Graphical abstract
Keywords: Microwave, Ultrasound, Combined reactor
Highlights
-
•
A hybrid US and MW reactor is reported.
-
•
The system uses monomode MW and US convertors operating at different frequencies.
-
•
Continuously high-power densities can be used with fully controlled temperature.
-
•
Transesterification of vegetable oil was used as a model for process intensification.
Abstract
Ultrasound (US) and Microwaves (MW) are effective methods for processes intensification. Their combined use in the same reactor can lead to remarkable results. Recently there has been a resurgence of interest in this field for new synthetic applications using reactors based upon existing technologies. We describe here a new type of apparatus in which the thermal energy is continuously removed from the system making possible the use of high power and adjustable ultrasonic and microwave densities throughout the process. The installation consists of a glass reactor located in a monomode applicator which is immersed at the same time in an ultrasonic device which can be operated at different frequencies and powers. A liquid, transparent to microwaves, was used to couple ultrasonic energy to the reactor and to remove the heat generated. Comsol software was used to get information about the distribution of ultrasonic and microwave energy between the reactor liquid and the coupling fluid. The performance was assessed using the conversion of p-nitrophenol into 4-nitrocatechol as a chemical dosimeter and a transesterification.
1. Introduction
To a chemist, process intensification can be expressed as: faster reactions, better conversions, improved or new product and fewer by-products. Such improvements should, of course, be accompanied by simplification of the production line and lower production costs. Ultrasound and Microwaves are both considered to be effective methods for process intensification due to the special ways in which each of these energy sources can activate reactions but in rather different ways [1]:
-
•
The effects of ultrasound are linked to the alternating movement of the molecules in a liquid phase. When the amplitudes of this movement are sufficiently high greater than a critical value, the rarefaction cycle which drags molecules apart may exceed the attractive forces between these molecules in the liquid and cavitation bubbles will form. It is the fate of these cavities when they collapse in succeeding compression cycles which generates the energy for chemical and mechanical effects [2]. This is a remarkable phenomenon induced throughout the liquid and in the case of aqueous systems each cavitation bubble acts as a localised “hotspot” generating temperatures of about 4,000 K and pressures in excess of 100 MPa with lifetimes shorter than 0.1 µs and cooling rates above 1010 Ks−1 [3]. Within heterogeneous media the collapse of such bubbles is asymmetric which produces different effects depending on the medium involved but with particular effects in reactions involving liquid reactions involving metal surfaces, powders or other particulate matter and emulsification. In homogeneous systems sonochemical reactions involve more than simply the mechanical effects of cavitation [4]. They may also enhance electron transfer and cause temporary effects on solvent structure [5], [6].
-
•
The specific effects of microwaves for heating compared with conventional heating include higher heating rates, selective heating of the components in a heterogeneous reaction mixture and heating of a whole volume rather than from the outside in (convection). An early example of this in synthesis was microwave activation of phase transfer catalysis in solvent-free conditions [7]. With such advantages microwave irradiation (usually at a frequency of 2.45 GHz) produces efficient internal heating for most chemical reactions, delivering energy exactly where it is needed even under exothermic conditions [8]. This results in a more economical use of electricity with a higher efficiency than thermal methods. Microwave installations do not produce dust, noise, exhaust gas, vibrations or ambient temperature increase, they are smaller and occupy less floor space, are modular in design allowing easy scaling up and scaling down [9].
