Abstract
Microbially produced gamma poly glutamic acid (γ–PGA) is a commercially important biopolymer with many applications in foods and various other substances and are abundantly used in different parts of the world. With an aim to study the potent γ–PGA producing Bacillus species, a total of 47 different samples (Kinema, soil, and water) were randomly collected from different locations across the country, and Bacillus sp. were selectively isolated, screened, and characterized by performing physiological, biochemical, morphological, and 16S rRNA gene sequencing. The microbial production of γ–PGA was assayed with the selected isolates on the PGA medium and the metabolite obtained was recovered by ethanol precipitation method and further characterized by thin-layer chromatography (TLC). Thermotolerance (25–60 °C), pH tolerance (4–9), and NaCl tolerance (1–9%) tests were performed to optimize the bacterial growth and γ–PGA production and its viscosity were measured by Ostwald's viscometer. Out of 145 randomly selected colonies, 63 isolates were Gram-positive, rods, and endospore producers and were presumptively confirmed as genus Bacillus. Higher growth of γ–PGA producers were reported in 22 isolates and was found at optimum conditions such as temperature (30–37 °C), pH (6.5–7), incubation time (3 days), and NaCl concentration (3%) and γ–PGA thus produced was further verified by TLC with the retention factor (RF) value 0.27. The potent isolates were closely similar to Bacillus subtilis subsp. stercoris, Bacillus cereus, Bacillus paranthracis, and Bacillus licheniformis etc. Based on the findings of the study, B. licheniformis is the most potent γ–PGA producing Bacillus sp. which can further be used for the commercial production of γ–PGA. To the best of our knowledge, there is yet no published research from Nepal showing the production of the γ–PGA although microbially produced γ–PGA are the major constituents in some popular foods in particular communities of the country.
Keywords: γ–PGA, Bacillus species, Optimization, 16S rRNA gene
γ–PGA, Bacillus species, Optimization, 16S rRNA gene.
1. Introduction
Polyglutamic acid (PGA), a poly amino acid is a naturally presenting anionic, biodegradable, water-soluble, non-toxic, gluey, consumable biopolymer comprising the residues of D and L-glutamic acids with a wide range of commercial applications [1, 2, 3]. Gamma poly glutamic acid (γ–PGA), a type of PGA is a naturally occurring poly (amino acid) polymerized by γ–amide linkages that consist of numerous free carboxyl groups in the principal chain of γ–PGA synthesized by some strains of Bacillus [4]. γ–PGA, the biodegradable and non-toxic substance with superior absorbency and moisture retention, has been synthesized for a variety of industrial uses, including flocculants, hydrogels, cosmetics, thickeners, medication delivery, dispersants and feed additives [5]. Bacillus sp. is one of the most investigated microbial groups because they can produce varieties of biotechnological applicable substances such as γ–PGA, amylase, protease, etc. [6, 7, 8, 9].
Ivanovics and co-workers first discovered γ–PGA [10, 11] by releasing Bacillus anthracis into the medium upon autoclaving or maturing and autolysis of the cells. There are various sources for producing γ–PGA such as mucilage “natto” (fermented soybean, a type of traditional cuisine in Japan), which comprises a blend of γ–PGA and fructan synthesized by Bacillus subtilis var sawamura [12, 13]. Previously, it was reported that Fusobacterium nucleatum–a Gram-negative bacterium, some archaea and eukaryotes have been reported to synthesize γ–PGA [14, 15, 16]. However, for commercial production of γ–PGA, the chiefly used strains included Bacillus licheniformis and Bacillus subtilis [17, 18]. In many Asian countries, Bacillus sp. ferment soybean products naturally, producing γ–PGA, which gives the product its characteristic sticky texture [19] such as Natto of Japan [20, 21], Tungrymbai and Bekang of India [22] and Kinema of eastern Nepal and southern parts of Bhutan, Kalimpong, Darjeeling, and Sikkim in India [23]. Kinema is a naturally fermented soybean product with gluey, mild ammoniacal flavor and non-salted nature consumed in Nepal among Rai, Limbu, Sherpa, and Gurung communities. The synthesis of γ–PGA by Bacillus sp. is likely to be the cause of relative viscosity and stickiness in Kinema [24].
The formation of γ–PGA in submerged fermentation (SMF) and solid-state fermentation (SSF) has been researched by a number of researchers [2], and they also explored the nutritional needs for bacteria synthesizing γ–PGA to improve productivity and discovered that these properties differed depending on the strains employed. Based on nutrient requirements, γ–PGA producing bacteria are divided into two groups: glutamic acid-dependent bacteria and glutamic acid-independent bacteria. Several other parameters, in addition to glutamic acid, influenced the productivity and quality of γ–PGA, including carbon and nitrogen supplies, ionic strength, aeration, agitation, and medium pH [2]. The γ–PGA production is significantly influenced by the activity of the existing gene of Bacillus sp. The γ–PGA synthesizing genes such as pgsB, pgsC, pgsA, and pgsE were first detected in B. subtilis and B. anthracis [25, 26, 27]. The ratio of γ–PGA molecular weight and D-/L-glutamic acid depend on species and growth conditions of microorganisms. B. licheniformis, for example, produces several forms of γ-(D,L)-PGA, with D-glutamate concentrations varying from 10% to 100% [28, 29] whereas B. anthracis mainly produces D-glutamic acid-type γ–PGA (γ-(D)-PGA) [30].
γ–PGA and its derivatives have broad applications and they are widely used in the food industry as a food supplement for the promotion of adsorption of bioavailable minerals such as Ca2+ [31], in medicine as a drug carrier [32], tissue engineering [33], bioremediation [34] and other purposes such as moisturizer [35], biocontrol agent [36], biodegradable plastic [3], antibacterial agent [9] and many more. Various substrates such as cane molasses, glucose, etc. can be used for synthesizing γ–PGA. For the synthesis of γ–PGA, it is possible to use cheaper substrates such as cane molasses instead of glucose [37]. In Nepal, cane molasses is abundantly available, which could pave the way for the commercial production of γ–PGA. Furthermore, residual monosodium glutamate can be found in the waste liquor of the glutamic acid during the fermentation, which might also be used to produce more γ–PGA.
This is the first research in Nepal exploring the γ–PGA production using Bacillus sp. γ–PGA can be used in Nepal to solve different problems such as treatment of wastewater as well as for developing biodegradable plastic to reduce pollution, making dippers as well as food additives. These could be the milestones in the innovation and commercial production of γ–PGA which ultimately might contribute to the country's economy. Keeping these things at the central focus, the study targeted to screen and identify potent γ–PGA producing Bacillus sp. from Nepalese soybean fermented food (Kinema), soil and water.
