Abstract
Emerging evidence suggests mild traumatic brain injury related headache (MTBI-HA) is a form of neuropathic pain state. Previous supraspinal mechanistic studies indicate patients with MTBI-HA demonstrate a dissociative state with diminished levels of supraspinal prefrontal pain modulatory functions and enhanced supraspinal sensory response to pain in comparison to healthy controls. However, the relationship between supraspinal pain modulatory functional deficit and severity of MTBI-HA is largely unknown. Understanding this relationship may provide enhanced levels of insight about MTBI-HA and facilitate the development of treatments. This study assessed pain related supraspinal resting states among MTBI-HA patients with various headache intensity phenotypes with comparisons to controls via functional magnetic resonance imaging (fMRI). Resting state fMRI data was analyzed with self-organizing-group-independent-component-analysis in three MTBI-HA intensity groups (mild, moderate, and severe) and one control group (n = 16 per group) within a pre-defined supraspinal pain network based on prior studies. In the mild-headache group, significant increases in supraspinal function were observed in the right premotor cortex (T = 3.53, p < 0.001) and the left premotor cortex (T = 3.99, p < 0.0001) when compared to the control group. In the moderate-headache group, a significant (T = −3.05, p < 0.01) decrease in resting state activity was observed in the left superior parietal cortex when compared to the mild-headache group. In the severe-headache group, significant decreases in resting state supraspinal activities in the right insula (T = −3.46, p < 0.001), right premotor cortex (T = −3.30, p < 0.01), left premotor cortex (T = −3.84, p < 0.001), and left parietal cortex (T = −3.94, p < 0.0001), and an increase in activity in the right secondary somatosensory cortex (T = 4.05, p < 0.0001) were observed when compared to the moderate-headache group. The results of the study suggest that the increase in MTBI-HA severity may be associated with an imbalance in the supraspinal pain network with decline in supraspinal pain modulatory function and enhancement of sensory/pain decoding.
Keywords: MTBI, traumatic brain injury, chronic post-traumatic headache, pain modulation, functional magnetic resonance imaging, resting state functional activity
Introduction
In patients with mild traumatic brain injury (MTBI), headaches are a common and often debilitating symptom. Emerging clinical and mechanistic evidences suggest MTBI related headache (MTBI-HA) is a form of neuropathic pain state.1–7 The supraspinal pain processing network is known to involve the thalamus and pons, which relay sensory afferent signs to other supraspinal regions including: 1) sensory discriminatory regions such as the primary and secondary somatosensory cortices and the inferior parietal lobe; 2) affective regions such as the anterior cingulate cortex and the insula; and 3) modulatory regions involving various regions of the prefrontal cortices.8 Decreases of medial prefrontal cortical activities and other motor cortical functions are known to be associated with central hyperalgesia.9 As pain perception and relief relies heavily on the balance between the affective and modulatory/adaptive functions of the pain network, a disruption in the intra-dynamic of the network, such as diminished modulatory/adaptive function as demonstrated in previously conducted experimental pain model and chronic pain studies by others, can often lead to the development of maladaptive central pain states with associated neurological symptoms (chronic headache), and neuropsychological dysfunction (attention deficit and depression).10–12 In the area of neurophysiological assessments, MTBI patients appear to suffer from long lasting elevation of resting motor threshold, suggesting a deficiency in cortical excitability and connectivity with brain areas associated with pain modulation/adaptation in this patient population.13 In addition, previous supraspinal mechanistic studies indicate patients with MTBI-HA demonstrate a diminished level of supraspinal prefrontal pain modulatory functions and an enhanced supraspinal sensory response to pain in comparison to healthy controls.1,2 These previous findings highly support the notion that MTBI-HA is a neuropathic pain state and suggests MTBI-HA may result in a similar maladaptive central pain state.
Though there is evidence indicating that supraspinal maladaptation/imbalance can lead to the development of persistent MTBI-HA, no study has been conducted to assess how the maladaptive state may vary with different headache severity phenotypes. Understanding the differences in supraspinal maladaptive states in various headache severity-based phenotypes can provide an enhanced level of insight on the underlying mechanisms leading to the various MTBI-HA presentations, and thus facilitate the development of treatment options. Here, we hypothesized that headache severity phenotype is associated with the degree of impairment in supraspinal pain modulatory functions, with the severe MTBI-HA phenotype demonstrating the highest degree of impairment. To test this hypothesis, the current study aims to compare supraspinal resting state pain sensory discriminatory, affective, and modulatory functions among MTBI-HA patients with various headache intensity phenotypes (mild, moderate, and severe) and controls.
