Abstract
Dracocephalum moldavica L. is a significant component in the Iranian food basket. This study aimed to investigate the bioactive compounds and biological activities of different extracts obtained from D. moldavica aerial parts. From the aerial parts, a crude methanolic (MeOH) extract and its four sub‐fractions, that is, petroleum ether (Pet), ethyl acetate (EtOAc), n‐butanol (n‐BuOH), and aqueous (water) extracts were obtained. The total phenolic and flavonoid contents as well as the antioxidant and cytotoxic activities of the extracts were determined. Moreover, the phytochemical profiles of the essential oil (EO) and of those extracts with the highest antioxidant activity measured by GC/MS and UPLC–PDA‐ESI–QTOF–MS/MS. Results showed that the highest concentrations of phenols and flavonoids as well as the most potent antioxidant potential according to the DPPH method were determined in the EtOAc and MeOH extracts with IC50 values of 22.0 and 34.4 µg.ml‐1, respectively. Quantitative analysis of these extracts was subsequently performed by UPLC–PDA‐ESI–QTOF–MS/MS. Both extracts contained mainly rosmarinic acid, caffeic acid, and 2‐hydroxycinnamic acid, which may be responsible for their high antioxidant activity. Moreover, none of the extracts showed cytotoxic effects against MCF7, SW48, and a normal cell line of mouse embryonic fibroblast cells (NIH/3T3) in the tested concentrations (up to 400 μg.ml‐1). Additionally, GC‐MS analysis showed that oxygenated monoterpenes (55.4%) were the main constituents of the EO of D. moldavica.
Keywords: antioxidant activity, cytotoxic activity, Dracocephalum moldavica, essential oil, GC‐MS, UPLC‐MS
Dracocephalum moldavica is a significant component in the Iranian food basket. The total phenolic and flavonoid contents as well as antioxidant and cytotoxic activities of D. moldavica extracts were determined. In addition, the phytochemical profile of the essential oil (EO) using GC/MS and those extracts with the highest antioxidant activity by UPLC–DAD‐ESI–QTOF–MS/MS were measured.
1. INTRODUCTION
The daily intake of sufficient vegetables has an important role in preventing several diseases (Barends et al., 2019). D. moldavica (Moldavian balm) is a common edible vegetable used daily for the preparation of many Iranian dishes. It belongs to the Lamiaceae family, is up to 80 cm tall, and is native to central Asia (Yousefzadeh et al., 2018). D. moldavica preparations are used in food and in pharmaceutical industries as food additive, tea, and herbal remedy. Traditionally, the plant is applied as analgesic, anticonvulsive, anti‐inflammatory, sedative, wound healing, and in the treatment of cardiovascular disorders (Yousefzadeh et al., 2013). In the Mexican traditional medicine, it is used for the treatment of nervous diseases (Martinez‐Vazquez et al., 2012), while in traditional Chinese medicine (TCM), it is mainly used in the treatment of liver disorders, headache, stomach problems, and congestion (Jiang et al., 2014). Furthermore, in TCM in a clinical trial the aqueous extract of D. moldavica was shown to be effective in the treatment of cardiovascular disease, asthma, fatigue insomnia, and neurasthenia (N. Yu et al., 2015).
Phytochemical investigations on the aerial parts of D. moldavica have demonstrated the presence of several bioactive compounds, including terpenoids, phenolic compounds (rosmarinic and caffeic acid derivatives), flavonoids (kaempferol, quercetin, esculetin, diosmetin, acacetin, apigenin, luteolin, cirsimaritin, salvigenin, santa flavone, agastachoside, and their glycosides), alkaloids, iridoids, and coumarins (Sultan et al., 2008; Yang et al., 2014; Zeng et al., 2010). Phenolic compounds, especially phenolic acid derivatives, such as rosmarinic and caffeic acids, were associated with the high antioxidant potential of D. moldavica (Weremczuk‐Jeżyna et al., 2013). Various analytical methods are developed for the identification and quantification of bioactive compounds in medicinal plants. However, in these samples, there are some limitations, including the complexity, the structural diversity, and the low content of bioactive compounds (Adnani et al., 2012). In this regard, the choice of an appropriate technique is important. The application of UPLC‐ESI‐MS in the identification of natural compounds has attracted much attention because of its high resolution for the separation of complicated samples, analysis speed, sensitivity, selectivity, specificity, and reduced solvent consumption (Chen et al., 2010). As it is a significant component in the Iranian food basket, D. moldavica was selected for this study. To the best of our knowledge, there is no comprehensive study on this edible vegetable plant. Therefore, for the comprehensive identification and quantification of the chemical composition of D. moldavica, UPLC–DAD‐ESI–QTOF–MS/MS was used as a powerful tool for the separation of low molecular weight and nonvolatile samples, and GC/MS for the separation of volatile and thermally stable compounds. As biological activities, we evaluated the antioxidant and cytotoxic abilities of different plant extracts. Our study established a new approach to explore comprehensively the chemical components of D. moldavica extracts using UPLC–PDA‐ESI–QTOF–MS/MS. The obtained results broaden our knowledge about the structural diversity of the components in Moldavian balm for a better understanding of the possible role of the constituents on biological properties as well as for further research in food and pharmaceutical issues.
2. MATERIAL AND METHODS
2.1. Plant material
D. moldavica was purchased from a local market in Mashhad city (Khorasan Razavi province, Northeastern of Iran) in September 2017. The plant material was identified by M. Souzani (Department of Pharmacognosy, Mashhad University of Medical Sciences) and a voucher specimen (10,169) was deposited in the herbarium of the Department of Pharmacognosy, Mashhad University of Medical Sciences.
2.2. Preparation of the extracts
The aerial parts were washed with tap water and dried. For extraction of plant materials, all solvents were purchased from Dr. Mojallali Industrial Chemical Complex Co. 400 g dried material was powdered and macerated in methanol (analytical grade, 99.5%) for 24 hr (3 times, 1 L) at room temperature. The obtained extract was filtered using filter papers (Whatman® No.1, Merck) and the organic solvent concentrated under a vacuum. Then, the entire extract was suspended in water (50 ml) and partitioned with Pet (200 ml), EtOAc (200 ml), and n‐BuOH (200 ml), successively. Afterward, the solvents were evaporated under reduced pressure to get the different sub‐fractions. To prepare the EO, the aerial parts of the plant were subjected to hydrodistillation (Clevenger‐type apparatus, Pyrexfan Co) for 3 hr. The obtained EO was dried over anhydrous sodium sulfate (Merck) and stored in the dark until further testing.