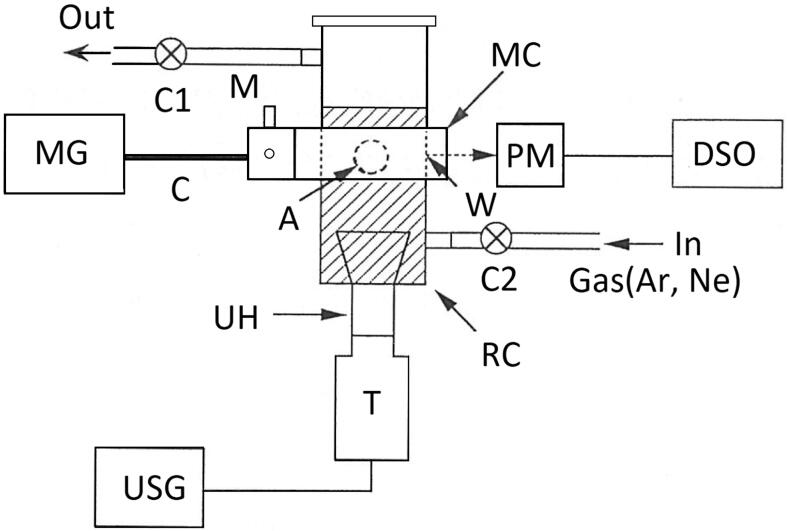
The rationale behind merging ultrasound and microwaves as an energy source stems from the differences between them. Although microwaves provide rapid heating of a reaction mixture, mass transfer is a limiting factor in the overall rate of the process. Ultrasound provides good mass transfer but with limited heating. Thus a combination of US and MW in one machine (or process) may overcome limitations of mass transfer and heating [10]. The first published results on the use of this combination was published in 1995 by Maeda and Amemiya from the Institute of Physical and Chemical Research in Japan [11]. Fig. 1 shows a schematic diagram of the experimental apparatus. The microwave apparatus is composed of a microwave cavity (MC) (2.8 cm OD × 7 cm in length), a matching element (M) and a microwave oscillator unit (MG). Microwaves of 2.45 GHz are guided through a coaxial cable (C) to MC. The reaction cup (RC) is covered with black paper in order to shield it from light. The RC is also covered with aluminium foil. The ultrasound apparatus consists of an ultrasonic horn (UH) attached to the RC (Cup-horn type), a transducer (T) and an ultrasonic generator (USG) of 20 kHz (Shimadzu USP-600 type). The RC is filled with liquid (50 mL), including the shadow region. The cavitation field is produced by ultrasound within the RC.
Fig. 1.
Maeda et. al. schematic diagram of microwave-ultrasonic device [11].
The effects were monitored through observations of sonoluminescence (SL) and chemiluminescence (luminol). In the case of ultrasound irradiation only, the SL emission occurred predominantly at the compressed phase of bubbles however, with simultaneous irradiation by microwaves and ultrasound, SL occurred during not only the contraction of the bubble but also the expansion of bubble. In the case of chemiluminescence of luminol solution, a distinct amplification of the effect of ultrasound and microwaves seems to occur suggesting the possibility of promoting chemical reactions. In the conclusion to the article the authors suggested that “It is expected that the present method could open a new research field of microwave-sonochemistry“.
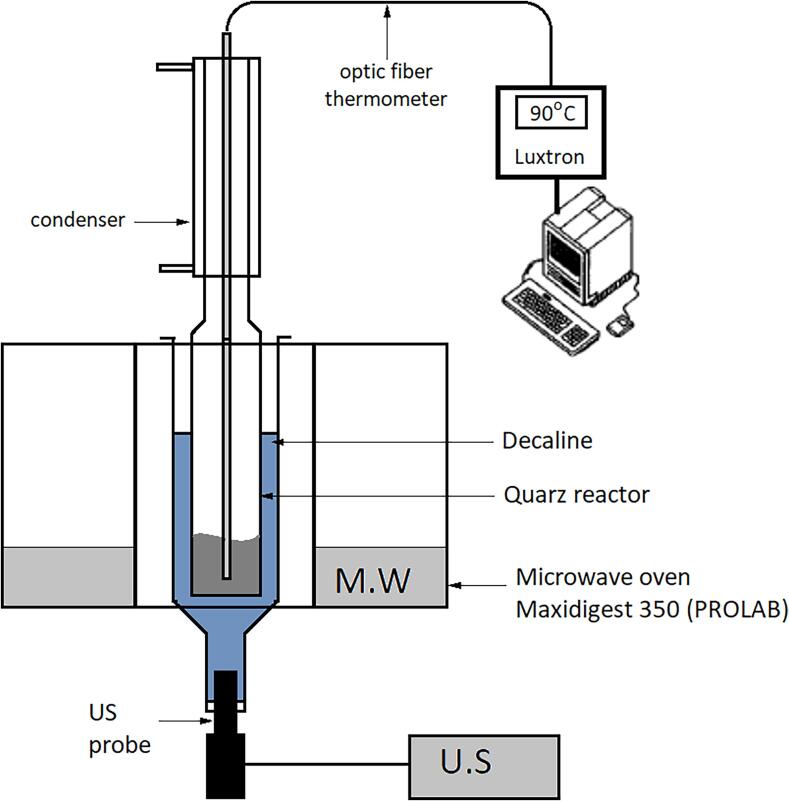
During that same period Jacques Berlan at ENSIGC, Toulouse was also engaged in pioneering work on the combination of the two techniques and had developed a different prototype hybrid reactor [12]. A Prolabo Maxidigest 350 was used as the MW oven (2.45 GHz) with a hole in the top to allow the quartz reactor in the oven to connect to a condenser. Ultrasound was provided through a cuphorn device (20 kHz) inserted through a hole in the base and with the US probe placed some distance below the oven itself. The ultrasound was conducted from the horn through a coupling fluid (decalin, which is impervious to MW) into which the quartz reactor was dipped (Fig. 2)
Fig. 2.
