Abstract
Background:
Successful treatment of major depressive disorder (MDD) can be challenging, and failures ("treatment-resistant depression" [TRD]) are frequent. Steps to address TRD include increasing antidepressant dose, combining antidepressants, adding adjunctive agents, or using nonpharmacological treatments. Their relative efficacy and tolerability remain inadequately tested. In particular, the value and safety of increasingly employed second-generation antipsychotics (SGAs) and new esketamine, compared to lithium as antidepressant adjuncts remain unclear.
Methods:
We reviewed randomized, placebo-controlled trials and used random-effects meta-analysis to compare odds ratio (OR) versus placebo, as well as numbers-needed-to-treat (NNT) and to-harm (NNH), for adding SGAs, esketamine, or lithium to antidepressants for major depressive episodes.
Results:
Analyses involved 49 drug-placebo pairs. By NNT, SGAs were more effective than placebo (NNT = 11 [CI: 9–15]); esketamine (7 [5–10]) and lithium (5 [4–10]) were even more effective. Individually, aripiprazole, olanzapine+fluoxetine, risperidone, and ziprasidone all were more effective (all NNT < 10) than quetiapine (NNT = 13), brexpiprazole (16), or cariprazine (16), with overlapping NNT CIs. Risk of adverse effects, as NNH for most-frequently reported effects, among SGAs versus placebo was 5 [4–6] overall, and highest with quetiapine (NNH = 3), lowest with brexpiprazole (19), 5 (4–6) for esketamine, and 9 (5–106) with lithium. The risk/benefit ratio (NNH/NNT) was 1.80 (1.25–10.60) for lithium and much less favorable for esketamine (0.71 [0.60–0.80]) or SGAs (0.45 [0.17–0.77]).
Conclusions:
Several modern antipsychotics and esketamine appeared to be useful adjuncts to antidepressants for acute major depressive episodes, but lithium was somewhat more effective and better tolerated.
Limitations:
Most trials of adding lithium involved older, mainly tricyclic, antidepressants, and the dosing of adjunctive treatments were not optimized.
Keywords: Antidepressants, antipsychotics, combination, depression, efficacy, esketamine, lithium
Introduction
Major depressive disorder (MDD) is a highly prevalent, episodic, or sometimes chronic illness associated with potentially severe functional impairment, co-occurring psychiatric and general medical morbidity, and excess mortality from suicide as well as from general medical conditions (Baldessarini and Tondo, 2020; Celano et al., 2018; Seligman and Nemeroff, 2015). The lifetime prevalence of MDD is approximately 4%–14% of the general population and mixed features are present in a quarter of patients with MDD (Ferrari et al., 2013; Tondo et al., 2018; Vázquez et al., 2018; Zimmermann et al., 2009). Major mood disorders generally produce high illness burdens, with substantial risks of sustained disability (Ferrari et al., 2013; World Health Organization, 2012).
Modern antidepressants, with or without psychotherapy, are the leading form of treatment provided to MDD patients (Baldessarini, 2013; Bauer et al., 2013; Kennedy et al., 2016). However, response rates with commonly employed antidepressants for acute episodes of major depression are moderate (40%–60%), and remission rates are even lower (30%–45%) (Baldessarini, 2013; Rush et al., 2006; Yuan et al., 2020). Moreover, long-term levels of treatment-unresponsive depression in MDD and bipolar disorder (BD) are surprisingly high and typically involve more than 40% of the time in follow-up, despite treatment by community standards (Forte et al., 2015). The limited efficacy of antidepressant therapy, with correspondingly prevalent "treatment-resistant" depression (TRD), encourages clinical trials of alternatives, including increased doses of antidepressants, changing to different antidepressants, adding other drugs, or use of nonpharmacological (psychological and physical) treatments (Bauer et al., 2013; Davies et al., 2019; MacQueen et al., 2017; Milev et al., 2016; Parikh et al., 2009). Particularly striking is the relatively infrequent use of lithium in acute unipolar depression, despite its prolonged clinical acceptance and extensive support for use in nonbipolar major depression, particularly as an adjunct to antidepressants, in addition to representing a fundamental treatment for BD (Bauer et al., 2013; Kennedy et al., 2016; Undurraga et al., 2019). Also, addition of second-generation antipsychotic drugs (SGAs) to antidepressants has been increasing (Mulder et al., 2018), and esketamine is emerging as a novel, rapidly acting agent that can be added safely to antidepressants (Bahji et al., 2020, 2021).
Despite extensive clinical experience in the use of adjunctive treatments with antidepressants, greater clarity is required regarding the relative efficacy and tolerability of specific drug combinations and their doses for major depression. This need led us to evaluate trials testing short-term efficacy and tolerability of a currently prevalent option: SGAs, and their comparison with adjunctive esketamine as another innovative option, and with lithium as one of the oldest such adjunctive options (Haddad et al., 2015; Undurraga et al., 2019). Our assessments and comparisons are based on meta-analytic estimates of odds ratio (OR) as well as number-needed-to-treat (NNT) to indicate efficacy, and number-needed-to-harm (NNH) arising from commonly clinically encountered adverse effects. NNT and NNH are convenient and clinically readily interpretable measures that also can express relative risk-benefit relationships as the NNH/NNT ratio (Citrome and Ketter, 2013) ].
Methods
Aims and eligibility criteria
We carried out a systematic review and meta-analysis and prepared this report adhering the preferred reporting items for systematic reviews and meta-analyses (PRISMA) (Liberati et al., 2009). We limited inclusion to peer-reviewed reports of randomized, nominally double-blinded, short-term (⩽12 weeks), placebo-controlled trials of selected agents of interest, including SGAs (those encountered were: aripiprazole, brexpiprazole, cariprazine, olanzapine+fluoxetine, risperidone, quetiapine, or ziprasidone; all inhibitors of serotonin 5-HT2 and dopamine D2 receptors), or intranasal esketamine, for comparison with lithium (usually as the carbonate), all combined with standard antidepressants to treat mainly unipolar major depressive episodes in adults diagnosed by modern criteria. We excluded reports involving special populations, such as juveniles, the elderly, or persons with major general medical or neurological illnesses.
Information sources and search
We systematically searched research literature in three electronic databases (PubMed, Google Scholar, and Medline) through October 2020 with combinations of the search terms: “major depression,” “controlled,” “randomized,” “clinical trial,” and “efficacy” (Appendix 1). We also examined previously published, partially relevant, systematic reviews (Bahji et al., 2020, 2021; Nelson and Papakostas, 2009; Ruberto et al., 2020; Spielmans et al., 2013; Undurraga et al., 2019) and references identified in them.
Of 4631 initially identified potential studies based on review of titles and abstracts, 124 required more detailed examination by two coauthors (GHV and RJB), resulting in 43 trials (with 49 drug-placebo pairs) meeting study inclusion criteria (Appendix Figure A1).
Summary measures
To combine the results of studies, we used a random-effects meta-analysis to pool effect sizes to obtain OR with 95% confidence intervals (CI), based on previously described methods (Bahji et al., 2020). We measured heterogeneity using the I2 statistic (Higgins and Thompson, 2002). Most identified studies defined “response” as at least a 50% reduction in scores with standardized depressive symptom rating-scales, commonly the Hamilton depression rating scale [HDRS17] or Montgomery-Åsberg Depression Rating Scale [MADRS]) (Hamilton, 1960; Montgomery and Åsberg, 1979). We summarized response rates using pooled ORs and their CIs.
We also computed initial depression severity ratings as the percentage of maximum possible scale scores (52 with HDRS17, 60 with MADRS), and tested for their similarity between subjects randomized to active treatments versus to placebo, using paired-t tests.
In addition to response rates, clinical efficacy of individual agents and drug types was expressed semi-quantitatively as the estimated “NNT” (with CI), computed as the reciprocal of meta-analytically pooled differences in proportions of patients responding to an active drug versus placebo. NNT indicates the approximate number of patients treated to encounter a patient with superior benefit with a test treatment over a control condition (smaller NNT demonstrating greater efficacy), typically based on response rates with a drug versus with placebo.