2.3. Total phenolic content (TPC)
The TPC was measured colorimetrically with a standard Folin–Ciocalteu method (Slinkard & Singleton, 1977). The extract (20 μl) was mixed with 1,160 μl distilled water and 100 μl Folin‐Ciocalteu reagent (Merck). After 5 min, 300 μl sodium bicarbonate (20%, Merck) solution was added to the mixture and kept at room temperature for 2 hr. Absorbance was read at 760 nm using a Biotech Plate Reader (BioTek Instruments). A calibration curve (5–80 μg/ml) was built with gallic acid (Sigma‐Aldrich), and TPC expressed in mg gallic acid per gram dried extract (mg GAE g‐1).
2.4. Total flavonoid content
The TFC was determined by the aluminum chloride colorimetric method (Chang et al., 2002). After mixing 500 μl extract with 100 μl aluminum chloride (10%, Merck)), 1,500 μl ethanol (95%), 100 μl potassium acetate (1 M, Merck), and 2,800 μl distilled water, the mixture was kept at room temperature for 30 min and the absorbance measured at 415 nm. The results were expressed as mg quercetin (≥95%, Merck) equivalents per gram dried extract (mg QE g‐1).
2.5. Antioxidant activity
2.5.1. 2,2‐Diphenyl‐1‐picrylhydrazyl (DPPH) radical scavenging
The free radical scavenging activity of extracts was tested by a DPPH test (Mensor et al., 2001). Briefly, 100 μl of different extract concentrations (12.5–400 μg.ml‐1) was added to 100 μl freshly prepared 0.1 mM DPPH (Merck) solution in methanol. After 30 min of reaction at 37℃ in the darkness, the absorbance of the sample was measured at 518 nm. Ascorbic acid was applied as positive control. In this method, DPPH (100 μl) + methanol (100 μl) are used as blank. The antioxidant capacity was then calculated using the following Equation (1):
| (1) |
2.5.2. β‐carotene linoleic acid bleaching (BCB) assay
The BCB assay was conducted according to the standard method (Kulisic et al., 2004). In brief, β‐carotene (0.1 mg, ≥93%, Merck) was dissolved in 0.5 ml chloroform and mixed with 10 mg linoleic acid (≥99%, Merck) and 100 mg Tween‐40. Then, the chloroform was evaporated at 50℃, distilled water (25 ml) was added and the mixture sonicated for 1 min. An initial absorbance was recorded at 470 nm (time =0 min). Aliquots of the β‐carotene/linoleic acid solution (200 μl) were mixed with the prepared extracts (50 μl) and incubated at 50℃. The absorbance was measured at 470 nm after 120 min incubation. Antioxidant activity of the extracts was calculated by Equation (2):
| (2) |
where AA(0) and AA(120) are the absorbances of sample at times 0 and 120 min, while AC(0) and AC(120) are the absorbances of control after 0 and 120 min.
2.6. Cytotoxic activity
Human breast cancer cell line MCF7, colorectal cancer cell line SW48, and a normal cell line mouse embryonic fibroblast cells NIH 3T3 were provided by the National Cell Bank of Iran (Pasteur Institute). They were kept with 10% (v/v) fetal bovine serum (FBS) (Gibco), penicillin/streptomycin at 100 IU/ml and 2 mM L‐glutamine. Cultures were incubated with 5% CO2 in a humidified atmosphere at 37ºC. The cytotoxic effect of the prepared extracts was assessed using the AlamarBlue® (BioSource Invitrogen) proliferation assay. Briefly, cells were seeded in 96‐well plates at a density of 1 × 104. The cells were treated with different concentrations of extract (100 μl, 50–400 μg.ml‐1) after overnight growth. After 48 hr treatment, 20 μl AlamarBlue® reagent was added to each well. After 2 to 4 hr, the absorbance at 600 nm was measured on a Biotech Plate Reader (BioTek Instruments). Doxorubicin (0.1, 0.5 and 2 μg.ml‐1) was chosen as a positive control. IC50 values were calculated from Boltzmann sigmoidal concentration–response curve nonlinear regression fitting models (Lyles et al., 2008).
2.7. Chemical profiles and phytochemical content
2.7.1. Gas chromatography–mass spectrometry (GC‐MS)
The GC‐MS analyses were performed using a Agilent 5,975 apparatus with a HP‐5ms column (30 m × 0.25 mm i.d., 0.25 µm film thickness) interfaced with a quadruple mass detector and a computer equipped with Wiley 7n.l library. Instrumental conditions: oven temperature gradient: 50℃ during 5min, 50℃–250℃ at 3℃ /min and 250℃ during 10 min; injector temperature 250℃; injection volume, 1 µl; split ratio, 1:20; carrier gas, Helium at 1.0 ml/min; ionization potential, 70 eV; ionization current, 150 µA; ion source temperature, 280℃; mass range, 35–465 m/z. The constituents of the oils were identified by calculation of their retention indices under temperature programmed conditions for n‐alkanes (C8‐C23) and the oil on the HP‐5ms column (van Den Dool & Dec. Kratz, 1963). Identification of individual compounds was made by comparison of their mass spectra and retention indices (RI) with those of authentic samples and those given in the literature (Adams, 2007).
2.7.2. Ultra‐performance liquid chromatography coupled with a photo diode array detector and electrospray ionization quadrupole time‐of‐flight mass spectrometry (UPLC‐PAD/ESI‐QTOF/MS)
An Acquity Ultra‐Performance Liquid Chromatograph (UPLC, Waters) coupled to a photo diode array detector (PDA, Waters) and an electrospray ionization quadrupole time‐of‐flight tandem mass‐spectrometer (ESI–QTOF/MS; Waters) was used. Chromatographic separation was done using an Acquity UPLC column (UPLC® BEH C18, 100 mm × 2.1 mm, 1.7 μm, Waters). A binary mobile phase was used, mobile phase A (ultra‐pure water with 0.1% formic acid) and mobile phase B (acetonitrile with 0.1% formic acid). Formic acid and acetonitrile were UPLC‐MS grade from Actu, OSS, The Netherlands. A gradient separation was applied; 10% B, 0 min; 70% B, 30 min; 100% B, 33.33 min; 100% B, 38.33 min; 10% B, 41.67 min; 10% B, 50 min. The column temperature was maintained at 40℃, flow rate at 0.5 ml/min, wavelength range between 210 and 400 nm, and 10 µl sample was injected.
