Summary
Iron deficiency anaemia is a global health problem, which particularly affects pregnant women. Iron deficiency anaemia during pregnancy is associated with increased maternal and perinatal morbidity and mortality. Maternal iron deficiency may also be associated with neurocognitive deficits in infants. Iron requirements increase during pregnancy and are influenced by hepcidin, the master regulator of iron homeostasis. The enduring global burden of maternal anaemia suggests that currently employed iron supplementation strategies are suboptimal. Recent developments in our understanding of systemic and placental iron homeostasis may improve therapeutic effectiveness by altering the dose and frequency of oral iron. Intravenous iron appears to be a safe treatment to correct maternal anaemia rapidly but research on patient-centred outcomes and cost-effectiveness is needed. Future trials should be adequately powered to assess outcomes relevant to pregnant women.
Keywords: Anaemia, pregnancy, iron deficiency, iron
Introduction
Approximately 1.24 billion people worldwide are affected by iron deficiency anaemia (IDA).1 It is one of the leading global causes of years lived with disability, particularly affecting women, low–middle social demographic groups and populations of Asia and sub-Saharan Africa.1 Maternal anaemia is thought to affect 32 million women worldwide.2,3 A large, UK cohort study reported that 46% of women were anaemic at some point during pregnancy.4 Iron deficiency (ID) is by far the most common cause of maternal anaemia but other causes include haemoglobinopathies, such as sickle-cell anaemia and thalassaemia; deficiencies of folate, B12 or both; hookworm infection; schistosomiasis and HIV infection.
The association between anaemia and poor maternal, fetal and neonatal outcomes is now well established.5,6 Anaemia is increasingly recognised as a potentially modifiable risk factor for postpartum haemorrhage – a leading cause of maternal morbidity and mortality.7–9 Adverse fetal and neonatal outcomes include preterm labour, growth restriction and increased mortality.
This review discusses iron homeostasis and current definitions of IDA in pregnancy, adverse maternal and neonatal outcomes associated with anaemia, and the most recent treatment recommendations for IDA during pregnancy and the postpartum period. Management of other causes of anaemia is beyond the scope of this review and is reviewed elsewhere.10–14
Methods
We searched MEDLINE, PubMed, Cochrane Central Register of Controlled Trials, EMBASE and Google Scholar. Titles and abstracts were screened and references of all identified systematic reviews, randomised controlled trials, observational studies, review articles and current treatment guidelines were checked for further relevant literature. The search was restricted to literature from 1 January 2000, but older important publications were not excluded. The end date of the literature search was 12 January 2020. Topics beyond the scope of this review were referenced by relevant narrative reviews, systematic reviews or clinical guidelines where applicable. The literature searches included the following terms: iron; anaemia/anemia; haemoglobin/haemoglobin; outcomes; pregnancy; obstetric; neonatal; fetal/foetal; and parturient. The results are shown in Supplementary Figure 1.
Iron homeostasis during pregnancy
Iron requirements during pregnancy
Iron is an essential element required by almost all organisms due to the indispensable roles it plays in processes such as DNA synthesis, cell growth and differentiation, immunity, mitochondrial function and responses to hypoxia.15–19 Iron requirements increase approximately 10-fold during pregnancy from 0.8 mg/day in the first trimester to 7.5 mg/day in the third trimester in order to support the increase in maternal red cell mass, sustain placental and fetal growth, and accommodate blood loss during delivery.20,21 The placenta requires around 90 mg of iron in its own right and transports approximately 270 mg of iron to the fetus over the course of a normal pregnancy.22
The role of hepcidin
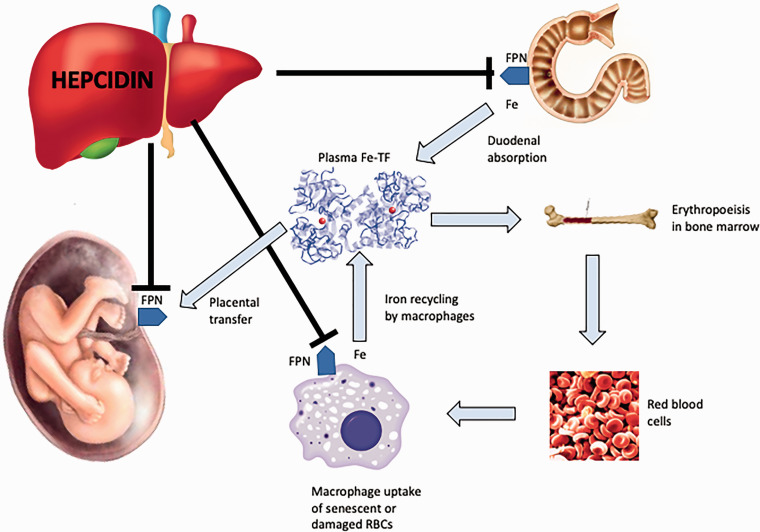
Systemic iron homeostasis is controlled by hepcidin – a peptide hormone predominantly produced in the liver and excreted by the kidneys.16 Hepcidin works by regulating the activity of ferroportin – the sole mammalian iron transport protein.16 Ferroportin delivers stored, dietary or recycled iron to blood plasma and is expressed at all sites involved in iron–plasma exchange, including the basolateral membrane of duodenal enterocytes, macrophages, hepatocytes and the basal surface of placental syncytiotrophoblasts facing the fetal circulation.16,23 At each of these sites hepcidin causes intracellular degradation of ferroportin, thereby preventing iron export to blood plasma (Figure 1). Changes in hepcidin levels can lead to rapid fluctuations in plasma iron concentrations.
Figure 1.
Hepcidin–ferroportin interaction and major systemic iron pathways. Hepcidin expression results in degradation of ferroportin which impairs release of iron from macrophages and duodenal enterocytes. Reduction in hepcidin during pregnancy increases the availability of iron for placental transfer.
Fe-TF: iron-transferrin; FPN: ferroportin; RBC: red blood cell.
Expression of hepcidin is increased in response to inflammation, infection, malignancy and iron overload.19 Hepcidin-mediated degradation of ferroportin causes iron to remain trapped within macrophages and duodenal enterocytes and therefore unavailable for those tissues that require it. Inhibition of duodenal absorption of iron – known as the ‘hepcidin block’ – may explain why oral iron is ineffective in inflammatory states. Hepcidin expression is reduced in states of ID, anaemia, hypoxaemia and increased erythropoietic drive.19
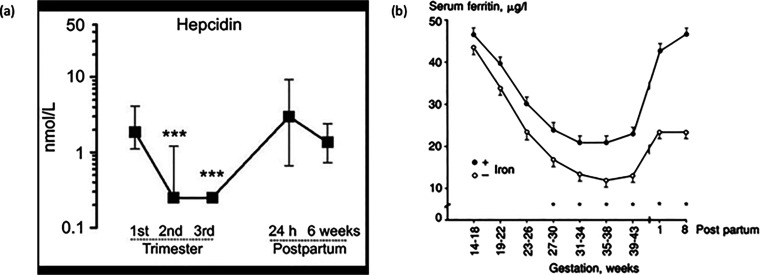
In healthy pregnancy, hepcidin increases in the first trimester when compared with the non-pregnant state, but then decreases in the second and third trimesters (Figure 2).24,25 It is presumed that this pattern facilitates increased absorption of dietary iron and release of iron from stores. However, the mechanism underlying maternal hepcidin suppression as pregnancy progresses is still unknown.22 The development of ID may be the key driver, although low hepcidin concentrations are reported even in women who are iron replete at delivery.26
Figure 2.
