Abstract
Allium species are medically important plants, rich in bioactive molecules with antitumoral properties. In this study, we aimed to investigate the molecular composition and in vitro anticancer activities of Allium willeanum Holmboe, an endemic Allium species of the island of Cyprus. GC-MS analysis of ethanolic extracts of A. willeanum H. bulb (AWB) showed bioactive molecules, octadecanoic acid 2-hydroxy-1-(hydroxymethyl)ethyl ester (21.99 %), hexadecanoic acid (20.42 %), pentadecanoic acid (9.19 %), 1,2-benzenedicarboxylic acid, diethyl ester (8.79 %), with known anticancer activities. AWB exerted significant reduction in mitochondria dependent metabolic activity as a measure of cell growth on MCF-7 (weakly metastatic) and MDA-MB-231 (strongly metastatic) human breast cancer (BCa) cell lines with a more prominent effect on highly metastatic BCa cells. Both trypan blue and lactate dehydrogenase (LDH) cytotoxicity assays quantitatively revealed that exposure to AWB extract significantly reduced cancer cell viability in both tested cell types. Differential activation of caspases in the tested cell lines indicated faster apoptotic activity on MDA-MB-231 cells compared to MCF-7. A significant reduction in cell motility was demonstrated upon AWB exposure of MCF-7 and MDA-MB-231 cells. AWB bioactive molecules collectively act on cancer cells to exert significant cytotoxic, apoptotic activities on both MCF-7 and MDA-MB-231 cells and significantly reduce their lateral motility, elucidating AWB as a promising agent to further be utilized in anticancer studies.
Keywords: Allium willeanum Holmboe, Breast cancer, MDA-MB-231, MCF-7, Anticancer activity
Allium willeanum Holmboe; Breast cancer; MDA-MB-231; MCF-7; Anticancer activity.
1. Introduction
Breast cancer is among the most prevalent cancer types globally observed in women, causing the highest number of cancer related deaths worldwide [1]. Chemotherapy, the currently preferred method, used along with surgical procedures have severe side effects that can reduce patients' quality of life [2] emphasizing the need to develop novel, direct and integrative therapies. Such developments can help improve disease outcomes and patients’ quality of life [3].
Genus Allium (including garlic) has been historically used as therapeutic plants because of their anticancer, antiinflammatory, antiobesity, antidiabetic, antioxidant, antibacterial, antifungal, neuroprotective, immunomodulatory effects and in prevention of cardiovascular diseases [4, 5]. Specifically, Allium sativum is of interest due to its various therapeutic properties including its anticoagulant, antihistaminic, antiparasitic, antifungal, antiprotozoan and antiviral functions [6, 7]. Allium sativum's anticancer properties have also been widely demonstrated, revealing that increased consumption of garlic decreases the risk of several cancer types such as colon, pancreas, breast cancer [8] and increased intake of Allium class vegetables decrease stomach cancer risk [9]. Further, Allium associated molecular activities are involved in control of several cancers like BCa, including suppression of deoxyribonucleic acid (DNA) adduct formation, activation of metabolizing enzymes responsible for detoxification of carcinogens and induction of apoptotic mechanisms [8, 10].
Natural flora of the island of Cyprus reserves several plants with therapeutic value, all of which are understudied. A. willeanum H. is one of eight Allium species endemic to the island of Cyprus belonging to the Amaryllidaceae family [11]. A. willeanum H. belongs to the same section as Allium sativum, which already has well established antitumoral activities [6], leading us to hypothesize that A. willeanum H. also has anticancer properties. So, in this study we sought to determine the in vitro anticancer activities of AWB on MDA-MB-231 (strongly metastatic BCa cell line) and MCF-7 (weakly metastatic BCa cell line) and revealed cytotoxic, antimetastatic, and apoptotic properties.
2. Materials and methods
2.1. Collection of wild Allium willeanum H. Samples
Fresh forms of A. willeanum H. were collected from Pentadactylos Mountains of North Cyprus in early summer. Identification of wild A. willeanum H. plant samples were performed by Prof. Dr. Mehmet Koyuncu, Chair of Pharmaceutical Botany Department, Cyprus International University, Faculty of Pharmacy. A specimen of Allium willeanum H. was stored in the Public Herbarium of Cyprus International University with “CIU38” herbarium number by Prof. Dr. Mehmet Koyuncu. No special permission is needed for collection purposes.
2.2. Extraction of Allium willeanum Holmboe
Collected and separated AWB bulb were dried at room temperature. Dried plant material was then powdered in mixer grinder. Maceration was carried out by mixing powdered material with ethanol (95 %) with 1:10 w/v ratio. Mixture was macerated at room temperature for 24 h and filtered through Whatman No. 1 filter paper. Concentration process of filtrates was performed at 40 °C by Rotary-evaporator (Heidolph, Germany). AWB yield was 0.634 %. Extract was labelled accordingly and stored at 4 °C for further analysis.
2.3. Culture conditions of breast cancer cells
MDA-MB-231 and MCF-7 BCa cells which were obtained from Imperial College, London, UK were grown in 4 mmol/l L-glutamine and 10 % foetal bovine serum supplemented Dulbecco's Modified Eagle Medium (DMEM) (Gibco by Life Technologies™, USA). Cells were incubated at 37 °C temperature and 100 % relative humidity with 5 % CO2 [12].
2.4. Cytotoxicity assays
Cytotoxic effect of Allium willeanum H. bulb extract on MCF-7 and MDA-MB-231 breast cancer cell lines were determined based on results from trypan blue exclusion (section 2.4.1), 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-2H-tetrazolium bromide (MTT) (section 2.4.2) and lactate dehydrogenase (LDH) assays (section 2.4.3).
2.4.1. Trypan blue exclusion assay
Trypan blue exclusion assay was used for or a direct identification and enumeration of live and dead MDA-MB-231 and MCF-7 breast cancer cells. Cells were plated in 35mm cell culture dishes (Thermo Scientific Nunc Cell Culture Dishes; Thermo Scientific; USA) with 3 × 104/ml density and incubated overnight before the AWB extract treatment (10000, 5000, 2500, 1250, 625, 312.5 and 156.25 μg/ml for 24, 48, 72 h period). Controls received only cell culture media. Percentage of alive cells in the assay was calculated from 20 randomly selected areas [13] upon investigation under inverted light microscope (Leica Microsystems, Germany).
