Abstract
Background
In the randomised phase III KEYNOTE-062 study, pembrolizumab was non-inferior to chemotherapy for overall survival in patients with programmed death-ligand 1 (PD-L1)-positive [combined positive score (CPS) ≥1] advanced gastric/gastroesophageal junction (GEJ) cancer. We present findings of prespecified health-related quality-of-life (HRQOL) analyses for pembrolizumab versus chemotherapy in this population.
Materials and methods
HRQOL, a secondary endpoint, was measured in patients who received ≥1 dose of study treatment and completed ≥1 HRQOL questionnaire [European Organisation for the Research and Treatment of Cancer (EORTC) 30-question quality-of-life (QLQ-C30), EORTC 22-question quality-of-life gastric-cancer-specific module (QLQ-STO22)]. Least squares mean (LSM) change (baseline to week 18) in global health status/quality of life (GHS/QOL; EORTC QLQ-C30) and time to deterioration (TTD) in GHS/QOL, nausea/vomiting and appetite loss scores (EORTC QLQ-C30) and abdominal pain/discomfort scores (EORTC QLQ-STO22) were evaluated.
Results
The HRQOL population comprised 495 patients with CPS ≥1 (pembrolizumab, 252; chemotherapy, 243). Compliance rates at week 18 were similar for pembrolizumab and chemotherapy (EORTC QLQ-C30, 87.9% and 81.9%; EORTC QLQ-STO22, 87.9% and 81.3%, respectively). There was no between-arm difference in LSM score change in GHS/QOL [−0.16; 95% confidence interval (CI) −5.01 to 4.69; P = 0.948]. The LSM score change for most subscales showed comparable worsening in both arms. TTD for GHS/QOL [hazard ratio (HR), 0.96; 95% CI, 0.67-1.38; P = 0.826], appetite loss (HR, 0.83; 95% CI, 0.58-1.20; P = 0.314) and pain (HR, 1.22; 95% CI, 0.78-1.91; P = 0.381) were similar between arms. Longer TTD was observed for pembrolizumab versus chemotherapy for nausea/vomiting (HR, 0.61; 95% CI, 0.44-0.85; P = 0.003).
Conclusions
HRQOL was maintained with first-line treatment with pembrolizumab in patients with PD-L1–positive advanced gastric/GEJ cancer and was similar between pembrolizumab and chemotherapy in this population.
Key words: gastric cancer, gastroesophageal cancer, pembrolizumab, quality of life, patient-reported outcomes
Highlights
-
•
HRQOL was similar between pembrolizumab and chemotherapy in patients with PD-L1-positive advanced gastric/GEJ cancer.
-
•
General HRQOL as measured by QLQ-C30 GHS/QOL scores was comparable between treatment arms from baseline to week 18.
-
•
The EQ-5D-3L visual analogue scale was also equivalent between arms from baseline to week 18.
Introduction
Gastric cancer is among the most commonly diagnosed cancers globally, with an incidence of >1 million new cases (sixth most common among cancer types) and almost 800 000 deaths (third most leading cause of cancer deaths) annually.1 The 5-year survival rate for patients with advanced stage gastric/gastroesophageal junction (GEJ) cancer is 5%-10%.2 Advanced gastric/GEJ cancer is also marked by worsening overall health-related quality of life (HRQOL) as measured by validated questionnaires. Physical, social and emotional functioning and the disease-related symptom profile (weight loss, abdominal pain, vomiting, gastric obstruction, bleeding) are more burdensome in the advanced stage of gastric/GEJ cancer than in earlier stages. In addition, chemotherapy is known to cause drug-related symptoms (abdominal pain, fatigue, nausea/vomiting, diarrhoea) in patients with advanced gastric/GEJ cancer.2 For most patients with advanced gastric/GEJ cancer, the standard of care first-line treatment is doublet chemotherapy,3, 4, 5, 6 which offers a modest but statistically significant benefit of about 1 month in overall survival (OS) compared with single-agent chemotherapy.7 Chemotherapy regimens used as first-line therapy, regardless of type, have been found to maintain HRQOL over time.8
The programmed death 1 (PD-1) inhibitor pembrolizumab has demonstrated antitumour activity and a manageable toxicity profile in patients with programmed death-ligand 1 (PD-L1)–positive [combined positive score (CPS) ≥1] advanced gastric/GEJ adenocarcinoma.9,10 In cohort 1 of the phase II KEYNOTE-059 trial (NCT02335411), pembrolizumab monotherapy was given to patients with gastric/GEJ adenocarcinoma in the third-line or later setting. Among patients whose tumours express CPS ≥1, the objective response rate (ORR) was 16% and the median duration of response (DOR) was 16 months.10 Pembrolizumab also demonstrated antitumour activity in patients with previously untreated gastric/GEJ adenocarcinoma enrolled in cohorts 2 (pembrolizumab plus chemotherapy) and 3 (pembrolizumab monotherapy) of the KEYNOTE-059 study; patients in cohort 3 were required to have CPS ≥1 tumours. The ORR was 60% in cohort 2 and 26% in cohort 3, and safety was tolerable with both regimens.11 The KEYNOTE-062 trial (NCT02494583) was a randomised, active-controlled, phase III study of pembrolizumab as monotherapy or in combination with chemotherapy compared with placebo plus chemotherapy as first-line treatment of patients with advanced gastric or GEJ cancer. At the final analysis, pembrolizumab monotherapy was non-inferior to chemotherapy for OS among patients with CPS ≥1 tumours [median OS, 10.6 versus 11.1 months; hazard ratio (HR), 0.91; 99.2% confidence interval (CI), 0.69-1.18 (prespecified non-inferiority margin = 1.2)].12 Although not formally tested, there was a clinically meaningful improvement in OS in patients with CPS ≥10 tumours (median OS, 17.4 versus 10.8 months; HR, 0.69; 95% CI, 0.49-0.97). Pembrolizumab monotherapy offered an improved safety profile with a lower incidence of any-grade (54% versus 92%) and grade 3-4 (16% versus 68%) treatment-related adverse events (AEs) compared with chemotherapy.12
Here we present findings from the prespecified secondary and exploratory HRQOL analyses in patients with CPS ≥1 gastric/GEJ cancer who received pembrolizumab monotherapy versus chemotherapy in the KEYNOTE-062 trial.
