Abstract
Many studies have reported that dietary fibers play a crucial role in promoting intestinal health of the host, since it strengthens functions of epithelial barrier and meanwhile maintains intestinal homeostasis of the host by modulating gut microbiota and short‐chain fatty acid (SCFA) production. Pig is a good animal model to study effects of dietary fiber on gut health and microbial community. This review has summarized the relevant knowledge available based on roles of various dietary fibers in gut health and energy metabolism of pigs and humans. Evidences summarized in our review indicated that modulating intestinal microbial composition and SCFA production by consuming specific dietary fibers properly could be conducive to health improvement and disease prevention of the host. However, types of dietary fiber from edible foods exert divergent impacts on gut health, energy metabolism, microbial composition, and SCFA production. Therefore, more attention should be focused on different responses of various dietary fibers intake on host metabolism and health.
Keywords: dietary fiber, gut health, microbiota, pig, short‐chain fatty acids
Modulating intestinal microbial composition and short‐chain fatty acid (SCFA) production by regulating specific dietary fibers intake properly could be conducive to host health improvement and disease prevention. Types of dietary fiber from edible foods exert divergent impacts on gut health, microbial composition, and SCFA production.
1. INTRODUCTION
Prebiotics are food components that can be selectively fermented, leading to changes in composition and activity of gut microbiota, then contributing to improvement of host health (Gibson et al., 2017). Dietary fibers derived from foods, which include cellulose, hemicelluloses, pectin, gums, mucilage, undigested oligosaccharide, and resistant starch, are usually considered to behave as prebiotics in human health and nutrition (Mudgil & Barak, 2013). Dietary fibers are polysaccharides which linked with more than 10 glycosidic bonds, and they are partially or completely fermented by gut microbiota in the hindgut of pigs and humans to synthesize short‐chain fatty acids (SCFA). Dietary fibers could decrease transit time of digesta, increase stool bulk, and reduce blood cholesterol and glucose (Jarrar et al., 2019; Kerckhoffs et al., 2003; Mudgil & Barak, 2013). Apart from those directly physiological responses originated from physiochemical properties of dietary fibers, it could also improve growth and activity of the intestinal microbiota, which underlie some prebiotic effects on host health and disorders prevention (Gensollen et al., 2016). Intestinal microbiota community shaped via microbial fermentation of dietary fiber is beneficial to host health through regulating physiological processes of the intestine and functions of mucosal immunity. More specifically, gut microbiota intensify integrity of the gut barrier comprised by intestinal epithelial cells, suppress colonization of enteric pathogens, and produce antibacterial peptides in the mucus layer of host intestine (Bäumler & Sperandio, 2016; Natividad & Verdu, 2013).
The SCFA produced by microbial fermentation of dietary fibers mainly include acetate, propionate, and butyrate, which play an important role in regulating energy metabolism, immunological function, and gut cell proliferation of the host (Koh et al., 2016). Butyrate is a source of energy for colonocytes to maintain the gut barrier, whereas acetate and propionate are delivered to peripheral circulation through the portal vein to participate in metabolisms of the liver and peripheral tissues (Liu, Wang, et al., 2018; Liu, Zhao, et al., 2018). In addition, several studies have demonstrated that SCFA has diverse metabolic and regulatory activities, such as modulating immune functions, providing energy for cell turnover, and being a histone deacetylase (HDAC) inhibitor (Flint et al., 2012; Thangaraju et al., 2009). Furthermore, there is a broad consensus that SCFA act as physiological signaling molecules to adjust biological processes associated with host health and nutrition. Many researchers reported that SCFA mediates glucose homeostasis by activating G protein‐coupled receptors (GPR 41 and 43) and stimulating enteroendocrine L‐cells to produce glucagon‐like peptide 1 (GLP‐1) and peptide YY (PYY), resulting in an increase insulin sensitivity (Mudgil & Barak, 2013; Tolhurst et al., 2012). The SCFA promotes secretion of inflammatory cytokines, such as interleukin‐6 (IL‐6), tumor necrosis factor‐α (TNF‐α), interleukin‐10 (IL‐10), and chemokine monocytes chemotactic protein‐1 (MCP‐1) to enhance intestinal immune barrier function by inhibiting activity of HDAC and stimulating expression of G protein‐coupled receptors (Montagne et al., 2003; Smith et al., 2013). Nicolucci et al. (2017) reported that obese patients who consumed inulin reduced plasma triglyceride IL‐6 concentrations. Therefore, SCFA plays a crucial role to regulate responses of dietary fiber fermentation by gut microbiota on host metabolisms and health.
As reported by Cappai et al. (2013), a higher starch digestibility from cereals is positively related to a lower amylose to amylopectin ratio in the starch composition under a same starch concentration condition, as starch with high amylose content is less digestible. Therefore, structure and composition of dietary fibers may play an important role in fiber fermentability by gut microbiota. Evidences showed effects of different types of dietary fibers derived from edible foods on gut health and energy metabolism of the host were associated with their physical characteristics and fiber composition (Zhao et al., 2019). Microbial metabolites produced from microbial fermentation of fiber are also varying when pigs and humans consume different types of dietary fiber. A higher proportion of valeric acid accounted for total SCFA was observed when hulled shredded acorns are fed to pigs (Cappai et al., 2020). Illustrating these effects on development of gastrointestinal tract in humans is challenging because of difficulty in sample collection. Alternatively, pig is a good model to study effects of dietary fibers on gut health and microbial composition in humans, considering high similarity of the intestinal biology and gut microbiota community between pigs and humans (Lee et al., 2011). Our hypothesis is that roles of different types of dietary fiber in regulating gut health and host metabolism vary. Therefore, this review summarizes effects of different dietary fibers with varying physicochemical properties derived from commercial diets on energy metabolism, gut morphology, gut barrier function, intestinal microbiota, and SCFA production in both pigs and humans, and practice of dietary intervention using dietary fibers to maintain host health and metabolism. Considering various proportions of dietary fibers derived from different fiber‐rich foods and their different physicochemical properties, it is crucial to ingest a variety of fiber‐rich foods to benefit animal and human health.
2. DEFINITION AND CLASSIFICATION OF DIETARY FIBERS
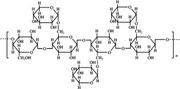
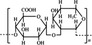
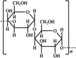
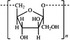
A widely accepted definition is that dietary fiber is one of the carbohydrates that are indigestible by endogenous enzymes in pigs and humans and meanwhile exert vital impacts on maintaining normal physiological function and energy metabolism of the host (Cummings & Stephen, 2007). Dietary fiber is mainly divided into oligosaccharides and polysaccharides. Oligosaccharides are nondigestible carbohydrates composed of 3–9 monosaccharides which are connected with either α 1–4 or α 1–6 glycosidic bonds, and mainly include fructo‐oligosaccharides, galacto‐oligosaccharides and isomalto‐oligosaccharides, human milk oligosaccharides and xylo‐oligosaccharides (Borderías et al., 2005). Edible foods provide many oligosaccharides to pigs and humans, which usually have sweet taste and exhibit prebiotic effects on gut microbiota and host health (Cheng et al., 2017). Polysaccharides are complex carbohydrates, composed of 10 up to several thousand monosaccharides, which are primarily composed of resistant starch, cellulose, hemicellulose, and β‐glucan. Cellulose is the most abundant polysaccharides consisting of up to 10,000 glucose monomer units per molecule, and it is the major component of the plant cell wall, which is only partially fermented in the intestine of pigs and humans. Hemicellulose is also a component of the plant cell wall, but it has both linear and branched molecules containing 50~200 pentose units and hexose units, such as β‐glucan, glucomannan, arabinoxylan, and xylan (Adebowale et al., 2019). β‐glucan is a branched polysaccharide with glucose polymers which lead to its high solubility and fermentability in the intestine (Bashir & Choi, 2017). Resistant starch, which is divided into four types based on its structure and fermentability, is a kind of starch that can pass through small intestine of humans without being fully digested, and reach large intestine to be degraded by beneficial gut bacteria (Sajilata et al., 2006). Pectin, characterized by high viscosity and fermentability, is composed of three different polysaccharides: homogalacturonans, rhamnogalacturonans, and galactomannans (Rejaii & Salehi, 2016). Different kinds of dietary fibers derived from edible foods are showed in Table 1. Generally, polysaccharides in legume seeds primarily consist of raffinose, stachyose, and verbascose, and cereal‐derived polysaccharides are made up of xylo‐oligosaccharide and β‐glucan. Fructo‐oligosaccharide is a main fiber component in fruits, and resistant starch is primary nondigestible carbohydrates in root vegetables. Chemical compositions and physical properties of the common cereal and cereal by‐products are presented in Tables 2 and 3, respectively. Overall, there is large variation in composition of chemical constituents and physical properties among cereal and cereal by‐products.
TABLE 1.
Main compounds and sources of dietary fibers in foods
| Categories | Degree of polymerization | Type | Structure | Source |
|---|---|---|---|---|
| Oligosaccharides | 3–9 | |||
| Malto‐Oligosaccharides | α‐glucans |
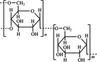
|
Starch hydrolysis process | |
| Non‐α‐glucan oligosaccharides | Raffinose |
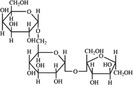
|
Soybean meal, peas, rapeseed meal, sunflower meal, cottonseed meal | |
| Stachyose |
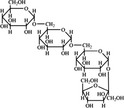
|
|||
| Verbascose |
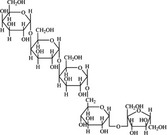
|
|||
| Fructo‐oligosaccharides |
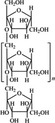
|
Banana, onion, barley | ||
| Galacto‐oligosaccharides |
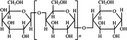
|
Milk | ||
| Xylo‐oligosaccharides |
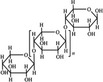
|
Cereals Bardana, onion | ||
| Polysaccharides | ≥10 | |||
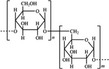
| Resistant starch | RS1, RS2, RS3, RS4 |
|
Peas, fava beans, raw potato | |
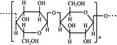
| Cell wall | Cellulose |
|
Most foods | |
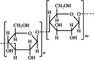
| Mixed linked β‐glucan |
|
Barley, oats, rye | ||
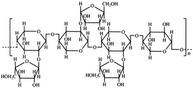
| Arabinoxylan |
|
Rye, wheat, barley, cereal by‐products | ||
| Xyloglucans |
|
Pea hulls | ||
| Rhamnogalacturans |
|
Soybean meal, sugar beet pulp | ||
| Noncell wall | Galactans |
|
Lupins | |
| Fructans |
|
Chicory roots, rye | ||
| Mannan |
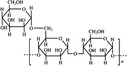
|
Coconut cake, palm cake | ||
| Pectin |
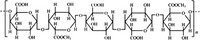
|
Fruits | ||
| Guar gums |
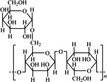
|
Guar |
TABLE 2.