2.4.2. 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-2H-tetrazolium bromide (MTT) assay
MTT assay was used in order to determine reduction of a 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-2H-tetrazolium bromide into formazan product by the mitochondria of viable breast cancer cell lines tested. Growth rate of the tested cell lines were detected by evaluating the linear relationship between cellular activity (via mitochondrial activity) and absorbance for the quantitative determination of cell proliferation. The change in the MDA-MB-231 and MCF-7 cell numbers was determined by colorimetric MTT assay as previously described by Isbilen and coworkers [14]. Both BCa cell lines were plated with 3 × 104/ml density overnight and then treated with AWB extract (10000, 5000, 2500, 1250, 625, 312.5 and 156.25 μg/ml) for designated time periods (24, 48, 72 h). Control experiments received only cell culture media. Measurements were taken at 490 nm on a multiwell plate reader (Biotek ELx800, Labx; Canada) [14]. Excel was used to calculate IC50 value for the extract concentration that killed 50 % of the cells.
2.4.3. LDH cytotoxicity assay
Lactate Dehydrogenase (LDH) is a cytosolic enzyme released as a result of damaged plasma membrane which is directly proportional to cellular cytotoxicity. Effect of AWB exposure (10000, 5000, 2500, 1250, 625, 312.5 and 156.25 μg/ml) on LDH release was quantitatively measured by incubating MCF-7 and MDA-MB-231 cells (0.5 × 106/ml) for 24 h and assessed by using LDH Cytotoxicity Assay Kit (Thermo Scientific, USA) according to the manufacturers’ instructions. Control experiments received only cell culture media.
2.5. Wound healing assay
For wound healing assay, BCa cells were plated with 4 × 105/ml density and incubated at 37 °C, 5 % CO2 overnight. Wounds were formed by using 200μl Gilson pipette tips with 0.5–0.8 mm width. The media was replaced with fresh media containing Allium extract and wounds widths were measured (margin to margin) under inverted light microscope camera (Leica, Germany) via ImageJ software. After 24h of treatment with AWB extract, wound widths were re-measured and re-evaluated [14]. Motility index (MoI) was used for calculation of lateral motility of breast cancer cell lines.
| MoI = 1-(W1/W0) |
which W1 stands for the width of the wound at time t (24h) while W0 is the starting wound width (time = 0 h) [14]. Control experiments received only cell culture media.
2.6. Caspase-3 and Caspase-9 activity
Determination of caspase-3 and -9 activation in MCF-7 and MDA-MB-231 breast cancer cell lines (0.5 × 106/ml) upon 24h AWB exposure (10000, 5000, 2500, 1250, 625, 312.5 and 156.25 μg/ml) was carried out quantitatively by Caspase-3 Colorimetric Activity Assay Kit (Merck Millipore, USA) and Caspase-9 Colorimetric Activity Assay Kit (Merck Millipore, USA) according to the manufacturer's instructions. Control experiments received only cell culture media.
2.7. Gas chromatography mass spectroscopy (GC-MS) analysis
Gas chromatography mass spectroscopy analysis was performed as previously described by Leikshmi and coworkers [15]. 1ml of A. willeanum H. bulb extract was analysed using a GCMS-QP2010 PlusSystem (Shimadzu, Japan). Helium was used as carrier gas in the constant flow mode at 1 ml/min. GC-MS oven initial temperature was 50 °C (for 2 min) which was gradually increased by rate of 5 °C/min up to 280 °C and maintained for 9 min. Injection port temperature was ensured as 250 °C and detector temperature was 300 °C. The ionization voltage was 70eV. 30 m long RTS volatile column was used for separation of compounds. Quadrupole Mass Detector was employed to detect compounds when they were vented from the column. Using MS data library WILEY7.LIB the spectrum was analysed and compounds were identified [15].
2.8. Statistical analysis
Experiments were performed in triplicates. All the figures and tables were created using Excel. Data were presented as mean ± S.E.M. Data were analysed by Student's unpaired t-test or Newman-Keuls post hoc comparison (GraphPad InStat).
3. Results and discussion
3.1. AWB extract induces cytotoxicity on MCF-7 and MDA-MB-231 BCa cells
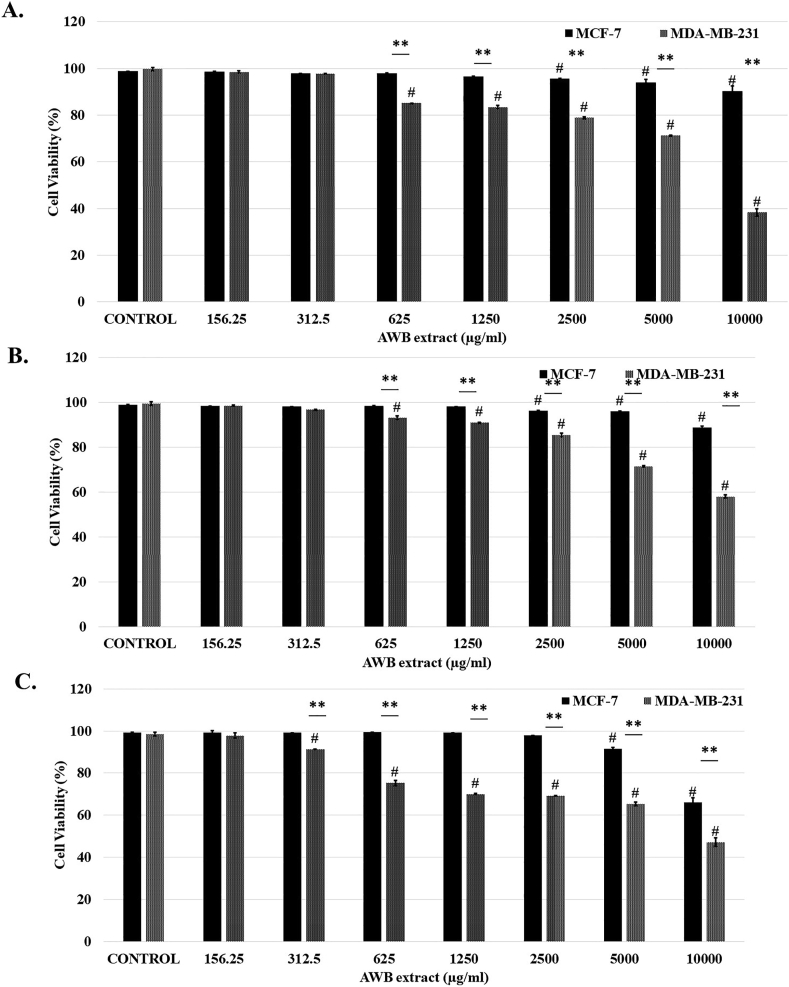
Trypan blue exclusion assays were performed to determine cytotoxic effects of AWB on MCF-7 and MDA-MB-231 at different time points (24, 48 and 72h). Results were used to comparatively analyze the toxicity of AWB extract on MCF-7 vs MDA-MB-231 cells (Figure 1). AWB extract exhibited significantly higher toxicity on MDA-MB-231 cells (24h: 60.4 %; 48h: 41.39 %; 72h: 51.32 % decrease in viability; control vs 10000 μg/ml; p < 0.01) compared to MCF-7 cells (24h: 8.52 %; 48h: 10.25 %; 72h: 33.25 % decrease in viability; control vs 10000 μg/ml; p < 0.01) revealing the preferential targeting of highly metastatic BCa cells by the AWB for all the tested time points (24, 48, 72h) (Figure 1).
