Abstract
Lumbar pars interarticularis (PI) injury or spondylolysis occurs only in humans. This represents a stress fracture of the PI. Excessive loading in repetitive hyperextension is a significant risk factor and occurs most commonly at L5 followed by L4. It is bilateral in 80% of symptomatic cases but can be unilateral defect as well which runs a more benign course. Symptoms of low back pain relating to this lesion are more common in young athletes involved in trunk twisting sports. Like other stress fractures, the pain may come on abruptly or more insidiously over time and only related to certain activities. The pathologic progression starts with a stress reaction in the pars, progressing to an incomplete stress fracture, and then a complete pars fracture. Diagnosis is dependent on clinical examination and radiological imaging studies (plain radiography, computed tomography (CT) scans and magnetic resonance imaging (MRI) scans). Treatment is dependent on symptoms as well as radiographic stage of the lesion. Conservative management is the mainstay of treating early lesions. A comprehensive rehabilitation program incorporates core spinal stabilization exercises. Athletes should not return to sports until pain free. Professional sporting individuals are at increased risk of failure of resolution of symptoms that may require early surgical repair of the PI defect. Modified Buck's technique & pedicle screw-hook constructs for direct repair has a high success rate in patients who have persistent low back pain. Minimally invasive lumbar pars defect repair has given similar successful outcome with added advantage of minimizing muscle injury, preserving the adjacent joint and reduced hospital stay. Functional outcome is evaluated using the Visual Analogue Scale (VAS) for back pain, Oswestry Disability Index (ODI) and 36-Item Short-Form Health Survey (SF-36). Preoperative ODI and SF-36 physical component scores (PCS) are significant predictor of a good functional outcome.
Keywords: Lumbar spondylolysis, Pars interarticularis defect, Pars defect, Pars stress injury, Direct repair
1. Etiology
Spondylolysis is derived from the Greek spondylo meaning spine and lysis meaning to dissolve. Lumbar Spondylolysis is defined as a defect or abnormality of the pars interarticularis. Only humans have lumbar pars interarticularis defect (PI).1, 2, 3 The first reflection of genetic influence in lumbar spondylolysis was shown in families by Wiltse in 1962.4 During childhood the incidence is 4.4% which increases to 6% in adulthood.5 The incidence is approximately 15–47%6, 7, 8 in the young sporting population. Both hereditary and acquired risk factors have been suggested with an increased prevalence in young sporting men actively involved in certain high-risk sports.
Lumbar PI defect runs an unpredictable course.9 The first sign of stress fracture is a subtle stress reaction within the pars and the mode of failure is fatigue.1 Repetitive hyperextension and excessive loading of pars in the sporting population has been suggested as a significant risk factor.
The fact that pars defect was not found in other primates suggested the theory of congenital weakness of the pars in humans.10 The incidences of PI defects in Eskimos are as high as 20–50%.11 But it was highly unlikely that the PI defects caused severe disability in these Eskimos. In the Canadian Eskimos, the incidence of PI defect doubles by early adulthood with equal incidence of complete and incomplete spondylolysis.12
The current consensus favors PI defect as a developmental lesion occurring sometime in childhood. There are two peaks of presentation, i.e. one occurs between 5 and 7 years of age and another peak in teenage years.13 In early teenage period it presents as a continuum of stress reaction which can progress to incomplete and then a complete defect. It is suggested that two factors may contribute towards the developmental PI lesion. Firstly, ossification of lower lumbar segment is not uniform.14 Secondly, a differential bone density between PI and base of pedicle in lower lumbar spine.14 This change in trabecular bone density may result in an area of weakness or stress riser. Further loading may lead to fatigue failure of an already weak PI. Another theory suggests that the insufficient increases in the inter-facet distances may increase the pressure during hyperextension leading to development of pars defect.2 A recent biomechanical analysis suggested that combination of flexion, axial rotation and compression induced highest stress concentration increasing the risk of lumbar spondylolysis.15 In patients with higher pelvic incidence and sacral slope the stresses on the PI intensify.15 This abnormal spino-pelvic balance alters the biomechanical stresses at the lumbo-sacral junction.16
In a large comparative study of single segment PI defect against multi-level lumbar PI defects, it was observed that subjects with multi-level spondylolysis were characterized by insufficiency of lower lumbar segmental lordosis and retroverted pelvis.17
1.1. Historical review
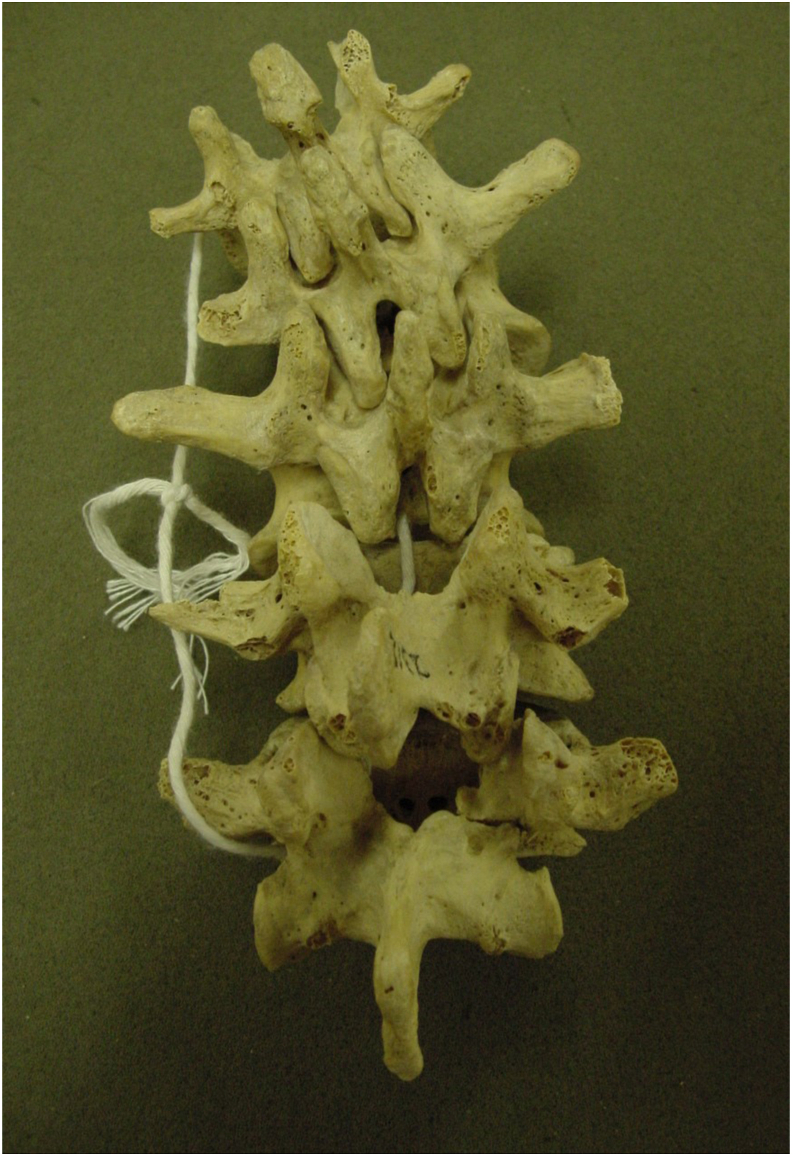
‘Spondylolysis’ was first described by George Murray Humphrey (1858), a surgeon from Cambridge.18,19 In 1855, Prof Robert du Coblenz from Marburg, Germany described the importance of PI integrity in prevention of vertebral olisthesis.19 Three decades later, in 1884 Franz Neugebauer from Warsaw, first published the link between PI defect and erect human posture.18 The theory of “fatigue failure” was suggested by Wiltse (1957) which was supported by subsequent researchers.1 The prevalence of PI defect was about 6% in archeological skeletons of Romano-British populations20 (Fig. 1). In Native American skeletons from 6000 to 1000BC, the prevalence was 20%.18 This prevalence rises to 50% in skeletal remains of Eskimos.11 There was no significant variation between race and gender in 485 caucasian skeletons studied by Eisenstein.21
Fig. 1.
Five lumbar vertebrae in a 13th century skeleton with a unilateral defect of the right pars at L5 vertebra. (From Hamann Todd Collection, Natural History Museum, Cleveland, USA).
