Abstract
Objective:
Current guidelines have different recommendations on applying pharmacological interventions for managing cancer-related fatigue (CRF) among cancer survivors. This systematic review aims to synthesize clinical evidence on pharmacological interventions for managing CRF.
Methods:
Five databases were searched for potential randomized controlled trials (RCTs) from their inception until October 2020. RCTs assessing the effect of pharmacological treatments for CRF among cancer survivors were considered eligible. Clinical significance was determined by comparing the estimated effect with that of minimal important difference (MID). The risk of bias of each included RCT was appraised using the Cochrane risk of bias tool for randomized trials 2. Data were synthesized using random-effect pairwise meta-analyses.
Results:
A total of 15 RCTs (1238 participants) were included. The majority presented some concerns of bias arising from the randomization process and selection of the reported results. Meta-analysis showed that psychostimulant and wakefulness agents had statistically significant while clinically insignificant effects on the treatment of CRF (pooled weighted mean difference [WMD]: 2.8, 95% confidence interval [CI]: 0.2-5.4, I2: 0%, 3 RCTs, MID: 3.0-6.0). Three natural products, including Renshen Yangrong Tang (mean difference [MD]: −16.1, 95% CI: −8.9 to −23.3, MID: −17.3 to −11.4), Tualang honey (MD: 11.2, 95% CI: 7.1-15.3, MID: 3.0-6.0), and Shenmai injection plus Peptisorb (MD: −1.6, 95% CI: −2.1 to −1.1, MID: −1.1 to −0.8) demonstrated statistically and clinically significant effect in reducing CRF.
Conclusions:
Existing evidence showed promising effects of 3 natural products in reducing CRF among cancer survivors. The results from this study need to be further confirmed with well-designed and adequately powered RCTs that use validated instruments for the measurement of CRF.
Keywords: fatigue, cancer survivors, drug therapy, systematic review, meta-analysis
Introduction
With the current rise in cancer incidence, technological advances in cancer screening, diagnosis, and treatment have led to a growing number of cancer survivors around the world.1 Cancer survivors refer to a group of patients who have successfully completed the initial cancer treatment, and they are generally expected to regain their functions at the level before their cancer diagnosis.2 However, symptoms experienced by cancer survivors may worsen their function and quality of life,3 of which cancer-related fatigue (CRF) is one of the frequently reported symptoms.2 Current studies reported that 17% to 53% of cancer survivors experienced fatigue which affects their daily living.4-6 Variations in scales used for measuring CRF might contribute to the discrepancy reported in the publications,4 as several scales have been developed to measure CRF, with no standardized measurement scale for CRF.
As the first step for management, current guidelines recommend identifying and treating the potential causes of fatigue.4,7 If fatigue persists after addressing all potential root causes (eg, anemia, sleep disturbance, pain, and mental disorders), non-pharmacologic interventions including physical activity and psychosocial interventions should be considered.1,4,7 As a second-line treatment, both the American Society of Clinical Oncology1 and the National Comprehensive Cancer Network (NCCN)4 recommend psychostimulants (eg, methylphenidate) if non-pharmacologic interventions fail.
Indeed, the latest version of the NCCN guidelines mentioned that evidence supporting the effectiveness of methylphenidate in reducing CRF is limited.4 The current NCCN recommendation is based on evidence from randomized controlled trials (RCTs), nonetheless, a systematic review that summarizes the most up-to-date evidence is lacking.4 That might due to the following factors: (1) systematic reviews on pharmacological treatments for managing CRF are outdated,8-13 with the latest literature search conducted in 201711; (2) there is only one systematic review summarizing evidence on pharmacological interventions specifically for CRF among cancer survivors, and only thyroid cancer survivors were included.13 Indeed, pharmacological interventions are not recommended by the Pan-Canadian guideline as they are considered experimental.7
Besides methylphenidate, the American Society of Clinical Oncology1 also recommends wakefulness agents (eg, modafinil), but it is not recommended by the NCCN in 20204 as two RCTs failed to demonstrate a significant effect.10,14 Based on evidence summarized by the Oncology Nursing Society, there are multiple pharmacological interventions (eg, psychostimulant, natural products, donepezil) that have been tested for managing CRF, but their effectiveness is yet to be confirmed.15
In view of these conflicting recommendations, there is a need to clarify the potential role of pharmacological interventions based on up-to-date clinical evidence. This systematic review and meta-analysis aims to synthesize the latest evidence on pharmacological interventions for reducing CRF among cancer survivors.
Methods
The protocol of this systematic review has been registered in PROSPERO (registration number: CRD42018102347).
Eligible Criteria
Types of RCTs
To be included in this systematic review, RCTs must report quantitative results and satisfy the eligibility criteria in the aspects of participants, interventions, and outcome of interest, as described below.
Types of participants
RCTs recruited patients with any type of cancer who had completed cancer treatment, with ≤50% of recruited patients in Stage III/IV or Grade III/IV of the TNM Classification of Malignant Tumors, were eligible for inclusion. With this criterion, we would be able to focus mainly on cancer survivors.
Types of interventions
We included RCTs evaluating six common pharmacological interventions14,16 including (i) psychostimulants and wakefulness agents, (ii) natural products, (iii) hormonal therapies, (iv) acetylcholinesterase inhibitors, (v) antidepressants, and (vi) somatostatins. Comparisons with placebo, no treatment, and any other pharmacological interventions or non-pharmacological interventions are considered eligible.
Types of outcome measures
The eligible RCTs should report CRF as a primary or secondary outcome measured with any of the validated instruments listed in Supplemental File, Appendix 1.17
Search Strategy
Five electronic databases (CENTRAL, MEDLINE, EMBASE, CINAHL Plus, and AMED) were searched for potential RCTs from their inception until October 2020. Validated, sensitivity maximized search filters for RCTs were applied in MEDLINE and EMBASE searches.18,19 The searches were limited to human studies and no language restriction was applied. Seven additional sources were searched to identify additional records, including Global Health, NHS Health Technology Assessment Database, Digital Dissertation Consortium, ClinicalTrials.gov, Drugs@FDA, European Medicines Agency Public Assessment Reports, and Pharmaceuticals and Medical Devices Agency of Japan. The search strategies of this systematic review are presented in Supplemental File, Appendix 2.
Literature Selection
Two reviewers (XS and YC) independently screened titles and abstracts of search results, evaluated potential full texts, and determined eligibility. Discrepancies were resolved by consensus between 2 reviewers. A third reviewer (IW) was invited for consensus adjudication when unresolvable discrepancies occurred.