Figure 1.
Allium willeanum H. bulb (AWB) extract significantly reduces the viability of MCF-7 and MDA-MB-231 cells at 24 h (A), 48h (B) and 72h (C) of incubation. Statistical significance of MCF-7/MDA-MB-231 vs control represented as “#”.∗, p < 0.05 and ∗∗, p < 0.01 MCF-7 vs MDA-MB-231. Controls received only culture media. Data represent mean ± S.E.M.
Overall, AWB incubation resulted in a consistent, concentration dependent decrease in cell viability. Significant cytotoxic activity was shown at 625 μg/ml and onwards at 24 and 48h and 312.5 μg/ml and onwards at 72h of incubation with MDA-MB-231 cells (p < 0.05). However, significant toxicity in MCF-7 cells was observed at 2500 μg/ml and onwards at 24 and 48h; 5000 μg/ml and onwards at 72h of incubation (p < 0.05, Figure 1). The increased cytotoxic activity of AWB on MDA-MB-231 cells at lower concentrations compared to MCF-7 cells reveals the cytotoxic efficiency of AWB on strongly metastatic cancer cells.
3.2. AWB extract impacts mitochondrial integrity dependent cell growth of MCF-7 and MDA-MB-231 cells
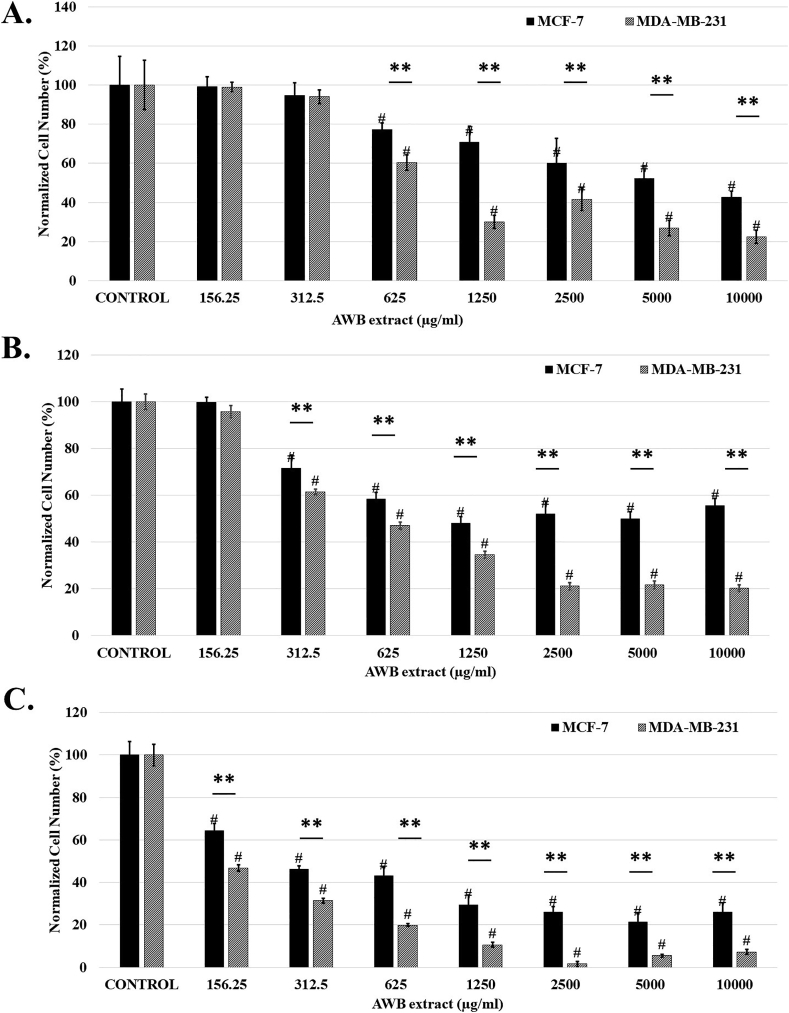
Metabolic viability based assays like MTT is extensively used in determining cell viability. Alterations in both mitochondrial content and metabolism can influence the metabolic viability of cells. Therefore, we tested to see if ethanolic AWB extract induced cell death in MCF-7 and MDA-MB-231 breast cancer cell lines via a mitochondria dependent pathway. Upon incubating BCa cell lines with AWB extract for 24, 48 and 72 h periods, AWB incubations revealed a significant decrease in MTT absorbance of cells, as a measure for reduction in cell growth in both MCF-7 and MDA-MB-231 BCa cells, in a concentration dependent manner. Interestingly, a more significant effect was observed in MDA-MB-231 cells (24 h: 39.06 % decrease at 625 μg/ml; 48 h: 38.52 % decrease at 312.5 μg/ml; 72 h: 53.27 % decrease at 156 μg/ml; p < 0.01; n = 3; Figure 2) compared to MCF-7 cells (24 h: 29.14 % decrease at 625 μg/ml; 48 h: 28.54 % decrease at 325 μg/ml, 72 h: 35.62 % decrease at 156 μg/ml; p < 0.01; n = 3; Figure 2) demonstrating an especially effective decrease in cell growth for strongly metastatic cancer cells.
Figure 2.