1.2. Pain source
The usual clinical presentation is complains of low back pain (LBP) on extension without radicular pain. Soft tissues in PI defect termed ‘spondylolysis ligament’ has been suggested a distinct source of pain since it contains nociceptive pain fibres.22 These neural elements may be stretched which may lead to defective healing of the PI defect.23 Associated degenerate disc or facet joint may be the other sources of back pain. Ciullo (1985) suggested that pain may be due to acute stress fractures or segmental instability at the PI defect.10 However, pain due to instability remains a controversial issue.24
1.3. Evolution of symptoms
Athletes have a greater frequency of symptomatic lesions presenting at an earlier age than asymptomatic subjects. However, in many young sportsmen, repeated microtrauma may present as a new acute PI defect. In one study, 95% patients with spondylolysis were sporting subjects (i.e. American footballers, gymnasts, weight lifters, wrestlers and tennis players).5,9 Lumbar pars defect is also prevalent in soccer players, cricketers, baseball players, rowers, swimmers and divers.7,25 Repetitive hyperextension leads to stress on the inferior articular facet e.g. fast bowling in cricket and many gymnastic exercises. One recent study suggested sports-specific movements and lower limb dominance should be considered in young sporting subjects with symptomatic spondylolysis.25 Cortical fatigue resulting in PI stress fractures has been reproduced in vitro.26 One must remember that a physiological stress reaction within PI precedes the fatigue failure. It is also important for the clinician to appreciate that not every initial stress reaction develop into a fracture and pain occurs in a select few.
The young sporting subjects may present with early onset LBP with complains of more pain than general population. Sporting activities and age of the PI defect has direct relationship with the development of pain. Functionally, the sporting subjects suffer more disability due to pain than the general population with the lumbar PI defect. Although many think that the incidence of LBP increases with age, on the contrary a long term study observed LBP did not increase with aging.8 This observation is debatable since a degenerate disc at L4/5 is present frequently in a L5 pars defect even without a vertebral slip.27 The strong ilio-lumbar ligaments render the L5/S1 segment more stable with a PI defect at L5. If LBP continues to progress with aging in spondylolysis, it is suggested that the pain generator may be either the L4/5 disc or the spondylolytic defect itself.
1.4. Natural history
PI defects are staged as early, progressive and terminal on CT scans.28 In an early stage, a hairline crack is visible in the pars. When a gap occurs it is in progressive stage. The terminal stage is visualized as pseudoarthrosis. Early stage defects have high potential for healing on its own. Many subjects in progressive and terminal stage may not heal but may remain asymptomatic. The unpredictable natural history of the progression of the spondylolytic defect to vertebral slip puts the clinician in a difficult situation. Moreover, the severity of spondylolysis varies with location. Majority of L5 spondylolysis exhibits terminal-stage defects (63%), while most L3/L4 spondylolysis exhibits early-stage defects.29
1.5. Development of olisthesis
Unilateral PI defects did not develop into spondylolisthesis or significant disability.8 The risk of the progression of spondylolisthesis in patients with bilateral pars defect is similar to that of the general population, but there is marked slowing of slip progression with each decade.8 In immature lumbar spine of children (9–15year), slippage was more prevalent.9 This slippage halts with vertebral maturity.30 When a child or adolescent presents with bilateral PI defect of L5, it is recommended to counsel the parents that a 5% incidence of progression of vertebral slip may occur in adulthood.8 There is no evidence that competitive sports increase the risk of progression of olisthesis.31,32
1.6. Diagnosis
A typical presentation is unilateral LBP. The incidence of LBP is higher in athletes who are involved in twisting or hyperextension of lumbar spine.6,33 Most common location is at L5 (85–95%) and is usually bilateral. Second most common site is L4 (10–15%).3, 4, 5,7,34 The pain is accentuated by the standing one-leg lumbar extension stance i.e. Stork test. Few symptomatic patients may complain of lumbar paraspinous spasms and pain throughout the range of motion. A high index of suspicion is required for early diagnosis in a young sporting subject complaining of LBP increased by sporting activity. It is imperative to rule out other causes for LBP e.g. discogenic, facet mediated, myofascial or sacro-iliac origin of pain.
1.7. Imaging
Radiological imaging helps the clinician in diagnosis as well as prognostication of PI defect. It is recommended to diagnose early PI injuries which have a higher potential for complete healing. Magnetic Resonance Imaging (MRI) and Computerised Tomography (CT) scans can detect stage of the pars defect dictating the need for conservative treatment or surgical intervention.35 Planar bone scintigraphy (PBS) and single-photon- emission computed tomographies (SPECT) are quite sensitive in detecting PI lesions but their use has declined in clinical practice.36, 37, 38
The reverse gantry CT scan is most specific imaging for demonstrating a pars defect where the scan plane is perpendicular to the defect.39 The morphology of PI defects i.e. site, orientation and width of the defect are detailed well on CT scans. These help in preoperative planning for internal fixation.39,40 For identifying occult lesions, CT scan is again the imaging of choice.
Currently, routine use of MRI scans allows for early diagnosis and treatment of patients with suspected lumbar PI stress injuries.41 MRI provides more sensitive imaging in PI lesions since subtle bone marrow edema in early stress injuries can be easily detected. Based on MRI scans, Hollenberg et al. (2002) classified the PI defect into five grades i.e. grade 0 - normal to grade 4 – complete spondylolysis.42 MRI scans can predict which lesions would heal.35 Although, MRI scans are not accurate in visualizing the cortical integrity of incomplete fractures.43
2. Management
2.1. Non operative treatment
The aim of managing acute painful spondylolysis is to eliminate movement across the PI defect. Conservative treatment depends on the clinical severity, age, type of sports and level of sporting activity. Non operative management includes: pain relief medication (NSAIDs), avoiding sporting activities, core muscle stabilization & training and brace treatment. At least three to six months of bracing and rest from sporting activities has been recommended.
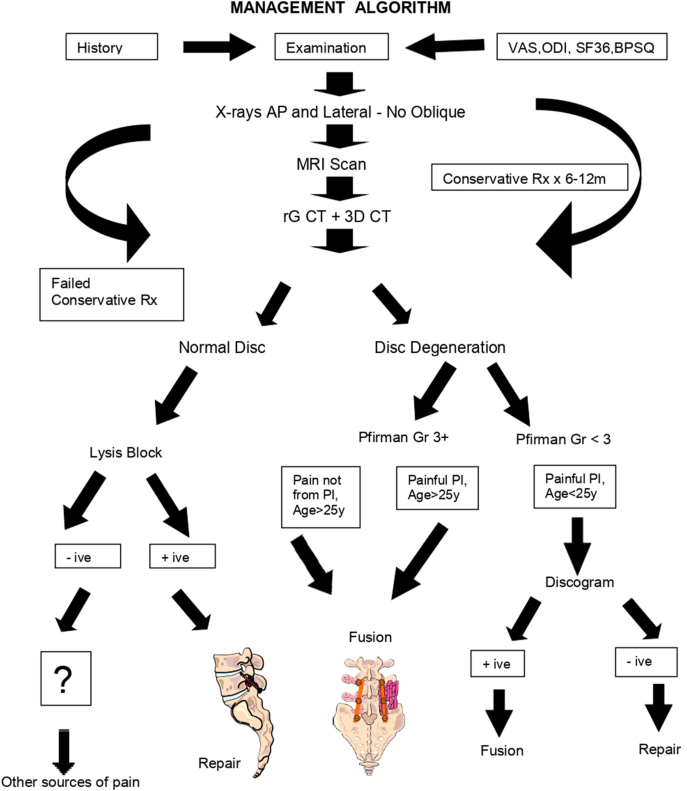
A period of physical therapy followed the initial bracing.44 The rehabilitation program focuses on hamstring stretching, core strengthening, and pelvic tilts. Progressive increase in lumbar range of motion to normal limits without symptoms may allow the clinician to decide on return to sports. Most often the decision is not based on healing of the PI defect. Healing potential for symptomatic unilateral defects was higher than bilateral defects.8 Eleven (11) athletes with unilateral spondylolysis had a 100% healing rate.45 The most important predictor of successful union was dependent on the stage of the PI defect.28 Non-surgical treatment for six to twelve months is the gold standard for managing patients with either unilateral or bilateral spondylolysis.46, 47, 48 An algorithm for management of LBP due to lumbar PI injuries is suggested (Fig. 2).
