Highlights
-
•
Risks with tight adaptive RT margins.
-
•
Cancer control may be poorer if margins tight.
-
•
Prospective studies required.
Keywords: Radiation oncology, Bladder cancer, Adaptive radiation therapy, Online image guidance, Clinical outcomes
Abstract
Background and Purpose
To report long-term outcomes of online image-guided (IG) adaptive radiation therapy (aRT) versus conventional IG radiation therapy (cRT) for bladder preservation in muscle-invasive bladder cancer (MIBC).
Materials and Methods
A retrospective review of patients with histologically proven MIBC who were prescribed radical intent radiation therapy (RT) following trans-urethral resection of bladder tumour (TURBT) was conducted. There were three groups based on their RT treatment modality: conventional RT (cRT), margin 5 mm adaptive RT (aRT5mm) and margin 7 mm adaptive RT (aRT7mm).
Results
171 patients were included in this study, with median age of 79.4 years (41–90). Approximately half of all patients received concurrent chemotherapy. N = 57 underwent cRT, n = 39 underwent aRT5mm, and n = 75 underwent aRT7mm. Response evaluable patients in all three groups (n = 133) had high rates of complete response (CR, 83%) on first post-RT cystoscopy with no significant differences between the groups. At a median follow-up of 54 months, the 5-year freedom from muscle-invasive failure survival (FFMIFS) in the cRT, aRT5mm, and aRT7mm groups were 75%, 59%, and 98%, respectively. The estimated cancer specific survival (CSS) at 5 years were 60%, 30%, and 59%, respectively. The estimated overall survival (OS) at 5 years were 43%, 26%, and 38%, respectively. The incidence of late grade 3 or 4 toxicity was n = 5 in aRT5mm, n = 2 in cRT group, and n = 1 in aRT7mm.
Conclusion
IG aRT with 7 mm expansion for MIBC provides higher rates of FFMIFS, similar 5-year CSS and OS, as well as toxicity outcomes when compared to cRT. aRT with 5 mm expansion with this RT protocol is not recommended for treatment.
Introduction
Bladder cancer is the ninth most common cancer in the world, with the highest incidence seen in the European male population [1].
Maximal safe trans-urethral resection of bladder tumour (TURBT) followed by radical radiation therapy (RT) with concurrent radiation-sensitising chemotherapy is an accepted alternative to radical cystectomy for muscle invasive bladder cancer (MIBC) [2], [3].
Bladder size is prone to both inter- and intra-fraction motion [4]. Therefore, ideal clinical target volume (CTV) to planning target volume (PTV) expansion margins have been extensively analysed. Empirically, a 1.5 to 2.0 cm margin from CTV to PTV will encompass the CTV > 93% of the time [5]. Meijer et al [4] recommended an anisotropic margin expansion from CTV to PTV after considering set-up errors, organ motion, and small interobserver variations.
To account for the widely fluctuating sizes of the bladder, tumour coverage, and unnecessarily excessive doses to organs at risk (OAR), adaptive radiation therapy (aRT) techniques have been developed around the world [6], [7].
We report on the long-term clinical outcomes in patients with MIBC treated using either conventional RT (cRT) or aRT as part of bladder conserving therapy at the Peter MacCallum Cancer Centre (PMCC) in Australia.
Materials and methods
Inclusion Criteria
Patients with histologically MIBC proven urothelial cancer and its variants, T2-T4N0M0, who were prescribed radical intent RT following TURBT at a PMCC site were identified. Patients with co-existing primary malignancies were excluded. Institutional Human Research Ethics Committee approval was obtained prior to commencement of this study. Patient data were retrospectively retrieved from institution electronic medical records and paper records from November 2006 to 30th September 2019.
Technical considerations: Radiation Therapy
Conventional RT (cRT) technique
The conventional treatment plan incorporated a 1.5 cm isotropic expansion of the CTV (whole bladder) to construct a PTV. A 3D conformal technique (3DCRT) was employed with 6 – 18 megavoltage (MV) beams, using a minimum of three fields. Image guidance (IG) was performed with daily kilovoltage (KV) or MV imaging and with cone beam computed tomography (CBCT) only when available.
Online adaptive “plan of the day (PoD)” RT technique
The online adaptive process was based on an institutional pilot study [8] and also defined in the Trans-Tasman Radiation Oncology Group (TROG) multi-centre feasibility study [9]. Further details of the RT planning and treatment technique, including staff training and credentialing, and quality assurance are in previous technical publications [8], [9], [10], [11], [12]. Elective nodal irradiation was not undertaken [5], [13]. RT boost of gross tumour or partial bladder volume, whether sequential or simultaneous integrated, was not applied to this study population.
In brief, patients were asked to void immediately prior to entering the CT simulation room. During CT acquisition, patients were positioned supine with pelvic immobilisation. A 3D conformal technique (3DCRT) was employed with 6 – 18 megavoltage (MV) beams, using a minimum of three fields. Intensity modulated radiation therapy (IMRT) was not used at the time due to the increased time required for planning and quality assurance, particularly in view of the need for development of the adaptive plans during the second week of treatment.