To assess the acceptability and tolerability of treatments, we used NNH, which is the reciprocal of differences in proportions of patients reporting a common adverse effect with drug versus placebo; larger NNH values indicate greater tolerability (Andrade, 2015). The most prevalent adverse effects with antipsychotics were excessive sedation or somnolence, weight gain, extrapyramidal neurological symptoms, and akathisia; with intranasal esketamine the most commonly noted adverse effect was dizziness; and with lithium, tremor. NNT and NNH values for individual drugs and drug types were computed by random-effects meta-analysis and reported with 95% CI. They were compared statistically by contingency tables (χ2) based on pooled responder rates and pooled rates of experiencing specified adverse effects.
Finally, we computed the likelihood to be harmed or helped (LHH) as the ratio of NNH to NNT (Citrome and Ketter, 2013). LHH reflects the balance between harm and benefits (risk/benefit ratio) and is reported for each drug and drug type for which data were available. Other measures are reported as means with 95% CI. Statistical significance required two-tailed p < 0.05. Analyses employed commercial software: Statview.5 (SAS Institute, Cary, NC, USA) for spreadsheets, and R Studio (RStudio PBC, Boston, MA, USA) and Stata.13 (StataCorp, College Station, TX, USA) for analyses.
Results
Overall findings
The PRISMA-guided process of selecting reports for inclusion is summarized in Appendix Figure A1. Of the 49 included trials (from 43 reports), four (with SGAs) involved more than one drug-arm, yielding 28 trials for SGAs, 14 for lithium carbonate, and 7 for intranasal esketamine, for a total of 49 drug-placebo pairs.
A total of 8104 subjects were included in the 28 add-on SGA trials: 4030 randomized to combination with an SGA, and 4074 (3008 unique participants owing to repeated use of some controls) with added placebo. Trial-duration averaged 7.07 (6.49–7.65) weeks, subject-age averaged 44.7 (44.3–45.1) years, and 67.2% (67.1–67.3) of participants were women (Appendix Table A1). Mean baseline depression severity ratings, expressed as percentage of maximum attainable score, ranked: 51.5 (44.7–47.4) with lithium, 46.0 (44.7–47.4) with SGAs, and 37.6 (36.3–47.4) with esketamine. These initial scores differ highly significantly (overall t = 3.68, p < 0.0001), and each Scheffé post-hoc pairwise comparison also differs significantly (lithium vs. esketamine, p < 0.0001; esketamine vs. SGA, p = 0.001; lithium vs. SGA, p = 0.04).
In the seven trials for intranasal esketamine as an add-on to antidepressant treatment, there were 1287 subjects: 711 randomized to added esketamine and 576 to added placebo. Trial duration was 4 weeks for all trials of esketamine, subject-age averaged 46.0 (40.1–46.0) years, and 63.0% (55.5–76.3) of participants were women (Appendix Table A2).
Of the 14 trials for lithium carbonate as an add-on to antidepressant treatment, there were 640 subjects: 292 randomized to added lithium and 348 to added placebo. Trial duration averaged 3.4 (2.0–4.8) weeks, subject-age averaged 43.7 (40.0–47.0) years, and 63.0% (55.5–76.3) of participants were women (Appendix Table A3).
Meta-analyses
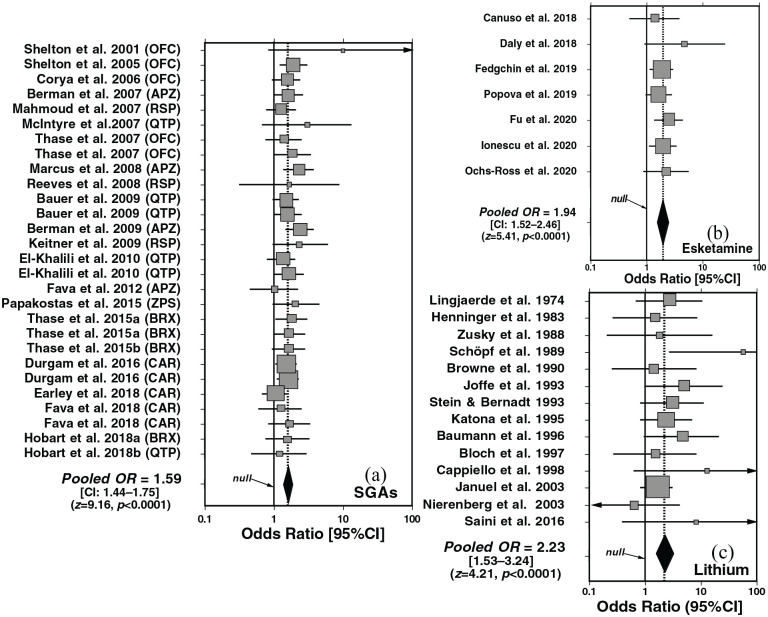
Random-effects meta-analysis of trials of adding SGAs versus placebo to antidepressants yielded highly significant superiority of SGAs overall (OR = 1.59 [CI: 1.44–1.75]; z = 9.16, p < 0.0001; Figure 1(a)). The efficacy of intranasal esketamine was intermediate between SGAs and lithium (OR = 1.94 [1.52–2.46]; z = 4.98, p < 0.0001; Figure 1(b)), and the efficacy of lithium was highest (OR = 2.22 [1.44–3.43]; z = 3.59, p=0.0003; Figure 1(c)).
Figure 1.
Forest plots of random-effects meta-analyses for clinical trials testing the efficacy of supplementing antidepressants with active agents or placebo for major depression: (a) second-generation antipsychotics (SGAs, 28 trials), (b) intranasal esketamine (7 trials), or (c) lithium carbonate (13 trials). SGAs tested were: APZ, aripiprazole; BRX, brexpiprazole; CAR, cariprazine; OFC, olanzapine+fluoxetine combination; QTP, quetiapine; RSP, risperidone; ZPS, ziprasidone. Adding all three types of active treatments were much more effective than adding placebo: (a) SGAs: pooled OR = 1.59 [CI: 1.44–1.75]; z-score = 9.16, p < 0.0001; (b) esketamine: pooled OR = 1.85 [1.45–2.35]; z-score = 4.98, p < 0.0001; Lithium: pooled OR = 2.12 [1.46–3.09]; z = 3.92, p < 0.0001. Heterogeneity ratings (I2) all were <1.0%.
NNT
NNT values for response among individual drugs or types (Table 1) did not differ significantly (overlapping CIs), but tended to be lower (more favorable) with lithium (NNT = 5 [4–10]) than with esketamine (NNT = 7 [5–10]) or SGAs overall (11 [9–15]). NNT among particular SGAs ranked: risperidone (6 [3–13]) = olanzapine/fluoxetine (which includes an antidepressant; 6 [4–19]) ⩽ ziprasidone (7 [3–∞]) ⩽ aripiprazole (9 [5–24]) ⩽ cariprazine (16 [8–52]) = brexpiprazole (16 [10–34]; Table 1). Based on responder rates, lithium was significantly superior to SGAs (χ2 = 19.6, p < 0.0001), as was esketamine (χ2 = 30.9, p < 0.0001), whereas lithium and esketamine did not differ significantly (χ2 = 0.340, p = 0.561).
Table 1.