Materials and methods
Study design and patients
KEYNOTE-062 (ClinicalTrials.gov: NCT02494583) was a global, randomised, phase III clinical trial of first-line pembrolizumab monotherapy or pembrolizumab plus chemotherapy versus chemotherapy in patients with advanced gastric/GEJ cancer (Supplementary Figure S1, available at https://doi.org/10.1016/j.esmoop.2021.100189). In the primary analysis, pembrolizumab versus chemotherapy met the criteria for non-inferiority, whereas pembrolizumab-chemotherapy versus chemotherapy did not meet the criteria for superiority.12 Therefore, we focused on the HRQOL outcomes for pembrolizumab monotherapy compared with chemotherapy in this HRQOL analysis. Details of this comparison in the primary study have been reported elsewhere and are briefly summarised here.12 Eligible patients had locally advanced unresectable or metastatic gastric/GEJ adenocarcinoma that was human epidermal growth factor receptor 2 (HER2)/neu-negative and PD-L1–positive (CPS ≥1) and Eastern Cooperative Oncology Group performance status 0 or 1. PD-L1 positivity was tested using PD-L1 IHC 22C3 pharmDx (Agilent Technologies, Carpinteria, CA, USA) and was measured using CPS [defined as the number of PD-L1–positive cells (tumour cells, lymphocytes, macrophages) as a proportion of the total number of viable tumour cells × 100]. Patients were randomly assigned 1 : 1 : 1 to pembrolizumab 200 mg, pembrolizumab-chemotherapy [cisplatin 80 mg/m2/day on day 1 plus 5-fluorouracil (FU) 800 mg/m2/day on days 1 to 5 or capecitabine 1000 mg/m2 twice daily] or placebo-chemotherapy every 3 weeks for a maximum of 35 cycles (∼2 years). The chemotherapy regimen was decided by the investigator before randomisation.
The study protocol and all amendments were approved by the appropriate ethics committee at each centre. The study was conducted in accordance with the protocol, its amendments and the standards of Good Clinical Practice. All patients provided written informed consent.
HRQOL outcomes and assessments
HRQOL outcomes reported here were prespecified secondary and exploratory endpoints from KEYNOTE-062. Secondary endpoints included mean change from baseline to week 18 in the global health status/quality-of-life (GHS/QOL), functioning and symptom scores of the European Organisation for the Research and Treatment of Cancer (EORTC) core 30 quality-of-life questionnaire (QLQ-C30) and the EORTC QLQ 22-question quality-of-life gastric cancer-specific module (QLQ-STO22) as well as time to deterioration (TTD) in the GHS/QOL, nausea/vomiting and appetite loss scores of the EORTC QLQ-C30 and the abdominal pain/discomfort scores of the EORTC QLQ-STO22. Week 18 was selected based on a blinded review of compliance/completion rates where rates were 60%-80%. Health status from the EuroQol five-dimension, three-level (EQ-5D-3L) questionnaire was an exploratory endpoint.
HRQOL questionnaires were administered by qualified site personnel and completed electronically by the patient, before study drug administration or AE/disease status evaluation and in the following order: EQ-5D-3L, EORTC QLQ-C30, EORTC QLQ-STO22. The EQ-5D-3L is a standardised instrument that has been translated into 170 languages and provides data for use in economic models and analyses of health utility or quality-adjusted life-years and addresses five health state dimensions: mobility, self-care, usual activities, pain/discomfort and anxiety/depression.13 The EORTC QLQ-C30 is a widely used and well-validated cancer-specific HRQOL instrument that has been translated to and validated in 81 languages and comprises a GHS/QOL scale, five functional dimensions (physical, role, emotional, cognitive and social), three symptom scales (fatigue, nausea/vomiting and pain) and six single-item measures (dyspnoea, sleep disturbance, appetite loss, constipation, diarrhoea and financial difficulties).14 The EORTC QLQ-STO22, designed for use in clinical trials in addition to the EORTC QLQ-C30 to assess disease-specific treatment measures and translated into 60 languages, consists of 22 items: symptoms of dysphagia (three items), pain (four items), reflux symptoms (three items), eating restrictions (five items), anxiety (three items), dry mouth, taste, body image and hair loss.15 Questionnaires were administered at baseline; at weeks 3, 6, 9 and 12; every 6 weeks thereafter up to 1 year or end of treatment (whichever came first); and at the 30-day post-treatment discontinuation follow-up visit.
As previously defined for the EORTC QLQ-C30, a mean change of 5 to 10 points in EORTC QLQ-C30 scores represents a small change (‘little’ change as reported by patients), 10 to 20 points represents a moderate change and >20 points represents a large change (‘very much’ as reported by patients); a decline from baseline of ≥10 points on the functional or global health and QOL scales was considered a clinically meaningful deterioration.16 Therefore, deterioration was defined as a ≥10-point decline from baseline when measuring TTD.
Statistical analysis
The HRQOL analysis population comprised all patients who received ≥1 dose of study treatment and completed ≥1 HRQOL questionnaire. Compliance and completion rates were summarised by treatment arm and visit and were reported for all three HRQOL questionnaires. Compliance rate was defined as the proportion of patients who completed ≥1 HRQOL questionnaire among those expected to complete the questionnaires at each visit (excluding patients missing by design because they discontinued study treatment). Completion rate was defined as the proportion of patients who completed ≥1 HRQOL questionnaire among the total HRQOL analysis population at each visit. Change in least squares mean (LSM) score from baseline to week 18 was assessed using a constrained longitudinal data analysis model based on the missing-at-random assumption. Descriptive analyses of mean score and mean score changes from baseline [±standard error (SE)] in the GHS/QOL and subscale scores of the EORTC QLQ-C30 and nausea/vomiting subscale scores for the EORTC QLQ-STO22 were summarised through week 48 to further depict trends. The Kaplan–Meier method was used to estimate the TTD survival curve for the GHS/QOL score and subscales of the EORTC QLQ-C30 and EORTC QLQ-STO22.17 The TTD analysis was censored at death. Cox proportional hazards model with treatment as a covariate was used to assess the magnitude of treatment differences. The median TTD HR and 95% CI were reported.
