Abstract
Objective
To evaluate the magnitude of the association between risk factors and premature myocardial infarction (MI) (men aged 18-55 years; women aged 18-65 years).
Patients and Methods
We searched MEDLINE and other databases from inception through April 30, 2020, as well as bibliography of articles selected for data extraction. We selected observational studies reporting the magnitude of the association of at least 1 risk factor (demographic characteristics, lifestyle factors, clinical risk factors, or biomarkers) with premature MI and a control group. Pooled risk estimates (random effects) from all studies unadjusted and adjusted for risk factors were reported as summary odds ratios (ORs) with 95% CIs.
Results
From 35,320 articles of 12.7 million participants, we extracted data on 19 risk factors from 77 studies across 58 countries. Men had a higher risk of premature MI (OR, 2.39; 95% CI, 1.71 to 3.35) than did women. Family history of cardiac disease was associated with a higher risk of premature MI (OR, 2.67; 95% CI, 2.29 to 3.27). Major modifiable risk factors associated with higher risk were current smoking (OR, 4.34; 95% CI, 3.68 to 5.12 vs no/former), diabetes mellitus (OR, 3.54; 95% CI, 2.69 to 4.65), dyslipidemia (OR, 2.94; 95% CI, 1.76 to 4.91), and hypertension (OR, 2.85; 95% CI, 2.48 to 3.27). Higher body mass index carried higher risk (OR, 1.46; 95% CI, 1.24 to 1.71 for ≥25 kg/m2 vs <25 kg/m2). Biomarkers associated with 2- to 3-fold higher risk were total cholesterol levels greater than 200 mg/dL, triglyceride levels higher than 150 mg/dL, and high-density lipoprotein cholesterol levels less than 60 mg/dL (to convert to mmol/L, multiply by 0.0259).
Conclusion
Major risk factors for premature MI are mostly amenable to patient, population, and policy level interventions. Mild elevations in body mass index and triglyceride levels were associated with higher risk, which has implications for the growing worldwide epidemic of cardiometabolic diseases.
Abbreviations and Acronyms: BMI, body mass index; HDL, high-density lipoprotein; IHD, ischemic heart disease; LDL, low-density lipoprotein; MI, myocardial infarction; OR, odds ratio; RoB, risk of bias
Noncommunicable diseases, which include ischemic heart disease (IHD), are a major cause of premature mortality and claim 15 million lives annually.1 This has prompted organizations worldwide to adopt resolutions to tackle premature mortality, defined as mortality in adults aged 30 to 70 years.1, 2, 3 Premature IHD mortality is driven by premature myocardial infarction (MI). Despite this, there is no comprehensive synthesis of risk factors associated with premature MI, a limitation that impairs strategies to curb the burden of IHD and premature mortality.
Premature MI generally refers to MI in men aged 18 to 55 years or women aged 18 to 65 years.4, 5, 6 In an early study of premature MI, American adults younger than 40 years with coronary thrombosis were observed to have a higher prevalence of tobacco use, sedentary habits, and possibly higher dietary intake of cholesterol-containing foods such as milk and eggs when compared with healthy adults.7 Since then, studies in different world regions have described risk factors associated with premature MI and generally observed a higher prevalence of diabetes, hypertension, and smoking compared with adults without MI.8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19 INTERHEART was the largest global study to describe risk factors associated with premature MI. Although not exclusively focused on premature MI, age-stratified analysis in INTERHEART using age cutoff points defined above revealed that the population attributable risk of premature MI was more than 90% for a combination of 9 factors: smoking, low consumption of fruit and vegetables, inadequate exercise, low consumption of alcohol, psychosocial stress, hypertension, diabetes mellitus, abdominal obesity, and elevated ratio of blood levels of apolipoprotein B/apolipoprotein A1.20 Despite the vast literature on premature MI, studies vary by selection criteria of participants, age cutoff point for premature MI, and operational definition of risk factors. Therefore, synthesis of the literature has typically focused on the association of individual risk factors with MI without stratification by age.21, 22, 23 To our knowledge, there is no synthesis of diverse risk factors associated with premature MI.
To address this knowledge gap, we conducted a systematic review and meta-analysis of demographic characteristics, lifestyle factors, clinical risk factors, and biomarkers associated with premature MI. Our findings highlight the contribution of risk factors to premature MI and could guide the development of interventions to reduce the burden of risk factors associated with premature MI and premature mortality.
Patients and Methods
The study protocol (PROSPERO: CRD42018076862) has been described.24 This study is reported according to the Preferred Reporting Items for Systematic Reviews and Meta-analysis guidelines (Supplemental Table 1, available online at http://www.mcpiqojournal.org).25,26
Eligibility Criteria
We selected studies that reported the magnitude of the association of at least 1 risk factor (demographic characteristics, lifestyle factors, clinical risk factors, or biomarkers) with premature MI and age-matched non-MI referent groups. We included case-control, cohort, and cross-sectional studies and excluded conference abstracts, review articles, research theses, editorials, commentaries, opinions, viewpoints, and case reports. We excluded studies with fewer than 100 individuals with premature MI as well as studies without a non-MI referent group.
Risk Factors
We evaluated 19 potential risk factors from information provided on demographic characteristics, lifestyle factors, clinical risk factors, and biomarkers. We obtained information on demographic characteristics (sex, race or ethnicity, and family history of any cardiac disease), lifestyle factors (tobacco and alcohol), and clinical risk factors (diabetes mellitus, hypertension, body mass index [BMI, calculated as the weight in kilograms divided by the height in meters squared], and dyslipidemia). We obtained information on concentration/levels of biomarkers such as cholesterol (total cholesterol, low-density lipoprotein [LDL] cholesterol, high-density lipoprotein [HDL] cholesterol, non-HDL cholesterol), triglycerides, apolipoprotein A and B, C-reactive protein, and related measures (eg, HDL cholesterol/total cholesterol ratio).
Outcome
Premature MI was defined as first or recurrent MI in men aged 18 to 55 years or women aged 18 to 65 years.
Search Methods
The search strategy combined electronic and manual searches (Supplemental Table 2, available online at http://www.mcpiqojournal.org). The search strategy was developed by an academic librarian (A.P.A.) with input from 3 investigators (S.B.D., A.A.A., and S.M.). We searched MEDLINE (Ovid), AMED (Ovid), Embase (Ovid), EBSCO CINAHL Plus, ClinicalTrials.gov, and Cochrane Central Register of Controlled Trials from inception through September 20, 2017 and updated our search in June 22, 2018 and April 30, 2020 to identify subsequent citations. Searches were limited to the English language. We conducted a manual search of the bibliography of articles selected for data extraction.