Serial changes in serum hepcidin and ferritin concentrations during pregnancy and postpartum. (a) Median (IQR) serum hepcidin concentrations in 31 women during pregnancy and postpartum. ***Compared with first-trimester values, P < 0.0001. (b) Geometric mean ± SEM serum ferritin concentrations during pregnancy in women given oral iron supplementation (filled circles; n = 63) compared with controls (open circles; n = 57).
Source: Reproduced with permission from van Santen et al.25
Placental and fetal iron homeostasis
The majority of iron transfer to the fetus occurs during the third trimester, which coincides with the period of lowest hepcidin expression.22 This transfer is unidirectional. Iron is bound to transferrin in the maternal circulation and is taken up by transferrin receptor 1 located on the apical membrane of the syncytiotrophoblast.22 This complex is then endocytosed and eventually exported to the basal surface of the syncytiotrophoblasts where, through ferroportin, iron enters the fetal circulation.21,22 Relatively little is known about fetal hepcidin and the responses of the maternal–placental–fetal unit to changes in maternal iron status. A recent study evaluating mechanisms in murine and in vitro trophoblastic models of severe ID observed an unexpected response that prioritised placental iron retention over fetal iron transfer.23 This response may have an evolutionary benefit to protect iron-dependent placental processes, and may provide overall benefit for the fetus despite diminishing fetal iron availability. This work also suggests that the fetus may be unable to compensate for maternal ID by increasing placental iron transfer.23 Further studies are required to clarify the significance of these findings.
Changes in other markers of iron status during pregnancy
Concentrations of serum ferritin and iron, along with transferrin saturation, gradually fall to a nadir in the third trimester.22,24 The decrease is less marked in pregnant women receiving iron supplementation.22 However, since ferritin is an acute phase protein, and transferrin a negative acute phase protein, these parameters become unreliable as indices of iron status in pregnancies complicated by inflammatory pathologies.16
Defining IDA in pregnant women
IDA is a composite diagnosis based on haemoglobin (Hb) and ferritin concentrations. The World Health Organisation (WHO) has defined anaemia in pregnancy as a Hb concentration less than 110 g/L irrespective of trimester, but recognises that Hb may fall physiologically by around 5 g/L during the second trimester.27 This is due to a rise in plasma volume of ∼50%, disproportionate to the concomitant ∼25% rise seen in red cell mass.28,29
The WHO definition has been questioned as it is derived from studies conducted in the 1950s and 1960s that did not include pregnant women and used now superseded methods for measuring Hb. Recent evidence has also questioned the validity of the figure of 5 g/L for the physiological fall in Hb during the second trimester. A large, multi-ethnic, observational study of 7054 pregnant women found that the fall in Hb was in the order of 14 g/L, or 11% of the first trimester value.27 Such findings have significant implications for the diagnosis and management of maternal anaemia.30,31 The use of a lower Hb threshold compared with men for the diagnosis of anaemia in non-pregnant women has been recently challenged,32 and guidelines now recommend targeting an Hb > 130 g/L in patients undergoing major surgery, irrespective of sex.33
The most recent British Committee for Standards in Haematology (BCSH) guidelines on the management of ID have defined anaemia as Hb less than 110 g/L in the first trimester, less than 105 g/L in the second and third trimesters, and less than 100 g/L in the immediate postpartum period.34 It is recommended that Hb routinely be measured at the initial booking consultation with a healthcare professional in the first trimester and at around 28 weeks’ gestation. Although not supported by high-quality evidence, these timepoints were felt to be practically feasible.
Most stored iron is located in the liver, bound to the iron storage protein ferritin. The most common test of iron status, serum ferritin, provides a convenient measure of storage iron. Current BCSH guidelines recommend using a ferritin of less than 30 µg/L to diagnose ID in pregnancy, but this is not unified globally.34,35 The ferritin threshold of 30 µg/L is derived from two studies published in the 1990s that compared serum ferritin with histochemical assessments of bone marrow iron stores.36,37 These studies had limitations including small sample sizes and inclusion of women with possible coexisting inflammatory conditions. As already mentioned, ferritin is an acute phase protein. Ferritin may even be elevated as a result of pregnancy itself, so while a low ferritin almost invariably indicates ID, a normal ferritin cannot reliably be used to exclude it. To date, no high-quality studies have been undertaken to investigate pregnancy-specific ferritin thresholds.
Most experience with other biomarkers of iron status such as transferrin saturation, soluble transferrin receptor, reticulocyte Hb content, mean cell Hb concentration and hepcidin comes from non-pregnant populations; experience in pregnancy is largely limited to research purposes.38–41
Non-anaemic iron deficiency
Anaemia is the final manifestation of ID as erythropoiesis is often preserved until the advanced stages of ID.42 Therefore, much of the burden of ID in pregnant women will go unnoticed if the absence of anaemia is taken to imply adequate iron stores. Non-anaemic iron deficiency (NAID) is increasingly being recognised as a disease but the clinical relevance in pregnancy is unclear.42 A recent study of 102 non-anaemic pregnant women found that 42% had evidence of ID as determined by a ferritin less than 30 µg/L or transferrin saturation less than 20%, but data on maternal and fetal outcomes were lacking.43
A recent systematic review of iron supplementation in healthy non-pregnant women suffering from NAID showed lower levels of subjective fatigue in participants who received iron.44 However, there were no improvements in objective measures of physical capacity such as time trials, time to exhaustion tests or maximal oxygen consumption. Furthermore, the overall quality of evidence was judged to be low to moderate. Small exploratory studies in patients undergoing elective colorectal and cardiac surgery have suggested worse postoperative outcomes in patients with NAID when compared with iron-replete individuals.45,46
Relying on the presence of anaemia to prompt assessment of iron status may result in large numbers of pregnant women with ID being missed. Further research into the diagnosis of NAID and its impact on maternal and fetal outcomes is needed. Routine screening of pregnant women using serum ferritin has been suggested; however, cost implications and lack of well-designed prospective studies to support this approach mean that a more targeted approach of identifying and treating at-risk pregnant women is currently recommended.34
Outcomes associated with maternal anaemia
Maternal outcomes
The clinical signs and symptoms of IDA include fatigue, pallor, angular cheilitis, weakness, palpitations, shortness of breath, restless legs, pica syndrome, irritability and poor concentration. These may also be present in NAID.
Observational studies47,48 have demonstrated an association between maternal anaemia and mortality, with one study demonstrating a 29% linear increase in maternal mortality with each 10 g/L decrease in maternal Hb.48 A recent study reported that severe anaemia, defined as Hb < 70 g/L during pregnancy or postpartum, doubled the risk of death.49 Of particular concern, maternal anaemia – with or without ID – increases the risk of developing postpartum haemorrhage.7,50 A large, two-centre, UK study, involving 10,213 women, found that 62% of women with Hb < 85 g/L developed postpartum haemorrhage, and 26% progressed to develop severe (greater than 1500 mL) postpartum haemorrhage.51
It is important to note that the majority of these data come from low- and middle-income countries.48,49 The timing of Hb measurement is not always clear and therefore it is difficult to determine whether postpartum haemorrhage led to severe anaemia, or whether severe anaemia during pregnancy increased the risk of postpartum haemorrhage. In addition, despite attempts to control for multiple confounders such as haemorrhage, sepsis and critical care admission, it is possible that a weaker association between anaemia and poor maternal outcomes exists than is initially suggested by such data.