Data Extraction and Management
The following data were extracted from each included RCT by two reviewers (XS and WC) independently: year of publication, the number of patients randomized and analyzed, patient characteristics, types of cancer, the scale of fatigue measurement, and characteristics of interventions in treatment and control groups, duration after cancer diagnosis and treatment, follow-up duration, and adverse events. Discrepancies were resolved by consensus between the two reviewers, with reference to the original publications.
Risk of Bias Assessment
Two reviewers (XS and YC) independently assessed the risk of bias of each included RCT using the Cochrane risk of bias tool for randomized trials 2 (RoB 2).20 Discrepancies in assessments were resolved by discussion and consensus between the two reviewers, adjudication was sought from the third reviewer (IW) when disagreement persisted. Domains of the risk of bias assessment included: bias arising from the randomization process, bias due to deviations from intended interventions, bias due to missing outcome data, bias in the measurement of the outcome, and bias in the selection of the reported result. Grading judgment of “low,” “high,” or “some concerns” risk of bias was made for each domain based on the information provided by the included publications.
Data Synthesis
Using Review Manager Version 5.3, a random effect pairwise meta-analysis was performed to synthesize data extracted from included RCTs with the same interventions, similar follow-up duration (differences in follow-up duration ≤4 weeks), and the same assessment scales. The pooled weighted mean difference (WMD) with its 95% confidence interval (CI) was used to synthesize continuous outcomes. Conversions were applied so that a positive WMD can represent the superiority of the pharmacological treatment group. I-square (I2) values were calculated for quantifying heterogeneity among RCTs. I2 values of <30%, 30% to 60%, >60% were regarded as low, moderate, and high heterogeneity respectively.21
Minimal important difference (MID) was extracted from a related systematic review,22 which generally summarized MID for a specific scale (eg, Multidimensional Fatigue Inventory [MFI], Functional Assessment of Chronic Illness Therapy [FACIT]) from a range of related primary studies. When the systematic review22 did not provide MID for a specific scale, an alternative approach for calculating MID, which was taken half of the standard deviation of the baseline value.23 Through the aforementioned methods, we determined all the MIDs as shown in Table 2. With the presence of statistical significance, the clinical significance of each intervention was assessed by comparing mean difference (MD) or pooled WMD with the MID of the corresponding scales. If the MD/pooled WMD was larger than the upper limit of the range of the MID, we concluded that the intervention effect had clinical significance. On the other hand, if the MD/pooled WMD was within the range of the MID, we concluded that the intervention effect was considered to be of potential clinical significance.
Table 2.
Effectiveness of Pharmacological Interventions for Cancer-Related Fatigue among Cancer Survivors.
| Authors | Comparison | Fatigue measurement scale | Follow-up duration (weeks) | Within treatment group | Within control group | MD (95% CI) | MID/MID value range for fatigue improvementa | ||
|---|---|---|---|---|---|---|---|---|---|
| MD (SD) | N | MD (SD) | N | ||||||
| Laigle-Donadey et al26 | Dexamphetamine vs placebo | MFI-20 (20-100) | 13.0 | −12.3 (12.8) | 22 | −8.9 (8.7) | 19 | −3.4 (−10.0 to 3.2) | −9.6 to −6.8 |
| Berenson et al34 | Armodafinil vs placebo | FACIT-F (0-52) | 4.0 | 7.8 (10.6) | 18 | 7.7 (10.3) | 23 | 0.1 (−6.4 to 6.6) | 3.0 to 6.0 |
| Richard et al35 | Methylphenidate vs placebo | FACIT-F (0-52) | 6.0 | 4.8 (8.2) | 11 | 2.4 (5.0) | 12 | 2.4 (−8.0 to 3.2) | 3.0 to 6.0 |
| 10.0 | 7.7 (7.8) | 11 | 1.4 (7.6) | 12 | 6.3 (−0.0 to 12.6) | ||||
| Boele et al28 | Modafinil vs placebo | CIS (0-140) | 6.0 | −14.1 (23.0) | 26 | −11.3 (23.1) | 29 | −2.8 (−15.0 to 9.4) | −10.1 |
| Lower et al29 | Dexmethylphenidate vs placebo | FACIT-F (0-52) | 8.0 | 10.5 (8.8) | 54 | 6.8 (10.0) | 69 | 3.7 (0.4 to 7.0) | 3.0 to 6.0 |
| Xu et al30 | Renshen Yangrong Tang vs Huangqi | QLQ C-30 (0-100) | 6.0 | −23.6 (18.8) | 41 | −7.5 (14.5) | 42 | −16.1 (−8.9 to −23.3) | −17.3 to −11.4 |
| Guglielmo et al31 | American ginseng vs placebo | BFI (0-10) | 8.0 | −1.8 (1.8) | 17 | −2.2 (1.6) | 15 | −0.4 (−0.8 to 1.6) | −1.1 |
| Ramasamy et al32 | Tualang honey vs vitamin C | FACIT-F (0-52) | 8.0 | 14.9 (7.2) | 21 | 3.7 (6.0) | 19 | 11.2 (7.11 to 15.3) | 3.0 to 6.0 |
| Jeong et al27 | Bojungikki-tang vs no treatment | FACIT-F (0-160) | 2.0 | 8.0 (23.7) | 20 | −2.1 (22.7) | 20 | 10.1 (−4.3 to 24.5) | 5.5 to 8.5 |
| Dong et al33 | Shenmai injection plus peptisorb vs placebo | VAS-F (0-10) | 1.3 | −5.7 (0.6) | 20 | −4.1 (0.9) | 19 | −1.6 (−2.1 to −1.1) | −1.1 to −0.8 |
| Peptisorb vs placebo | VAS-F (0-10) | 1.3 | −4.4 (0.8) | 19 | −4.1 (0.9) | 19 | −0.3 (−0.9 to 0.3) | −1.1 to −0.8 | |
| Kamath et al36 | Thyrotropin-releasing hormone vs placebo | FACIT-F (0-52) | 4.0 | 35.6 (8.8) | 8 | 28.7 (11.1) | 8 | 6.9 (−2.9 to 16.7) | 3.0 to 6.0 |
| Fahlén et al37 | Menopausal hormone therapy vs no treatment | QLQ C-30 (0-100) | 24.0 | −2.6 (22.7) | 27 | −6.3 (22.2) | 21 | 3.8 (−9.0 to 16.6) | −17.3 to −11.4 |
| 52.0 | −9.8 (19.9) | 27 | −5.8 (23.4) | 21 | −4.0 (−16.5 to 8.5) | ||||
| Lawrence et al39 | Donepezil vs placebo | FACIT-F (0-52) | 12.0 | 2.9 (12.7) | 23 | 5.7 (13.8) | 27 | −2.8 (−10.4 to 4.8) | 3.0 to 6.0 |
| 24.0 | 6.8 (12.3) | 22 | 8.3 (13.6) | 25 | −1.5 (−8.9 to 5.9) | ||||
| Salehifar et al38 | Bupropion vs placebo | FACIT-F (0-52) | 6.0 | 7.0 (6.7) | 13 | 1.9 (7.2) | 14 | 5.1 (−0.1 to 10.3) | 3.0 to 6.0 |
| Eaton et al40 | Pasireotide vs placebo | QLQ C-30 (0-100) | 8.6 | NR | 152 | NR | 148 | NR (P > .05) | −17.3 to −11.4 |
Abbreviations: BFI, Brief Fatigue Inventory; CI, confidence interval; CIS, checklist individual strength; FACIT-F, Functional Assessment of Chronic Illness Therapy-Fatigue Scale; MD, mean difference; MFI-20, Multidimensional Fatigue Inventory; MID, minimal important difference; N, number of patients; NR, not reported; QLQ C-30, Cancer Quality of Life Questionnaire; SD, standard deviation; VAS-F, Visual Analog Scale for Fatigue.