Berlan et al schematic diagram of microwave-ultrasonic device [12].
The equipment was used for two reactions the pyrolysis of urea and the esterification of propanol with acetic acid. In both cases there was a significant improvement in organic synthesis through the combination of microwaves and ultrasound
There are two ways of combining ultrasounds and microwaves for chemical processing [13]:
-
•
Sequential use of two separate reactors, one for ultrasound and one for microwaves, between which the reaction mixture is recirculated using a pump, or
-
•
Use of a single reactor in which microwaves and ultrasounds are injected simultaneously.
Although the first version is simpler to build, because existing equipment can be used, the second is much more attractive because it allows for the simultaneous combination of kinetic effects with mass transfer to ensure the increase of the overall rate of the chemical process. Early attempts to develop the single reactor option involved a ceramic or glass horn providing the sonication inserted in the microwave field but this has a significant limitation because of the characteristics of ceramic materials which cannot operate at high vibrational amplitudes [12]. Nevertheless, both methods of combination have been used and the results reviewed [13], [14], [15].
Recently there has been a resurgence of interest in the use of combined US and MW reactors for chemical syntheses. In two papers published in 2020 [16], [17] the equipment used was referred to as CW-2000 Ultrasound Microwave Cooperative Extractor/Reactor, which is not described in detail, but it appears to consist of a probe system inserted into a reactor inside of a microwave oven. In other papers published [18], [19] the US/MW device provides ultrasonic irradiation via a glass horn and MW via a multimode applicator. The design was similar to that described by Berlan [12]. In the most recent paper in 2021 the ultrasound is again introduced via a glass (Pyrex) horn into the microwave cavity.
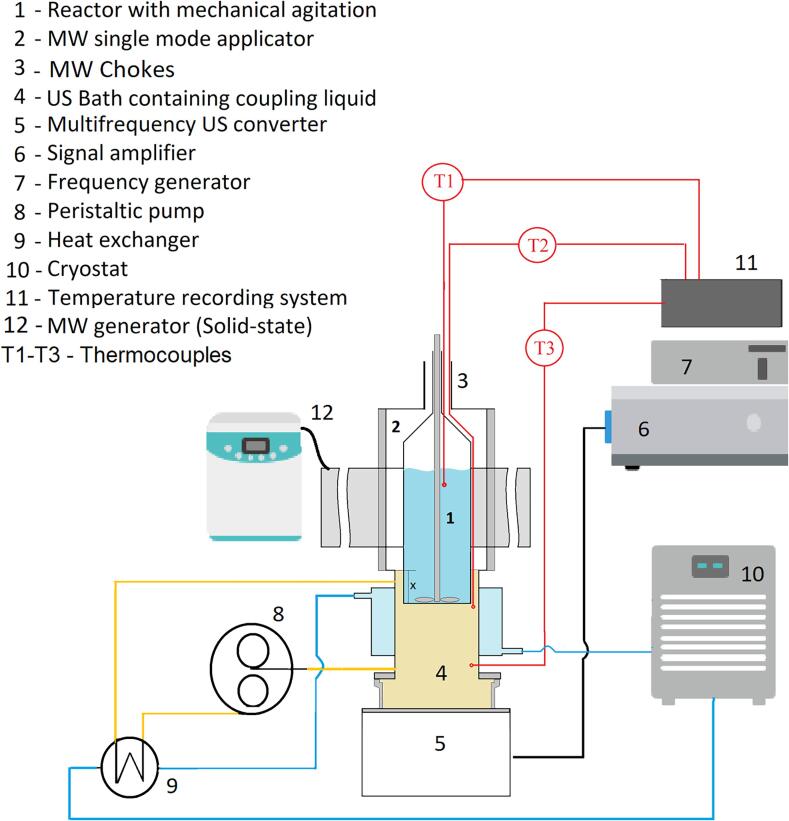
The single reactor approach as described by Leonelli and Mason [13] was chosen by our group to build a new type of MW and multifrequency US, combined reactor (see Fig. 3). However, our equipment design differs significantly from those described in recent publications because:
-
•
The equipment is fully controllable from the point of view of both sources of energy US and MW.