Postpartum anaemia has been linked to depression,52–54 fatigue,55 impaired cognition,56 impaired lactation and early cessation of breast feeding.57,58 Trials of postpartum iron therapy have demonstrated improvements in Hb and serum ferritin concentration but data on patient-centred outcomes are lacking.59
Fetal and neonatal outcomes
Maternal IDA is a recognised risk factor for preterm labour, low birthweight and small-for-gestational age (SGA) babies.5,7,60,61 The Baby’s Vascular health and Iron in Pregnancy (BABY VIP) study showed that maternal ID in the first trimester was associated with a two-fold increase risk of having a fetus with SGA. Every 10 g/L decrease in maternal Hb before 20 weeks’ gestation was associated with a 30% increase in the relative risk (RR) of SGA.61 A systematic review of 48 randomised controlled trials (a total of 17,793 women) and 44 cohort studies (a total of 1,851,682 women) found that iron supplementation resulted in a modest increase in birthweight (weighted mean difference (WMD) 41–69 g) with a small reduction in the risk of delivering a low birthweight infant.60 There was no evidence of an effect on gestation length, preterm birth or SGA infants.
Maternal anaemia is also associated with increased perinatal and neonatal mortality.4,7 A large, multi-ethnic, UK observational cohort study which evaluated over 14,000 pregnant women, found that women with Hb < 100 g/L had a three-fold increased risk of perinatal death and a five-fold increased risk of stillbirth when compared with women who had Hb > 110 g/L.4 The authors controlled for multiple confounders including advanced maternal age, ethnicity, body mass index, smoking status and a range of medical comorbidities.
There is increasing interest in the impact of maternal ID on the neonatal brain and cognitive development.62 Fetal brain growth accelerates rapidly in the last trimester and continues for the first 2 years after birth,63 by the end of which total brain volume reaches 80%–90% of adult volume.64 The iron-dependent processes occurring during this period include monoamine neurotransmission, myelination and hippocampal development.65 ID has been shown to alter expression of genes critical for hippocampal development and function.66 Infants with evidence of ID in utero have been demonstrated to have abnormal neural maturation, poor memory, altered interactions with caregivers and an increased incidence of abnormal neurological reflexes.65,67 Low ferritin levels in utero have also been linked with lower IQ, worse language ability and poorer tractability at up to 5 years of age.68 Studies addressing the effects of iron supplementation have yielded mixed results. In one study in Nepal, prenatal iron supplementation for women at high risk of developing ID resulted in improved intellectual and fine motor functioning in subsequent children aged 7–9 years.69 However, in other studies, the effects of iron supplementation on children whose mothers have established ID have been less clear,70 implying that timing of the intervention is critical; earlier supplementation during the antenatal period may be necessary for a beneficial effect on the developing brain. Future maternal intervention trials should incorporate neurodevelopmental outcomes of offspring.
Treatment strategies
The recommendations from the most recent BCSH guidelines34 are summarised in Table 1. Guidelines from other countries make broadly similar recommendations, with some modifications tailored towards their respective population.71–73
Table 1.
Summary of key recommendations from the 2019 UK guidelines on the management of iron deficiency anaemia.
| Recommendation | Grade of recommendation |
|---|---|
| Antenatal | |
| Healthcare professionals should be aware that iron deficiency anaemia in pregnancy is common and associated with increased risk of maternal morbidity and mortality | 1B |
| Healthcare professionals should be aware that iron deficiency anaemia in pregnancy is associated with increased risk of perinatal morbidity and mortality, with implications for infant neurocognitive development | 2B |
| Haemoglobin should be routinely measured at booking and at around 28 weeks’ gestation | 1D |
| If anaemia without an obvious other cause is detected, a diagnostic trial of oral iron should be given without delay, with a repeat full blood count in 2–3 weeks | 1D |
| Non-anaemic women at risk of iron deficiency should be identified and either started on prophylactic iron empirically or have serum ferritin checked first | 1D |
| A serum ferritin level of less than 30 μg/L in pregnancy is indicative of iron deficiency. Levels higher than this do not rule out iron deficiency or depletion | 2C |
| The optimal dose of elemental oral iron of 40–80 mg every morning is suggested, checking haemoglobin at 2–3 weeks to ensure an adequate response. Further research is warranted. | 2C |
| For nausea and epigastric discomfort, alternate day dosing or preparations with lower iron content should be tried. | 1A |
| Once the Hb is in the normal range, replacement should continue for 3 months and until at least 6 weeks postpartum to replenish iron stores | 1D |
| If response to oral iron is poor, compliance should be checked, and consideration given to alternative causes of anaemia | 1A |
| Intravenous iron should be considered in women who present after 34 weeks’ gestation with confirmed iron deficiency anaemia and an Hb of less than 100 g/L | 1C |
| Intrapartum and postpartum | |
| Women with iron deficiency anaemia with an Hb of less than 100 g/L should deliver in an obstetrician-led unit and should have active management of the third stage of labour | 1D |
| After delivery, women with blood loss greater than 500 mL, those with uncorrected anaemia detected in the antenatal period or those with symptoms suggestive of anaemia postnatally should have their Hb checked within 48 h of delivery | 2A |
| Women with Hb less than 100 g/L within 48 h of delivery, who are haemodynamically stable, asymptomatic or mildly symptomatic, should be offered oral elemental iron 40–80 mg daily for at least 3 months | 2A |
| Use of intravenous iron postpartum should be considered in women who are previously intolerant of, or do not respond to, oral iron and/or where the severity of symptoms of anaemia requires prompt management | 2B |
Hb: haemoglobin.
Prenatal
A careful history is required to identify women at risk of developing ID with or without anaemia (Table 2). All women should be offered dietary advice, though this is invariably insufficient to correct established ID. Dietary iron is absorbed in two forms – non-haem (inorganic) iron and haem-bound iron. Non-haem iron exists predominantly in the oxidised ferric (Fe3+) form and needs to be reduced to the ferrous (Fe2+) form in order to be absorbed efficiently.34
Table 2.
