Summary
Background
Many smokers do not use existing free or low-cost smoking cessation services, cost-effective interventions to increase use are needed.
Methods
We did a 2-armed cluster randomised controlled trial (cRCT) in Hong Kong, China, to evaluate the effectiveness of active referral plus a small financial incentive on abstinence. Chinese adult smokers who smoked at least 1 cigarette per day were proactively recruited from 70 community sites (clusters). Random allocation was concealed until the recruitment started. The intervention group received an offer of active referral to cessation services at baseline plus an incentive (HK$300/US$38) after using any cessation services within 3 months. The control group received general brief cessation advice. The primary outcomes were biochemically validated abstinence at 3 and 6 months. Operating costs in real-world implementation was calculated. Trial Registry: ClinicalTrials.gov NCT03565796.
Findings
Between June and September 2018, 1093 participants were randomly assigned to the intervention (n=563) and control (n=530) groups. By intention-to-treat, the intervention group showed higher validated abstinence than the control group at 3 months (8.4% vs. 4.5%, risk ratio [RR] 1.88, 95% CI 1.01-3.51, P=0.046) and 6 months (7.5% vs. 4.5%, RR 1.72, 95% CI 1.01-2.93, P=0.046). Average cost per validated abstinence was lower in the intervention (US$ 421) than control (US$ 548) group.
Interpretation
This cRCT has first shown that a simple, brief, and low-cost intervention with active referral plus a small monetary incentive was effective in increasing smoking abstinence and smoking cessation service use in community smokers.
Funding
Hong Kong Council on Smoking and Health.
Keywords: smoking cessation, service use, financial incentives, active referral, community, smoker, Chinese
Research in context.
Evidence before this study
We searched PubMed and Google Scholar from December 31, 2000 to March 15, 2021, with the search terms “financial incentives”, “monetary incentives”, “contingent payment”, “reward”, “smoking”, and “tobacco” for randomised trials published in English and Chinese. We identified two relevant Cochrane reviews of incentive-based interventions for smoking cessation: offering financial incentives upon abstinence (including 33 trials) and reducing financial burden of tobacco dependence treatment (including 17 trials). High-certainty evidence showed financial incentives increased abstinence and moderate-certainty evidence supported full financial coverage of tobacco dependence treatment directed at smokers. However, incentive amounts varied considerably (US$45US$1185) and high-quality trials mostly used large incentives (≥ US$500) as a sole intervention to reward abstinence. Recent 2 trials showed supportive evidence of modest incentives (US$60US$190) contingent upon service use in low-income smokers. In Chinese smokers, a stand-alone incentive (US$64) increased quit attempts but did not increase quitting. Our sequential trials showed effectiveness of an active referral model in which briefly trained lay advisors connected smokers to smoking cessation services. We found no trial combined active referral model with a small incentive to increase cessation services use. Most incentive-based trials were in Western countries while Chinese accounts for one-third of the world’s smokers. There is a lack of evidence on the effect of incentives, with or without referral assistance, especially in Chinese smokers.
Added value to this study
Our trial shows that a small monetary incentive (US$38) combined with an active referral model for cessation services is effective to increase abstinence in community settings. The moderate effect size of the validated abstinence at 6 months is consistent with recent Cochrane reviews of financial incentives for abstinence and tobacco dependence treatment, supporting the benefit of small incentives in combination with existing cessation assistance. The combined intervention is simple, brief, low cost, and scalable in the real-world setting.
Implications of all the available evidence
To our knowledge, we have provided the first robust evidence to support the use of a small incentive contingent upon the use of cessation services as an adjuvant to active referral to smoking cessation services. As one of the sequential trials using active referral, we improved referral model by incentivising services use. Such intervention can be easily disseminated with brief training of lay persons to reach a large proportion of community smokers with low intention to quit, in whom the intervention effect seemed to be stronger than in those with higher intention to quit at baseline. Our findings suggested that smoking cessation service providers can consider offering small financial incentives to promote treatment uptake and abstinence cost-effectively.
Alt-text: Unlabelled box
Introduction
One-third of the world's population are covered by smoking cessation services at best practice level.1 Motivating community smokers to use smoking cessation service is challenging, although the benefits of smoking cessation treatments (medications, nicotine replacement therapies, and counselling) are well-recognised.2,3 In Hong Kong, the most westernised and developed city of China, smoking prevalence is low (10.2% in 2019) and many smokers were hard-core.4 Although cessation services are mostly free and easily accessible,5 many smokers (63.3%) were unmotivated to quit and very few (2.7%) had ever used any smoking cessation services.4 Government and smoking cessation service providers need cost-effective strategies to increase use and uptake of treatment.
Innovative interventions connecting smokers to cessation services have been studied with promising results. These included providing personalised risk information and taster sessions of smoking cessation service,6 patient navigators (buddy),7 connecting electronic health records to cessation providers,8 and referring smokers to cessation services.9 Our community-based trials showed the effectiveness of active referral approaches that lay advisors with a short period of training linking smokers to smoking cessation services on abstinence.10,11 However, two-thirds of participants who received the referral failed to use the chosen services, probably due to the mismatched time and schedule and low interest in using the services.11
By leveraging insights from behavioural economic, financial incentives are increasingly used to encourage smokers to quit.12,13 Meta-analyses showed high‐certainty evidence of offering monetary incentives upon quitting (including 33 trials, risk ratio [RR] 1.49)12 and moderate‐certainty evidence of covering expenses of tobacco dependence treatment (including 17 trials, RR 1.77)13 on abstinence at 6 months or longer in diverse populations and settings. Incentive trials mainly focused on patients7,14,15 with few focusing on smokers in the community.16,17 The amount of incentives varied considerably among trials (ranges US$45–US$1185) and whether larger incentives produced larger effects was unclear.12 Emerging evidence suggested the use of modest incentives (US$60–US$190) contingent upon treatment use18,19 and small amount of monetary incentives are more sustainable for application in large community-based smoking cessation programs.20 Our previous trial found a small, abstinence-contingent incentive (HK$500/US$64) effective in increasing quit attempt, but the effects on service use and abstinence was inconclusive.17 By different design features, incentives of similar amount varied in type, frequency, condition, and delivery of payment produced various effectiveness.21,22 Built on our prior work,10,11,17 we evaluated the effect of a small monetary incentive (HK$300/US$38) combined with an active referral on smoking cessation service use and abstinence in community smokers.
