Abstract
Background:
Off-label disease-modifying therapies (DMTs) for multiple sclerosis (MS) are used in at least 89 countries. There is a need for structured and transparent evidence-based guidelines to support clinical decision-making, pharmaceutical policies and reimbursement decisions for off-label DMTs.
Objectives/Results:
The authors put forward general principles for the ethical use of off-label DMTs for treating MS and a process to assess existing evidence and develop recommendations for their use.
Conclusion:
The principles and process are endorsed by the World Federation of Neurology (WFN), American Academy of Neurology (AAN), European Academy of Neurology (EAN), Americas Committee for Treatment and Research in Multiple Sclerosis (ACTRIMS), European Committee for Treatment and Research in Multiple Sclerosis (ECTRIMS), Middle-East North Africa Committee for Treatment and Research in Multiple Sclerosis (MENACTRIMS) and Pan-Asian Committee for Treatment and Research in Multiple Sclerosis (PACTRIMS), and we have regularly consulted with the Brain Health Unit, Mental Health and Substance Use Department at the World Health Organization (WHO).
Keywords: Disease-modifying therapies, access to treatment, off-label treatment, guideline
Introduction
All people with multiple sclerosis (MS) should have access to effective, safe and affordable disease-modifying therapies (DMTs). A range of DMTs is required for use in different clinical scenarios and personal circumstances, but these are not always available or affordable.1,2
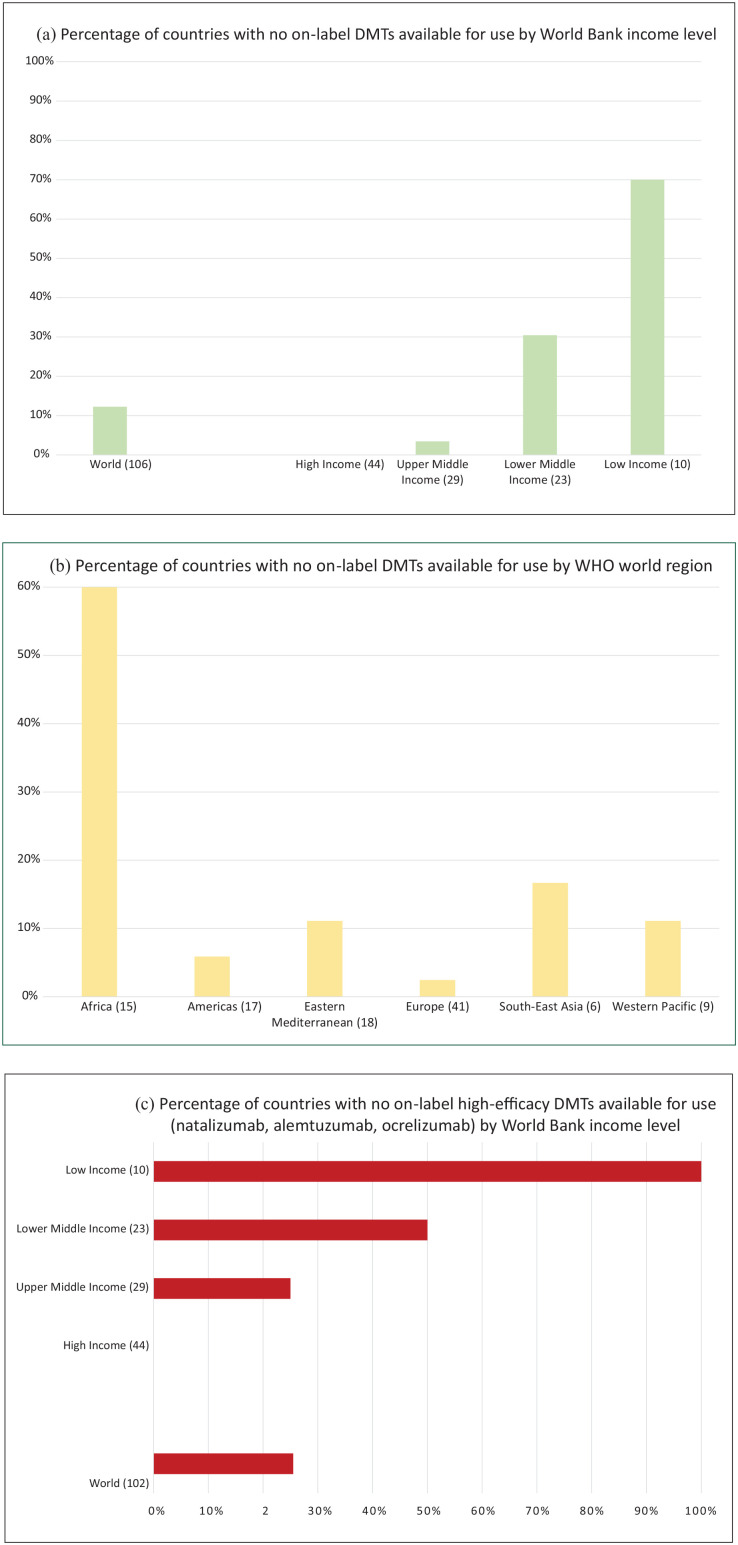
MSIF’s recent Atlas of MS survey showed that 72% of countries have major barriers for accessing DMTs. The countries were categorised according to the World Bank’s income groups for analysis: 70% of low-income countries and 60% of countries in the WHO Africa region report no on-label MS DMTs available for use (Figure 1(a)–(b)).3 The quality of the data from these regions is variable and only a proportion of countries were able to supply data for the Atlas, suggesting the percentages may be even higher due to the lack of awareness of MS and existing treatment options. On-label high-efficacy monoclonal antibodies are particularly poorly available (Figure 1(c)).3
Figure 1.