Compositions of dietary fibers in common cereals (on dry matter basis)
| Item, g/kg | Maize | Wheat | Rye | Barley | Oat |
|---|---|---|---|---|---|
| Soluble NSP | 9 | 25 | 42 | 56 | 40 |
| Rhamnose | 0 | 0 | 0 | 0 | 0 |
| Arabinose | 3 | 7 | 12 | 6 | 3 |
| Xylose | 2 | 9 | 20 | 6 | 2 |
| Mannose | 2 | 2 | 2 | 2 | 2 |
| Galactose | 1 | 2 | 1 | 1 | 2 |
| Glucose | 1 | 4 | 6 | 39 | 28 |
| Uronic acids | 1 | 1 | 1 | 2 | 3 |
| Insoluble NSP | 66 | 74 | 94 | 88 | 110 |
| Rhamnose | 0 | 0 | 0 | 0 | 0 |
| Arabinose | 19 | 22 | 24 | 22 | 15 |
| Xylose | 28 | 38 | 41 | 50 | 78 |
| Mannose | 1 | 1 | 3 | 2 | 1 |
| Galactose | 4 | 2 | 4 | 2 | 5 |
| Glucose | 9 | 7 | 20 | 8 | 5 |
| Uronic acids | 6 | 4 | 3 | 4 | 7 |
| Cellulose | 22 | 20 | 16 | 43 | 82 |
Abbreviation: NSP, nonstarch polysaccharides.
TABLE 3.
The compositions of dietary fibers in common cereal by‐products (on dry matter basis)
| Item, g/kg | Maize | Wheat | Rye | Barley | Oat | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Flour | Bran | Gluten | Flour | Bran | Middlings | Bran | Middlings | Dehulled | Hull meal | Feed meal | Hull meal | |
| Soluble NSP | 8 | 32 | 6 | 16 | 29 | 71 | 63 | 62 | 50 | 20 | 42 | 13 |
| Rhamnose | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Arabinose | 3 | 6 | 1 | 3 | 7 | 21 | 11 | 17 | 4 | 3 | 2 | 2 |
| Xylose | 3 | 5 | 1 | 7 | 10 | 31 | 33 | 30 | 7 | 0 | 1 | 0 |
| Mannose | 1 | 1 | 3 | 0 | 1 | 2 | 1 | 1 | 1 | 0 | 2 | 1 |
| Galactose | 1 | 2 | 0 | 2 | 2 | 3 | 2 | 2 | 2 | 1 | 2 | 0 |
| Glucose | 0 | 6 | 2 | 2 | 8 | 11 | 13 | 10 | 34 | 13 | 33 | 8 |
| Uronic acids | 1 | 12 | 0 | 2 | 2 | 3 | 2 | 3 | 1 | 3 | 2 | 1 |
| Insoluble NSP | 13 | 240 | 14 | 17 | 273 | 101 | 321 | 199 | 58 | 267 | 39 | 295 |
| Rhamnose | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Arabinose | 3 | 66 | 3 | 6 | 83 | 27 | 67 | 53 | 17 | 48 | 8 | 26 |
| Xylose | 3 | 111 | 3 | 8 | 138 | 36 | 180 | 89 | 29 | 184 | 15 | 212 |
| Mannose | 0 | 3 | 0 | 1 | 4 | 6 | 2 | 6 | 2 | 3 | 1 | 1 |
| Galactose | 0 | 18 | 0 | 0 | 7 | 4 | 10 | 7 | 0 | 5 | 1 | 9 |
| Glucose | 5 | 10 | 6 | 4 | 27 | 21 | 53 | 40 | 8 | 12 | 10 | 12 |
| Uronic acids | 2 | 32 | 2 | 0 | 13 | 7 | 8 | 5 | 2 | 15 | 3 | 35 |
| Cellulose | 0 | 83 | 5 | 3 | 72 | 19 | 39 | 27 | 19 | 192 | 8 | 196 |
Abbreviation: NSP, nonstarch polysaccharides.
3. PHYSICAL CHARACTERISTICS OF DIETARY FIBERS
Major physical characteristics of dietary fibers include the following aspects: solubility, water‐holding capacity, viscosity, swelling capacity, and bulk density. Based on solubility, dietary fibers in edible foods are divided into two categories: soluble and insoluble chemical components (Ferrario et al., 2017). Water‐holding capacity is ability of dietary fibers to combine with water for forming colloidal suspensions, and this ability depends on types of glycosidic bonds and compositions of polysaccharides (Lan et al., 2012). Viscosity is an important physical characteristic that affects physiological function of dietary fibers. Viscosity of pectin and glucan is greater than that of the cellulose and lignin in edible foods for pigs and humans (Dikeman & Fahey, 2006). Moreover, dietary fibers with long chains are easier to form net structures than short‐chain fractions, leading to greater viscosity of long‐chain dietary fibers. Swelling occurs when structure of dietary fibers solubilizes and is dispersed by incoming water, and therefore, swelling degree is dependent on water‐binding capacity of dietary fiber (Knudsen et al., 2013). Expansion and dispersion of dietary fibers allow more rapid access by microbial enzymes, resulting in increased fiber fermentability and SCFA production. Bulk density is defined as the degree of consistency measured by the quantity of mass per unit volume occupied by the fibrous materials (Elleuch et al., 2011). A lower bulk density would lead to more fullness in the gastrointestinal tract, resulting in a reduced appetite and feed intake. In the future, there will be less variation on physical characteristics of different dietary fibers, but the relationship between their physical characteristics and host health and metabolism have been barely studied.
4. DIETARY FIBERS AND GUT HEALTH AND ENERGY METABOLISMS OF THE HOST
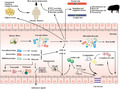
Increasingly, researchers have proven that dietary fibers improve gut health of the host (Figure 1 and Table 4). One reason for improvement in gut health is direct responses of dietary fibers on gut development and intestinal motility, resulting in improving gut morphology and capacity of nutrient absorption. Additionally, dietary fibers shape gut microbial compositions to regulate gut health of the host. Dietary fibers are fermented by gut bacteria to produce SCFA, such as acetate, propionate, and butyrate, which enhance gut health and immune function in pigs and humans. However, different results have been reported focusing on effects of different types of dietary fibers on SCFA production, gut microbiota composition, as well as gut health and disease of the host.
FIGURE 1.
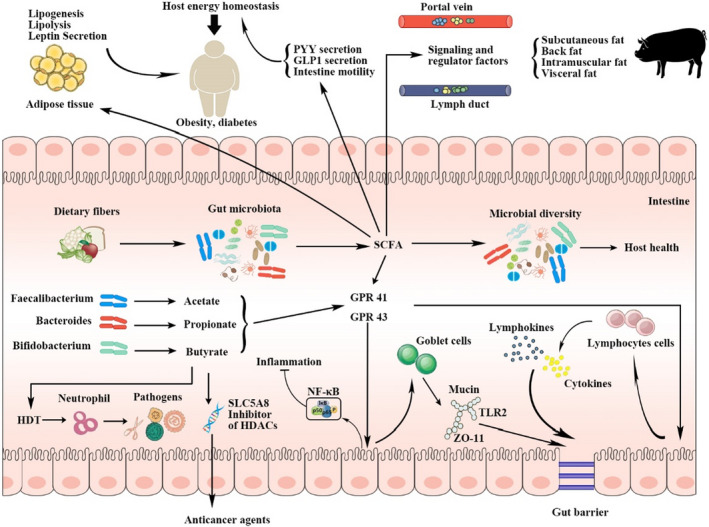
Trophic mechanisms of dietary fibers fermented by gut microbiota on energy metabolism and gut health in pigs and humans. GLP‐1, glucagon‐like peptide‐1; GPR, G protein‐coupled receptors; HDAC, histone deacetylase; HDP, human defense peptides; NF‐κB, nuclear factor‐κB; PYY, peptide YY; SCFA, short‐chain fatty acids; TLR2, Toll‐like receptor
TABLE 4.