Sensitivity analysis was planned by including only RCTs with a low overall risk of bias. However, this was not performed due to the unavailability of RCTs with a low risk of bias. The presence of publication bias was evaluated by Begg’s test and Egger’s test when more than 10 RCTs were included in a meta-analysis.24,25 A two-tailed statistical level of 0.05 was considered as significant.
Results
Study Selection
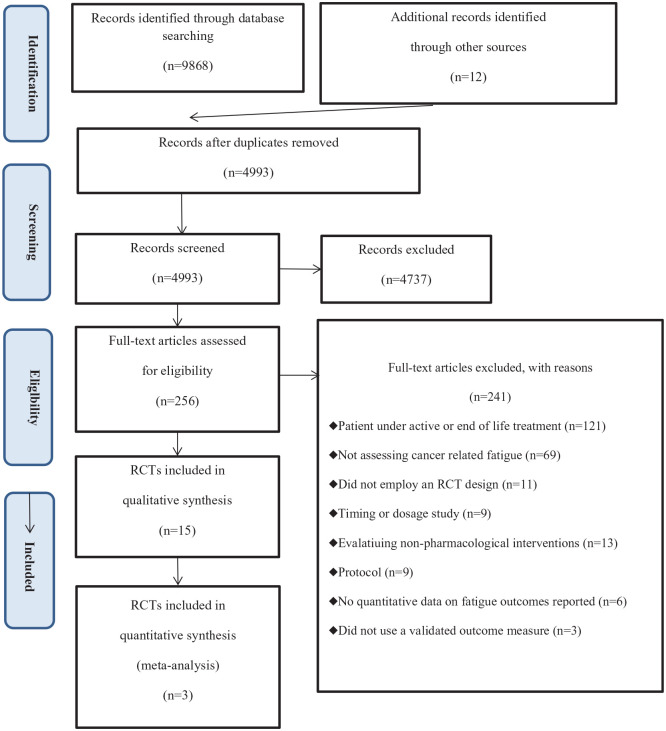
The literature search identified 9880 records. Eventually, 15 RCTs were included in the qualitative synthesis,26-40 of which five were included in the meta-analysis.26,28,29,34,35 Details of the literature search and RCT selection can be found in Figure 1.
Figure 1.
PRISMA flow diagram for literature search and selection.
Characteristics of Included RCTs
Participants
Characteristics of included RCTs are summarized in Table 1. The 15 RCTs included a total of 1238 patients. The average age of participants ranged from 48.2 to 74.0 years. The sample size among included RCTs ranged from 18 to 443, with 13 RCTs (86.7%) recruiting less than 100 participants.26-28,30-39 Among three RCTs providing related information, the average duration after completing cancer treatment ranged from 1.9 to 6.5 years.
Table 1.
Basic Characteristics of Included RCTs.
| Authors (country) | Types of intervention | Treatments | Number of participants (R/A) | Mean age (SD) (y) | Male (%) | Mean duration after Dx/Tx (y) | Types of cancer | Follow-up duration | Fatigue measurement scale | Baseline fatigue score (SD) |
|---|---|---|---|---|---|---|---|---|---|---|
| Laigle-Donadey et al26 (France) | Psychostimulant and wakefulness agent | Dexamphetamine, 5 mg b.d. for 10 d, then 10 mg b.d. for 10 d, and then 15 mg b.d. for 10 W | 23/22 | 55.0 (16.6) | 13 (59.0) | (NR)/5.2 | Primary brain tumors | 13.0 W | MFI-20 (20-100), QLQ C-30 (0-100) | MFI-20: 72.0 (12.1) |
| Placebo | 23/19 | 49.0 (10.3) | 8 (42.0) | (NR)/6.5 | MFI-20: 72.3 (8.3) | |||||
| Berenson et al34 (USA) | Psychostimulant and wakefulness agent | Armodafinil, 150 mg o.d. for 4 W | 25/18 | 63.0 (1.8) | 14 (56.0) | NR | Multiple myeloma | 4.0 W, 8.0 Wa | BFI (0-10), FACIT-F (0-52) | FACIT-F: 19.2 (8.7) |
| Placebo | 25/23 | 67.0 (2.3) | 15 (60.0) | FACIT-F: 18.8 (8.9) | ||||||
| Richard et al35 (Canada) | Psychostimulant and wakefulness agent | Methylphenidate, 5 mg o.d. for 2 W, then 5 mg b.d. for 8 W | 12/11 | 63.0 (NR) | 24 (100.0) | NR/2.4 | Prostate cancer | 6.0 W, 10.0 W | FACIT-F (0-52) | 36.0 (10.4) |
| Placebo | 12/12 | 74.0 (NR) | NR/3.3 | 37.0 (14.8) | ||||||
| Boele et al28 (Netherlands) | Psychostimulant and wakefulness agent | Modafinil, 100 mg b.d. for 1 W, 200 mg b.d. for 5 W | 37/26 | 48.2 (12.0) | 14 (37.8) | 4.1/NR | Primary brain tumor | 6.0 W | CIS (0-140) | 93.8 (20.2) |
| Placebo | 37/29 | 93.8 (20.2) | ||||||||
| Lower et al29 (USA) | Psychostimulant and wakefulness agent | Dexmethylphenidate, ≤50 mg o.d. for 8 W | 76/75 | 52.5 (10.2) | 4 (5.3) | NR/2.6 | Variousb | 8.0 W | FACIT-F (0-52) | 30.9 (10.2) |
| Placebo | 78/77 | 53.2 (8.4) | 5 (6.5) | NR/2.2 | 30.0 (10.1) | |||||
| Xu et al30 (China) | Natural product | Renshen Yangrong Tang, 10 g b.d. for 6 W | 45/41 | 53.3 (6.6) | 7 (17.0) | NR | Variousb | 2.0 W, 4.0 W, 6.0 W | MDASI (0-10), QLQ C-30 (0-100) | MDASI: 6.2 (NR) |
| Huangqi, 5 g b.d. for 6 W | 45/42 | 56.3 (7.9) | 10 (24.0) | MDASI: 5.4 (NR) | ||||||