2. Material and methods
2.1. Collection and processing of samples
Samples were obtained from different locations in Nepal from November 2018 to April 2019. Altogether 47 samples (7 Kinema, 20 soil and 20 water samples) were collected in zip-lock sterile plastic bags and transported to the Food Microbiology Laboratory of National College, Khusibu, Kathmandu, and stored at room temperature. The selective isolation of Bacillus sp. was performed according to the protocols followed by Sapkota et al. [7]. The sample (10 g) was mixed with sterile water (90 mL) and heated in a water bath at 80 °C for about 10 min with constant stirring. Later, it was serially diluted and spread plating was performed on nutrient agar (NA) and incubated at 37 °C for 24 h.
2.2. Isolation and screening of bacteria
The typically Bacillus like bigger colonies was randomly selected, isolated, and further sub-cultured in NA (Hi-Media Pvt. Ltd, Bombay, India), and was preserved in 50% (v/v) glycerol stock solution at –20 °C for further processing. The culture was grown on Luria-Bertani (LB) agar at 37 °C for 24 h according to Tork et al. [38]. Only mucoid colonies were selected as PGA producers [38].
2.3. Synthesis of PGA
Mucoid colonies were incubated for 24 h at 37 °C in a conical flask comprising 100 mL of PGA medium (Hi-Media Pvt. Ltd, Bombay, India) consisting of C₅H₈NO₄Na (2%), C6H12O6 (2%), (NH4)2SO4 (1%), Na2HPO4 (0.1%), KH2PO4 (0.1%), MgSO4.7H2O (0.05%), Mn(Cl2).4H2O (0.002%), and FeCl3.7H2O (0.005%) [39]. After incubation, the culture was centrifuged for 20 min at 10,000 rpm to extract insoluble material from the supernatant. To obtain fibrous precipitate–presumably the γ–PGA, an equal volume of ethanol was added to the supernatant.
2.4. Morphological, biochemical, physiological, and molecular characterization of isolates
The morphological, physiochemical, and biochemical characterization of isolates were performed based on methods proposed by Manual Methods for Common Bacteriology [40]. Colonies obtained were subjected to Gram's staining and relevant biochemical tests (catalase, oxidase, Voges–Proskauer, gelatin hydrolysis, casein hydrolysis, citrate utilization, and sugars fermentation) for confirmation of Bacillus sp. [8]. For the extraction of genomic DNA from the isolates, the cells were boiled for 15 min before being centrifuged at 10,000 rpm for 20 min at 4 °C. Polymerase chain reaction (PCR) was performed with the cell-free supernatant, and the obtained products were purified using the QIA quick PCR Purification Kit (Qiagen) following the manufacturer's guidelines. DNA denaturation (30 cycles) at 98 °C for 10 seconds, primer annealing at 55 °C for 5 s, and elongation at 72 °C for 1 min were performed during the PCR. Amplification of the 16S rRNA gene was performed using the following universal primer sets: 8F (5′-AGAGTTTGATCCCTCAG-3′) and 1492R (5′-GGTTACCTTGTTACGACTT-3′) [41, 42, 43]. By loading 7 μL of each PCR product with 2 μL of loading dye into the wells of 0.8% agarose gel containing 2 μL/mL EtBr (ethidium bromide), the amplified DNA fragments were separated using gel electrophoresis. The size of DNA fragments was calculated using DNA markers as the visual reference. The bands were visualized using a gel documentation system. For the 16S rRNA gene sequencing, amplified DNA was sent to Macrogen (Korea). The raw sequence was analyzed by Bio Lign 4.03. The MEGA version X program was used to build the phylogenetic tree, which included neighbor-joining, maximum-parsimony, and maximum-likelihood approaches [44, 45], with bootstrap values computed from 1000 replications.
2.5. Effect of incubation temperature and time on growth condition
The growth conditions for each isolate were optimized with variation in pH, temperature, NaCl, and incubation period. At the end of the incubation period, bacterial growth was observed under a UV spectrophotometer at 610 nm. The viscosity of fermented broth was measured using Ostwald's viscometer [38]. The effect of temperature on bacterial growth was optimized at various temperatures (25–60 °C) for up to 5 days. Likewise, the effect of incubation time on bacterial growth was optimized at various time intervals (24–96 h) up to 5 days. The growth of isolates and relative viscosity was measured every 24 h using a spectrophotometer.
2.6. Effect of pH and NaCl concentrations on bacterial growth
The effect of pH on bacterial growth was optimized at a pH range of 4–9 up to 5 days. Likewise, the effect of NaCl concentration on bacterial growth was optimized at various concentrations of salt (1–9%) for up to 5 days. The growth of isolates and relative viscosity (ʎ = 610 nm) was measured every 24 h using a spectrophotometer.
2.7. Measurement of viscosity
Following bacterial cells removal, the relative viscosity of the culture filtrate was determined with a conventional Ostwald viscometer at 30 °C [38]. Estimation of flow time was carried out, and the relative viscosity–the ratio of polymer viscosity to solvent viscosity was calculated using the formula as given below: [38, 46]
where μr denotes relative viscosity, μL is the viscosity of de-ionized water, and μ is the viscosity of the sample [38].
Where ts is the sample's falling time (sec.) at 30 °C and to (sec.) is the falling time of de-ionized water at similar conditions.
2.8. Amino acid analysis
The amino acid analysis was done by the TLC (thin-layer chromatography) according to the protocol developed by Song et al. [47] with some modifications. For γ–PGA analysis, obtained γ–PGA was hydrolyzed with 6N HCl at 100 °C for 60 min based on the quantity of γ–PGA in an airtight tube followed by evaporation to eliminate of residual HCL, and then the hydrolyzed product was dissolved in 1 mL distilled water and the 5 μL of this solution was loaded onto a TLC plate. TLC was conducted on a silica plate with solvent systems of butanol–acetic acid-water (4:1:3, w/w) or ethanol-water (7:3 v/v). Detection of amino acids was accomplished by spraying the samples with 0.2% ninhydrin. The purity of γ–PGA is confirmed by the presence of glutamic acid.
2.9. Data analysis
Analysis of data as well as plots’ construction was done using the ggplot2 (grammar of graphics, version 3.3.2) package of R-programming Language (version 1.2.5033) (https://cran.r-project.org/). The assigned Genbank accession number for the isolates B2-9, CO4-1, D2-5, and KK7-12 are LC537275, LC537276, LC537277, and LC537278 respectively.