-
•
It can be operated at different frequencies (24, 580, 864 and 1146 kHz)
-
•
The MW generator is solid-state (not magnetron) allowing a well-controlled adjustment of power (from 0 to 200 W) not possible with magnetron type MW. In addition, the frequency can be adjusted within the range of 2.43 up to 2.47 GHz.
-
•
The MW cavity is monomode not a multimode, allowing a more uniform treatment of reaction mixtures.
-
•
It can be used continuously at a constant reactor temperature regardless the MW or US powers used because the reactor is partially immersed in the liquid of the US bath which is continuously cooled by means of a jacket through which a coolant can circulate.
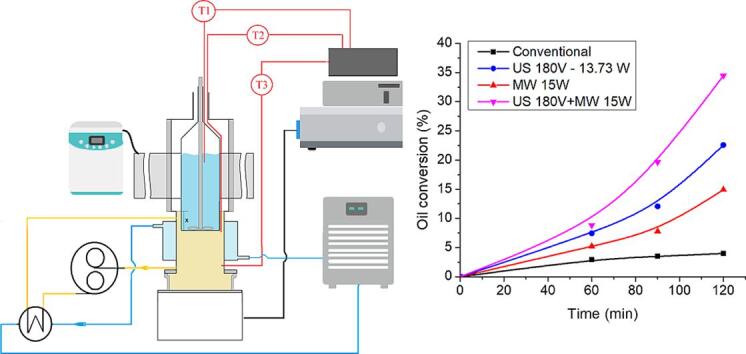
Fig. 3.
Ultrasound and Microwave hybrid installation.
The aim of this paper is to determine experimentally several parameters critical for the correct application of these two energy sources in this recently designed reactor. These include the optimal position of the reactor as defined by the calorimetric determination of US and MW absorbed powers and real examples of transformations (such as the transesterification of vegetal oil with ethanol) to illustrate the potential for this system to be used for the intensification of chemical processes.
2. Materials and methods
2.1. Materials and equipment
The experiments were performed using dodecane as the coupling and cooling fluid (>95%), 4-nitrophenol (analytical standard), ethanol (absolute) all from Sigma-Aldrich and edible sunflower oil.
The equipment is shown in Fig. 3 and is the subject of a patent application [20]. It consists of a single glass reactor with a volume of 100 mL (1) equipped with a mechanical stirrer and three K type thermocouples T1 in the reactor with T2 and T3 monitoring the inlet and outlet temperatures of the coupling fluid. The coupling fluid is cooled by a jacket of circulating liquid and the reactor is partially immersed in the coupling fluid in order to control the reaction temperature. The reactor can receive at the same time microwaves and ultrasounds via the coupling liquid (4). Ultrasonic power is provided through a multifrequency transducer (5), from a frequency generator (7) and a power amplifier (6). Microwave energy is supplied from a solid-state MW generator (12) via a single mode applicator (2) with a volume of 282 mL (components of Sairem - Miniflow 200 SS system).
2.2. Procedure and experimental setup
The reactor is loaded with the reaction mixture without catalyst or any other key component. All ultrasonic and microwave generation systems are started at the desired power levels with coupling fluid and coolant recirculation systems in place. By adjusting the system thermal equilibrium can be established at the desired reaction temperature (50 °C in our case for both reactions studied), the catalyst or key component is added, and the process is monitored through sampling and analysis at predetermined time intervals. The control (conventional) reaction was performed under conventional heating at 50 °C. In case of p-nitrophenol (PNP) degradation, a solution with a concentration of 100 µmol/L was used, with corrected pH to 5 with 1 N HCl. The degradation of PNP was followed by monitoring its concentration via spectrophotometric method [21].