Methods
Study design
We conducted a parallel 2-arm, pragmatic, community-based cluster-randomised controlled trial (cRCT) nested within an annual smoking cessation campaign (Quit-to-Win Contest) organised by the Hong Kong Council on Smoking and Health.10,11,17,23,24 The trial protocol approved by the Institutional Review Board (IRB) of the University of Hong Kong/Hospital Authority Hong Kong West Cluster (UW18–318) has been registered with ClinicalTrials.gov (NCT03565796) and published elsewhere.25 Consolidated Standards of Reporting Trials (CONSORT) reporting guideline was followed.
Recruitment and participants
From June to September 2018, participants were individually recruited from 70 community sites in all 18 districts of Hong Kong. Eligible community sites were public spaces that allowed health promotion activities and had large numbers of pedestrians and smokers to ensure adequate recruitment. Recruitment took place in the vicinity of shopping malls, housing estates, pedestrian pavements, community centres, lottery centres, and commercial buildings. Similar to our previous trials,10,11,17,23,24 university students and volunteers from nongovernmental organisations (n=99) were trained as smoking cessation advisors through a 6-hour intensive workshop. Trained smoking cessation advisors proactively approached smokers using the “foot-in-the-door” approach by initiating a smaller and more acceptable request to increase the likelihood of agreeing to a second, larger request.26 Smoking cessation advisors first chatted with smokers a few simple questions, such as daily cigarette consumption, number of years of smoking, and past quitting attempts, to arouse their interest. Smokers who were willing to talk and eligible were invited to participate in the trial. Eligibility criteria included Hong Kong residents aged 18 years or older; smoking at least 1 cigarette per day in the past 3 months, validated by an exhaled carbon monoxide (CO) level of ≥4 parts per million; being able to communicate in Cantonese; owning a mobile phone for follow-up; and having intention to quit or reduce smoking. We excluded smokers who were participating in other smoking cessation programs and had communication barriers.
Interventions
Participants in the intervention and control groups received brief advice guided by the AWARD model (ask, warn, advise, refer, do-it-again; appendix p 1), which was modified from the 5As (ask, advise, assess, assist, and arrange) for use in community settings.10,11,17,23,24 Participants in the intervention group were actively referred to cessation services (Refer) and received a pocket-sized referral card describing the features of cessation services (available therapies, operational hours, and locations). Smoking cessation advisors assisted participants in choosing preferred service providers using the referral card. Participants were also informed of a small monetary incentive (HK$300/US$38) for using any types of cessation services (appendix p 1) within 3 months. We chose the fixed contingent incentive because of a standard amount could simplify the procedures for such a large group of community smokers recruited in a short period. As pre-commitment had been shown to motivate behavioural change,27,28 participants agreed to be referred were asked to sign a referral form to show commitment to quit and willingness to use the chosen cessation services. The contact details of signed participants were sent to the selected service providers within 1 week after enrolment. The service providers proactively called back the participants for scheduling phone counselling appointment or clinic visit afterwards. At 1- and 2-month booster calls, research staff encouraged the participants to use the services and provided referral assistance again if they failed to quit (Do-it-again). Participants who self-reported using the cessation services within 3 months received the cash coupons (HK$300/US$38). The incentives were delivered through registered mails with a cover letter acknowledging their participation and explaining the purpose of the reward.
Randomisation and blinding
The unit of randomisation was eligible community sites. An investigator (MPW) who was not involved in participant recruitment computer-generated the allocation sequence (1:1 ratio) using blocks of random size (2, 4, or 6). The randomisation database was password-protected (solely accessed by MPW) and allocation sequence was concealed from the smoking cessation advisors until the beginning of each recruitment session. Blinding of the advisors and participants was not possible, although all outcome assessors and statistical analysts remained blinded until the primary analyses were completed.
Outcomes
The primary outcomes were biochemically validated abstinence at 3 months (end of treatment) and 6 months after treatment initiation. Self-reported cessation was confirmed by the exhaled CO (<4 parts per million) and salivary cotinine (<10 ng/mL) tests.29,30 Secondary outcomes included self-reported 7-day point-prevalence abstinence (PPA), smoking reduction defined by at least a 50% reduction in daily cigarette consumption compared with that at baseline, quit attempt, and cumulative use of cessation service at 1, 2, 3 and 6 months. Research staff members visited self-reported quitters who had completed abstinence (not even a puff) for at least 7 days at 3 and 6 months for confirming abstinence biochemically. Exhaled carbon monoxide samples were measured using a piCO Smokerlyzer (Bedfont Scientific) and saliva cotinine samples were measured using a NicAlert test strip (Nymos Pharmaceutical Corporation) (appendix p 1). Participants were classified as service users if they reported having attended at least one treatment session delivered by any cessation service providers. The research staff regularly cross-checked the participants’ attendance records from cessation service providers.