Efficacy of lithium or second-generation antipsychotics (SGAs) versus placebo (PBO) added to antidepressants for major depression.
| Treatment | Studies (n) | Responders (%) |
Response measures [95%CI] |
||
|---|---|---|---|---|---|
| Drug | Placebo | OR | NNT | ||
| Lithium | 14 | 148/292 (50.7) | 111/348 (32.0) | 2.04 [1.42–2.93] | 5 [4–10] |
| Esketamine | 7 | 346/711 (48.7) | 188/576 (32.6) | 1.96 [1.55–2.50] | 7 [5–10] |
| All SGAs | 28 | 1516/4030 (37.6) | 1100/4074 (27.0) | 1.59 [1.44–1.75] | 11 [9–15] |
| Risperidone | 3 | 104/211 (49.3) | 53/172 (30.8) | 2.12 [1.39–3.25] | 6 [4–13] |
| Olanzapine/fluoxetine | 5 | 255/593 (43.0) | 163/541 (30.1) | 1.72 [1.27–2.34] | 6 [4–19] |
| Ziprasidone | 1 | 25/71 (35.2) | 14/68 (20.6) | 2.10 [0.98–4.50] | 7 [3–∞] |
| Aripiprazole | 4 | 216/608 (35.5) | 151/709 (21.3) | 1.89 [1.38–2.57] | 9 [5–24] |
| Quetiapine | 6 | 369/745 (49.5) | 304/843 (36.1) | 1.50 [1.21–1.86] | 13 [8–42] |
| Cariprazine | 5 | 390/967 (40.3) | 315/952 (33.1) | 1.38 [1.14–1.66] | 16 [8–52] |
| Brexpiprazole | 4 | 157/790 (19.9) | 100/789 (12.7) | 1.70 [1.29–2.24] | 16 [10–34] |
Data are ranked in ascending order of number-needed-to-treat (NNT).
NNH
NNH for lithium was highest (lowest risk) at 9 [5–106], and greater than with intranasal esketamine (5 [4–6]) or all SGAs pooled (5 [4–6]). For individual SGAs, NNH ranged from 19 with brexpiprazole to 3 with quetiapine (Table 2). Based on adverse event rates, lithium was safer than either SGAs or esketamine (χ2 = 1567 and 158, respectively; both p ⩽ 0.0001), and risk was lower with esketamine than with SGAs (χ2 = 13.0, p = 0.0003).
Table 2.
Relative risk of adverse events associated with second-generation antipsychotics (SGAs, esketamine or lithium versus placebo (PBO) added to antidepressants for major depression.
| Treatment | Trials (n) | Adverse event | Adverse events/person (%) |
NNH [95%CI] | LHH (NNH/NNT) [95%CI] | |
|---|---|---|---|---|---|---|
| Drug | PBO | |||||
| Lithium | 14 | Tremor | 120/140 (80.5) | 99/142 (69.7) | 9 [5–106] | 1.80 [1.25–10.60] |
| Esketamine | 7 | Dizziness | 216/736 (22.4) | 56/576 (7.6) | 5 [4-6] | 0.71 [0.60–0.80] |
| All SGAs | 25 | Various | 969/4178 (23.2) | 202/3311 (6.10) | 5 [4–6] | 0.45 [0.17–0.77] |
| Brexpiprazole | 4 | Akathisia | 83/1032 (8.04) | 21/819 (2.56) | 19 [14–29] | 1.19 [1.04–1.66] |
| Olanzapine/fluoxetine | 5 | Weight-gain >10% | 109/584 (18.7) | 4/537 (0.74) | 9 [5–20] | 1.50 [1.08–3.34] |
| Cariprazine | 3 | Akathisia | 131/962 (13.6) | 17/605 (2.8) | 9 [7–12] | 0.56 [0.30–0.80] |
| Risperidone | 2 | Sedation/somnolence | 15/211 (7.11) | 10/175 (5.71) | 5 [4–6] | 0.83 [0.36–1.00] |
| Ziprasidone | 1 | Sedation/somnolence | 24/71 (33.8) | 8/68 (11.7) | 5 [3–11] | 0.71 [0.29–0.96] |
| Aripiprazole | 4 | EPS/akathisia | 243/662 (36.7) | 90/769 (11.7) | 4 [3–5] | 0.44 [0.14–0.79] |
| Quetiapine | 6 | Sedation/somnolence | 364/656 (55.5) | 52/338 (15.4) | 3 [2–3] | 0.23 [0.05–0.54] |
Data are ranked by descending NNH.
CI: confidence interval; EPS: extrapyramidal signs or symptoms; LLH: likelihood of help or harm or risk/benefit ratio (NNH/NNT); NNH: number-needed-to-harm; NNT: number needed to treat; PBO: placebo; SGA: second-generation antipsychotic.
In addition, the LHH or risk/benefit ratio (NNH/NNT) was more favorable (larger) with lithium (LHH = 1.50 [1.08–3.34] than with intranasal esketamine (LHH = 0.71 [0.60–0.80]) or SGAs-combined (LLH = 0.45 [0.17–0.77]; Table 2), with nonoverlapping CIs.
Discussion
This systematic review compared efficacy (as OR vs. placebo in random-effects meta-analyses and as NNT) and tolerability (as NNH) and their risk/benefit ratio (NNH/NNT, or LHH) in placebo-controlled, randomized, add-on trials of SGAs, intranasal esketamine, or lithium to supplement standard antidepressants. Literature searching yielded 43 peer-reviewed reports meeting study criteria, with 49 drug-placebo pairs (Figure 1).
SGAs overall were more effective than placebo (OR = 1.59 [1.44–1.75]; NNT = 11 [9–15]), but esketamine (OR = 1.96 [1.55-2.50]; NNT = 7 [5–10]) and lithium (OR = 2.04 [1.42–2.93]; NNT = 5 [4–10]) were even more effective. Individually, compared to placebo, aripiprazole, olanzapine+fluoxetine, risperidone, and ziprasidone were more effective than placebo in attaining an antidepressant response (all NNT < 10), and more so than quetiapine (NNT = 13), brexpiprazole (NNT = 16), or cariprazine (NNT = 16). However, the CIs of NNTs for individual added SGAs treatments overlapped.
Apparent risk of adverse effects, as NNH (higher value with lower risk) for most frequently reported effects among SGAs versus placebo, was highest with quetiapine (NNH = 3) and lowest with brexpiprazole (NNH = 19). In addition, the NNH was lower (higher risk) with intranasal esketamine (NNH = 5 [4–6] and all SGAs-pooled (5 [4–6]) than with lithium (9 [5–106]). The benefit/risk ratio (NNH/NNT, or LHH; Table 2) was 1.50 [1.08–3.34] for lithium and much lower, or less favorable, with intranasal esketamine (0.71 [0.60–0.80]) and all SGAs (0.45 [0.17–0.77]).
These findings support the efficacy of SGAs, intranasal esketamine, and lithium over placebo in supplementing antidepressant treatment of acute major depression in adults. However, the trials included are heterogeneous, and computed values of NNT for individual SGAs had overlapping CIs, limiting their potential value in guiding recommendations regarding which drug should be used as a first choice. Moreover, initial depression severity ratings normalized as the percentage of maximum scale scores differed significantly and ranked: lithium (51.1%) > SGAs (46.0%) > esketamine (37.6%). The same order was found regarding efficacy as OR in meta-analyses (Figure 1) and as NNT (Table 1). This ranking may suggest preferential efficacy with higher initial depression severity, favoring lithium, or possibly an artifact of contrasts between higher initial to end-point depression ratings.
We also found similar results regarding tolerability as NNH, ranking: lithium ⩾ intranasal esketamine ⩾ SGAs (Table 2). Tolerability and efficacy is essential in deciding which treatment should be used, as adverse effects can reduce subjective well-being and treatment-adherence and adversely affect treatment outcomes (Solmi et al., 2017). Use of SGAs can lead to a range of adverse effects, including excessive sedation, akathisia, and risks of weight gain and adverse cardiometabolic effects with some SGAs (Baldessarini, 2013; Centorrino et al., 2012; Gierisch et al., 2014; Solmi et al., 2017). Use of intranasal esketamine at approved doses can lead to other adverse events, including dizziness, dissociation, headaches, paraesthesia, nausea, vomiting, and somnolence, with even potential risks of psychosis at higher doses (Bahji et al., 2021). Of note, mean duration of lithium trials was shorter than for esketamine and SGAs; this difference may imply a faster antidepressant action with lithium, and might also limit appearance of side effects.
Lithium is effective for treating affective disorders with evidence of reduction of suicidal risk and mortality, but is underutilized (Baldessarini, 2013; Undurraga et al., 2019) especially in MDD. Concerns that limit the use of lithium include a narrow therapeutic index, with risks of intoxication at circulating concentrations only 2–3 times above therapeutic levels, as well as of adverse long-term effects on thyroid and renal function (Baldessarini, 2013). In addition, lithium salts have lacked commercial promotion as unpatentable minerals in competition with other treatments.