All patients were prescribed 64 Gy in 32 daily fractions over 6 ½ weeks to the middle of the bladder as defined by the ICRU50 reference point [14]; complete coverage of the PTV by the 95% isodose was required. Daily CBCT imaging were obtained prior to each fraction to verify bladder size and position. Patients were asked to re-void if their bladder extended beyond the largest PTV, and the CBCT IG process was repeated.
For each patient, four plans were generated for the entire course of treatment: conventional, small, medium and large. The adaptive plans were created from a composite of the planning CT and the first 5 daily on-treatment CBCTs. Conventional RT treatment plan, as outlined above, was utilised to deliver the first 7 fractions. For fractions 8 to 32, credentialed radiation therapy staff selected from one of the three adaptive plans (small, medium and large).
In August 2010 the margin used for adaptive RT to the bladder changed from 5 mm to 7 mm. This change occurred just as the TROG 10.01 BOLART study began recruitment. The decision for this was made after retrospective review of post-treatment verification CBCT images had shown some geographical misses. Therefore, for this study, all analysis will be done according to three groups: conventional RT (cRT), 5 mm adaptive RT (aRT5mm) and 7 mm adaptive RT (aRT7mm).
Statistical analysis
Complete response (CR) was defined as no disease seen at cystoscopy or present at random biopsy. CR rate was described as a percentage with 95% confidence interval (Clopper-Pearson method). Response rate was compared between treatments using Fisher exact test.
Overall survival (OS) and cancer specific survival (CSS) were measured from date of commencement of RT until the date of death due to any cause, or date of death from bladder cancer. Time to local muscle-invasive bladder failure was measured in the subset of patients who achieved CR, from the date of response assessment to the date of muscle-invasive bladder failure (death was a censoring event). Kaplan-Meier curves were used to describe all time to event endpoints. Cox proportional hazard model was used to estimate the hazard ratio with 95% confidence interval and to compare RT techniques.
Treatment-related grade 3 or 4 bladder and bowel side effects were tabulated. Fisher exact test was used to compare the rate of grade 3 or 4 toxicities between RT techniques. New grade 3 or 4 adverse events were defined as grade 3 or 4 adverse events reported any time after commencing RT that were not present before commencing treatment. If a grade 3 or 4 symptom was reported before and after RT, these are regarded as being due to symptoms of the tumour rather than from the treatment itself, and thus were not included in our results. However, if there was a worsening of a symptom from a grade 1–2, to a grade 3 or higher, they would be included. All statistical analyses were performed in R version 3.6.3.
Results
Between November 2006 and December 2015, 171 patients were identified. 57 (33%) received cRT, 39 (23%) received aRT5mm, and 75 (44%) received aRT7mm. cRT treatment start dates ranged from September 2007 to September 2015. aRT5mm start dates ranged from November 2006 to August 2010, after which aRT7mm was used. Approximately half of all patients received concurrent radio-sensitising chemotherapy, while the other half were deemed medically unfit and had RT alone. The proportion of these patients in each group were not statistically significantly different.
Patient and tumour characteristics are summarised in Table 1, together with the RT technique, and concurrent chemotherapy use data. All patients except one, were prescribed 64 Gy in 32 fractions of RT, with or without concurrent radio-sensitising chemotherapy. One patient received 60 Gy in 30 fractions in order to avoid excessive dose to his transplanted pelvic kidney. He was included in the study in the cRT group as his treatment was of radical intent. One patient (in aRT5mm group) did not complete the prescribed RT due to other illness, ceasing at 62 Gy in 31 fractions.
Table 1.
Patient characteristics by RT technique.
| RT Technique |
|||||
|---|---|---|---|---|---|
| Characteristic |
Conventional n = 57 (33%) |
Adaptive 5 mm n = 39 (23%) |
Adaptive 7 mm n = 75 (44%) |
Total n = 171 (100%) |
|
| Treatment period | Sep 2007 - Sep 2015 | Nov 2006 - Aug 2010 | Sep 2010 – Dec 2015 | Nov 2006 - Dec 2015 | |
| Age | |||||
| Mean (SD) | 75.9 (8.8) | 75.2 (11.1) | 78.4 (8.5) | 76.9 (9.3) | |
| Median [range] | 77.5 [53.2–88.7] | 77.7 [41.0–87.6] | 80.9 [57.3–89.9] | 79.4 [41.0–89.9] | |
| IQR | 70.0–82.8 | 70.2–82.8 | 74.7–84.1 | 71.8–83.6 | |
| T stage | |||||
| T2 | 43 (75%) | 28 (72%) | 63 (84%) | 134 (78%) | |
| T3 | 11 (19%) | 8 (21%) | 9 (12%) | 28 (16%) | |
| T4 | 3 (5%) | 3 (8%) | 3 (4%) | 9 (5%) | |
| N stage | |||||
| N0 | 57 (100%) | 39 (100%) | 75 (100%) | 171 (100%) | |
| M stage | |||||
| M0 | 57 (100%) | 39 (100%) | 75 (100%) | 171 (100%) | |
| ECOG | |||||
| 0 | 13 (23%) | 11 (28%) | 27 (36%) | 51 (30%) | |
| 1 | 38 (67%) | 25 (64%) | 35 (47%) | 98 (58%) | |
| 2 | 4 (7%) | 3 (8%) | 12 (16%) | 19 (11%) | |
| 3 | 1 (2%) | 0 | 0 | 1 (1%) | |
| 4 | 1 (2%) | 0 | 0 | 1 (1%) | |
| Missing | 0 | 0 | 1 | 1 | |
| Concurrent chemotherapy | |||||
| No | 29 (51%) | 22 (56%) | 36 (48%) | 87 (51%) | |
| Yes | 28 (49%) | 17 (44%) | 39 (52%) | 84 (49%) | |
Of the total 171 patients, 133 (78%) had documented post treatment response assessment data on retrospective review of the available medical records. Their response assessment results are in Table 2. The numbers within the square brackets denote the 95% confidence interval of the percentage value.