Sample size
Power calculations were based on the results of our prior trial (6-month validated abstinence of 9.0% in the active referral group; 5.0% in the control group),10 and we conservatively assumed an additional effect size of 1.25 for a small incentive added to active referral (RR of full financial coverage for tobacco dependence treatment on abstinence was 1.7713). To have 80% power to detect a between group difference with a 5% two-sided type I error, 286 participants were needed per group. After adjusting for the clustering effect (design effect =1.24) of individuals being group recruited from the same recruitment site (an intra-cluster correlation coefficient [ICC] of 0.015,31 mean cluster size of 17, and 33 clusters in each group) and accounting for a retention rate of 70% at follow-ups, 10,11 a total of 1134 participants were required. A total of 70 clusters were eligible and agreed to participate in the trial, we decided to include them all.
Statistical analyses
The primary analyses were done by intention-to-treat analysis with missing outcomes assumed to have not changed from baseline. We used Generalised Estimating Equation (GEE) models by Poisson distribution with a log link and an exchangeable correlation structure to examine the intervention effect, accounting for potential clustering effect of recruitment sessions. We calculated ICCs to assess the proportion of variance in study outcomes. We pre-specified subgroup analyses to examine whether intervention effects differed by sex, age group, education level, household income, past quit attempt, nicotine dependence, and intention to quit.32,33 Interaction terms between these variables and study groups were included in multivariable models to calculate P value for multiplicative interaction. Intervention adherence analyses were conducted by comparing whether participants had received referral, used cessation service, and received the financial incentive. Post-hoc analyses explored the characteristics of adherent participants. The cost-minimisation analyses presented operating costs from the provider perspective only. We calculated the cost per abstinence at 6 months including costs of training, recruitment, and intervention materials and delivery (within 3-month treatment period), and excluded cost for implementing the Quit-to-Win contest (e.g., expenses on mass-media campaigns, lucky draw prizes). All costs were expressed in 2018-19 US$ using the official average annual exchange rate (US$1=HK$7.8). A two-sided P value of <0.05 was taken as statistically significant. Sensitivity analysis was conducted using Poisson regression without and with multiple imputation (appendix p 1). Bayesian multilevel modelling using Poisson regression was performed to address multiplicity concerns on co-primary outcomes (appendix p 1). Data was entered using SPSS version 25.0 and verified and analysed using Stata/MP version 15.1.
Role of the funding source
The funding source had no role in the study design, data collection, data analysis, data interpretation, or writing of the manuscript. MPW and XW had full access to all the data in the study. MPW had final responsibility for the decision to submit for publication.
Results
Participant characteristics
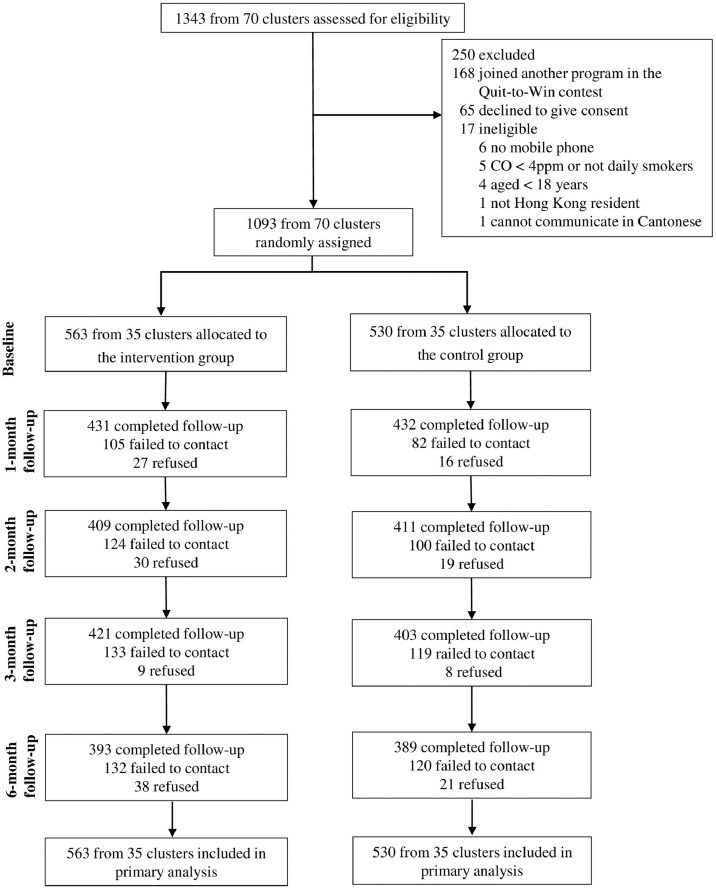
Of the 1343 smokers screened for eligibility in 70 community sites, 1093 participants (82.9% male) were eligible and consented to participate (Fig. 1). The participants were cluster‐randomised to the intervention group (n=563; 35 clusters) or control group (n=530; 35 clusters). Demographic characteristics and smoking profile were similar between the 2 groups (Table 1). At baseline, almost half of the participants (45.9%) had a low level of nicotine dependence, 9.6% had used cessation services, and over two-thirds (69.5%) were not ready to quit within 60 days. Overall retention rates were 79.0% (n=863) at 1 month, 75.0% (n=820) at 2 months, 75.4% (n=824) at 3 months, and 71.6% (n=782) at 6 months, which were similar between the 2 groups at all follow-ups (P=0.098–0.890).
Figure 1.
The Consolidated Standards of Reporting Trials (CONSORT) flow diagram
Table 1.