Study Selection
Articles identified from electronic literature databases were merged into a single database and imported into Covidence, an online software platform that streamlines the study using the Cochrane Review process. For primary screening, articles were screened by title and abstract independently by 2 investigators. For secondary screening, full-text articles were independently reviewed by 2 investigators. If full-text articles were unavailable online or through the library (Mayo Clinic in Rochester, Minnesota), we made 2 attempts to contact the authors by e-mail before excluding the study. The bibliography of selected studies was screened (by title and abstract, and full text if necessary) for inclusion by 1 investigator (S.B.D. or Y.M.H.).
Data Extraction and Management
Two investigators independently extracted data including study characteristics, risk factors, and measures of the association with premature MI using a piloted standardized data extraction form in Microsoft Excel. At the conclusion of data extraction, all articles and extracted data were reviewed by 1 investigator. If 2 or more articles reported results using the same data set or cohort, the study with the largest sample size for a given risk factor was selected. To quantify the association between a risk factor and premature MI, we extracted study level estimates (eg, odds ratio [OR] and 95% CI). We did not include studies that reported only concentrations/levels of biomarkers as we could not derive risk estimates. All studies reported 95% CIs except for 1 study, which reported 99% CI.20 If risk estimates were not reported, we extracted a 2×2 table from the study (frequency of risk factors with or without premature MI) and calculated the OR and 95% CI.
Risk of Bias Assessment
Two investigators independently assessed the risk of bias (RoB) using the Newcastle-Ottawa Scale for case-control and cohort studies and adapted it for cross-sectional studies (Supplemental Method 1, available online at http://www.mcpiqojournal.org).27 As information on risk factors may be obtained from different sources (eg, self-reported and medical records) and are associated with different risks of bias, we reported the RoB for each risk factor from individual studies (Supplemental Tables 3 and 4, available online at http://www.mcpiqojournal.org). The RoB for risk factors was evaluated on the basis of the reported result (eg, frequency or risk estimate), method of ascertainment (eg, self-reported or verified from medical records), and whether the risk estimate was adjusted for cardiovascular risk factors (Supplemental Table 3). Accordingly, risk factors were categorized as having high, moderate, or low RoB. Therefore, the RoB assessment accounts for risk estimates that were unadjusted or adjusted for risk factors (Supplemental Table 3).
Resolution of Conflicts
Conflicts between investigators were resolved by discussion, and if necessary, through consultation with a third investigator.
Data Synthesis
Given variability in operational definitions across studies, risk factors were approximated to conventional definitions for inclusion in meta-analysis (Supplemental Table 4). We conducted a random effects meta-analysis to pool results across studies as we expected variation in study setting, adjusted covariates, and populations.28 The association between risk factors and premature MI is reported as summary OR (95% CI). Where applicable, we provided separate estimates for risk factors on the basis of RoB (low, moderate, and high). Pooled risk estimates include data with or without adjustment for cardiovascular risk factors. We reported the I2 index test for heterogeneity.29 Analyses were conducted with STATA V.15 (StataCorp LLC). Statistical significance was defined as 2-tailed P<.05.
Patient and Public Involvement
There was no patient or public involvement in the study design, execution, or analysis.
Ethics Approval
This study analyzed data from published studies without individual level participant identifiers and was exempt from review by the Mayo Clinic Institutional Review Board (Rochester, MN).
Results
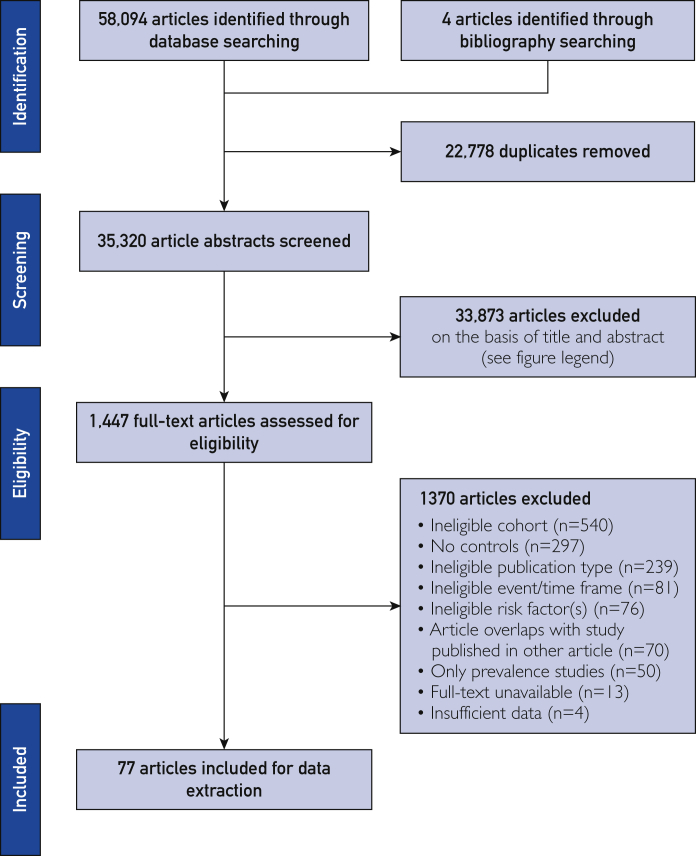
Of 35,320 unique articles, 1447 articles met criteria for full-text screening, of which 77 were selected for data extraction (Figure 1). The selected studies reported 19 risk factors in 12.7 million participants from 58 countries in all world regions; 96% of studies (n=74 of 77) were single country, with 24% (n=18 of 74) in the United States. Most studies (n=44 of 77) provided non–sex-stratified results, whereas others were limited only to men (n=16 of 77) or women (n=16 of 77).
Figure 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analysis flowchart of articles included for data extraction. The search strategy is provided in Supplemental Table 2 (available online at http://www.mayoclinicproceedings.org). From 35,320 unique articles, we screened titles and abstracts and excluded 33,873 articles that did not include all 3 concepts (risk factor, premature, and myocardial infarction) (primary screening). The full-text of eligible articles (n=1447) was reviewed using criteria for primary screening to identify articles for data extraction (n=77). All stages were conducted independently by 2 investigators.