Allium willeanum H. bulb (AWB) extract impacts viability of MCF-7 and MDA-MB-231 cells in mitochondria dependent manner. Statistical significance of MCF-7/MDA-MB-231 vs control represented as “#”.∗, p < 0.05 and ∗∗, p < 0.01 MCF-7 vs MDA-MB-231. Controls received only culture media. Data represent mean ± S.E.M.
In order to gain insight towards the differential effect of AWB on the two cell lines, the IC50 values for 24, 48 and 72 h incubations were calculated based on the 50 % alive cells compared to the control experiments obtained from the MTT assays. IC50 values for AWB treatment on both BCa cells for all time periods (24, 48 and 72 h incubations) were evaluated. Data revealed that AWB treated MDA-MB-231 BCa cells have lower IC50 values indicating stronger effect on highly metastatic MDA-MB-231 cell line compared to the MCF-7 cell line (Table 1).
Table 1.
IC50 values of MCF-7 and MDA-MB-231 cells incubated with AWB extract. Data represent mean ± S.E.M.
| IC50 (μg/ml) | ||
|---|---|---|
| MCF-7 | MDA-MB-231 | |
| 24 h | 2972.2 ± 234 (p < 0.05) | 1207.3 ± 189 (p < 0.05) |
| 48 h | 1076.7 ± 118 (p < 0.05) | 862.6 ± 112 (p < 0.05) |
| 72 h | 397.1 ± 59 (p < 0.05) | 276.8 ± 38 (p < 0.05) |
3.3. AWB extract induced cytotoxicity determined by LDH release
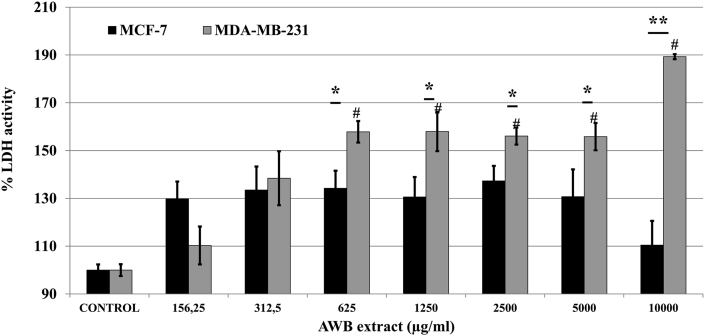
LDH release levels were evaluated for both MCF-7 and MDA-MB-231 cells, to quantitatively assess cytotoxicity upon AWB treatment. Cells were incubated with AWB extract for 24h where control experiments were taken as 100 %. It was revealed that incubations with AWB caused significant increase in LDH release on MDA-MB-231 cells (24h: 189.29 ± 1.05; at 10000 μg/ml; p < 0.05; Figure 3). On the other hand, AWB incubations did not cause significant levels of LDH release on MCF-7 cells (24 h: 110.47 ± 10.13; at 10000 μg/ml; p > 0.05; Figure 3). These results quantitatively support the consistently observed robust cytotoxic effects of the AWB specifically on highly metastatic breast cancer cell line MDA-MB-231.
Figure 3.
Allium willeanum H. bulb (AWB) extract significantly induces LDH release on MDA-MB-231 BCa cells. Statistical significance of MCF-7/MDA-MB-231 vs control represented as “#”.∗, p < 0.05 and ∗∗, p < 0.01 MCF-7 vs MDA-MB-231. Controls received only culture media. Data represent mean ± S.E.M.
AWB cytotoxicity on cancer cells was quantitatively assessed using L-Lactate dehydrogenase (LDH) release. Upon cellular exposure to endogenous or exogenous damage, LDH is released from cytoplasm to the extracellular milieu [16]. Measuring LDH release can provide quantitative insight towards the damage exerted on the cancer cells by AWB. LDH cytotoxicity assay results revealed that incubation with AWB extract had no significant increase in LDH release in MCF-7 cells where MDA-MB-231 cells showed elevated levels of LDH through the 24 h incubation (Figure 3). This result is consistent with the higher levels of cytotoxicity observed in MDA-MB-231 with Trypan Blue Exclusion assay (Figure 1) emphasizing the increased toxicity exerted on the strongly metastatic cell line MDA-MB-231 compared to the weakly metastatic cell line MCF-7. Molecular investigation of AWB composition revealed 20.42 % hexadecanoic acid which was previously revealed to exhibit cytotoxic activities on MCF-7 and MDA-MB-231 breast cancer cells [17] likely contributing to the cytotoxic activities associated with AWB extract.
3.4. AWB extract induces Caspase-3 and -9 activities in MCF-7 and MDA-MB-231
Caspase-3 and -9 activation studies were carried out in order to gain insight towards the mechanism associated with the observed cell death. Caspases are a family of endonucleases that are responsible for sustaining homeostasis by regulating inflammation and apoptotic cell death [18]. Caspase family comprises of the initiator caspases (caspase-8 and -9) which are responsible for activation of the executioner caspases (caspases-3, -6, and -7) that subsequently organize their activities to demolish key structural proteins and activate other enzymes where this activation in the caspase cascade leads to programmed cell death mechanisms [18, 19].