NNT is a convenient and clinically readily interpretable measure of therapeutic effect-size and may support comparisons of different treatments given under comparable conditions, but it has important limitations (Andrade, 2015; Mendes et al., 2017). In the present analyses, lithium yielded an NNT of 5 (4–10), indicating that approximately one out of five patients treated would respond to adding lithium to an antidepressant in comparison with adding placebo. Generally, small NNTs are preferable, although larger values (>10) may be acceptable if the outcome is the prevention of mortality or severe morbidity (Katsanos et al., 2015). Also, meaningful interpretation of NNT requires consideration of response rate: a relatively low response rate with an active treatment may be significantly superior to that with placebo, but not be clinically valuable. NNH and the risk/benefit ratio (NNH/NNT) are also subject to limitations, notably including the clinical significance of the adverse effect being considered.
Limitations
Most trials of adding lithium to antidepressants for major depressive episodes involved older, mainly tricyclic, antidepressants, and dosing of all reported adjunctive treatments were not optimized. We also found poor systematization of the adverse effect profile in some trials, especially involving lithium. Use of NNT and NNH to compare treatments is limited by the comparability of different trials and further limited by the rarity of desirable, head-to-head comparisons of different active treatments under identical conditions.
Conclusions
Based on meta-analyses to determine the OR and NNT, several modern drugs developed as antipsychotics as well as intranasal esketamine were effective as adjuncts to antidepressants for acute major depressive episodes, but lithium was somewhat more effective and better tolerated. The findings encourage clinical consideration of lithium as a particularly attractive adjunct in the treatment of major depression.
Appendix
Figure A1.
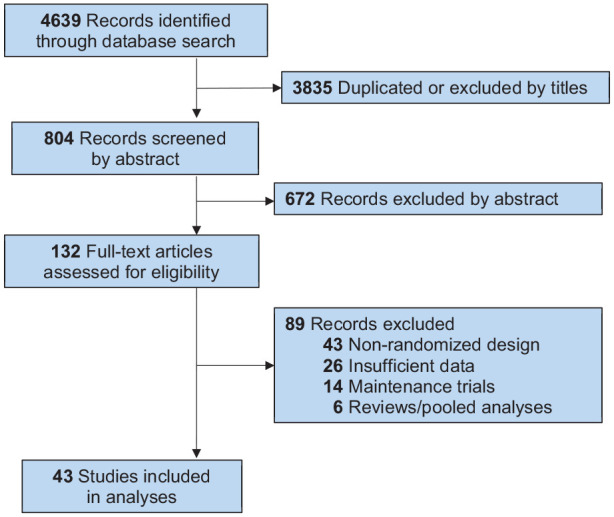
Flow-chart of selection of reports for inclusion in study, based on PRISMA recommendations (http://www.prisma-statement.org/PRISMAStatement/PRISMAStatement) to yield 39 reports (with 43 trials) included for analysis.
Table A1.
Characteristics of second-generation antipsychotics (SGAs) vs. placebo (PBO) add-on trials for major depressive episodes.
| Report | SGAs | ADs | Mean SGA dose (mg/day) | Subjects (N) |
Mean age (years) | Female (%) | Time (weeks) | Failed AD trials | Ratings | Responders [n (%)] |
Response OR | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SGA | PBO | SGA | PBO | ||||||||||
| Shelton et al. (2001) | OFC | FLX | Flexible (13.5/52) | 9 | 9 | 42 | 75 | 8 | 2 prior, 1 pros | MADRS | 5 (55.6) | 1 (11.1) | 10.0 |
| Shelton et al. (2005) | OFC | FLX/NRT | Flexible (8.5/35.6) | 146 | 210 | 44 | 64 | 8 | 1 prior, 1 pros | MADRS | 65 (44.5) | 62 (29.5) | 1.92 |
| Corya et al. (2006) | OFC | FLX/VNX | Fixed (6, 12/25, 50) | 243 | 119 | 46 | 72 | 12 | 11 prior, 1 pros | MADRS | 105 (43.2) | 40 (33.6) | 1.50 |
| Berman et al. (2007) | APZ | Various | Flexible (11.8) | 184 | 178 | 45 | 63 | 6 | 1–3 prior, 1 pros | MADRS | 62 (33.7) | 42 (23.6) | 1.65 |
| Mahmoud et al. (2007) | RSP | Various | Flexible (1 or 2) | 137 | 131 | 46 | 74 | 6 | 1 pros | HDRS | 63 (46.0) | 38 (29.0) | 1.27 |
| McIntyre et al. (2007) | QTP | Various | Flexible (182) | 18 | 16 | 44 | 62 | 8 | 1 prior | HDRS | 9 (50.0) | 4 (25.0) | 3.00 |
| Thase et al. (2007) | OFC | FLX | Fixed (6, 12, 18/50) | 101 | 102 | 44 | 60 | 8 | 1 prior, 1 pros | MADRS | 37 (36.6) | 30 (29.4) | 1.39 |
| Thase et al. (2007) | OFC | FLX | Fixed (6, 12, 18/50) | 97 | 101 | 45 | 68 | 8 | 1 prior, 1 pros | MADRS | 43 (44.3) | 30 (29.7) | 1.89 |
| Marcus et al. (2008) | APZ | Various | Flexible (11.0) | 191 | 190 | 44 | 70 | 6 | 1–3 prior, 1 pros | MADRS | 62 (32.5) | 33 (17.4) | 2.29 |
| Reeves et al. (2008) | RSP | Various | Flexible (1.2) | 12 | 11 | 44 | 70 | 8 | 1 pros | MADRS | 7 (58.3) | 5 (45.4) | 1.68 |
| Bauer et al. (2009) | QTP | Various | Fixed (150 or 300) | 167 | 163 | 45 | 68 | 6 | 1 prior | MADRS | 93 (55.7) | 75 (46.0) | 1.47 |
| Bauer et al. (2009) | QTP | Various | Fixed (150 or 300) | 163 | 163 | 45 | 68 | 6 | 1 prior | MADRS | 94 (57.7) | 75 (46.0) | 1.60 |
| Berman et al. (2009) | APZ | Various | Flexible (13.9) | 177 | 172 | 45 | 73 | 6 | 1–3 prior, 1 pros | MADRS | 82 (46.3) | 46 (26.7) | 2.36 |
| Keitner et al. (2009) | RSP | Various | Flexible (1.6) | 62 | 30 | 45 | 57 | 4 | 1 pros | MADRS | 34 (54.8) | 10 (33.3) | 2.43 |
| El-Khalili et al. (2010) | QTP | Various | Fixed (150 or 300) | 148 | 148 | 46 | 72 | 6 | 1 prior | MADRS | 77 (52.0) | 68 (46.0) | 1.28 |
| El-Khalili et al. (2010) | QTP | Various | Fixed (150 or 300) | 150 | 148 | 46 | 72 | 6 | 1 prior | MADRS | 88 (58.7) | 68 (46.0) | 1.67 |
| Fava et al. (2012) | APZ | Various | Fixed (2–5) | 56 | 169 | 45 | 65 | 8 | 1–3 prior, 1 pros | MADRS | 10 (17.9) | 30 (17.8) | 1.01 |
| Papakostas et al. (2015) | ZPS | sCTP | Flexible (98) | 71 | 68 | 44 | 70 | 8 | 1 pros | HDRS | 25 (35.2) | 14 (20.6) | 2.09 |
| Thase et al. (2015a) | BRX | Various | Fixed (1 or 3) | 211 | 203 | 46 | 68 | 6 | 1–3 prior, 1 pros | MADRS | 49 (23.2) | 29 (14.3) | 1.81 |
| Thase et al. (2015a) | BRX | Various | Fixed (1 or 3) | 213 | 203 | 46 | 68 | 6 | 1–3 prior, 1 pros | MADRS | 47 (22.1) | 29 (14.3) | 1.70 |
| Thase et al. (2015b) | BRX | Various | Fixed (2) | 175 | 178 | 45 | 70 | 6 | 1–3 prior, 1 pros | MADRS | 41 (23.4) | 28 (15.7) | 1.64 |
| Durgam et al. (2016) | CAR | Various | Fixed (1–2) | 273 | 266 | 45 | 68 | 8 | 1 pros | MADRS | 131 (48.0) | 101 (38.0) | 1.51 |