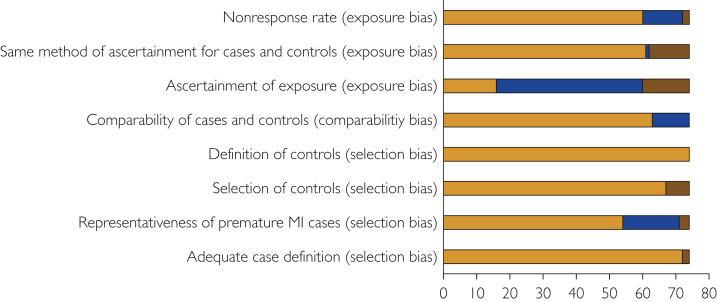
Studies varied in participant selection criteria, operational definition of risk factors, and covariates adjusted in risk models (Supplemental Tables 4-6, available online at http://www.mcpiqojournal.org). Of these, 29 studies reported risk estimates with adjustment for 1 or more cardiovascular risk factor (Supplemental Table 6). Most studies had a low risk of selection bias, a low risk of comparability bias, but a high risk of exposure bias (Figure 2). High and moderate RoBs for risk factors mostly resulted from studies that provided frequencies of self-reported risk factors rather than adjusted risk estimates of verified risk factors (Supplemental Table 4, available online at http://www.mcpiqojournal.org).
Figure 2.
Risk of bias of case-control studies selected for data extraction on the basis of the Newcastle-Ottawa Scale. Criteria to determine low (orange), medium (brown), and high (blue) risk of bias using selection, comparability, and exposure/outcome factors are described in Supplemental Method 1 (available online at http://www.mayoclinicproceedings.org). The Newcastle-Ottawa Scale was adapted for cohort studies (n=3). All studies had a low risk of bias on all criteria (described in Supplemental Method 1) except 1 study, which had a high risk of comparability bias. MI, myocardial infarction.
Demographic Characteristics
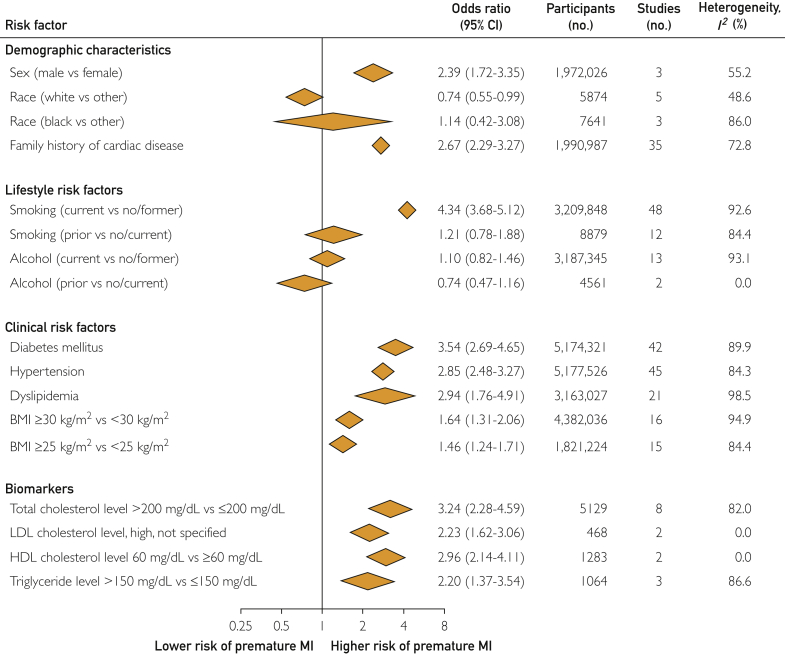
Men had a higher risk of premature MI (OR, 2.39; 95% CI, 1.71 to 3.35) than did women (Figure 3). Overall, white vs nonwhite individuals had a lower risk of premature MI (OR, 0.74; 95% CI, 0.55 to 0.99). A similar trend was noted in the smaller number of studies reporting results for nonwhite vs white women (OR, 0.73; 95% CI, 0.51 to 1.06) (Figure 3; Supplemental Tables 7 and 8 and Supplemental Figures 1 and 2, available online at http://www.mcpiqojournal.org).
Figure 3.
Pooled risk estimates for risk factors associated with the risk of premature MI. Pooled risk estimates include data with or without adjustment of cardiovascular risk factors. Given variability in the operational definition of risk factors across studies, risk factors were approximated to conventional definitions for inclusion in the meta-analysis (Supplemental Table 4, available online at http://www.mayoclinicproceedings.org). Details on study characteristics, risk factors, risk estimates by sex, and risk estimates by risk of bias are given in Supplemental Tables 4 to 15 and Supplemental Figures 1 to 14 (available online at http://www.mayoclinicproceedings.org). For family history of cardiac disease, diabetes mellitus, hypertension, dyslipidemia, and LDL cholesterol level, pooled risk estimates are based on the presence vs absence of the risk factor. Diabetes mellitus includes type 1 and/or type 2 diabetes. BMI, body mass index; HDL, high-density lipoprotein; LDL, low-density lipoprotein; MI, myocardial infarction. SI conversion factor: To convert mg/dL values to mmol/L, multiply by 0.0259.
Individuals reporting a positive family history of any cardiac disease had a nearly 3-fold higher risk of premature MI (OR, 2.67; 95% CI, 2.29 to 3.27) compared with individuals reporting no cardiac family history (Figure 3; Supplemental Figure 3, available online at http://www.mcpiqojournal.org).
Lifestyle Risk Factors
Individuals who were current smokers vs no/former smokers had a more than 4-fold higher risk of premature MI (OR, 4.34; 95% CI, 3.68 to 5.12) (Figure 3; Supplemental Table 9 and Supplemental Figures 4 and 5, available online at http://www.mcpiqojournal.org). The risk of premature MI in individuals with current alcohol use vs no/former alcohol use varied by RoB: there was a higher risk in high RoB studies and no association in moderate RoB studies. In men, current alcohol use vs no/former alcohol use as well as no alcohol vs 0.1 to 30.0 g of alcohol per day were associated with a higher risk of premature MI (Figure 3; Supplemental Tables 9 and 10 and Supplemental Figure 6, available online at http://www.mcpiqojournal.org).30
Clinical Risk Factors
Individuals with diabetes mellitus had a 4- to 5-fold higher risk of premature MI in men (OR, 5.04; 95% CI, 2.56 to 9.91) and women (OR, 3.99; 95% CI, 2.74 to 5.83) than did individuals without diabetes mellitus. Individuals with hypertension and dyslipidemia had a nearly 3-fold higher risk of premature MI than did individuals without these risk factors (Figure 3; Supplemental Table 11 and Supplemental Figures 7, 8, and 9, available online at http://www.mcpiqojournal.org).