Fig. 2.
Algorithm for management of Lumbar Spondylolysis.
2.2. Surgical treatment
Usually most patients recover from pain following conservative management. However, few young sporting individuals experience disabling symptoms who do not respond to non-surgical management preventing them from sporting activity. These patients may require surgical intervention (approximately 9–15% cases undergo surgery).49 About 5.4% athletes required surgery.34 Postero-lateral arthrodesis (fusion) with or without excision of posterior elements was performed till early 70s before introduction of direct repair technique. Fusion resulted in loss of motion segment which increased the risk of adjacent segment degeneration.
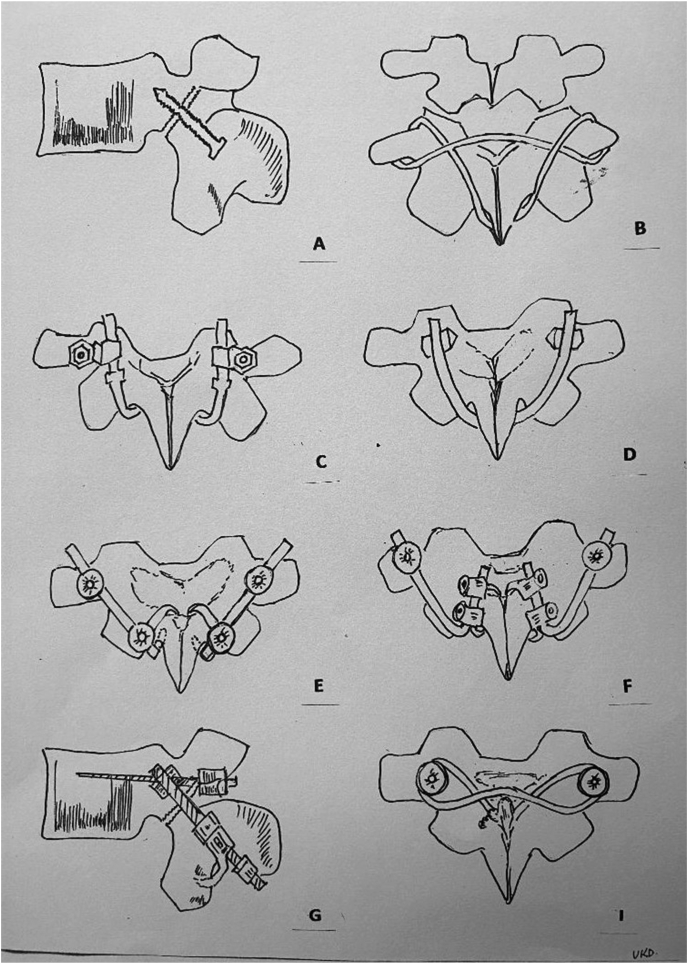
The rationale for direct repair of PI defect is to eliminate the pain locus. The pars defect as the primary pain generator is confirmed by image guided local anaesthetic injection.50 The credibility for pars injection as diagnostic modality is due to the presence of nociceptive free nerve endings in the PI defect.22,23 Several techniques to stabilize a lumbar spondylolytic defect are described (Fig. 3A–I). Previous authors have published the techniques of direct repair: Buck (1970), Morscher hook screw (1984), Scott wiring (1987) and many other methods.51, 52, 53 Screw fixation for PI defects resulted in good outcome in non-sporting as well as sporting population.54, 55, 56, 57
Fig. 3.
Schematic diagram of different types of direct repair. A: Buck's direct repair: use of a single cortical screw across the pars defect, B: Scott's repair: A SS wire is passed circumferentially around the transverse and spinous process like a tension band wiring, C: Morscher's technique: Pedicle screw-laminar hook construct, D: Gillet & Petit technique: Pedicle screw with an U shaped rod under the spinous process, E: Tokuhashi technique: A titanium pedicle screw hook system (standard or polyaxial pedicle screw and a rod–laminar hook complex) either single inferior laminar hook or a claw construct, F: Ishida technique: Pedicle screw- Claw hook construct (superior and inferior laminar hook were connected to form a claw), G: Kakiuchi technique: Rod is attached to pedicle screw with variable angle eyebolt and the laminar hook is attached to the rod, I: Songer & Rovin technique: Pedicle screws inserted superiorly and a 1mm double cable passed through the pedicle screw holes on opposite sides with passage of cable sublamilarly from caudal to cranial followed by tensioning and crimping under spinous process.
3. Biomechanical basis of surgical treatment
The intervertebral mobility at the level of PI defect as well as at the upper adjacent level was increased in a biomechanical study of calf lumbar spines.58 Buck's screw repair stabilizes both the lytic and the adjacent segment simultaneously.51 Buck's fixation with screws provided the stiffest and strongest fixation.59 Comparison of techniques of direct repair showed Buck's and Morscher's hook-rod-screw system stabilized the motion segments in flexion.60 The properties of ideal fixation device for repair of PI defects are: 1) stabilize the motion segment 2) negate the extension & torsional forces, 3) must be of low profile to avoid the irritation of adjacent facet joint, and 4) allows for bone grafting.
3.1. Direct repair
Direct repair of lumbar spondylolysis with bone grafts alone was first published by Kimura (1968).61 This technique showed preservation of motion segment although the patient was subjected to lie in bed for 2months followed by lumbosacral support for 4–6months. Buck (1970) described for the first time, bilateral screw fixation across the PI defect.51 This was a difficult technique especially for L5 defects since there was a wide variation in the orientation of the defect. In this technique, PI defect is decorticated and a cortical bone screw is inserted like fixing a fracture (Fig. 4A, Fig. 4CA–D). This technique was followed by Morscher hook-screw52 and Scott's tension band wiring.53 Scott reported good results in seven (7) patients.53 Scott wiring has many disadvantages such as less stiffness, intraoperative complications, and wire breakage. Also, bony union across the defect is a suspect with Scott wiring.60
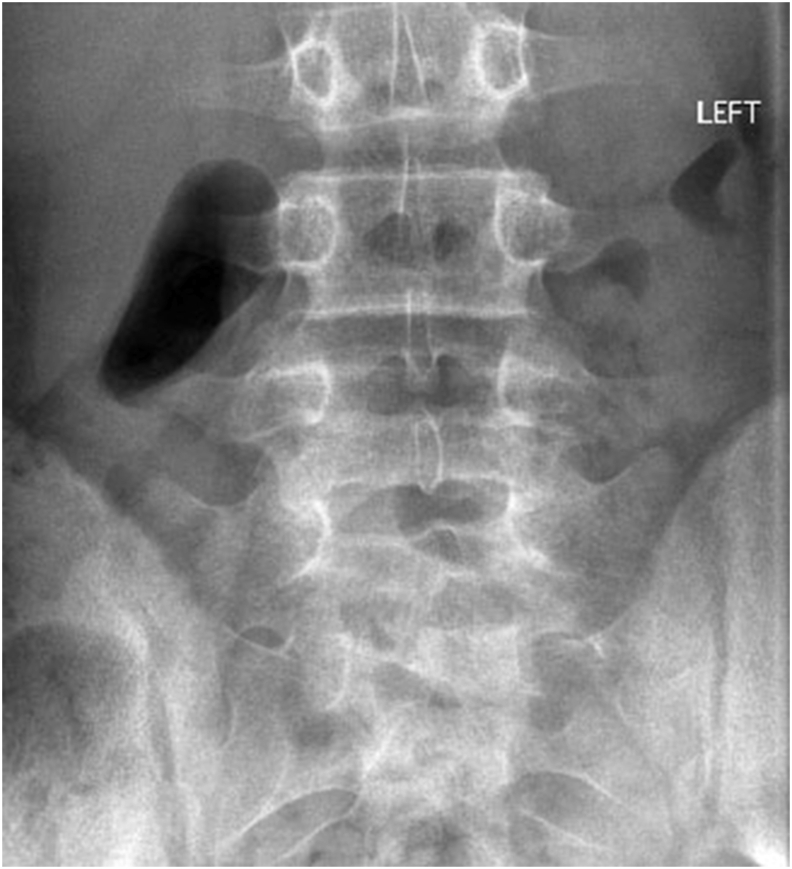
Fig. 4A.