The ESI operating conditions for MS spectra acquisition in negative mode were as follows: capillary voltage, 2.6 kV; cone, 40 V; desolvation temperature 500℃; and source temperature, 150℃. The desolvation and cone gas flow rates were 0 and 1,000 L/h, respectively. Nitrogen (99.80% N28, Air Liquide, Auderghem, Belgium) was used for both desolvation and cone gas. Sample analysis was done independently in MSE acquisition (E is the collision energy) applying a full scan mode (50–1200 m/z range), in 1 s scan time. The precursor mass spectra acquisition was done in two continuous modes, a no collision energy mode, and a high collision energy (15–35 eV). Leucine enkephalin (Sigma‐Aldrich) was used as internal reference (LockSpray™) to calibrate the ESI source. The data were acquired by a MassLynx™ 4.1 software (Waters).
2.7.3. Sample preparation
Plant extract, 4 mg, was dissolved in 2.0 ml water/methanol (1:1; v/v) and then mixed for 10 min. Then, the sample was filtered using a membrane filter (0.20‐μm) prior to injection.
2.7.4. Identification and quantification of compounds
Compounds were identified and quantified in accordance to the retention times and mass spectral data (mass‐to‐charge (m/z), molecular peaks and their fragmentation) of the calibration standards. The analyte concentration was calculated using calibration curves of pure standards (Sigma‐Aldrich). Stock solution of each pure calibration standard (1 mg.ml‐1) was prepared in methanol, and dilutions were made at 6 levels (1, 5, 10, 25, 50, 100 μg.ml‐1) for the calibration curves. Results were expressed as μg.g‐1 pure extract. The quantification was done in duplicate.
3. RESULTS AND DISCUSSION
3.1. Essential oil composition
Seventy compounds, representing 99.6% of the EO of D. moldavica, were identified (Table 1). The main components were geranial (25.5%), estragole (16.0%), and geranyl acetate (15.2%). The majority of the compounds in the EO were oxygenated monoterpenes (55.4%). Golparvar et al., (2016) reported that D. moldavica EO collected from Kamu Mountain, Isfahan province, Iran, was dominated by geranyl acetate (36.62%), geraniol (24.3%), neral (16.2%), and geranial (11.2%). In a study by Yousefzadeha et al. (2018), geraniol, geranial, nerol, and geranyl acetate were the major constituents of the EO of D. moldavica collected from five habitats in the north‐west of Iran (Salmas, Urmia, Khoy, Maragheh, and Tabriz). Fallah et al., (2018) found that the major components of the EO of D. moldavica were geranyl acetate, neral, linalool acetate and geraniol. In another study (Fallah et al., 2018), geranial (29.0%–41.5%), geranyl acetate (24.7%–34.8%), and neral (21.9%–28.6%) were the main components of the EO of D. moldavica. Still different results were reported by some other researchers, who found that linalool (Hussein et al., 2006) and citral (Nikitina et al., 2008; Shuge et al., 2009) are the predominant components of D. moldavica EO. Such differences in EO composition are common and might be due to physiological variations as well as ecological and genetic factors, seasonal and climatic conditions, harvest period, and the distillation technique applied (Shakeri et al., 2019).
TABLE 1.
Volatile components in the EO of Deracocephalum moldavica
| No | Compound | RI1 | Percentage (%) |
|---|---|---|---|
| 1 | Benzaldehyde | 962 | t 2 |
| 2 | 1‐octen‐3‐ol | 982 | 0.1 |
| 3 | 6‐methyl‐5‐hepten‐2‐one | 988 | 0.4 |
| 4 | Myrcene | 992 | 0.1 |
| 5 | 2E,4E‐heptadienal | 1,011 | T |
| 6 | ρ‐cymene | 1,026 | T |
| 7 | Limonene | 1,030 | 0.1 |
| 8 | cis‐ocimene | 1,041 | 0.2 |
| 9 | Benzene acetaldehyde | 1,045 | 0.1 |
| 10 | trans‐ocimene | 1,052 | 0.1 |
| 11 | Bergamal | 1,058 | 0.1 |
| 12 | cis‐linalool oxide | 1,074 | 0.1 |
| 13 | Terpinolene | 1,089 | 0.1 |
| 14 | trans‐linalool oxide | 1,090 | 0.1 |
| 15 | Linalool | 1,101 | 1.3 |
| 16 | 1‐octen‐3‐yl acetate | 1,115 | 0.1 |
| 17 | Allo‐ocimene | 1,133 | 0.1 |
| 18 | trans‐chrysanthemal | 1,154 | 0.1 |