Baseline characteristics of the intention-to-treat population (N=1093)
| Intervention | Control | |
|---|---|---|
| Cluster level | ||
| Number of clusters | 35 | 35 |
| Mean cluster size (SD) | 16 (9.79) | 15 (7.17) |
| Individual levela | ||
| Number of participants | 563 | 530 |
| Sex | ||
| Male | 472 (83.84) | 434 (81.89) |
| Female | 91 (16.16) | 96 (18.11) |
| Ethnicity | ||
| Chinese | 563 (100.00) | 530 (100.00) |
| Age, years | ||
| ≤ 39 | 208 (38.73) | 225 (43.10) |
| 40-59 | 211 (39.29) | 193 (36.97) |
| ≥ 60 | 118 (21.97) | 104 (19.92) |
| Marital status | ||
| Single | 154 (32.22) | 159 (34.87) |
| Married/Cohabited | 302 (63.18) | 276 (60.53) |
| Divorced/separated/Widowed | 22 (4.60) | 21 (4.61) |
| Have a child | 171 (38.51) | 160 (38.83) |
| Education level | ||
| Primary or below | 73 (17.22) | 51 (13.04) |
| Secondary | 275 (64.86) | 248 (63.43) |
| Tertiary | 76 (17.92) | 92 (23.53) |
| Monthly household income, HK$7.8 = US$1.0 | ||
| Less than HK$25,000 | 193 (53.76) | 158 (48.47) |
| HK$25,000- HK$59,999 | 148 (41.23) | 138 (42.33) |
| HK$60,000 or more | 18 (5.01) | 30 (9.20) |
| Age at starting smoking, years | ||
| ≤ 17 | 270 (51.04) | 245 (49.00) |
| 18-25 | 229 (43.29) | 224 (44.80) |
| ≥ 26 | 30 (5.67) | 31 (6.20) |
| Daily cigarettes consumption, mean (SD) | 15.20 (10.33) | 14.95 (10.38) |
| Nicotine dependency (HSI)b | ||
| Light (≤ 2) | 243 (43.39) | 255 (48.66) |
| Moderate (3-4) | 270 (48.21) | 226 (43.13) |
| Heavy (5-6) | 47 (8.39) | 43 (8.21) |
| Past quit attempt(s) | ||
| No | 200 (36.76) | 207 (39.96) |
| Yes | 344 (63.24) | 311 (60.04) |
| Ever used cessation methods | ||
| Never tried to reduce or quit | 166 (31.86) | 177 (35.47) |
| Never used | 124 (23.80) | 112 (22.44) |
| On my own | 179 (34.36) | 164 (32.87) |
| Used smoking cessation service/medications | 52 (9.98) | 46 (9.22) |
| Intention to quit | ||
| Within 7 days | 124 (22.38) | 91 (17.40) |
| Within 30 days | 62 (11.19) | 52 (9.94) |
| Within 60 days | 27 (4.87) | 28 (5.35) |
| Undetermined | 341 (61.55) | 352 (67.30) |
| Perception of quitting, mean (SD)c | ||
| Importance | 6.58 (2.58) | 6.58 (2.48) |
| Confidence | 5.31 (2.59) | 5.30 (2.36) |
| Difficulty | 6.60 (2.57) | 6.58 (2.41) |
Data are n (%) unless otherwise stated. Sample size varied because of missing data.
HSI=Heaviness of Smoking Index.
Score: 0-10, higher scores indicating more.
Smoking cessation outcomes
Table 2 shows that by intention-to-treat, the intervention group had statistically significantly higher validated abstinence than the control group at 3 months (8.4% vs. 4.5%, RR 1.88, 95% CI 1.01-3.51, P=0.046) and 6 months (7.5% vs. 4.5%, RR 1.72, 95% CI 1.01-2.93, P=0.046). The self-reported 7-day PPA was also statistically significantly higher in the intervention group at 3 months (17.9% vs. 12.3%, RR 1.42, 95% CI 1.01-1.99, P=0.043) and 6 months (18.5% vs. 11.7%, RR 1.58, 95% CI 1.18-2.12, P=0.002). The 2 groups had similar smoking reduction rates and quit attempts. Cumulative use rate of cessation services was statistically significantly and much higher in the intervention group (from 12.1% to 21.5%) than in the control group (from 1.1% to 6.8%) at 1, 2, 3 and 6 months (all P<0.001). These results were similar in the regression and multiple imputation models (appendix p 2). The ICC for primary outcomes (0.03, appendix p 3) was slightly higher than anticipated. Intervention effect on primary outcomes remained robust in Bayesian analysis (appendix p 4).
Table 2.
Primary and secondary outcomes (N=1093)
| Intervention (N=563) | Control (N=530) | Generalised Estimating Equation model | |||||
|---|---|---|---|---|---|---|---|
| n | % | n | % | RR (95% CI) | P value | ||
| Primary outcomes | |||||||
| Validated abstinence | |||||||
| 3-month | 47 | 8.35 | 24 | 4.53 | 1.88 (1.01-3.51) | 0.046 | |
| 6-month | 42 | 7.46 | 24 | 4.53 | 1.72 (1.01-2.93) | 0.046 | |
| Secondary outcomes | |||||||
| Self-reported 7-day PPA | |||||||
| 1-month | 73 | 12.97 | 52 | 9.81 | 1.31 (0.89-1.93) | 0.17 | |
| 2-month | 83 | 14.74 | 53 | 10.00 | 1.47 (1.03-2.10) | 0.03 | |
| 3-month | 101 | 17.94 | 65 | 12.26 | 1.42 (1.01-1.99) | 0.043 | |
| 6-month | 104 | 18.47 | 62 | 11.70 | 1.58 (1.18-2.12) | 0.002 | |
| Smoking reductiona | |||||||
| 1-month | 110 | 22.45 | 106 | 22.18 | 1.00 (0.71-1.42) | 1.00 | |
| 2-month | 104 | 21.67 | 118 | 24.74 | 0.84 (0.60-1.17) | 0.30 | |
| 3-month | 94 | 20.35 | 117 | 25.16 | 0.75 (0.52-1.09) | 0.13 | |
| 6-month | 95 | 20.70 | 112 | 23.93 | 0.84 (0.59-1.19) | 0.33 | |
| Quit attempt(s) | |||||||
| 1-month | 110 | 19.54 | 112 | 21.13 | 0.92 (0.70-1.20) | 0.54 | |
| 2-month (cumulative) | 167 | 29.66 | 168 | 31.70 | 0.92 (0.75-1.13) | 0.45 | |
| 3-month (cumulative) | 193 | 34.28 | 186 | 35.09 | 0.96 (0.79-1.17) | 0.70 | |
| 6-month (cumulative) | 247 | 43.87 | 242 | 45.66 | 0.96 (0.84-1.09) | 0.51 | |
| Use of smoking cessation services | |||||||
| 1-month | 68 | 12.08 | 6 | 1.13 | 10.79 (4.32-26.94) | <0.001 | |
| 2-month (cumulative) | 97 | 17.23 | 9 | 1.70 | 10.18 (4.79-21.65) | <0.001 | |
| 3-month (cumulative) | 108 | 19.18 | 11 | 2.08 | 9.26 (5.08-16.87) | <0.001 | |
| 6-month (cumulative) | 121 | 21.49 | 36 | 6.79 | 3.17 (2.16-4.67) | <0.001 | |
RR: risk ratio; CI: confidence interval; PPA: point prevalence of abstinence.