| Guglielmo et al31 (Italy) | Natural product | American ginseng, 500 mg b.d. for 8 W | 17/17 | 57.4 (32.5) | 13 (76.0) | NR | Head and neck cancer | 8.0 W | BFI (0-10) | 6.4 (1.2) |
| Placebo | 15/15 | 56.5 (36.0) | 9 (60.0) | 4.6 (2.1) | ||||||
| Ramasamy et al32 (Malaysia) | Natural product | Tualang honey, 20 mg o.d. for 8 W | 21/21 | 54.6 (11.0) | 17 (80.9) | NR | Head and neck malignancy | 4.0 W, 8.0 W | FACIT-F (0-52) | 24.7 (7.5) |
| Vitamin C, 100 mg o.d. for 8 W | 21/19 | 54.4 (11.7) | 13 (68.4) | 24.5 (6.5) | ||||||
| Jeong et al27 (Korea) | Natural product | Bojungikki-tang, 2.5 g t.d.s. for 2 W | 20/20 | 49.4 (10.8) | 8 (40.0) | NR/2.2 | Variousb | 2.0 W | VAS-F (0-100), FACIT-F(0-160) | FACT-F: 92.8 (23.5) |
| No treatment | 20/20 | 53.4 (8.0) | 7 (35.0) | NR/1.9 | FACT-F: 94.9 (23.2) | |||||
| Dong et al33 (China) | Natural product | Shenmai (Ginseng-Ophiopogon) injection plus Peptisorb, Shenmai: 50 ml IV for 1 W, Peptisorb: 20-25 kcal/kg per day n.i.t. for 1 W | 20/20 | 61.6 (10.5) | 16 (80.0) | NR | Gastric cancer | 1.3 W | VAS-F (0-10), POMS-F (0-28) | VAS-F: 9.0 (0.4) |
| Peptisorb, 20-25 kcal/kg per day n.i.t. for 1 W | 19/19 | 61.7 (8.5) | 16 (84.2) | VAS-F: 8.9 (0.4) | ||||||
| Placebo (IV injection) | 19/19 | 60.2 (12.2) | 16 (84.2) | VAS-F: 9.0 (0.7) | ||||||
| Kamath et al36 (USA) | Hormone | Thyrotropin-releasing hormone, 0.5-1.5 mg infusion for 4 W | 9/8 | 58.0 (9.4) | 2 (22.2) | NR | Variousb | 0.4 W, 4.0 W | VAS-E (0-100), POMS-F (0-28), FACIT-F (0-52) | NR |
| Placebo (saline) | 9/8 | |||||||||
| Fahlén et al37 (Sweden) | Hormone | Menopausal hormone therapy, 2 mg estradiol o.d. and various progestogens for 52 W | 38/27 | 57.0 (5.6) | 0 (0) | NR | Breast cancer | 24.0 W, 52.0 W | QLQ C-30 (0-100) | 28.6 (21.6) |
| No treatment | 37/21 | 32.8 (24.2) | ||||||||
| Lawrence et al39 (USA) | Acetylcholinesterase inhibitor | Donepezil, 5 mg o.d. for 6 W, then ≤18 mg o.d. for 18 W | 31/22 | 55.8 (NR) | 0 (0) | NR | Breast cancer | 12.0 W, 24.0 W | FACIT-F (0-52) | 32.2 (12.8) |
| Placebo | 31/25 | 28.5 (14.0) | ||||||||
| Salehifar et al38 (Iran) | Aminoketone antidepressants | Bupropion, 75 mg o.d. for first 3 d, then 75 mg b.d. for 6 W | 15/13 | 53.7 (12.9) | 4 (31.0) | NR | Variousb | 2.0 W, 6.0 W | BFI (0-10), FACIT-F (0-52) | FACIT-F: 23.2 (7.2) |
| Placebo | 15/14 | 60.2 (9.9) | 7 (50.0) | FACIT-F: 24.6 (7.5) | ||||||
| Eaton et al40 (USA) | Somatostatin analog | Pasireotide, 900 µg SQ b.d. for 1 W | 219/152 | 64.0 (11.0) | 82 (53.9) | NR | Pancreatic cancer | 2.0 W, 8.6 W | QLQ C-30 (0-100) | 23.7 (NR) |
| Placebo | 224/148 | 64.0 (13.0) | 83 (56.1) | 24.8 (NR) |
Abbreviations: A, number of patients analyzed; BFI, Brief Fatigue Inventory; CIS, checklist individual strength; Dx, diagnosis; FACIT-F, Functional Assessment of Chronic Illness Therapy-Fatigue Scale; IV, intravenous administration; MDASI, MD Anderson Symptom Inventory; MFI-20, Multidimensional Fatigue Inventory; NR, not reported; POMS-F, Profile of Mood States-Fatigue; QLQ C-30, Cancer Quality of Life Questionnaire; R, number of patients randomized; RCT, randomized controlled trial; SD, standard deviation; SQ, subcutaneous administration; Tx, treatment; VAS-F, Visual Analog Scale for Fatigue; VAS-E, Visual Analog Scale for energy; W, weeks; o.d., once daily; b.d., twice daily; t.d.s., 3 times daily; n.i.t., nasointestinal tube.
Treatment group result only.