The association of BMI 30 kg/m2 or higher vs less than 30 kg/m2 with premature MI was reported in 16 studies (non–sex-stratified, n=9; men, n=4; women, n=3) and varied by RoB and sex. One high RoB study31 found no association with premature MI (OR, 0.86; 95% CI, 0.60 to 1.23), whereas 15 moderate or low RoB studies found higher risk. This positive association was preserved in sex-stratified analysis of men (OR, 1.94; 95% CI, 1.47 to 2.56), with a nonsignificant association in women (OR, 1.28; 95% CI, 0.95 to 1.73). Notably, the association of BMI 25 kg/m2 or higher vs less than 25 kg/m2 with premature MI was similar to that of BMI 30 kg/m2 or higher vs less than 30 kg/m2 with premature MI (Figure 3; Supplemental Table 11 and Supplemental Figures 9-11, available online at http://www.mcpiqojournal.org).
Two studies evaluated the association of waist-to-hip ratio with the risk of premature MI. In men, waist-to-hip ratio greater than 0.90, compared with 0.90 or less, was associated with a 10-fold higher risk of premature MI (adjusted OR, 9.57; 95% CI, 5.52 to 16.6)30; in another study, waist-to-hip ratio (top 2 tertiles vs lowest tertile) in non–sex-stratified analysis was associated with a higher risk of premature MI (OR, 1.79; 99% CI, 1.52 to 2.09), albeit to a lesser magnitude.20
Biomarkers
Thirteen studies reported the association of measured lipids with premature MI (Figure 3; Supplemental Tables 12-15 and Supplemental Figures 12-14, available online at http://www.mcpiqojournal.org). Individuals with total cholesterol levels greater than 200 mg/dL (to convert to mmol/L, multiply by 0.0259) had a higher risk of premature MI (OR, 3.24; 95% CI, 2.28 to 4.59) than did individuals with total cholesterol levels 200 mg/dL or lower. Similarly, individuals with elevated LDL cholesterol levels (cutoff point not defined) had a higher risk of premature MI (OR, 2.23; 95% CI, 1.62 to 3.06) as did individuals with triglyceride levels greater than 150 mg/dL vs 150 mg/dL or less (OR, 2.20; 95% CI, 1.37 to 3.54) and individuals with HDL cholesterol levels less than 60 mg/dL vs 60 mg/dL or higher (OR, 2.96; 95% CI, 2.14 to 4.11). Biomarker measurements reported per 0.1 increment in HDL/cholesterol ratio and per SD increment in LDL cholesterol level, HDL cholesterol level, and non-HDL cholesterol level had associations similar to those based on biomarker cutoff points.32,33 Based on 1 study (INTERHEART) for each biomarker, apolipoprotein A1 (OR, 0.69; 95% CI, 0.64 to 0.74) and apolipoprotein B (OR, 1.59; 95% CI, 1.50 to 1.69) levels indicated the magnitude of risk similar to their associated cholesterol, HDL, and LDL levels. When combined, the apolipoprotein B/apolipoprotein A1 ratio indicated a higher risk of premature MI (OR, 4.35; 99% CI, 3.49 to 5.42).20 Based on 1 study for each biomarker, total cholesterol, LDL cholesterol, and triglyceride levels (top quartile vs lowest quartile, for all) were associated with a higher risk of premature MI whereas HDL cholesterol level was associated with a lower risk.34
Discussion
In this meta-analysis of 77 studies of 12.7 million participants, modifiable risk factors associated with a higher risk of premature MI included diabetes mellitus and smoking (3- to 4-fold higher risk), dyslipidemia (2- to 3-fold higher risk), and obesity (1.5-fold higher risk). Individuals with higher levels of total cholesterol and lower levels of HDL cholesterol had a 2- to 3-fold higher risk of premature MI. Notably, mild elevations in BMI (≥25 kg/m2 vs <25 kg/m2) and triglyceride levels (>150 mg/dL vs lower) were associated with a higher risk of premature MI. Major nonmodifiable risk factors associated with a 2- to 3-fold higher risk of premature MI were male sex and a positive family history of cardiac disease. White vs nonwhite individuals had a lower risk of premature MI. The higher risk of premature MI associated with elevation in BMI and cholesterol levels is concerning because their global prevalence is increasing, with substantial implications for the burden of premature MI.35,36
Our study evaluated the association of 19 risk factors with premature MI, identified articles from various electronic databases from database inception to April 2020, and had 2 investigators independently screen articles, extract data, and assess RoB. This allowed us to apply a uniform rigorous methodology and summarize risk estimates for several risk factors. Our study differs from studies that evaluated single risk factors, applied time restrictions to the search, had independent evaluations at select stages in the review process, or were narrative descriptions. One study compared the prognosis of smokers vs nonsmokers in adults 45 years and younger with MI.37 This study included articles from 2001 to 2017 and included only adults with MI; based on 4 studies, there were no differences between smokers and nonsmokers with in-hospital cardiac events and major adverse cardiovascular events.37 Recent narrative reviews have highlighted the cardiovascular risk factor burden in young adults.38, 39, 40 These reviews focused on primary and secondary cardiovascular prevention and management in women, on risk factors for diverse cardiovascular conditions (eg, IHD and atrial fibrillation), and on differences between young and older adults with IHD.38, 39, 40 Consequently, the search strategy and study selection criteria differed from our study. Recently, the Atherosclerosis Risk in Communities Study based in the United States reported that from 1995 to 2014, the prevalence of hypertension and diabetes mellitus has increased in adults aged 35 to 54 years hospitalized with MI.41 Taken together, these studies support our results on risk factors associated with a higher risk of premature MI.