| Durgam et al. (2016) | CAR | Various | Fixed (2–4.5) | 271 | 266 | 45 | 73 | 8 | 1 pros | MADRS | 134 (49.4) | 101 (38.0) | 1.60 |
| Earley et al. (2018) | CAR | Various | Fixed (1.5–4.5) | 269 | 258 | 44 | 65 | 8 | 1–2 prior, 1 pros | MADRS | 75 (27.9) | 71 (27.5) | 1.02 |
| Fava et al. (2018) | CAR | Various | Fixed (0.1–0.3) | 76 | 81 | 46 | 52 | 8 | 1–2 prior, 1 pros | MADRS | 23 (30.3) | 21 (25.9) | 1.24 |
| Fava et al. (2018) | CAR | Various | Fixed (1–2) | 73 | 81 | 44 | 51 | 8 | 1–2 prior, 1 pros | MADRS | 27 (37.0) | 21 (25.9) | 1.68 |
| Hobart et al. (2018a) | BRX | Various | Flexible (2.2) | 191 | 205 | 43 | 74 | 6 | 1–3 prior, 1 pros | MADRS | 20 (10.5) | 14 (6.83) | 1.60 |
| Hobart et al. (2018b) | QTP-xr | Various | Fixed (150 or 300) | 99 | 205 | 43 | 69 | 6 | 1–3 prior, 1 pros | MADRS | 8 (8.08) | 14 (6.83) | 1.20 |
| Totals/averages [95%CI] | – | – | – | 3983 | 4074* | 44.7 [44.3–45.1] | 67.2 [67.1–67.3] | 7.07 [6.49–7.65] | – | – | 1516/3983 (38.1%) [36.6–39.6] | 1100/4074 (27.0%) [25.6–28.4] | 1.59 [1.44–1.75] |
Data are derived from 28 trials in 22 reports. The pooled Response Ratio is based on pooled rates for SGAs/PBO (38.1%/28.9%). The mean response ratio = 1.57 [1.22–1.92], based on ratios in individual trials, but based on pooled counts of responders/subjects, the ratio is 1.41 (38.1%/27.0%), based on 1516/3983 = 38.1% with SGAs versus 1100/4074 = 27.0% with PBO. There are 28 SGA trials. OR was determined from random-effects meta-analysis. Of the 28 trials, 11 (39.3%) independently favored SGA over PBO significantly.
ADs: antidepressants; APZ: aripiprazole; BRX: brexpiprazole; CAR: cariprazine; FLX: fluoxetine; NRT: nortriptyline; OFC: olanzapine+fluoxetine combination; PBO: placebo; pros: prospective; QTP: quetiapine; Rem: remission; xr: extended release.
Corrected for repeated use, the placebo total N = 3008.
Table A2.
Characteristics of esketamine (sKet) versus placebo (PBO) add-on trials for major depressive episodes.
| Study | sKet (nasal dose) | Age (years) | Females (%) | Subjects (N) |
Responders (%) |
Response OR | ||
|---|---|---|---|---|---|---|---|---|
| sKet | PBO | sKet | PBO | |||||
| Canuso et al. (2018) | 84 mg twice/week | 35.9 | 65.2 | 35 | 31 | 24 (68.6) | 19 (61.3) | 1.38 |
| Daly et al. (2018) | 28–84 mg twice/week | 45.4 | 57.0 | 34 | 33 | 8 (23.5) | 2 (6.06) | 4.77 |
| Fedgchin et al. (2019) | 56–84 mg twice/week | 46.6 | 71.1 | 229 | 113 | 123 (53.7) | 44 (38.9) | 1.82 |
| Popova et al. (2019) | 56–84 mg twice/week | 45.7 | 61.9 | 114 | 109 | 70 (61.4) | 52 (47.7) | 1.74 |
| Fu et al. (2020) | 84 mg twice/week | 39.3 | 61.6 | 113 | 112 | 51 (45.1) | 28 (25.0) | 2.47 |
| Ionescu et al. (2021) | 84 mg twice/week | 40.8 | 59.9 | 114 | 113 | 53 (46.5) | 35 (31.0) | 1.94 |
| Ochs-Ross et al. (2020) | 28–84 mg twice/week | 70.0 | 62.0 | 72 | 65 | 17 (23.6) | 8 (12.3) | 2.20 |
| Totals/averages [95%CI] | 28–84 mg twice weekly | 46.2 [35.9–56.5] | 62.7 [58.6–66.8] | 711 | 576 | 346/711 (48.7%) [44.9–52.4] | 188/576 (32.6%) [28.8–36.6] | 2.23 [1.53–3.24] |
OR was determined from random-effects meta-analysis. Of the seven trials, three (42.9%) independently favored esketamine over placebo significantly.
Table A3.
Characteristics of lithium versus placebo (PBO) add-on trials for major depressive episodes.
| Study | ADs | Age (years) | Females (%) | Lithium dose | Duration (weeks) | Subjects (N) |
Responders (%) |
Response OR | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Lithium | Placebo | Lithium | Placebo | |||||||
| Lingjærde et al. (1974) | TCAs | 49 | 78 | 0.8–1.3 mM | 6 | 20 | 25 | 8 (40.0) | 5 (20.0) | 2.67 |
| Heninger et al. (1983) | TCAs, MIA | 51 | 80 | 900–1200 mg/day (0.5–1.1 mM) | 2 | 8 | 17 | 5 (62.5) | 9 (52.9) | 1.48 |
| Zusky et al. (1988) | TCAs, MAOIs | 45 | 81 | 900 mg/day | 2 | 8 | 8 | 3 (37.5) | 2 (25.0) | 1.80 |
| Schöpf et al. (1989) | TCAs | 54 | 70 | 600–800 mg/day (0.6–0.8 mM) | 1 | 14 | 13 | 9 (64.3) | 0 (0.00) | 57.0 |
| Browne et al. (1990) | TCAs | 42 | 59 | 900 mg/day | 2 | 15 | 15 | 4 (26.7) | 3 (20.0) | 1.45 |
| Joffe et al. (1993) | TCAs | 37 | 55 | 900 mg/day | 2 | 17 | 16 | 9 (52.9) | 3 (18.8) | 4.88 |
| Stein and Bernadt (1993) | TCAs | 47 | 79 | 250 mg/day | 3 | 16 | 34 | 7 (43.8) | 7 (20.6) | 3.00 |
| Katona et al. (1995) | FLX, LFP | 40 | 57 | 800 mg/day (0.6–1 mM) | 6 | 29 | 32 | 15 (51.7) | 10 (31.3) | 2.36 |
| Baumann et al. (1996) | CTP | 41 | 71 | 800 mg/day (0.5–0.8 mM) | 1 | 10 | 32 | 6 (60.0) | 8 (25.0) | 4.50 |
| Bloch et al. (1997) | DMI | 47 | 55 | 0.7–1.0 mM | 5 | 16 | 15 | 9 (56.3) | 10 (66.7) | 1.50 |
| Cappiello et al. (1998) | DMI | 40 | 66 | 900 mg/day | 4 | 14 | 15 | 4 (28.6) | 0 (0.0) | 13.3 |
| Januel et al. (2003) | CMI | 44 | 62 | 750 mg/day | 2 | 74 | 75 | 42 (56.8) | 34 (45.3) | 1.58 |
| Nierenberg et al. (2003) | NRT | 38 | 46 | 900 mg/day | 6 | 18 | 17 | 2 (11.1) | 3 (17.6) | 0.58 |
| Saini et al. (2016) | IMI | 37 | 23 | 0.6–0.8 mM | 4 | 20 | 20 | 20 (100.0) | 17 (85.0) | 8.20 |
| Totals/averages [95%CI] | – | 43.7 [40.6–46.8 | 63.0 [53.8–72.2] | 0.6–1.3 mM | 3.29 [1.57–5.01] | 279 | 364 | 143/279 (51.3%) [45.2–57.3] | 111/364 (30.5%) [25.8–35.5] | 2.23 [1.53–3.24] |
OR was determined from random-effects meta-analysis. Of the 14 trials, three (21.4%) significantly favored Li over placebo independently.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Supported in part by an award from the Aretæus Foundation of Rome and Centro Bini Private Donors Research Fund (to LT), by ANID-PIA-ACT192064, ANID-FONDECYT 1180358, 1200601, Clínica Alemana de Santiago ID 863 (to JU), and by a grant from the Bruce J. Anderson Foundation and by the McLean Private Donors Research Fund (to RJB).