Please refer to Supplemental File, Appendix 3 for details.
Types of cancer
Ten out of the 15 included RCTs focused on a specific type of cancer patients, including breast cancer37,39 (2 RCTs), primary brain tumors26,28 (2 RCTs), head and neck cancer31,32 (2 RCTs), pancreatic cancer40 (1 RCT), gastric cancer33 (1 RCT), prostate cancer35 (1 RCT), and multiple myeloma34 (1 RCT). The remaining five RCTs27,29,30,36,38 included patients with multiple cancer diagnosis. Supplemental File, Appendix 3 displays for details on types of cancer each trial has recruited.
Interventions
Types of pharmacological interventions included psychostimulant and wakefulness agents (5 RCTs),26,28,29,34,35 natural products (5 RCTs),27,30-33 hormonal therapies (2 RCTs),36,37 acetylcholinesterase inhibitor (1 RCT),39 antidepressants (1 RCT),38 and somatostatin analog (1 RCT).40 Placebo was adopted as the control in all but two RCTs,26,28-36,38-40 of which no treatment was offered to control group patients in these two RCTs.27,37 Intervention duration ranged from 1 to 52 weeks. Detailed descriptions of the treatments are reported in Table 1.
Outcome measurement
Nine instruments were used for assessing CRF among the included RCTs, including FACIT-F (8 RCTs),27,29,32,34-36,38,39 Cancer Quality of Life Questionnaire (QLQ C-30, 4 RCTs),26,30,37,40 Brief Fatigue Inventory (BFI, 3 RCTs),31,34,38 Visual Analogue Scale for Fatigue (VAS-F, 2 RCTs),27,33 Profile of Mood States-Fatigue (POMS-F, 2 RCTs),33,36 MD Anderson Symptom Inventory (MDASI, 1 RCT),30 MFI-20 (1 RCT),26 Checklist Individual Strength (CIS, 1 RCT),28 and Visual Analog Scale for Energy (VAS-E, 1 RCT).36 Follow-up duration for the outcome assessment ranged from three days to 52 weeks, with only three RCTs (20%) reporting a follow-up duration >12 weeks.26,37,39 Results on the estimation of treatment effects of each included RCTs are presented in Table 2.
Risk of Bias of Included RCTs
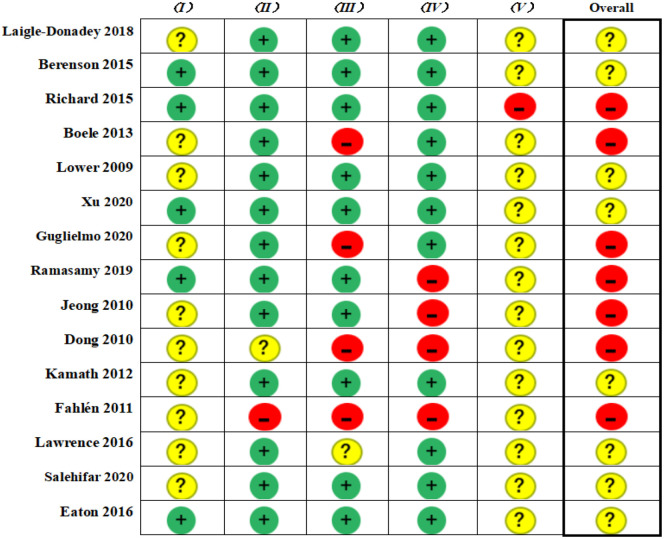
The results of the risk of bias assessment are presented in Figure 2. Among these 15 RCTs, seven had a high overall risk of bias27,28,31-33,35,37 and the remaining eight had some concerns.26,29,30,34,36,38-40 No RCT was rated as having low overall risk of bias. The majority of RCTs presented some concerns of bias arising from the randomization process and selection of the reported results. Detailed results on risk of bias assessment along with supports for judgments are reported in Supplemental File, Appendix 4.
Figure 2.
Risk of bias among included randomized controlled trials.
I, bias arising from the randomization process; II, bias due to deviations from intended interventions; III, bias due to missing outcome data; IV, bias in the measurement of the outcome; V, bias in the selection of the reported result.
Effectiveness of Pharmacological Interventions for Cancer-Related Fatigue Among Cancer Survivors
Psychostimulant and wakefulness agents
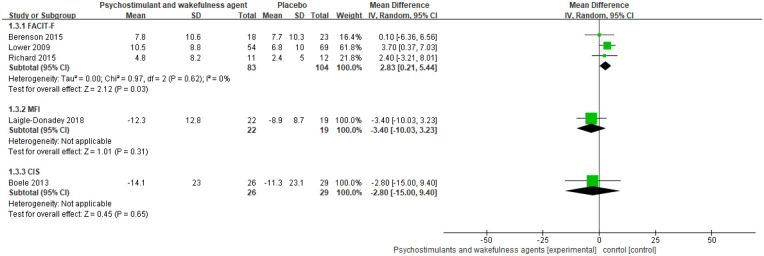
The psychostimulant and wakefulness agents evaluated by the five RCTs were dexamphetamine, armodafinil, methylphenidate, modafinil, and dexmethylphenidate (Table 2).26,28,29,34,35 Result of a pairwise meta-analysis of three RCTs with four to eight weeks follow-up duration measured by FACIT-F showed that psychostimulant and wakefulness agents statistically reduced CRF when compared to placebo (pooled WMD: 2.8, 95% CI: 0.2-5.4, I2: 0%) (Figure 3). However, the magnitude of effect was below the MID value of the FACIT-F (3.0-6.0).23 The two remaining RCTs did not show any significant effect of psychostimulant or wakefulness agents in reducing CRF when compared to placebo. Due to the limited number of included RCTs (<10), we were not able to assess the potential of publication bias.
Figure 3.
Psychostimulants and wakefulness agents versus placebo in reducing fatigue among cancer survivors: meta-analysis.
Follow-up duration: trials used FACIT-F: 4 to 8 weeks; trial used MFI-20: 13 weeks; trial used CIS: 6 weeks.
Abbreviations: FACIT-F, Functional Assessment of Chronic Illness Therapy-Fatigue Scale; MD, mean difference; MFI-20, Multidimensional Fatigue Inventory; CIS, checklist individual strength.