3. Results and discussion
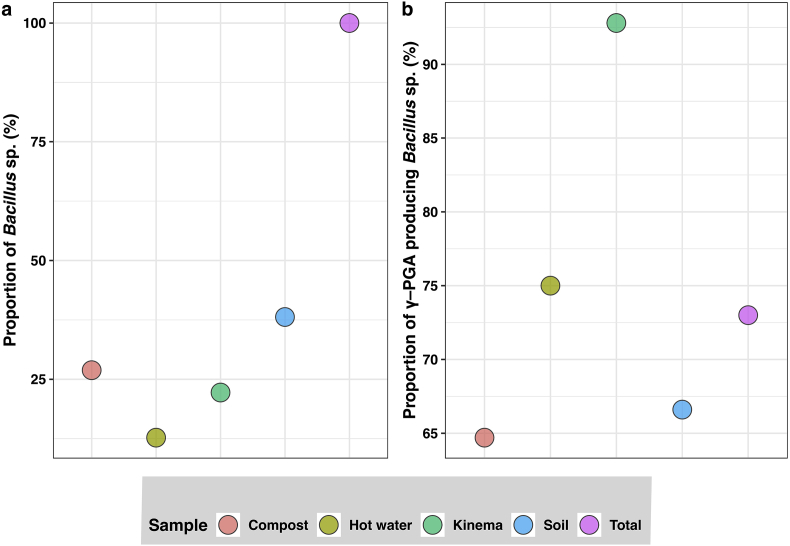
Colonies exhibiting the typical characteristics of the Bacillus sp. (creamy white color, soft consistency, mucoid appearance, convex and flat elevation, and irregular shape) were selected for further studies [7, 8, 48]. After performing Gram's staining of all 145 isolates from different locations of Nepal, 63 isolates were found to be Gram-positive, rods, spore-forming whereas the remaining 82 bacteria in total were Gram-negative and non-spore forming (Table 1, Figure 1c and d). The γ–PGA production was shown in Figure 1a and b. The total numbers of Bacillus sp. isolated from different samples are Kinema (14), soil (24), hot water (8), compost (17) (Figure 2). Although lower proportions of Bacillus sp. (22.2% (14/63)) were isolated from the Kinema sample, the higher proportions of γ–PGA producers (92.8% (13/14)) were reported indicating that γ–PGA producing Bacillus were the dominantly present in Kinema. This finding is in tune with Chettri et al. [39] who reported that the γ–PGA producing Bacillus sp. were predominantly present in Kinema. Similarly, B. licheniformis, B. subtilis, B. circulans, B. thuringiensis and B. cereus were isolated from the Kinema samples in previous studies [49, 50, 51]. Likewise, the soil is also the common habitat for Bacillus sp. In the current study, 38.1% (24/63) Bacillus sp. was isolated from the soil amongst which 66.6% (16/24) Bacillus sp. are γ–PGA producers. Sy et al. [52] also reported the potent γ–PGA producing Bacillus sp. from the soil. Similarly, in the present study, the proportion of Bacillus sp. isolated from hot water is 12.7% (8/63) with 75% (6/8) γ–PGA producers, compost is 26.9% (17/63) with 64.7% (11/17) γ–PGA producers. Previous studies also reported the γ–PGA producing Bacillus sp. from hot water samples [53]. However, there are very few studies on the isolation of γ–PGA producing Bacillus sp. from compost samples. All the findings indicate that Bacillus sp. capable of producing γ–PGA is abundantly distributed in a different environment.
Table 1.
Sample description and gram-positive isolated strain.
| Location | Sample code | Sample type (n) | Isolated strain |
|---|---|---|---|
| Tatopani | T | Water (n = 2) | 2 |
| Bhotekoshi | B | Water (n = 1) | 3 |
| Dharan | KD | Kinema (n = 1) | 2 |
| Kathmandu | KK | Kinema (n = 1) | 2 |
| Solukhumbu | KS | Kinema (n = 1) | 5 |
| Kathmandu | CM | Soil, Compost (n = 10) | 3 |
| Syangja | SY, G, WK | Soil, water (n = 5) | 5 |
| Butwal | BUT | Soil, water (n = 3) | 5 |
| Pokhara | PKr | Soil, water (n = 2) | 9 |
| Chitwan | CO | Soil, water (n = 7) | 4 |
| Kathmandu | DS, D, JD | Kinema (n = 4) | 9 |
| Nawalparasi | N | Soil (n = 4) | 5 |
| Bharatpur |
Bhw |
Soil (n = 6) |
9 |
| Total isolates | n = 47 | 63 |
Figure 1.
γ-PGA biopolymer production on PGA medium (note: isolates indication from left to right B2-10, CO4-1, DS4-1, D2- 5, B2-9, CM6-1, S6- 3, KK7-12, T2-3 respectively) (a); Clumping of insoluble material presumably γ-PGA biopolymer produced by isolate strain S6-3 after addition of ethanol into PGA medium (b); Bacillus licheniformis (KK7-12) on NA plate after incubation for 24 h at 37 °C (c); Gram staining of Bacillus licheniformis (KK7-12) (Gram-positive, rod) (d).
Figure 2.
Proportion of Bacillus sp. isolated from different samples (a); the proportion of γ–PGA producing Bacillus sp. isolated from different samples (b).
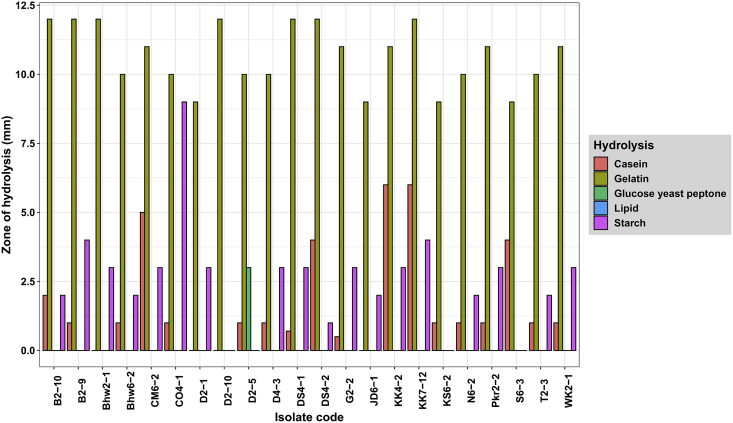
All isolates were catalase positive while the oxidase test gave variable results. It can be noted that 22 isolates could produce higher γ–PGA and all isolates were found to be endospore former except three isolates B2-9, N6-2, and WK2-1 were found to be non-spore former. Figure 3 demonstrates the zone of the starch, gelatin, and casein hydrolysis ranging from 1-9 mm, 9–12 mm, and 0.5–6 mm respectively. After 24 h incubation at 37 °C, all 22 isolates had the ability of hydrolysis, but all isolates did not show lipid hydrolysis and GYP hydrolysis except one isolate D2-5 showed GYP hydrolysis. This showed that Bacillus sp. produced γ–PGA along with the amylase and protease enzyme. The study showed that some bacterial isolates had a high zone of starch hydrolysis (9 mm diameter), which could be used in amylase industries, while some showed a high zone of casein hydrolysis (6 mm diameter), which also could be used in dairy industries as milk clotting enzyme. Additionally, different sugar utilization test was also conducted for the identification of Bacillus (Table 2). All isolates were capable of utilizing glucose and sucrose amongst which only two isolates were gas producers. Likewise, only 3 isolates were found to be lactose fermenters with only one of them producing gas. Sapkota et al. [7] reported that Bacillus sp. are capable of fermenting various sugars such as glucose, lactose, sucrose, etc., which is consistent with the findings of this study.