The percentage of countries that do not have access to on-label DMTs for use, stratified for (a) income level and (b) region, as well as (c) the percentage of countries with no access to on-label high-efficacy DMTs according to income level. Total number of countries that supplied data for this question in the Atlas survey in parentheses (n = number of countries). Please note that we lack data from a number of countries around the world and that World Bank income classification comes with limitations of accuracy and heterogeneity within countries.
Despite 17 different on-label DMTs having marketing authorisation for MS, a number of off-label DMTs are also used in practice for the treatment of MS, for example, azathioprine (has approval in Germany), rituximab, cladribine (non-oral forms), mitoxantrone (has approval in the United States), methotrexate, leflunomide, fludarabine, cyclophosphamide, minocycline, mycophenolate mofetil and intravenous immunoglobulin (IVIG). Some of these were used off-label before on-label DMTs came on the market, but many are still used to treat MS. The amount and quality of clinical evidence supporting the use of these DMTs varies greatly.
The drivers for off-label prescribing can be complex4 and are influenced by local health care infrastructure, access to health insurance, and availability of innovator drugs, generics and biosimilars. In low-resource settings, off-label prescribing is often driven by a lack of appropriate options or cost5 (see Box 1).
Box 1.
Case study from Malaysia.
|
Clinical features of patient: A 24-year-old woman develops recurrent multifocal attacks of vertigo, unsteady gait, double vision and weakness of limbs. Neurologically, she has an internuclear ophthalmoplegia, ataxia of limbs and gait with pyramidal signs in the lower limbs and spastic paraparesis. She doesn’t recover well from her attacks and her EDSS within a short period of time increases to 5.0 due to attack-related disability. Her MRI of brain and spine shows multiple active lesions in the cortex, periventricular, juxtacortical, brainstem, cerebellar and spinal cord regions some of which are enhancing. She has a highly aggressive form of relapsing remitting multiple sclerosis with significant residual disability. Treatment options: Her clinical features warrant high-efficacy treatments such as fingolimod, alemtuzumab, natalizumab, ocrelizumab, cladribine or off-label rituximab. Personal circumstances: She is considered a non-resident due to her family circumstances, and therefore unable to access treatment through local hospital funding or non-governmental organisation support. She has no medical insurance coverage and has economic constraints. She paid for plasma exchange through a donation and was started on off-label therapy with azathioprine as this was the only treatment option she could afford (approximate prices in Malaysia in 2020: azathioprine €135/year vs rituximab €1430/year, fingolimod €13,240/year, alemtuzumab €73,315/year and cladribine €18,330/year). In 2020, ocrelizumab and natalizumab were not available in Malaysia. |
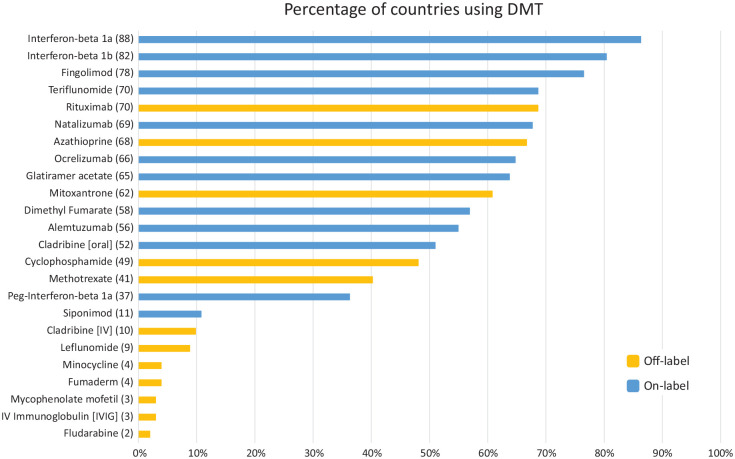
A total of 89 countries (87%) use at least one off-label DMT to treat MS. Rituximab and azathioprine were mentioned by most countries (Figure 2): 69% and 67%, respectively. There is lack of data on the scale, i.e., the number of people being treated with these DMTs in each country.