Summary of dietary interventions with dietary fibers to regulate gut health and alleviate metabolic syndrome in pig model and humans
| Subject of study | Types of dietary fibers | Prebiotic response | References |
|---|---|---|---|
| Pig | Wheat, barley | Colon weight | Serena et al. (2008) |
| Pig | Sunflower meal, sugar beet pulp, wheat fiber | Intestine weight, amylase activity | Bikker et al. (2006) |
| Pig | Pearl barley | Colon weight | Hopwood et al. (2004) |
| Pig | Sugar beet pulp | Gut morphology | Schiavon et al. (2004) |
| Pig | Corn bran, wheat bran | Enzyme activity | Chen et al. (2014) |
| Pig | Wheat bran, pea fiber | Tight junction protein and toll‐like receptor expression | Chen et al. (2013) |
| Pig | Pea fiber | Colonic mucin level | Che et al. (2014) |
| Pig | Arabinoxylan | Goblet cell number and sIgA secretion | Chen et al. (2015) |
| Pig | Chicory fiber | HSP27 expression | Liu et al. (2012) |
| Pig | Resistant starch | Mucin secretion | Zhou et al. (2016) |
| Pig | Corn bran, wheat bran | Mucin‐2 expression | Vila (2017) |
| Hypercholesterolemic patient | β‐glucan from oat bran | Serum cholesterol | Kerckhoffs et al. (2003) |
| Healthy individual | Cereal fiber | Serum cholesterol | Jarrar et al. (2019) |
| Healthy individual | Resistant starch | Inulin sensitivity | Giles et al. (2019) |
| Younger and middle‐aged patient | Cereal fiber with low glucose index | Alleviate type Ⅱ diabetes | Schulze et al. (2004) |
| Diabetic patient | Whole grain | GLP‐1 expression, hemoglobin A1c levels | Zhao et al. (2018) |
| Diabetic patient | Whole grain | Plasma glucose, insulin, and ghrelin responses | Silva et al. (2015) |
| Healthy individual | Whole grain | Glycemic control | Venn and Mann (2004) |
| Healthy individual | Barley Kernel‐based bread | Gut hormones and insulin sensitivity index | Nilsson et al. (2015) |
| Nonobese healthy individual | Oligofructose | Hormones to regulate appetite | Pedersen et al. (2013) |
| Nonobese healthy individual | Fruits and vegetables | Limit long‐term weight gain | Mozaffarian et al. (2011) |
| Obese patient | Inulin | Serum triglyceride and IL‐6 | Nicolucci et al. (2017) |
Abbreviations: CRC, colorectal cancer; GLP‐1, glucagon‐like peptide‐1; HSP, heat shock protein; IL‐6, interleukin‐6.
4.1. Dietary fibers and intestinal development
Intake of dietary fibers reduces energy density in edible foods. To compensate, small intestines of pigs and humans need to expand area intestinal villi for improved nutrient absorption, which stimulates intestinal development to meet the nutrient requirements of the host, leading to the increased gastrointestinal tract weight (Serena et al., 2008). Physiological basis for intestinal digestion and nutrient absorption is morphology of the intestinal mucosa, including villus height and the crypt depth. Mucosa morphology reflects intestinal capacity of nutrient absorptions. A decreased ratio of villus height to crypt depth usually relates to impaired digestion and absorption of nutrients by intestinal mucosa. On the contrary, increased ratio of villus height to crypt depth usually indicates improved intestinal mucosal function, and enhanced digestion and absorption of nutrients (Furuse, 2010). Serena et al. (2008) used gestating sows to demonstrate that diets based on wheat and barley with high concentrations of dietary fibers increased colon weight of gestation pig. Bikker et al. (2006) observed that neonatal piglets fed high concentration of soluble dietary fibers sourced from wheat middlings, sunflower meal, and sugar beet pulp tended to increase length of small intestine and improve amylase activity in the small intestinal brush border. Hopwood et al. (2004) also found that pearl barley, rich in glucan and resistant starch, increased weight of colon and caecum in neonatal piglets. Similarly, dietary fibers intake increased villus height to crypt depth ratio and improved absorptive ability of the small intestine in pigs (Jha et al., 2019). Schiavon et al. (2004) showed that an improvement in viscosity of intestinal digesta accelerated cell exfoliation in the apical part of the intestinal villus by fiber intake, resulting in reduced villus height and deep crypt depth. However, intake of dietary fiber derived from corn bran and wheat bran did not affect villus height and crypt depth of the jejunum and ileum in pigs, but enhanced activities of sucrase and maltase (Chen et al., 2014). One important reason that dietary fibers can benefit development of the gastrointestinal tract is that they directly disrupt surface structure of the mucosal layer and increase speed of cell shedding, which causes compensatory growth of mucosal cells. In addition, SCFA produced by gut microbiota through microbial fermentation of dietary fibers in the large intestine of the host reduces pH of the gut, and stimulates cell division and cell proliferation. Specifically, butyrate provides energy for proliferation of the epithelial cells in the host intestine, modifies gene expression for epidermal growth factor, and repairs damaged epithelial cells, which all promote intestinal growth and development (Koh et al., 2016; Liu, Wang, et al., 2018; Liu, Zhao, et al., 2018). Furthermore, SCFA simulates secretion of gastrin and glucagon‐like peptides, which boost proliferation of the epithelial cells in the host intestine (Tolhurst et al., 2012). Overall, dietary fiber accelerates motility and development of gastrointestinal tract through its physical function and prebiotic responses of SCFA produced by microbial fermentation of dietary fiber.
4.2. Dietary fibers and intestinal mucosal barrier
Mucosal barrier of intestine is composed of the epithelial cell barrier and the mucosal barrier attached to the epithelial cells. Mucosal barrier mainly consists of mucins, intestinal trefoil peptide, antimicrobial peptide, cytokines, and secretory immunoglobulin A (Bai et al., 2010). Zhou et al. (2016) showed that resistant starch intake significantly increased secretion of mucin by colonic goblet cells of pigs. Vila (2017) observed that intake of cereal foods, corn bran, and wheat bran significantly improved mucin‐2 level in the ileum and colon of pigs. Che et al. (2014) demonstrated that colonic mucin level in pigs fed pea fiber diet was 16% higher than that fed a control diet. Chen et al. (2013) reported that diets supplied with 10% wheat bran or pea fiber improved gut barrier functions of the neonatal piglets, which was caused by enhanced expression of the tight junction protein between the ileum and colonic epithelial cells (ZO‐11) and the Toll‐like receptor (TLR2) mRNA (Chen et al., 2013). However, intake of corn bran and soy fiber did not affect intestinal barrier functions compared with a control group. Addition of arabinoxylan in neonatal piglet's diets significantly increased an amount of IgA secreted in the intestine and a number of goblet cells, and reduced intestinal permeability (Chen et al., 2015). Under the condition of high temperature, pigs and humans can synthesize heat shock protein (HSP) to alleviate heat stress and support stability of the mucosal barrier in the host intestine. A recent study illustrated that chicory fiber intake significantly increased expression of HSP27 in the ileum and colon of pigs, and expression of HSP27 in the ileum was positively correlated with the soluble uronic acid intake (Liu et al., 2012). Furthermore, many researchers have reported that SCFA production is beneficial to secretion of mucosal proteins in pig intestine, but concentrations and types of SCFA can influence expression of mucosal proteins (Barcelo et al., 2000; Hatayama et al., 2007). Fundamentally, high levels of dietary fibers supplementation can decrease energy density in diets, leading to an increase in food intake and greater digesta flow in the intestine, which promotes renewal of mucosal protein, thus affecting intestinal mucosal layer. Moreover, dietary fibers stimulate intestinal epithelial cells to secret mucosal protein, and produce growth factors and metabolites such as arachidonic acid, all of which are beneficial to goblet cell proliferation and mucosal protein secretion. Furthermore, dietary fibers modify intestinal barrier by altering microbial community. Desai et al. (2016) reported that there was an interactive relationship between dietary fibers and mucosal barrier of the colon. With inadequate amount of dietary fibers, gut bacteria could only maintain their own growth by using colonic mucin as a nutrient source, inevitably leading to the erosion of the mucosal barrier. It was suggested that gut bacteria play a key role in interactive relationships between dietary fibers and mucosal layer or intestinal epithelium. However, different dietary fibers have discrete structures, such as monosaccharide type, glycosidic bond, and physicochemical property, thus exerting different impacts on intestinal barrier in pigs and humans (Hamakerb & Tuncil, 2014).
4.3. Dietary fibers and short‐chain fatty acids production
Dietary fiber fermented by gut microbiota leads to the production of SCFA, including acetate, propionate, and butyrate, along with lactate and some gases like hydrogen, carbon dioxide, and methane (den Besten et al., 2013). Process of microbial fermentation of dietary fiber to produce SCFA involves a series of principle reactions, and is mediated by compositions and abundances of gut microbiota (Koh et al., 2016; Louis et al., 2014). Acetate accounts for about 60% of the total SCFA produced in the large intestine, whereas small quantities of propionate and butyrate are produced by microbial fermentation (Lunn & Buttriss, 2010). Lactate is primarily produced in the upper gut, but acetate, propionate, and butyrate are synthesized in the colon and cecum, which are associated with specific microbial community localization in the foregut and hindgut of the host (Zhao et al., 2019). In the foregut, Lactobacillus is primary lactate‐producing bacteria, and an abundance of Lactobacillus would reduce in the hindgut due to gut environment, such as pH and oxygen concentration. In addition, Firmicutes and Bacteroides are dominant bacteria in the hindgut and account for more than 90% of total bacteria, and Prevotellaceae, Ruminococcaceae, and Lachnospiraceae are primarily bacteria on the family level to produce SCFA by microbial fermentation of dietary fiber (Liu et al., 2017). The SCFA can be absorbed rapidly by intestinal epithelial cells and influence gene expression, cell differentiation, and proliferation (Mu et al., 2017). Acetate is absorbed by the portal vein and acts on energy source to muscle tissues while propionate is converted to glucose in the liver (Makki et al., 2018; Williams et al., 2001). Butyrate is easily metabolized by β‐oxidation in the mitochondria and provides from 60% to 70% of the total energy demand of colonic epithelial cells (Corrêa‐Oliveira et al., 2016; Mentschel & Claus, 2003). In addition to being an important respiratory fuel, butyrate is considered beneficial for gut health of the host because it promotes proliferation of mucosa, differentiation of epithelial cells, and function of colonic barrier in the host intestine (Mu et al., 2017).