Figure 3.
Hydrolysis of different substrates by isolated strains.
Table 2.
Screening of γ–PGA and endospore-forming isolate with sugars utilizing abilities.
| S.N. | Strain code | γ–PGA production (pH 6.5) | Endospore staining | Glucose | Sucrose | Lactose |
|---|---|---|---|---|---|---|
| 1 | B2-9 | ++++ | Non-spore former | Y,- | Y,- | R,- |
| 2 | D2-5 | ++++ | Spore former | Y,- | Y,+ | R,- |
| 3 | S6-3 | ++++ | Spore former | Y,- | Y,- | Y,- |
| 4 | CO4-1 | ++++ | Spore former | Y,- | Y,- | R,- |
| 5 | DS4-1 | ++++ | Spore former | Y,- | Y,- | R,- |
| 6 | CM6-2 | ++++ | Spore former | Y,- | Y,- | R,- |
| 7 | Bhw6-2 | ++++ | Spore former | Y,- | Y,- | R,- |
| 8 | Bhw2-1 | ++++ | Spore former | Y,- | Y,- | R,- |
| 9 | DS4-2 | ++++ | Spore former | Y,- | Y,- | R,- |
| 10 | KK4-2 | ++++ | Spore former | Y,- | Y,- | R,- |
| 11 | N6-2 | ++++ | Non-spore former | Y,- | Y,- | R,- |
| 12 | Pkr2-2 | ++++ | Spore former | Y,- | Y,- | R,- |
| 13 | T2-3 | ++++ | Spore former | Y,- | Y,- | Y,+ |
| 14 | B2-10 | ++++ | Spore former | Y,- | Y,- | Y,- |
| 15 | G2-2 | ++++ | Spore former | Y,- | Y,+ | R,- |
| 16 | D4-3 | ++++ | Spore former | Y,- | Y,- | R,- |
| 17 | JD6-1 | ++++ | Spore former | Y,- | Y,- | R,- |
| 18 | KS6-2 | ++++ | Spore former | Y,- | Y,- | R,- |
| 19 | WK2-1 | ++++ | Non-spore former | Y,- | Y,- | R,- |
| 20 | D2-10 | ++++ | Spore former | Y,- | Y,- | R,- |
| 21 | D2-1 | ++++ | Spore former | Y,- | Y,- | R,- |
| 22. | KK7-12 | ++++ | Spore former | Y,- | Y,- | R,- |
S.N: Serial number, Note: (++++): high γ –PGA Production, Y: yellow color and fermentative, R: red color and No fermentation, +: Gas production, – No gas production.
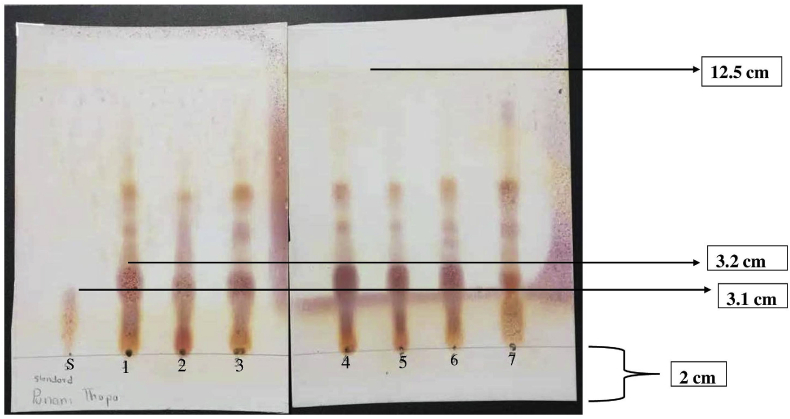
TLC is a significant and cost-effective method used to identify amino acids [54]. In this study, TLC was used to confirm that the compound produced by Bacillus sp. is poly glutamic acid. TLC is also based on the separation principle which relies on the relative affinity of the compounds to the stationary and mobile phases [55]. Based on this principle, the present study identified the amino acid present in the compound produced by the Bacillus sp. Figure 4 demonstrates eight distinct (S,1–7) spots of the amino acid (γ–PGA) produced by the selected Bacillus strain. Standard glutamic acid codes as S and other strains codes from 1-7 such as B2-9, CO4-1, D2-5, KK7-12, DS4-1, CM6-2, and Bhw2-1 respectively. The amino acids in γ–PGA produced from Bacillus strains were characterized using TLC. The distance travelled by all the compounds was found to be 3.2 cm and that of solvent was found to be 12.5 on the TLC plate. Likewise, the distance travelled by the standard glutamic acid (positive control) was found to be 3.1 cm and the color spots obtained were compared with γ–PGA producing isolates. The retention factor of control (S) was found to be 0.25 and the rest of the compounds produced by selected bacterial strains were found to be 0.27, with the comparing retention factor all compounds were found to be γ–PGA. The previous study by Song et al. [47] also used TLC for the detection of the γ–PGA synthesized by the Bacillus sp., which is in tune with the present study.
Figure 4.
Amino acid analysis (γ–PGA) by TLC method produced by different selected strains. (Note: (S): Control glutamic acid 1: B2-9, 2: CO4-1, 3: D2-5, 4: KK7-12, 5: DS4-1, 6: CM6-2, 7: Bhw2-1).