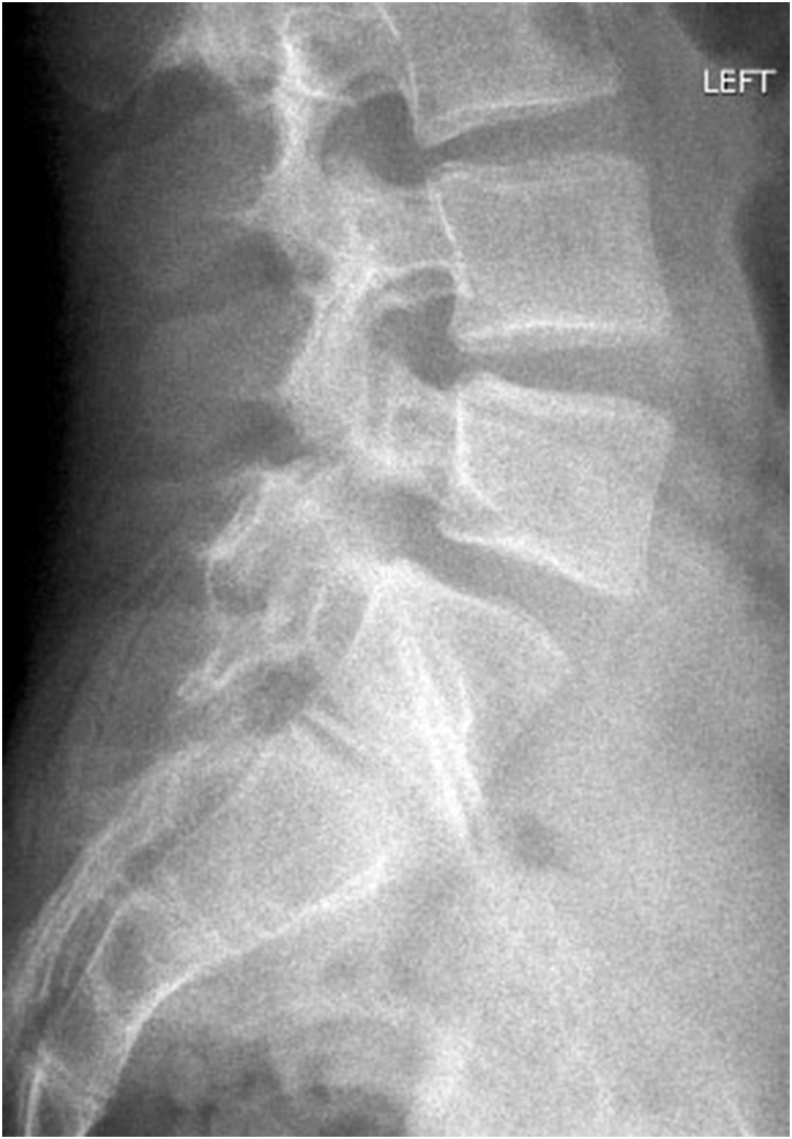
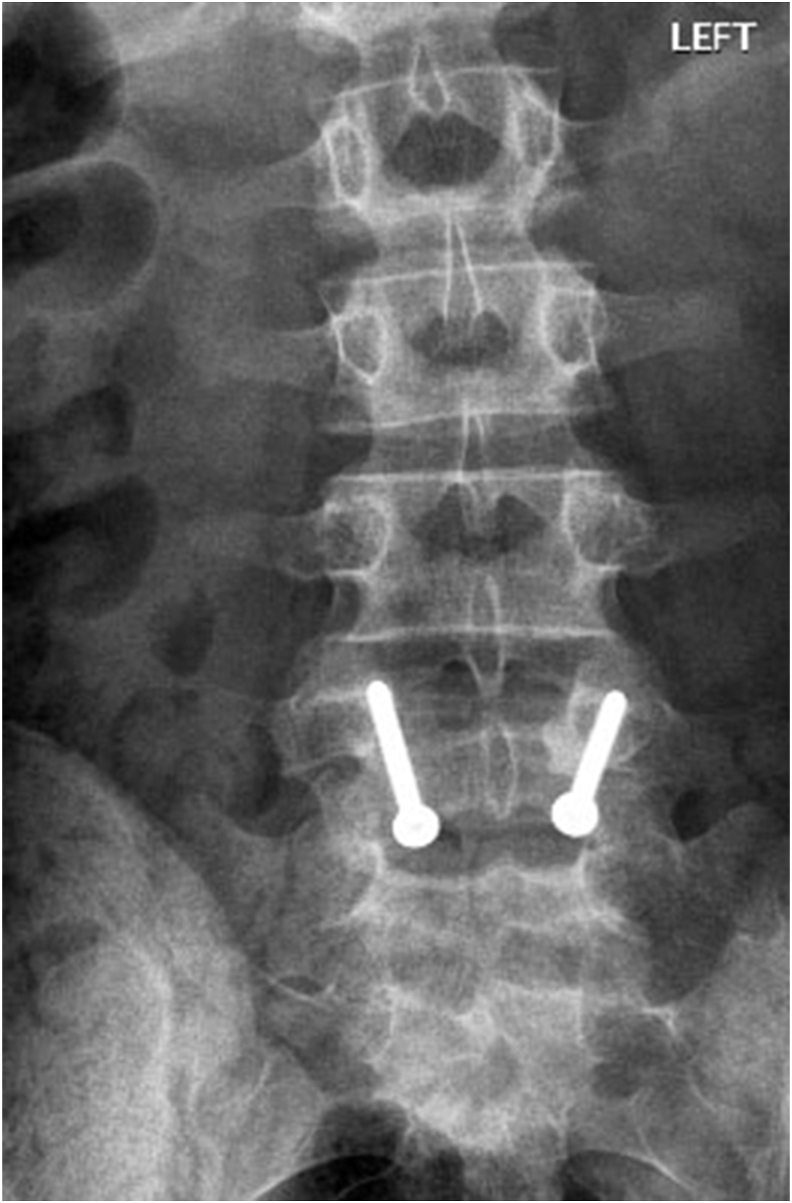
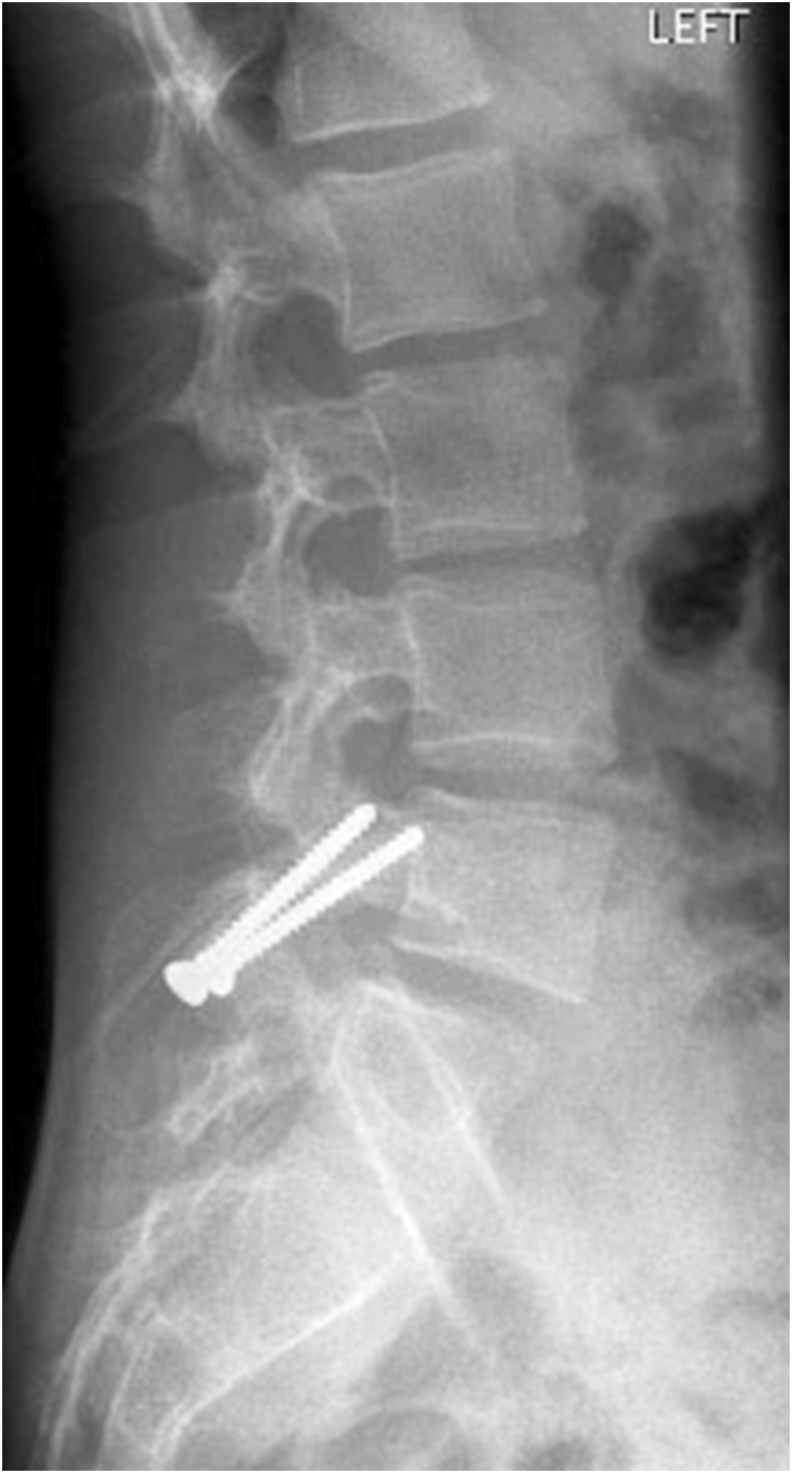
Fig 4A & B: X-rays of Lumbosacral spine (AP and Lateral view) showing bilateral pars defect in L4 vertebrae in an 18year old male.