The SCFA facilitates microbial growth and bacteriocin secretion in the intestine, and then enhances immune barrier and microbial community structure of the intestine, resulting in improving gut health of the host (Liu, Wang, et al., 2018; Liu, Zhao, et al., 2018). During process of microbial fermentation to dietary fibers, SCFA produced decreases pH of the intestinal environment and promotes proliferation of the intestinal epithelial cells (Morrison & Preston, 2016). A decreased pH provides a suitable growth environment for the beneficial bacteria, such as Bifidobacterium and Lactobacillus, and further reduces intestinal pH value and bacteria susceptible to acidic conditions, resulting in inhibiting growth of harmful bacteria and invasion of pathogens. In addition, many beneficial bacteria shaped by SCFA can secret bacteriocin to kill harmful bacteria and then improve health of the host. Bacteriocins lacticin, nisin, and bioengineered nisin variants, which are bacteriocins produced by strains of Lactococcus lactis, have been shown to be effective in vitro against clinically relevant diseases and disorders (Rea et al., 2013). The SCFA produced by microbial fermentation decreases secretion of proinflammatory cytokines, such as interleukin‐6 (IL‐6) and tumor necrosis factor‐α (TNF‐α), and promotes anti‐inflammatory cytokine interleukin‐10 (IL‐10) and chemokine monocytes chemotactic protein‐1 (MCP‐1), to enhance intestinal immune barrier function (Montagne et al., 2003). The SCFA production was also reported to modulate the immune function of the host by inhibiting the activity of HDAC and stimulating the expression of G protein‐coupled receptors (Smith et al., 2013). Sodium butyrate regulates release of interleukin‐2 (IL‐2), IL‐6, interleukin‐8 (IL‐8), and TNF‐α by inhibiting HDAC activity and activating the activator protein 1 (AP‐1) signaling pathway in intestinal epithelial cells to enhance the intestinal immune function of the host (Cox et al., 2009; Tan et al., 2014). At the same time, sodium butyrate effectively regulates function of T lymphocytes through motivating G protein‐coupled receptors (GPR43) to reduce the level of inflammatory factor IL‐2 and to increase the secretion of anti‐inflammatory factor interleukin‐4 (IL‐4) and antimicrobial peptide LL‐37, which ultimately inhibits the inflammation response of the host (Cleophas et al., 2016; Macpherson et al., 2008). Overall, these results indicate that dietary fiber plays a crucial part in immune function of the host by increasing the SCFA concentration.
Many researchers reported that SCFA mediates glucose homeostasis and fat acids metabolism in the host by activating GPR41, GPR43, and stimulating enteroendocrine L‐cells to produce GLP‐1 and peptide YY, resulting in improved insulin sensitivity (Mudgil & Barak, 2013; Tolhurst et al., 2012). Pedersen et al. (2013) reported that oligofructose stimulated GLP‐1 and insulin secretion to increase host appetite, resulting in depressing intake of food and incidence of obesity. Furthermore, a consumption of barley kernel‐based bread to healthy human volunteers, which is rich in β‐glucan, improved glucose metabolism and prevented the risk of obese disease (Nilsson et al., 2015). Similar, Mozaffarian et al. (2011) reported that nonobese healthy individual who consumed fibers from fruits and vegetables could limit weight gain for a long time. In addition, a recent study assessed the relationship between various types of dietary carbohydrates and insulin resistance, and results showed adolescents with dietary fiber intervention reduced possibility of insulin resistance, but no significantly associations were observed for rest of the carbohydrate variables (Castro‐Quezada et al., 2019). Resistant starch consumption decreased digestible carbohydrates oxidation and serum glucose concentration with improved insulin sensitivity after meals (Giles et al., 2019). Therefore, it is a promising strategy to regulate glucose and fatty acids metabolism, and prevent human obesity and diabetes by specific dietary fiber consumption by modulating cellular receptors of GPRs (Schulze et al., 2004; Venn & Mann, 2004). However, Haenen et al. (2013) did not find an improvement in intestinal expression of GPR41 and GPR43 when pigs were fed diets high in resistant starch. Nielsen et al. (2015) observed a lower expression of GPR41 when feeding a diet supplemented with high concentration of arabinoxylan. Furthermore, Hooda et al. (2010) reported that oat β‐glucan intake increased the net SCFA absorption in the portal vein of catheterized pigs, but reduced the production of insulin mediated by GLP‐1 activity, which is consistent with the previous finding in diabetic patients (Silva et al., 2015). Ingerslev et al. (2014) did not observe a link between SCFA absorption and the portal GLP‐1 flux. Discrepancy of observations above should be primarily associated with types and structures of dietary fibers.
Among the dietary fiber fractions, β‐glucan is receiving more attention because it is an easily fermentable energy source for intestinal microbiota. β‐glucan is certainly fermented by most of gut microbiota, except for Enterobacteriaceae (Stack et al., 2010). Intestinal microbiota could produce lactic acid to reduce intestinal pH and further selectively facilitate proliferation of Lactobacillus and Bifidobacterium (Stack et al., 2010). Oat bran, a soluble dietary fiber source rich in β‐glycan, produces almost twice the amount of SCFA per gram of dietary fiber compared with wheat bran during microbial fermentation (Zhao et al., 2020). Bach Knudsen and Canibe (2000) detected higher concentration of lactate (11.6 vs. 3.4 mmol/ kg of digested feed) and greater proportion of butyrate accounted for total organic acid (9.1% vs. 6.1%) in the small intestine of pig's model after feeding diets supplemented with oat bran than wheat bran. Zhao et al. (2019) reported that intake of oat bran by pigs had significantly distinct improvement on amounts of lactic acid produced in the foregut, and soybean hulls and sugar beet pulp fed to pigs. They also observed acetate, propionate, and butyrate concentrations in the hindgut in oral bran treatment were higher than corn bran and wheat bran. Freire et al. (2000) investigated the effects of dietary wheat bran, sugar beet pulp, soybean hulls, or alfalfa meal intake on total SCFA production in the cecum of pigs. They found that dietary soybean hulls consumption increased total SCFA concentration by 11.2%, 30.5%, and 27.2% compared with dietary wheat bran, sugar beet pulp, and alfalfa consumption, respectively (Freire et al., 2000). Overall, variation in fermentability and SCFA production among different types of dietary fibers could be mainly ascribed to the differences in their chemical compositions and physicochemical properties. There is great potential to improve gut health and immune function of humans by regulating the intake of different kinds of dietary fibers to manipulate the production of SCFA.
The present argument is that SCFA produced in the intestine not only derived from microbial fermentation of dietary fibers, but also resulted from the secretion of the nondietary fiber components (Montoya et al., 2016). Montoya et al. (2017) reported that SCFA sourced from in vitro fermentation of dietary fibers in kiwifruit using fecal microbiota of humans only accounted for 30% of the total SCFA production. They explained that the main endogenous nondietary fiber components are soluble dietary fibers derived from the intestinal mucin in the small intestine, whereas microbial cell was the main component of the main endogenously losses as an insoluble dietary fiber in the whole gastrointestinal tract (Montoya et al., 2017).
In addition, the SCFA concentration measured in many in vivo studies only came from microbial fermentation, but a part of SCFA absorption by epithelial cells in the gut of the host was usually ignored. A common method to quantify the net absorption of SCFA is to collect blood samples from the portal vein and mesenteric artery simultaneously, and then analyze the SCFA concentrations via a portal vein‐catheterized pig model. Net portal absorption and concentration of SCFA in the portal vein or mesenteric artery estimated via catheterized pigs depend on the types of dietary fibers in cereal‐based diets fed to pigs. For instance, diets rich in arabinoxylan can stimulate proliferation of butyrate‐producing microorganisms and butyrate production in the large intestine, and increase the net portal absorption of butyrate compared with diets high in resistant starch with equal amount of dietary fibers (Ingerslev et al., 2014; Nielsen et al., 2015). Therefore, it would be extremely difficult to measure actual produced SCFA in vivo, as produced SCFA are rapidly metabolized by gut microbiota or absorbed by the host. The absorption and net production of SCFA, rather than a real‐time concentration of SCFA in the gut, derived from gut microbiota to ferment different types of dietary fibers should be quantified to understand the fermentable capacity of dietary fibers and the metabolic pathway of SCFA in the gut of the host.
4.4. Dietary fibers and gut microbiota
Microbial community in the host intestine is a complex and dynamic ecosystem that produces crucial metabolites to regulate host metabolism, such as SCFA, 5‐hydroxytryptamine, polymyxin, and bacitracin. Microbial metabolites depress proliferation of harmful bacteria and balance interactive competition between “beneficial bacteria” and “harmful bacteria.” In addition, microbial metabolites play important roles in maintaining intestinal barrier, facilitating immunological function, and modulating gene expression of host metabolism (Cani, 2016; Guo et al., 2008). An increment of microbial activity was found in the intestine of pigs fed diets containing a high content of dietary fiber, as indicated by increased bacterial counts and ATP concentration (Liu, Wang, et al., 2018; Liu, Zhao, et al., 2018). It indicates that dietary fibers can activate microbial activity, resulting in producing more microbial metabolites. Many reports have indicated that Firmicutes and Bacteroidetes are the two dominant phyla in the gastrointestinal tract of pigs and humans, which account for about 90% of the gut microbiota (Bian et al., 2016; Liu, Wang, et al., 2018; Liu, Zhao, et al., 2018). Firmicutes utilize dietary fibers to produce SCFA, especially butyrate. Bacteroidetes have a great capacity for degradation of dietary fibers to produce propionate, such as Prevotella (Flint et al., 2008). Mu et al. (2017) reported that dietary supplementation with alfalfa meal increased populations of Firmicutes and Bacteroidetes compared to wheat bran in neonatal piglets. Similarly, diets rich in resistant starch increased relative populations of some specific members of Firmicutes, as well as a ratio of Firmicutes to Bacteroidetes in human's intestine (Maier et al., 2017). In contrast, Ferrario et al. (2017) detected an increase in numbers of Bacteroidetes and a decrease in populations of Firmicutes in subjects supplied with dietary inulin. Further, specific bacteria in phylum of Firmicutes and Bacteroidetes to ferment different types of dietary fibers has not been known well.