Methodology
A total of 118 subjects (83 with MTBI-HA and 35 Veteran controls) were screened and enrolled according to the study protocol approved by the human subject protection committee. The patients in the MTBI-HA groups were enrolled if they met the MTBI diagnosis based on the published criteria from both the 1993 American Congress of Rehabilitation Medicine and recent (2009) recommendations from the Department of Defense and presented with headaches due to their brain injury. All MTBI-HA subjects must have persistent headache symptom for >3 months since the most recent injury. Controls were healthy subjects who did not have a history of MTBI or persistent headaches. All subjects were between the ages of 18–50.
Exclusion criteria for both MTBI-HA subjects and controls included: history of pacemaker implant; pregnancy; ferromagnetic material such as shrapnel, bullet fragments, or implanted devices in the body, incompatible with magnetic resonance imaging (MRI) procedures; history of life-threatening diseases, dementia, or serious psychiatric illnesses; history of suicidality; presence of any other chronic neuropathic pain states or neurological diseases such as seizure; involvement of litigation; inability to understand the study instruction or communicate in English; and history of ongoing substance abuse.
Headache intensity grouping
Subjects from the pool of 83 MTBI Veterans (72 males) were organized into three different headache intensity (mild, moderate, and severe) groups based on their headache severity rating on the Neurobehavioral Symptom Inventory (NSI) and Numerical Rating Scale and a total of 48 subjects (n = 16 in each group) were selected to allow for age and gender matching. In addition, 16 subjects with similar age and gender were selected from the pool of 35 Veterans (24 males) with no prior history of headache and TBI as controls.
Demographic information and headache history
Both MTBI-HA and control subjects’ demographic information (age, gender, ethnicity, education) were recorded. MTBI injury type (blast or non-blast), duration of headaches in months, and medication usage were also recorded. In addition, information regarding depressive symptomatology was assessed via the Beck Depression Inventory-II (BDI-II) and Hamilton Rating Scale for Depression (HAMD), with the HAMD scores converted to BDI-II for subsequent data analysis,14 and presence of a clinical Post-traumatic Stress Disorder (PTSD) diagnosis (based off of clinical diagnosis in medical charts) were gathered.
Data analyses for demographics and headache history
A univariate analysis of variance (ANOVA) with Bonferroni corrections was conducted for age, education, and duration of headaches since MTBI. Pearson’s-Chi Squared Test with Bonferroni Corrections were utilized for ethnicity, MTBI type, depression, PTSD, and medication comparisons. A two-sided Fisher’s Exact Test with Bonferroni corrections was used for data where appropriate (e.g., small sample sizes).
Imagining acquisition
Head movements during scanning were minimized by instructing the subjects to hold their head still during the scanning, applying padding between the subjects’ head and the head-coil, or having subjects wear a cervical collar to minimize both lateral and axial head movements.15,16 A 5 to 6-minute resting state functional MRI (fMRI) scan was conducted in a 3 T GE scanner with T2*-weighted EPI-sequences (TE = 30 ms, TR = 2.0 s, a = 90°, TH = 4.0 mm, 31–32 slices, FOV = 220×220–240×240 mm2). Anatomical brain scans were obtained with fast spoiled gradient echo sampling: 168–176 slices T1, 256×256 and 1 mm-1.2 mm slice thickness.
Anatomical MRI data preprocessing
Anatomical data was processed using BrainVoyager’s (BV) software (Masstricht, Netherlands) for homogenous processing.17,18 Subject scans were iso-voxeled to 1 mm resolution and underwent intensity inhomogeneity correction before being transformed to AC-PC Talairach standard space.