Risk factors for developing iron deficiency anaemia during pregnancy.
| Lifestyle factors |
| • Vegetarian or vegan diets |
| • Diets low in haem iron or high in substances impairing iron absorption |
| Patient factors |
| • Previous anaemia |
| • Malabsorptive conditions (e.g. inflammatory bowel disease, coeliac disease) |
| • Known haemoglobinopathy |
| • Jehovah’s Witness |
| Obstetric factors |
| • Multiparity |
| • Multiple gestation (e.g. twins) |
| • High risk of bleeding during pregnancy or during labour |
| • Short interpregnancy interval (less than 1 year) |
Meat, fish and poultry are rich sources of haem iron, which is absorbed much more readily than non-haem iron. Non-haem iron is predominantly derived from plant-based foods, where it is complexed in insoluble forms, which significantly contributes to the high prevalence of ID observed in societies that consume vegetarian diets. Iron absorption is inhibited by tannins in tea and coffee, and phytate, a substance found in cereals and legumes. Co-ingestion of vitamin C (ascorbic acid) significantly increases iron uptake from non-haem sources.74
Oral iron
Oral iron is an effective, cheap and safe way to treat ID. The large systematic review discussed earlier found that prenatal iron increased maternal Hb by a mean of 46 g/L (95% Confidence Interval (CI): 37–55 g/L), with concomitant reductions in the RR of developing (1) anaemia (RR 0.50; 95% CI: 0.42–0.59); (2) ID (RR 0.59; 95% CI: 0.46–0.79) and (3) iron-deficiency anaemia (RR 0.40; 95% CI: 0.26–0.60).60
Current pregnancy guidelines recommend 40–80 mg of elemental oral iron once daily each morning,34 which differs from previous guidance recommending 100–200 mg daily.74 The WHO recommendations on antenatal care for a positive pregnancy experience recommend 30–60 mg of oral iron once daily or intermittent doses of 120 mg if daily iron is not acceptable due to side effects.75,76 These recommendations have largely been driven by recent advances in our understanding of iron homeostasis. Studies in non-pregnant women have shown that a once daily or alternate day dosing strategy may be more effective and better tolerated by women compared with the traditional higher total daily doses.77,78 Oral ingestion of a single dose of ferrous sulphate results in a rapid rise in circulating hepcidin, which can take up to 48 h to return to normal.77 Subsequent oral doses may not be absorbed during this period because of this ‘hepcidin block’, and may expose women to well-recognised gastrointestinal side effects such as nausea, constipation and epigastric pain. A recent meta-analysis reported an incidence of up to 70% associated with oral iron.79 Since hepcidin levels are also lowest in the morning, dosing at this time is advised.80
Ferrous salts such as ferrous sulphate, ferrous fumarate and ferrous gluconate (Table 3) are preferable to ferric salts due to better oral bioavailability but differ with respect to available elemental iron. Multivitamins and ‘off the shelf’ preparations are not recommended as they contain insufficient amounts of iron and may include other substances, particularly calcium, that impair iron absorption. Slow release and enteric-coated formulations are undesirable as the majority of iron in these preparations is carried past the duodenum and therefore not absorbed.81
Table 3.
Characteristics of different oral iron formulations.
| Ferrous sulphate | Ferrous fumarate | Ferrous gluconate | |
|---|---|---|---|
| Brand name | Feospan®, FeroSul®Ferrograd® | Galfer® | Fergon®, Ferralet® |
| Preparation, mg | 200 | 210 | 300 |
| Elemental iron, mg | 65 | 65 | 35 |
| Common side effects | Constipation; diarrhoea; nausea; gastrointestinal discomfort | ||
| Uncommon side effects | Discolouration of stools; decreased appetite | ||
| Product cost per 28 packet tablet, NHS indicative price as per BNF, £ | 1.60 | 1.00 | 3.35 |
BNF: British National Formulary; NHS: UK National Health Service.
Once commenced on oral iron, Hb should be measured after 2–3 weeks to assess response. Once Hb has normalised, replacement should continue for three further months, and, if near term, up to 6 weeks postpartum.34 If the response is poor, compliance should be explored. Other causes for failure to respond to oral therapy include iron sequestration driven by elevated hepcidin as a result of inflammation or infection; ongoing blood loss and other causes of anaemia such as folate deficiency.
Intravenous iron
Intravenous iron is efficacious in replenishing iron stores and treating anaemia in a wide range of clinical situations with or without the presence of inflammation.82,83 Intravenous iron should be considered from the second trimester for women with confirmed IDA who do not respond to, or who fail to tolerate, oral iron. Given the proximity to delivery, it should also be considered for women presenting after 34 weeks’ gestation with Hb < 100 g/L and confirmed ID.34
Recent systematic reviews have shown intravenous iron to be more effective at improving maternal Hb (WMD 6.6 g/L; 95% CI: 3.1–10.1 g/L) and ferritin (WMD 45.6 µg/L; 95% CI: 26.21–65.16 µg/L) at delivery when compared with oral iron supplementation; intravenous therapy is also associated with fewer mild medication reactions than oral treatment (RR 0.34; 95% CI: 0.20–0.57).84,85 Intravenous iron is also associated with higher neonatal birthweight (WMD 58 g; 95% CI: 6–111 g). However, there was no evidence of an effect of intravenous iron to reduce maternal blood transfusion requirements or improve neonatal Hb. It is worth noting that the majority of the studies included in these systematic reviews were small and of low methodological quality, as evidenced by the wide confidence intervals. There was also heterogeneity with regards to the timing and dosage of iron supplementation and a lack of reporting of maternal and neonatal-centred outcomes.
Four intravenous iron preparations are currently available in the UK. The properties of these are summarised in Table 4.Newer iron preparations such as ferric carboxymaltose (Ferinject®) and iron isomaltoside (Monofer®) form stable carbohydrate complexes that permit controlled delivery of iron to the reticuloendothelial system (liver macrophages, spleen and bone marrow).86,87 Iron is subsequently delivered in a slow and controlled manner to iron binding proteins such as ferritin. These mechanisms limit the amount of freely available and potentially toxic unbound iron circulating in the bloodstream, while also bypassing the ‘hepcidin block’ in inflammatory states.87
Table 4.
Characteristics of different intravenous iron formulations available in the UK.
| Ferric carboxymaltose | Iron isomaltoside | Iron (III) hydroxide sucrose | Iron (III) hydroxide dextran | |
|---|---|---|---|---|
| Brand name | Ferinject® | Monofer® | Venofer® | CosmoFer® |
| Iron content, mg/mL | 50 | 100 | 20 | 50 |
| Labile iron (% injected dose) | 0.6 | 1.0 | 3.5 | 3.5 |
| Route of administration | Intravenous, slow infusion | Intravenous, slow infusion | Intravenous, slow infusion | Intravenous, slow infusion; intramuscular (gluteal) |
| Test dose required | No | No | First dose for new patients | Yes, before every dose |
| Maximal single dose | 20 mg/kg diluted in 100 mL 0.9% saline. Maximum weekly dose of 1000 mg | 20 mg/kg diluted in maximum 500 mL 0.9% saline | 200 mg, can be repeated up to three times in 1 week | 20 mg/kg, diluted in maximum 500 mL 0.9% saline or 5% glucose |
| Half-life | 7–12 h | 5 h | 6 h | 20 h |
| Infusion time, minimum | 15 min | 15 min, Doses greater than 1000 mg should be administered over greater than 30 min | 30 min | Over 4–6 h |
| Use in pregnancy | Avoid in first trimester | Avoid in first trimester | Avoid in first trimester | Avoid in first trimester |
| Lactation | Less than 1% iron passed into breastmilk; doubtful clinical significance | Low transfer into breastmilk; doubtful clinical significance | No available data | No available data |
| Adverse drug reactions | Common: nausea (2.9%) headache, dizziness, injection site reactions, transient hypophosphataemia | Common: nausea, injection site reactions | Common: nausea, injection site reactions, hypotension, hypertension | Approximately 5% will experience dose-dependent adverse reactions |
| Anaphylactic reactions | Rare (≥1/10,000 to <1/1000) | Rare (≥1/10,000 to <1/1000) | Rare (≥1/10,000 to <1/1000) | Rare (≥1/10,000 to <1/1000) |
| Product cost per 1000 mg, NHS indicative price as per BNF, £ | 154.23 | 169.50 | 109.09 | 159.40 |
BNF: British National Formulary; NHS: UK National Health Service.