Risk factors identified in our meta-analysis are a useful starting point for discussions between providers and patients but will require formal evaluation and integration into risk prediction scores. Current cardiovascular risk scores such as pooled cohort equations (adults aged 40-79 years), Reynolds risk score (adults aged 45-80 years), Framingham risk score (adults aged 30-74 years), QResearch risk score (adults aged 25-84 years), Systematic COronary Risk Evaluation (adults aged 45-80 years), and Coronary Artery Risk Development in Young Adults risk score (adults aged 18-30 years) use different risk factors and cardiovascular end points, yield different estimates of cardiovascular risk, and are not validated in adults below the age cutoff points of their cohorts.42, 43, 44, 45, 46, 47 This is relevant as coronary atherosclerosis begins to develop in late adolescence or early adulthood, below the age cutoff point of cohorts used for current risk scores.48,49 In addition, current risk scores emphasize conventional MI events (ie, MI in men older than 55 years and in women older than 65 years), but predominant risk factors in premature and conventional MI may be different. Recently, there were a few reports that compared risk factors associated with premature and conventional MI. Studies such as Framingham Heart Study and INTERHEART found the age-related association of biomarkers (eg, total cholesterol and apolipoprotein B) and cardiovascular risk.33,50 Genetic studies have found that familial hypercholesterolemia mutations and polygenic scores are associated with a higher risk of premature MI, suggesting that the pathophysiology of premature and conventional MI may differ.51,52 Further studies are required to identify these differences and guide the development of targeted therapies, in particular for young adults. Compared with conventional MI, premature MI was associated with a higher prevalence of smoking, obesity, and family history of cardiac disease but a lower prevalence of diabetes mellitus and hypertension.38,53 Consistent with these findings, a recent study found that the 25-year risk of premature atherosclerotic cardiovascular disease (cardiovascular and cerebrovascular) events could be predicted on the basis of simple lifestyle factors (eg, diet and physical activity).46 These results support observations from the present study and stress the need for risk prediction scores specific to premature MI. Most risk prediction scores were developed and validated in adults older than 40 years. In adults aged 25 to 40 years, further research is required to identify risk factors that predict the risk of premature MI. The present study identified demographic characteristics, lifestyle risk factors, clinical risk factors, and biomarkers associated with odds of premature MI. Whether these or other factors (eg, lipoproteins, inflammatory biomarkers, or metabolic biomarkers) can predict the risk of premature MI should be the topic of future studies.
Recent studies found that young women compared with older women or similarly aged men have a higher prevalence of myocardial infarction in the absence of obstructive coronary artery disease, a clinical entity of MI with less than 50% coronary occlusion.54 The risk factors for myocardial infarction in the absence of obstructive coronary artery disease are not well understood and may explain part of the burden of MI in younger people. Although the risk factors identified in the present study may not explain causative factors in atherosclerosis, future studies will be required to identify whether other risk factors (eg, novel biomarkers) reveal pathways involved in accelerated atherosclerosis.
At the global level, the higher burden of premature MI has affected several countries including Australia, Canada, the United Kingdom, and the United States, in which the rates of incidence and mortality for premature MI appear to be increasing.55, 56, 57, 58, 59, 60 From 2019 to 2035, the cost of managing premature cardiovascular disease in the United States is projected to increase from US$270 billion to US$370 billion, and much more globally.61 Recently, the Disease Control Priorities 3 working group provided policymakers with broad recommendations on cost-effective cardiovascular interventions.62 Although not specific to premature MI, these recommendations as well as findings from our meta-analysis may guide local and regional interventions for premature MI. Targeting key risk factors highlighted in this and other studies could reduce the number of premature cardiovascular deaths in men (from >5 million to 3.5 million) and women (from 2.8 million to 2 million) by the year 2025.63
Our study has potential limitations. We included only English language articles, which may alter the precision of our estimates but not necessarily cause systematic bias.64 During primary screening, we excluded abstracts that did not mention risk factors or age groups of interest; therefore, studies may have been excluded inadvertently if this information was not reported in the abstract. To mitigate this, we used a broad search strategy as evidenced by the large number of articles obtained for primary screening and had 2 authors independently screen articles. Furthermore, few studies included in the meta-analysis used unconventional operational definitions for risk factors or cutoff points for biomarkers, which we approximated to conventional definitions and cutoff points. Also, studies differed by design (eg, cross-sectional and cohort) and whether the risk estimates were adjusted for cardiovascular risk factors and by the number of risk factors adjusted, which could potentially alter the magnitude of the association between risk factors and premature MI. To mitigate potential bias, we incorporated information on whether risk estimates were adjusted for cardiovascular risk factors into the RoB assessment (Supplemental Tables 3 and 6). These were determined a priori, and we provided pooled and subtotal estimates on the basis of RoB. Further, we did not include studies that report only concentrations/levels of biomarkers as they could not be used to derive risk estimates. Despite this, our study has several strengths as it comprehensively analyzed the association of several risk factors with premature MI, applied rigorous methodology, and identified several risk factors amenable to interventions. To our knowledge, this study is the most comprehensive evaluation of risk factors associated with premature MI and identifies risk factors amenable to individual and population level interventions.
In this study, most data were from high-income countries. Future studies should focus on improving risk prediction in young adults, particularly in regions such as Latin America, Middle East and North Africa, and sub-Saharan Africa, in which the burden of premature MI is high and there is a paucity of studies of risk factors. Future studies should also consider combining individual level data from studies of biomarkers to estimate differences in premature MI and controls. Studies should also focus on long-term outcomes of individuals with premature MI. In this regard, the Young-MI cohort based on 2 hospitals in Boston, Massachusetts, has characterized risk factors and short- and long-term outcomes in adults younger than 55 years with MI.11,19,65, 66, 67, 68 Similar studies in other regions/countries may guide clinical practice and monitoring of individuals with premature MI. Ultimately, success will require coordination between governmental and nongovernmental stakeholders to place “people first” on any agenda that strives to reduce premature cardiovascular mortality by the year 2030, as prioritized in the United Nations Sustainable Development Goals.2
Conclusion
This meta-analysis identified major modifiable risk factors for premature MI, including diabetes mellitus, smoking, dyslipidemia, and obesity. We also found that higher levels of total cholesterol, LDL cholesterol, and triglycerides and lower levels of HDL cholesterol were associated with a higher risk of premature MI. The significance of these risk factors to the burden of premature MI is concerning because the global prevalence of most of these risk factors is increasing, with significant implications for the burden of premature MI. However, most of these risk factors are modifiable and amenable to interventions. We did not identify articles related to socioeconomic status and the risk of premature MI, and further studies are required to determine whether socioeconomic status is associated with the risk of premature MI. Further research is required to develop interventions at the person, population, and policy levels to reduce the burden of these risk factors and of premature MI.
Acknowledgments
We are grateful to Danielle J. Gerberi, MLS, AHIP, and Marla A. Battey, BCEd, (both from Saint Marys Staff Library, Mayo Clinic, Rochester, MN) for assistance in procuring full-text articles. We are grateful to Mallory Heath, MLS, (from Brigham and Women’s Hospital, Boston, MA) for assistance with editing the manuscript.
Drs Alsheikh-Ali and Mora contributed equally to this work.
Footnotes
Dr Hydoub is now with the Division of Medicine, Sheikh Shakhbout Medical City, Abu Dhabi, United Arab Emirates; Mr Reka is now with the Leddy Library, University of Windsor, Windsor, Ontario, Canada; Dr Alzuabi is now with the Department of Neurology, Medical University of South Carolina, Charleston; and Dr Farwati is now with the Department of Internal Medicine, Cleveland Clinic, Cleveland, OH.