| 19 | Citronellal | 1,156 | 0.1 |
| 20 | Nerol oxide | 1,159 | 0.1 |
| 21 | Methyl chavicol (estragole) | 1,204 | 16.0 |
| 22 | 4‐methylene isophorone | 1,220 | 0.1 |
| 23 | Nerol | 1,232 | 0.3 |
| 24 | Neral | 1,254 | 9.7 |
| 25 | Geraniol | 1,258 | 0.5 |
| 26 | Geranial | 1,280 | 25.5 |
| 27 | Unknown | 1,302 | 0.2 |
| 28 | Geranyl formate | 1,306 | 0.4 |
| 29 | Neryl acetate | 1,365 | 1.2 |
| 30 | α‐copaene | 1,378 | 1.0 |
| 31 | Nerolic acid | 1,378 | 0.2 |
| 32 | β‐bourbonene | 1,389 | 0.3 |
| 33 | Geranyl acetate | 1,390 | 15.2 |
| 34 | Geranic acid | 1,406 | 0.2 |
| 35 | Methyl eugenol | 1,410 | 0.2 |
| 36 | β‐caryophyllene | 1,423 | 0.6 |
| 37 | Unknown | 1,430 | 0.1 |
| 38 | β‐copaene | 1,434 | T |
| 39 | Dihydro‐β‐ionone | 1,443 | T |
| 40 | Aromadendrene | 1,446 | T |
| 41 | α‐humulene | 1,457 | 0.2 |
| 42 | E‐β‐farnesene | 1,461 | T |
| 43 | α‐amorphene | 1,483 | 0.1 |
| 44 | Germacrene D | 1,486 | 0.3 |
| 45 | E‐β‐ionone | 1,490 | 0.5 |
| 46 | E,E‐α‐farnesene | 1,510 | 0.1 |
| 47 | γ‐cadinene | 1517 | 0.1 |
| 48 | δ‐cadinene | 1526 | 0.3 |
| 49 | β‐thujaplicinol | 1537 | 0.2 |
| 50 | α‐calacorene | 1547 | 0.2 |
| 51 | E‐ρ‐methoxy cinnamaldehyde | 1572 | 0.5 |
| 52 | Spathulenol | 1583 | 1.8 |
| 53 | Caryophyllene oxide | 1588 | 1.3 |
| 54 | n‐hexadecane | 1601 | T |
| 55 | Ledol | 1606 | 0.2 |
| 56 | 1,10‐di‐epi‐cubenol | 1622 | 1.1 |
| 57 | Epi‐α‐muurolol | 1648 | 0.2 |
| 58 | 3‐thujopsanone | 1655 | 0.2 |
| 59 | 2Z,6E‐farnesol | 1,730 | 0.5 |
| 60 | 2E,6E‐farnesol | 1748 | 0.6 |
| 61 | Tetradecanoic acid | 1,770 | 0.4 |
| 62 | Neophytadiene | 1842 | 0.6 |
| 63 | Hexahydrofarnesyl acetone | 1851 | 0.5 |
| 64 | Methyl hexadecanoate | 1925 | 0.1 |
| 65 | Isophytol | 1952 | 0.2 |
| 66 | Dibutyl phthalate | 1968 | 0.3 |
| 67 | n‐hexadecanoic acid | 1972 | 3.2 |
| 68 | Eicosane | 2001 | 0.1 |
| 69 | cis‐phytol | 2,122 | 9.7 |
| 70 | Ethyl linoleate | 2,161 | 1.7 |
| Major Compound Groups | |||
| Monoterpene hydrocarbon | 0.6 | ||
| Oxygenated monoterpene | 55.4 | ||
| Sesquiterpen hydrocarbon | 3.4 | ||
| Oxygenated sesquiterpene | 6.7 | ||
| Diterpenoide | 9.9 | ||
| Phenyl propanoides | 16.7 | ||
| Miscellaneous | 6.9 | ||
| Total Identified | 99.6 | ||
Major compounds are shown in bold.
RI: Retention Index on the HP‐5 MS column.
t: trace (<0.1%).
3.2. Total phenolic (TPC) and total flavonoid contents (TFC)
The total phenolic content (TPC) of extracts from D. moldavica is most commonly estimated by the Folin‐Ciocalteu method. In this analytical method, phenolic compounds are deprotonated and form phenolate ions that react with the Folin–Ciocalteu reagent (phosphomolybdate and phosphotungstate), resulting in a blue color, which absorbs visible light with a maximum around 765 nm (Vazquez et al., 2015), while the method for the determination of TFC is based on the formation of flavonoid–aluminum complexes with a maximum absorbance at 410–430 nm (Pękal, 2014). TPC and TFC of the extracts are presented in Figure 1a in aqueous and EtOAc extracts, respectively. The highest TPC was determined in EtOAc extract (96.8 ± 1.5 mg GAE g‐1), followed by the MeOH extract, 80.1 ± 2.3 mg GAE g‐1. The lowest TPC was measured in the aqueous extract, 68.8 ± 2.4 mg GAE g‐1. TFC was in the range from 23.9 ± 1.2 (in aqueous extract) to 79.3 ± 2.5 mg QE g‐1 (in EtOAc extract). In the literature, the antioxidant activity and TPC of a 70% aqueous MeOH extract of D. moldavica was evaluated by (Weremczuk‐jeżyna et al., 2017). The TPC of the aerial parts of D. moldavica was 110.1 mg GAE g‐1, which was higher than observed in our study. In another study, by Aprotosoaiea et al. (2016), the TPC of the aerial parts of D. moldavica was 289.55 ± 2.63 mg of GAE g‐1, which was also higher than found for the MeOH extract in our study. Furthermore, Dastmalchi et al., (2007) observed a higher TPC for the 80% MeOH extract of the aerial parts of Iranian D. moldavica (488.4 ± 1.8 mg/g), but lower amounts for the EtOAc extracts compared to our samples.
FIGURE 1.