Newer preparations also enable delivery of higher doses of iron over short time frames, typically 15–20 min (Table 4) and are increasingly being offered in an outpatient setting. True anaphylaxis and severe hypersensitivity reactions are rare with these modern intravenous iron preprations,88,89 but facilities and staff trained in the management of anaphylaxis should be present. Common side effects include nausea, headaches, dizziness, hypertension, flushing and injection/infusion site reactions. These are managed by slowing the infusion rate and providing symptomatic care, such as antiemetics. Transient hypophosphataemia has been reported in several studies, and this may be more pronounced in pregnant compared with non-pregnant women.90,91 Whether this hypophosphataemia has any clinically relevant consequences is uncertain. Permanent haemosiderin skin pigmentation has been reported from extravasation,92 and women should therefore be advised to report any pain at the infusion site. An increased risk of infection was a concern with older intravenous iron preparations, driven by the availability of unbound circulating iron for invading bacterial pathogens. Recent systematic reviews, which have included critically ill patients at high risk of sepsis, have demonstrated no increased risk of bacterial infection.93,94
Intravenous iron preparations are considerably more expensive than oral iron, even without including the costs of administration such as infusion kits and staff time. Although trials have reported costs of treatment, no formal cost-effectiveness analysis has been undertaken comparing intravenous iron with oral iron in pregnant women. Given the improved effectiveness and favourable side-effect profile of newer intravenous iron preparations discussed above, it remains possible that the overall economic cost might favour intravenous therapy in this setting.
During labour and postpartum
Women who enter labour with IDA are at increased risk of postpartum haemorrhage and have lower iron stores in reserve to support compensatory erythropoiesis following significant blood loss. Mode of delivery should be determined by obstetric indications but appropriate intravenous access, availability of a group and screen, birth in an obstetrician-led unit and active management of the third stage of labour should be considered.34
Appropriate management of IDA in the antenatal period reduces the likelihood of developing postpartum anaemia. Current guidelines recommend measuring Hb within 48 h of delivery in women with uncorrected anaemia in the antenatal period, blood loss of over 500 mL, or signs and symptoms suggestive of anaemia. Oral iron may be sufficient in women without active bleeding and asymptomatic or mildly symptomatic anaemia. The recommended daily dose is 40–80 mg of elemental iron for 3 months.34
Women requiring urgent correction of symptomatic anaemia, or those who are intolerant of oral iron, should be offered intravenous iron. A recent systematic review demonstrated a mean improvement of 9 g/L (95% CI: 4–13 g/L) at 6 weeks postpartum in women who received intravenous iron compared with oral iron.59 The reported rate of anaphylaxis in women receiving intravenous iron was 0.6%. Low-quality evidence suggests that intravenous iron may lower fatigue and depression scores at up to 12 weeks postpartum.95 There is a need for well-designed, adequately powered, randomised controlled trails.
Allogeneic red blood cell transfusion should be reserved for women with severe active bleeding, imminent cardiac compromise or symptoms of anaemia requiring urgent attention. Women should be fully consented regarding the potential risks of transfusion,96 including being unable to donate blood in the future, and potential alternative treatments.
Conclusion and directions for future research
ID remains the most common cause of maternal anaemia worldwide, with detrimental consequences for both mother and baby. Large-scale epidemiological studies have demonstrated that antenatal anaemia is a risk factor for maternal mortality, perinatal mortality, preterm labour, low birthweight infants and postpartum haemorrhage. Maternal ID may also be associated with poor infant neurodevelopmental outcomes. Postpartum anaemia is linked with lactation failure and low maternal quality of life scores.
The WHO aims to reduce the prevalence of anaemia in women of reproductive age by 50% between 2010 and 2025.6 Currently, interventions such as oral or intravenous iron do not appear to be working at the scale required to meet the WHO aims. This may be partly due to uncertainty regarding how best to investigate, prevent and treat maternal anaemia. Importantly, few trials so far have reported maternal and neonatal-centred outcomes; the vast majority have instead focused on haematological parameters. Despite this limitation being highlighted in a systematic review in 2012,97 little progress appears to have been made since. Advances in our understanding of iron physiology have led to the potential for considerable improvement by altering the dosing strategy for oral iron. Newer laboratory indices of iron status such as hepcidin may ultimately help to guide iron therapy, but a recent study found no benefit of using a hepcidin-guided screen-and-treat approach when compared with the WHO’s recommended regimen.98
Over a 5-year period, the Primary prevention of maternal ANaemia to avoid preterm Delivery and other Adverse outcomes (PANDA)99 research programme, recently funded by the National Institute for Health Research (NIHR), will attempt to address some of the research uncertainties highlighted in this review. Initial work will include identifying barriers and enablers to iron supplementation, development of a behavioural intervention to promote compliance and a feasibility randomised controlled trial to identify the optimal dosing schedule for oral iron. This will be followed by a larger, two-arm, multicentre trial of oral-iron supplementation versus placebo which will aim to enrol 11,020 pregnant women across 20 maternity units. The primary outcome is a composite of preterm birth, still birth, neonatal death and SGA infants. Long-term maternal and infant neurodevelopmental outcomes will also be evaluated. Future research is also needed to identify effective screening strategies during the antenatal and postnatal periods; determining the optimal dose of oral iron treatments; developing core outcome sets; and exploring the utility of universal iron supplementation to prevent maternal IDA.
Supplemental Material
Supplemental material, sj-pdf-1-obm-10.1177_1753495X20932426 for Iron deficiency anaemia in pregnancy: A contemporary review by Charlotte S Benson, Akshay Shah, Matthew C Frise and Charlotte J Frise in Obstetric Medicine
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: AS is currently being supported by an NIHR Doctoral Research Fellowship (DRF-2017–10-094).
Ethical approval: Not applicable.
Informed consent: Not applicable.
Guarantor: CSB is the guarantor of the present work.
Contributorship: CJF and MCF conceived the idea for the review. The literature searches, drafting and editing of all article versions were undertaken by CB and AS. All authors have contributed to, reviewed and approved the final version of the draft.