Grant Support: The work was supported by a career development award (K23MD016230) from the National Institute on Minority Health and Health Disparities (S.B.D.), grant K24 HL136852 (S.M.) from the National Heart, Lung, and Blood Institute, and by UAE-HMS Cooperative Research Award (United Arab Emirates) (A.A.A. and S.M.). The funders had no role in the study design; in the collection, analysis, and interpretation data; in the writing of report; and in the decision to submit the article for publication. The authors worked independently of the funders.
Potential Competing Interests: Dr Murad is an employee of Mayo Clinic (outside the submitted work). Dr Mora has received consultancy fees from Quest Diagnostics and Pfizer (outside the submitted work). The other authors report no competing interests.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Supplemental material can be found online at: http://www.mcpiqojournal.org. Supplemental material attached to journal articles has not been edited, and the authors take responsibility for the accuracy of all data.
Supplemental Online Material
References
- 1.Noncommunicable Diseases Progress Monitor 2017. World Health Orgnaization website. https://www.who.int/publications/i/item/9789241513029. Accessed June 3, 2021.
- 2.Sustainable Development Knowledge Platform. United Nations Sustainable Development website. https://sustainabledevelopment.un.org/index.html. Accessed June 3, 2021.
- 3.Global strategy for the prevention and control of noncommunicable diseases. World Health Organization website. http://apps.who.int/gb/archive/pdf_files/WHA53/ea14.pdf. Accessed April 15, 2020.
- 4.De Sutter J., De Bacquer D., Kotseva K. Screening of family members of patients with premature coronary heart disease; results from the EUROASPIRE II family survey. Eur Heart J. 2003;24(3):249–257. doi: 10.1016/s0195-668x(02)00386-x. [DOI] [PubMed] [Google Scholar]
- 5.Kotseva K., Wood D., De Backer G., De Bacquer D., Pyörälä K., Keil U., EUROASPIRE Study Group EUROASPIRE III: a survey on the lifestyle, risk factors and use of cardioprotective drug therapies in coronary patients from 22 European countries. Eur J Cardiovasc Prev Rehabil. 2009;16(2):121–137. doi: 10.1097/HJR.0b013e3283294b1d. [DOI] [PubMed] [Google Scholar]
- 6.Lloyd-Jones D.M., Nam B.H., D’Agostino R.B. Parental cardiovascular disease as a risk factor for cardiovascular disease in middle-aged adults: a prospective study of parents and offspring. JAMA. 2004;291(18):2204–2211. doi: 10.1001/jama.291.18.2204. [DOI] [PubMed] [Google Scholar]
- 7.Glendy R.E., Levine S.A., White P.D. Coronary disease in youth: comparison of 100 patients under 40 with 300 persons past 80. J Am Med Assoc. 1937;109(22):1775. [Google Scholar]
- 8.Avezum A., Makdisse M., Spencer F., GRACE Investigators Impact of age on management and outcome of acute coronary syndrome: observations from the Global Registry of Acute Coronary Events (GRACE) Am Heart J. 2005;149(1):67–73. doi: 10.1016/j.ahj.2004.06.003. [DOI] [PubMed] [Google Scholar]
- 9.El-Menyar A., Zubaid M., Shehab A. Prevalence and impact of cardiovascular risk factors among patients presenting with acute coronary syndrome in the Middle East. Clin Cardiol. 2011;34(1):51–58. doi: 10.1002/clc.20873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Callachan E.L., Alsheikh-Ali A.A., Wallis L.A. Analysis of risk factors, presentation, and in-hospital events of very young patients presenting with ST-elevation myocardial infarction. J Saudi Heart Assoc. 2017;29(4):270–275. doi: 10.1016/j.jsha.2017.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Singh A., Collins B.L., Gupta A. Cardiovascular risk and statin eligibility of young adults after an MI: partners YOUNG-MI registry. J Am Coll Cardiol. 2018;71(3):292–302. doi: 10.1016/j.jacc.2017.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ahmed E., Alhabib K.F., El-Menyar A. Age and clinical outcomes in patients presenting with acute coronary syndromes. J Cardiovasc Dis Res. 2013;4(2):134–139. doi: 10.1016/j.jcdr.2012.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bucholz E.M., Strait K.M., Dreyer R.P. Sex differences in young patients with acute myocardial infarction: a VIRGO study analysis. Eur Heart J Acute Cardiovasc Care. 2017;6(7):610–622. doi: 10.1177/2048872616661847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lu Y., Zhou S., Dreyer R.P. Sex differences in lipid profiles and treatment utilization among young adults with acute myocardial infarction: results from the VIRGO study. Am Heart J. 2017;183:74–84. doi: 10.1016/j.ahj.2016.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pelletier R., Humphries K.H., Shimony A., GENESIS-PRAXY Investigators Sex-related differences in access to care among patients with premature acute coronary syndrome. CMAJ. 2014;186(7):497–504. doi: 10.1503/cmaj.131450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moccetti T., Malacrida R., Pasotti E., Investigators of the Gruppo Italiano per lo Studio della Sopravvivenza nell'Infarto Miocardico (GISSI-2) Epidemiologic variables and outcome of 1972 young patients with acute myocardial infarction: data from the GISSI-2 database. Arch Intern Med. 1997;157(8):865–869. [PubMed] [Google Scholar]
- 17.Morillas P., Bertomeu V., Pabón P., PRIAMHO II Investigators Characteristics and outcome of acute myocardial infarction in young patients: the PRIAMHO II study. Cardiology. 2007;107(4):217–225. doi: 10.1159/000095421. [DOI] [PubMed] [Google Scholar]
- 18.Bostom A.G., Cupples L.A., Jenner J.L. Elevated plasma lipoprotein(a) and coronary heart disease in men aged 55 years and younger: a prospective study. JAMA. 1996;276(7):544–548. doi: 10.1001/jama.1996.03540070040028. [DOI] [PubMed] [Google Scholar]
- 19.DeFilippis E.M., Singh A., Divakaran S. Cocaine and marijuana use among young adults presenting with myocardial infarction. J Am Coll Cardiol. 2018;71(22):2540–2551. doi: 10.1016/j.jacc.2018.02.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yusuf S., Hawken S., Ounpuu S., INTERHEART Study Investigators Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case-control study. Lancet. 2004;364(9438):937–952. doi: 10.1016/S0140-6736(04)17018-9. [DOI] [PubMed] [Google Scholar]
- 21.Liu S.C., Xia L., Zhang J. Gout and risk of myocardial infarction: a systematic review and meta-analysis of cohort studies. PLoS One. 2015;10(7):e0134088. doi: 10.1371/journal.pone.0134088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sacco S., Ornello R., Ripa P. Migraine and risk of ischaemic heart disease: a systematic review and meta-analysis of observational studies. Eur J Neurol. 2015;22(6):1001–1011. doi: 10.1111/ene.12701. [DOI] [PubMed] [Google Scholar]
- 23.Armstrong E.J., Harskamp C.T., Armstrong A.W. Psoriasis and major adverse cardiovascular events: a systematic review and meta-analysis of observational studies. J Am Heart Assoc. 2013;2(2):e000062. doi: 10.1161/JAHA.113.000062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dugani S.B., Ayala Melendez A.P., Reka R. Risk factors associated with premature myocardial infarction: a systematic review protocol. BMJ Open. 2019;9(2):e023647. doi: 10.1136/bmjopen-2018-023647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guides and handbooks. Cochrane Training website. https://training.cochrane.org/handbooks Accessed December 18, 2019.