Resting state fMRI preprocessing
Resting state fMRI data was also processed using BV software. Subject scans were preprocessed with cubic spline slice scan time correction, trilinear/sinc-interpolation for 3 D motion correction, a 4 mm Gaussian filter for spatial smoothening, and a 2-cycle high-pass (GLM-Fourier) temporal filtering. Each subject’s resting state fMRI was co-registered to its corresponding anatomical scan and then transformed into Talairach space, resulting in a normalized 4 D volume time course data set.19,20
Resting state fMRI between-group comparison
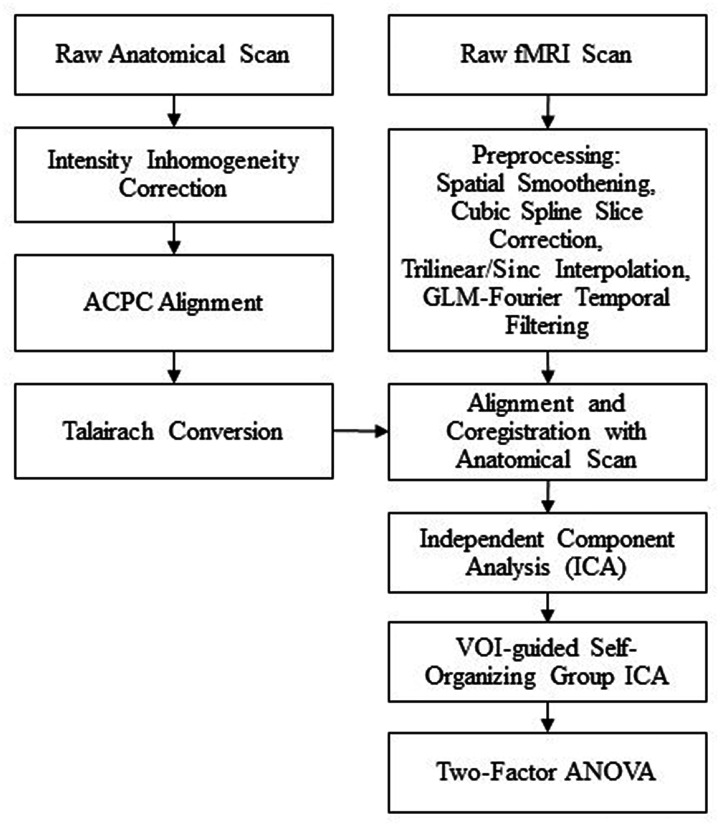
Independent component analysis (ICA) was conducted on each individual subject using BV’s FastICA algorithm with deflation approach and a Tanh nonlinearity function to produce 30 components per subject. BV’s self-organizing group ICA (SOGICA) plugin was then run using the individual subject ICAs and guided by our previous study’s volume of interest (VOI) of cortical regions known to be associated with pain processing.2,19 The SOGICA settings were as follows: group components = 30, absolute threshold before similarity processing = 0, and a degree of temporal similarity in the clustering = 0. A two-factor (cluster x within/between HA Severity group) ANOVA was then conducted to determine the interaction and followed with a student’s T-test to evaluate the differences in functional connectivity among the VOI between each group. Any supraspinal regions with significant (p < 0.01 and cluster threshold > 150voxels) activation or deactivation difference in activity were recorded while the other regions were filtered out.2 The spatial coordinates of the significant clusters were identified and named via Talairach Client and confirmed using BrainVoyager Tutor’s probabilistic anatomical map (see Figure 1).
Figure 1.
Imaging data acquisition, processing and analysis flow chart. ACPC: Anterior Commissure-Posterior Commissure; fMRI: functional magnetic resonance imaging; GLM: general linear model; VOI: volume of interest; ANOVA: analysis of variance.
Results
Demographic
The average ages (years old±SD) of the subjects were 32.06 ± 5.86, 32.75 ± 5.56, 33.06 ± 6.10, 32.63 ± 5.74, for control, mild, moderate, and severe groups (all males), respectively (Table 1). Overall, there were no significant age, ethnicity, or headache duration differences among the groups (Table 2). No female Veterans were included in the analyses due to difficulty in group age matching for the same gender. There was a significant (p = 0.036) difference in the years of education between the control and moderate headache intensity groups (Table 2). There was a significant difference (p = 0.004) in MTBI type between moderate and severe headache groups (Table 2), such that the severe headache group contained significant more subjects that had suffered a TBI from a combined blast/non-blast mechanism, and significantly less subjects who experienced a non-blast only TBI. For clinical PTSD diagnosis, a significant (p = 0.001) overall difference was found among all groups and subsequent corrected pair-wise comparisons demonstrated significant differences in control vs. severe (p = 0.002), and mild vs. severe (p = 0.007) groups (Table 2). While a significant (p = 0.031) overall difference was found in the prevalence of depression subtypes among all headache intensity groups and control, secondary pair-wise comparisons demonstrated no significant differences in all comparisons (Table 2). There were no significant differences in medication use among the headache groups (Table 3).