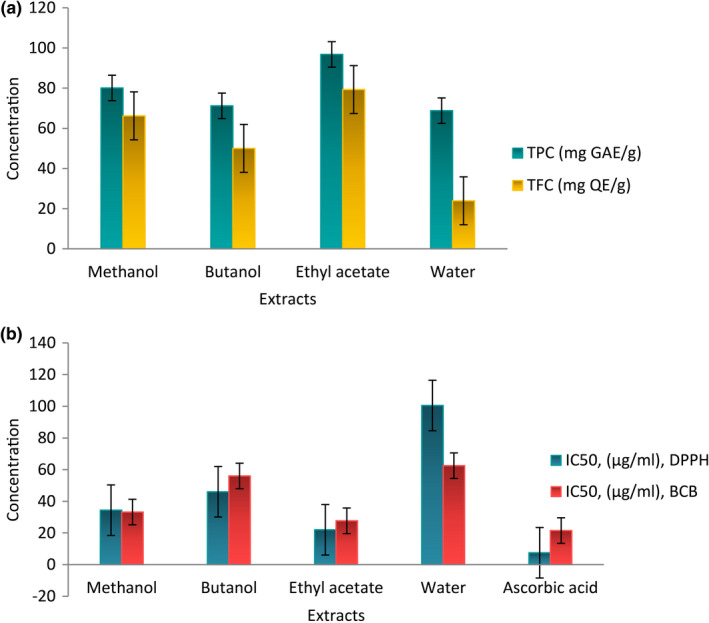
Total phenolic and total flavonoid contents (a) and antioxidant activities (b) of Deracocephalum moldavica extracts
3.3. Antioxidant activity and UPLC/ESI‐QTOF‐MS analysis
Among the extracts of D. moldavica, the EtOAc one exhibited the strongest scavenging activity with an IC50 value of 22.0 ± 2.1 µg.ml‐1 which is less active than ascorbic acid as positive control (IC50 = 7.5 ± 0.2 μg.ml‐1) (Figure 1b). Antioxidant activity was also found in the MeOH extract (IC50 = 34.4 ± 2.5 µg.ml‐1). The potent free radical scavenging activity of the MeOH extract of D. moldavica confirmed Dastmalchi et al., (2007), who revealed that the MeOH extract was a significantly better scavenger than quercetin. It is also in accordance with another study which reported scavenging effects of the MeOH extract of D. moldavica in the DPPH assay (EC50 = 23.10 ± 0.10 μg.ml‐1) (Aprotosoaie et al., 2016). In the BCB method, the EtOAc extract again exerted the strongest β‐carotene inhibition activity (94% inhibition, at 150 µg.ml‐1) followed by the MeOH (82%), n‐BuOH (75%), and aqueous (59%) extracts (Figure 1b). In the present study, UPLC/ESI‐QTOF‐MS was carried out on the extracts with the highest antioxidant activity to find the compounds potentially responsible for the antioxidant activity. The antioxidant activity of the MeOH and especially the EtOAc extracts of D. moldavica was in accordance with their amounts of phenolic acids. The UPLC/ESI‐QTOF‐MS analysis (Table 2) revealed that the MeOH extract of D. moldavica contains high amounts of phenolic acids, including rosmarinic acid (34,407 ± 694 µg.g‐1) and 2‐hydroxycinnamic acid (15,124 ± 2000 µg.g‐1), and of 4‐hydroxycoumarin (5,216 ± 95 µg.g‐1). In the literature, rosmarinic acid was also found to have the highest concentration in a MeOH extract of an Iranian D. moldavica (89,083 ± 1,380 μg.g‐1) (Dastmalchi et al., 2007). In our study, a much higher concentration of rosmarinic acid (75,508 ± 1,044 μg.g‐1) than in the MeOH extract was found in the EtOAc extract, followed by caffeic acid (69,678 ± 5,578 μg.g‐1), 3‐hydroxybenzoic acid (35,368 ± 2,803 μg.g‐1), and 2‐hydroxycinnamic acid (23,466 ± 2,122 μg.g‐1). It is evident from our results that the compounds most responsible for high antioxidant capacity of D. moldavica were phenolic acids such as rosmarinic acid, caffeic acid, hydroxycinnamic acids, and hydroxycinnamic acid. The antioxidant activity of rosmarinic acid, an ester of caffeic acid and 3,4‐dihydroxyphenyllactic acid, has already been demonstrated both in vitro and in vivo by many researchers (Adomako‐Bonsu et al., 2017; Nicolai et al., 2016; Tsai et al., 2019).
TABLE 2.
Phenolic compounds quantified in the evaluated extracts from Deracocephalum moldavica, presented as mean ±standard deviation (μg.g‐1)
| Compounds | MeOH extract | EtOAC extract | Molecular formula | Molecular weight (M) | HPLC ESI‐MS (m/z) | ||
|---|---|---|---|---|---|---|---|
| RT (min) | [M‐H]‐ | ||||||
| 1 | Malic acid | 255 ± 59 | 73.7 ± 46.6 | C4H6O5 | 134.087 | 0.94 | 133.014 |
| 2 | Quinic acid | 463 ± 29 | 72.3 ± 3.8 | C7H12O6 | 192.167 | 0.96 | 191.120 |
| 3 | Succinic acid | 4,527 ± 902 | 5,072 ± 131.2 | C4H6O4 | 118.088 | 1.21 | 117.018 |
| 4 | Citric acid | 5,101 ± 397 | 46.2 ± 3.8 | C6H8O7 | 192.123 | 1.22 | 191.102 |
| 5 | Pyrogallol | 2.4 ± 0.7 | 11.75 ± 0.2 | C6H6O3 | 126.111 | 1.24 | 125.024 |
| 6 | Gallic acid | 17 ± 1.2 | 79.4 ± 2.4 | C7H6O5 | 170.022 | 1.33 | 168.90 |
| 7 | Pyrocatechol | 6.7 ± 0.04 | 141 ± 8 | C6H6O2 | 110.112 | 1.95 | 109.028 |
| 8 | 3–4‐Hydroxybenzoic acid | 56.7 ± 0.15 | 1,151 ± 62.7 | C7H6O4 | 154.121 | 2.01 | 153.010 |
| 9 | Catechin | 0.76 ± 0.24 | 0.20 ± 0.03 | C15H14O6 | 290.271 | 2.21 | 289.064 |
| 10 | Chlorogenic acid | 1,359 ± 100 | 288 ± 16 | C16H18O9 | 354.311 | 2.37 | 353.202 |
| 11 | 4‐Hydroxybenzoic acid | 70 ± 5.2 | 2,867 ± 240 | C7H6O3 | 138.122 | 2.8 | 137.050 |
| 12 | 3‐Hydroxybenzoic acid | 535 ± 486 | 35,368 ± 2,803 | C7H6O3 | 138.122 | 2.83 | 137.025 |
| 13 | Esculetin | 31 ± 1.7 | 888.9 ± 0.52 | C9H6O4 | 178.143 | 3.03 | 177.018 |
| 14 | Vanillic acid | 97.8 ± 29 | 755.5 ± 29.65 | C8H8O4 | 168.148 | 3.13 | 167.036 |
| 15 | Syringic acid | 39 ± 1.9 | 107.4 ± 2.7 | C9H10O5 | 198.174 | 3.17 | 197.045 |
| 16 | Caffeic acid | 3,019 ± 44 | 69,678 ± 5,578 | C9H8O4 | 180.159 | 3.19 | 179.035 |