3. Results and discussions
3.1. The optimal position of the reactor within the installation
In order to determine the optimal position of the glass reactor within this installation with respect to the input of ultrasound and microwave energy together with effective heat withdrawal by the coupling fluid Comsol software was employed (see supporting material). This modelling suggested that the reactor should be immersed in the coupling fluid (dodecane) at 20 mm and this was used throughout the experiments. The MW loss in the chokes was also found to be low and less than the limits of EU Directive 2013/35, which states a much higher limit of 50 W/m2 [22].
3.2. Calorimetric determination of US and MW absorbed powers
The calorimetric determination of the US power absorbed by liquid in the glass reactor and separately by the coupling fluid were carried out using procedures described in the literature [23], [24]. Distilled water (100 mL) was added to the reactor, and dodecane (150 mL) to the US bath. The US and MW were switched on for a short time (10–30 s). By measuring the temperature rise in the coupling fluid (T2) and in the reactor liquid (T3) respectively, the calorimetric values of the dissipated powers in the reactor liquid and in the coupling fluid were determined.
The measurements were performed for the US multifrequency transducer from Meinhardt Ultrasonics (580; 864 and 1146 kHz) and the 24 kHz device from REUS. The position of the reactor in the coupling fluid was at a depth of 20 mm. The values obtained for the power absorbed in the reactor itself (1) and in the coupling fluid in the US bath (4) at different frequencies are presented in Table 1, Table 2.
Table 1.
Calorimetric measurement of ultrasonic power for multifrequency US convertor.
| Amplitude | Power, W |
|||||
|---|---|---|---|---|---|---|
| 580 kHz |
864 kHz |
1146 kHz |
||||
| In the US bath | In the reactor | In the US bath | In the reactor | In the US bath | In the reactor | |
| 5 | 3.7 | 5.3 | 4.2 | 4.9 | 3.9 | 5.3 |
| 6 | 7.5 | 8.8 | 7.0 | 8.4 | 10.2 | 10.0 |
| 7 | 11.7 | 13.2 | 12.3 | 12.6 | 16.1 | 13.0 |
Table 2.
Calorimetric measurement of ultrasonic power for 24 kHz transducer.
| % of full power (supply voltage) | Power, W |
|
|---|---|---|
| In the US bath | In the reactor | |
| 15 (100 V) | 5.0 | 1.9 |
| 20 (120 V) | 10.0 | 5.0 |
| 30 (140 V) | 19.3 | 11.6 |
An important advantage of this installation is that the microwave and/or ultrasound energy can be continuously supplied and controlled to the same reaction mixture in such a way as to not exceed a prescribed temperature. This is possible because the heat absorbed in the reactor is transferred to the coupling liquid and this, in turn, to the coolant flowing through the jacket (as can be seen in Fig. 3).
Dodecane is an excellent coupling liquid because it combines two remarkable properties: it is a non-polar solvent that has a very small dielectric constant (fv = 2.01) [25] and is almost transparent in the microwave range. It is also unaffected by sonication over a wide temperature range [23]. This makes it entirely suitable for use as a coolant for the removal of excess heat from a microwave cavity, besides coupling ultrasound with the reactor [26].
Microwave energy was applied using the same configuration as for ultrasonic energy. The power absorbed by the liquid in the reactor was nearly the same as the power provided by the MW generator (confirming microwave energy absorption efficiencies of over 96%). In addition, the temperature in the US bath did not rise. This shows that the MW applicator is well tuned, there is no reflected MW power and that the coupling fluid was well chosen in that it did not absorb MW energy.
When used in the hybrid configuration (simultaneously applied US and MW) a simple additive effect of the absorbed powers was observed.