ORCID iDs
Charlotte S Benson https://orcid.org/0000-0002-3900-1049
Matthew C Frise https://orcid.org/0000-0001-5575-2531
References
- 1.Theo V, et al. Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet 2017; 390: 1211–1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stevens GA, Finucane MM, De-Regil LM, et al. Global, regional, and national trends in haemoglobin concentration and prevalence of total and severe anaemia in children and pregnant and non-pregnant women for 1995-2011: a systematic analysis of population-representative data. Lancet Glob Health 2013; 1: e16–e25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Daru J, Cooper NA, Khan KS.Systematic review of randomized trials of the effect of iron supplementation on iron stores and oxygen carrying capacity in pregnancy. Acta Obstet Gynecol Scand 2016; 95: 270–279. [DOI] [PubMed] [Google Scholar]
- 4.Nair M, Churchill D, Robinson S, et al. Association between maternal haemoglobin and stillbirth: a cohort study among a multi-ethnic population in England. Br J Haematol 2017; 179: 829–837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rahman MM, Abe SK, Rahman MS, et al. Maternal anemia and risk of adverse birth and health outcomes in low- and middle-income countries: systematic review and meta-analysis. Am J Clin Nutr 2016; 103: 495–504. [DOI] [PubMed] [Google Scholar]
- 6.Young MF.Maternal anaemia and risk of mortality: a call for action. Lancet Glob Health 2018; 6: e479–e480. [DOI] [PubMed] [Google Scholar]
- 7.Nair M, Choudhury MK, Choudhury SS, et al. Association between maternal anaemia and pregnancy outcomes: a cohort study in Assam, India. BMJ Glob Health 2016; 1: e000026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lucas DN, Bamber JH.UK confidential enquiry into maternal deaths - still learning to save mothers’ lives. Anaesthesia 2018; 73: 416–420. [DOI] [PubMed] [Google Scholar]
- 9.Nelson-Piercy C.The UK maternal death report. Obstet Med 2015; 8: 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Balarajan Y, Ramakrishnan U, Ozaltin E, et al. Anaemia in low-income and middle-income countries. Lancet 2011; 378: 2123–2135. [DOI] [PubMed] [Google Scholar]
- 11.Naik RP, Lanzkron S.Baby on board: what you need to know about pregnancy in the hemoglobinopathies. Hematol-Am Soc Hematol Educ Program 2012; 2012: 208–214. [DOI] [PubMed] [Google Scholar]
- 12.Devalia V, Hamilton MS, Molloy AM, British Committee for Standards in Hematology. Guidelines for the diagnosis and treatment of cobalamin and folate disorders. Br J Haematol 2014; 166: 496–513. [DOI] [PubMed] [Google Scholar]
- 13.Roy NBA, Pavord S.The management of anaemia and haematinic deficiencies in pregnancy and post-partum. Transfus Med 2018; 28: 107–116. [DOI] [PubMed] [Google Scholar]
- 14.Tolentino K, Friedman JF.An update on anemia in less developed countries. Am J Trop Med Hyg 2007; 77: 44–51. [PubMed] [Google Scholar]
- 15.Zhang C.Essential functions of iron-requiring proteins in DNA replication, repair and cell cycle control. Protein Cell 2014; 5: 750–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ganz T.Systemic iron homeostasis. Physiol Rev 2013; 93: 1721–1741. [DOI] [PubMed] [Google Scholar]
- 17.Frise MC, Cheng HY, Nickol AH, et al. Clinical iron deficiency disturbs normal human responses to hypoxia. J Clin Invest 2016; 126: 2139–2150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Frise MC, Robbins PA.Iron, oxygen, and the pulmonary circulation. J Appl Physiol 2015; 119: 1421–1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Drakesmith H, Prentice AM.Hepcidin and the iron-infection axis. Science 2012; 338: 768–772. [DOI] [PubMed] [Google Scholar]
- 20.Koenig MD, Tussing-Humphreys L, Day J, et al. Hepcidin and iron homeostasis during pregnancy. Nutrients 2014; 6: 3062–3083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bothwell TH.Iron requirements in pregnancy and strategies to meet them. Am J Clin Nutr 2000; 72: 257S–264S. [DOI] [PubMed] [Google Scholar]
- 22.Fisher AL, Nemeth E.Iron homeostasis during pregnancy. Am J Clin Nutr 2017; 106: 1567S–1574S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sangkhae V, Fisher AL, Wong S, et al. Effects of maternal iron status on placental and fetal iron homeostasis. J Clin Invest 2020; 130: 625–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bah A, Pasricha SR, Jallow MW, et al. Serum hepcidin concentrations decline during pregnancy and may identify iron deficiency: analysis of a longitudinal pregnancy cohort in the Gambia. J Nutr 2017; 147: 1131–1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.van Santen S, Kroot JJ, Zijderveld G, et al. The iron regulatory hormone hepcidin is decreased in pregnancy: a prospective longitudinal study. Clin Chem Lab Med 2013; 51: 1395–1401. [DOI] [PubMed] [Google Scholar]
- 26.Rehu M, Punnonen K, Ostland V, et al. Maternal serum hepcidin is low at term and independent of cord blood iron status. Eur J Haematol 2010; 85: 345–352. [DOI] [PubMed] [Google Scholar]
- 27.Churchill D, Nair M, Stanworth SJ, et al. The change in haemoglobin concentration between the first and third trimesters of pregnancy: a population study. BMC Pregnancy Childb 2019; 19: 359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Costantine MM.Physiologic and pharmacokinetic changes in pregnancy. Front Pharmacol 2014; 5: 65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.de Haas S, Ghossein-Doha C, van Kuijk SM, et al. Physiological adaptation of maternal plasma volume during pregnancy: a systematic review and meta-analysis. Ultrasound Obstet Gynecol 2017; 49: 177–187. [DOI] [PubMed] [Google Scholar]
- 30.Pasricha SR, Colman K, Centeno-Tablante E, et al. Revisiting WHO haemoglobin thresholds to define anaemia in clinical medicine and public health. Lancet Haematol 2018; 5: e60–e62. [DOI] [PubMed] [Google Scholar]
- 31.Daru J, Sobhy S, Pavord S.Revisiting the basis for haemoglobin screening in pregnancy. Curr Opin Obstet Gynecol 2019; 31: 388–392. [DOI] [PubMed] [Google Scholar]
- 32.Butcher A, Richards T, Stanworth SJ, et al. Diagnostic criteria for pre-operative anaemia-time to end sex discrimination. Anaesthesia 2017; 72: 811–814. [DOI] [PubMed] [Google Scholar]
- 33.Munoz M, Acheson AG, Auerbach M, et al. International consensus statement on the peri-operative management of anaemia and iron deficiency. Anaesthesia 2017; 72: 233–247. [DOI] [PubMed] [Google Scholar]
- 34.Pavord S, Daru J, Prasannan N, et al. UK guidelines on the management of iron deficiency in pregnancy. Br J Haematol 2020; 188: 819–830. [DOI] [PubMed] [Google Scholar]
- 35.Daru J, Colman K, Stanworth SJ, et al. Serum ferritin as an indicator of iron status: what do we need to know? Am J Clin Nutr 2017; 106: 1634S–1639S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.van den Broek NR, Letsky EA, White SA, et al. Iron status in pregnant women: which measurements are valid? Br J Haematol 1998; 103: 817–824. [DOI] [PubMed] [Google Scholar]