Table 2.
Response assessment.
| RT Technique |
||||
|---|---|---|---|---|
| Response | cRT (n = 47) | aRT5mm (n = 31) | aRT7mm (n = 55) | Total (n = 133) |
| CR | 37 (79% [64, 89]) | 28 (90% [74, 98]) | 45 (82% [69, 91]) | 110 (83% [75, 89]) |
| PR | 5 (11% [4, 23]) | 1 (3% [0, 17]) | 2 (4% [0, 13]) | 8 (6% [3, 12]) |
| PD | 5 (11% [4, 23]) | 2 (6% [1, 21]) | 8 (15% [6, 27]) | 15 (11% [6, 18]) |
There was no significant difference in response when comparing aRT5mm vs cRT (p = 0.23) nor for aRT7mm vs cRT (p = 0.80). There was also no significant difference in response when any aRT was compared with cRT (p = 0.47).
Of the 38 who were not included, n = 10 were in the cRT group, n = 8 in the aRT5mm group, and n = 20 in the aRT7mm group. Two had refused to have check cystoscopy procedure, four had developed metastatic disease and did not proceed to check cystoscopy, six had died prior to check cystoscopy (n = 2 related cause, n = 3 unrelated cause, n = 1 unknown cause), ten had missing data, and the remaining 16 were lost to follow-up.
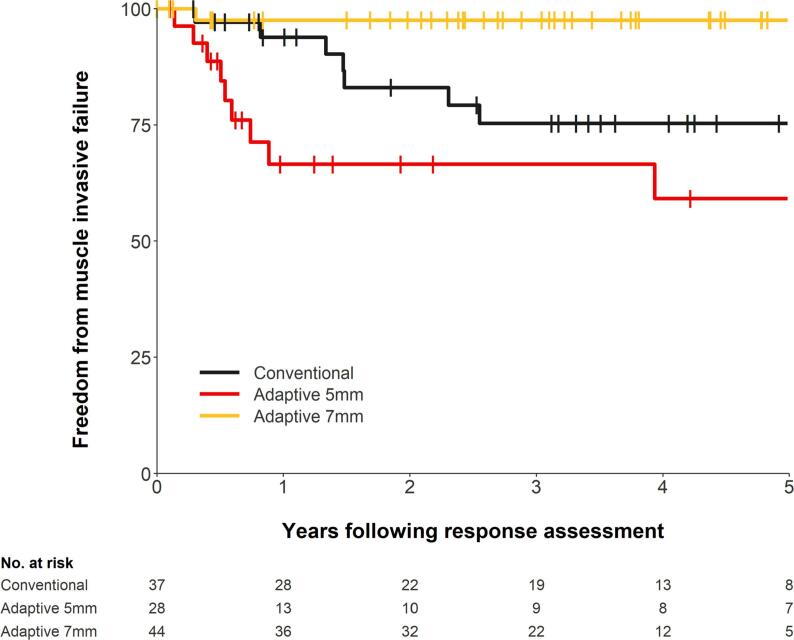
The median follow-up period of this study was 54 months. Patients who achieved CR at their response assessment had their time to local muscle invasive failure measured. Fig. 1 shows the time to local muscle invasive failure according to RT technique. One patient had no date of response assessment and was excluded from the analysis. The 5-year freedom from muscle invasive failure survival (FFMIFS) for the cRT, aRT5mm, and aRT7mm groups were 75% (95% CI: 55% – 87%), 59% (95% CI: 35% – 77%), and 98% (95% CI: 84% – 100%), respectively. The hazard ratio (HR) for aRT5mm vs cRT was 1.9 (95% CI: 0.7 – 5.0, p = 0.19) and the HR for aRT7mm vs cRT was 0.1 (95% CI: 0.0 – 0.8, p = 0.032).
Fig. 1.
Time to local muscle invasive failure on CR patients according to RT technique.
Six patients underwent cystectomy following initial CR at response assessment. Three were in the cRT group, two in aRT5mm group, and one in aRT7mm group.