3.3. Determination of the effectiveness of the hybrid reactor
3.3.1. Measurement of p-nitrophenol degradation
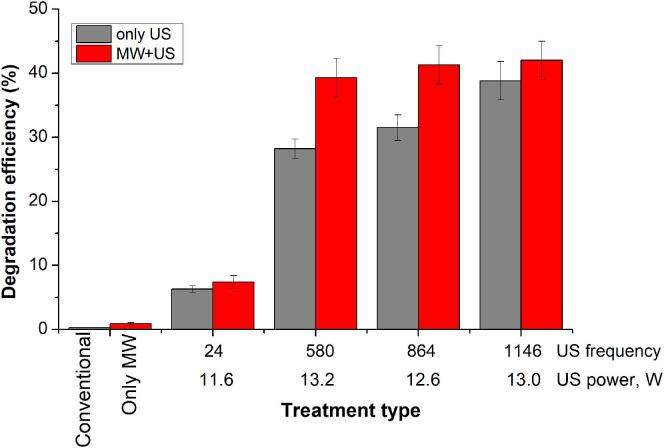
The degradation of p-nitrophenol (PNP) is one of the methods used to measure the efficiency of generating active radical species (especially OH radicals) by cavitation during sonochemistry [21], [27]. Fig. 4 shows the degradation efficiency of PNP under various conditions in the Hybrid Reactor: conventional, microwave only (10 W), US only (12–13 W) and combined MW and US at different frequencies. One would not anticipate any effect of normal stirring or MW alone plus stirring on the decomposition of PNP in the absence of ultrasound. However, MW and stirring does produce a small degradation (less than 0.9 %). Whether this is due to hydroxy radical formation under microwave heating is unclear one might even say unlikely since at the same temperature with stirring alone PNP shows essentially zero degradation within experimental error. In 2015 there was a report of a microwave assisted Fenton-like process but this was a catalysed reaction unlike the one studied here [28]. The results obtained with ultrasound alone are as expected in terms of US frequency and power with increasing frequency leading to more efficient degradation i.e. consistent with literature data [29].
Fig. 4.
Degradation efficiency of PNP depending on the treatment conditions (reaction time 50 min).
It is the experiments in which US and MW are combined that produce the most interesting results in that MW can be seen to significantly increased the degradation of PNP compared with US alone. We believe that this might be the first clear chemical evidence for a synergism of US + MW.
3.3.2. The combined effects of US and MW on transesterification
The transesterification of vegetal oil with ethanol, under heterogenous acidic catalysis, is of great interest in the production of biofuels and has been well documented and provides a challenge in terms of process optimization [30], [31]. Ultrasonic energy can emulsify the reactants to reduce the catalyst requirement, alcohol-oil ratio, reaction time and reaction temperature [32].
Sunflower oil (52.5 mL) and ethanol (17.5 mL) were placed in the Hybrid Reactor and subjected to a series of experiments involving separately US and MW irradiation and in each case 3 g of Amberlite IR 120 (H form, corresponding to 0.1 mol H+/L) was added but only when the temperature stabilized. The oil/ethanol mixture was analysed by GC-FID according to the EN 14,103 standard method for biodiesel [33]. The results, for 24 kHz ultrasound frequency, are shown in the Fig. 5 below:
Fig. 5.
Transesterification of sunflower oil with ethanol over Amberlite IR 120.
Once again there are the results obtained when US and MW are combined which provide the most interesting data. It can be seen that there is a high synergetic effect for this transesterification reaction which itself is known to be slow under normal acidic catalysis [34].
4. Conclusions
A new combined (hybrid) ultrasonic and microwave installation was designed, built and characterized. This new system maintains a low temperature in the reaction medium even when using high-power densities (of the order of 1–2 W/mL) which is extremely important for the:
-
•
extraction of valuable active principles from plants;
-
•
reduction in degradation of thermolabile compounds;
-
•
intensification of the ultrasonic and microwave processes in solution
In addition, synergetic effects on the degradation of p-nitrophenol and the transesterification of sunflower oil under heterogenous acidic catalysis shows the synergism in this ultrasound and microwave hybrid device.
CRediT authorship contribution statement
Ioan Călinescu: Investigation, Writing - review & editing. Mircea Vinatoru: Methodology, Validation, Writing - review & editing. Daniela Ghimpețeanu: Investigation. Vasile Lavric: Software, Formal analysis. Timothy J. Mason: Conceptualization, Investigation, Writing - original draft.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgment
The authors acknowledge the financial support received from the Competitiveness Operational Program 2014-2020, Action 1.1.4: Attracting high-level personnel from abroad in order to enhance the RD capacity, project: P_37_471, “ULTRA_MINT Technologies”, financed by contract: 47/05.09.2016.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ultsonch.2021.105701.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Stankiewicz A., Mouljin J. Process Intensification Transforming Chemical Engineering. Chem. Eng. Prog. 2000:22–34. [Google Scholar]
- 2.Mason T.J., Lorimer J.P. JAI Press Inc.; Weinheim: 2002. Applied Sonochemistry: Uses of Power Ultrasound in Chemistry and Processing. [Google Scholar]
- 3.M. Ashokkumar, T.J. Mason, Sonochemistry Kirk-Othmer Encyclopedia of Chemical Technology, in, John Wiley & Sons New York, 2007.