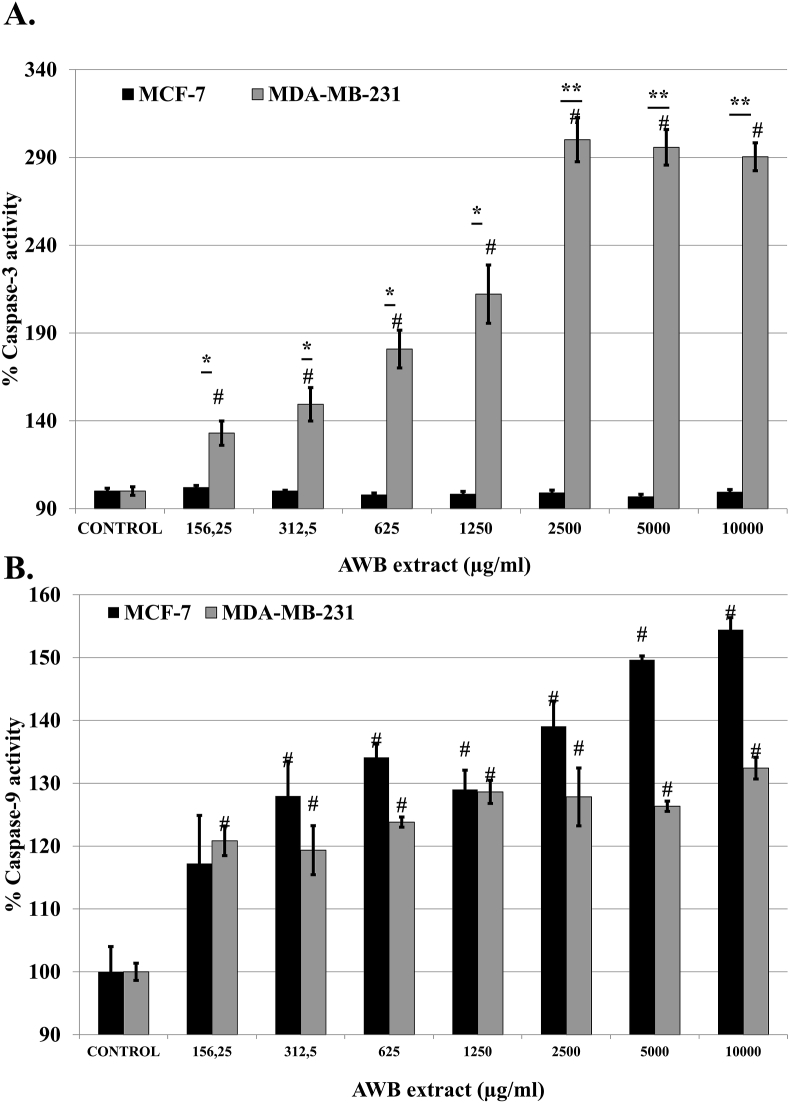
In order to investigate if cytotoxic properties associated with AWB are mechanistically based on apoptosis, caspase-3 and -9 activities were investigated. % relative caspase-3 activity was evaluated compared to the negative control for AWB extract on MCF-7 and MDA-MB-231 BCa cell lines. Control experiments were taken as 100 %. In comparison to untreated MDA-MB-231 BCa cells, AWB treatment caused significantly increased caspase-3 activity (24h: 290 ± 7.97 %; at 10000 μg/ml; p < 0.05; Figure 4A) for the 24h period. On the other hand, AWB treatments on MCF-7 cells did not show any significant increase in caspase-3 activity (24h: 99.38 ± 1.48 %; at 10000 μg/ml; p > 0.05; Figure 4A) revealing the apoptotic activity of the extract exerted specifically on aggressively metastatic cancer cell line, MDA-MB-231. Similarly, AWB incubations significantly increased caspase-9 activation in MDA-MB-231 BCa cells (24h: 132.41 ± 1.73 %; at 10000 μg/ml; p < 0.05; Figure 4B) as well as causing a significant increase in caspase-9 activity in MCF-7 cells (24h: 154.43 ± 1.92 %; at 10000 μg/ml; p < 0.05; Figure 4B). These results cumulatively indicate that apoptotic activity takes place in a faster, more robust way in the MDA-MB-231 cell line compared to the MCF-7 cell line. This was observed by the activation of only the initiator caspase-9 in MCF-7 cells, while both the initiator and executioner caspases (-9 and -3) carried on with activity in MDA-MB-231 cells, suggesting an advanced cascade.
Figure 4.
Allium willeanum H. bulb (AWB) extract significantly increases the activity of (A) caspase-3 and (B) caspase-9 in MCF-7 and MDA-MB-231 BCa cells. Statistical significance of MCF-7/MDA-MB-231 vs control represented as “#”.∗, p < 0.05 and ∗∗, p < 0.01 MCF-7 vs MDA-MB-231. Controls received only culture media. Data represent mean ± S.E.M.
It was previously shown that octadecanoic acid and hexadecanoic acid incubations induced caspase-8 and caspase-3 mediated cell death in PC12 cells [20, 21]. Incubation with AWB caused a significant increase (p < 0.05) in caspase-3 in MDA-MB-231 cells where no significant increase was observed in MCF-7 cells (p > 0.05) (Figure 4A). Conversely, AWB incubation led to a higher induction in caspase-9 activation in MCF-7 cells compared to the MDA-MB-231 cells (Figure 4B). This phenomenon suggest that presence of these bioactive molecules may activate caspase-dependent cell death mechanism in both MCF-7 and MDA-MB-231 breast cancer cell lines. Albeit a faster and more robust activation of apoptotic pathways in MDA-MB-231 cells was observed indicating that this cell line moved forward to the activation of executioner caspases like caspase-3 whereas MCF-7 cells were at the beginning stages of a lagging apoptotic process, only achieving the initiator caspase-9 activation at the time point tested. Morphological investigation of AWB extract treated MDA-MB-231 cells via microscopy also revealed presence of membrane blebs (data not shown) which are associated with apoptotic cascade activation [22]. Membrane blebbing observed during apoptosis depends on caspase-3 mediated cleavage of ROCK-I. Rho-activated serine/threonine kinase (ROCK-I) protein regulates actomyosin filament assembly as well as myosin contractility by inducing increased phosphorylation of the myosin regulatory light chain (MLC) [22]. Previous studies revealed that the activation of caspase-3 induces ROCK-I cleavage, leading to removal of its inhibitor domain. Cleavage of ROCK-I protein activates MLC phosphorylation along with cell contraction and membrane blebbing [22]. The differential activation of caspases by AWB, associated with a delay or inability to activate caspase-3 in MCF-7 cells may provide an explanation towards the decreased viability observed in weakly metastatic MCF-7 cell line.