A population of Lachnoclostridium in fecal samples is influenced by dietary fibers supplementation, which has a connection with the obesity in humans (Amadou et al., 2016). The abundance of Ruminococcus_1 increased when pigs were fed diets containing soybean hulls, and it was reported that Ruminococcus_1 can ferment dietary fibers to produce SCFA (Pryde et al., 2002). Che et al. (2014) and Chen et al. (2014) showed that diets containing pea fiber increased the number of Lactobacillus in the colon of pigs, but pea fiber had no significant effects on the number of Bifidobacteria and Escherichia coli, while diets containing wheat bran significantly increased the number of Bifidobacteria in pigs. The relative abundances of Lactobacillus, the dominant species of the lactate‐producing bacteria in the ileum, and Prevotella in the colon were positively correlated with the concentration of dietary chicory fiber (Liu et al., 2012). Furthermore, high resistant starch supplementation in diets of adult pigs increased the relative abundances of Faecalibacterium prausnitzii and Ruminococcus bromii, and reduced the population of pathogenic bacteria Escherichia coli and Pseudomonas (Haenen et al., 2013).
Improved numbers of Bifidobacteria spp. and a decrease populations of total anaerobes or Clostridia in the feces were observed when the pigs were fed diets containing 0.5% fructo‐oligosaccharide in combination with Bifidobacterium longum (Clarke et al., 2017). Zhao et al. (2018) reported that dietary barley supplementation increased Lactobacilli spp. and Bifidobacterium spp. populations, but decreased numbers of Enterobacteria spp. in the cecum of pigs in comparison with a corn‐based diet, which may be attributed to greater β‐glucan levels in barley diet compared with a corn diet (Zhao et al., 2018). Oat fiber and β‐glucan isolates, coming from fermented oat‐based products containing both native and microbial β‐glucan, promote growth of Bifidobacteria spp. in the intestine of pigs and humans (Martensson et al., 2005). Pieper et al. (2008) found that dehulling barley with high amounts of soluble nonstarch polysaccharides favored growth of xylan‐degarding and glucan‐degrading bacteria (Pieper et al., 2008), whereas β‐glucan from hulled barleys favored growth of Lactobacilli spp. in weaned pigs (Pieper et al., 2008). These results suggest that different sources of β‐glucan have inconsistent responses on microbial composition and abundance in the intestine of the host, which could be associated with the structure and physical properties of different β‐glucan source, such as solubility and molecule weight. Moreover, an increase of Bifidobacteria spp. and Enterobacteria spp. abundances in ileal digesta were observed when growing pigs were fed diets supplemented with guar gum or cellulose (Owusu‐Asiedu et al., 2006). Nielsen et al. (2014) showed that diets supplemented with arabinoxylan increased populations of Bifidobacterium spp. and Lactobacillus spp. in the colon of pigs.
Small intestine is mainly responsible for food digestion and absorption, while large intestine is important for microbial fermentation of substances (Healey et al., 2020). Hindgut of pigs and humans contain a larger proportion of Firmicutes than small intestine, indicating that large intestine might undertake a crucial role in microbial fermentation of dietary fiber (Flint et al., 2008). Escherichia‐Shigella, Lactobacillus, Streptococcus, and Enterococcus, dominant genera of intestinal microbiota in ileal digesta, are the major bacterial species with greater abundances compared with large intestine of the pig (Zhao et al., 2019). Greater populations of Escherichia‐Shigella and Streptococcus are always considered as pathogenic bacteria related to host infection and enteric diseases, such as diarrhea symptoms. Thus, occurrences of enteropathies are primarily associated with microbial composition in the upper gut (Healey et al., 2020). Lactobacillus is a beneficial bacterial specie to improve gut health of the host, so that it is extremely crucial to maintain balance of different bacteria in the gut by dietary nutrients intervention. An altered gut microbiota composition derived from lack of low dietary fibers could lead to a severe deterioration of mucus layer and increased susceptibility to infections and chronic inflammatory diseases (Desai et al., 2016; Makki et al., 2018). Therefore, dietary fiber ingested from foods has a potential to prevent against metabolic diseases in humans by reshaping composition of gut microbiota. However, effects of dietary fiber on intestinal bacterial community vary among different studies, which could be attributed to different types of dietary fiber types, as well as variation on quantity of dietary fiber, available for microbial fermentation in the gut. Furthermore, a study reported consuming inulin at breakfast after a longer fasting period had a greater response on fecal microbiota of individuals (Sasaki et al., 2019), which indicated dietary habit play an important role in regulation of microbial community after ingesting dietary fibers.
5. SUMMARY AND PERSPECTIVE
There are many complicated and subtle interactions between types of dietary fiber and gut microbiota, SCFA production, and host health. It has been widely accepted that SCFA, especially butyrate, plays a critical role in modulating and improving gut health. However, there are still many vague aspects about underlying relationships between dietary fiber and host health. Major challenges related to dietary fiber research may include (1) to identify the specific mechanisms of SCFA produced from dietary fiber fermented by gut microbiota on functions of animal tissues and organs, (2) to clarify the relationship between host health and gut microbiota shaped by the dietary intervention with dietary fibers, (3) to further illustrate different responses of various dietary fibers derived from edible foods and their combinations on host health. Overall, we suggest that more attention should be focused on specific chemical constituents and physical characteristics of various dietary fibers when they take actions in regulating host metabolism and health.
CONFLICT OF INTEREST
None of the authors had a financial or personal conflict of interest in relation to the present study.
AUTHOR CONTRIBUTIONS
Pan Yang: Conceptualization (supporting); Data curation (supporting); Formal analysis (lead); Investigation (lead); Visualization (lead); Writing‐original draft (lead). Jinbiao Zhao: Conceptualization (lead); Data curation (lead); Formal analysis (supporting); Funding acquisition (lead); Project administration (lead); Writing‐review & editing (lead).
ETHICAL APPROVAL AND CONSENT TO PARTICIPATE
Not applicable.
DATA AVAILABILITY STATEMENT
All authors consent that raw data presented in this review are available after publication. Please contact author for data requests.
ACKNOWLEDGMENTS
The authors are grateful to the professional suggestions from Dr. Zhaokai Yang (College of Science, China Agricultural University) for carbohydrate structure and to the Postdoctoral Innovative Talent Support Program (No. BX20200365).
Yang, P., & Zhao, J. Variations on gut health and energy metabolism in pigs and humans by intake of different dietary fibers. Food Science & Nutrition, 2021;9: 4639–4654. 10.1002/fsn3.2421
REFERENCES
- Adebowale, T. O., Yao, K., & Oso, A. O. (2019). Major cereal carbohydrates in relation to intestinal health of monogastric animals: A review. Animal Nutrition, 5, 331–339. 10.1016/j.aninu.2019.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amadou, T., Hosny, M., La Scola, B., & Cassir, N. (2016). “Lachnoclostridium bouchesdurhonense,” a new bacterial species isolated from human gut microbiota. New Microbes and New Infections, 13, 69–70. 10.1016/j.nmni.2016.06.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bach Knudsen, K. E., & Canibe, N. (2000). Breakdown of plant carbohydrates in the digestive tract of pigs fed on wheat‐ or oat‐based rolls. Journal of the Science of Food and Agriculture, 80, 1253–1261. [DOI] [Google Scholar]
- Bai, Z., Zhang, Z., Ye, Y., & Wang, S. (2010). Sodium butyrate induces differentiation of gastric cancer cells to intestinal cells via the PTEN/phosphoinositide 3‐kinase pathway. Cell Biology International, 34, 1141–1145. 10.1042/CBI20090481 [DOI] [PubMed] [Google Scholar]
- Barcelo, A., Claustre, J., Moro, F., Chayvialle, J. A., Cuber, J. C., & Plaisancié, P. (2000). Mucin secretion is modulated by luminal factors in the isolated vascularly perfused rat colon. Gut, 46, 218–224. 10.1136/gut.46.2.218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bashir, K. M. I., & Choi, J. (2017). Clinical and physiological perspectives of β‐glucans: The past, present, and future. International Journal of Molecular Sciences, 18, 1906. 10.3390/ijms18091906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bäumler, A. J., & Sperandio, V. (2016). Interactions between the microbiota and pathogenic bacteria in the gut. Nature, 535, 85–93. 10.1038/nature18849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bian, G., Ma, S., Zhu, Z., Su, Y., Zoetendal, E. G., Mackie, R., Liu, J., Mu, C., Huang, R., Smidt, H., & Zhu, W. (2016). Age, introduction of solid feed and weaning are more important determinants of gut bacterial succession in piglets than breed and nursing mother as revealed by a reciprocal cross‐fostering model. Environmental Microbiology, 18, 1566–1577. 10.1111/1462-2920.13272 [DOI] [PubMed] [Google Scholar]