Analyses were conducted using the final analysis data cut-off date of 26 March 2019 [median follow-up, 11.3 months (range, 0.2-41.2 months)].
Results
Patients
A total of 763 patients were randomly assigned in KEYNOTE-062 (pembrolizumab monotherapy, n = 256; pembrolizumab plus chemotherapy, n = 257; chemotherapy, n = 250).12 The HRQOL population in the current analysis of pembrolizumab monotherapy versus chemotherapy comprised 495 patients who received treatment and completed ≥1 HRQOL questionnaire by the final analysis (26 March 2019): 252 in the pembrolizumab arm and 243 in the chemotherapy arm.
HRQOL compliance and completion
Among patients in the HRQOL analysis population, compliance rates at week 18 were similar in the pembrolizumab arm and the chemotherapy arm for EORTC QLQ-C30 (87.9% and 81.9%), EORTC QLQ-STO22 (87.9% and 81.3%) and EQ-5D-3L (87.9% and 82.5%) questionnaires, respectively. Completion rates of all three questionnaires decreased from baseline because of treatment discontinuation attributed to disease progression, death or AEs (Supplementary Tables S1-S3, available at https://doi.org/10.1016/j.esmoop.2021.100189).
Change in HRQOL from baseline to week 18
EORTC QLQ-C30 and EORTC QLQ-STO22
Baseline GHS/QOL scores of the EORTC QLQ-C30 were well balanced between the pembrolizumab and chemotherapy arms (Table 1).
Table 1.
Change from baseline in EORTC QLQ-C30 GHS/QOL symptom scores and EORTC QLQ-STO22 symptom subscale scores at week 18
| Treatment | Baseline score, mean (SD) | Week 18 score, mean (SD) | Change from baseline at week 18, LSM (95% CI)a | Difference in LSM (95% CI) |
|---|---|---|---|---|
| EORTC QLQ-C30 GHS/QOL | ||||
| Pembrolizumab |
n = 239 62.7 (21.8) |
n = 102 66.4 (20.2) |
n = 251 −1.9 (−5.8 to 2.0) |
−0.16 (−5.0 to 4.7) P = 0.948 |
| Chemotherapy |
n = 234 62.4 (21.1) |
n = 140 63.6 (20.2) |
n = 243 −1.8 (−5.2 to 1.7) |
|
| EORTC QLQ-C30 nausea/vomiting | ||||
| Pembrolizumab |
n = 239 16.1 (21.9) |
n = 102 15.9 (23.3) |
n = 251 5.8 (1.3-10.3) |
−2.1 (−7.8 to 3.7) P = 0.477 |
| Chemotherapy |
n = 234 16.8 (21.9) |
n = 140 21.2 (25.2) |
n = 243 7.9 (4.0-11.8) |
|
| EORTC QLQ-C30 appetite loss | ||||
| Pembrolizumab |
n = 239 33.9 (33.9) |
n = 102 21.9 (29.1) |
n = 251 −3.5 (−9.4 to 2.3) |
−4.6 (−11.9 to 2.7) P = 0.217 |
| Chemotherapy |
n = 234 36.6 (33.3) |
n = 140 32.9 (30.7) |
n = 243 1.0 (−4.1 to 6.2) |
|
| EORTC QLQ-STO22 pain | ||||
| Pembrolizumab |
n = 239 30.4 (22.1) |
n = 102 22.0 (20.2) |
n = 251 −1.1 (−4.7 to 2.4) |
2.4 (−2.2 to 6.9) P = 0.308 |
| Chemotherapy |
n = 233 28.8 (21.3) |
n = 139 22.3 (20.4) |
n = 243 −3.5 (−6.6 to −0.4) |
|
CI, confidence interval; GHS, global health status; LSM, least squares mean; EORTC QLQ-C30, European Organisation for the Research and Treatment of Cancer core 30 quality-of-life questionnaire; EORTC QLQ-STO22, European Organisation for the Research and Treatment of Cancer 22-question quality-of-life gastric cancer-specific module; QOL, quality of life; SD, standard deviation.