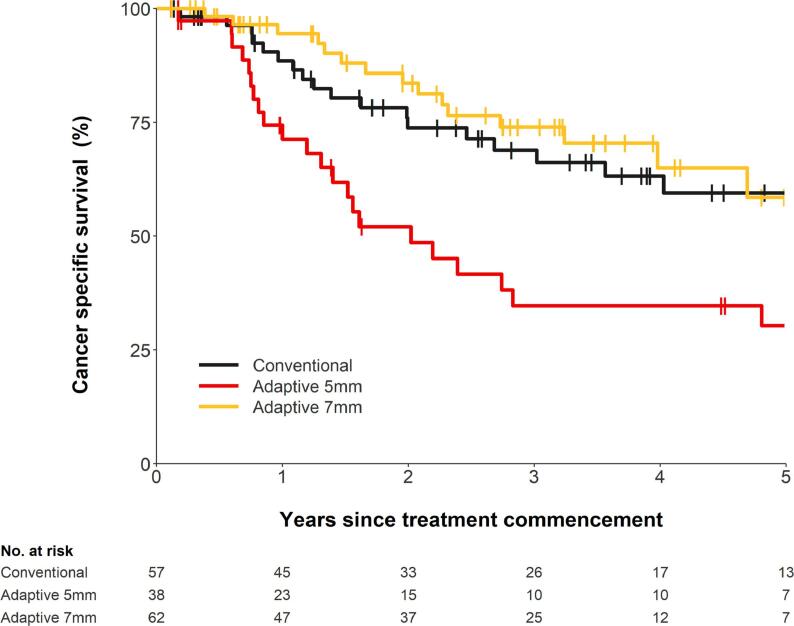
Of the total 171 patients, cancer specific survival (CSS) was assessed in 157 (92%) patients. Fourteen (8%) patients had their cause of death missing and were not included in the analysis. This is shown in Fig. 2. The 5-year CSS for the cRT, aRT5mm, and aRT7mm groups were 60% (95% CI: 43% − 73%), 30% (95% CI: 15% − 47%), and 59% (37% − 75%), respectively. The HR for aRT5mm vs cRT was 2.4 (95% CI: 1.3–4.4, p = 0.007) and the HR for aRT7mm vs cRT was 0.8 (95% CI: 0.4–1.6, p = 0.558).
Fig. 2.
Cancer specific survival according to RT technique.
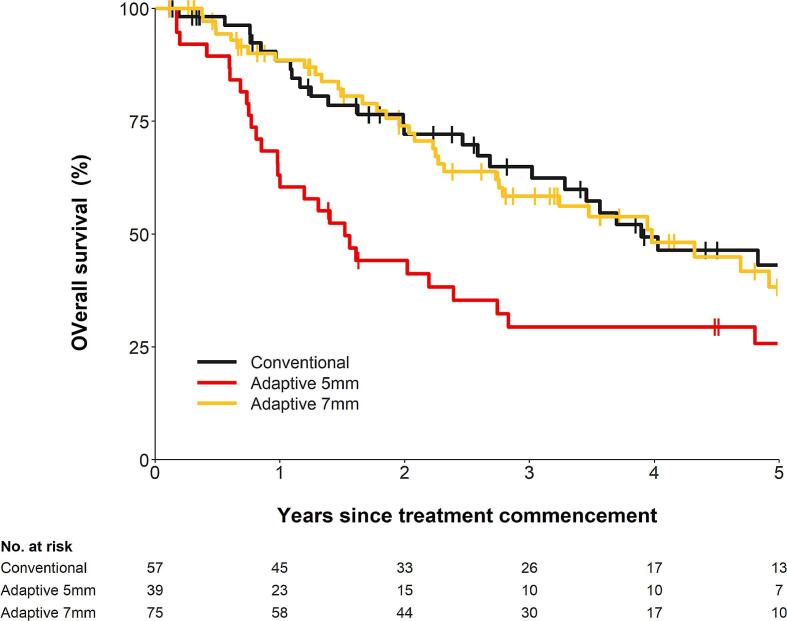
Overall survival was assessed on all 171 patients. This is shown in Fig. 3. The 5-year OS for the cRT, aRT5mm, and aRT7mm groups were 43% (95% CI: 28% − 58%), 26% (95% CI: 13% − 41%), and 38% (95% CI: 24% − 53%), respectively. The HR for aRT5mm vs cRT was 1.9 (95% CI: 1.1–3.1, p = 0.015) and the HR for aRT7mm vs cRT was 1.1 (95% CI: 0.7–1.9, p = 0.612).
Fig. 3.
Overall survival according to RT technique.
Toxicity
Adverse events were assessed on all patients. Table 3, Table 4 shows grade 3 or 4 adverse events after start of RT occurring in approximately eighteen percent of the whole cohort: acute (occurred within 90 days of start of RT) and late (occurred after 90 days from start of RT).
Table 3.
Treatment-related acute (<90 days from start of RT) grade 3 or 4 adverse events by RT technique.
| RT Technique |
|||
|---|---|---|---|
| Acute Adverse Event | Conventional |
Adaptive 5 mm |
Adaptive 7 mm |
| (n = 57) | (n = 39) | (n = 75) | |
| GU Toxicities | 6 (11%) | 11 (28%) | 4 (5%) |
| Anorexia | 0 | 0 | 1 (1%) |
| Rash | 0 | 2 (5%) | 0 |
| Fatigue | 0 | 1 (3%) | 0 |
| Number of patients experiencing at least one grade 3 or 4 toxicity | 6 (11%) | 12 (31%) | 5 (7%) |
Table 4.