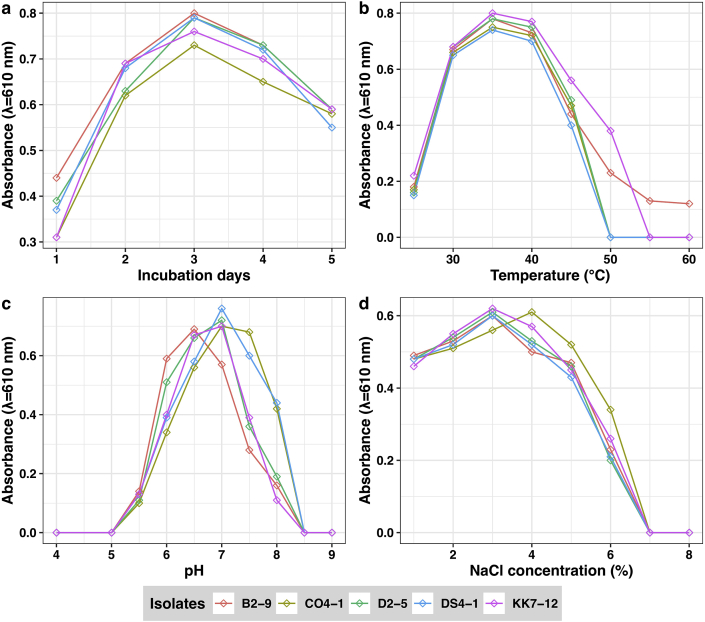
Optimization of bacterial growth at different temperatures, salt concentrations, and pH is illustrated in Figure 5. As depicted in Figure 5a, the highest growth of isolates was observed after 3 days of incubation at 37 °C on the PGA medium. The optimal temperature for the growth was reported to be 30–37 °C while some bacterial isolates showed the highest growth at 37 °C on the PGA medium (Figure 5b). These findings were commensurate with the study conducted by Song et al. [47] who also reported that the growth of Bacillus sp. and maximum γ–PGA synthesis was obtained at 37 °C. Likewise, B. subtilis F-2-01 was reported to multiply and synthesize maximum γ–PGA at 37 °C in the research conducted by Kubota et al. [4]. However, multiplication of B. licheniformis and γ–PGA production was reported at the temperature range of 30–50 °C by Cheng et al. [17]. This showed the different Bacillus sp. has a variable optimum temperature for the growth and maximum production of γ–PGA. Similarly, Ju et al. [18] reported the growth of Bacillus sp. and the highest γ–PGA production for 3 days of cultivation, which is consistent with our findings. No growth was observed at 55 °C and 60 °C and poor growth were detected at 25 °C, 45 °C, and 50 °C in the present study, revealing that the extreme higher and lower temperature is not suitable for the growth of Bacillus sp. producing γ–PGA.
Figure 5.
Growth of selected Bacillus isolates at different incubation period (a); temperatures (b); pH (c); NaCl concentration (d).
The maximum bacterial growth was recorded with a pH ranging from 6.5 to 7 (Figure 5c). Also, isolate CO4-1 and some other isolates showed the highest bacterial growth at pH 7. No growth was observed at pH 4, 5, 8.5, and 9, but poor growth was observed at pH 5.5 and 8.5. In a study conducted by Chettri et al. [39], 30 Bacillus isolates were reported to produce PGA at pH 7.5, which showed that the pH can be varied among different species of the Bacillus for the optimized production of γ–PGA. The extreme pH affects the structure of all macromolecules resulting in the breakage of hydrogen bonds holding together strands of DNA break. Any changes in the pH affect the ionization of functional amino acid groups, disrupting hydrogen bonding, which causes the folding of the molecule, inducing denaturation and loss of the activity [[56], [57]], thereby decreasing the production of γ–PGA. Hence, optimum pH is very crucial for the higher yield of γ–PGA. Likewise, the cell growth was hindered with rising NaCl concentration, and no growth was observed beyond 7% during γ–PGA production (Figure 5d), but most of the bacterial strain showed the highest bacterial growth at a concentration ranging from 3% NaCl concentration on the PGA medium. This could be the case since higher salt concentrations can kill bacteria by extracting water via osmosis. Bacterial proteins and enzymes are unable to function in the absence of water, resulting in cell death [58]. The results of this study are consistent with Ogawa et al. [6] who also reported that the bacterial growth and most efficient γ–PGA production was achievable with 3% (w/v) NaCl in the case of B. subtilis MR-141, yielding 22 g/L γ–PGA. In contrast to our study, Wei et al. [59] found that the bacterial growth and maximum γ–PGA production at 8% NaCl in halotolerant Bacillus licheniformis WX-02.
The viscosities of γ–PGA produced by selected bacterial isolates increased with incubation days and were found to be maximum at 4 days of incubation (Figure 6a). The viscosity of PGA produced by selected bacterial isolates increases at a temperature ranging from 35–37 °C (Figure 6b). At low temperature and high temperature, the lowest viscosity was observed. However, in the study conducted by Tork et al. [38], the relative viscosity of the γ–PGA was found to be higher at 30 °C Figure 6c demonstrates that the viscosity of PGA produced by selected bacterial isolates increases at pH ranging from 6.5–7. At low pH and high pH, the lowest viscosity was observed. According to Kedia et al. [60], the viscosity of γ–PGA is heavily reliant on both pH and ionic strength due to sudden changes in the biopolymer's conformation caused by both parameters. Furthermore, for the isolates in this study, the optimal incubation duration for growth and γ–PGA synthesis was 3–5 days. The culture broth was found highly viscous at 37 °C and decreased at 45 °C. This indicates that the viscosity is dependent on the temperature.
Figure 6.
Change of relative viscosity on PGA medium produced by selected strains at different incubation days (a); temperature (b); pH (c).
Based on high (++++) fibrous precipitate at 37 °C, four selected Bacillus isolates (B2-9, D2-5, CO4-1, and KK7-12) were identified by 16S rRNA gene sequencing and matched to the GenBank database of the National Center for Biotechnology Information (NCBI), Rockville Pike, Bethesda MD, USA. Isolates KK7-12, B2-9, CO4-1, and D2-5 were identified as Bacillus licheniformis, Bacillus subtilis, Bacillus paranthracis, and Bacillus cereus respectively. Likewise, using 16S rRNA gene sequencing, Chettri et al. [39] also characterized the PGA-producing Bacillus sp. as Bacillus subtilis, B. licheniformis and B. sonorensis. The phylogenetic tree showed that all the isolates clustered with the respective group of Bacillus sp. with more than 50% bootstrap values calculated from 1000 replications (Figure 7). The 16S rRNA gene sequence similarity percentage of potent γ–PGA producing isolates with the nearest reference strains is mentioned in Table 3.
Figure 7.