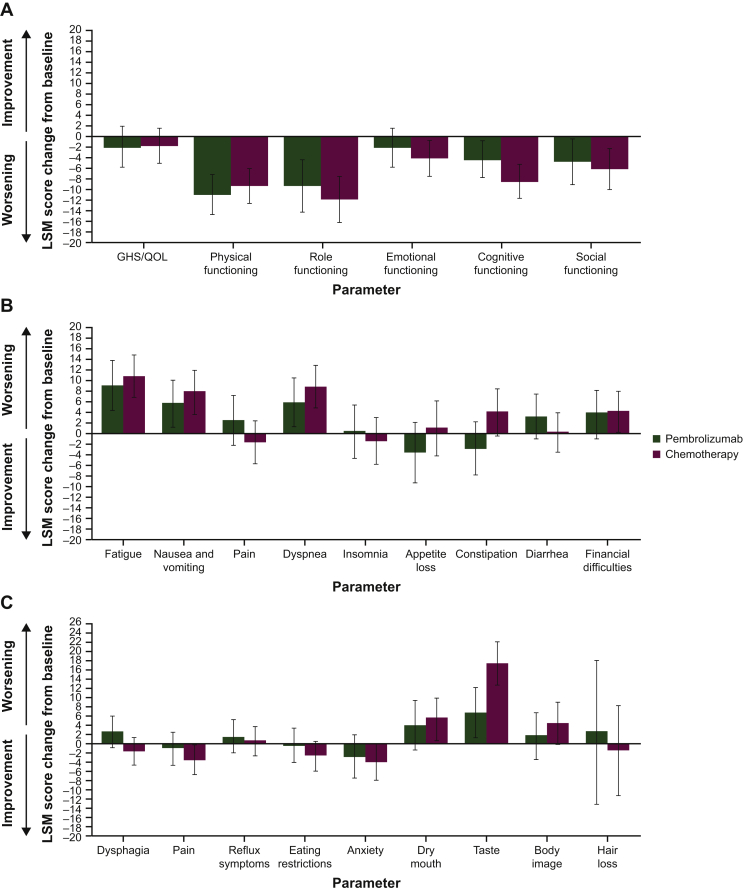
At week 18, mean GHS/QOL scores were 66.4 [standard deviation (SD) ±20.2] with pembrolizumab and 63.6 (SD ±20.2) with chemotherapy. No clinically meaningful between-arm differences in LSM score were observed (LSM difference, −0.16; 95% CI, −5.0 to 4.7; nominal P = 0.948). Results were similar for the EORTC QLQ-C30 symptom subscales of nausea/vomiting and appetite loss and the EORTC QLQ-STO22 symptom subscale of pain (Table 1). The LSM score change from baseline to week 18 for most of the EORTC QLQ-C30 and EORTC QLQ-STO22 function and symptom subscales showed comparable worsening in both treatment arms (Figure 1). The largest between-arm difference in LSM score was for the taste subscale of the EORTC QLQ-STO22, whereas patients in the chemotherapy arm reported greater worsening.
Figure 1.
LSM (95% CI) change from baseline to week 18 in (A) EORTC QLQ-C30 GHS/QOL and functional subscale scores, (B) EORTC QLQ-C30 symptom subscale scores and (C) EORTC QLQ-STO22 symptom subscale scores.
CI, confidence interval; GHS, global health status; LSM, least squares mean; EORTC QLQ-C30, European Organisation for the Research and Treatment of Cancer core 30 quality-of-life questionnaire; EORTC QLQ-STO22, European Organisation for the Research and Treatment of Cancer 22-question quality-of-life gastric cancer-specific module; QOL, quality of life.
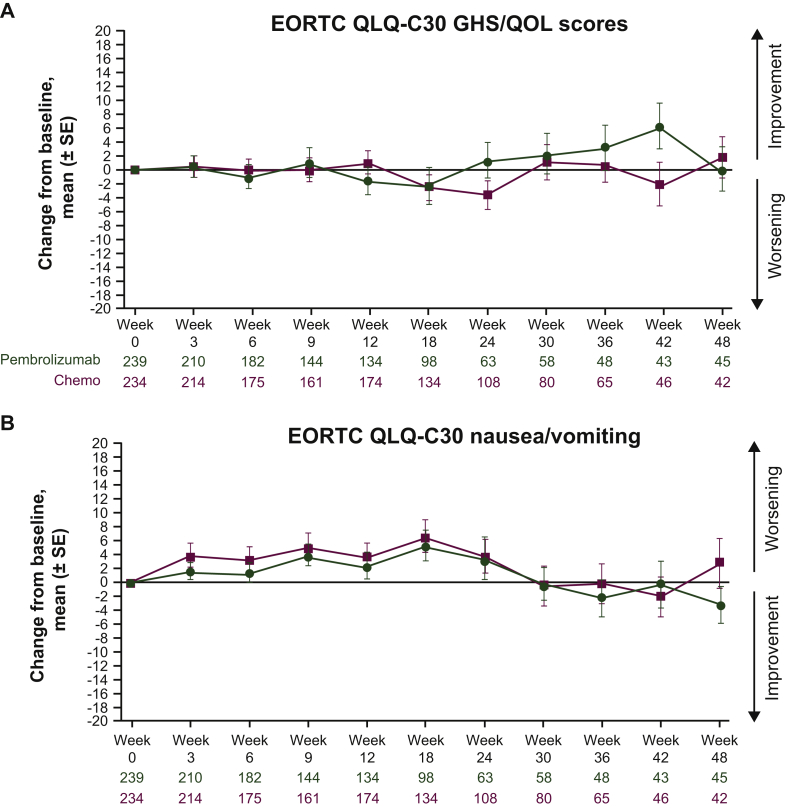
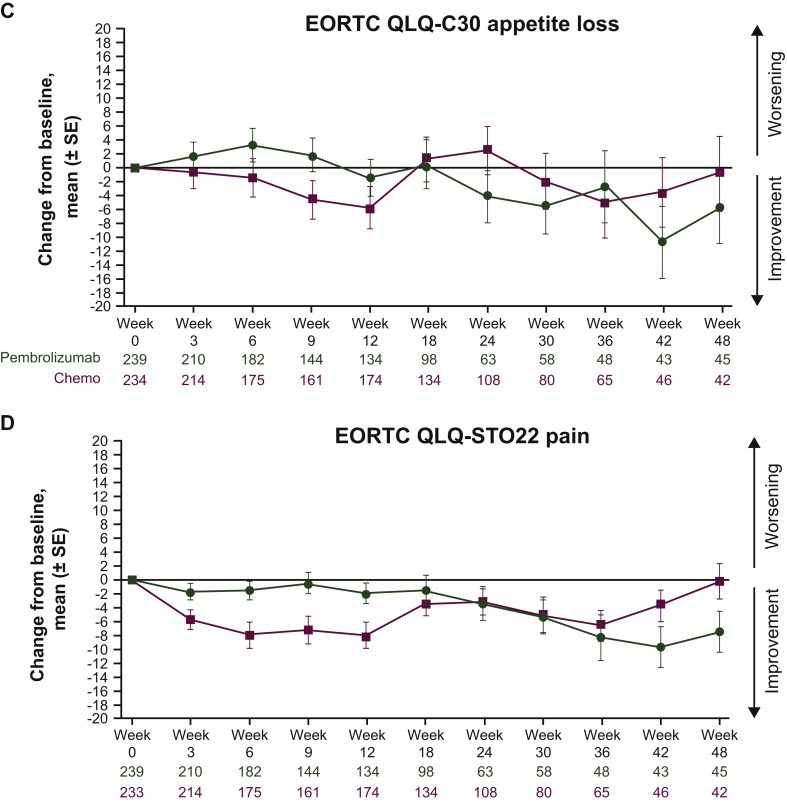
Descriptive analyses of mean change from baseline revealed that GHS/QOL scores remained relatively stable for both arms (Figure 2A). A slight improvement was observed in the pembrolizumab arm between weeks 18 and 42. Descriptive analyses of mean score change from baseline through week 48 of follow-up demonstrated a general trend in improvement for the EORTC QLQ-C30 nausea/vomiting and appetite loss subscales and the EORTC QLQ-STO22 pain subscale (Figure 2B-D). However, these changes from baseline were not clinically meaningful.