- 4.Suslick K. Sonochemistry. Science. 1990;247:1439–1445. doi: 10.1126/science.247.4949.1439. [DOI] [PubMed] [Google Scholar]
- 5.T.J. Mason, J.-L. Luche, Ultrasound as a New Tool for Synthetic Chemists in: R.v. Eldik, C.D. Hubbard (Eds.) Chemistry Under Extreme and Non-Classical Conditions, Wiley, 1996.
- 6.Vinatoru M., Mason T.J. Can sonochemistry take place in the absence of cavitation? – A complementary view of how ultrasound can interact with materials. Ultrason Sonochem. 2019;52:2–5. doi: 10.1016/j.ultsonch.2018.07.036. [DOI] [PubMed] [Google Scholar]
- 7.Deshayes S., Liagre M., Loupy A., Luche J.-L., Petit A. Microwave activation in phase transfer catalysis. Tetrahedron. 1999;55(36):10851–10870. [Google Scholar]
- 8.Loupy A., editor. Microwaves in Organic Synthesis. Wiley; 2006. [Google Scholar]
- 9.Stefanidis G.D., Muñoz A.N., Sturm G.S.J., Stankiewicz A. A helicopter view of microwave application to chemical processes: reactions, separations, and equipment concepts. Rev. Chem. Eng. 2014;30 doi: 10.1515/revce-2013-0033. [DOI] [Google Scholar]
- 10.Gude V.G. Synergism of microwaves and ultrasound for advanced biorefineries. Resour.-Effic. Technol. 2015;1(2):116–125. doi: 10.1016/j.reffit.2015.10.001. [DOI] [Google Scholar]
- 11.Maeda M., Amemiya H. Chemical effects under simultaneous irradiation by microwaves and ultrasound. New J. Chem. 1995;19:1023–1028. [Google Scholar]
- 12.F. Chemat, M. Poux, J.L.D. Martino, J. Berlan, An Original Microwave-Ultrasound Combined Reactor Suitable for Organic Synthesis: Application to Pyrolysis and Esterification, Journal of Microwave Power and Electromagnetic Energy, 31 (1996) 19-22 10.1080/08327823.1996.11688288.
- 13.Leonelli C., Mason T.J. Microwave and ultrasonic processing: Now a realistic option for industry. Chem. Eng. Process. Process Intensif. 2010;49(9):885–900. doi: 10.1016/j.cep.2010.05.006. [DOI] [Google Scholar]
- 14.G. Cravotto, P. Cintas, The Combined Use of Microwaves and Ultrasound: Improved Tools in Process Chemistry and Organic Synthesis, Chemistry – A European Journal, 13 (2007) 1902-1909 10.1002/chem.200601845. [DOI] [PubMed]
- 15.Vinatoru M., Calinescu I. AMPERE 2019–17th International Conference on Microwave and High Frequency Heating. 2019. Microwave and ultrasounds together – A challenge; pp. 105–112. [Google Scholar]
- 16.Yan J., Zhao Y.u., Li K., Zhang H., Fan L., Lu Z. Efficient production of biodiesel from ionic liquid catalyzed esterification using ultrasonic-microwave combined intensification. Chem. Eng. Process. - Process Intensification. 2020;149:107870. doi: 10.1016/j.cep.2020.107870. [DOI] [Google Scholar]
- 17.Habinshuti I., Mu T.-H., Zhang M. Ultrasound microwave-assisted enzymatic production and characterisation of antioxidant peptides from sweet potato protein. Ultrason Sonochem. 2020;69:105262. doi: 10.1016/j.ultsonch.2020.105262. [DOI] [PubMed] [Google Scholar]
- 18.Zuliani A., Cano M., Calsolaro F., Puente Santiago A.R., Giner-Casares J.J., Rodríguez-Castellón E., Berlier G., Cravotto G., Martina K., Luque R. Improving the electrocatalytic performance of sustainable Co/carbon materials for the oxygen evolution reaction by ultrasound and microwave assisted synthesis, Sustainable. Energy Fuels. 2021;5(3):720–731. doi: 10.1039/D0SE01505A. [DOI] [Google Scholar]
- 19.Moran M.J., Martina K., Baricco F., Tagliapietra S., Manzoli M., Cravotto G. Tuneable Copper Catalysed Transfer Hydrogenation of Nitrobenzenes to Aniline or Azo Derivatives. Adv. Synth. Catal. 2020;362(13):2689–2700. doi: 10.1002/adsc.202000127. [DOI] [Google Scholar]
- 20.I. Calinescu, D. Ghimpeteanu, M. Vinatoru, V. Lavric, N.D. Ignat, Installation for combined use of ultrasounds and microwaves for intensifying physico-chemical processes, RO134747-A0 RO000236 04 May 2020, <Go to ISI>://DIIDW:2021239950.