Natural products
Five types of natural products were assessed in five separate RCTs (Table 2).27,30-33 Meta-analysis was not conducted for a significantly high level of heterogeneity observed (I2 = 85%) among the five RCTs. Renshen Yangrong Tang (MD: −16.1, 95% CI: −8.9 to −23.3, MID: −17.3 to −11.4), Tualang honey (MD: 11.2, 95% CI: 7.1-15.3, MID:3.0-6.0), and Shenmai (Ginseng-Ophiopogon) injection plus Peptisorb (MD: −1.6, 95% CI: −2.1 to −1.1, MID: −1.1 to −0.8) were significantly and clinically more effective than control intervention; The MD value of Renshen Yangrong Tang was very close to the upper limit of MID, so we considered its effectiveness had clinical significance. On the other hand, results from small sample size RCTs (n ≤ 40) indicated that American ginseng and Bojungikki-tang (also known as Bu-Zhong-Yi-Qi-Tang or Hochu-ekki-to) did not offer statistically significant alleviation in CRF when compared to control, although point estimation on the effect of Bojungikki-tang (MD = 10.1) was larger than the upper limit of MID (8.5).
Hormonal therapies
Two hormonal therapies were assessed by two RCTs with a small sample size (n < 50) (Table 2).36,37 Neither thyrotropin-releasing hormone nor menopausal hormone therapy showed statistically significant improvement in CRF when compared to control treatment, although the point effect estimation (MD = 6.9) on thyrotropin-releasing hormone was higher than the upper limit of MID (6.0).
Other pharmacological interventions
The effect of acetylcholinesterase inhibitor (donepezil),39 antidepressants (bupropion),38 and somatostatin analog (pasireotide)40 for CRF was assessed by comparing to placebo separately in three different RCTs (Table 2). No statistically significant alleviation effect was found for the three aforementioned interventions, although bupropion (MD: 5.1, 95% CI: −0.1 to 10.3) showed a potential clinically significant effect (MID: 3.0-6.0) in reducing CRF when compared to placebo.
Adverse Events
Adverse events were explicitly reported in twelve RCTs.26-30,32,34-36,38-40 Among those twelve RCTs, two cases of serious adverse events (ptosis and pneumonia) were reported by one RCT, and these adverse events were deemed to be unrelated to the treatment.34 Details of adverse events reported among included RCTs can be found in Supplemental File, Appendix 5.
Discussion
This systematic review comprehensively summarized the updated evidence on pharmacological interventions for CRF among cancer survivors, with only one third of the RCTs overlapping with the previous systematic review,27,29,34,35,39 and ten additional RCTs were identified in this systematic review. Our updated meta-analysis indicating that psychostimulant and wakefulness agents, including armodafinil, methylphenidate, and dexmethylphenidate, only provide slight, clinically insignificant benefit over placebo at four to eight weeks follow-up. Three natural products, including Renshen Yangrong Tang, Tualang honey, and Shenmai injection plus Peptisorb demonstrated statistically and clinically significant effects on reducing CRF among cancer survivors. It is worth noted that the risk of bias of the included RCTs is not satisfactory, with no RCT being judged as low overall risk of bias. In addition, information related to side effects of Shenmai injection plus Peptisorb was not mentioned in the RCT.39
It is worth noted that preliminary results from small RCTs indicated potential clinically significant improvement in CRF among patients treated with Bojungikki-tang, thyrotropin-releasing hormone, and bupropion when compared to placebo or no treatment,35,36,38 although statistical significance was not reached. Future well-designed RCTs with appropriate power are needed to confirm these findings as the aforementioned RCTs included sample sizes ≤40 patients. Indeed, lack of statistical power is a common limitation among the included RCTs, with 13 out of 15 RCTs reporting a sample size less than 100.
The follow-up duration of included RCTs is generally short, with 80% lasting for less than 9 weeks. However, CRF might affect the quality of life among cancer survivors for years. For example, it is reported that CRF lasted for more than 5 years.41 Future research should explore and define a clinically relevant follow-up duration for assessing the effect of pharmacological interventions on CRF.
Fatigue is a complex symptom that covers multiple aspects, including physical, psychological, and mental components.5 Single item instruments such as BFI, which has been used in three out of the 15 included RCTs, are not recommended as outcome measurement as it will not be able to assess CRF in a comprehensive manner.8,17 However, there is no international consensus on what constitutes a core outcome set for CRF, and thus a wide range of instruments have been used among RCTs. In this systematic review, three RCTs were excluded for not using a validated instrument on fatigue.42-44 Although the QLQ C-30 was recommended by the European Organization for Research and Treatment of Cancer for its feasibility, it might not be sensitive enough to detect changes between groups.17 This may explain the lack of significant difference between pasireotide and placebo in one of the included RCTs.40 The FACIT-F was recommended when a unidimensional instrument is considered, and The Fatigue Questionnaire (FQ) (also called the Chalder Fatigue Scale) was recommended when a multidimensional instrument is considered.17
It is well recognized that safety is an important issue in clinical decision-making for adopting a new intervention. However, information about adverse events was not reported in three out of the 15 included RCTs.31,33,37 For example, an RCT indicated that Shenmai injection plus Peptisorb showed statistically and clinically significant effects when compared to placebo. However, the lack of safety information prohibits recommendations on their use, or whether further large-scale RCT on this intervention should be conducted.33 Future RCTs are suggested to report safety related information in accordance with the extension of CONSORT statement on harms.45
This systematic review has several strengths. First, a comprehensive literature search was conducted to identify the most up-to-date RCTs. Second, we only focused on cancer patients in the survivor stage, which is in line with the guideline recommendation.1,4 Third, meta-analysis was conducted among RCTs with similar follow-up duration, same CRF assessment scale, and within the same drug classes, so as to ensure the homogeneous and clinical interpretation of the results.
Several limitations among included RCTs should be addressed in future studies. First, although comprehensive literature searches were conducted, we were unable to assess the potential of publication bias due to the limited number of included RCTs for meta-analysis (<10). Second, there is no head-to-head comparison across different treatments among the included RCTs. Although placebo was used as the control across many included RCTs, variation in follow-up duration and measurements for CRF prohibited the potential of conducting network meta-analysis for comparing the comparative effect across different treatments. Third, MID varies across different scales, or even within the same scale based on methods used for determining MID. Hence, there was no uniform MID. In this study, we extracted scale-specific MID from a systematic review,22 which was presented as a range extracted from different primary studies. That might affect the assessment and interpretation of clinical significance. Last but not the least, risk of bias among the included RCTs might jeopardize the validity of the results generated from this systematic review.