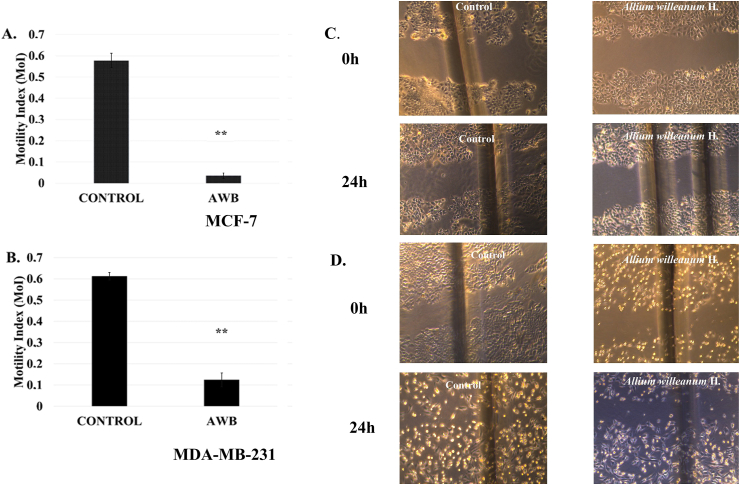
3.5. AWB significantly reduces the lateral motility of MCF-7 and MDA-MB-231 cells
Wound healing assay also known as lateral motility assay is classically performed in order to evaluate the closure of the manually created scratch on a monolayer of cells. This assay was used to determine the effect of AWB extract on lateral motility of breast cancer cell lines MCF-7 and MDA-MB-231. 156.25 μg/ml extract concentration was selected as it had no significant toxicity effect on either cell line. The extract reduced the MoI (Motility Index) in both tested cell lines (Figure 5). Treatment of the MCF-7 cells with control revealed an MoI of 0.57 ± 0.03 where upon AWB extract treatment (24h), the MoI of MCF-7 cells was significantly reduced to 0.035 ± 0.01 (p < 0.05) (Figures 5A, 5C) demonstrating a decrease in motility. MDA-MB-231 cells control group MoI was 0.40 ± 0.01 such that upon AWB extract treatment (24h), the MoI of MDA-MB-231 cells were reduced to 0.12 ± 0.03 (p < 0.01) (Figures 5B, 5D). The significant reduction observed in the motility index indicates that AWB extract interferes with the lateral motility of the cancer cells, MCF-7 and MDA-MB-231. The data revealed that the AWB extract exhibited stronger antimetastatic capacity on MCF-7 cells compared to MDA-MB-231 cells. This result is in line with the metastatic nature and motility of both cell lines. Since one of the prevailing mechanisms of cancer leading to high mortality and morbidity is metastasis, AWB extract interfering with lateral motility demonstrates its potential as a possible protective, antimetastatic agent, especially effective against nonaggressive cancers like weakly metastatic MCF-7 cells as well as highly aggressive cells like MDA-MB-231.
Figure 5.
Allium willeanum H. (AWB) extract inhibits lateral motility of MCF-7 and MDA-MB-231 cells. Bar graph is illustrating lateral motility data for (A) MCF-7 and (C) MDA-MB-231 cells for 24 h incubation. Representative image is showing effects of AWB on lateral motility of (B) MCF-7 and (D) MDA-MB-231 cells. Controls received only culture media. Data shown mean ± S.E.M. Statistical significance: ∗, p < 0.05 and ∗∗, p < 0.01.
3.6. Gas chromatography mass spectroscopy (GC-MS) analysis of AWB
GC-MS characterization of A. willeanum H. bulb (AWB) lead to the identification of a number of bioactive compounds with known biological activities (Table 2). Bioactive molecules such as octadecanoic acid 2-hydroxy-1-(hydroxymethyl)ethyl ester (21.99 %), hexadecanoic acid (20.42 %), tetradecanoic acid (5.57 %), pentadecanoic acid (9.19 %) 1,2-benzenedicarboxylic acid, diethyl ester (8.79 %) were previously shown to have anticancer effects likely contributing to the cytotoxic, apoptotic and antimotility effects observed in our study [23, 24, 25].
Table 2.
Bioactive compounds determined by GC-MS analysis and the biological activities of the molecules identified in A. willeanum H. bulb (AWB) ethanolic extract.
| Compound Name | Molecular Formula | AWB extract %∗ | Retention Time (min) | Biological Activity | |
|---|---|---|---|---|---|
| 1 | 1,2,3-Propanetriol (CAS) Glycerol | C3H8O3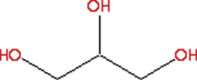
|
9.78 | 3.37 | Dehydrating agent [26]. |
| 2 | 1,2Benzenedicarboxylic acid, diethyl ester (CAS) Ethyl phthalate,2-Benzenedicarboxylic acid | C24H38O4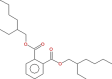
|
8.79 | 26.05 | Anticancer [23], antimicrobial, antifungal, antimalarial [27, 28] |
| 3 | Pentadecanoic acid (CAS) Pentadecylic acid | C15H30O12
|
9.19 | 34.44 | Antimicrobial, antifungal [29] antioxidant [30]. |
| 4 | Tetradecanoic acid (CAS) Myristic acid | C14H28O2
|
5.57 | 31.44 | Antioxidant, hypercholesterolemic, cancer-preventive, cosmetic [30]. |
| 5 | Hexadecanoic acid (CAS) Palmitic acid | C16H32O2
|
20.42 | 44.44 | Antitumoral [31, 32], antimicrobial, antioxidant, decrease blood cholesterol, anti-inflammatory [27], hypocholesterolemicnematicide, pesticide, antiandrogenic flavor, hemolytic, 5-Alpha reductase inhibitor [33]. |
| 6 | Octadecanoic acid, 2-hydroxy-1-(hydroxymethyl)ethyl ester (CAS) 2-Monostearin, Stearin | C21H42O4
|
21.99 | 47.69 | Anticancer [27, 28, 33] antioxidantant, antimicrobial [34]. |
| 7 | Octadecamethyl-cyclononasiloxane; Cyclononasiloxane, octadecamethyl- (CAS) | C18H54O9Si9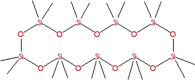
|
6.49 | 39.90 | Antifungal [35]. |
| 8 | 9-Octadecenamide, (Z)- (CAS) Oleamide, Oleic acid | C18H35NO
|
3.68 | 48.65 | Antifungal and antibacterial [35]. |
| 9 | 2H-Azonin-2-one, octahydro- (CAS) Capryllactam C8H15NO |
C8H15NO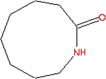
|
5.87 | 26.59 | _ |
The molecules are obtained from Wiley.7 MS Library.
% peak area relative to GC-MS chromatogram.