| 17 | Epicatechin | 0.33 ± 0.01 | 0.48 ± 0.28 | C15H14O6 | 290.271 | 3.84 | 289.064 |
| 18 | 4‐Hydroxycinnamic acid | 80 ± 3.6 | 1587 ± 80.8 | C9H8O3 | 164.160 | 4.54 | 163.042 |
| 19 | 3‐Hydroxycinnamic acid | 121 ± 10.6 | 2,146 ± 90 | C9H8O3 | 164.160 | 4.56 | 163.042 |
| 20 | Rutin | 668 ± 8.8 | 530 ± 43.3 | C27H30O16 | 610.153 | 4.71 | 609.1 |
| 21 | Sinapic acid | 0.96 ± 0.2 | 16.7 ± 1.10 | C11H12O5 | 224.212 | 4.88 | 223.061 |
| 22 | Ferulic acid | 10 ± 7.0 | 416 ± 0.80 | C10H10O4 | 194.186 | 5.05 | 193.050 |
| 23 | 2‐ Hydroxycinnamic acid | 15,124 ± 2000 | 23,466 ± 2,122 | C9H8O3 | 164.160 | 5.14 | 163.042 |
| 24 | Tannic acid | 4,069 ± 2,101 | 73.45 ± 11.50 | C76H52O46 | 1701.206 | 5.31 | 1,700.080 |
| 25 | Naringin | 965 ± 17.7 | 11 ± 2.4 | C27H32O14 | 580.539 | 5.84 | 579.173 |
| 26 | Benzoic acid | 662 ± 54.25 | 3,268 ± 20 | C7H6O2 | 122.123 | 5.97 | 121.031 |
| 27 | Quercitrin | 26 ± 8.4 | 321.4 ± 42 | C21H20O11 | 448.38 | 6.02 | 447.120 |
| 28 | Hesperidin | 1,030 ± 251 | 789.7 ± 513 | C28H34O15 | 610.565 | 6.24 | 609.172 |
| 29 | Rosmarinic acid | 34,407 ± 694 | 75,508 ± 1,044 | C18H16O8 | 360.318 | 6.94 | 359.054 |
| 30 | 4‐Hydroxycoumarin | 5,216 ± 95 | 7,215 ± 158 | C9H8O3 | 164.160 | 7.04 | 163.042 |
| 31 | Salicylic acid | 3.20 ± 0.10 | 20.92 ± 0.07 | C7H6O3 | 138.122 | 7.38 | 137.025 |
| 32 | Resveratrol acid | 1.3 ± 0.04 | 53.13 ± 3.3 | C14H12O3 | 228.247 | 8.24 | 227.072 |
| 33 | Luteolin | 5.6 ± 2.85 | 7.5 ± 0.2 | C15H10O6 | 286.239 | 8.87 | 285.040 |
| 34 | Quercitin | 1.5 ± 0.12 | 12.4 ± 1.2 | C15H10O7 | 302.238 | 9.11 | 301.000 |
| 35 | Naringenin | 33.4 ± 4.2 | 114.9 ± 0.6 | C15H12O5 | 272.256 | 10.77 | 271.061 |
| 36 | Hesperetin | 9.9 ± 0.36 | 19.9 ± 9.8 | C16H14O6 | 302.282 | 11.04 | 301.015 |
| 37 | Kaempferol | 38.7 ± 17.6 | 134 ± 4.5 | C15H10O6 | 286.239 | 11.12 | 285.040 |
Abbreviation: ND, not detected.
3.4. Cytotoxic activity
Extracts of D. moldavica in a concentration range from 50 to 400 μg.ml‐1 were assayed for their cytotoxic activity against two human cancer cell lines, SW‐48 and MCF‐7, and against a normal cell line, NIH/3T3. None of the extracts (50–400 μg.ml‐1) exhibited cytotoxic activity, suggesting potential safety of the plant. This is in accordance with a study by Yu et al., (2019) who did not found a significant cytotoxic effect of the EtOAc extract of D. moldavica (33.3% growth inhibition at 100 μg.ml‐1) against human epidermal keratinocyte (HaCaT) cells. To the best of our knowledge, there is no other published data on the cytotoxicity of D. moldavica extracts.
4. CONCLUSION
The antioxidant and cytotoxic activities of different extracts of D. moldavica, that is, EtOAc, MeOH, n‐BuOH and aqueous extracts, the total phenolic and flavonoid contents as well as the phytochemical profiles of the EO and the extracts were determined. The EtOAc and MeOH extracts were found to possess remarkable antioxidant activity in the DPPH and BCB assays. GC‐MS analysis showed that the majority of the compounds in the EO were oxygenated monoterpenes (55.4%). Further, UPLC–QTOF–MS analysis allowed identifying 37 metabolites, mainly pertaining to phenolic acids. Rosmarinic acid occurs in high amounts in the EtOAc and MeOH extracts of D. moldavica and may be responsible for most of the antioxidant activity. Our UPLC/PDA‐MS analysis focused on the quantification of some specific phenolic compounds. Thus, further studies are required to identify other compounds that may be present in significant amounts, but were not determined. None of the extracts, even at high concentrations (400 μg.ml‐1), showed considerable cytotoxicity, which suggests potential safety of the plant to be used as a natural preservative in food.
CONFLICT OF INTEREST
No conflict of interest was reported by the authors.
AUTHOR CONTRIBUTION
Azin Fattahi: Investigation (equal). Abolfazl Shakeri: Conceptualization (equal); Investigation (equal); Writing‐original draft (lead). Zahra Tayarani‐Najaran: Software (equal). Mourad Kharbach: Methodology (equal). Karen Segers: Methodology (equal). Yvan Vander Heyden: Methodology (equal). Seyedeh Faezeh Taghizadeh: Formal analysis (equal); Software (equal). Hanieh Rahmani: Investigation (equal). Javad Asili: Conceptualization (equal); Funding acquisition (equal).
ACKNOWLEDGMENTS
This research was financially supported by grants from the Mashhad University of Medical Sciences (Mashhad, Iran) Research Council [grant number 960960].