Table 1.
Group demographics.
|
Severity of MTBI-HA |
||||
|---|---|---|---|---|
| Controls(n = 16) | Mild(n = 16) | Moderate(n = 16) | Severe(n = 16) | |
| Demographic characteristics | ||||
| Age (years) | 32.06 (5.86) | 32.75 (5.56) | 33.06 (6.10) | 32.63 (5.74) |
| Sex (N male) | 16 | 16 | 16 | 16 |
| Education (years) | 14.81 (1.60) | 14.19 (2.17) | 13.16 (0.93) | 14.06 (1.65) |
| Ethnicity | ||||
| % Hispanic/Latino | 19% | 25% | 25% | 44% |
| % Not Hispanic/Latino | 81% | 75% | 75% | 56% |
| MTBI characteristics | ||||
| Headache duration since most significant MTBI (months) | N/A | 81.25 (53.57) | 103.06 (59.71) | 90.63 (53.80) |
| MTBI mechanism | ||||
| % non-blast | N/A | 44% | 50% | 13% |
| % blast | N/A | 31% | 50% | 31% |
| % both | N/A | 25% | 0% | 56% |
| Mood | ||||
| Depression | ||||
| % minimal | 56% | 50% | 13% | 13% |
| % mild | 19% | 13% | 19% | 19% |
| % moderate | 13% | 31% | 38% | 19% |
| % severe | 13% | 6% | 31% | 50% |
| % clinical PTSD diagnosis | 25% | 31% | 69% | 88% |
All variables reported means and standard deviations unless otherwise indicated; N = number.
Table 2.
Group demographic comparison P-values (all pair-wise comparisons were corrected).
| Overall | Control vs mild | Control vs moderate | Control vs severe | Mild vs moderate | Mild vs severe | Moderate vs severe | |
|---|---|---|---|---|---|---|---|
| Demographic characteristics | |||||||
| Age | 0.969 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 |
| Education | 0.050 | 1.000 | 0.036* | 1.000 | 0.489 | 1.000 | 0.749 |
| Ethnicity | 0.426 | 1.000 | 1.000 | 0.763 | 1.000 | 1.000 | 1.000 |
| MTBI characteristics | |||||||
| MTBI headache Duration | 0.545 | N/A | N/A | N/A | 0.823 | 1.000 | 1.000 |
| MTBI type | 0.007* | N/A | N/A | N/A | 0.278 | 0.286 | 0.004* |
| Mood | |||||||
| Depression | 0.031* | 1.000 | 0.310 | 0.246 | 0.525 | 0.125 | 1.000 |
| Clinical PTSD diagnosis | 0.001* | 1.000 | 0.079 | 0.002* | 0.203 | 0.007* | 1.000 |
* indicates the bold typed P value is less than 0.05.N/A - Control subjects did not have TBI therefore statistical analysis was not conducted.
Table 3.
Pairwise group medication comparison (P-values) between various MTBI-HA intensity groups.
| Medication | All MTBI-HA | Mild vs moderate | Mild vs severe | Moderate vs severe |
|---|---|---|---|---|
| GABA | 0.344 | 1.000 | 1.000 | 1.000 |
| SSRI | 0.102 | 0.304 | 1.000 | 1.000 |
| TCA | 0.102 | 1.000 | 1.000 | 0.304 |
| ACET | 0.344 | 1.000 | 1.000 | 1.000 |
| NSAIDS | 0.438 | 1.000 | 1.000 | 1.000 |
| TeCA | 0.148 | 1.000 | 1.000 | 0.677 |
| TRIPTAN | 0.106 | 0.998 | 0.249 | 1.000 |
| TOP | 0.360 | ‡ | 1.000 | 1.000 |
| AED | 0.210 | 0.677 | 1.000 | 1.000 |
| Benzo | 0.344 | 1.000 | 1.000 | 1.000 |
| OPIOID | 0.593 | 1.000 | 1.000 | 1.000 |
| NERI | 0.124 | ‡ | 1.000 | 1.000 |
| AP | 0.593 | 1.000 | 1.000 | 1.000 |
| AMP | 0.360 | 1.000 | ‡ | 1.000 |
Values based on number of medications taken.