Figure 2.
Mean (±SE) change from baseline by study visit in (A) EORTC QLQ-C30 GHS/QOL scores, (B) EORTC QLQ-C30 symptom subscale nausea/vomiting scores, (C) EORTC QLQ-C30 subscale appetite loss scores and (D) EORTC QLQ-STO22 symptom pain subscale scores. Parts C and D are continued on next page.
GHS, global health status; EORTC QLQ-C30, European Organisation for the Research and Treatment of Cancer core 30 quality-of-life questionnaire; EORTC QLQ-STO22, European Organisation for the Research and Treatment of Cancer 22-question quality-of-life gastric cancer-specific module; QOL, quality of life; SE, standard error.
EQ-5D-3L visual analogue scale
The EQ-5D-3L visual analogue scale (VAS) score decreased from baseline to week 18 in the pembrolizumab arm (LSM score change, −5.98; 95% CI, −9.28 to −2.69) and the chemotherapy arm (LSM score change, −4.78; 95% CI, −7.70 to −1.87), indicating worsening. There was no difference in LSM between arms (LSM difference, −1.20; 95% CI, −5.41 to 3.00; nominal P = 0.574) (Supplementary Figure S2, available at https://doi.org/10.1016/j.esmoop.2021.100189).
Time to deterioration at week 18
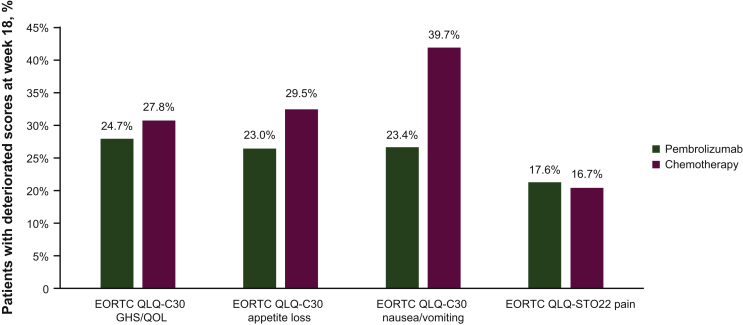
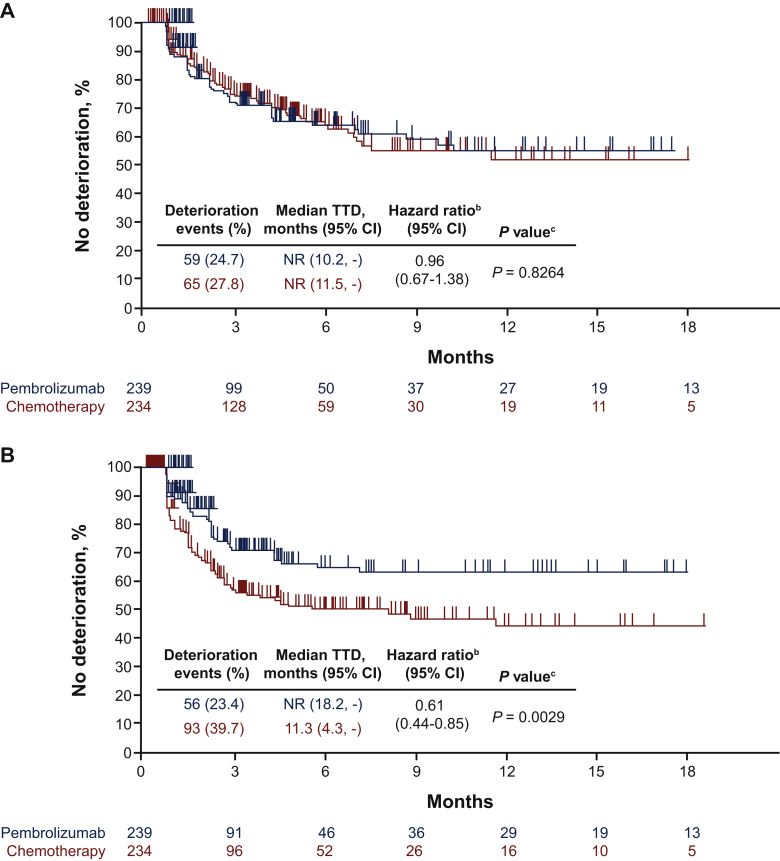
The proportion of patients with deterioration at week 18 (defined as a ≥10-point worsening from baseline) was similar between the pembrolizumab and chemotherapy arms for the GHS/QOL score, the EORTC QLQ-C30 appetite loss subscale and the EORTC QLQ-STO22 pain subscale. In the EORTC QLQ-C30 nausea/vomiting subscale, substantially fewer patients experienced deterioration at week 18 with pembrolizumab (23.4%) than with chemotherapy (39.7%) (Figure 3).
Figure 3.
Proportion of patients with deteriorated EORTC QLQ-C30 and EORTC QLQ-STO22 scores at week 18.