Quitting not included as reduction.
Subgroup analyses
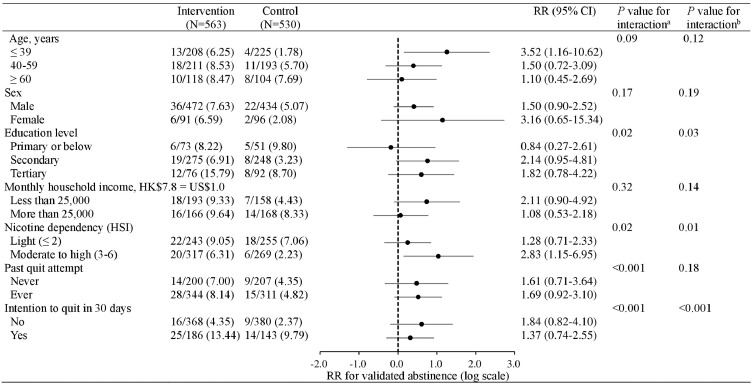
Figure 2 shows that by intention-to-treat, the RR of the intervention was greater in participants with secondary education (vs. elementary or below education, P for interaction=0.02), moderate to high nicotine dependent smokers (vs. light nicotine dependent, P for interaction=0.02), and those who had no intention to quit within 30 days at baseline (vs. intention to quit within 30 days, P for interaction <0.001). In 367 intervention group participants who were proactively referred to cessation services, 107 (29.2%) attended at least one session of services within 3 months and 74 (74/107, 69.2%) received the incentive eventually (appendix pp 5-6). Validated abstinence at 6 months were higher in adherent subgroups (i.e., received referral only, received referral and used the services, used the service with incentives) than those who refused referral, with adjusted RRs ranging from 1.46 to 6.37 (appendix p 6). Post-hoc analyses showed that adherent participants were more determined to quit within 30 days (vs. no intention to quit in 30 days) and had ever used service or medications (vs. never tried to reduce or quit) (appendix p 7).
Figure 2.
Validated abstinence in subgroups at 6 months
RR: risk ratio.
aP-value calculated by Poisson regression model.
bP-value calculated by multiple imputation model.
Cost-minimisation analyses
The total operating cost was slightly higher in the intervention group (US$17684) than in the control group (US$13146) (Table 3 and appendix p 8). The clinical impact of the intervention outweighed its higher cost as the average cost per validated abstinent at 6 months was 30% lower in the intervention group (US$421.0 [315.7-564.9]) than the control group (US$547.5 [371.9-813.2]).
Table 3.
Cost-minimisation analyses at 6 months
| Total costa | Average cost per validated abstinent participant | Average cost per self-reported abstinent participant | |
|---|---|---|---|
| US$ | US$ (95% CI) | US$ (95% CI) | |
| Intervention group | 17684 | 421.0 (315.7-564.9) | 170.1 (143.4-203.0) |
| Control group | 13146 | 547.5 (371.9-813.2) | 212.0 (168.4-269.0) |
| Difference between groups | 4538 | 126.5 (115.7-137.3) | 41.9 (39.4-44.4) |
Included expenses on manpower and materials needed for training, recruitment and intervention delivery. Detailed expenses are shown in Table S6 in the supplementary appendix.
Discussion
In this large, community-based cRCT, we have first found small monetary incentives combined with an active referral model increased validated abstinence and cessation service use at the end of the intervention (3 months after baseline) and 3 months post-treatment (6 months after baseline). The increases of about 75% in the validated abstinence and 239% in the actual usage rate of cessation services from our simple, brief, and low-cost intervention were substantial. The average cost per validated abstinence was 30% lower in the intervention group. The moderate effect size of the validated abstinence at 6 months (RR, 1.72) appears consistent with recent Cochrane reviews of financial incentive for smoking cessation (RR, 1.49)12 and tobacco dependence treatment (RR, 1.77),13 although a direct comparison is not feasible due to differences in participants’ characteristics, settings, and the intervention intensity and components. Compared with prior trials which were on patients[6], [7], [8], [9] or low-income smokers,18,19,34 we targeted and proactively recruited smokers in the general population who are less motivated than those who actively seek help. We trained laypeople as smoking cessation advisors, who are not healthcare professionals but are capable of providing the intervention. Hence, such intervention can be disseminated with brief training of volunteers, especially from medical and nursing schools.