‡No statistics computed because medication is a constant.
All medication measures were non-significant with p > 0.05.
p-values calculated with chi-square tests or Fisher’s exact test where appropriate.
GABA, gamma-Aminobutyric acid; SSRI, Selective serotonin reuptake inhibitors; TCA, Tricyclic antidepressants; ACET, Acetaminophen; NSAIDS, Nonsteroidal anti-inflammatory drugs, TeCA, Tetracyclic antidepressants; TRIPTAN, Triptans; TOP, Topical Lidocaine: AED, Anti-epileptic drugs; Benzo, Benzodiazepines; OPIOID, Opioids; NERI, norepinephrine reuptake inhibitor; AP, Antipsychotic; AMP, Amphetamine.
Resting state fMRI
Control vs mild headache group resting state comparison
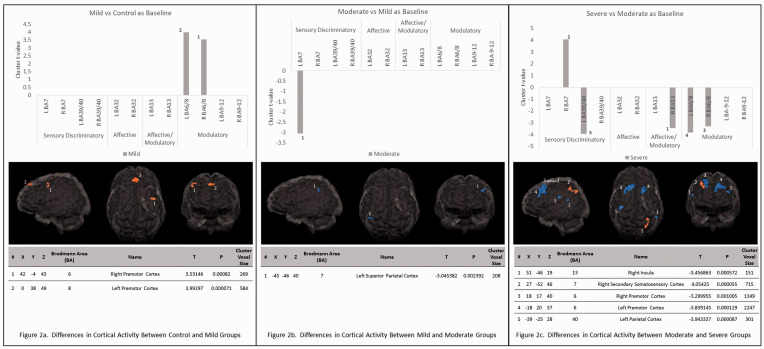
In the mild-headache group, a T-test demonstrated significant increases in activity in the right premotor cortex (T = 3.53, p < 0.001) and the left premotor cortex (T = 3.99, p < 0.0001) when compared to the control group at resting state (see Figure 2(a)).
Figure 2.
Differences in cortical activity between headache severity groups. Glass brains illustrate areas of more activation in orange and areas of less activation in blue. (a) The areas of activation in the mild vs. control headache severity with the control group as baseline for comparison. (b) The areas of activation in the moderate vs. mild headache severity with the mild group as baseline for comparison. (c) The areas of activation in the severe vs. moderate headache severity with the moderate group as baseline for comparison.
Mild vs moderate headache group resting state comparison
In the moderate-headache group, a T-test demonstrated a significant (T = −3.05, p < 0.01) decrease in activity in the left superior parietal cortex when compared to the mild-headache group at resting state (see Figure 2(b)).
Moderate vs severe headache group resting state comparison
In the severe-headache group, a T-test demonstrated significant decreases in resting state activities in the right insula (T = −3.46, p < 0.001), right premotor cortex (T = −3.30, p < 0.01) , left premotor cortex (T = −3.84, p < 0.001), and left parietal cortex (T = −3.94, p < 0.0001), and an increase in activity in the right secondary somatosensory cortex (T = 4.05, p < 0.0001) when compared to the moderate-headache group at resting state (see Figure 2(c)).