ORCID iD: Gustavo H. Vázquez  https://orcid.org/0000-0002-2918-3336
https://orcid.org/0000-0002-2918-3336
References
- Andrade C. (2015) The numbers needed to treat and harm (NNT, NNH) statistics: What they tell us and what they do not. J Clin Psychiatry 76: e330–e333. DOI: 10.4088/JCP.15f09870. [DOI] [PubMed] [Google Scholar]
- Bahji A, Ermacora D, Stephenson C, et al. (2020) Comparative efficacy and tolerability of pharmacological treatments for the treatment of acute bipolar depression: Systematic review and network meta-analysis. J Affect Disord 269: 154–184. DOI: 10.1016/j.jad.2020.03.030. [DOI] [PubMed] [Google Scholar]
- Bahji A, Vázquez GH, Zarate CA. (2021) Comparative efficacy of racemic ketamine and esketamine for depression: Systematic review and meta-analysis. J Affect Disord 271: 542–555. DOI: 10.1016/j.jad.2020.09.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldessarini RJ. (2013) Chemotherapy in Psychiatry: Pharmacologic Basis of Treatments for Major Mental Illness, 3rd edn. New York, NY: Springer-Verlag. [Google Scholar]
- Baldessarini RJ, Tondo L. (2020) Suicidal risks in 12 DSM-5 psychiatric disorders. J Affect Disord 271: 66–73. DOI: 10.1016/j.jad.2020.03.083. [DOI] [PubMed] [Google Scholar]
- Bauer M, Pretorius HW, Constant EL, et al. (2009) Extended-release quetiapine as adjunct to an antidepressant in patients with major depressive disorder: Results of a randomized, placebo-controlled, double blind study. J Clin Psychiatry 70: 540–549. [DOI] [PubMed] [Google Scholar]
- Bauer M, Pfennig A, Severus E, et al. (2013) World Federation of Societies of Biological Psychiatry (WFSBP) guidelines for biological treatment of unipolar depressive disorders, part 1: Update 2013 on the acute and continuation treatment of unipolar depressive disorders. World J Biol Psychiatry 14: 334–385. DOI: 10.3109/15622975.2013.804195. [DOI] [PubMed] [Google Scholar]
- Baumann P, Nil R, Souche A, et al. (1996) Double-blind, placebo-controlled study of citalopram with and without lithium in the treatment of therapy-resistant depressive patients: Clinical, pharmacokinetic, and pharmacogenetic investigation. J Clin Psychopharmacol 16: 307–314. [DOI] [PubMed] [Google Scholar]
- Berman RM, Marcus RN, Swanink R, et al. (2007) The efficacy and safety of aripiprazole as adjunctive therapy in major depressive disorder: A multicenter, randomized, double-blind, placebo-controlled study. J Clin Psychiatry 68: 843–853. [DOI] [PubMed] [Google Scholar]
- Berman RM, Fava M, Thase ME, et al. (2009) Aripiprazole augmentation in major depressive disorder: A double-blind, placebo-controlled study in patients with inadequate response to antidepressants. CNS Spectr 14: 197–206. [DOI] [PubMed] [Google Scholar]
- Bloch M, Schwartzman Y, Bonne O, et al. (1997) Concurrent treatment of nonresistant major depression with desipramine and lithium: Double-blind, placebo-controlled study. J Clin Psychopharmacol 17: 44–48. DOI: 10.1097/00004714-199702000-00008. [DOI] [PubMed] [Google Scholar]
- Browne M, Lapierre YD, Hrdina PD, et al. (1990) Lithium as an adjunct in the treatment of major depression. Int Clin Psychopharmacol 5: 103–110. DOI: 10.1097/00004850-199004000-00004. [DOI] [PubMed] [Google Scholar]
- Canuso CM, Singh JB, Fedgchin M, et al. (2018) Efficacy and safety of intranasal esketamine for the rapid reduction of symptoms of depression and suicidality in patients at imminent risk for suicide: Results of a double-blind, randomized, placebo-controlled study. Am J Psychiatry 175: 620–630. DOI: 10.1176/appi.ajp.2018.17060720. [DOI] [PubMed] [Google Scholar]
- Cappiello A, McDougle CJ, Delgado PL, et al. (1998) Lithium and desipramine vs. desipramine alone in the treatment of severe major depression: a preliminary study. Int Clin Psychopharmacol 13: 191–198. DOI: 10.1097/00004850-199809000-00001. [DOI] [PubMed] [Google Scholar]
- Celano CM, Villegas AC, Albanese AM, et al. (2018) Depression and anxiety in heart failure: Review. Harv Rev Psychiatry 26: 175–184. DOI: 10.1097/HRP.0000000000000162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centorrino F, Masters GA, Talamo A, et al. (2012) Metabolic syndrome in psychiatrically hospitalized patients treated with antipsychotics and other psychotropics. Hum Psychopharmacol 27: 521–526. DOI: 10.1002/hup.2257. [DOI] [PubMed] [Google Scholar]
- Citrome L, Ketter TA. (2013) When does a difference make a difference? Interpretation of number needed to treat, number needed to harm, and likelihood to be helped or harmed. Int J Clin Pract 67: 407–411. DOI: 10.1111/ijcp.12142. [DOI] [PubMed] [Google Scholar]
- Corya SA, Williamson D, Sanger TM, et al. (2006) A randomized, double-blind comparison of olanzapine/fluoxetine combination, olanzapine, fluoxetine, and venlafaxine in treatment-resistant depression. Depress Anxiety 23: 364–372. [DOI] [PubMed] [Google Scholar]
- Daly EJ, Singh JB, Fedgchin M, et al. (2018) Efficacy and safety of intranasal esketamine adjunctive to oral antidepressant therapy in treatment-resistant depression: Randomized clinical trial. JAMA Psychiatry 75: 139–148. DOI: 10.1001/jamapsychiatry.2017.3739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies P, Ijaz S, Williams CJ, et al. (2019) Pharmacological interventions for treatment-resistant depression in adults. Cochrane Database Syst Rev 12: CD010557. DOI: 10.1002/14651858.CD010557.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durgam S, Earley W, Guo H, et al. (2016) Efficacy and safety of adjunctive cariprazine in inadequate responders to antidepressants: A randomized, double-blind, placebo-controlled study in adult patients with major depressive disorder. J Clin Psychiatry 77: 371–378. [DOI] [PubMed] [Google Scholar]
- Earley WR, Guo H, Nemeth G, et al. (2018) Cariprazine augmentation to antidepressant therapy in major depressive disorder: Results of a randomized, double-blind, placebo-controlled trial. Psychopharmacol Bull 48: 62–80. [PMC free article] [PubMed] [Google Scholar]
- El-Khalili N, Joyce M, Atkinson S, et al. (2010) Extended release quetiapine fumarate (quetiapine XR) as adjunctive therapy in major depressive disorder (MDD) in patients with an inadequate response to ongoing antidepressant treatment: A multicentre, randomized, double-blind, placebo controlled study. Int J Neuropsychopharmacol 13: 917–932. [DOI] [PubMed] [Google Scholar]
- Fava M, Mischoulon D, Iosifescu D, et al. (2012) A double-blind, placebo-controlled study of aripiprazole adjunctive to antidepressant therapy among depressed outpatients with inadequate response to prior antidepressant therapy (ADAPT-A Study). Psychother Psychosom 81(2): 87–97. [DOI] [PubMed] [Google Scholar]
- Fava M, Durgam S, Earley W, et al. (2018) Efficacy of adjunctive low-dose cariprazine in major depressive disorder: A randomized, double-blind, placebo-controlled trial. Int Clin Psychopharmacol 33: 312–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedgchin M, Trivedi M, Daly EJ, et al. (2019) Efficacy and safety of fixed-dose esketamine nasal spray combined with a new oral antidepressant in treatment-resistant depression: results of a randomized, double-blind, active-controlled study (TRANSFORM-1). Int J Neuropsychopharmacol 22: 616–630. DOI: 10.1093/ijnp/pyz039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari AJ, Somerville AJ, Baxter AJ, et al. (2013) Global variation in the prevalence and incidence of major depressive disorder: Systematic review of the epidemiological literature. Psychol Med 43: 471–481. DOI: 10.1017/S0033291712001511. [DOI] [PubMed] [Google Scholar]