- Bikker, P., Dirkzwager, A., Fledderus, J., Trevisi, P., le Huërou‐Luron, I. , Lallès, J. P., & Awati, A. (2006). The effect of dietary protein and fermentable carbohydrates levels on growth performance and intestinal characteristics in newly weaned piglets. Journal of Animal Science, 84, 3337–3345. 10.2527/jas.2006-076 [DOI] [PubMed] [Google Scholar]
- Borderías, A. J., Sánchez‐Alonso, I., & Pérez‐Mateos, M. (2005). New applications of fibres in foods: Addition to fishery products. Trends in Food Science & Technology, 16, 458–465. 10.1016/j.tifs.2005.03.011 [DOI] [Google Scholar]
- Cani, P. D. (2016). Interactions between gut microbes and host cells control gut barrier and metabolism. International Journal of Obesity Supplements, 6, S28–S31. 10.1038/ijosup.2016.6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cappai, M. G., Alesso, G. A., Nieddu, G., Sanna, M., & Pinna, W. (2013). Electron microscopy and composition of raw acorn starch in relation to in vivo starch digestibility. Food & Function, 4, 917. 10.1039/c3fo60075k [DOI] [PubMed] [Google Scholar]
- Cappai, M. G., Wolf, P., Pinna, W., Rust, P., & Kamphues, J. (2020). Pre‐caecal disappearance of starch and volatile fatty acid (VFA) content in digesta of caecum of growing pigs fed with ripe hulled shredded acorns in their diet. Agriculture, 10, 508. 10.3390/agriculture10110508 [DOI] [Google Scholar]
- Castro‐Quezada, I., Flores‐Guillén, E., Núñez‐Ortega, P. E., Irecta‐Nájera, C. A., Sánchez‐Chino, X. M., Mendez‐Flores, O. G., Olivo‐Vidal, Z. E., García‐Miranda, R., Solís‐Hernández, R., & Ochoa‐Díaz‐López, H. (2019). Dietary carbohydrates and insulin resistance in adolescents from marginalized areas of Chiapas, México. Nutrients, 11(12), 3066. 10.3390/nu11123066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Che, L., Chen, H., Yu, B., He, J., Zheng, P., Mao, X., Yu, J., Huang, Z., & Chen, D. (2014). Long‐term intake of pea fiber affects colonic barrier function, bacterial and transcriptional profile in pig model. Nutrition and Cancer, 66(3), 388–399. 10.1080/01635581.2014.884229 [DOI] [PubMed] [Google Scholar]
- Chen, H., Mao, X. B., Che, L. Q., Yu, B., He, J., Yu, J., Han, G., Huang, Z., Zheng, P., & Chen, D. (2014). Impact of fiber types on gut microbiota, gut environment and gut function in fattening pigs. Animal Feed Science and Technology, 195, 101–111. 10.1016/j.anifeedsci.2014.06.002 [DOI] [Google Scholar]
- Chen, H., Mao, X., He, J., Yu, B., Huang, Z., Yu, J., Zheng, P., & Chen, D. (2013). Dietary fibre affects intestinal mucosal barrier function and regulates intestinal bacteria in weaning piglets. British Journal of Nutrition, 110, 1837–1848. 10.1017/S0007114513001293 [DOI] [PubMed] [Google Scholar]
- Chen, H., Mao, X., Yin, J., Yu, B., He, J., Che, L., Yu, J., Huang, Z., Zheng, P., Michiels, J., De Smet, S., & Chen, D. (2015). Comparison of jejunal digestive enzyme activities, expression of nutrient transporter genes, and apparent fecal digestibility in weaned piglets fed diets with varied sources of fiber. Journal of Animal and Feed Science, 24, 41–47. 10.22358/jafs/65651/2015 [DOI] [Google Scholar]
- Cheng, W., Lu, J., Li, B., Lin, W., Zhang, Z., Wei, X., Sun, C., Chi, M., Bi, W., Yang, B., Jiang, A., & Yuan, J. (2017). Effect of functional oligosaccharides and ordinary dietary fiber on intestinal microbiota diversity. Frontiers in Microbiology, 8, 1750. 10.3389/fmicb.2017.01750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke, S. T., Brooks, S., Inglis, G. D., Yanke, L. J., Green, J., Petronella, N., Ramdath, D. D., Bercik, P., Green‐Johnson, J. M., & Kalmokoff, M. (2017). Impact of β2‐1 fructan on faecal community change: Results from a placebo‐controlled, randomised, double‐blinded, cross‐over study in healthy adults. The British Journal of Nutrition, 118(6), 441–453. 10.1017/S0007114517002318 [DOI] [PubMed] [Google Scholar]
- Cleophas, M. C., Crişan, T. O., Lemmers, H., Toenhake‐Dijkstra, H., Fossati, G., Jansen, T. L., Dinarello, C. A., Netea, M. G., & Joosten, L. A. (2016). Suppression of monosodium urate crystal‐induced cytokine production by butyrate is mediated by the inhibition of class I histone deacetylases. Annals of the Rheumatic Diseases, 75, 593–600. 10.1136/annrheumdis-2014-206258 [DOI] [PubMed] [Google Scholar]
- Corrêa‐Oliveira, R., Fachi, J. L., Vieira, A., Sato, F. T., & Vinolo, M. A. (2016). Regulation of immune cell function by short‐chain fatty acids. Clinical & Translational Immunology, 5, e73. 10.1038/cti.2016.17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox, M. A., Jackson, J., Stanton, M., Rojas‐Triana, A., Bober, L., Laverty, M., Yang, X., Zhu, F., Liu, J., Wang, S., Monsma, F., Vassileva, G., Maguire, M., Gustafson, E., Bayne, M., Chou, C. C., Lundell, D., & Jenh, C. H. (2009). Short‐chain fatty acids act as antiinflammatory mediators by regulating prostaglandin E(2) and cytokines. World Journal of Gastroenterology, 15, 5549–5557. 10.3748/wjg.15.5549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings, J. H., & Stephen, A. M. (2007). Carbohydrate terminology and classification. European Journal of Clinical Nutrition, 61(1), S5–S18. 10.1038/sj.ejcn.1602936 [DOI] [PubMed] [Google Scholar]
- den Besten, G. , van Eunen, K. , Groen, A. K., Venema, K., Reijngoud, D. J., & Bakker, B. M. (2013). The role of short‐chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. Journal of Lipid Research, 54(9), 2325–2340. 10.1194/jlr.R036012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desai, M. S., Seekatz, A. M., Koropatkin, N. M., Kamada, N., Hickey, C. A., Wolter, M., Pudlo, N. A., Kitamoto, S., Terrapon, N., Muller, A., Young, V. B., Henrissat, B., Wilmes, P., Stappenbeck, T. S., Núñez, G., & Martens, E. C. (2016). A dietary fiber‐deprived gut microbiota degrades the colonic mucus barrier and enhances pathogen susceptibility. Cell, 167, 1339–1353.e21. 10.1016/j.cell.2016.10.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dikeman, C. L., & Fahey, G. C. (2006). Viscosity as related to dietary fiber: A review. Critical Reviews in Food Science and Nutrition, 46(8), 649–663. 10.1080/10408390500511862 [DOI] [PubMed] [Google Scholar]
- Elleuch, M., Bedigian, D., Roiseux, O., Besbes, S., Blecker, C., & Attia, H. (2011). Dietary fibre and fibre‐rich by‐products of food processing: Characterisation, technological functionality and commercial applications: A review. Food Chemistry, 124(2), 411–421. 10.1016/j.foodchem.2010.06.077 [DOI] [Google Scholar]
- Ferrario, C., Statello, R., Carnevali, L., Mancabelli, L., Milani, C., Mangifesta, M., Duranti, S., Lugli, G. A., Jimenez, B., Lodge, S., Viappiani, A., Alessandri, G., Dall'Asta, M., Del Rio, D., Sgoifo, A., van Sinderen, D. , Ventura, M., & Turroni, F. (2017). How to feed the mammalian gut microbiota: Bacterial and metabolic modulation by dietary fibers. Frontiers in Microbiology, 8, 1749. 10.3389/fmicb.2017.01749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flint, H. J., Bayer, E. A., Rincon, M. T., Lamed, R., & White, B. A. (2008). Polysaccharide utilization by gut bacteria: Potential for new insights from genomic analysis. Nature Reviews Microbiology, 6, 121–131. 10.1038/nrmicro1817 [DOI] [PubMed] [Google Scholar]
- Flint, H. J., Scott, K. P., Louis, P., & Duncan, S. H. (2012). The role of the gut microbiota in nutrition and health. Nature Reviews Gastroenterology & Hepatology, 9(10), 577–589. 10.1038/nrgastro.2012.156 [DOI] [PubMed] [Google Scholar]
- Freire, J. P. B., Guerreiro, A. J. G., Cunha, L. F., & Aumaitre, A. (2000). Effect of dietary fibre source on total tract digestibility, caecum volatile fatty acids and digestive transit time in the weaned piglet. Animal Feed Science and Technology, 87, 71–83. 10.1016/S0377-8401(00)00183-8 [DOI] [Google Scholar]
- Furuse, M. (2010). Molecular basis of the core structure of tight junctions. Cold Spring Harbor Perspectives in Biology, 2, a002907. 10.1101/cshperspect.a002907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gensollen, T., Iyer, S. S., Kasper, D. L., & Blumberg, R. S. (2016). How colonization by microbiota in early life shapes the immune system. Science, 352, 539–544. 10.1126/science.aad9378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson, G. R., Hutkins, R., Sanders, M. E., Prescott, S. L., Reimer, R. A., Salminen, S. J., Scott, K., Stanton, C., Swanson, K. S., Cani, P. D., Verbeke, K., & Reid, G. (2017). Expert consensus document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nature Reviews Gastroenterology & Hepatology, 14, 491–502. 10.1038/nrgastro.2017.75 [DOI] [PubMed] [Google Scholar]