Treatment-related late (90 + days from start of RT) grade 3 or 4 adverse events by RT technique.
| RT Technique |
|||
|---|---|---|---|
| Late Adverse Event | Conventional |
Adaptive 5 mm |
Adaptive 7 mm |
| (n = 57) | (n = 39) | (n = 75) | |
| GU Toxicities | 1 (2%) | 4 (10%) | 1 (1%) |
| GI Toxicity | 1 (2%) | 0 | 0 |
| Pain - Other | 0 | 2 (5%) | 0 |
| Fatigue | 0 | 1 (3%) | 0 |
| Number of patients experiencing at least one grade 3 or 4 toxicity | 2 (4%) | 5 (13%) | 1 (1%) |
When treatment-related acute and late grade 3 or 4 adverse events were combined, aRT5mm group had a higher rate of adverse events compared to cRT group (p = 0.015). This rate of adverse events for aRT7mm group was not statistically different than the cRT group (p = 0.238).
Discussion
For delineation of RT target volumes in MIBC, use of anisotropic CTV to PTV margins are recommended [15] given it has been shown that inter-fraction motion of the target is anisotropic, with greatest amplitude of motion in the cranial and anterior directions [16], [17], [18]. Choice of the size of CTV to PTV expansion margin largely depends on setup, verification, and treatment techniques. Foroudi et al. [18] demonstrated that when daily set-up is based on soft tissue imaging, a margin of 15 mm will provide coverage over 96%.
Adaptive RT with PoD technique based on patient specific CBCT allows for intra-fraction bladder size variability, with the ultimate goal of ensuring coverage of the target while sparing OAR. There have been five other publications describing different methods of individualised PoD based on CBCT approach. Three of these studies were retrospective planning studies [19], [20], [21], one study only evaluated two patients [22], and one study used their adaptive RT technique to treat patients in a phase II study evaluating MRI guided online adaptive re-optimisation of RT in MIBC [23]. A range of expansion margins to create PTV were used in these studies.
Adaptive Radiotherapy with strict IG for MIBC was first implemented at the PMCC in late 2007 [8]. In the pilot study, a 5 mm CTV to PTV expansion margin was initially used. This was then increased to 7 mm margin in the Trans-Tasman Radiation Oncology Group (TROG) 10.01 study, which reported on the feasibility of aRT when applied to 50 patients [9]. Considering the clinical outcomes of this study, despite it having all the caveats of a retrospective analysis, we can infer that 5 mm margin is too tight and the inferior results are likely related to geographical misses of the PTV due to intra-fraction motion.
In the modern era of IMRT, particularly volumetric modulated arc therapy (VMAT), the use of anisotropic expansion margins and daily IG with CBCT to ensure accuracy of treatment may be considered an ideal.
While we await results of the phase II RAIDER study, to the best of the authors’ knowledge, this is the first study that attempts to provide long term clinical data of personalised adaptive RT with PoD based on CBCT for radical intent whole bladder RT treatment for MIBC, as well as directly comparing this to non-adaptive RT technique.
When attempting to compare the outcomes of this study to other published radical RT in MIBC studies, care must be taken due to several differences in patient and treatment factors.
The overall rates of CR in all groups of patients in this study were comparable with those reported in the pooled analysis of multiple prospective RTOG studies which evaluated long term outcomes of bladder preserving combined modality therapy for MIBC [24]. However, the 5-year cancer specific survival (CSS) and overall survival (OS) rates in this retrospective study were lower. The cRT and aRT7mm groups had similar estimated 5-year CSS of 60% and 59%, respectively, while the pooled analysis had 5-year disease specific survival of 71%. This is likely due to the differences in patient characteristics as well as rates of inclusion of chemotherapy in their treatment, and thus difference in their systemic relapse risk. The median age of patients was significantly older in this retrospective study (79.4 years) than in the pooled analysis (66 years). Approximately half of the patients in this retrospective study did not receive any chemotherapy as part of their treatment, while all patients in the prospective RTOG studies did. The RTOG studies also included pelvic nodal volumes in the RT, while this study did not. The estimated 5-year OS in the cRT and aRT7mm groups were again similar to each other, at 43% and 38%, respectively, with a hazard ratio of 1.1 and p-value of 0.612.
Murthy et al. [25] reported on clinical outcomes following their adaptive PoD approach in bladder carcinoma using intensity modulated radiation therapy (IMRT) with a shorter median follow up period than this study. Their PoDs were generated from the planning CT, using concentric anisotropic margins around the primary CTV, thus not individualised to patients’ daily CBCT. Their 3-year disease free survival rate and OS rates were 62.9% and 67.7%, respectively. Results from this study also cannot be directly compared to the results of our study as there were many differences including the median age of patients being younger in the Murthy et al. study (65.5 years), a greater proportion of the evaluable 106 patients received chemotherapy as part of their treatment, and the RT technique included elective nodal volumes and allowed for simultaneous integrated boost (SIB) for selected patients.