The positions of strains B2-9, D2-5, CO4-1, and KK7-12 are shown in a phylogenetic tree based on 16S rRNA gene sequences. At the branch points, bootstrap values of more than 50% (expressed as percentages of 1000 replications) are displayed. Neighbour-joining, maximum-parsimony, and maximum-likelihood methods were used to build the tree. A 0.05 nucleotide substitution per position is represented by the scale bar.
Table 3.
16S rRNA gene sequencing similarity of potent poly γ-glutamic acid-producing strains.
| Isolate Code | Close taxon name | Strain's name | Similarity (%) |
|---|---|---|---|
| B2-9 | Bacillus subtilis subsp. stercoris | D7XPN1 | 98.9% |
| D2-5 | Bacillus cereus | ATCC 14579 | 99.8% |
| CO4-1 | Bacillus paranthracis | Mn5 | 99.93% |
| KK7-12 | Bacillus licheniformis | ATCC 14580 | 99.8% |
4. Conclusion
Bacillus isolates B2-9, D2-5, CO4-1, and KK7-12 were found as potent γ–PGA producers. They were identified as Bacillus subtilis subsp. stercoris, Bacillus cereus, Bacillus paranthracis, and Bacillus licheniformis based on physiological, morphological, biochemical, and 16S rRNA gene sequencing techniques. The conditions for bacterial growth and γ–PGA production varied based on their adaptability at different incubation periods, temperatures, pH, and NaCl concentrations. Herein, under proper optimization, B. licheniformis could be the promising agent for commercial production of γ–PGA at an industrial scale for various purposes. Further research is mandatory for the quantification, purification, and characterization of γ–PGA synthesized by the Bacillus sp.
Declarations
Author contribution statement
Punam Thapa: Performed the experiments; Contributed reagents, materials, analysis tools or data.
Alina Thapa, Sujan Khadka, Tika Bahadur Karki: Conceived and designed the experiments; Analyzed and interpreted the data; Wrote the paper.
Sanjeep Sapkota, Om Prakash Panta, Suprina Sharma: Analyzed and interpreted the data; Wrote the paper.
Pramod Poudel: Conceived and designed the experiments; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability statement
Data will be made available on request.
Declaration of interests statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
The authors are grateful to the Department of Microbiology, National College, Tribhuvan University, Kathmandu, Nepal, for providing the necessary facilities to conduct this study.
References
- 1.Park S.-B., Sung M.-H., Uyama H., Han D.K. Poly(glutamic acid): production, composites, and medical applications of the next-generation biopolymer. Prog. Polym. Sci. 2021;113:101341. [Google Scholar]
- 2.Bajaj I., Singhal R. Poly (glutamic acid) – an emerging biopolymer of commercial interest. Bioresour. Technol. 2011;102:5551–5561. doi: 10.1016/j.biortech.2011.02.047. [DOI] [PubMed] [Google Scholar]
- 3.Ogunleye A., Bhat A., Irorere V.U., Hill D., Williams C., Radecka I. Poly-γ-glutamic acid: production, properties and applications. Microbiology. 2015;161:1–17. doi: 10.1099/mic.0.081448-0. [DOI] [PubMed] [Google Scholar]
- 4.Kubota H., Matsunobu T., Uotani K., Takebe H., Satoh A., Tanaka T., Taniguchi M. Production of Poly(y-glutamic acid) by Bacillus subtilis F-2-01. Biosci. Biotechnol. Biochem. 1993;57:1212–1213. doi: 10.1271/bbb.57.1212. [DOI] [PubMed] [Google Scholar]
- 5.Shi F., Xu Z., Cen P. Microbial production of natural poly amino acid. Sci. China, Ser. B Chem. 2007;50:291–303. [Google Scholar]
- 6.Ogawa Y., Yamaguchi F., Yuasa K., Tahara Y. Efficient production of γ-polyglutamic acid by Bacillus subtilis (natto) in jar fermenters. Biosci. Biotechnol. Biochem. 1997;61:1684–1687. doi: 10.1271/bbb.61.1684. [DOI] [PubMed] [Google Scholar]
- 7.Sapkota S., Khadka S., Gautam A., Maharjan R., Shah R., Dhakal S., Panta O.P., Khanal S., Poudel P. Screening and optimization of thermo-tolerant Bacillus sp. For amylase production and antifungal activity. J. Instr. Sci. Technol. 2019;24:47–56. [Google Scholar]
- 8.Khadka S., Adhikari S., Thapa A., Panday R., Adhikari M., Sapkota S., Regmi R.S., Adhikari N.P., Proshad R., Koirala N. Screening and optimization of newly isolated thermotolerant Lysinibacillus fusiformis strain SK for protease and antifungal activity. Curr. Microbiol. 2020;77:1558–1568. doi: 10.1007/s00284-020-01976-7. [DOI] [PubMed] [Google Scholar]
- 9.Ajayeoba T.A., Dula S., Ijabadeniyi O.A. Properties of poly-γ-glutamic acid producing-Bacillus species isolated from ogi liquor and lemon-ogi liquor. Front. Microbiol. 2019;10 doi: 10.3389/fmicb.2019.00771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ivanovics G., Bruckner V. Chemische und immunologische Studien uber den Mechanimus der Milzbrandinfektion und Im- munitat; die chemische Struktur der Kapdelsubstanz des Milz- brandbasillus und der serologisch identischen spezischen Substanz des Bacillus mesentericus. Z. Immunitatsforsch. 1937;90:304–318. [Google Scholar]
- 11.Ivanovics G., Erdos L. Ein Beitrag zum Wesen der Kapsel- substanz des Milzbrandbazillus. Z. Immunitatsforsch. 1937;90:5–19. [Google Scholar]
- 12.Shih I.L., Van Y.T. The production of poly-(γ-glutamic acid) from microorganisms and its various applications. Bioresour. Technol. 2001;79:207–225. doi: 10.1016/s0960-8524(01)00074-8. [DOI] [PubMed] [Google Scholar]
- 13.Candela T., Fouet A. Poly-gamma-glutamate in bacteria. Mol. Microbiol. 2006;60:1091–1098. doi: 10.1111/j.1365-2958.2006.05179.x. [DOI] [PubMed] [Google Scholar]