About two-thirds of the participants in the intervention group were referred to cessation services. The improvement in more intensive treatment exposure is noteworthy given that 90% of participants had never used any cessation services or medications before and 70% were not willing to quit in the short term. Compared to those who refused referral, the most adherent participants had 6-fold validated abstinence at 6 months. We found that participants who had moderate to high nicotine dependence and had no intention to quit within 30 days were more responsive to our intervention.
The strategy of tailoring referral assistance and incentivising treatment use may help smokers overcome some of the barriers of using the service (e.g., time mismatch and low interest) and costs (travel, taking leave from work).11 Smoking cessation advisors introduced existing cessation services and the participants were free to choose services according to their preference and availability (e.g., treatment, time, location). Autonomy to choose services increased intrinsic motivation to change which might be further enhanced by small incentive.35 Participants agreed to be referred were also required to sign a commitment contract, which has been found to increase adherence and abstinence in prior trials.27,28 These mechanisms, however, needed to be further dissected properly.
Our previous trial had shown the effectiveness of active referral in the community setting on smoking cessation.10,11 Other trials in high-income countries mostly used large incentive (over US$500).14,21,34,36 But overly large incentives may limit intervention scalability to the population level and financial sustainability in the real-world setting.12 The present trial has provided the first evidence of effectiveness of a small incentive (US$38) contingent upon the use of cessation services as an adjuvant to active referral. Given Hong Kong has one of the highest gross domestic product per capita in the world (US$48676 in 2018),37 the amount was relatively small, probably the smallest compared to previous trials. Hence our findings converge with recent evidence that the size of the incentive does not have to be large, and its effect can be augmented when combined with other cessation interventions.18,19 The total cost of per validated abstinent in program operating costs (US$421.0) only accounted for 60% of costs per bed-day for public acute inpatients (US$707).38 Contingent reinforcement techniques39 to provide immediate incentives (i.e., presented at the same time of clinic visit) to strengthen the intervention effect should be tested.
Our study had other strengths. The proactive, multi-cluster Quit-to-Win campaign allowed us to recruit from all districts for a reasonably representative group of daily smokers mostly not ready to quit. Such a pragmatic design improves the applicability of intervention trials in real-world practices, given the small incentive and brief training of lay advisors (volunteers). We enrolled about 80% of eligible smokers and successfully followed up 72% at 6 months. Sensitivity analyses with the use of multiple imputation showed our findings were robust.
This trial had several limitations. First, the secondary outcome on the use of cessation services was self-reported. A concern of financial incentives was that participants could be untruthful about their behaviour to secure rewards.40 We checked the attendance records from the service providers and confirmed that 97% (105/108) of self-reported service users in the intervention group had attended the services. Second, as the incentives were sent by post, the delayed mode of remuneration might not be salient enough to overcome the present bias (the tendency of pursuing instant gratification).39 We also failed to deliver some incentives as 26 participants were unwilling to provide postal addresses because of privacy and personal concerns. Future trials may transfer monetary incentives through electronic payment methods to optimise the immediacy of incentive delivery. Third, as active referral and financial incentives were bundled, we cannot completely disentangle the effects of each intervention component and the treatments from the cessation service providers. Our previous trial had shown the effectiveness of active referral model,10,11 but not the stand-alone monetary incentive.17 A factorial trial is warranted to evaluate the unique contributions of each intervention component and the combined effects. Fourth, we only calculated operating costs and used simple cost-minimisation analyses. A more comprehensive analysis assessing the clinical and societal benefits of the combined intervention from a lifetime perspective may be more informative.41 Fifth, smoking behaviour change may result from being investigated,42 which was more likely in intensive intervention group. Sixth, the observed effect was slight lower than anticipated. The difference could be attributable to random error or differences between the present study and the prior trial used for effect size estimation.10 Lastly, the trial was conducted in Hong Kong, where smokers are predominantly male4 and cessation services are mostly free of charge.5 Generalisability to other populations where female smoking is more prevailing and free smoking cessation services are not available is uncertain.
Future research should test the long-term effects of using small incentives on cessation outcomes. Examining the underlying mechanisms of monetary incentives is also needed. Qualitative research exploring the mechanisms may explain the positive effect of monetary incentives on adherence and whether the social interactions (with lay advisors and treatment therapists) could further improve adherence and thus abstinence. Design features of monetary incentives warrant further investigation, such as the frequency and delivery of incentives, and whether the incentive is fixed or can be tailored to non-adherent smokers. Other incentive structures, such as social incentives within a gamification design, could be a scalable, low-cost approach to increase participants’ motivation and adherence.43
Smoking cessation services are free and effective but underused in Hong Kong. This pragmatic trial has shown that a proactive referral model combining a small incentive could increase validated abstinence by about 70% and tripled the use of smoking cessation service at 6 months. Our findings suggested that smoking cessation service providers can consider offering small financial incentives to promote treatment uptake and abstinence cost-effectively.
To conclude, this cRCT has first shown that a simple, brief, and low-cost intervention with active referral plus a small monetary incentive was effective in increasing smoking abstinence and smoking cessation service use in community smokers. Further studies on adherence, the mechanism, cost-effectiveness analysis, and longer-term abstinence are warranted.
Contributors
MPW, XW and THL conceived and design the study. XW coordinated the field work. XW did the statistical analysis with support from YW. XW wrote the first draft of the manuscript. All authors provided critical comments and approved the final manuscript for publication submission.
Data sharing statement
The study protocol, statistical analysis plan, and de-identified data are available on reasonable request to the corresponding author.
Editor note: The Lancet Group takes a neutral position with respect to territorial claims in published maps and institutional affiliations.
Declaration of Competing Interest
We declare no competing interests.