GHS, global health status; EORTC QLQ-C30, European Organisation for the Research and Treatment of Cancer core 30 quality-of-life questionnaire; EORTC QLQ-STO22, European Organisation for the Research and Treatment of Cancer 22-question quality-of-life gastric cancer-specific module; QOL, quality of life.
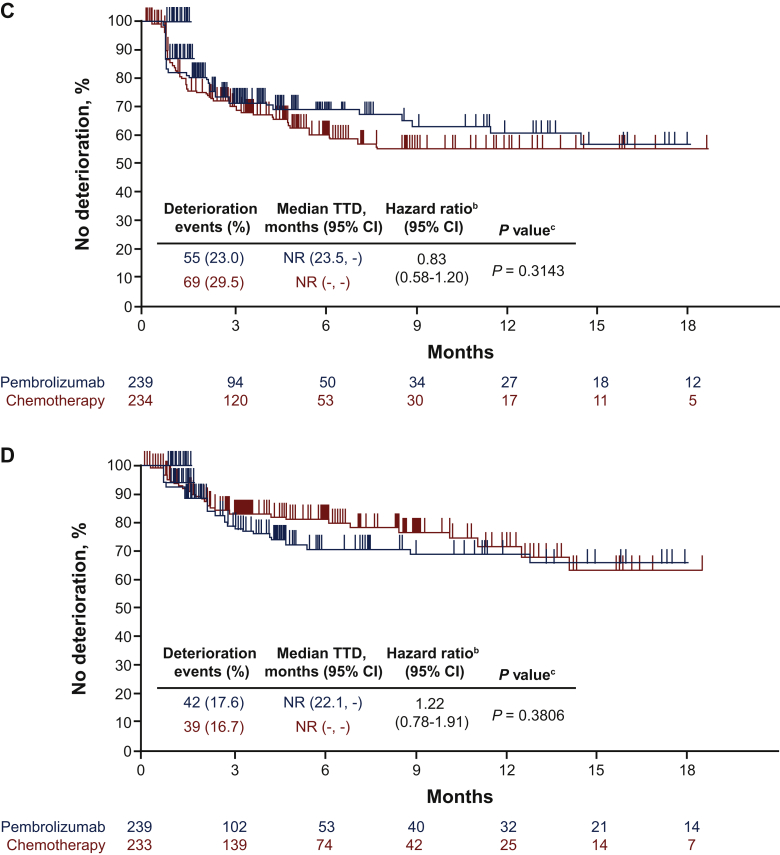
TTD in GHS/QOL (HR, 0.96; 95% CI, 0.67-1.38; nominal P = 0.826), EORTC QLQ-C30 appetite loss subscale (HR, 0.83; 95% CI, 0.58-1.20; nominal P = 0.314), and EORTC QLQ-STO22 pain subscale (HR, 1.22; 95% CI, 0.78-1.91; nominal P = 0.381) were similar between arms (Figure 4A, C, D). Longer TTD was observed for pembrolizumab than for chemotherapy for the EORTC QLQ-C30 nausea/vomiting subscale (HR, 0.61; 95% CI, 0.44-0.85; nominal P = 0.003) (Figure 4B).
Figure 4.
TTD in (A) EORTC QLQ-C30 GHS/QOL, (B) EORTC QLQ-C30 nausea/vomiting subscale, (C) EORTC QLQ-C30 appetite loss subscale and (D) EORTC QLQ-STO22 pain subscale. Parts C and D are continued on next page.
CI, confidence interval; GHS, global health status; NR, not reached; EORTC QLQ-C30, European Organisation for the Research and Treatment of Cancer core 30 quality-of-life questionnaire; EORTC QLQ-STO22, European Organisation for the Research and Treatment of Cancer 22-question quality-of-life gastric cancer-specific module; QOL, quality of life; TTD, time to deterioration; -, the median was not reached.
a From product-limit (Kaplan–Meier) method for censored data.
b Based on stratified Cox regression model with treatment as a covariate.
c Two-sided P value based on stratified log-rank test.
Discussion
This analysis showed that HRQOL was similar in patients with PD-L1−positive (CPS ≥1) advanced gastric/GEJ tumours who received first-line pembrolizumab and those who received chemotherapy. Compliance rates of the three HRQOL questionnaires were high (≥81%) and generally equivalent between treatment arms at week 18, the primary analysis time point for HRQOL. General HRQOL as measured by EORTC QLQ-C30 GHS/QOL scores were comparable between treatment arms from baseline to week 18. The EQ-5D-3L VAS, which measures general HRQOL regardless of disease status, was also equivalent between arms from baseline to week 18.
All five functional subscales and nearly all symptom subscales of the EORTC QLQ-C30 showed comparable scores between arms. The same was true of the EORTC QLQ-STO22 symptom subscales with the exception of taste, which showed a worsening trend for patients treated with chemotherapy than for patients treated with pembrolizumab. TTD for the EORTC QLQ-C30 nausea/vomiting subscale was longer for pembrolizumab-treated patients than for chemotherapy-treated patients. The primary analysis of the KEYNOTE-062 trial demonstrated a favourable safety profile for pembrolizumab monotherapy compared with chemotherapy. The incidence of treatment-related AEs associated with symptom and single-item subscales (fatigue, nausea, vomiting, decreased appetite, constipation, diarrhoea) was lower for pembrolizumab-treated patients than for chemotherapy-treated patients.12 Not all symptoms that occurred more frequently among chemotherapy-treated patients resulted in an observable change in HRQOL; no clinically meaningful differences in LSM score from baseline to week 18 were observed in appetite loss, constipation and diarrhoea scales or in TTD for appetite loss. Taken together, pembrolizumab offers favourable safety compared with chemotherapy and maintains HRQOL.