- Forte A, Baldessarini RJ, Tondo L, et al. (2015) Long-term morbidity in bipolar-I, bipolar-II, and unipolar major depressive disorders. J Affect Disord 178: 71–78. DOI: 10.1016/j.jad.2015.02.011. [DOI] [PubMed] [Google Scholar]
- Fu D-J, Ionescu DF, Li X, et al. (2020) Esketamine nasal spray for rapid reduction of major depressive disorder symptoms in patients who have active suicidal ideation with intent: Double-blind, randomized study (ASPIRE I). J Clin Psychiatry 81: 19m13191. DOI: 10.4088/JCP.19m13191. [DOI] [PubMed] [Google Scholar]
- Gierisch JM, Nieuwsma JA, Bradford DW, et al. (2014) Pharmacologic and behavioral interventions to improve cardiovascular risk factors in adults with serious mental illness: A systematic review and meta-analysis. J Clin Psychiatry 75: e424–440. DOI: 10.4088/JCP.13r08558. [DOI] [PubMed] [Google Scholar]
- Haddad PM, Talbot PS, Anderson IM, et al. (2015) Managing inadequate antidepressant response in depressive illness. Br Med Bull 115: 183–201. DOI: 10.1093/bmb/ldv034. [DOI] [PubMed] [Google Scholar]
- Hamilton M. (1960) A rating scale for depression. J Neurol Neurosurg Psychiatry 23: 56–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heninger GR, Charney DS, Sternberg DE. (1983) Lithium carbonate augmentation of antidepressant treatment: An effective prescription for treatment-refractory depression. Arch Gen Psychiatry 40: 1335–1342. DOI: 10.1001/archpsyc.1983.01790110077013. [DOI] [PubMed] [Google Scholar]
- Higgins JPT, Thompson SG. (2002) Quantifying heterogeneity in a meta-analysis. Stat Med 21: 1539–1558. DOI: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- Hobart M, Skuban A, Zhang P, et al. (2018. a) A randomized, placebo-controlled study of the efficacy and safety of fixed-dose brexpiprazole 2 mg/d as adjunctive treatment of adults with major depressive disorder. J Clin Psychiatry 79(4): 17m12058. [DOI] [PubMed] [Google Scholar]
- Hobart M, Skuban A, Zhang P, et al. (2018. b) Efficacy and safety of flexibly dosed brexpiprazole for the adjunctive treatment of major depressive disorder: A randomized, active-referenced, placebo-controlled study. Curr Med Res Opin 34(4): 633–642. [DOI] [PubMed] [Google Scholar]
- Ionescu DF, Fu D-J, Qiu X, et al. (2021) Esketamine nasal spray for rapid reduction of depressive symptoms in patients with major depressive disorder who have active suicide ideation with intent: results of a Phase 3, double-blind, randomized study (ASPIRE II). Int J Neuropsychopharmacol 24: 22–31. DOI: 10.1093/ijnp/pyaa068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Januel D, Poirier M-F, D’alche-Biree F, et al. (2003) Multicenter double-blind randomized parallel-group clinical trial of efficacy of the combination clomipramine (150 mg/day) plus lithium carbonate (750 mg/day) versus clomipramine (150 mg/day) plus placebo in the treatment of unipolar major depression. J Affect Disord 76: 191–200. DOI: 10.1016/s0165-0327(02)00086-1. [DOI] [PubMed] [Google Scholar]
- Joffe RT, Levitt AJ, Bagby RM, et al. (1993) Predictors of response to lithium and triiodothyronine augmentation of antidepressants in tricyclic non-responders. Br J Psychiatry 163: 574–578. DOI: 10.1192/bjp.163.5.574. [DOI] [PubMed] [Google Scholar]
- Katona CL, Abou-Saleh MT, Harrison DA, et al. (1995) Placebo-controlled trial of lithium augmentation of fluoxetine and lofepramine. Br J Psychiatry 166: 80–86. DOI: 10.1192/bjp.166.1.80. [DOI] [PubMed] [Google Scholar]
- Katsanos K, Spiliopoulos S, Saha P, et al. (2015) Comparative efficacy and safety of different antiplatelet agents for prevention of major cardiovascular events and leg amputations in patients with peripheral arterial disease: Systematic review and network meta-analysis. PLoS One 10: e0135692. DOI: 10.1371/journal.pone.0135692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keitner GI, Garlow SJ, Ryan CE, et al. (2009) A randomized, placebo-controlled trial of risperidone augmentation for patients with difficult-to-treat unipolar, non-psychotic major depression. J Psychiatr Res 43: 205–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy SH, Lam RW, McIntyre RS, et al. (2016) Canadian network for mood and anxiety treatments (CANMAT) 2016 clinical guidelines for the management of adults with major depressive disorder. Can J Psychiatry 61: 540–560. DOI: 10.1177/0706743716659417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberati A, Altman DG, Tetzlaff J, et al. (2009) The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation andelaboration. PLoS Med 6: e1000100. DOI: 10.1371/journal.pmed.1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lingjærde O, Edlund AH, Gormsen CA, et al. (1974) Effect of lithium carbonate in combination with tricyclic antidepressants in endogenous depression: Double-blind, multicenter trial. Acta Psychiatr Scand 50: 233–242. DOI: 10.1111/j.1600-0447.1974.tb08212.x. [DOI] [PubMed] [Google Scholar]
- MacQueen G, Santaguida P, Keshavarz H, et al. (2017) Systematic review of clinical practice guidelines for failed antidepressant treatment response in major depressive disorder, dysthymia, and subthreshold depression in adults. Can J Psychiatry 62: 11–23. DOI: 10.1177/0706743716664885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahmoud RA, Pandina GJ, Turkoz I, et al. (2007) Risperidone for treatment-refractory major depressive disorder: A randomized trial. Ann Intern Med 147: 593–602. [DOI] [PubMed] [Google Scholar]
- Marcus RN, McQuade RD, Carson WH, et al. (2008) The efficacy and safety of aripiprazole as adjunctive therapy in major depressive disorder: A second multicenter, randomized, double-blind, placebo-controlled study. J Clin Psychopharmacol 28: 156–165. [DOI] [PubMed] [Google Scholar]
- McIntyre A, Gendron A, McIntyre A. (2007) Quetiapine adjunct to selective serotonin reuptake inhibitors or venlafaxine in patients with major depression, comorbid anxiety, and residual depressive symptoms: A randomized, placebo controlled pilot study. Depress Anxiety 24: 487–494. [DOI] [PubMed] [Google Scholar]
- Mendes D, Alves C, Batel-Marques F. (2017) Number needed to treat (NNT) in clinical literature: An appraisal. BMC Med 15: 112. DOI: 10.1186/s12916-017-0875-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milev RV, Giacobbe P, Kennedy SH, et al. (2016) Canadian network for mood and anxiety treatments (CANMAT) clinical guidelines for the management of adults with major depressive disorder: Section 4. Neurostimulation treatments. Can J Psychiatry 61: 561–575. DOI: 10.1177/0706743716660033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montgomery SA, Åsberg M. (1979) A new depression scale designed to be sensitive to change. Br J Psychiatry 134: 382–389. [DOI] [PubMed] [Google Scholar]