- 37.Hallberg L, Bengtsson C, Lapidus L, et al. Screening for iron deficiency: an analysis based on bone-marrow examinations and serum ferritin determinations in a population sample of women. Br J Haematol 1993; 85: 787–798. [DOI] [PubMed] [Google Scholar]
- 38.Choi JW, Im MW, Pai SH.Serum transferrin receptor concentrations during normal pregnancy. Clin Chem 2000; 46: 725–727. [PubMed] [Google Scholar]
- 39.Vora SM, Messina G, Pavord S.Utility of erythrocyte indices in identifying iron deficiency in pregnancy. Obstet Med. Epub ahead of print 8 November 2019. DOI: 10.1177/1753495X19878617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pasricha SR, Atkinson SH, Armitage AE, et al. Expression of the iron hormone hepcidin distinguishes different types of anemia in African children. Sci Transl Med 2014; 6: 235re3. [DOI] [PubMed] [Google Scholar]
- 41.Shah A, Wray K, James T, et al. Serum hepcidin potentially identifies iron deficiency in survivors of critical illness at the time of hospital discharge. Br J Haematol 2019; 184: 279–281. [DOI] [PubMed] [Google Scholar]
- 42.Pratt JJ, Khan KS.Non-anaemic iron deficiency - a disease looking for recognition of diagnosis: a systematic review. Eur J Haematol 2016; 96: 618–628. [DOI] [PubMed] [Google Scholar]
- 43.Auerbach M, Abernathy J, Juul S, et al. Prevalence of iron deficiency in first trimester, nonanemic pregnant women. J Matern Fetal Neonatal Med 2019; 1–4. [DOI] [PubMed] [Google Scholar]
- 44.Houston BL, Hurrie D, Graham J, et al. Efficacy of iron supplementation on fatigue and physical capacity in non-anaemic iron-deficient adults: a systematic review of randomised controlled trials. BMJ Open 2018; 8: e019240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Miles LF, Sandhu RN, Grobler AC, et al. Associations between non-anaemic iron deficiency and outcomes following surgery for colorectal cancer: an exploratory study of outcomes relevant to prospective observational studies. Anaesth Intensive Care 2019; 47: 152–159. [DOI] [PubMed] [Google Scholar]
- 46.Miles LF, Kunz SA, Na LH, et al. Postoperative outcomes following cardiac surgery in non-anaemic iron-replete and iron-deficient patients - an exploratory study. Anaesthesia 2018; 73: 450–458. [DOI] [PubMed] [Google Scholar]
- 47.Black RE, Victora CG, Walker SP, et al. Maternal and child undernutrition and overweight in low-income and middle-income countries. Lancet 2013; 382: 427–451. [DOI] [PubMed] [Google Scholar]
- 48.Say L, Chou D, Gemmill A, et al. Global causes of maternal death: a WHO systematic analysis. Lancet Glob Health 2014; 2: e323–e333. [DOI] [PubMed] [Google Scholar]
- 49.Daru J, Zamora J, Fernandez-Felix BM, et al. Risk of maternal mortality in women with severe anaemia during pregnancy and post partum: a multilevel analysis. Lancet Glob Health 2018; 6: e548–e554. [DOI] [PubMed] [Google Scholar]
- 50.Kavle JA, Stoltzfus RJ, Witter F, et al. Association between anaemia during pregnancy and blood loss at and after delivery among women with vaginal births in Pemba Island, Zanzibar, Tanzania. J Health Popul Nutr 2008; 26: 232–240. [PMC free article] [PubMed] [Google Scholar]
- 51.Briley A, Seed PT, Tydeman G, et al. Reporting errors, incidence and risk factors for postpartum haemorrhage and progression to severe PPH: a prospective observational study. BJOG 2014; 121: 876–888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Corwin EJ, Murray-Kolb LE, Beard JL.Low hemoglobin level is a risk factor for postpartum depression. J Nutr 2003; 133: 4139–4142. [DOI] [PubMed] [Google Scholar]
- 53.Maeda Y, Ogawa K, Morisaki N, et al. Association between perinatal anemia and postpartum depression: a prospective cohort study of Japanese women. Int J Gynaecol Obstet 2020; 148: 48–52. [DOI] [PubMed] [Google Scholar]
- 54.Wassef A, Nguyen QD, St-Andre M.Anaemia and depletion of iron stores as risk factors for postpartum depression: a literature review. J Psychosom Obstet Gynaecol 2019; 40: 19–28. [DOI] [PubMed] [Google Scholar]
- 55.Lee KA, Zaffke ME.Longitudinal changes in fatigue and energy during pregnancy and the postpartum period. J Obstet Gynecol Neonatal Nurs 1999; 28: 183–191. [DOI] [PubMed] [Google Scholar]
- 56.Beard JL, Hendricks MK, Perez EM, et al. Maternal iron deficiency anemia affects postpartum emotions and cognition. J Nutr 2005; 135: 267–272. [DOI] [PubMed] [Google Scholar]
- 57.Henly SJ, Anderson CM, Avery MD, et al. Anemia and insufficient milk in first-time mothers. Birth 1995; 22: 86–92. [DOI] [PubMed] [Google Scholar]
- 58.Rioux FM, Savoie N, Allard J.Is there a link between postpartum anemia and discontinuation of breastfeeding? Can J Diet Pract Res 2006; 67: 72–76. [DOI] [PubMed] [Google Scholar]
- 59.Sultan P, Bampoe S, Shah R, et al. Oral vs intravenous iron therapy for postpartum anemia: a systematic review and meta-analysis. Am J Obstet Gynecol 2019; 221: 19–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Haider BA, Olofin I, Wang M, et al. Anaemia, prenatal iron use, and risk of adverse pregnancy outcomes: systematic review and meta-analysis. BMJ 2013; 346: f3443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Alwan NA, Cade JE, McArdle HJ, et al. Maternal iron status in early pregnancy and birth outcomes: insights from the Baby’s Vascular health and Iron in Pregnancy study. Br J Nutr 2015; 113: 1985–1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Radlowski EC, Johnson RW.Perinatal iron deficiency and neurocognitive development. Front Hum Neurosci 2013; 7: 585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhang Y, Shi J, Wei H, et al. Neonate and infant brain development from birth to 2 years assessed using MRI-based quantitative susceptibility mapping. Neuroimage 2019; 185: 349–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Knickmeyer RC, Gouttard S, Kang C, et al. A structural MRI study of human brain development from birth to 2 years. J Neurosci 2008; 28: 12176–12182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Doom JR, Georgieff MK.Striking while the iron is hot: understanding the biological and neurodevelopmental effects of iron deficiency to optimize intervention in early childhood. Curr Pediatr Rep 2014; 2: 291–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Carlson ES, Stead JD, Neal CR, et al. Perinatal iron deficiency results in altered developmental expression of genes mediating energy metabolism and neuronal morphogenesis in hippocampus. Hippocampus 2007; 17: 679–691. [DOI] [PubMed] [Google Scholar]
- 67.Amin SB, Orlando M, Eddins A, et al. In utero iron status and auditory neural maturation in premature infants as evaluated by auditory brainstem response. J Pediatr 2010; 156: 377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tamura T, Goldenberg RL, Hou J, et al. Cord serum ferritin concentrations and mental and psychomotor development of children at five years of age. J Pediatr 2002; 140: 165–170. [DOI] [PubMed] [Google Scholar]
- 69.Christian P, Murray-Kolb LE, Khatry SK, et al. Prenatal micronutrient supplementation and intellectual and motor function in early school-aged children in Nepal. JAMA 2010; 304: 2716–2723. [DOI] [PubMed] [Google Scholar]