Intensification of chemotherapy for gastric/GEJ cancer can provide modest gains in OS,7 and first-line chemotherapy regimens in gastric/GEJ cancer tend to maintain HRQOL as measured by GHS/QOL.8 Evidence from immunotherapy trials has demonstrated that monotherapy with PD-1 inhibitors can provide efficacy benefits and tolerable safety while maintaining or improving HRQOL in patients with solid tumours.18,19 In a systematic review of HRQOL outcomes, there was a consistent prolongation of the time to symptom deterioration and better symptom control at different follow-up points with nivolumab, pembrolizumab and atezolizumab in patients with lung cancer, melanoma, head and neck cancer and urothelial cancer.18 Findings from the present analysis of KEYNOTE-062 demonstrate the ability of pembrolizumab monotherapy to maintain HRQOL in the first-line setting in patients with gastric/GEJ cancer.
One limitation of these HRQOL analyses is the partially blinded design of the study. Patients were not fully blinded to pembrolizumab monotherapy because only one type of study treatment was administered in that arm of the trial. Conversely, administration of pembrolizumab or placebo was blinded in the combination chemotherapy arms. Future data releases will elucidate the HRQOL profile of double-blinded pembrolizumab or placebo compared with chemotherapy.
Conclusion
In this study in patients who had advanced gastric/GEJ adenocarcinoma with PD-L1 CPS ≥1 tumours, HRQOL was similar between the pembrolizumab and the chemotherapy arms in the first-line setting. Longer TTD was observed for pembrolizumab than for chemotherapy for the nausea/vomiting subscale in EORTC QLQ-C30, which aligned with the increased incidence of nausea and vomiting treatment-related AEs from chemotherapy in the primary safety analysis. Overall, HRQOL was similar in patients with PD-L1-positive advanced gastric/GEJ adenocarcinoma, whether they received pembrolizumab or chemotherapy as first-line treatment.
Acknowledgements
We thank the patients and their families, as well as the investigators and site personnel involved in the study. Medical writing and/or editorial assistance was provided by Tim Peoples, MA, ELS, Traci Stuve, MA, and Holly C. Cappelli PhD, CMPP, of ApotheCom (Yardley, PA, USA). This assistance was funded by Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA.
Funding
This work was supported by Merck Sharp & Dohme Corp. (no grant number), a subsidiary of Merck & Co., Inc. (no grant number), Kenilworth, NJ, USA.
Disclosure
EVC reports advisory/consultancy fees from Array, AstraZeneca, Bayer, BioPharma, Bristol Myers Squibb, Celgene, Halozyme, Lilly, Merck KGaA, Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA, Novartis, Roche and Servier; and researching grant/funding (institution) from Amgen, Bayer, Bristol Myers Squibb, Boehringer Ingelheim, Celgene, Ipsen, Lilly, Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA., Merck KGaA, Novartis, Roche and Servier. AV is an employee of Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA. Y-JB reports consulting/advisory role for Astellas, AstraZeneca, Bayer, BeiGene, Bristol Myers Squibb, Daichii-Sankyo, Eli Lilly, Genentech/Roche, Genexine, Green Cross, Hanmi, Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA, Merck Serono, Novartis, Samyang Biopharmaceuticals and Taiho; and grants (to the institution for clinical trials) from Astellas, AstraZeneca, Bayer, BeiGene, Bristol Myers Squibb, Boehringer Ingelheim, Boston Biomedical, CKD Pharma, Curis, Daiichi Sankyo, Eli Lilly, Five Prime, Genentech/Roche, Genexine, Green Cross, GSK, MacroGenics, Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA, Merck Serono, Novartis, Ono, Pfizer, Taiho and Takeda. CSF reports consulting role for Agios, Amylin Pharmaceuticals, Bain Capital, CytomX Therapeutics, Daiichi Sankyo, Eli Lilly, Entrinsic Health, EvolveImmune Therapeutics, Genentech, Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA, Taiho and Unum Therapeutics. He also serves as a director for CytomX Therapeutics and owns unexercised stock options for CytomX and Entrinsic Health. He is a co-founder of EvolveImmune Therapeutics and has equity in this private company. KS reports grants and personal fees from Astellas Pharma, Eli Lilly and Company, Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA, Ono Pharmaceutical and Taiho Pharmaceutical; personal fees from AbbVie, Bristol Myers Squibb, GlaxoSmithKline, Novartis, Pfizer Inc., Takeda Pharmaceuticals and Yakult; and grants from Chugai Pharma, Daiichi Sankyo, Dainippon Sumitomo Pharma and Medi Science. YYJ reports advisory fees from Bristol Myers Squibb, Lilly, Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA, Merck Serono and Pfizer; and research expenses from Amgen, Bayer, Boehringer Ingelheim, Genentech, Lilly and Roche. VS reports research funding from Bristol Myers Squibb, EMD-Serono and Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA. SSt reports advisory/consultancy role for Bayer, Bristol Myers Squibb, Exelixis, Genentech, Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA, and QED. JMN is an employee of Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA. UK is an employee of Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA. SSh is an employee and a stockholder of Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA. MA reports scientific consultancy role for Bristol Myers Squibb, Lilly, Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA, and Servier; and honoraria for speaking for Bristol Myers Squibb, Lilly, Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA, and Servier. All other authors have declared no conflicts of interest.
Data sharing
Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA (MSD) is committed to providing qualified scientific researchers access to anonymised data and clinical study reports from the company's clinical trials for the purpose of conducting legitimate scientific research. MSD is also obligated to protect the rights and privacy of trial participants and, as such, has a procedure in place for evaluating and fulfilling requests for sharing company clinical trial data with qualified external scientific researchers. The MSD data-sharing website (available at: http://engagezone.msd.com/ds_documentation.php) outlines the process and requirements for submitting a data request. Applications will be promptly assessed for completeness and policy compliance. Feasible requests will be reviewed by a committee of MSD subject matter experts to assess the scientific validity of the request and the qualifications of the requestors. In line with data privacy legislation, submitters of approved requests must enter into a standard data-sharing agreement with MSD before data access is granted. Data will be made available for request after product approval in the US and EU or after product development is discontinued. There are circumstances that may prevent MSD from sharing requested data, including country or region-specific regulations. If the request is declined, it will be communicated to the investigator. Access to genetic or exploratory biomarker data requires a detailed, hypothesis-driven statistical analysis plan that is collaboratively developed by the requestor and MSD subject matter experts; after approval of the statistical analysis plan and execution of a data-sharing agreement, MSD will either perform the proposed analyses and share the results with the requestor or will construct biomarker covariates and add them to a file with clinical data that is uploaded to an analysis portal so that the requestor can perform the proposed analyses.