Fig. 4C.
Fig 4C & D: 2years post-operative X-rays of Lumbosacral spine (AP and Lateral view) showing healed bilateral pars defect in L4 vertebrae in the same patient.
Kakiuchi described a pedicle screw-sublaminar hook technique with 100% union of PI defect.56 In their series, 43% patients were beyond 30 years of age and concomitant degenerate disc was not an exclusion criterion.56 This view of higher age with disc degeneration is in contradiction to many advocates of direct repair. In a prospective study, 92% (12/13) patients had united PI defect below 20years of age while non-union was recorded in 6 patients beyond the age of 20years with a hook screw construct. Despite the non-union, 4/6 had excellent clinical outcome.62 In a series of 23 patients (mean age 34years) who were treated with pedicle screw hook technique, reported 87% good outcome (100% radiological union) below the age of 30years against 73% good outcome (82% radiological union) above the age of 30years. There was no degenerate disc disease in patients below the age of 30years.63 It was suggested patients with moderate degenerative disc disease can undergo direct repair.63 A study observed the rate of pseudoarthrosis in patients below the age of 20years was less as compared to patients above the age of 20years (8.6% vs 35%).64 The presence of disc degeneration (Pfirmann grade 3 and above) on MRI scan is a contraindication to direct repair.65
Songer & Rovin reported of seven (7) patients with PI defects treated successfully by a pedicle screw-cable construct.65 The authors suggested that this construct provided the strongest anchors at the pedicle and lamina to compress and stabilize the defect.65 Disadvantage of this technique were wide surgical exposure and sublaminar wire passage. In another study, three (3) patients returned to sports after cable-screw construct.66 In a multicentre study, Ishida et al. compared the pedicle screw claw-hook fixation versus pedicle screw hook fixation technique. The outcome was better with pedicle screw claw-hook fixation method.67 In sporting subjects, who had direct repair of either unilateral or bilateral PI defects, the return to sports was reported in 80–90%.46,47 Rajasekaran et al. had reported 78% good outcome68 and Snyder et al. had reported on 16 patients of direct repair with complete resolution of symptoms.69 However, implant loosening or screw breakage was frequently reported. To avoid this problem many surgeons are using some form of segmental pedicle screw hook fixation which is a stronger construct and decreases the risk of hardware failure.63,70
Buck's screw technique had the best clinical outcome in general population as well as in well-motivated professional athletes as reported from multiple centers around the world. Although technically difficult with little room for error, the technique is safe, reliable and reproducible.46,47,51,54,55,71,72 In a recent meta-analysis comparing all the techniques, it was suggested that the pedicle screw based constructs had the best fusion rates and lowest complication rates.70
3.2. Postoperative rehabilitation
The post-operative protocol is customized according to the patient profile but generally core strengthening and extremity flexibility exercises in neutral spine are recommended by the end of second week. Non-impact aerobic exercises can be started by the end of four weeks. Sport related exercises can be started by 4–6months when patient has no pain.73
3.3. Predictors of surgical outcome
Low back pain is the single most important predictor for one having a surgical intervention.74,75 Most surgeons prefer to perform direct repair if the spondylolytic gap is less than 4mm46,51 and if there was no adjacent level disc degeneration. A positive response to pars injection had good outcome after direct fixation of PI defect.71 Owing to these factors, surgical repair of lumbar spondylolysis is not considered by many beyond the age of 25years.
For predicting prognosis in young sporting subjects who underwent direct repair of lumbar spondylolysis, a model was suggested following a clinical and statistical analysis.74 Patients younger than 25years run a predictable course after surgery of PI defect.75 Therefore, successful surgical outcome may be predicted preoperatively when the following factors were considered: below 25years of age, pars defect gap is less than 4 mm, minimal disc degeneration (Pfirmann grade 1–3), positive pars injection, type of direct repair and psychological motivation of the individual.28,45,46,51,71,74,75
These factors were accounted for in the regression model which predicted the outcome in 80.9% sporting subjects undergoing Buck's direct repair.74,75 Other significant prognostic predictors for a successful outcome were preoperative ODI (Oswestry Disability Index) & SF-36 PCS (physical component) scores.75
3.4. Minimal access percutaneous direct repair
In the last few years, many surgeons are advocating minimally invasive (MISS) repair of symptomatic pars defect. This technique is found to be effective, especially in well-motivated and physically fit athletes. Wilson used a tubular retractor system for curettage and bone grafting of the PI defect, followed by a threaded dynamic compression screw percutaneously.76
Jia et al. reported on eight (8) patients who had MISS direct repair technique (percutaneous bilateral intralaminar screws), had 81% successful union and good outcome.77 The success of MISS direct repair depends on the fracture morphology and orientation78 and minimizes paraspinal muscle damage which supports early rehabilitation.79 The application of endoscopy and percutaneous screws lessens soft tissue disruption allowing for performing the surgery under local anaesthesia.80
3.5. Outcome measures
An outcome assessment in a patient with spinal pain improves the understanding of relief of pain and recovery of function. The VAS (Visual Analogue scoring) for LBP was responsive enough to detect the minimal clinical change after treatment.81 ODI is a time tested valid measurement of condition-specific disability which was developed for both assessment and outcome.82 ODI and VAS scoring together evaluates functional status that is affected by LBP, rendering them appropriate primary outcome measure in pain due to lumbar PI defect.
The 36-Item Short Form Health Survey questionnaire (SF-36) is a popular generic instrument measuring Health-Related Quality of Life.83 Generic tools may not always be able to detect subtle effects of a specific condition on quality of life. Modified ODI and SF-36 (PCS) measurements were reliable and have necessary width scale to reliably detect a change of health status in most subjects with disability due to LBP.84 A prospective interventional study showed good clinical outcomes measured by using ODI and SF12 after a year of surgery, despite poor union rate of the pars defect (55%).80
Thus, combining the three outcome measures (VAS, ODI and SF-36) provides a robust responsiveness after treatment for symptomatic lumbar spondylolysis.46,47,74,75
These outcome measures in combination allow one to understand the pain status, functional disability, motivation, physical abilities, and the sporting abilities. Although the disease specific and generic measures give an idea about the functional ability, there is a lacuna in the outcome measurements for return to active sports. The type of information needed to select the optimal instrument for sporting subjects with LBP is unavailable in the literature. A questionnaire for sporting subjects with LBP was proposed which would guide the clinician regarding outcome after surgical treatment of lumbar pars defect. This questionnaire is a valuable and valid measurable instrument.75
4. Summary
A history of athletic participation with clinical symptoms of LBP leads the clinician to high suspicion of lumbar pars defect. The unilateral pars fractures typically heal, especially among acute fractures. Bilateral PI defects are painful in patients who are in active sports which involves hyperextension of spine. Diagnosis is established through clinical and radiological imaging studies.