- 70.Pasricha SR, Drakesmith H, Black J, et al. Control of iron deficiency anemia in low- and middle-income countries. Blood 2013; 121: 2607–2617. [DOI] [PubMed] [Google Scholar]
- 71.Munoz M, Pena-Rosas JP, Robinson S, et al. Patient blood management in obstetrics: management of anaemia and haematinic deficiencies in pregnancy and in the post-partum period: NATA consensus statement. Transfus Med 2018; 28: 22–39. [DOI] [PubMed] [Google Scholar]
- 72.Breymann C, Bian XM, Blanco-Capito LR, et al. Expert recommendations for the diagnosis and treatment of iron-deficiency anemia during pregnancy and the postpartum period in the Asia-Pacific region. J Perinat Med 2011; 39: 113–121. [DOI] [PubMed] [Google Scholar]
- 73.American College of Obstetricians and Gynecologists. ACOG Practice Bulletin No. 95: anemia in pregnancy. Obstet Gynecol 2008; 112: 201–207. [DOI] [PubMed] [Google Scholar]
- 74.Pavord S, Myers B, Robinson S, et al. UK guidelines on the management of iron deficiency in pregnancy. Br J Haematol 2012; 156: 588–600. [DOI] [PubMed] [Google Scholar]
- 75.Pena-Rosas JP, De-Regil LM, Dowswell T, et al. Intermittent oral iron supplementation during pregnancy. Cochrane Database Syst Rev 2012; 7: CD009997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tuncalp O, Pena-Rosas JP, Lawrie T, et al. WHO recommendations on antenatal care for a positive pregnancy experience – going beyond survival. BJOG 2017; 124: 860–862. [DOI] [PubMed] [Google Scholar]
- 77.Moretti D, Goede JS, Zeder C, et al. Oral iron supplements increase hepcidin and decrease iron absorption from daily or twice-daily doses in iron-depleted young women. Blood 2015; 126: 1981–1989. [DOI] [PubMed] [Google Scholar]
- 78.Stoffel NU, Cercamondi CI, Brittenham G, et al. Iron absorption from oral iron supplements given on consecutive versus alternate days and as single morning doses versus twice-daily split dosing in iron-depleted women: two open-label, randomised controlled trials. Lancet Haematol 2017; 4: e524–e533. [DOI] [PubMed] [Google Scholar]
- 79.Tolkien Z, Stecher S, Mander AP, et al. Ferrous sulfate supplementation causes significant gastrointestinal side-effects in adults: a systematic review and meta-analysis. PLoS ONE 2015; 10: e0117383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Schaap CC, Hendriks JC, Kortman GA, et al. Diurnal rhythm rather than dietary iron mediates daily hepcidin variations. Clin Chem 2013; 59: 527–535. [DOI] [PubMed] [Google Scholar]
- 81.Tapiero H, Gate L, Tew KD.Iron: deficiencies and requirements. Biomed Pharmacother 2001; 55: 324–332. [DOI] [PubMed] [Google Scholar]
- 82.Clevenger B, Gurusamy K, Klein AA, et al. Systematic review and meta-analysis of iron therapy in anaemic adults without chronic kidney disease: updated and abridged Cochrane review. Eur J Heart Fail 2016; 18: 774–785. [DOI] [PubMed] [Google Scholar]
- 83.Litton E, Xiao J, Ho KM.Safety and efficacy of intravenous iron therapy in reducing requirement for allogeneic blood transfusion: systematic review and meta-analysis of randomised clinical trials. BMJ 2013; 347: f4822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lewkowitz AK, Gupta A, Simon L, et al. Intravenous compared with oral iron for the treatment of iron-deficiency anemia in pregnancy: a systematic review and meta-analysis. J Perinatol 2019; 39: 519–532. [DOI] [PubMed] [Google Scholar]
- 85.Qassim A, Grivell RM, Henry A, et al. Intravenous or oral iron for treating iron deficiency anaemia during pregnancy: systematic review and meta-analysis. Med J Aust 2019; 211: 367–373. [DOI] [PubMed] [Google Scholar]
- 86.Kalra PA, Bhandari S.Efficacy and safety of iron isomaltoside (Monofer) in the management of patients with iron deficiency anemia. Int J Nephrol Renovasc Dis 2016; 9: 53–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Keating GM.Ferric carboxymaltose: a review of its use in iron deficiency. Drugs 2015; 75: 101–127. [DOI] [PubMed] [Google Scholar]
- 88.Ehlken B, Nathell L, Gohlke A, et al. Evaluation of the reported rates of severe hypersensitivity reactions associated with ferric carboxymaltose and iron (III) isomaltoside 1000 in Europe based on data from Eudravigilance and Vigibase between 2014 and 2017. Drug Saf 2019; 42: 463–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Myers B, Myers O, Moore J.Comparative efficacy and safety of intravenous ferric carboxymaltose (Ferinject) and iron(III) hydroxide dextran (Cosmofer) in pregnancy. Obstet Med 2012; 5: 105–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zoller H, Schaefer B, Glodny B.Iron-induced hypophosphatemia: an emerging complication. Curr Opin Nephrol Hypertens 2017; 26: 266–275. [DOI] [PubMed] [Google Scholar]
- 91.Huang LL, Lee D, Troster SM, et al. A controlled study of the effects of ferric carboxymaltose on bone and haematinic biomarkers in chronic kidney disease and pregnancy. Nephrol Dial Transplant 2018; 33: 1628–1635. [DOI] [PubMed] [Google Scholar]
- 92.El-Zaatari MS, Hassan-Smith ZK, Reddy-Kolanu V.Extravasation and pigmentation post iron infusion. Br J Hosp Med (Lond). Epub ahead of print 2 April 2019. DOI: 10.12968/hmed.2019.80.4.ii. [DOI] [PubMed] [Google Scholar]
- 93.Avni T, Bieber A, Grossman A, et al. The safety of intravenous iron preparations: systematic review and meta-analysis. Mayo Clin Proc 2015; 90: 12–23. [DOI] [PubMed] [Google Scholar]
- 94.Shah A, Fisher SA, Wong H, et al. Safety and efficacy of iron therapy on reducing red blood cell transfusion requirements and treating anaemia in critically ill adults: a systematic review with meta-analysis and trial sequential analysis. J Crit Care 2019; 49: 162–171. [DOI] [PubMed] [Google Scholar]
- 95.Holm C, Thomsen LL, Langhoff-RoosJ. Intravenous iron isomaltoside treatment of women suffering from severe fatigue after postpartum hemorrhage. J Matern Fetal Neonatal Med 2018; 32: 2797–2804. [DOI] [PubMed] [Google Scholar]
- 96.Delaney M, Wendel S, Bercovitz RS, et al. Transfusion reactions: prevention, diagnosis, and treatment. Lancet 2016; 388: 2825–2836. [DOI] [PubMed] [Google Scholar]
- 97.Parker JA, Barroso F, Stanworth SJ, et al. Gaps in the evidence for prevention and treatment of maternal anaemia: a review of systematic reviews. BMC Pregnancy Childb 2012; 12: 56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Bah A, Muhammad AK, Wegmuller R, et al. Hepcidin-guided screen-and-treat interventions against iron-deficiency anaemia in pregnancy: a randomised controlled trial in The Gambia. Lancet Glob Health 2019; 7: e1564–e1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.National Institute for Health Research. Funding and awards, https://www.fundingawards.nihr.ac.uk/award/NIHR200869 (2019, accessed 3 December 2019).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-obm-10.1177_1753495X20932426 for Iron deficiency anaemia in pregnancy: A contemporary review by Charlotte S Benson, Akshay Shah, Matthew C Frise and Charlotte J Frise in Obstetric Medicine