Fattahi A, Shakeri A, Tayarani‐Najaran Z, et al. UPLC–PDA‐ESI–QTOF–MS/MS and GC‐MS analysis of Iranian Dracocephalum moldavica L.. Food Sci Nutr. 2021;9:4278–4286. 10.1002/fsn3.2396
Azin Fattahi and Abolfazl Shakeri contributed equally to this work.
REFERENCES
- Adams, R. P. (2007). Identification of essential oil components by gas chromatography/mass spectrometry (Vol. 456). Allured Publishing Corporation. [Google Scholar]
- Adnani, N., Michel, C. R., & Bugni, T. S. (2012). Universal quantification of structurally diverse natural products using an evaporative light scattering detector. Journal of Natural Products, 75(4), 802–806. 10.1021/np300034c [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adomako‐Bonsu, A. G., Chan, S. L., Pratten, M., & Fry, J. R. (2017). Antioxidant activity of rosmarinic acid and its principal metabolites in chemical and cellular systems: Importance of physico‐chemical characteristics. Toxicology in Vitro, 40, 248–255. 10.1016/j.tiv.2017.01.016 [DOI] [PubMed] [Google Scholar]
- Aprotosoaie, A. C., Mihai, C. T., Vochita, G., Rotinberg, P., Trifan, A., Luca, S. V., & Miron, A. (2016). Antigenotoxic and antioxidant activities of a polyphenolic extract from European Dracocephalum moldavica L. Industrial Crops and Products, 79, 248–257. 10.1016/j.indcrop.2015.11.004 [DOI] [Google Scholar]
- Barends, C., Weenen, H., Warren, J., Hetherington, M. M., de Graaf, C. , & de Vries, J. H. M. (2019). A systematic review of practices to promote vegetable acceptance in the first three years of life. Appetite, 137, 174–197. 10.1016/j.appet.2019.02.003 [DOI] [PubMed] [Google Scholar]
- Chang, C.‐C., Yang, M.‐H., Wen, H.‐M., & Chern, J.‐C. (2002). Estimation of total flavonoid content in propolis by two complementary colorimetric methods. Journal of Food and Drug Analysis, 10(3), 178–182. [Google Scholar]
- Chen, Z., Wu, J. B., Liao, X. J., Yang, W., & Song, K. (2010). Development and validation of an UPLC‐DAD‐MS method for the determination of leonurine in Chinese motherwort (Leonurus japonicus). Journal of Chromatographic Science, 48(10), 802–806. 10.1093/chromsci/48.10.802 [DOI] [PubMed] [Google Scholar]
- Dastmalchi, K., Damien Dorman, H. J., Laakso, I., & Hiltunen, R. (2007). Chemical composition and antioxidative activity of Moldavian balm (Dracocephalum moldavica L.) extracts. LWT ‐ Food Science and Technology, 40(9), 1655–1663. 10.1016/j.lwt.2006.11.013 [DOI] [Google Scholar]
- Fallah, S., Rostaei, M., Lorigooini, Z., & Abbasi Surki, A. (2018). Chemical compositions of essential oil and antioxidant activity of dragonhead (Dracocephalum moldavica) in sole crop and dragonhead‐ soybean (Glycine max) intercropping system under organic manure and chemical fertilizers. Industrial Crops and Products, 115, 158–165. 10.1016/j.indcrop.2018.02.003 [DOI] [Google Scholar]
- Golparvar, A. R., Hadipanah, A., Gheisari, M. M., & Khaliliazar, R. (2016). Chemical constituents of essential oil of Dracocephalum moldavica L. and Dracocephalum kotschyi Boiss. from Iran. Acta Agriculturae Slovenica, 107(1), 25–31. [Google Scholar]
- Hussein, M. S., El‐Sherbeny, S. E., Khalil, M. Y., Naguib, N. Y., & Aly, S. M. (2006). Growth characters and chemical constituents of Dracocephalum moldavica L. plants in relation to compost fertilizer and planting distance. Scientia Horticulturae, 108(3), 322–331. 10.1016/j.scienta.2006.01.035 [DOI] [Google Scholar]
- Jiang, J., Yuan, X., Wang, T., Chen, H., Zhao, H., Yan, X., Wang, Z., Sun, X., & Zheng, Q. (2014). Antioxidative and cardioprotective effects of total flavonoids extracted from Dracocephalum moldavica L. against acute ischemia/reperfusion‐induced myocardial injury in isolated rat heart. Cardiovascular Toxicology, 14(1), 74–82. 10.1007/s12012-013-9221-3 [DOI] [PubMed] [Google Scholar]
- Kulisic, T., Radonic, A., Katalinic, V., & Milos, M. (2004). Use of different methods for testing antioxidative activity of oregano essential oil. Food Chemistry, 85(4), 633–640. 10.1016/j.foodchem.2003.07.024 [DOI] [Google Scholar]
- Lyles, R. H., Poindexter, C., Evans, A., Brown, M., & Cooper, C. R. (2008). Nonlinear model‐based estimates of IC(50) for studies involving continuous therapeutic dose‐response data. Contemporary Clinical Trials, 29(6), 878–886. 10.1016/j.cct.2008.05.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez‐Vazquez, M., Estrada‐Reyes, R., Martinez‐Laurrabaquio, A., Lopez‐Rubalcava, C., & Heinze, G. (2012). Neuropharmacological study of Dracocephalum moldavica L. (Lamiaceae) in mice: Sedative effect and chemical analysis of an aqueous extract. Journal of Ethnopharmacology, 141(3), 908–917. 10.1016/j.jep.2012.03.028 [DOI] [PubMed] [Google Scholar]
- Mensor, L. L., Menezes, F. S., Leitão, G. G., Reis, A. S., Santos, T. C., Coube, C. S., & Leitão, S. G. (2001). Screening of Brazilian plant extracts for antioxidant activity by the use of DPPH free radical method. Phytotherapy Research, 15(2), 127–130. 10.1002/ptr.687 [DOI] [PubMed] [Google Scholar]