The impact of adding chemotherapy to RT is well known and shown in the randomised phase 3 BC2001 trial to be associated with a 33% relative reduction in the risk of locoregional recurrence, and reduction of almost 50% in invasive recurrence [26]. Extended median 10-year follow-up of this study population has been presented in abstract form only at the time of writing this paper, which further confirms the improvement in locoregional control and invasive locoregional control in the chemoradiotherapy arm [27]. Other chemotherapy regimens have also been shown to be acceptable [24].
Overall rates of reported acute grade 3 or 4 toxicity in this retrospective study was 13% in the entire cohort of patients, while the reported treatment-related late grade 3 or 4 toxicity was even lower at 5%. This implies that the treatment was generally well tolerated. These values are lower than those reported in other published studies [28], [29], [30], acknowledging that the treatment received in these studies all included concurrent chemotherapy, and the RTOG studies would have also encompassed pelvic nodal volumes in the RT delivered. Another likely factor is that toxicities in retrospective studies are more frequently underreported compared to clinical trials.
It is unclear the reasons for the observed higher rate of toxicity in the aRT5mm group. We suspect that a bigger proportion of patients in the aRT5mm group were treated as part of a prospective pilot study [8] than in the cRT and aRT7mm patient groups. The prospective study would have specified stricter recording of toxicity data, and thus leading to an imbalance of results when compared to retrospective collection of general medical records where toxicity data is more likely to be under-reported. Care must therefore also be taken when considering these toxicity results given the added bias.
One of the main purposes of adopting an adaptive protocol was to reduce the dose to OAR and therefore reduce the chance of toxicities. The rate of grade 3 or 4 toxicity in the aRT7mm group was 8%, while it was 14% in the cRT group. This study did not provide clear evidence that aRT7mm indeed reduces the toxicity rate, however the results suggest this may be true and a larger study is required to confirm.
Several inherent limitations apply to this retrospective study, most importantly relating to missing data, whether it is due to patients being lost to follow-up, or non-compliance with attending appointments, patients dying or developing metastatic disease prior to the first response cystoscopy assessment. There would be variability of inter-clinician toxicity reporting. However, we would expect this variation to be lower with higher grades of toxicity that require intervention.
Progression in technology and radiation techniques allowing for dynamic volumetric intensity modulated radiation therapy, as well as magnetic resonance imaging (MRI) guidance and implanted fiducial markers also offers a means of more accurate, rapid, image-guided delivery of the daily dose of radiation that can overcome the concerns of bladder filling and organ motion [31], [32], [33], [34], [35], [36].
Conclusion
This retrospective study shows that image guided adaptive RT using isotropic 7 mm CTV to PTV expansion margin can be considered safe and effective to treat MIBC when compared to non-adaptive, conventional RT. It also highlights the importance of selecting an adequate expansion margin when planning adaptive RT, as a smaller 5 mm margin has been shown to lead to inferior cancer control outcomes.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Antoni S., Ferlay J., Soerjomataram I., Znaor A., Jemal A., Bray F. Bladder Cancer Incidence and Mortality: A Global Overview and Recent Trends. Eur Urol. 2017;71(1):96–108. doi: 10.1016/j.eururo.2016.06.010. [DOI] [PubMed] [Google Scholar]
- 2.Giacalone N.J., Shipley W.U., Clayman R.H., Niemierko A., Drumm M., Heney N.M. Long-term Outcomes After Bladder-preserving Tri-modality Therapy for Patients with Muscle-invasive Bladder Cancer: An Updated Analysis of the Massachusetts General Hospital Experience. Eur Urol. 2017;71(6):952–960. doi: 10.1016/j.eururo.2016.12.020. [DOI] [PubMed] [Google Scholar]
- 3.Vashistha V., Wang H., Mazzone A., Liss M.A., Svatek R.S., Schleicher M. Radical Cystectomy Compared to Combined Modality Treatment for Muscle-Invasive Bladder Cancer: A Systematic Review and Meta-Analysis. Int J Radiat Oncol Biol Phys. 2017;97(5):1002–1020. doi: 10.1016/j.ijrobp.2016.11.056. [DOI] [PubMed] [Google Scholar]