- 14.Candela T., Moya M., Haustant M., Fouet A. Fusobacterium nucleatum, the first Gramnegative bacterium demonstrated to produce polyglutamate. Can. J. Microbiol. 2009;55:627–632. doi: 10.1139/w09-003. [DOI] [PubMed] [Google Scholar]
- 15.Weber J. Poly(y-glutamic Acid)s are the major constituents of nematocysts in Hydra (Hydrozoa, Cnidaria)∗. J. Biol. Chem. 1990;265:9664–9669. [PubMed] [Google Scholar]
- 16.Hezayen F.F., Rehm B.H.A., Tindall B.J., Steinbüchel A. Transfer of Natrialba asiatica B1T to Natrialba taiwanensis sp. nov. and description of Natrialba aegyptiaca sp. nov., a novel extremely halophilic, aerobic, non-pigmented member of the Archaea from Egypt that produces extracellular poly(glutamic acid) Int. J. Syst. Evol. Microbiol. 2001;51:1133–1142. doi: 10.1099/00207713-51-3-1133. [DOI] [PubMed] [Google Scholar]
- 17.Cheng C., Asada Y., Aida T. Production of γ-polyglutamic acid by Bacillus licheniformis A35 under denitrifying conditions. Agric. Biol. Chem. 1989;53:2369–2375. [Google Scholar]
- 18.Ju W.T., Song Y.S., Jung W.J., Park R.D. Enhanced production of poly-γ-glutamic acid by a newly-isolated Bacillus subtilis. Biotechnol. Lett. 2014;36:2319–2324. doi: 10.1007/s10529-014-1613-3. [DOI] [PubMed] [Google Scholar]
- 19.Nishito Y., Osana Y., Hachiya T., Popendorf K., Toyoda A., Fujiyama A., Itaya M., Sakakibara Y. Whole genome assembly of a natto production strain Bacillus subtilis natto from very short read data. BMC Genom. 2010;11:243. doi: 10.1186/1471-2164-11-243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nagai T. Overview of studies on Bacillus subtilis (natto) bacteriophages and the prospects. Japan Agric. Res. Q. 2012;46:305–310. [Google Scholar]
- 21.Kada S., Ishikawa A., Ohshima Y., Yoshida K.I. Alkaline serine protease AprE plays an essential role in poly-γ-glutamate production during natto fermentation. Biosci. Biotechnol. Biochem. 2013;77:802–809. doi: 10.1271/bbb.120965. [DOI] [PubMed] [Google Scholar]
- 22.Chettri R., Tamang J.P. Functional properties of tungrymbai and bekang , naturally fermented soybean foods of north East India. Int. J. Fermented Foods. 2014;3:87. doi: 10.1016/j.ijfoodmicro.2014.12.021. [DOI] [PubMed] [Google Scholar]
- 23.Tamang J.P. Naturally fermented ethnic soybean foods of India. J. Ethn. Foods. 2015;2:8–17. doi: 10.1016/j.ijfoodmicro.2014.12.021. [DOI] [PubMed] [Google Scholar]
- 24.Chettri R., Tamang J.P. Bacillus species isolated from tungrymbai and bekang, naturally fermented soybean foods of India. Int. J. Food Microbiol. 2015;197:72–76. doi: 10.1016/j.ijfoodmicro.2014.12.021. [DOI] [PubMed] [Google Scholar]
- 25.Ashiuchi M., Kamei T., Misono H. Poly-γ-glutamate synthetase of Bacillus subtilis. J. Mol. Catal. B Enzym. 2003;23:101–106. [Google Scholar]
- 26.Ashiuchi M., Soda K., Misono H. A poly-γ-glutamate synthetic system of Bacillus subtilis IFO 3336: gene cloning and biochemical analysis of poly-γ-glutamate produced by Escherichia coli clone cells. Biochem. Biophys. Res. Commun. 1999;263:6–12. doi: 10.1006/bbrc.1999.1298. [DOI] [PubMed] [Google Scholar]
- 27.Candela T. CapE, a 47-amino-acid peptide, is necessary for. Society. 2005;187:7765–7772. doi: 10.1128/JB.187.22.7765-7772.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cromwick A.M., Gross R.A. Effects of manganese (II) on Bacillus licheniformis ATCC 9945A physiology and γ-poly(glutamic acid) formation. Int. J. Biol. Macromol. 1995;17:259–267. doi: 10.1016/0141-8130(95)98153-p. [DOI] [PubMed] [Google Scholar]
- 29.Throne C.B., Leonard C.G. Isolation of D- and L-glutamyl polypeptides from culture filtrates of Bacillus subtilis. J. Biol. Chem. 1958;233:1109–1112. [PubMed] [Google Scholar]
- 30.Hanby W.E., Rydon H.N. The capsular substance of Bacillus anthracis. Biochem. J. 1946;40:297–309. [PubMed] [Google Scholar]
- 31.Tanimoto H., Fox T., Eagles J., Fairweather-Tait S.J., Tanimoto H., Satoh H., Nozawa H., Okiyama A., Morinaga Y. Acute effect of poly-γ-glutamic acid on calcium absorption in post-menopausal women. J. Am. Coll. Nutr. 2007;26:645–649. doi: 10.1080/07315724.2007.10719642. [DOI] [PubMed] [Google Scholar]
- 32.Ye H., Jin L., Hu R., Yi Z., Li J., Wu Y., Xi X., Wu Z. Poly(γ,l-glutamic acid)-cisplatin conjugate effectively inhibits human breast tumor xenografted in nude mice. Biomaterials. 2006;27:5958–5965. doi: 10.1016/j.biomaterials.2006.08.016. [DOI] [PubMed] [Google Scholar]
- 33.Tsao C.T., Chang C.H., Lin Y.Y., Wu M.F., Wang J.L., Han J.L., Hsieh K.H. Antibacterial activity and biocompatibility of a chitosan-γ- poly(glutamic acid) polyelectrolyte complex hydrogel. Carbohydr. Res. 2010;345:1774–1780. doi: 10.1016/j.carres.2010.06.002. [DOI] [PubMed] [Google Scholar]
- 34.Sheu Y.-T., Tsang D.C.W., Dong C.-D., Chen C.-W., Luo S.-G., Kao C.-M. Enhanced bioremediation of TCE-contaminated groundwater using gamma poly-glutamic acid as the primary substrate. J. Clean. Prod. 2018;178:108–118. [Google Scholar]
- 35.Ben-Zur N., Goldman D.M. g-Poly glutamic acid: a novel peptide for skin care, Cosmet. Toilet. 2007;122:65–74. [Google Scholar]
- 36.Wang Q., Chen S., Zhang J., Sun M., Liu Z., Yu Z. Co-producing lipopeptides and poly-γ-glutamic acid by solid-state fermentation of Bacillus subtilis using soybean and sweet potato residues and its biocontrol and fertilizer synergistic effects. Bioresour. Technol. 2008;99:3318–3323. doi: 10.1016/j.biortech.2007.05.052. [DOI] [PubMed] [Google Scholar]