- Giles, E. D., Brown, I. L., MacLean, P. S., Pan, Z., Melanson, E. L., Heard, K. J., Cornier, M. A., Marden, T., & Higgins, J. A. (2019). The in vivo net energy content of resistant starch and its effect on macronutrient oxidation in healthy adults. Nutrients, 11, 2484. 10.3390/nu11102484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo, X., Xia, X., Tang, R., Zhou, J., Zhao, H., & Wang, K. (2008). Development of a real‐time PCR method for Firmicutes and Bacteroidetes in faeces and its application to quantify intestinal population of obese and lean pigs. Letters in Applied Microbiology, 47, 367–373. 10.1111/j.1472-765X.2008.02408.x [DOI] [PubMed] [Google Scholar]
- Haenen, D., Zhang, J., Souza da Silva, C., Bosch, G., van der Meer, I. M. , van Arkel, J. , van den Borne, J. J. , Pérez Gutiérrez, O., Smidt, H., Kemp, B., Müller, M., & Hooiveld, G. J. (2013). A diet high in resistant starch modulates microbiota composition, SCFA concentrations, and gene expression in pig intestine. The Journal of Nutrition, 143, 274–283. 10.3945/jn.112.169672 [DOI] [PubMed] [Google Scholar]
- Hamakerb, R., & Tuncil, Y. E. A. (2014). Perspective on the complexity of dietary fiber structures and their potential effect on the gut microbiota. Journal of Molecular Biology, 426, 3838–3850. 10.1016/j.jmb.2014.07.028 [DOI] [PubMed] [Google Scholar]
- Hatayama, H., Iwashita, J., Kuwajima, A., & Abe, T. (2007). The short chain fatty acid, butyrate, stimulates MUC2 mucin production in the human colon cancer cell line, LS174T. Biochemical and Biophysical Research Communications, 356, 599–603. 10.1016/j.bbrc.2007.03.025 [DOI] [PubMed] [Google Scholar]
- Healey, G. R., Celiberto, L. S., Lee, S. M., & Jacobson, K. (2020). Fiber and prebiotic interventions in pediatric inflammatory bowel disease: What role does the gut microbiome play? Nutrients, 12(10), 3204. 10.3390/nu12103204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooda, S., Matte, J. J., Vasanthan, T., & Zijlstra, R. T. (2010). Dietary oat beta‐glucan reduces peak net glucose flux and insulin production and modulates plasma incretin in portal‐vein catheterized grower pigs. The Journal of Nutrition, 140, 1564–1569. 10.3945/jn.110.122721 [DOI] [PubMed] [Google Scholar]
- Hopwood, D. E., Pethick, D. W., Pluske, J. R., & Hampson, D. J. (2004). Addition of pearl barley to a rice‐based diet for newly weaned piglets increases the viscosity of the intestinal contents, reduces starch digestibility and exacerbates post‐weaning colibacillosis. British Journal of Nutrition, 92, 419–427. 10.1079/bjn20041206 [DOI] [PubMed] [Google Scholar]
- Ingerslev, A. K., Theil, P. K., Hedemann, M. S., Lærke, H. N., & Bach Knudsen, K. E. (2014). Resistant starch and arabinoxylan augment SCFA absorption, but affect postprandial glucose and insulin responses differently. British Journal of Nutrition, 111, 1564–1576. 10.1017/S0007114513004066 [DOI] [PubMed] [Google Scholar]
- Jarrar, A. H., Beasley, J. M., Ohuma, E. O., Ismail, L. C., Qeshta, D. A., Mohamad, M. N., & Dhaheri, A. S. (2019). Effect of high fiber cereal intake on satiety and gastrointestinal symptoms during Ramadan. Nutrients, 11, 939. 10.3390/nu11040939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jha, R., Fouhse, J. M., Tiwari, U. P., Li, L., & Willing, B. P. (2019). Dietary fiber and intestinal health of monogastric animals. Frontiers in Veterinary Science, 6, 48. 10.3389/fvets.2019.00048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerckhoffs, D. A. J. M., Hornstra, G., & Mensink, R. P. (2003). Cholesterol‐lowing effect of β‐glucan from oat bran in midly hypercholesterolemic subjects may decrease when β‐glucan is incorporated into bread and cookies. American Journal of Clinical Nutrition, 78, 221–227. 10.1093/ajcn/78.2.221 [DOI] [PubMed] [Google Scholar]
- Knudsen, K. E. B., Lærke, H. N., & Jørgensen, H. (2013). Carbohydrates and carbohydrate utilization in swine. In Chiba L. I. (Ed.), Sustainable swine nutrition (pp. 109–137). Wiley‐Blackwell. [Google Scholar]
- Koh, A., De Vadder, F., Kovatcheva‐Datchary, P., & Bäckhed, F. (2016). From dietary fiber to host physiology: Short‐chain fatty acids as key bacterial metabolites. Cell, 165(6), 1332–1345. 10.1016/j.cell.2016.05.041 [DOI] [PubMed] [Google Scholar]
- Lan, G., Chen, H., Chen, S., & Tian, J. (2012). Chemical composition and physicochemical properties of dietary fiber from Polygonatum odoratum as affected by different processing methods. Food Research International, 49(1), 406–410. 10.1016/j.foodres.2012.07.047 [DOI] [Google Scholar]
- Lee, J. E., Lee, S., Sung, J., & Ko, G. (2011). Analysis of human and animal fecal microbiota for microbial source tracking. The ISME Journal, 5, 362–365. 10.1038/ismej.2010.120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, H. Y., Lundh, T., Dicksved, J., & Lindberg, J. E. (2012). Expression of heat shock protein 27 in gut tissue of growing pigs fed diets without and with inclusion of chicory fiber. Journal of Animal Science, 90, 25–27. 10.2527/jas.53724 [DOI] [PubMed] [Google Scholar]
- Liu, H., Wang, J., He, T., Becker, S., Zhang, G., Li, D., & Ma, X. (2018). Butyrate: A double‐edged sword for health? Advances in Nutrition, 9, 21–29. 10.1093/advances/nmx009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, P., Zhao, J., Guo, P., Lu, W., Geng, Z., Levesque, C. L., Johnston, L. J., Wang, C., Liu, L., Zhang, J., Ma, N., Qiao, S., & Ma, X. (2017). Dietary corn bran fermented by Bacillus subtilis MA139 decreased gut cellulolytic bacteria and microbiota diversity in finishing pigs. Frontiers in Cellular and Infection Microbiology, 7, 526. 10.3389/fcimb.2017.00526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, P., Zhao, J., Wang, W., Guo, P., Lu, W., Wang, C., Liu, L., Johnston, L. J., Zhao, Y., Wu, X., Xu, C., Zhang, J., & Ma, X. (2018). Dietary corn bran altered the diversity of microbial communities and cytokine production in weaned pigs. Frontiers in Microbiology, 9, 2090. 10.3389/fmicb.2018.02090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louis, P., Hold, G. L., & Flint, H. J. (2014). The gut microbiota, bacterial metabolites and colorectal cancer. Nature Reviews Microbiology, 12, 661–672. 10.1038/nrmicro3344 [DOI] [PubMed] [Google Scholar]
- Lunn, J., & Buttriss, J. L. (2010). Carbohydrates and dietary fibre. Nutrition Bulletin, 32, 21–64. 10.1111/j.1467-3010.2007.00616.x [DOI] [Google Scholar]
- Macpherson, A. J., McCoy, K. D., Johansen, F. E., & Brandtzaeg, P. (2008). The immune geography of IgA induction and function. Mucosal Immunology, 1, 11–22. 10.1038/mi.2007.6 [DOI] [PubMed] [Google Scholar]
- Maier, T. V., Lucio, M., Lee, L. H., VerBerkmoes, N. C., Brislawn, C. J., Bernhardt, J., Lamendella, R., McDermott, J. E., Bergeron, N., Heinzmann, S. S., Morton, J. T., González, A., Ackermann, G., Knight, R., Riedel, K., Krauss, R. M., Schmitt‐Kopplin, P., & Jansson, J. K. (2017). Impact of dietary resistant starch on the human gut microbiome, metaproteome, and metabolome. Mbio, 8, e01343‐17. 10.1128/mBio.01343-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makki, K., Deehan, E. C., Walter, J., & Bäckhed, F. (2018). The impact of dietary fiber on gut microbiota in host health and disease. Cell Host & Microbe, 23, 705–715. 10.1016/j.chom.2018.05.012 [DOI] [PubMed] [Google Scholar]
- Martensson, O., Biorklund, M., Lambo, A. M., Duenas‐Chasco, M., Irastorz, A., Holst, O., Norin, E., Welling, G., Oste, R., & Onning, G. (2005). Fermented, ropy, oat‐based products reduce cholesterol levels and stimulate the Bifidobacteria flora in humans. Nutrition Research, 25, 429–442. 10.1016/j.nutres.2005.03.004 [DOI] [Google Scholar]
- Mentschel, J., & Claus, R. (2003). Increased butyrate formation in the pig colon by feeding raw potato starch leads to a reduction of colonocyte apoptosis and a shift to the stem cell compartment. Metabolism, 52, 1400–1405. 10.1016/s0026-0495(03)00318-4 [DOI] [PubMed] [Google Scholar]
- Montagne, L., Pluske, J. R., & Hampson, D. J. (2003). A review of interactions between dietary fibre and the intestinal mucosa, and their consequences on digestive health in young non‐ruminant animals. Animal Feed Science and Technology, 108, 95–117. 10.1016/S0377-8401(03)00163-9 [DOI] [Google Scholar]
- Montoya, C. A., Henare, S. J., Rutherfurd, S. M., & Moughan, P. J. (2016). Potential misinterpretation of the nutritional value of dietary fiber: Correcting fiber digestibility values for nondietary gut‐interfering material. Nutrition Reviews, 74, 517–533. 10.1093/nutrit/nuw014 [DOI] [PubMed] [Google Scholar]
- Montoya, C. A., Rutherfurd, S. M., & Moughan, P. J. (2017). Ileal digesta nondietary substrates from cannulated pigs are major contributors to in vitro human hindgut short‐chain fatty acid production. The Journal of Nutrition, 147, 264–271. 10.3945/jn.116.240564 [DOI] [PubMed] [Google Scholar]
- Morrison, D. J., & Preston, T. (2016). Formation of short chain fatty acids by the gut microbiota and their impact on human metabolism. Gut Microbes, 7, 189–200. 10.1080/19490976.2015.1134082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mozaffarian, D., Hao, T., Rimm, E. B., Willett, W. C., & Hu, F. B. (2011). Changes in diets and lifestyle and long‐term weight gain in woman and men. The New England Journal of Medicine, 364, 2392–2404. 10.1056/NEJMoa1014296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mu, C., Zhang, L., He, X., Smidt, H., & Zhu, W. (2017). Dietary fibres modulate the composition and activity of butyrate‐producing bacteria in the large intestine of suckling piglets. Antonie van Leeuwenhoek, 110, 687–696. 10.1007/s10482-017-0836-4 [DOI] [PubMed] [Google Scholar]