- 4.Meijer G.J., Rasch C., Remeijer P., Lebesque J.V. Three-dimensional analysis of delineation errors, setup errors, and organ motion during radiotherapy of bladder cancer. Int J Radiat Oncol Biol Phys. 2003;55(5):1277–1287. doi: 10.1016/s0360-3016(02)04162-7. [DOI] [PubMed] [Google Scholar]
- 5.Hindson B.R. Australian & New Zealand Faculty of Radiation Oncology Genito-Urinary Group: 2011 consensus guidelines for curative radiotherapy for urothelial carcinoma of the bladder. J Med Imaging Radiat Oncol. 2012;56(1):18–30. doi: 10.1111/j.1754-9485.2011.02336.x. [DOI] [PubMed] [Google Scholar]
- 6.Kibrom A.Z., Knight K.A. Adaptive radiation therapy for bladder cancer: a review of adaptive techniques used in clinical practice. J Med Radiat Sci. 2015;62(4):277–285. doi: 10.1002/jmrs.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kong V.C., Taylor A., Rosewall T. Adaptive Radiotherapy for Bladder Cancer-A Systematic Review. J Med Imaging Radiat Sci. 2017;48(2):199–206. doi: 10.1016/j.jmir.2016.10.014. [DOI] [PubMed] [Google Scholar]
- 8.Foroudi F., Wong J., Kron T., Rolfo A., Haworth A., Roxby P. Online adaptive radiotherapy for muscle-invasive bladder cancer: results of a pilot study. Int J Radiat Oncol Biol Phys. 2011;81(3):765–771. doi: 10.1016/j.ijrobp.2010.06.061. [DOI] [PubMed] [Google Scholar]
- 9.Foroudi F., Pham D., Rolfo A., Bressel M., Tang C.I., Tan A. The outcome of a multi-centre feasibility study of online adaptive radiotherapy for muscle-invasive bladder cancer TROG 10.01 BOLART. Radiother Oncol. 2014;111(2):316–320. doi: 10.1016/j.radonc.2014.02.015. [DOI] [PubMed] [Google Scholar]
- 10.Pham D., Roxby P., Kron T., Rolfo A., Foroudi F. Introduction of online adaptive radiotherapy for bladder cancer through a multicentre clinical trial (Trans-Tasman Radiation Oncology Group 10.01): Lessons learned. J Med Phys. 2013;38(2):59. doi: 10.4103/0971-6203.111308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Foroudi F., Pham D., Bressel M., Tongs D., Rolfo A., Styles C. The utility of e-Learning to support training for a multicentre bladder online adaptive radiotherapy trial (TROG 10.01-BOLART) Radiother Oncol. 2013;109(1):165–169. doi: 10.1016/j.radonc.2012.10.019. [DOI] [PubMed] [Google Scholar]
- 12.Kron T., Pham D., Roxby P., Rolfo A., Foroudi F. Credentialing of radiotherapy centres for a clinical trial of adaptive radiotherapy for bladder cancer (TROG 10.01) Radiother Oncol. 2012;103(3):293–298. doi: 10.1016/j.radonc.2012.03.003. [DOI] [PubMed] [Google Scholar]
- 13.Tunio M.A., Hashmi A., Qayyum A., Mohsin R., Zaeem A. Whole-pelvis or bladder-only chemoradiation for lymph node-negative invasive bladder cancer: single-institution experience. Int J Radiat Oncol Biol Phys. 2012;82(3):e457–e462. doi: 10.1016/j.ijrobp.2011.05.051. [DOI] [PubMed] [Google Scholar]
- 14.Landberg, T., et al., Report 50. Journal of the International Commission on Radiation Units and Measurements, 2016. os26(1): p. NP-NP.
- 15.Khalifa J., Supiot S., Pignot G., Hennequin C., Blanchard P., Pasquier D. Recommendations for planning and delivery of radical radiotherapy for localized urothelial carcinoma of the bladder. Radiother Oncol. 2021;161:95–114. doi: 10.1016/j.radonc.2021.06.011. [DOI] [PubMed] [Google Scholar]
- 16.Yee D., Parliament M., Rathee S., Ghosh S., Ko L., Murray B. Cone beam CT imaging analysis of interfractional variations in bladder volume and position during radiotherapy for bladder cancer. Int J Radiat Oncol Biol Phys. 2010;76(4):1045–1053. doi: 10.1016/j.ijrobp.2009.03.022. [DOI] [PubMed] [Google Scholar]
- 17.Lalondrelle S., Huddart R., Warren-Oseni K., Hansen V.N., McNair H., Thomas K. Adaptive-predictive organ localization using cone-beam computed tomography for improved accuracy in external beam radiotherapy for bladder cancer. Int J Radiat Oncol Biol Phys. 2011;79(3):705–712. doi: 10.1016/j.ijrobp.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 18.Foroudi F., Pham D., Bressel M., Wong J., Rolfo A., Roxby P. Bladder cancer radiotherapy margins: a comparison of daily alignment using skin, bone or soft tissue. Clin Oncol (R Coll Radiol) 2012;24(10):673–681. doi: 10.1016/j.clon.2012.06.012. [DOI] [PubMed] [Google Scholar]
- 19.Vestergaard A., Søndergaard J., Petersen J.B., Høyer M., Muren L.P. A comparison of three different adaptive strategies in image-guided radiotherapy of bladder cancer. Acta Oncol. 2010;49(7):1069–1076. doi: 10.3109/0284186X.2010.501813. [DOI] [PubMed] [Google Scholar]
- 20.Tolan S., Kong V., Rosewall T., Craig T., Bristow R., Milosevic M. Patient-specific PTV margins in radiotherapy for bladder cancer - a feasibility study using cone beam CT. Radiother Oncol. 2011;99(2):131–136. doi: 10.1016/j.radonc.2011.04.008. [DOI] [PubMed] [Google Scholar]