Conclusion
Results from this meta-analysis suggested that psychostimulant and wakefulness agents had limited effect in reducing CRF among cancer survivors. Preliminary results showed beneficial effects of Renshen Yangrong Tang, Tualang honey, and Shenmai injection plus Peptisorb for treating CRF among cancer survivors. Further RCTs are suggested to (i) confirm the results identified in this study with adequate sample size, proper instruments that cover multiple aspects of fatigue, and appropriate follow-up duration, and (ii) improve the internal validity by taking strategies to ensure allocation concealment, blinding of outcome assessment, and nonselective reporting of results.
Supplemental Material
Supplemental material, sj-docx-1-ict-10.1177_15347354211038008 for Pharmacological Interventions for the Management of Cancer-Related Fatigue Among Cancer Survivors: Systematic Review and Meta-Analysis by Xuemei Sun, Yancong Chen, William KW Cheung, Irene XY Wu, Fang Xiao and Vincent CH Chung in Integrative Cancer Therapies
Acknowledgments
The authors acknowledge Ms. Lang Qin from Xiangya School of Public Health, Central South University for editing the language of the manuscript.
Footnotes
Author Contributions: FX and IW developed the research question. WC and IW did the literature search. XS, YC, and WC selected the literature, extracted the data, and assessed the risk of bias. VC and WC analyzed the data. VC, FX, and IW interpreted the results, and XS, YC, IW, FX, and VC wrote the manuscript. All the authors contributed to the interpretation and revision of the manuscript. All the authors have approved the version to be published and have participated sufficiently in the work to take public responsibility for appropriate portions of the content.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by Direct Grant (no. 4054330) from the Chinese University of Hong Kong; the High-level Talents Introduction Plan (no. 202045003) of Central South University, Changsha, China; the Special Funding for the Construction of Innovative Provinces in Hunan (no. 2019SK2141); and the China Oceanwide Holding Group Project Fund (no. 143010100).
ORCID iDs: Xuemei Sun  https://orcid.org/0000-0002-4501-7098
https://orcid.org/0000-0002-4501-7098
William KW Cheung  https://orcid.org/0000-0001-5011-8080
https://orcid.org/0000-0001-5011-8080
Supplemental Material: Supplemental material for this article is available online.
References
- 1.Bower JE, Bak K, Berger A, et al. Screening, assessment, and management of fatigue in adult survivors of cancer: an American Society of Clinical Oncology clinical practice guideline adaptation. J Clin Oncol. 2014;32:1840-1850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fong DY, Ho JW, Hui BP, et al. Physical activity for cancer survivors: meta-analysis of randomised controlled trials. BMJ. 2012;344:e70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pearson EJM, Morris ME, di Stefano M, McKinstry CE. Interventions for cancer-related fatigue: a scoping review. Eur J Cancer Care. 2018;27(1). doi:10.1111/ecc.12516. [DOI] [PubMed] [Google Scholar]
- 4.NCCN Guidelines Version 2. Cancer-related fatigue. www.nccn.org. Accessed May 4, 2020.
- 5.Thong MSY, van Noorden CJF, Steindorf K, Arndt V. Cancer-related fatigue: causes and current treatment options. Curr Treat Options Oncol. 2020;21:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jones JM, Olson K, Catton P, et al. Cancer-related fatigue and associated disability in post-treatment cancer survivors. J Cancer Surviv. 2016;10:51-61. [DOI] [PubMed] [Google Scholar]
- 7.Howell D, Keller-Olaman S, Oliver TK, et al. A pan-Canadian practice guideline and algorithm: screening, assessment, and supportive care of adults with cancer-related fatigue. Curr Oncol. 2013;20:e233-e246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Minton O, Richardson A, Sharpe M, Hotopf M, Stone P. Drug therapy for the management of cancer-related fatigue. Cochrane Database Syst Rev. 2010;7:CD006704. [DOI] [PubMed] [Google Scholar]
- 9.Qu D, Zhang Z, Yu X, Zhao J, Qiu F, Huang J. Psychotropic drugs for the management of cancer-related fatigue: a systematic review and meta-analysis. Eur J Cancer Care. 2016;25:970-979. [DOI] [PubMed] [Google Scholar]
- 10.Mustian KM, Alfano CM, Heckler C, et al. Comparison of pharmaceutical, psychological, and exercise treatments for cancer-related fatigue: a meta-analysis. JAMA Oncol. 2017;3:961-968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tomlinson D, Robinson PD, Oberoi S, et al. Pharmacologic interventions for fatigue in cancer and transplantation: a meta-analysis. Curr Oncol. 2018;25:e152-e167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bohlius J, Tonia T, Nüesch E, et al. Effects of erythropoiesis-stimulating agents on fatigue- and anaemia-related symptoms in cancer patients: systematic review and meta-analyses of published and unpublished data. Br J Cancer. 2014;111:33-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.To J, Goldberg AS, Jones J, et al. A systematic review of randomized controlled trials for management of persistent post-treatment fatigue in thyroid cancer survivors. Thyroid. 2015;25:198-210. [DOI] [PubMed] [Google Scholar]
- 14.Page BR, Shaw EG, Lu L, et al. Phase II double-blind placebo-controlled randomized study of armodafinil for brain radiation-induced fatigue. Neuro Oncol. 2015;17:1393-1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mitchell SA, Hoffman AJ, Clark JC, et al. Putting evidence into practice: an update of evidence-based interventions for cancer-related fatigue during and following treatment. Clin J Oncol Nurs. 2014;18(Suppl):38-58. [DOI] [PubMed] [Google Scholar]
- 16.Ebede CC, Jang Y, Escalante CP. Cancer-related fatigue in cancer survivorship. Med Clin North Am. 2017;101:1085-1097. [DOI] [PubMed] [Google Scholar]
- 17.Minton O, Stone P. A systematic review of the scales used for the measurement of cancer-related fatigue (CRF). Ann Oncol. 2009;20:17-25. [DOI] [PubMed] [Google Scholar]
- 18.Wong SS, Wilczynski NL, Haynes RB. Developing optimal search strategies for detecting clinically sound treatment studies in EMBASE. J Med Libr Assoc. 2006;94:41-47. [PMC free article] [PubMed] [Google Scholar]