Discussion
While emerging evidence supports the notion that MTBI-HA should be addressed as a neuropathic pain state involving abnormality in central pain processing and modulation functions, little is known about how these functional abnormalities may vary with severity of headache. Previous supraspinal mechanistic studies indicated patients with MTBI-HA demonstrated a diminished level of supraspinal prefrontal pain modulatory functions and enhanced supraspinal sensory response to pain in comparison to healthy controls.1,2 This functional abnormality was found to be correlated with structural (white matter) deficits vital to the functional connectivity of these relevant regions.3 These structurally related functional deficits were also found in a recent blood perfusion study in which MTBI patients presented with hypoperfusion in the basal ganglion, a key relay center between the cortical areas (particularly the prefrontal cortical area and parietal cortices) and the hyperperfusion of the limbic system, suggesting a dissociative state between the sensory discrimatory/affective (hyperactive) and modulatory (hypoactive) aspects of supraspinal activities.21 Behaviorally, this dissociative state is found to be associated with peripheral tactile sensitivity known as allodynia in which non-noxious tactile stimulus is perceived as painful in patients with correlated peripheral sensory and supraspinal prefrontal cortical modulatory dysfunction.22
The current study further demonstrated that subjects with mild severity MTBI-HA displayed enhanced resting state supraspinal pain modulatory function in the premotor area in the presence of headache in comparison with the controls (Figure 2(a)). This observation suggests when the intensity of headache is mild, the supraspinal modulatory function is reactively enhanced to counteract the symptom. However, when the mild headache severity group was compared to the moderate headache severity group, the modulatory functions did not appear to be improved accordingly suggesting that the supraspinal modulatory response was unable to react proportionally with an increase in headache severity (Figure 1(b)). In addition, the somatosensory cortex demonstrated a degree of hypoactivity in the moderate headache intensity group in comparison with the mild headache intensity group. This imbalance of resting state pain modulatory functions became even more prominent as the severe headache group demonstrated a significant decrease in resting state activities in a wide area of supraspinal modulatory areas and an increase of activity in areas of sensory discriminatory functions in the primary somatosensory cortices when compared with their moderate severity counterpart (Figure 2(c)). Furthermore, the severe headache group also exhibited a decrease of activity in the insula, a key related region for pain modulation in comparison with moderate headache group, suggesting an added degree of impairment in pain modulation increased headache severity. Overall, the anterior cingulate cortex which previously has been shown to have an enhanced activity in patients with MTBI-HA in comparison to healthy controls did not demonstrate any significant differences in activities among the different headache intensity groups.2 Thus, this finding supports the notion that the increase in MTBI-HA severity is mainly associated with an enhanced imbalance in pain related supraspinal functions between modulatory and sensory discriminatory brain areas.
Mood disorders, such as depression and PTSD, are common comorbid symptoms in patients with traumatic brain injuries, with overlapping cognitive and functional impairments.23,24 In this study, the severe headache intensity group indicated greater incidence of clinical PTSD as compared to the control and mild headache intensity groups. Given that PTSD is known to be associated with enhanced function of the sympathetic nervous system as found in neuropathic pain states, the result of the current study confirms and further demonstrates such a correlation becomes more significant with the increase of headache intensity. However, there is no direct correlation between depression and headache intensity phenotype after correction of multiple comparisons based on the current sample size. Thus, the authors recommend future studies with larger sample sizes are required to further ascertain this issue.
Some limitations of the current study are worthy of discussion. First, although a significant difference was observed in the type of TBI between moderate and severe headache intensity groups, it is not believed that these findings confound the inference of the imaging data as previous studies have indicated that headache and co-morbid symptom presentations related to the two different MTBI types are indistinguishable.25,26 Furthermore, the current comparisons are conducted with a cross sectional design with subjects matched in age and gender with no difference in their headache durations and did not look at the longitudinal progression of supraspinal changes in the same cohort. Thus, future studies may focus on correlating changes in MTBI-HA intensity with resting state functional alterations over time. Likewise, similar studies should be conducted to assess change in cortical functions with interventions for headache improvement such as transcranial magnetic stimulation.27–29 While the sample size of the current study may be considered as adequate in referencing previously conducted studies in this area, future studies with larger sample sizes will certainly provide more definitive confirmation.