Phytochemical profiling of AWB carried out using GC-MS analysis provided insight towards its chemical composition and bioactive compounds associated with in vitro anticancer activities. The obtained results were in line with the observed inhibitory effect of the AWB on both breast cancer cell lines as 21.99 % octadecanoic acid was detected in its composition (Table 2). Octadecanoic acid (stearic acid) was previously shown to have inhibitory activities against viability of various BCa cells while triggering apoptosis of MDA-MB-361, MDA-MB-231 and MCF-7 human BCa cells by induction of apoptosis via caspase-3 activation [18, 36]. The presence of a high percentage of octadecanoic acid has likely contributed to the observed apoptotic activities. GC-MS analysis also revealed the presence of hexadecanoic acid (20.42 %) as well as 9-octadecenamide (13.68 %) (Table 2) in AWB extract, which were previously reported to have antimetastatic potential [36, 37]. Hexadecanoic acid induces caspase-8 and caspase-3 mediated apoptosis [22], generation of reactive oxygen species (ROS) by interacting with Toll like receptor 4 (TLR4)/ROS/p53 pathway, causes alterations in structure and function of mitochondria [38], also prevents tumor cell invasion and metastasis [36]. Similarly, 9-octadecenamide was demonstrated to inhibit the spontaneous metastasis of BL6 skin melanoma cells [36].
4. Conclusion
Overall, our study reveals significant antitumoral potency against strongly metastatic BCa cells with minor effects on weakly metastatic cells in a concentration dependent manner. The cytotoxic effects associated with AWB suggest, a more potent activity of AWB on concentration-dependent reduction of cell viability via mitochondrial activity of highly metastatic BCa cells MDA-MB-231 compared to the weakly metastatic cells MCF-7. Moreover, lateral motility studies revealed the inhibitory effects of AWB on motility of BCa cells where a strong inhibition was observed on the motility of both cell lines with a prominent effect on MCF-7 cells. Caspase-3 and -9 activation revealed the apoptotic mechanism underlying the anticancer properties exerted by AWB with significantly high activity of caspase-3 and -9 on MDA-MB-231 cells whereas only significantly high caspase-9 activity on MCF-7 cells. Hence, AWB extract has been demonstrated to trigger a faster apoptotic activity at 24h incubations on MDA-MB-231 cells compared to the MCF-7 cells. Collectively, our results demonstrate anticancer properties of the never before studied endemic species A. willeanum H. revealing it as a potential natural product to be further studied against metastatic cancers.
Declarations
Author contribution statement
Ovgu Isbilen: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Ender Volkan: Conceived and designed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
Work was supported by Cyprus International University Biotechnology Research Center.
Data availability statement
Data included in article/supplementary material/referenced in article.
Declaration of interests statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
Authors would like acknowledge Prof. Dr. Mehmet Koyuncu for his support in collection and identification of plant specimen.
References
- 1.Bray F., Ferlay J., Soerjomataram I., Siegel R.L., A Torre L., Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J. Clin. 2018;68(6):394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Roth A.J., Weinberger M.I., Nelson C.I. Prostate cancer: psychosocial implications and management. Future Oncol. 2008;4(4):561–568. doi: 10.2217/14796694.4.4.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Siegel R.L., Miller K.D., Jemal A. Cancer statistics, 2018. CA A Cancer J. Clin. 2018;68(1):7–30. doi: 10.3322/caac.21442. [DOI] [PubMed] [Google Scholar]
- 4.Sadiq A., Ahmad S., Ali R., Ahmad F., Ahmad S., Zeb A., Ayaz M., Ullah F., N Siddique A. Antibacterial and antifungal potentials of the solvents extracts from Eryngium caeruleum, Notholirion thomsonianum and Allium consanguineum. BMC Compl. Altern. Med. 2016;24(16):478. doi: 10.1186/s12906-016-1465-6. (1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zeng Y., Li Y., Yang J., Pu X., Du J., Yang X., Yang T., Yang S. Therapeutic role of functional components in alliums for preventive chronic disease in human being. Evid. Based Compl. Altern. Med. 2017;2017:9402849. doi: 10.1155/2017/9402849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Adak M., Teixeira J.A. Garlic [Allium sativum] and its beneficial effect on cardiovascular disease: a review. Int. J. Biomed. Pharmaceut. Sci. 2010;4:1–20. [Google Scholar]
- 7.Bayan L., Koulivand P.H., Gorji A. Garlic: a review of potential therapeutic effects. Avicenna J. Phytomed. 2014;4(1):1–14. [PMC free article] [PubMed] [Google Scholar]
- 8.Nouroz F., Mehboob M., Noreen S., Zaidi F., Mobin T.A. Review on anticancer activities of garlic (Allium sativum L). Middle-east. J. Sci. Res. 2015;23(6):1145–1151. [Google Scholar]
- 9.Mikaili P., Maadirad S., Moloudizargari M., Aghajanshakeri S., Sarahroodi S. Therapeutic uses and pharmacological properties of garlic, shallot, and their biologically active compounds. Iran J. Basic Med. Sci. 2013;16(10):1031–1048. [PMC free article] [PubMed] [Google Scholar]
- 10.Ranjani R., A Raju M. Anticancer properties of Allium sativum - a review. Asian J. Biochem. Pharmaceut. Res. 2012;2(3):190–196. [Google Scholar]
- 11.Meikle R.D. Vol. 2. The Bentham-Moxon Trust; Kew: 1985. pp. 1625–1626. (Flora of Cyprus 2). [Google Scholar]
- 12.Aydar E., Yeo S., Djamgoz M., Palmer C. Abnormal expression, localization and interaction of canonical transient receptor potential ion channels in human breast cancer cell lines and tissues: a potential target for breast cancer diagnosis and therapy. Cancer Cell Int. 2009;9:23. doi: 10.1186/1475-2867-9-23. Published 2009 Aug 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tiwary B.K., Bihani S., Kumar A., Chakraborty R., Ghosh R. The in vitro cytotoxic activity of ethno-pharmacological important plants of Darjeeling district of West Bengal against different human cancer cell lines. BMC Compl. Alternative Med. 2015;15:22. doi: 10.1186/s12906-015-0543-5. Published 2015 Feb 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Isbilen O., Rizaner N., Volkan E. E. Anti-proliferative and cytotoxic activities of Allium autumnale P. H. Davis (Amaryllidaceae) on human breast cancer cell lines MCF-7 and MDA-MB-231. BMC Compl. Alternative Med. 2018;18(1):30. doi: 10.1186/s12906-018-2105-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lekshmi N.C.J.P., Viveka S., Viswanathan M.B., Shobi T.M. GC-MS Characterization of organic sulphur compounds and other volatile odorous compounds from Allium sativum. Asian J. Microbiol. Biotechnol. Environ. Sci. 2015;17:73–78. [Google Scholar]