Non-operative treatment includes individualized physiotherapy program which are activity modification and a progressive course of specific exercises which usually leads to symptom resolution and return to sports. Failure of 6–12months of conservative treatment with the patient continuing to experience disabling LBP may be treated surgically. Once the pain locus is identified (i.e. PI as pain generator) and morphology of PI defect is assessed by CT scan and MRI scan, the PI defect may undergo direct repair. Direct repair can be performed using Buck's cortical screw technique, Morscher hook-screw technique or any methods using combination of hook, screws or cables. The motion segment is preserved by direct repair techniques using compression screws or pedicle screw–hook constructs along with bone grafts for enhancing bony or fibrous union of the defect. Currently, in many centers, endoscopic and advanced fluoroscopy has led surgeons to do percutaneous minimally invasive direct repair of lumbar spondylolysis with good functional outcome. Pedicle screw–based segmental fusion is advised if concurrent disc or facet joint degeneration is confirmed by imaging.
Contributions of author-
Ujjwal K Debnath – research, write manuscript, edit and revision of manuscript.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Wiltse L.L., Widell, Jackson D.W. Fatigue fracture: the basic lesion in isthmic spondylolisthesis. J Bone Jt Surg Am Vol. 1975;57:17–22. [PubMed] [Google Scholar]
- 2.Ward C.V., Latimer B. Human evolution and the development of spondylolysis. Spine. 2005;30(16):1808–1814. doi: 10.1097/01.brs.0000174273.85164.67. (Phila Pa 1976) [DOI] [PubMed] [Google Scholar]
- 3.Standaert C.J., Herring S.A. Spondylolysis: a critical review. Br J Sports Med. 2000;34:415–422. doi: 10.1136/bjsm.34.6.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wiltse L.L. The etiology of spondylolisthesis. J Bone Jt Surg Am Vol. 1962;44:539–560. [PubMed] [Google Scholar]
- 5.Fredrickson B.E., Baker D., McHolick W.J., Yuan H.A., Lubicky J.P. The natural history of spondylolysis and spondylolisthesis. J Bone Jt Surg Am Vol. 1984;66(5):699–707. [PubMed] [Google Scholar]
- 6.Rossi F., Dragoni S. Lumbar spondylolysis: occurrence in comnpetitive athletes. Updated achievements in a series of 390 cases. J Sports Med Phys Fit. 1990;30(4):450–452. [PubMed] [Google Scholar]
- 7.Micheli L.J., Wood R. Back pain in young athlete: significant differences from adults in causes and patterns. Arch Pediatr Adolesc Med. 1995;149:15–18. doi: 10.1001/archpedi.1995.02170130017004. [DOI] [PubMed] [Google Scholar]
- 8.Soler T., Calderon C. The prevalence of spondylolysis in the Spanish elite athlete. Am J Sports Med. 2000;28:57–62. doi: 10.1177/03635465000280012101. [DOI] [PubMed] [Google Scholar]
- 9.Beutler W.J., Fredrickson B.E., Murtland A., Sweeney C.A., Grant W.D., Baker D. The natural history of spondylolysis and spondylolisthesis: 45-year follow-up evaluation. Spine. 2003 May 15;28(10):1027–1035. doi: 10.1097/01.BRS.0000061992.98108.A0. (Phila Pa 1976) [DOI] [PubMed] [Google Scholar]
- 10.Ciullo J.V., Jackson D.W. Pars interarticularis stress reaction, spondylolysis, and spondylolisthesis in gymnasts. Clin Sports Med. 1985;4(1):95–110. [PubMed] [Google Scholar]
- 11.Kettlekamp D.B., Wright D.G. Spondylolysis in alaskan eskimo. J Bone Jt Surg Am Vol. 1971;53(3):563–566. [PubMed] [Google Scholar]
- 12.Merbs C.F. Incomplete spondylolysis and healing. Spine. 1995;20(21):2328–2333. doi: 10.1097/00007632-199511000-00011. [DOI] [PubMed] [Google Scholar]
- 13.Smith J.A., Hu S.S. Management of spondylolysis and spondylolisthesis in the pediatric and adolescent population. Orthop Clin N Am. 1999;30:487–498. doi: 10.1016/s0030-5898(05)70101-2. [DOI] [PubMed] [Google Scholar]
- 14.Sagi H.C., Jarviss J.G., Uhthoff H.K. Histomorphic analysis of the development of the pars interarticularis and its association with isthmic spondylolysis. Spine. 1998;23(15):1635–1639. doi: 10.1097/00007632-199808010-00002. (Phila Pa 1976) [DOI] [PubMed] [Google Scholar]
- 15.Sterba M., Arnoux P.J., Labelle H., Warner W.C., Aubin C.É. Biomechanical analysis of spino-pelvic postural configurations in spondylolysis subjected to various sport-related dynamic loading conditions. Eur Spine J. 2018 Aug;27(8):2044–2252. doi: 10.1007/s00586-018-5667-0. [DOI] [PubMed] [Google Scholar]
- 16.Mac-Thiong J.M., Labelle H., Wang Z., de Guise J.A. Postural model of sagittal spino-pelvic balance and its relevance for lumbosacral developmental spondylolisthesis. Spine. 2008;33(21):2316–2325. doi: 10.1097/BRS.0b013e318186b236. (Phila Pa 1976) [DOI] [PubMed] [Google Scholar]
- 17.Qing – Shuang Zhou M.M., Sun X., Chen X. How does sagittal spinopelvic alignment of lumbar multisegmental spondylolysis differ from monosegmental spondylolysis? J Neurosurg Spine. 2020 Apr 17:1–8. doi: 10.3171/2020.2.SPINE191415. [DOI] [PubMed] [Google Scholar]
- 18.Gumphry G.M. Macmillan & Co.; Cambridge, UK: 1858. A Treatise on the Human Skeleton; p. 143n. [Google Scholar]
- 19.Newell R.L.M. Historical perspective spondylolysis: an historical review. Spine. 1995;20(17):1950–1956. doi: 10.1097/00007632-199509000-00022. (Phila Pa 1976) [DOI] [PubMed] [Google Scholar]
- 20.Waldron H.A. Variations in the prevalence of spondylolysis in early British populations. J Roy Soc Med. 1991;84:547–549. doi: 10.1177/014107689108400915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Eisentstein S.M. Spondylolysis: a skeletal investigation of two population groups. J Bone Joint Surg. 1978;60(4):488–494. doi: 10.1302/0301-620X.60B4.361744. [DOI] [PubMed] [Google Scholar]
- 22.Eisenstein S.M., Ashton I.K., Roberts S. Innervation of the spondylolysis "ligament. Spine. 1994;19(8):912–916. doi: 10.1097/00007632-199404150-00008. (Phila Pa 1976) [DOI] [PubMed] [Google Scholar]
- 23.Nordström D., Santavirta S., Seitsalo S. Symptomatic lumbar spondylolysis. Neuroimmunologic studies. Spine. 1994;19(24):2752–2758. doi: 10.1097/00007632-199412150-00003. (Phila Pa 1976) [DOI] [PubMed] [Google Scholar]
- 24.Bogduk N. The anatomical basis for spinal pain syndromes. J Manip Physiol Ther. 1995;18(9):603–605. [PubMed] [Google Scholar]
- 25.Yokoe T., Tajima T., Sugimura H. Comparison of symptomatic spondylolysis in young soccer and baseball players. J Orthop Surg Res. 2020 Sep 3;15(1):378. doi: 10.1186/s13018-020-01910-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cyron B.M., Hutton W.C., Troup J.D. Spondylolytic fractures. J Bone Joint Surg. 1976;58(4):462–466. doi: 10.1302/0301-620X.58B4.1018032. [DOI] [PubMed] [Google Scholar]
- 27.Ishida Y., Ohmori K., Inoue H., Suzuki K. Delayed vertebral slip and adjacent disc degeneration with an isthmic defect of the fifth lumbar vertebra. J Bone Joint Surg. 1999;81(2):240–244. doi: 10.1302/0301-620x.81b2.9302. [DOI] [PubMed] [Google Scholar]