Supplementary data
References
- 1.Bray F., Ferlay J., Soerjomataram I., Siegel R.L., Torre L.A., Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Casamayor M., Morlock R., Maeda H., Ajani J. Targeted literature review of the global burden of gastric cancer. Ecancermedicalscience. 2018;12:883. doi: 10.3332/ecancer.2018.883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.National Comprehensive Cancer Network NCCN clinical practice guidelines in oncology (NCCN guidelines): gastric cancer (Version 4.2020) https://www.nccn.org/professionals/physician_gls/pdf/gastric.pdf Available at. [DOI] [PubMed]
- 4.Smyth E.C., Verheij M., Allum W., Cunningham D., Cervantes A., Arnold D. Gastric cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2016;27(suppl 5):v38–v49. doi: 10.1093/annonc/mdw350. [DOI] [PubMed] [Google Scholar]
- 5.Japanese Gastric Cancer Association Japanese gastric cancer treatment guidelines 2018 (5th ed.) Gastric Cancer. 2021;24:1–21. doi: 10.1007/s10120-020-01042-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Muro K., Lordick F., Tsushima T. Pan-Asian adapted ESMO clinical practice guidelines for the management of patients with metastatic oesophageal cancer: a JSMO-ESMO initiative endorsed by CSCO, KSMO, MOS, SSO and TOS. Ann Oncol. 2019;30:34–43. doi: 10.1093/annonc/mdy498. [DOI] [PubMed] [Google Scholar]
- 7.Wagner A.D., Syn N.L., Moehler M. Chemotherapy for advanced gastric cancer. Cochrane Database Syst Rev. 2017;8:Cd004064. doi: 10.1002/14651858.CD004064.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.van Kleef J.J., Ter Veer E., van den Boorn H.G. Quality of life during palliative systemic therapy for oesophagogastric cancer: systematic review and meta-analysis. J Natl Cancer Inst. 2020;112:12–29. doi: 10.1093/jnci/djz133. [DOI] [PubMed] [Google Scholar]
- 9.Muro K., Chung H.C., Shankaran V. Pembrolizumab for patients with PD-L1-positive advanced gastric cancer (KEYNOTE-012): a multicentre, open-label, phase 1b trial. Lancet Oncol. 2016;17:717–726. doi: 10.1016/S1470-2045(16)00175-3. [DOI] [PubMed] [Google Scholar]
- 10.Fuchs C.S., Doi T., Jang R.W. Safety and efficacy of pembrolizumab monotherapy in patients with previously treated advanced gastric and gastroesophageal junction cancer: phase 2 clinical KEYNOTE-059 trial. JAMA Oncol. 2018;4:e180013. doi: 10.1001/jamaoncol.2018.0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bang Y.J., Kang Y.K., Catenacci D.V. Pembrolizumab alone or in combination with chemotherapy as first-line therapy for patients with advanced gastric or gastroesophageal junction adenocarcinoma: results from the phase II nonrandomized KEYNOTE-059 study. Gastric Cancer. 2019;22:828–837. doi: 10.1007/s10120-018-00909-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shitara K., van Cutsem E., Bang Y.J. Efficacy and safety of pembrolizumab or pembrolizumab plus chemotherapy versus chemotherapy alone for patients with first-line, advanced gastric cancer: the KEYNOTE-062 phase 3 randomized clinical trial. JAMA Oncol. 2020;6:1571–1580. doi: 10.1001/jamaoncol.2020.3370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pickard A.S., Wilke C.T., Lin H.W., Lloyd A. Health utilities using the EQ-5D in studies of cancer. Pharmacoeconomics. 2007;25:365–384. doi: 10.2165/00019053-200725050-00002. [DOI] [PubMed] [Google Scholar]
- 14.Aaronson N.K., Ahmedzai S., Bergman B. The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. 1993;85:365–376. doi: 10.1093/jnci/85.5.365. [DOI] [PubMed] [Google Scholar]
- 15.Blazeby J.M., Conroy T., Bottomley A. Clinical and psychometric validation of a questionnaire module, the EORTC QLQ-STO 22, to assess quality of life in patients with gastric cancer. Eur J Cancer. 2004;40:2260–2268. doi: 10.1016/j.ejca.2004.05.023. [DOI] [PubMed] [Google Scholar]
- 16.Osoba D., Rodrigues G., Myles J., Zee B., Pater J. Interpreting the significance of changes in health-related quality-of-life scores. J Clin Oncol. 1998;16:139–144. doi: 10.1200/JCO.1998.16.1.139. [DOI] [PubMed] [Google Scholar]
- 17.Anota A., Hamidou Z., Paget-Bailly S. Time to health-related quality of life score deterioration as a modality of longitudinal analysis for health-related quality of life studies in oncology: do we need RECIST for quality of life to achieve standardization? Qual Life Res. 2015;24:5–18. doi: 10.1007/s11136-013-0583-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Abdel-Rahman O., Oweira H., Giryes A. Health-related quality of life in cancer patients treated with PD-(L)1 inhibitors: a systematic review. Expert Rev Anticancer Ther. 2018;18:1231–1239. doi: 10.1080/14737140.2018.1528146. [DOI] [PubMed] [Google Scholar]
- 19.Nishijima T.F., Shachar S.S., Muss H.B., Tamura K. Patient-reported outcomes with PD-1/PD-L1 inhibitors for advanced cancer: a meta-analysis. Oncologist. 2019;24:e565–e573. doi: 10.1634/theoncologist.2018-0449. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.