- 26.Moher D., Liberati A., Tetzlaff J., Altman D.G., PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. doi: 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.The Newcastle-Ottawa Scale. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp
- 28.DerSimonian R., Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 29.Higgins J.P.T., Thompson S.G., Deeks J.J., Altman D.G. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Oliveira A., Barros H., Azevedo A., Bastos J., Lopes C. Impact of risk factors for non-fatal acute myocardial infarction. Eur J Epidemiol. 2009;24(8):425–432. doi: 10.1007/s10654-009-9352-9. [DOI] [PubMed] [Google Scholar]
- 31.Qian G., Zhou Y., Liu H.B., Chen Y.D. Clinical profile and long-term prognostic factors of a young Chinese Han population (≤40 years) having ST-segment elevation myocardial infarction. Acta Cardiol Sin. 2015;31(5):390–397. doi: 10.6515/ACS20140929D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Montes R., Hurtado V., Alonso A. Autoantibodies against the endothelial receptor of protein C are associated with acute myocardial infarction in young women. J Thromb Haemost. 2005;3(7):1454–1458. doi: 10.1111/j.1538-7836.2005.01297.x. [DOI] [PubMed] [Google Scholar]
- 33.Sniderman A.D., Islam S., McQueen M. Age and cardiovascular risk attributable to apolipoprotein B, low-density lipoprotein cholesterol or non-high-density lipoprotein cholesterol. J Am Heart Assoc. 2016;5(10):e003665. doi: 10.1161/JAHA.116.003665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee H., Park J.B., Hwang I.C. Association of four lipid components with mortality, myocardial infarction, and stroke in statin-naïve young adults: a nationwide cohort study. Eur J Prev Cardiol. 2020;27(8):870–881. doi: 10.1177/2047487319898571. [DOI] [PubMed] [Google Scholar]
- 35.Farzadfar F., Finucane M.M., Danaei G., Global Burden of Metabolic Risk Factors of Chronic Diseases Collaborating Group (Cholesterol) National, regional, and global trends in serum total cholesterol since 1980: systematic analysis of health examination surveys and epidemiological studies with 321 country-years and 3.0 million participants. Lancet. 2011;377(9765):578–586. doi: 10.1016/S0140-6736(10)62038-7. [DOI] [PubMed] [Google Scholar]
- 36.GBD 2015 Obesity Collaborators. Afshin A., Forouzanfar M.H., Reitsma M.B. Health effects of overweight and obesity in 195 countries over 25 years. N Engl J Med. 2017;377(1):13–27. doi: 10.1056/NEJMoa1614362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu Y., Han T., Gao M. Clinical characteristics and prognosis of acute myocardial infarction in young smokers and non-smokers (≤45 years): a systematic review and meta-analysis. Oncotarget. 2017;8(46):81195–81203. doi: 10.18632/oncotarget.21092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dugani S.B., Murad W., Damilig K. Premature myocardial infarction in the Middle East and North Africa: rationale for the Gulf PREVENT study. Angiology. 2020;71(1):17–26. doi: 10.1177/0003319719849737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chandrasekhar J., Gill A., Mehran R. Acute myocardial infarction in young women: current perspectives. Int J Womens Health. 2018;10:267–284. doi: 10.2147/IJWH.S107371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Andersson C., Vasan R.S. Epidemiology of cardiovascular disease in young individuals. Nat Rev Cardiol. 2018;15(4):230–240. doi: 10.1038/nrcardio.2017.154. [DOI] [PubMed] [Google Scholar]
- 41.Arora S., Stouffer G.A., Kucharska-Newton A.M. Twenty year trends and sex differences in young adults hospitalized with acute myocardial infarction. Circulation. 2019;139(8):1047–1056. doi: 10.1161/CIRCULATIONAHA.118.037137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.D’Agostino R.B., Sr., Vasan R.S., Pencina M.J. General cardiovascular risk profile for use in primary care: the Framingham Heart Study. Circulation. 2008;117(6):743–753. doi: 10.1161/CIRCULATIONAHA.107.699579. [DOI] [PubMed] [Google Scholar]
- 43.SCORE risk charts: the European cardiovascular disease risk assessment model. European Society of Cardiology website. https://www.escardio.org/Education/Practice-Tools/CVD-prevention-toolbox/SCORE-Risk-Charts Accessed October 16, 2019.