- 19.Haynes RB, McKibbon KA, Wilczynski NL, Walter SD, Werre SR. Optimal search strategies for retrieving scientifically strong studies of treatment from Medline: analytical survey. BMJ. 2005;330:1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sterne JAC, Savović J, Page MJ, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. [DOI] [PubMed] [Google Scholar]
- 21.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557-560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nordin Å, Taft C, Lundgren-Nilsson Å, Dencker A. Minimal important differences for fatigue patient reported outcome measures—a systematic review. BMC Med Res Methodol. 2016;16:62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Norman GR, Sloan JA, Wyrwich KW. Interpretation of changes in health-related quality of life: the remarkable universality of half a standard deviation. Med Care. 2003;41:582-592. [DOI] [PubMed] [Google Scholar]
- 24.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629-634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thornton A, Lee P. Publication bias in meta-analysis: its causes and consequences. J Clin Epidemiol. 2000;53:207-216. [DOI] [PubMed] [Google Scholar]
- 26.Laigle-Donadey F, Ducray F, Boone M, et al. A phase III double-blind placebo-controlled randomized study of dexamphetamine sulfate for fatigue in patients suffering from primary brain tumors. Support Care Cancer. 2018;26:S131-S132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jeong JS, Ryu BH, Kim JS, Park JW, Choi WC, Yoon SW. Bojungikki-tang for cancer-related fatigue: a pilot randomized clinical trial. Integr Cancer Ther. 2010;9:331-338. [DOI] [PubMed] [Google Scholar]
- 28.Boele FW, Douw L, de Groot M, et al. The effect of modafinil on fatigue, cognitive functioning, and mood in primary brain tumor patients: a multicenter randomized controlled trial. Neurooncol. 2013;15:1420-1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lower EE, Fleishman S, Cooper A, et al. Efficacy of dexmethylphenidate for the treatment of fatigue after cancer chemotherapy: a randomized clinical trial. J Pain Symptom Manag. 2009;38:650-662. [DOI] [PubMed] [Google Scholar]
- 30.Xu Y, Wang XS, Chen Y, Shi Q, Chen TH, Li P. A phase II randomized controlled trial of Renshen Yangrong Tang herbal extract granules for fatigue reduction in cancer survivors. J Pain Symptom Manag. 2020;59:966-973. [DOI] [PubMed] [Google Scholar]
- 31.Guglielmo M, Di Pede P, Alfieri S, et al. A randomized, double-blind, placebo controlled, phase II study to evaluate the efficacy of ginseng in reducing fatigue in patients treated for head and neck cancer. J Cancer Res Clin Oncol. 2020;146:2479-2487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ramasamy V, Binti Mat Lazim N, Abdullah B, Singh A. Effects of Tualang honey on cancer related fatigue: a multicenter open-label trial of H&N cancer patients. Gulf J Oncol. 2019;1:43-51. [PubMed] [Google Scholar]
- 33.Dong QT, Zhang XD, Yu Z. Integrated Chinese and Western medical treatment on postoperative fatigue syndrome in patients with gastric cancer. Zhongguo Zhong Xi Yi Jie He Za Zhi. 2010;30:1036-1040. [PubMed] [Google Scholar]
- 34.Berenson JR, Yellin O, Shamasunder HK, et al. A phase 3 trial of armodafinil for the treatment of cancer-related fatigue for patients with multiple myeloma. Support Care Cancer. 2015;23:1503-1512. [DOI] [PubMed] [Google Scholar]
- 35.Richard PO, Fleshner NE, Bhatt JR, Hersey KM, Chahin R, Alibhai SM. Phase II, randomised, double-blind, placebo-controlled trial of methylphenidate for reduction of fatigue levels in patients with prostate cancer receiving LHRH-agonist therapy. BJU Int. 2015;116:744-752. [DOI] [PubMed] [Google Scholar]
- 36.Kamath J, Feinn R, Winokur A. Thyrotropin-releasing hormone as a treatment for cancer-related fatigue: a randomized controlled study. Support Care Cancer. 2012;20:1745-1753. [DOI] [PubMed] [Google Scholar]
- 37.Fahlén M, Wallberg B, von Schoultz E, et al. Health-related quality of life during hormone therapy after breast cancer: a randomized trial. Climacteric. 2011;14:164-170. [DOI] [PubMed] [Google Scholar]
- 38.Salehifar E, Azimi S, Janbabai G, et al. Efficacy and safety of bupropion in cancer-related fatigue, a randomized double blind placebo controlled clinical trial. BMC Cancer. 2020;20:158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lawrence JA, Griffin L, Balcueva EP, et al. A study of donepezil in female breast cancer survivors with self-reported cognitive dysfunction 1 to 5 years following adjuvant chemotherapy. J Cancer Surviv. 2016;10:176-184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Eaton AA, Gonen M, Karanicolas P, et al. Health-related quality of life after pancreatectomy: results from a randomized controlled trial. Ann Surg Oncol. 2016;23:2137-2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Eyl RE, Xie K, Koch-Gallenkamp L, Brenner H, Arndt V. Quality of life and physical activity in long-term (≥5 years post-diagnosis) colorectal cancer survivors – systematic review. Health Qual Life Outcomes. 2018;16:112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Capuron L, Gumnick JF, Musselman DL, et al. Neurobehavioral effects of interferon-alpha in cancer patients: phenomenology and paroxetine responsiveness of symptom dimensions. Neuropsychopharmacology. 2002;26:643-652. [DOI] [PubMed] [Google Scholar]
- 43.Semiglazov VF, Stepula VV, Dudov A, Schnitker J, Mengs U. Quality of life is improved in breast cancer patients by Standardised Mistletoe Extract PS76A2 during chemotherapy and follow-up: a randomised, placebo-controlled, double-blind, multicentre clinical trial. Anticancer Res. 2006;26:1519-1529. [PubMed] [Google Scholar]
- 44.Deshmukh V, Kulkarni A, Bhargava S, et al. Effectiveness of combinations of ayurvedic drugs in alleviating drug toxicity and improving quality of life of cancer patients treated with chemotherapy. Support Care Cancer. 2014;22:3007-3015. [DOI] [PubMed] [Google Scholar]
- 45.Schulz KF, Altman DG, Moher D. CONSORT 2010 statement: updated guidelines for reporting parallel group randomized trials. Ann Intern Med. 2010;152:726-732. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-ict-10.1177_15347354211038008 for Pharmacological Interventions for the Management of Cancer-Related Fatigue Among Cancer Survivors: Systematic Review and Meta-Analysis by Xuemei Sun, Yancong Chen, William KW Cheung, Irene XY Wu, Fang Xiao and Vincent CH Chung in Integrative Cancer Therapies