Acknowledgments
We would like to thank the participants for their participation in the study; the student helpers from universities and volunteers from non-governmental organisations for recruiting participants in the community; district partners and community organisations for supporting recruitment activities, and the research staff from the Smoking Cessation Research Team, School of Nursing, University of Hong Kong, Hong Kong SAR, China, for conducting the study.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.lanwpc.2021.100189.
Appendix. Supplementary materials
References
- 1.World Health Organization . Geneva: World Health Organization; 2019. WHO report on the global tobacco epidemic, 2019: offer help to quit tobacco use. [Google Scholar]
- 2.Lancaster T, Stead LF. Cochrane Database Syst Rev; 2017. Individual behavioural counselling for smoking cessation. CD001292. [DOI] [PubMed] [Google Scholar]
- 3.Stead LF, Carroll AJ, Lancaster T. Group behaviour therapy programmes for smoking cessation. Cochrane Database Syst Rev. 2017;(3) doi: 10.1002/14651858.CD001007.pub3. CD001007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Census and Statistics Department . Hong Kong SAR Government; Hong Kong SAR: 2020. Thematic Household Survey, Report No.70: Pattern of Smoking. [Google Scholar]
- 5.Tobacco and Alcohol Control Office. Smoking cessation clinics. https://www.taco.gov.hk/t/english/quitting/quitting_scc.html. Accessed April 28, 2021.
- 6.Gilbert H, Sutton S, Morris R. Effectiveness of personalised risk information and taster sessions to increase the uptake of smoking cessation services (Start2quit): a randomised controlled trial. Lancet. 2017;389(10071):823–833. doi: 10.1016/S0140-6736(16)32379-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lasser KE, Quintiliani LM, Truong V. Effect of patient navigation and financial incentives on smoking cessation among primary care patients at an urban safety-net hospital: a randomized clinical trial. JAMA Intern Med. 2017;177(12):1798–1807. doi: 10.1001/jamainternmed.2017.4372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fu SS, Van Ryn M, Sherman SE. Proactive tobacco treatment and population-level cessation: a pragmatic randomized clinical trial. JAMA Intern Med. 2014;174(5):671–677. doi: 10.1001/jamainternmed.2014.177. [DOI] [PubMed] [Google Scholar]
- 9.Vidrine JI, Shete S, Cao Y. Ask-Advise-Connect: a new approach to smoking treatment delivery in health care settings. JAMA Intern Med. 2013;173(6):458–464. doi: 10.1001/jamainternmed.2013.3751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang MP, Suen YN, Li WH. Intervention with brief cessation advice plus active referral for proactively recruited community smokers: a pragmatic cluster randomized clinical trial. JAMA Intern Med. 2017;177(12):1790–1797. doi: 10.1001/jamainternmed.2017.5793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Weng X, Luk TT, Suen YN. Effects of simple active referrals of different intensities on smoking abstinence and smoking cessation services attendance: a cluster-randomized clinical trial. Addiction. 2020;115:1902–1912. doi: 10.1111/add.15029. [DOI] [PubMed] [Google Scholar]
- 12.Notley C, Gentry S, Livingstone-Banks J, Bauld L, Perera R, Hartmann-Boyce J. Incentives for smoking cessation. Cochrane Database Syst Rev. 2019;(7) doi: 10.1002/14651858.CD004307.pub6. CD004307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van den Brand FA, Nagelhout GE, Reda AA. Cochrane Database Syst Rev; 2017. Healthcare financing systems for increasing the use of tobacco dependence treatment. CD004305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ladapo JA, Tseng C-H, Sherman SE. Financial incentives for smoking cessation in hospitalized patients: a randomized clinical trial. Am J Med. 2020 doi: 10.1016/j.amjmed.2019.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Volpp KG, Gurmankin Levy A, Asch DA. A randomized controlled trial of financial incentives for smoking cessation. Cancer Epidemiol Biomarkers Prev. 2006;15(1):12–18. doi: 10.1158/1055-9965.EPI-05-0314. [DOI] [PubMed] [Google Scholar]
- 16.White JS, Dow WH, Rungruanghiranya S. Commitment contracts and team incentives: a randomized controlled trial for smoking cessation in Thailand. Am J Prev Med. 2013;45(5):533–542. doi: 10.1016/j.amepre.2013.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cheung YTD, Wang MP, Li HCW. Effectiveness of a small cash incentive on abstinence and use of cessation aids for adult smokers: a randomized controlled trial. Addict Behav. 2017;66:17–25. doi: 10.1016/j.addbeh.2016.11.006. [DOI] [PubMed] [Google Scholar]
- 18.Fraser DL, Fiore MC, Kobinsky K. A randomized trial of incentives for smoking treatment in Medicaid members. Am J Prev Med. 2017;53(6):754–763. doi: 10.1016/j.amepre.2017.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Anderson CM, Cummins SE, Kohatsu ND, Gamst AC, Zhu SH. Incentives and patches for Medicaid smokers: an RCT. Am J Prev Med. 2018;55(6 Suppl 2) doi: 10.1016/j.amepre.2018.07.015. S138-s147. [DOI] [PubMed] [Google Scholar]