Footnotes
Authors’ Contributions: MF assisted in data collection, functional data analysis and manuscript preparation. AL and DS assisted in study design and manuscript preparation. VMS, LD, SS, AK, and AW assisted data collection. MV assisted in subject evaluation. SG assisted in the data analysis and manuscript preparation. RL assisted in neuroimaging assessment.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The authors thank the funding support from the VA Rehabilitation and Research Development SPIRE and Merit Awards to Dr. Leung (21RX001359, 21RX002366, IRX002506A), a Career Development Awards to Drs. Delano-Wood (CDA-2–034-07F) and Schiehser (CDA-2–065-10S), a Veterans Affairs Merit Award to Dr. Delano-Wood (CX000842-01A2) as well as a Department of Defense Investigator-Initiated Research Grant awarded to Dr. Delano-Wood (W81XWH-10–2-0169). Dr. Sorg received salary support from a VA Clinical Science Research & Development funded Career Development Award (IK2-CX001508) from the VA Clinical Science Research & Development Service.
ORCID iD: Alphonsa Kunnel https://orcid.org/0000-0002-6773-0863
References
- 1.Defrin R.Chronic post-traumatic headache: clinical findings and possible mechanisms. J Man Manip Ther 2014; 22: 36–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Leung A, Shukla S, Yang E, Canlas B, Kadokana M, Heald J, Davani A, Song D, Lin L, Polston G, Tsai A, Lee R.Diminished supraspinal pain modulation in patients with mild traumatic brain injury. Mol Pain 2016; 12: 174480691666266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Leung A, Yang E, Lim M, Metzger-Smith V, Theilmann R, Song D, Lin L, Tsai A, Lee R.Pain-related white matter tract abnormalities in mild traumatic brain injury patients with persistent headache. Mol Pain 2018; 14: 1744806918810297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Obermann M, Nebel K, Schumann C, Holle D, Gizewski ER, Maschke M, Goadsby PJ, Diener HC, Katsarava Z.Gray matter changes related to chronic posttraumatic headache. Neurology 2009; 73: 978–983. [DOI] [PubMed] [Google Scholar]
- 5.Jang SH, Park SM, Kwon HG.Relation between injury of the periaqueductal gray and Central pain in patients with mild traumatic brain injury: Observational study. Medicine 2016; 95: e4017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gironda RJ, Clark ME, Ruff RL, Chait S, Craine M, Walker R, Scholten J.Traumatic brain injury, polytrauma, and pain: challenges and treatment strategies for the polytrauma rehabilitation. Rehabil Psychol 2009; 54: 247–258. [DOI] [PubMed] [Google Scholar]
- 7.Ashman TA, Cantor JB, Gordon WA, Spielman L, Flanagan S, Ginsberg A, Engmann C, Egan M, Ambrose F, Greenwald BA.Randomized controlled trial of sertraline for the treatment of depression in persons with traumatic brain injury. Arch Phys Med Rehabil 2009; 90: 733–740. [DOI] [PubMed] [Google Scholar]
- 8.Neugebauer V, Galhardo V, Maione S, Mackey SC.Forebrain pain mechanisms. Brain Res Rev 2009; 60: 226–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Seifert F, Bschorer K, De Col R, Filitz J, Peltz E, Koppert W, Maihöfner C.Medial prefrontal cortex activity is predictive for hyperalgesia and pharmacological antihyperalgesia. J Neurosci 2009; 29: 6167–6175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tracey I.Neuroimaging of pain mechanisms. Curr Opin Support Palliat Care 2007; 1: 109–116. [DOI] [PubMed] [Google Scholar]
- 11.Cole MW, Schneider W.The cognitive control network: integrated cortical regions with dissociable functions. Neuroimage 2007; 37: 343–360. [DOI] [PubMed] [Google Scholar]
- 12.Leung A, Shukla S, Li E, Duann JR, Yaksh T.Supraspinal characterization of the thermal grill illusion with fMRI. Mol Pain 2014; 10: 18–03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tallus J, Lioumis P, Hamalainen H, Kahkonen S, Tenovuo O.Long-lasting TMS motor threshold elevation in mild traumatic brain injury. Acta Neurol Scand 2012; 126: 178–182. [DOI] [PubMed] [Google Scholar]