- 21.Kotronarou A., Mills G., Hoffmann M.R. Ultrasonic Irradiation of p-Nitrophenol in Aqueous Solution. J. Phys. Chem. 1991;95(9):3630–3638. [Google Scholar]
- 22.Directive 2013/35/EU of the European Parliament and of the Council, 2013.
- 23.Löning J.-M., Horst C., Hoffmann U. Investigations on the energy conversion in sonochemical processes. Ultrason. Sonochem. 2002;9(3):169–179. doi: 10.1016/S1350-4177(01)00113-4. [DOI] [PubMed] [Google Scholar]
- 24.Plattes M., Kohler C., Galle T. Disequilibrium calorimetry for determination of ultrasonic power in sonochemistry. MethodsX. 2017;4:274–278. doi: 10.1016/j.mex.2017.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.www.engineeringtoolbox.com, https://www.engineeringtoolbox.com/liquid-dielectric-constants-d_1263.html.
- 26.Gabriel C., Gabriel S., H. Grant E., H. Grant E., S. J. Halstead B., Michael P. Mingos D. Dielectric parameters relevant to microwave dielectric heating. Chem. Soc. Rev. 1998;27(3):213. doi: 10.1039/a827213z. [DOI] [Google Scholar]
- 27.Pradhan A.A., Gogate P.R. Degradation of p-nitrophenol using acoustic cavitation and Fenton chemistry. J Hazard Mater. 2010;173(1-3):517–522. doi: 10.1016/j.jhazmat.2009.08.115. [DOI] [PubMed] [Google Scholar]
- 28.Pan W., Zhang G., Zheng T., Wang P. Degradation of p-nitrophenol using CuO/Al2O3 as a Fenton-like catalyst under microwave irradiation. RSC Adv. 2015;5(34):27043–27051. doi: 10.1039/C4RA14516J. [DOI] [Google Scholar]
- 29.Barbier P. Study at 20 kHz and 500 kHz of the Ultrasound-Ozone Advanced Oxidation System : 4-Nitrophenol Degradation. J. Adv. Oxid. Technol. 1996;1:154–159. [Google Scholar]
- 30.Melero J.A., Iglesias J., Morales G. Heterogeneous acid catalysts for biodiesel production: current status and future challenges. Green Chem. 2009;11:1285. doi: 10.1039/b902086a. [DOI] [Google Scholar]
- 31.Brunschwig C., Moussavou W., Blin J. Use of bioethanol for biodiesel production. Prog. Energy Combust. Sci. 2012;38:283–301. doi: 10.1016/j.pecs.2011.11.001. [DOI] [Google Scholar]
- 32.Ramachandran K., Suganya T., Gandhi N.N., Renganathan S. Recent developments for biodiesel production by ultrasonic assist transesterification using different heterogeneous catalyst: a review. Renew. Sustain. Energy Rev. 2013;22:410–418. doi: 10.1016/j.rser.2013.01.057. [DOI] [Google Scholar]
- 33.Liquid petroleum products - Fatty acid methyl esters (FAME) for use in diesel engines and heating applications - Requirements and test methods (includes Amendment A1:2014), EN 14214, 2014.
- 34.Cabral N.M., Lorenti J.P., Plass W., Gallo J.M.R. Solid Acid Resin Amberlyst 45 as a Catalyst for the Transesterification of Vegetable Oil. Front Chem. 2020;8:305. doi: 10.3389/fchem.2020.00305. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.