- Mudgil, D., & Barak, S. (2013). Composition, properties and health benefits of indigestible carbohydrate polymers as dietary fiber: A review. International Journal of Biological Macromolecules, 61, 1–6. 10.1016/j.ijbiomac.2013.06.044 [DOI] [PubMed] [Google Scholar]
- Natividad, J. M., & Verdu, E. F. (2013). Modulation of intestinal barrier by intestinal microbiota: Pathological and therapeutic implications. Pharmacological Research, 69, 42–51. 10.1016/j.phrs.2012.10.007 [DOI] [PubMed] [Google Scholar]
- Nicolucci, A. C., Hume, M. P., Martínez, I., Mayengbam, S., Walter, J., & Reimer, R. A. (2017). Prebiotics reduce body fat and alter intestinal microbiota in children who are overweight or with obesity. Gastroenterology, 153, 711–722. 10.1053/j.gastro.2017.05.055 [DOI] [PubMed] [Google Scholar]
- Nielsen, T. S., Lærke, H. N., Theil, P. K., Sørensen, J. F., Saarinen, M., Forssten, S., & Knudsen, K. E. (2014). Diets high in resistant starch and arabinoxylan modulate digestion processes and SCFA pool size in the large intestine and faecal microbial composition in pigs. British Journal of Nutrition, 112, 1837–1849. 10.1017/S000711451400302X [DOI] [PubMed] [Google Scholar]
- Nielsen, T. S., Theil, P. K., Purup, S., Nørskov, N. P., & Bach Knudsen, K. E. (2015). Effects of resistant starch and arabinoxylan on parameters related to large intestinal and metabolic health in pigs fed fat‐rich diets. Journal of Agricultural and Food Chemistry, 63, 10418–10430. 10.1021/acs.jafc.5b03372 [DOI] [PubMed] [Google Scholar]
- Nilsson, A. C., Johansson‐Boll, E. V., & Björck, I. M. (2015). Increased gut hormones and insulin sensitivity index following a 3‐d intervention with a barley kernel‐based product: A randomised cross‐over study in healthy middle‐aged subjects. The British Journal of Nutrition, 114, 899–907. 10.1017/S0007114515002524 [DOI] [PubMed] [Google Scholar]
- Owusu‐Asiedu, A., Patience, J. F., Laarveld, B., Van Kessel, A. G., Simmins, P. H., & Zijlstra, R. T. (2006). Effects of guar gum and cellulose on digesta passage rate, ileal microbial populations, energy and protein digestibility, and performance of grower pigs. Journal of Animal Science, 84, 843–852. 10.2527/2006.844843x [DOI] [PubMed] [Google Scholar]
- Pedersen, C., Lefevre, S., Peters, V., Patterson, M., Ghatei, M. A., Morgan, L. M., & Frost, G. S. (2013). Gut hormone release and appetite regulation in healthy non‐obese participants following oligofructose intake. A dose‐escalation study. Appetite, 66, 44–53. 10.1016/j.appet.2013.02.017 [DOI] [PubMed] [Google Scholar]
- Pieper, R., Jha, R., Rossnagel, B., Van Kessel, A. G., Souffrant, W. B., & Leterme, P. (2008). Effect of barley and oat cultivars with different carbohydrate compositions on the intestinal bacterial communities in weaned piglets. FEMS Microbiology Ecology, 66, 556–566. 10.1111/j.1574-6941.2008.00605.x [DOI] [PubMed] [Google Scholar]
- Pryde, S. E., Duncan, S. H., Hold, G. L., Stewart, C. S., & Flint, H. J. (2002). The microbiology of butyrate formation in the human colon. FEMS Microbiology Letters, 217, 133–139. 10.1111/j.1574-6968.2002.tb11467.x [DOI] [PubMed] [Google Scholar]
- Rea, M. C., Alemayehu, D., Ross, R. P., & Hill, C. (2013). Gut solutions to a gut problem: Bacteriocins, probiotics and bacteriophage for control of Clostridium difficile infection. Journal of Medical Microbiology, 62, 1369–1378. 10.1099/jmm.0.058933-0 [DOI] [PubMed] [Google Scholar]
- Rejaii, M., & Salehi, E. A. (2016). Properties of sugar beet pulp pectin: A systemic review. International Journal of PharmTech Research, 9, 364–368. [Google Scholar]
- Sajilata, M. G., Singhal, R. S., & Kulkarni, P. R. (2006). Resistant starch‐A review. Comprehensive Reviews in Food Science and Food Safety, 1, 1–17. 10.1111/j.1541-4337.2006.tb00076.x [DOI] [PubMed] [Google Scholar]
- Sasaki, H., Miyakawa, H., Watanabe, A., Nakayama, Y., Lyu, Y., Hama, K., & Shibata, S. (2019). Mice microbiota composition changes by inulin feeding with a long fasting period under a two‐meals‐per‐day schedule. Nutrients, 11, 2802. 10.3390/nu11112802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiavon, S., Bortolozzo, A., Bailoni, L., & Tagliapetra, F. (2004). Effects of sugar beet pulp on growth and health status of weaned piglets. Italian Journal of Animal Science, 4, 337–351. 10.4081/ijas.2004.337 [DOI] [Google Scholar]
- Schulze, M. B., Liu, S., Rimm, E. B., Manson, J. E., Willett, W. C., & Hu, F. B. (2004). Glycemic index, glycemic load, and dietary fiber intake and incidence of type 2 diabetes in younger and middle‐aged women. American Journal of Clinical Nutrition, 80, 348–356. 10.1093/ajcn/80.2.348 [DOI] [PubMed] [Google Scholar]
- Serena, A., Hedemann, M. S., & Bach Knudsen, K. E. (2008). Influence of dietary fiber on luminal environment and morphology in the small and large intestine of sows. Journal of Animal Science, 86, 2217–2227. 10.2527/jas.2006-062 [DOI] [PubMed] [Google Scholar]
- Silva, F. M., Kramer, C. K., Crispim, D., & Azevedo, M. J. (2015). A high‐glycemic index, low‐fiber breakfast affects the postprandial plasma glucose, insulin, and ghrelin responses of patients with type 2 diabetes in a randomized clinical trial. Journal of Nutrition, 145, 736–741. 10.3945/jn.114.195339 [DOI] [PubMed] [Google Scholar]
- Smith, P. M., Howitt, M. R., Panikov, N., Michaud, M., Gallini, C. A., Bohlooly‐Y, M., Glickman, J. N., & Garrett, W. S. (2013). The microbial metabolites, short‐chain fatty acids, regulate colonic Treg cell homeostasis. Science, 341, 569–573. 10.1126/science.1241165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stack, H. M., Kearney, N., Stanton, C., Fitzgerald, G. F., & Ross, R. P. (2010). Association of beta‐glucan endogenous production with increased stress tolerance of intestinal lactobacilli. Applied and Environmental Microbiology, 76, 500–507. 10.1128/AEM.01524-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan, J., McKenzie, C., Potamitis, M., Thorburn, A. N., Mackay, C. R., & Macia, L. (2014). The role of short‐chain fatty acids in health and disease. Advances in Immunology, 121, 91–119. 10.1016/B978-0-12-800100-4.00003-9 [DOI] [PubMed] [Google Scholar]
- Thangaraju, M., Cresci, G. A., Liu, K., Ananth, S., Gnanaprakasam, J. P., Browning, D. D., Mellinger, J. D., Smith, S. B., Digby, G. J., Lambert, N. A., Prasad, P. D., & Ganapathy, V. (2009). GPR109A is a G‐protein‐coupled receptor for the bacterial fermentation product butyrate and functions as a tumor suppressor in colon. Cancer Research, 69, 2826–2832. 10.1158/0008-5472.CAN-08-4466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolhurst, G., Heffron, H., Lam, Y. S., Parker, H. E., Habib, A. M., Diakogiannaki, E., Cameron, J., Grosse, J., Reimann, F., & Gribble, F. M. (2012). Short‐chain fatty acids stimulate glucagon‐like peptide‐1 secretion via the G‐protein‐coupled receptor FFAR2. Diabetes, 61, 364–371. 10.2337/db11-1019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venn, B. J., & Mann, J. (2004). Cereal grains, legumes and diabetes. European Journal of Clinical Nutrition, 58, 1143–1161. 10.1038/sj.ejcn.1601995 [DOI] [PubMed] [Google Scholar]
- Vila, F. M. (2017). Effects of dietary fiber on swine intestinal epithelial and immune response. Master's Thesis, University of Minnesota, Minnesota. [Google Scholar]
- Williams, B. A., Verstegen, M. W., & Tamminga, S. (2001). Fermentation in the large intestine of single‐stomached animals and its relationship to animal health. Nutrition Research Reviews, 14, 207–228. 10.1079/NRR200127 [DOI] [PubMed] [Google Scholar]
- Zhao, J., Bai, Y., Tao, S., Zhang, G., Wang, J., Liu, L., & Zhang, S. (2019). Fiber‐rich foods affected gut bacterial community and short‐chain fatty acids production in pig model. Journal of Functional Foods, 57, 266–274. 10.1016/j.jff.2019.04.009 [DOI] [Google Scholar]
- Zhao, J., Bai, Y., Zhang, G., Liu, L., & Lai, C. (2020). Relationship between dietary fiber fermentation and volatile fatty acids' concentration in growing pigs. Animals, 10, 263. 10.3390/ani10020263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao, J., Liu, P., Wu, Y., Guo, P., Liu, L., Ma, X., Levesque, C., Chen, Y., Zhao, J., Zhang, J., & Ma, X. (2018). Dietary fiber increases butyrate‐producing bacteria and improves the growth performance of weaned piglets. Journal of Agricultural and Food Chemistry, 66, 7995–8004. 10.1021/acs.jafc.8b02545 [DOI] [PubMed] [Google Scholar]
- Zhou, L. P., Fang, L. D., Sun, Y., & Zhu, W. (2016). Effects of a diet high in resistant starch on fermentation end‐products of protein and mucin secretion in the colons of pigs. Starch, 69, 7–8. 10.1056/nejmoa1014296 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All authors consent that raw data presented in this review are available after publication. Please contact author for data requests.