- 20.Mundt MP, Baker TB, Piper ME, Smith SS, Fraser DL, Fiore MC. Financial incentives to Medicaid smokers for engaging tobacco quit line treatment: maximising return on investment. Tob Control. 2020;29(3):320. doi: 10.1136/tobaccocontrol-2018-054811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Halpern SD, French B, Small DS. Randomized trial of four financial-incentive programs for smoking cessation. NEJM. 2015;372(22):2108–2117. doi: 10.1056/NEJMoa1414293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.White JS, Lowenstein C, Srivirojana N, Jampaklay A, Dow WH. Incentive programmes for smoking cessation: cluster randomized trial in workplaces in Thailand. BMJ. 2020;371:m3797. doi: 10.1136/bmj.m3797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang MP, Li WH, Cheung YT. Brief advice on smoking reduction versus abrupt quitting for smoking cessation in chinese smokers: a cluster randomized controlled trial. Nicotine Tob Res. 2017;20(1):67–72. doi: 10.1093/ntr/ntx026. [DOI] [PubMed] [Google Scholar]
- 24.Wang MP, Luk TT, Wu Y. Chat-based instant messaging support integrated with brief interventions for smoking cessation: a community-based, pragmatic, cluster-randomised controlled trial. The Lancet Digital Health. 2019;1(4) doi: 10.1016/S2589-7500(19)30082-2. e183-e192. [DOI] [PubMed] [Google Scholar]
- 25.Weng X, Wang MP, Li HCW. Effects of active referral combined with a small financial incentive on smoking cessation: study protocol for a cluster randomised controlled trial. BMJ Open. 2020;10(10) doi: 10.1136/bmjopen-2020-038351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Freedman JL, Fraser SC. Compliance without pressure: the foot-in-the-door technique. J Pers Soc Psychol. 1966;4(2):195. doi: 10.1037/h0023552. [DOI] [PubMed] [Google Scholar]
- 27.Bosch-Capblanch X, Abba K, Prictor M, Garner P. Contracts between patients and healthcare practitioners for improving patients' adherence to treatment, prevention and health promotion activities. Cochrane Database Syst Rev. 2007;(2) doi: 10.1002/14651858.CD004808.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Giné X, Karlan D, Zinman J. Put your money where your butt is: a commitment contract for smoking cessation. Am Econ J Appl Econ. 2010;2(4):213–235. [Google Scholar]
- 29.Javors MA, Hatch JP, Lamb RJ. Cut-off levels for breath carbon monoxide as a marker for cigarette smoking. Addiction. 2005;100(2):159–167. doi: 10.1111/j.1360-0443.2004.00957.x. [DOI] [PubMed] [Google Scholar]
- 30.Cooke F, Bullen C, Whittaker R, McRobbie H, Chen M-H, Walker N. Diagnostic accuracy of NicAlert cotinine test strips in saliva for verifying smoking status. Nicotine Tob Res. 2008;10(4):607–612. doi: 10.1080/14622200801978680. [DOI] [PubMed] [Google Scholar]
- 31.Chan SS, Cheung YT, Wong YM, Kwong A, Lai V, Lam TH. A brief smoking cessation advice by youth counselors for the smokers in the Hong Kong Quit to Win Contest 2010: a cluster randomized controlled trial. Prev Sci. 2018;19(2):209–219. doi: 10.1007/s11121-017-0823-z. [DOI] [PubMed] [Google Scholar]
- 32.Abdullah ASM, Yam HK. Intention to quit smoking, attempts to quit, and successful quitting among Hong Kong Chinese smokers: population prevalence and predictors. Am J Health Promot. 2005;19(5):346–354. doi: 10.4278/0890-1171-19.5.346. [DOI] [PubMed] [Google Scholar]
- 33.Li L, Feng G, Jiang Y, Yong H-H, Borland R, Fong GT. Prospective predictors of quitting behaviours among adult smokers in six cities in China: findings from the International Tobacco Control (ITC) China Survey. Addiction. 2011;106(7):1335–1345. doi: 10.1111/j.1360-0443.2011.03444.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Etter JF, Schmid F. Effects of large financial incentives for long-term smoking cessation: a randomized trial. J Am Coll Cardiol. 2016;68(8):777–785. doi: 10.1016/j.jacc.2016.04.066. [DOI] [PubMed] [Google Scholar]
- 35.Ryan RM, Deci EL. Self-determination theory and the facilitation of intrinsic motivation, social development, and well-being. Am Psychol. 2000;55(1):68–78. doi: 10.1037//0003-066x.55.1.68. [DOI] [PubMed] [Google Scholar]
- 36.Volpp KG, Troxel AB, Pauly MV. A randomized, controlled trial of financial incentives for smoking cessation. N Engl J Med. 2009;360(7):699–709. doi: 10.1056/NEJMsa0806819. [DOI] [PubMed] [Google Scholar]
- 37.World Bank. GDP per capita (current US$). https://data.worldbank.org/indicator/NY.GDP.PCAP.CD?locations=HK. Accessed April 28, 2021.
- 38.Hospital Authority. Hospital Authority Fees and Charges Review Result. 2016; https://gia.info.gov.hk/general/201612/15/P2016121500672_249763_1_1481803448655.pdf. Accessed April 28, 2021.
- 39.Lussier JP, Heil SH, Mongeon JA, Badger GJ, Higgins ST. A meta-analysis of voucher-based reinforcement therapy for substance use disorders. Addiction. 2006;101(2):192–203. doi: 10.1111/j.1360-0443.2006.01311.x. [DOI] [PubMed] [Google Scholar]
- 40.Fernandez Lynch H, Joffe S, Thirumurthy H, Xie D, Largent EA. Association between financial incentives and participant deception about study eligibility. JAMA Netw Open. 2019;2(1) doi: 10.1001/jamanetworkopen.2018.7355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.van den Brand FA, Nagelhout GE, Winkens B, Chavannes NH, van Schayck OCP, Evers SMAA. Cost-effectiveness and cost–utility analysis of a work-place smoking cessation intervention with and without financial incentives. Addiction. 2020;115(3):534–545. doi: 10.1111/add.14861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McCambridge J, Witton J, Elbourne DR. Systematic review of the Hawthorne effect: new concepts are needed to study research participation effects. J Clin Epidemiol. 2014;67(3):267–277. doi: 10.1016/j.jclinepi.2013.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Asch DA, Rosin R. Engineering social incentives for health. N Engl J Med. 2016;375(26):2511–2513. doi: 10.1056/NEJMp1603978. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.