- 21.Tuomikoski L., Valli A., Tenhunen M., Muren L., Vestergaard A. A comparison between two clinically applied plan library strategies in adaptive radiotherapy of bladder cancer. Radiother Oncol. 2015;117(3):448–452. doi: 10.1016/j.radonc.2015.10.012. [DOI] [PubMed] [Google Scholar]
- 22.Wright P., Muren L.P., Høyer M., Malinen E. Evaluation of adaptive radiotherapy of bladder cancer by image-based tumour control probability modelling. Acta Oncol. 2010;49(7):1045–1051. doi: 10.3109/0284186X.2010.498431. [DOI] [PubMed] [Google Scholar]
- 23.Grønborg C., Vestergaard A., Høyer M., Söhn M., Pedersen E.M., Petersen J.B. Intra-fractional bladder motion and margins in adaptive radiotherapy for urinary bladder cancer. Acta Oncol. 2015;54(9):1461–1466. doi: 10.3109/0284186X.2015.1062138. [DOI] [PubMed] [Google Scholar]
- 24.Mak R.H., Hunt D., Shipley W.U., Efstathiou J.A., Tester W.J., Hagan M.P. Long-term outcomes in patients with muscle-invasive bladder cancer after selective bladder-preserving combined-modality therapy: a pooled analysis of Radiation Therapy Oncology Group protocols 8802, 8903, 9506, 9706, 9906, and 0233. J Clin Oncol. 2014;32(34):3801–3809. doi: 10.1200/JCO.2014.57.5548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Murthy V., Gupta P., Baruah K., Krishnatry R., Joshi A., Prabhash K. Adaptive Radiotherapy for Carcinoma of the Urinary Bladder: Long-term Outcomes With Dose Escalation. Clin Oncol (R Coll Radiol) 2019;31(9):646–652. doi: 10.1016/j.clon.2019.06.005. [DOI] [PubMed] [Google Scholar]
- 26.James N.D., Hussain S.A., Hall E., Jenkins P., Tremlett J., Rawlings C. Radiotherapy with or without chemotherapy in muscle-invasive bladder cancer. N Engl J Med. 2012;366(16):1477–1488. doi: 10.1056/NEJMoa1106106. [DOI] [PubMed] [Google Scholar]
- 27.Hall E., Hussain S.A., Porta N., Crundwell M., Jenkins P., Rawlings C.L. BC2001 long-term outcomes: A phase III randomized trial of chemoradiotherapy versus radiotherapy (RT) alone and standard RT versus reduced high-dose volume RT in muscle-invasive bladder cancer. J Clin Oncol. 2017;35(6_suppl):280. [Google Scholar]
- 28.Huddart R.A., Hall E., Hussain S.A., Jenkins P., Rawlings C., Tremlett J. Randomized noninferiority trial of reduced high-dose volume versus standard volume radiation therapy for muscle-invasive bladder cancer: results of the BC2001 trial (CRUK/01/004) Int J Radiat Oncol Biol Phys. 2013;87(2):261–269. doi: 10.1016/j.ijrobp.2013.06.2044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gogna N.K., Matthews J.H.L., Turner S.L., Mameghan H., Duchesne G.M., Spry N. Efficacy and tolerability of concurrent weekly low dose cisplatin during radiation treatment of localised muscle invasive bladder transitional cell carcinoma: a report of two sequential Phase II studies from the Trans Tasman Radiation Oncology Group. Radiother Oncol. 2006;81(1):9–17. doi: 10.1016/j.radonc.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 30.Efstathiou J.A., Bae K., Shipley W.U., Kaufman D.S., Hagan M.P., Heney N.M. Late pelvic toxicity after bladder-sparing therapy in patients with invasive bladder cancer: RTOG 89–03, 95–06, 97–06, 99–06. J Clin Oncol. 2009;27(25):4055–4061. doi: 10.1200/JCO.2008.19.5776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mangar S., Thompson A., Miles E., Huddart R., Horwich A., Khoo V. A feasibility study of using gold seeds as fiducial markers for bladder localization during radical radiotherapy. Br J Radiol. 2007;80(952):279–283. doi: 10.1259/bjr/54321311. [DOI] [PubMed] [Google Scholar]
- 32.Chai X., van Herk M., van de Kamer J.B., Remeijer P., Bex A., Betgen A. Behavior of lipiodol markers during image guided radiotherapy of bladder cancer. Int J Radiat Oncol Biol Phys. 2010;77(1):309–314. doi: 10.1016/j.ijrobp.2009.08.019. [DOI] [PubMed] [Google Scholar]
- 33.Pos F., Bex A., Dees-Ribbers H.M., Betgen A., van Herk M., Remeijer P. Lipiodol injection for target volume delineation and image guidance during radiotherapy for bladder cancer. Radiother Oncol. 2009;93(2):364–367. doi: 10.1016/j.radonc.2009.09.003. [DOI] [PubMed] [Google Scholar]
- 34.Whalley D. Promising results with image guided intensity modulated radiotherapy for muscle invasive bladder cancer. Radiat Oncol. 2015;10:205. doi: 10.1186/s13014-015-0499-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hunt A., Hanson I., Dunlop A., Barnes H., Bower L., Chick J. Feasibility of magnetic resonance guided radiotherapy for the treatment of bladder cancer. Clin Transl Radiat Oncol. 2020;25:46–51. doi: 10.1016/j.ctro.2020.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hijab A. MR-Guided Adaptive Radiotherapy for Bladder Cancer. Front Oncol. 2021;11 doi: 10.3389/fonc.2021.637591. [DOI] [PMC free article] [PubMed] [Google Scholar]