- 28.Fujii K., Katoh S., Sairyo K., Ikata T., Yosui N. Union of defects in the pars interarticularis of the lumbar spine in children and adolescents: the radiological outcome after conservative treatment. J Bone Joint Surg. 2004;86-B:225–231. doi: 10.1302/0301-620x.86b2.14339. [DOI] [PubMed] [Google Scholar]
- 29.Sakai T., Goda Y., Tezuka F. Characteristics of lumbar spondylolysis in elementary school age children. Eur Spine J. 2016;25:602–606. doi: 10.1007/s00586-015-4029-4. [DOI] [PubMed] [Google Scholar]
- 30.Sairyo K., Katoh S., Ikata T., Fujii K., Goel V.K. Development of spondylolytic olisthesis in adolescents. Spine J. 2001;1(3):171–175. doi: 10.1016/s1529-9430(01)00018-3. [DOI] [PubMed] [Google Scholar]
- 31.Seitsalo S., Osterman K., Hyvãrinen H., Tallroth K., Schlenzka D., Poussa M. Progression of spondylolisthesis in children and adolescents. A long-term follow-up of 272 patients. Spine. 1991;16(4):417–421. doi: 10.1097/00007632-199104000-00004. (Phila Pa 1976) [DOI] [PubMed] [Google Scholar]
- 32.Muschik M., Hahnel H., Robinson P.N., Perka C., Muschik C. Competitive sports and the progression of spondylolisthesis. J Pediatr Orthop. 1996;16(3):364–369. doi: 10.1097/00004694-199605000-00014. [DOI] [PubMed] [Google Scholar]
- 33.Overley S.C., McAnany S.J., Andelman S. Return to play in adolescent athletes with symptomatic spondylolysis without listhesis: a Meta-analysis. Global Spine J. 2018;8(2):190–197. doi: 10.1177/2192568217734520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tawfik S., Phan K., Mobbs R.J., Rao P.J. The incidence of pars interarticularis defects in athletes. Global Spine J. 2019:1–13. doi: 10.1177/2192568218823695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sairyo K., Sakai T., Yasui N. Conservative treatment of lumbar spondylolysis in childhood and adolescence: the radiological signs which predict healing. J Bone Joint Surg. 2009;91(2):206–209. doi: 10.1302/0301-620X.91B2.21256. [DOI] [PubMed] [Google Scholar]
- 36.Dutton J.A., Hughes S.P., Peters A.M. SPECT in the management of patients with back pain and spondylolysis. Clin Nucl Med. 2000 Feb;25(2):93–96. doi: 10.1097/00003072-200002000-00001. [DOI] [PubMed] [Google Scholar]
- 37.Debnath U.K., Freeman B.J.C., Solaymani-Dodaran M., Webb J.K. Role of SPECT imaging in posterior lumbar stress injuries. Indian J Orthop. 2005;39(4):228–231. [Google Scholar]
- 38.Gregory Pl, Batt M.E., Kerslake R.W., Scammell B.E., Webb J.K. The value of combining SPECT and CT in the investigation of spondylolysis. Eur Spine J. 2004;13:503–509. doi: 10.1007/s00586-004-0696-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Saifuddin A., White J., Tucker S., Taylor B.A. Orientation of lumbar pars defects: implications for radiological detection and surgical management. J Bone Joint Surg. 1998;80(2):208–211. doi: 10.1302/0301-620x.80b2.8219. [DOI] [PubMed] [Google Scholar]
- 40.Campbell R.S., Grainger A.J., Hide I.G., Papastefanou S., Greenough C.G. Juvenile spondylolysis: a comparative analysis of CT, SPECT and MRI. Skeletal Radiol. 2005;34(2):63–73. doi: 10.1007/s00256-004-0878-3. [DOI] [PubMed] [Google Scholar]
- 41.Udeshi U.L., Reeves D. Routine thin slice MRI effectively demonstrates the lumbar pars interarticularis. Clin Radiol. 1999;54(9):615–619. doi: 10.1016/s0009-9260(99)90024-7. [DOI] [PubMed] [Google Scholar]
- 42.Hollenberg G.M., Beattie P.F., Meyers S.P., Weinberg E.P., Adams M.J. Stress reactions of the lumbar pars interarticularis: the development of a new MRI classification system. Spine. 2002;27(2):181–186. doi: 10.1097/00007632-200201150-00012. (Phila Pa 1976) [DOI] [PubMed] [Google Scholar]
- 43.Wirtz D.C., Wildberger J.E., Rohrig H., Zilkens K.W. Early diagnosis of isthmic spondylolysis with MRI. Z Orthop Ihre Grenzgeb. 1999;137:508–511. doi: 10.1055/s-2008-1039380. [DOI] [PubMed] [Google Scholar]
- 44.McCleary M.D., Congeni J.A. Current concepts in the diagnosis and treatment of spondylolysis in young athletes. Curr Sports Med Rep. 2007 Jan;6(1):62–66. doi: 10.1007/s11932-007-0014-y. [DOI] [PubMed] [Google Scholar]
- 45.Sys J., Michielsen J., Bracke P., Martens M., Verstreken J. Nonoperative treatment of active spondylolysis in elite athletes with normal X-ray findings: literature review and results of conservative treatment. Eur Spine J. 2001;10(6):498–504. doi: 10.1007/s005860100326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Debnath U.K., Freeman B.J.C., Gregory P., de la Harpe D., Kerslake R.W., Webb J.K. Clinical outcome and return to sport after the surgical treatment of spondylolysis in young athlete. J Bone Joint Surg. 2003;85(2):244–249. doi: 10.1302/0301-620x.85b2.13074. [DOI] [PubMed] [Google Scholar]
- 47.Debnath U.K., Freeman B.J., Grevitt M.P., Sithole J., Scammell B.E., Webb J.K. Clinical outcome of symptomatic unilateral stress injuries of the lumbar pars interarticularis. Spine. 2007;32(9):995–1000. doi: 10.1097/01.brs.0000260978.10073.90. (Phila Pa 1976) [DOI] [PubMed] [Google Scholar]
- 48.Standaert C.J., Herring S.A. Expert opinion and controversies in sports and musculoskeletal medicine: the diagnosis and treatment of spondylolysis in adolescent athletes. Arch Phys Med Rehabil. 2007 Apr;88(4):537–540. doi: 10.1016/j.apmr.2007.01.007. [DOI] [PubMed] [Google Scholar]
- 49.Syrmou E., Tsitsopoulos P.P., Marinopoulos D. Spondylolysis: a review and reappraisal. Hippokratia. 2010 Jan-Mar;14(1):17–21. [PMC free article] [PubMed] [Google Scholar]
- 50.Suh P.B., Esses S.I., Kostuik J.P. Repair of pars interarticularis defect. The prognostic value of pars infiltration. Spine. 1991;16(8):S445–S448. (Phila Pa 1976) Suppl. [PubMed] [Google Scholar]
- 51.Buck J.E. Direct repair of the defect in spondylolisthesis – preliminary report. J Bone Joint Surg. 1970;52(3):432–438. [PubMed] [Google Scholar]
- 52.Morscher E., Gerber B., Fasel J. Surgical treatment of spondylolisthesis by bone grafting and direct stabilization of spondylolysis by means of a hook screw. Arch Orthop Trauma Surg. 1984;103(3):175–178. doi: 10.1007/BF00435550. [DOI] [PubMed] [Google Scholar]
- 53.Nicol R.O., Scott J.H. Lytic spondylolysis. Repair by wiring. Spine. 1986;11(10):1027–1030. doi: 10.1097/00007632-198612000-00011. [DOI] [PubMed] [Google Scholar]
- 54.Gillet P., Petit M. Direct repair of spondylolysis without spondylolisthesis, using a rod-screw construct and bone grafting of the pars defect. Spine. 1999;24(12):1252–1256. doi: 10.1097/00007632-199906150-00014. (Phila Pa 1976) [DOI] [PubMed] [Google Scholar]
- 55.Hardcastle P.H. Repair of spondylolysis in young fast bowlers. J Bone Joint Surg. 1993;75(3):398–402. doi: 10.1302/0301-620X.75B3.8496207. [DOI] [PubMed] [Google Scholar]