- 37.Zhang D., Feng X., Zhou Z., Zhang Y., Xu H. Economical production of poly(γ-glutamic acid) using untreated cane molasses and monosodium glutamate waste liquor by Bacillus subtilis NX-2. Bioresour. Technol. 2012;114:583–588. doi: 10.1016/j.biortech.2012.02.114. [DOI] [PubMed] [Google Scholar]
- 38.Tork S.E., Aly M.M., Alakilli S.Y., Al-Seeni M.N. Purification and characterization of gamma poly glutamic acid from newly Bacillus licheniformis NRC20. Int. J. Biol. Macromol. 2015;74:382–391. doi: 10.1016/j.ijbiomac.2014.12.017. [DOI] [PubMed] [Google Scholar]
- 39.Chettri R., Bhutia M.O., Tamang J.P. Poly-γ-Glutamic acid (PGA)-Producing Bacillus species isolated from kinema, Indian fermented soybean food. Front. Microbiol. 2016;7:1–7. doi: 10.3389/fmicb.2016.00971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vos P., Garrity G., Jones D., Krieg N.R., Ludwig W., Rainey F.A., Schleifer K.-H., Whitman W.B. second ed. Vol. 3. Springer-Verlag New York; 2009. Bergey’s Manual of Systematic Bacteriology.https://www.springer.com/gp/book/9780387950419 (The Firmicutes.). [Google Scholar]
- 41.Poudel P., Tashiro Y., Miyamoto H., Miyamoto H., Okugawa Y., Sakai K. Direct starch fermentation to l-lactic acid by a newly isolated thermophilic strain, Bacillus sp. MC-07. J. Ind. Microbiol. Biotechnol. 2015;42:143–149. doi: 10.1007/s10295-014-1534-0. [DOI] [PubMed] [Google Scholar]
- 42.Khadka S., Nshimiyimana J.B., Zou P., Koirala N., Xiong L. Biodegradation kinetics of diethyl phthalate by three newly isolated strains of Pseudomonas. Sci. Afr. 2020;8 [Google Scholar]
- 43.Nshimiyimana J.B., Khadka S., Zou P., Adhikari S., Proshad R., Thapa A., Xiong L. Study on biodegradation kinetics of di-2-ethylhexyl phthalate by newly isolated halotolerant Ochrobactrum anthropi strain L1-W. BMC Res. Notes. 2020;13:252. doi: 10.1186/s13104-020-05096-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stecher G., Tamura K., Kumar S. Molecular evolutionary genetics analysis (MEGA) for macOS. Mol. Biol. Evol. 2020 doi: 10.1093/molbev/msz312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kumar S., Stecher G., Li M., Knyaz C., Tamura K. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 2018;35:1547–1549. doi: 10.1093/molbev/msy096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jia-Fei Z., Zhong-Yang L., Ming-Jiang N., Ke-Fa C. Dependence of nanofluid viscosity on particle size and pH value. Chin. Phys. Lett. 2009;26:6–8. [Google Scholar]
- 47.Song D.Y., Reddy L.V., Charalampopoulos D., Wee Y.J. Poly-(γ-glutamic acid) production and optimization from agro-industrial bioresources as renewable substrates by bacillus sp. FBL-2 through response surface methodology. Biomolecules. 2019;9:754. doi: 10.3390/biom9120754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li Z., Chen R.W., Lei H.Y., Shan Z., Bai T., Yu Q., Li H.L. Characterization and flocculating properties of a novel bioflocculant produced by Bacillus circulans. World J. Microbiol. Biotechnol. 2009;25:745–752. [Google Scholar]
- 49.Sarkar P.K., Hasenack B., Nout M.J.R. Diversity and functionality of Bacillus and related genera isolated from spontaneously fermented soybeans (Indian Kinema) and locust beans (African Soumbala) Int. J. Food Microbiol. 2002;77:175–186. doi: 10.1016/s0168-1605(02)00124-1. [DOI] [PubMed] [Google Scholar]
- 50.Nout M.J.R., Bakshi D., Sarkar P.K. Microbiological safety of kinema, a fermented soya bean food. Food Contr. 1998;9:357–362. [Google Scholar]
- 51.Tamang J.P. Native microorganisms in the fermentation of kinema. Indian J. Microbiol. 2003;43:127–130. [Google Scholar]
- 52.Sy N., Thanh L., Kimura K., Tuyen D.T., Thi L., Anh N. Isolation , characterization of Bacillus sp . producing heavy metal absorption γ -PGA. J. Viet. Env. 2018;9:49–54. [Google Scholar]
- 53.Baxi N.N. Use of nonspecific, glutamic acid-free, media and high glycerol or high amylase as inducing parameters for screening Bacillus isolates having high yield of polyglutamic acid. Int. Sch. Res. Not. 2014;2014:1–8. doi: 10.1155/2014/608739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Stahl E. 3o ed. Springer-Verlag; New York: 1969. Thin-Layer Chromatography. A Laboratory Handbook. [Google Scholar]
- 55.Bheem . Owlcation; 2019. Thin Layer Chromatography (TLC): Principle and Procedure. [Google Scholar]
- 56.Kunioka M., Goto A. Biosynthesis of poly( ,-glutamic acid) from L-glutamic acid, citric acid, and ammonium sulfate in Bacillus subtilis IFO3335. Appl. Microbiol. Biotechnol. 1994;40:867–872. [Google Scholar]
- 57.Allen S., Auman A., Brelles-Mariño G., Feldman M.A., Flowers P., Franklund C., Paterson A., Pinchuk G., Rowley B., Sutherland M. 2016. The Effects of pH on Microbial Growth | Microbiology. [Google Scholar]
- 58.Davies Emma. 2019. Why Does Salt Have Antibacterial Properties?https://www.sciencefocus.com/nature/why-does-salt-have-antibacterial-properties/#:∼:text=Salt-kills-some-types-of,side-of-its-cell-membrane.&text=Some-bacteria-can-tolerate-salt%3B-they-are-halotolerant [Google Scholar]
- 59.Wei X., Ji Z., Chen S. Isolation of halotolerant Bacillus licheniformis WX-02 and regulatory effects of sodium chloride on yield and molecular sizes of poly-γ-glutamic acid. Appl. Biochem. Biotechnol. 2010;160:1332–1340. doi: 10.1007/s12010-009-8681-1. [DOI] [PubMed] [Google Scholar]
- 60.Kedia G., Hill D., Hill R., Radecka I. Production of poly-γ-glutamic acid by Bacillus subtilis and Bacillus licheniformis with different growth media. J. Nanosci. Nanotechnol. 2010;10:5926–5934. doi: 10.1166/jnn.2010.2614. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.