unwilling to participate; (3) the relatively small sample size, which increases the chances of type 2 errors, particularly considering the low incidence of the secondary outcomes; (4) the use of a questionnaire to assess PA, which is prone to recall bias and overreporting and is limited by the time frame of evaluation (i.e., 1 year); and (5) the lack of patient follow-up after hospital discharge.
5. Conclusion
In already hospitalized patients with COVID-19, PA did not independently associate with hospital length of stay or any other clinically relevant outcomes when adjusted for confounders. This does not mean that PA is futile in preventing COVID-19, particularly because being active may prevent several (if not all) comorbidities linked with poor prognosis for this disease. Rather, the current data should be interpreted as meaning that, among already hospitalized patients with more severe forms of COVID-19, being active is a potential protective factor likely outweighed by a cluster of comorbidities (e.g., type 2 diabetes, hypertension, weight excess) and older age, suggesting that the benefit of PA against the worsening of COVID-19 may vary across stages of the disease.
Acknowledgments
Acknowledgments
The authors are thankful to Monica Pinheiro, MD, MSc, and Roberta Costa, MSc, both from Ibirapuera Field Hospital, for the assistance with the study; all the staff members from both centers; and all the patients who participated in this study. AJP, KFG, IHM, ALF, RMRP, and BG were supported by São Paulo Research Foundation – FAPESP (grants No. 2015/26937-4, No. 2019/18039-7, No. 2019/24782-4, No. 2020/11102-2, No. 2016/00006-7 and No. 2020/05752-4, and No. 2017/13552-2). RMRP, HR, and BG were supported by Conselho Nacional de Desenvolvimento Científico e Tecnológico (grants No. 305556/2017-7, No. 301571/2017-1, and No. 301914/2017-6). Funding source had no involvement in study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the article for publication.
Authors' contributions
AJP participated in conception and design, participants’ recruitment, data collection, analysis and interpretation, statistical analysis, and manuscript writing; KFG participated in conception and design, participants’ recruitment, data collection, analysis and interpretation, and manuscript revision; ALF, IHM, LPS, BZR, and MDS, participated in participants’ recruitment, data collection, and manuscript revision; HR participated in data analysis and interpretation, statistical analysis, and manuscript revision; RMRP participated in data analysis and interpretation, obtaining funding, and manuscript revision; BG participated in conception and design, data analysis and interpretation, obtaining funding, and manuscript writing. All authors have read and approved the final version of the manuscript, and agree with the order of presentation of the authors.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Peer review under responsibility of Shanghai University of Sport.
Appendix. Supplementary materials
References
- 1.Hamer M, O'Donovan G, Stamatakis E. Lifestyle risk factors, obesity and infectious disease mortality in the general population: Linkage study of 97,844 adults from England and Scotland. Prev Med. 2019;123:65–70. doi: 10.1016/j.ypmed.2019.03.002. [DOI] [PubMed] [Google Scholar]
- 2.Damiot A, Pinto AJ, Turner JE, Gualano B. Immunological implications of physical inactivity among older adults during the COVID-19 pandemic. Gerontology. 2020;66:431–438. doi: 10.1159/000509216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Simpson RJ, Campbell JP, Gleeson M, et al. Can exercise affect immune function to increase susceptibility to infection? Exerc Immunol Rev. 2020;26:8–22. [PubMed] [Google Scholar]
- 4.Hamer M, Kivimaki M, Gale CR, Batty GD. Lifestyle risk factors, inflammatory mechanisms, and COVID-19 hospitalization: A community-based cohort study of 387,109 adults in UK. Brain Behav Immun. 2020;87:184–187. doi: 10.1016/j.bbi.2020.05.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sallis R, Young DR, Tartof SY, et al. Physical inactivity is associated with a higher risk for severe COVID-19 outcomes: A study in 48,440 adult patients. Br J Sports Med. 2021;55:1099–1105. doi: 10.1136/bjsports-2021-104080. [DOI] [PubMed] [Google Scholar]
- 6.Salgado-Aranda R, Pérez-Castellano N, Núñez-Gil I, et al. Influence of baseline physical activity as a modifying factor on COVID-19 mortality: A single-center, retrospective study. Infect Dis Ther. 2021;10:801–814. doi: 10.1007/s40121-021-00418-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Murai IH, Fernandes AL, Sales LP, et al. Effect of a single high dose of vitamin d3 on hospital length of stay in patients with moderate to severe COVID-19: A randomized clinical trial. JAMA. 2021;325:1053–1060. doi: 10.1001/jama.2020.26848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Joffe M, Gambhir M, Chadeau-Hyam M, Vineis P. Causal diagrams in systems epidemiology. Emerg Themes Epidemiol. 2012;9:1. doi: 10.1186/1742-7622-9-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Robins JM. Data, design, and background knowledge in etiologic inference. Epidemiology. 2001;12:313–320. doi: 10.1097/00001648-200105000-00011. [DOI] [PubMed] [Google Scholar]
- 10.Falagas ME, Kompoti M. Obesity and infection. Lancet Infect Dis. 2006;6:438–446. doi: 10.1016/S1473-3099(06)70523-0. [DOI] [PubMed] [Google Scholar]
- 11.de Souza FR, Motta-Santos D, Dos Santos Soares D, et al. Association of physical activity levels and the prevalence of COVID-19-associated hospitalization. J Sci Med Sport. 2021;24:913–918. doi: 10.1016/j.jsams.2021.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ek A, Kallings LV, Ekstrom M, Borjesson M, Ekblom O. Subjective reports of physical activity levels and sedentary time prior to hospital admission can predict utilization of hospital care and all-cause mortality among patients with cardiovascular disease. Eur J Cardiovasc Nurs. 2020;19:697–701. doi: 10.1177/1474515120921986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Woolcott JC, Ashe MC, Miller WC, Shi P, Marra CA, Team PR. Does physical activity reduce seniors' need for healthcare? A study of 24,281 Canadians. Br J Sports Med. 2010;44:902–904. doi: 10.1136/bjsm.2008.057216. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.