- Mulder R, Hamilton A, Irwin L, et al. (2018) Treating depression with adjunctive antipsychotics. Bipolar Disord 20: 17–24. DOI: 10.1111/bdi.12701. [DOI] [PubMed] [Google Scholar]
- Nelson JC, Papakostas GI. (2009) Atypical antipsychotic augmentation in major depressive disorder: Meta-analysis of placebo-controlled randomized trials. Am J Psychiatry 166: 980–991. DOI: 10.1176/appi.ajp.2009.09030312. [DOI] [PubMed] [Google Scholar]
- Nierenberg AA, Papakostas GI, Petersen T, et al. (2003) Lithium augmentation of nortriptyline for subjects resistant to multiple antidepressants. J Clin Psychopharmacol 23: 92–95. [DOI] [PubMed] [Google Scholar]
- Ochs-Ross R, Daly EJ, Zhang Y, et al. (2020) Efficacy and safety of esketamine nasal spray plus an oral antidepressant in elderly patients with treatment-resistant depression—TRANSFORM-3. Am J Geriatr Psychiatry 28: 121–141. DOI: 10.1016/j.jagp.2019.10.008. [DOI] [PubMed] [Google Scholar]
- Parikh SV, Segal ZV, Grigoriadis S, et al. (2009) Canadian network for mood and anxiety treatments (CANMAT) clinical guidelines for the management of major depressive disorder in adults. II. Psychotherapy alone or in combination with antidepressant medication. J Affect Disord 117: S15–S25. DOI: 10.1016/j.jad.2009.06.042. [DOI] [PubMed] [Google Scholar]
- Papakostas GI, Fava M, Baer L, et al. (2015) Ziprasidone augmentation of escitalopram for major depressive disorder: Efficacy results from a randomized, double-blind, placebo-controlled study. Am J Psychiatry 172(12): 1251–1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popova V, Daly EJ, Trivedi M, et al. (2019) Efficacy and safety of flexibly dosed esketamine nasal spray combined with newly initiated oral antidepressant in treatment-resistant depression: Randomized double-blind active-controlled study. Am J Psychiatry 176: 428–438. DOI: 10.1176/appi.ajp.2019.19020172. [DOI] [PubMed] [Google Scholar]
- Reeves H, Batra S, May RS, et al. (2008) Efficacy of risperidone augmentation to antidepressants in the management of suicidality in major depressive disorder: A randomized, double-blind, placebo-controlled pilot study. J Clin Psychiatry 69: 1228–1336. [DOI] [PubMed] [Google Scholar]
- Ruberto VL, Jha MK, Murrough JW. (2020) Pharmacological treatments for patients with treatment-resistant depression. Pharmaceuticals 13: 116–138. DOI: 10.3390/ph13060116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rush AJ, Kraemer HC, Sackeim HA, et al. (2006) Report by ACNP Task Force on response and remission in major depressive disorder. Neuropsychopharmacology 31: 1841–1853. DOI: 10.1038/sj.npp.1301131. [DOI] [PubMed] [Google Scholar]
- Saini R, Raju MSVK, Chaudhury S, et al. (2016) Accelerated antidepressant response to lithium augmentation of imipramine. Ind Psychiatry J 25: 93–100. DOI: 10.4103/0972-6748.196057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schöpf J, Baumann P, Lemarchand T, et al. (1989) Treatment of endogenous depressions resistant to tricyclic antidepressants or related drugs by lithium addition: Results of a placebo-controlled double-blind study. Pharmacopsychiatry 22: 183–187. DOI: 10.1055/s-2007-1014603. [DOI] [PubMed] [Google Scholar]
- Seligman F, Nemeroff CB. (2015) The interface of depression and cardiovascular disease: Therapeutic implications. Ann N Y Acad Sci 1345: 25–35. DOI: 10.1111/nyas.12738. [DOI] [PubMed] [Google Scholar]
- Shelton RC, Tollefson GD, Tohen M, et al. (2001) A novel augmentation strategy for treating resistant major depression. Am J Psychiatry 158: 131–134. [DOI] [PubMed] [Google Scholar]
- Shelton RC, Williamson DJ, Corya SA, et al. (2005) Olanzapine/fluoxetine combination for treatment-resistant depression: A controlled study of SSRI and nortriptyline resistance. J Clin Psychiatry 66: 1289–1297. [DOI] [PubMed] [Google Scholar]
- Solmi M, Murru A, Pacchiarotti I, et al. (2017) Safety, tolerability, and risks associated with first- and second-generation antipsychotics: A state-of-the-art clinical review. Ther Clin Risk Manag 13: 757–777. DOI: 10.2147/TCRM.S117321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spielmans GI, Berman MI, Linardatos E, et al. (2013) Adjunctive atypical antipsychotic treatment for major depressive disorder: A meta-analysis of depression, quality of life, and safety outcomes. PLoS Med 10: e1001403. DOI: 10.1371/journal.pmed.1001403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein G, Bernadt M. (1993) Lithium augmentation therapy in tricyclic-resistant depression: Controlled trial using lithium in low and normal doses. Br J Psychiatry 162: 634–640. DOI: 10.1192/bjp.162.5.634. [DOI] [PubMed] [Google Scholar]
- Thase ME, Corya SA, Osuntokun O, et al. (2007) A randomized, double-blind comparison of olanzapine/fluoxetine combination, olanzapine, and fluoxetine in treatment-resistant major depressive disorder. J Clin Psychiatry 68: 224–236. [DOI] [PubMed] [Google Scholar]
- Thase ME, Youakim JM, Skuban A, et al. (2015. a) Adjunctive brexpiprazole 1 and 3 mg for patients with major depressive disorder following inadequate response to antidepressants: A phase 3, randomized, double-blind study. J Clin Psychiatry 76(9): 1232–1240. [DOI] [PubMed] [Google Scholar]
- Thase ME, Youakim JM, Skuban A, et al. (2015. b) Efficacy and safety of adjunctive brexpiprazole 2 mg in major depressive disorder: A phase 3, randomized, placebo-controlled study in patients with inadequate response to antidepressants. J Clin Psychiatry 76(9): 1224–1231. [DOI] [PubMed] [Google Scholar]
- Tondo L, Vázquez GH, Pinna M, et al. (2018) Characteristics of depressive and bipolar disorder patients with mixed features. Acta Psychiatr Scand 138: 243–252. DOI: 10.1111/acps.12911. [DOI] [PubMed] [Google Scholar]
- Undurraga J, Sim K, Tondo L, et al. (2019) Lithium treatment for unipolar major depressive disorder: Systematic review. J Psychopharmacol 33: 167–176. DOI: 10.1177/0269881118822161. [DOI] [PubMed] [Google Scholar]
- Vázquez GH, Lolich M, Cabrera C, et al. (2018) Mixed symptoms in major depressive and bipolar disorders: Systematic review. J Affect Disord 225: 756–760. DOI: 10.1016/j.jad.2017.09.006. [DOI] [PubMed] [Google Scholar]
- World Health Organization (2012) Global burden of disease: Depression. Available at: https://www.who.int/news-room/fact-sheets/detail/depression (accessed 28 December 2020).
- Yuan Z, Chen Z, Xue M, et al. (2020) Application of antidepressants in depression: Systematic review and meta-analysis. J Clin Neurosci 80: 169–181. DOI: 10.1016/j.jocn.2020.08.013. [DOI] [PubMed] [Google Scholar]
- Zimmermann P, Brückl T, Nocon A, et al. (2009) Heterogeneity of DSM-IV major depressive disorder as a consequence of subthreshold bipolarity. Arch Gen Psychiatry 66: 1341–1352. DOI: 10.1001/archgenpsychiatry.2009.158. [DOI] [PubMed] [Google Scholar]
- Zusky PM, Biederman J, Rosenbaum JF, et al. (1988) Adjunct low dose lithium carbonate in treatment-resistant depression: Placebo-controlled study. J Clin Psychopharmacol 8: 120–124. DOI: 10.1097/00004714-198804000-00007. [DOI] [PubMed] [Google Scholar]