- 16.F Specian A., Serpeloni J.M., Tuttis K., Ribeiro D.L., Cilião H.L., Varanda E.A., Sannomiya M., Martinez-Lopez W., Vilegas W.I.M., Cólus L.D.H. Proliferation curves and cell cycle analysis are the most suitable assays to identify and characterize new phytotherapeutic compounds. Cytotechnology. 2016;68(6):2729–2744. doi: 10.1007/s10616-016-9998-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pacheco B.S., Dos Santos M.A.Z., Schultze E., Martins R.M., Lund R.G., Seixas F.K., Colepicolo P., Collares T., R Paula F., P De Pereira C.M. Cytotoxic activity of fatty acids from Antarctic macroalgae on the growth of human breast cancer cells. Front. Bioeng. Biotechnol. 2018;3(6):185. doi: 10.3389/fbioe.2018.00185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McIlwain D.R., Berger T., Mak T.W. Caspase functions in cell death and disease. Cold Spring Harb. Perspect. Biol. 2013;5(4):a008656. doi: 10.1101/cshperspect.a008656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li J., Yuan J. Caspases in apoptosis and beyond. Oncogene. 2008;27:6194–6206. doi: 10.1038/onc.2008.297. [DOI] [PubMed] [Google Scholar]
- 20.Evans L.M., Cowey S.L., Siegal G.P., W Hardy R. Stearate preferentially induces apoptosis in human breast cancer cells. Nutr. Cancer. 2009;61(5):746–753. doi: 10.1080/01635580902825597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ulloth J.E., Casiano C.A., De Leon M. Palmitic and stearic fatty acids induce caspase-dependent and -independent cell death in nerve growth factor differentiated PC12 cells. J. Neurochem. 2003;84(4):655–668. doi: 10.1046/j.1471-4159.2003.01571.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Leverrier Y Y., Ridley A.J. Apoptosis: caspases orchestrate the ROCK 'n' bleb. Nat. Cell Biol. 2001;3(4):E91–E93. doi: 10.1038/35070151. [DOI] [PubMed] [Google Scholar]
- 23.Tan J., Tian Y., Cai R., Luo R., Guo J. Chemical composition and antiproliferative effects of a methanol extract of aspongopus chinensis dallas. Evid. Based Compl. Altern. Med. 2019;30:2607086. doi: 10.1155/2019/2607086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.C Yoo Y., H Shin B., H Hong J., Lee J., Y Chee H., Song K.S., B Lee K. Isolation of fatty acids with anticancer activity from Protaetia brevitarsis larva. Arch Pharm. Res. (Seoul) 2007;30(3):361–365. doi: 10.1007/BF02977619. [DOI] [PubMed] [Google Scholar]
- 25.Krishnan K., Mani A., Jasmine S. Cytotoxic activity of bioactive compound 1, 2- benzene dicarboxylic acid, mono 2- ethylhexyl ester extracted from a marine derived streptomyces sp. VITSJK8. Int. J. Mol. Cell Med. 2014;3(4):246–254. [PMC free article] [PubMed] [Google Scholar]
- 26.Frank M.S., C Nahata M., Glycerol M.D. Hilty. A review of its pharmacology, pharmacokinetics, adverse reactions, and clinical use. Pharmacotherapy. 1981;1(2):147–160. doi: 10.1002/j.1875-9114.1981.tb03562.x. [DOI] [PubMed] [Google Scholar]
- 27.Belakhdar G., Benjouad A., H Abdennebi E. Determination of some bioactive chemical constituents from Thesium humile Vahl. J. Mater. Environ. Sci. 2015;6(10):2778–2783. [Google Scholar]
- 28.Win D.T. Oleic acid- the anti-breast cancer component in olive oil. Au J. Technol. 2005;9(2):75–78. [Google Scholar]
- 29.Chandrasekaran M., Senthilkumar A., Venkatesalu V. Antibacterial and antifungal efficacy of fatty acid methyl esters from the leaves of Sesuvium portulacastrum L. Eur. Rev. Med. Pharmacol. Sci. 2011;15(7):775–780. [PubMed] [Google Scholar]
- 30.Z Zayed M. GC-MS analysis of phytochemical constituents in leaf extracts of N Neolamarckia cadamba (rubiaceae) from Malasia. Int. J. Pharm. Pharmaceut. Sci. 2014;6(9):123–127. [Google Scholar]
- 31.Harada H., Yamashita U., Kurihara H., Fukushi E., Kawabata J., Kamei Y. Antitumor activity of palmitic acid found as a selective cytotoxic substance in a marine red alga. Anticancer Res. 2002;22(5):2587–2590. [PubMed] [Google Scholar]
- 32.Kumar R.S., Rajkapoor B., Perumal P. Antioxidant activities of Indigofera cassioides Rottl. Ex. DC. using various in vitro assay models. Asian Pac. J. Trop. Biomed. 2012;2(4):256–261. doi: 10.1016/S2221-1691(12)60019-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Asghar S.F., I Choudahry M. Gas chromatography-mass spectrometry (GC-MS) analysis of petroleum ether extract (oil) and bio-assays of crude extract of Iris germanica. Int. J. Genet. Mol. Biol. 2011;3(7):95–100. [Google Scholar]
- 34.Abubakar M.N., Majinda R. GC-MS analysis and preliminary antimicrobial activity of Albizia adianthifolia (schumach) and pterocarpus angolensis (DC) Medicine. 2016;3(1):3. doi: 10.3390/medicines3010003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.L Suriani N. Identification of the substance bioactive leaf extract piper caninum potential as botanical pesticides. Int. J. Pure Appl. Biosci. (IJPAB) 2016;4(4):24–32. [Google Scholar]
- 36.Hecht I., Natan S., Zaritsky A., Levine H., Tsarfaty I., Ben-Jacob E. The motility-proliferation-metabolism interplay during metastatic invasion. Sci. Rep. 2015;4(5):13538. doi: 10.1038/srep13538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Miura D., Kida Y., Nojima H. Camellia oil and its distillate fractions effectively inhibit the spontaneous metastasis of mouse melanoma BL6 cells. FEBS Lett. 2007;581(13):2541–2548. doi: 10.1016/j.febslet.2007.04.080. [DOI] [PubMed] [Google Scholar]
- 38.Yu G., Luo H., Zhang N. Loss of p53 sensitizes cells to palmitic acid-induced apoptosis by reactive oxygen species accumulation. Int. J. Mol. Sci. 2019;20(24):6268. doi: 10.3390/ijms20246268. Published 2019 Dec 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data included in article/supplementary material/referenced in article.