- 44.Hippisley-Cox J., Coupland C., Brindle P. Development and validation of QRISK3 risk prediction algorithms to estimate future risk of cardiovascular disease: prospective cohort study. BMJ. 2017;357:j2099. doi: 10.1136/bmj.j2099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Goff D.C., Lloyd-Jones D.M., Bennett G. American College of Cardiology/American Heart Association Task Force on Practice Guidelines. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines [published correction appears in Circulation. 2014;129(25)(suppl 2):S74-S75] Circulation. 2014;129(25 suppl 2):S49–S73. doi: 10.1161/01.cir.0000437741.48606.98. [DOI] [PubMed] [Google Scholar]
- 46.Gooding H.C., Ning H., Gillman M.W. Application of a lifestyle-based tool to estimate premature cardiovascular disease events in young adults: the Coronary Artery Risk Development in Young Adults (CARDIA) Study. JAMA Intern Med. 2017;177(9):1354–1360. doi: 10.1001/jamainternmed.2017.2922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ridker P.M., Buring J.E., Rifai N., Cook N.R. Development and validation of improved algorithms for the assessment of global cardiovascular risk in women: the Reynolds Risk Score [published correction appears in JAMA. 2007;297(13):1433] JAMA. 2007;297(6):611–619. doi: 10.1001/jama.297.6.611. [DOI] [PubMed] [Google Scholar]
- 48.McNamara J.J., Molot M.A., Stremple J.F., Cutting R.T. Coronary artery disease in combat casualties in Vietnam. JAMA. 1971;216(7):1185–1187. [PubMed] [Google Scholar]
- 49.Enos W.F., Holmes R.H., Beyer J. Coronary disease among United States soldiers killed in action in Korea; preliminary report. J Am Med Assoc. 1953;152(12):1090–1093. doi: 10.1001/jama.1953.03690120006002. [DOI] [PubMed] [Google Scholar]
- 50.Wilson P.W.F. Lipids and vascular disease: a Framingham perspective. Glob Heart. 2013;8(1):25–33. doi: 10.1016/j.gheart.2012.12.009. [DOI] [PubMed] [Google Scholar]
- 51.Thériault S., Lali R., Chong M., Velianou J.L., Natarajan M.K., Paré G. Polygenic contribution in individuals with early-onset coronary artery disease. Circ Genom Precis Med. 2018;11(1):e001849. doi: 10.1161/CIRCGEN.117.001849. [DOI] [PubMed] [Google Scholar]
- 52.Khera A.V., Chaffin M., Zekavat S.M. Whole-genome sequencing to characterize monogenic and polygenic contributions in patients hospitalized with early-onset myocardial infarction. Circulation. 2019;139(13):1593–1602. doi: 10.1161/CIRCULATIONAHA.118.035658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lei L., Bin Z. Risk factor differences in acute myocardial infarction between young and older people: a systematic review and meta-analysis. Int J Cardiovasc Sci. 2019;32(2):163–176. [Google Scholar]
- 54.Tamis-Holland J.E., Jneid H., Reynolds H.R., American Heart Association Interventional Cardiovascular Care Committee of the Council on Clinical Cardiology; Council on Cardiovascular and Stroke Nursing; Council on Epidemiology and Prevention; and Council on Quality of Care and Outcomes Research Contemporary diagnosis and management of patients with myocardial infarction in the absence of obstructive coronary artery disease: a scientific statement from the American Heart Association. Circulation. 2019;139(18):e891–e908. doi: 10.1161/CIR.0000000000000670. [DOI] [PubMed] [Google Scholar]
- 55.Wilmot K.A., O’Flaherty M., Capewell S., Ford E.S., Vaccarino V. Coronary heart disease mortality declines in the United States from 1979 through 2011: evidence for stagnation in young adults, especially women. Circulation. 2015;132(11):997–1002. doi: 10.1161/CIRCULATIONAHA.115.015293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ford E.S., Capewell S. Coronary heart disease mortality among young adults in the U.S. from 1980 through 2002: concealed leveling of mortality rates. J Am Coll Cardiol. 2007;50(22):2128–2132. doi: 10.1016/j.jacc.2007.05.056. [DOI] [PubMed] [Google Scholar]
- 57.Gupta A., Wang Y., Spertus J.A. Trends in acute myocardial infarction in young patients and differences by sex and race, 2001 to 2010. J Am Coll Cardiol. 2014;64(4):337–345. doi: 10.1016/j.jacc.2014.04.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Izadnegahdar M., Singer J., Lee M.K. Do younger women fare worse? Sex differences in acute myocardial infarction hospitalization and early mortality rates over ten years. J Womens Health (Larchmt) 2014;23(1):10–17. doi: 10.1089/jwh.2013.4507. [DOI] [PubMed] [Google Scholar]
- 59.Nedkoff L.J., Briffa T.G., Preen D.B. Age- and sex-specific trends in the incidence of hospitalized acute coronary syndromes in Western Australia. Circ Cardiovasc Qual Outcomes. 2011;4(5):557–564. doi: 10.1161/CIRCOUTCOMES.110.960005. [DOI] [PubMed] [Google Scholar]
- 60.O’Flaherty M., Ford E., Allender S., Scarborough P., Capewell S. Coronary heart disease trends in England and Wales from 1984 to 2004: concealed levelling of mortality rates among young adults. Heart. 2008;94(2):178–181. doi: 10.1136/hrt.2007.118323. [DOI] [PubMed] [Google Scholar]
- 61.Cardiovascular disease: a costly burden for America: projections through 2035. American Heart Association website. http://www.heart.org/idc/groups/heart-public/@wcm/@adv/documents/downloadable/ucm_491543.pdf Accessed April 19, 2019.
- 62.Prabhakaran D., Anand S., Watkins D., Disease Control Priorities-3 Cardiovascular, Respiratory, and Related Disorders Author Group Cardiovascular, respiratory, and related disorders: key messages from Disease Control Priorities, 3rd edition. Lancet. 2018;391(10126):1224–1236. doi: 10.1016/S0140-6736(17)32471-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sacco R.L., Roth G.A., Reddy K.S. The Heart of 25 by 25: achieving the goal of reducing global and regional premature deaths from cardiovascular diseases and stroke: a modeling study from the American Heart Association and World Heart Federation. Circulation. 2016;133(23):e674–e690. doi: 10.1161/CIR.0000000000000395. [DOI] [PubMed] [Google Scholar]
- 64.Morrison A., Polisena J., Husereau D. The effect of English-language restriction on systematic review-based meta-analyses: a systematic review of empirical studies. Int J Technol Assess Health Care. 2012;28(2):138–144. doi: 10.1017/S0266462312000086. [DOI] [PubMed] [Google Scholar]
- 65.DeFilippis E.M., Singh A., Gupta A. Long-term outcomes after out-of-hospital cardiac arrest in young patients with myocardial infarction. Circulation. 2018;138(24):2855–2857. doi: 10.1161/CIRCULATIONAHA.118.036506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yang J., Biery D.W., Singh A. Risk factors and outcomes of very young adults who experience myocardial infarction: the Partners YOUNG-MI Registry. Am J Med. 2020;133(5):605–612.e1. doi: 10.1016/j.amjmed.2019.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Divakaran S., Singh A., Biery D. Diabetes is associated with worse long-term outcomes in young adults after myocardial infarction: the Partners YOUNG-MI Registry. Diabetes Care. 2020;43(8):1843–1850. doi: 10.2337/dc19-0998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Singh A., Gupta A., DeFilippis E.M. Cardiovascular mortality after type 1 and type 2 myocardial infarction in young adults. J Am Coll Cardiol. 2020;75(9):1003–1013. doi: 10.1016/j.jacc.2019.12.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.