- Nicolai, M., Pereira, P., Vitor, R. F., Reis, C. P., Roberto, A., & Rijo, P. (2016). Antioxidant activity and rosmarinic acid content of ultrasound‐assisted ethanolic extracts of medicinal plants. Measurement, 89, 328–332. 10.1016/j.measurement.2016.04.033 [DOI] [Google Scholar]
- Nikitina, A. S., Popova, O. I., Ushakova, L. S., Chumakova, V. V., & Ivanova, L. I. (2008). Studies of the essential oil of Dracocephalum moldavica cultivated in the Stavropol region. Pharmaceutical Chemistry Journal, 42(4), 203–207. 10.1007/s11094-008-0092-z [DOI] [Google Scholar]
- Pękal, A. (2014). Evaluation of aluminium complexation reaction for flavonoid content assay. Food Analytical Methods, 7, 1776–1782. 10.1007/s12161-014-9814-x [DOI] [Google Scholar]
- Shakeri, A., D’Urso, G., Taghizadeh, S. F., Piacente, S., Norouzi, S., Soheili, V., Asili, J., & Salarbashi, D. (2019). LC‐ESI/LTQOrbitrap/MS/MS and GC–MS profiling of Stachys parviflora L. and evaluation of its biological activities. Journal of Pharmaceutical and Biomedical Analysis, 168, 209–216. 10.1016/j.jpba.2019.02.018 [DOI] [PubMed] [Google Scholar]
- Shuge, T., Xiaoying, Z., Fan, Z., Dongqing, A., & Tao, Y. (2009). Essential oil composition of the Dracocephalum moldavica L from Xinjiang in China. Pharmacognosy Research, 1(4), 172–174. [Google Scholar]
- Slinkard, K., & Singleton, V. L. (1977). Total phenol analysis: automation and comparison with manual methods. American Journal of Enology and Viticulture, 28(1), 49–55. [Google Scholar]
- Sultan, A., Aisa, H., & Eshbakova, K. (2008). Flavonoids from Dracocephalum moldavica . Chemistry of Natural Compounds, 44(3), 366–367. 10.1007/s10600-008-9065-4 [DOI] [Google Scholar]
- Tsai, C.‐F., Wu, J.‐Y., & Hsu, Y.‐W. (2019). Protective effects of rosmarinic acid against selenite‐induced cataract and oxidative damage in rats. International Journal of Medical Sciences, 16(5), 729–740. 10.7150/ijms.32222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Den Dool, H., & Dec. Kratz, P. (1963). A generalization of the retention index system including linear temperature programmed gas—liquid partition chromatography. Journal of Chromatography A, 11, 463–471. 10.1016/S0021-9673(01)80947-X [DOI] [PubMed] [Google Scholar]
- Vázquez, C. V., Rojas, M. G. V., Ramírez, C. A., Chávez‐Servín, J. L., García‐Gasca, T., Ferriz Martínez, R. A., García, O. P., Rosado, J. L., López‐Sabater, C. M., Castellote, A. I., Montemayor, H. M. A., & de la Torre Carbot, K. (2015). Total phenolic compounds in milk from different species. Design of an extraction technique for quantification using the Folin‐Ciocalteu method. Food Chemistry, 176, 480–486. 10.1016/j.foodchem.2014.12.050 [DOI] [PubMed] [Google Scholar]
- Weremczuk‐jeżyna, I., Grzegorczykkarolak, I., Frydrych, B., Hnatuszko‐konka, K., Gerszberg, A., & Wysokińska, H. (2017). Rosmarinic Acid Accumulation and Antioxidant Potential of Dracocephalum moldavica L. Cell Suspension Culture. Notulae Botanicae Horti Agrobotanici Cluj‐Napoca, 45(1), 215–219. 10.15835/nbha45110728 [DOI] [Google Scholar]
- Weremczuk‐Jeżyna, I., Grzegorczyk‐Karolak, I., Frydrych, B., Królicka, A., & Wysokińska, H. (2013). Hairy roots of Dracocephalum moldavica: Rosmarinic acid content and antioxidant potential. Acta Physiologiae Plantarum, 35(7), 2095–2103. 10.1007/s11738-013-1244-7 [DOI] [Google Scholar]
- Yang, L.‐N., Xing, J.‐G., He, C.‐H., & Wu, T. (2014). The phenolic compounds from Dracocephalum moldavica L. Biochemical Systematics and Ecology, 54, 19–22. 10.1016/j.bse.2013.12.009 [DOI] [Google Scholar]
- Yousefzadeh, S., Daryai, F., Mokhtassi‐Bidgoli, A., Hazrati, S., Yousefzadeh, T., & Mohammadi, K. (2018). Morphological, essential oil and biochemical variation of Dracocephalum moldavica L. populations. Journal of Applied Research on Medicinal and Aromatic Plants, 10, 59–66. 10.1016/j.jarmap.2018.06.005 [DOI] [Google Scholar]
- Yousefzadeh, S., Modarres‐Sanavy, S. A. M., Sefidkon, F., Asgarzadeh, A., Ghalavand, A., & Sadat‐Asilan, K. (2013). Effects of Azocompost and urea on the herbage yield and contents and compositions of essential oils from two genotypes of dragonhead (Dracocephalum moldavica L.) in two regions of Iran. Food Chemistry, 138(2), 1407–1413. 10.1016/j.foodchem.2012.11.070 [DOI] [PubMed] [Google Scholar]
- Yu, H., Liu, M., Liu, Y., Qin, L., Jin, M., & Wang, Z. (2019). Antimicrobial Activity and Mechanism of Action of Dracocephalum moldavica L. Extracts Against Clinical Isolates of Staphylococcus aureus . Frontiers in Microbiology, 10(1249), 1–10. 10.3389/fmicb.2019.01249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu, N., He, C., Awuti, G., Zeng, C., Xing, J., & Huang, W. (2015). Simultaneous determination of six active compounds in Yixin Badiranjibuya Granules, a Traditional Chinese Medicine, by RP‐HPLC‐UV Method. Journal of Analytical Methods in Chemistry, 2015, 974039–974047. 10.1155/2015/974039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng, Q., Jin, H. Z., Qin, J. J., Fu, J. J., Hu, X. J., Liu, J. H., Yan, L., Chen, M., & Zhang, W. D. (2010). Chemical constituents of plants from the genus Dracocephalum . Chemistry & Biodiversity, 7(8), 1911–1929. 10.1002/cbdv.200900188 [DOI] [PubMed] [Google Scholar]