- 14.Furukawa TA, Reijnders M, Kishimoto S, Sakata M, DeRubeis RJ, Dimidjian S, Dozois DJA, Hegerl U, Hollon SD, Jarrett RB, Lesperance F, Segal ZV, Mohr DC, Simons AD, Quilty LC, Reynolds CF, Gentili C, Leucht S, Engel RR, Cuijpers P.Translating the BDI and BDI-II into the HAMD and vice versa with equipercentile linking. Epidemiol Psychiatr Sci 2019; 29: e24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Leung A, Duann JR, Davis M, Li E, Fallah A, Yaksh TL.Facial-cervical collar restraint (FCCR) device in reducing head motion during a noxious stimulus study. Neuroimage 2006; 31: 504. [Google Scholar]
- 16.Shukla S, Torossian A, Duann JR, Leung A.The analgesic effect of electroacupuncture on acute thermal pain perception – a central neural correlate study with fMRI. Mol Pain 2011; 7: 45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Formisano E, Di Salle F, Goebel R.Fundamentals of data analysis methods in functional MRI. Advanced image processing in magnetic resonance imaging. Boca Raton: CRC Press, 2005, pp. 479–501. [Google Scholar]
- 18.Goebel R, Esposito F, Formisano E.Analysis of functional image analysis contest (FIAC) data with brainvoyager QX: from single-subject to cortically aligned group general linear model analysis and self-organizing group independent component analysis. Hum Brain Mapp 2006; 27: 392–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hyvarinen A.Fast and robust fixed-point algorithms for independent component analysis. IEEE Trans Neural Netw 1999; 10: 626–634. [DOI] [PubMed] [Google Scholar]
- 20.Hyvarinen A, Oja E.Independent component analysis: algorithms and applications. Neural Netw 2000; 13: 411–430. [DOI] [PubMed] [Google Scholar]
- 21.Lewine JD, Davis JT, Bigler ED, Thoma R, Hill D, Funke M, Sloan JH, Hall S, Orrison WW.Objective documentation of traumatic brain injury subsequent to mild head trauma: multimodal brain imaging with MEG, SPECT, and MRI. J Head Trauma Rehabil 2007; 22: 141–155. [DOI] [PubMed] [Google Scholar]
- 22.Defrin R, Riabinin M, Feingold Y, Schreiber S, Pick CG. Deficient pain modulatory systems in patients with mild traumatic brain and chronic post-traumatic headache: implications on its mechanism. J Neurotrauma. Epub ahead of print 30 July 2014. DOI: 10.1089/neu.2014.3359. [DOI] [PMC free article] [PubMed]
- 23.Jorge RE, Arciniegas DB.Mood disorders after TBI. Psychiatr Clin North Am 2014; 37: 13–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tanev KS, Pentel KZ, Kredlow MA, Charney ME.PTSD and TBI co-morbidity: scope, clinical presentation and treatment options. Brain Inj 2014; 28: 261–270. [DOI] [PubMed] [Google Scholar]
- 25.Faul M, Coronado V.Epidemiology of traumatic brain injury. Handb Clin Neurol 2015; 127: 3–13. [DOI] [PubMed] [Google Scholar]
- 26.Theeler BJ, Flynn FG, Erickson JC.Chronic daily headache in U.S. soldiers after concussion. Headache 2012; 52: 732–738. [DOI] [PubMed] [Google Scholar]
- 27.Leung A, Shukla S, Fallah A, Song D, Lin L, Golshan S, Tsai A, Jak A, Polston G, Lee R. Repetitive transcranial magnetic stimulation in managing mild traumatic brain injury-related headaches. Neuromodulation. Epub ahead of print 2015. DOI: 10.1111/ner.12364. [DOI] [PubMed]
- 28.Leung A, Metzger-Smith V, He Y, Cordero J, Ehlert B, Song D, Lin L, Shahrokh G, Tsai A, Vaninetti M, Rutledge T, Polston G, Sheu R, Lee R.Left dorsolateral prefrontal cortex rTMS in alleviating MTBI related headaches and depressive symptoms. Neuromodulation 2018; 21: 390–401. [DOI] [PubMed] [Google Scholar]
- 29.Stilling J, Paxman E, Mercier L, Gan LS, Wang M, Amoozegar F, Dukelow SP, Monchi O, Debert C.Treatment of persistent post-traumatic headache and post-concussion symptoms using repetitive transcranial magnetic stimulation: a pilot, double-blind, randomized controlled trial. J Neurotrauma 2020; 37: 312–323. [DOI] [PubMed] [Google Scholar]