- 56.Kakiuchi M. Repair of the defect in spondylolysis. Durable fixation with pedicle screws and laminar hooks. J Bone Jt Surg Am Vol. 1997;79(6):818–825. doi: 10.2106/00004623-199706000-00003. [DOI] [PubMed] [Google Scholar]
- 57.Tokuhashi Y., Matsuzaki H. Repair of defects in spondylolysis by segmental pedicular screw hook fixation. A preliminary report. Spine. 1976;21(17):2041–2045. doi: 10.1097/00007632-199609010-00023. (Phila Pa 1976) [DOI] [PubMed] [Google Scholar]
- 58.Mihara H., Onari K., Cheung B.C. The biomechanical effects of spondylolysis and its treatment. Spine. 2003;28:235–238. doi: 10.1097/01.BRS.0000042226.59713.0E. (Phila Pa 1976) [DOI] [PubMed] [Google Scholar]
- 59.Kip P.C., Esses S.I., Doherty B.I., Alexander J.W., Crawford M.J. Biomechanical testing of pars defect repairs. Spine. 1994;19(23):2692–2697. (Phila Pa 1976) [PubMed] [Google Scholar]
- 60.Deguchi M., Rapoff A.J., Zdeblick T.A. Biomechanical comparison of spondylolysis fixation techniques. Spine. 1999;24(4):328–333. doi: 10.1097/00007632-199902150-00004. (Phila Pa 1976) [DOI] [PubMed] [Google Scholar]
- 61.Kimura M. My method of filling the lesion with spongy bone in spondylolysis and spondylolisthesis (in Japanese) Orthop Surg. 1968;19:285–295. [PubMed] [Google Scholar]
- 62.Roca J., Iborra M., Cavanilles-Walker J.M., Albertí G. Direct repair of spondylolysis using a new pedicle screw hook fixation: clinical and CT-assessed study: an analysis of 19 patients. J Spinal Disord Tech. 2005 Feb;(18 Suppl):S82–S89. doi: 10.1097/01.bsd.0000123425.12852.3c. [DOI] [PubMed] [Google Scholar]
- 63.Debusscher F., Troussel S. Direct repair of defects in lumbar spondylolysis with a new pedicle screw hook fixation: clinical, functional and CT-assessed study. Eur Spine J. 2007;16(10):1650–1658. doi: 10.1007/s00586-007-0392-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ivanic G.M., Pink T.P., Achatz W. Direct stabilization of lumbar spondylolysis with a hook-screw. Mean 11-year follow-up period for 113 patients. Spine. 2003;28:255–259. doi: 10.1097/01.BRS.0000042251.62696.A5. (Phila Pa 1976) [DOI] [PubMed] [Google Scholar]
- 65.Songer M.N., Rovin R. Repair of the pars interarticularis defect with a cable-screw construct. A preliminary report. Spine. 1998;23(2):263–269. doi: 10.1097/00007632-199801150-00023. (Phila Pa 1976) [DOI] [PubMed] [Google Scholar]
- 66.Bozarth G.R., Fogel G.R., Toohey J.S., Neider A. Repair of pars interarticularis defect with a modified cable-screw construct. J Surg Orthop Adv. 2007;16(2):79–83. [PubMed] [Google Scholar]
- 67.Ishida K., Aota Y., Mitsugi N. Spondylolysis repair using a pedicle screw hook or claw-hook system -a comparison of bone fusion rates. Spine Surg Relat Res. 2018 Feb 28;2(2):135–139. doi: 10.22603/ssrr.2017-0011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rajasekaran S., Subbiah M., Shetty A.P. Direct repair of lumbar spondylolysis by Buck's technique. Indian J Orthop. 2011 Mar;45(2):136–140. doi: 10.4103/0019-5413.77133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Snyder L.A., Shufflebarger H., O'Brien M.F., Thind H., Theodore N., Kakarla U.K. Spondylolysis outcomes in adolescents after direct screw repair of the pars interarticularis. J Neurosurg Spine. 2014 Sep;21(3):329–333. doi: 10.3171/2014.5.SPINE13772. [DOI] [PubMed] [Google Scholar]
- 70.Mohammed N., Patra D.P., Narayan V. A comparison of the techniques of direct pars interarticularis repairs for spondylolysis and low-grade spondylolisthesis: a meta-analysis. Neurosurg Focus. 2018;44(1):1–11. doi: 10.3171/2017.11.FOCUS17581. E10. [DOI] [PubMed] [Google Scholar]
- 71.Wu S.S., Lee C.H., Chen P.Q. Operative repair of symptomatic spondylolysis following a positive response to diagnostic pars injection. J Spinal Disord. 1999;12(1):10–16. [PubMed] [Google Scholar]
- 72.Jeanneret B. Direct repair of spondylolysis. Acta Orthop Scan. 1993;251(Suppl):111–115. doi: 10.3109/17453679309160138. [DOI] [PubMed] [Google Scholar]
- 73.Radcliff K.E., Kalantar S.B., Reitman C.A. Surgical management of spondylolysis and spondylolisthesis in athletes: indications and return to play. Curr Sports Med Rep. 2009;8:35–40. doi: 10.1249/JSR.0b013e318194f89e. [DOI] [PubMed] [Google Scholar]
- 74.Debnath U.K. University of Nottingham; 2010. Factors Predicting the Outcome Following Treatment for Lumbar Spondylolysis.http://eprints.nottingham.ac.uk/id/eprint/12780/1 DM thesis. [Google Scholar]
- 75.Debnath U.K., Scammell B.E., Freeman B.J.C., McConnell J.R. Predictive factors for the outcome of surgical treatment of lumbar spondylolysis in young sporting individuals. Global Spine J. 2018 Apr;8(2):121–128. doi: 10.1177/2192568217713008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wilson L., Altaf F., Tyler P. Percutaneous pars interarticularis screw fixation: a technical note. Eur Spine J. 2016 Jun;25(6):1651–1654. doi: 10.1007/s00586-015-4152-2. [DOI] [PubMed] [Google Scholar]
- 77.Jia M., Wang J., Zhang Z., Zheng W., Zhou Y. Direct Repair of lumbar pars interarticularis defects by utilizing intraoperative O-Arm-based navigation and microendoscopic techniques. Spine. 2016 Oct;41(suppl 19):B6–B13. doi: 10.1097/BRS.0000000000001815. (Phila Pa 1976) [DOI] [PubMed] [Google Scholar]
- 78.Fayed I., Conte A.G., Voyadzis J.M. Success and failure of percutaneous minimally invasive direct pars repair: analysis of fracture morphology. World Neurosurg J. 2019 Jun;126:181–188. doi: 10.1016/j.wneu.2019.03.026. [DOI] [PubMed] [Google Scholar]
- 79.Widi G.A., Williams S.K., Levi A.D. Minimally invasive direct repair of bilateral lumbar spine pars defects in athletes. Case reports in medicine. 2013;2013 doi: 10.1155/2013/659078. pp.1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lee G.W., Lee S.M., Suh B.G. Direct repair surgery with screw fixation for young patients with lumbar spondylolysis: patient-reported outcomes and fusion rate in a prospective interventional study. Spine. 2015 Feb 15;40(4):E234–E241. doi: 10.1097/BRS.0000000000000714. (Phila Pa 1976) [DOI] [PubMed] [Google Scholar]
- 81.Hagg O., Ftzell P., Nordwall A. Swedish Lumbar Spine Study group. The clinical importance of changes in outcome scores after treatment for chronic low back pain. Eur Spine J. 2003;12(1):12–20. doi: 10.1007/s00586-002-0464-0. [DOI] [PubMed] [Google Scholar]
- 82.Fairbank J.C.T., Pynsent P.B. The Oswestry disability index. Spine. 2000;25(22):2940–2953. doi: 10.1097/00007632-200011150-00017. [DOI] [PubMed] [Google Scholar]
- 83.Ware J.E., Kosinski M., Keller S.K. The Health Institute; Boston, MA: 1994. SF-36 Physical and Mental Health Summary Scales. A User's Manual. [Google Scholar]
- 84.Davidson M., Keating J.L. A comparison of five low back disability questionnaires: reliability and responsiveness. Phys Ther. 2002 Jan;82(1):8–24. doi: 10.1093/ptj/82.1.8. [DOI] [PubMed] [Google Scholar]