Figure 2.
The percentage of countries that report using each DMT for MS. The number of countries for each DMT is in parentheses. Total number of countries that supplied data for this question in the Atlas survey is 102. Please note that mitoxantrone has regulatory approval in the United States and azathioprine in Germany.
Principles and process
There is a need to provide guidance for policymakers, clinicians and people affected by MS to ensure the best possible health outcomes. The highest ethical standard would be uniform access for all persons to a range of evidence-based therapies. Using off-label DMTs may thus be unethical if other, more appropriate, medications are available and affordable. It may also be considered unethical not to use off-label medication when on-label options are not available or affordable and when off-label treatments provide an effective and safe option.
(A) General principles for the ethical use of off-label DMTs for treating MS and long-term changes needed
Off-label use of DMTs to treat MS should be driven by the need to protect the person’s health.
Off-label use should be evidence-driven* and considered when on-label DMTs are not tolerated, unsuitable for the best clinical outcome, unavailable or unaffordable.
Shared decision-making between persons with MS and their health care professional is especially important when off-label DMTs are considered.
Appropriate information on health benefits and risks of the off-label DMT should be made available to persons with MS by their health care professional during the full disease management pathway.
Outcomes, effectiveness and adverse events when using off-label DMTs to treat MS should be carefully monitored.
Long-term changes needed:
To support evidence-driven clinical decision-making and reimbursement decisions, guidelines for the use of off-label DMTs to treat MS are needed. We outline in (B) a proposed process for evaluating the evidence-base for off-label DMTs and for developing recommendations.
The regulation of off-label prescribing for MS should be further considered and developed both nationally and internationally to support best clinical practice.
Regulatory agencies and other organisations should develop measures to facilitate official registration of off-label use of medicines with a positive benefit–harm balance based on adequate evidence.
*Please refer to point 1 in long-term changes needed for details on support for evidence-driven decision-making.
(B) Process for off-label DMT evaluation and recommendations
The evidence-base available for off-label DMTs is different from medicines that have been granted marketing authorisation, as large phase III trials for MS are often lacking. There is little economic incentive for the pharmaceutical industry to seek regulatory approval for these medicines as they are often off-patent and may compete with the approved DMTs to lower their economic value. The marketing of medicines for off-label use is illegal in many jurisdictions, while off-label prescribing is accepted as an essential part of normal medical practice. Availability and reimbursement of off-label medicines is often limited by health systems, deemed ‘experimental’ (lack of formal clinical trial evidence), or too expensive for the formularies (even if cheaper than on-label alternatives), irrespective of the health authority’s views on efficacy.
Guidelines are crucial for supporting the standardisation and improvement of care, and to inform policy and reimbursement decisions. MSIF, advised by its off-label treatment (MOLT) panel, puts forward the following process for developing guidelines.
Process
(a). The convening organisation needs to be committed to impartiality of the outcomes, and free from conflict of interest relating to the funding of the guidelines.
(b). A multidisciplinary and international guideline panel should be formed, as a large number of countries are using off-label DMTs. Conflicts of interest should be carefully assessed and managed throughout the process to avoid undue influence.
(c). Specific PICO (Population, Intervention, Comparator and Outcomes) questions should be selected to guide and support clinical practice, with consideration of relevant comparators in low-resource settings, for example, off-label use may be the only option available.
(d). All relevant evidence should be considered with an understanding of the limitations. An independent, systematic review using GRADE methodology is recommended. Additional considerations not captured in the systematic review may be collated, but considered separately in relation to source and quality.
(e). The development of recommendations needs to be structured and transparent. Considerations should also be given to values, equity, acceptability, feasibility and resource requirements (costs). The recommendations need to be clearly justified with emphasis on the criteria used, the importance of these criteria for the recommendations and the judgments made based on the evidence and additional considerations. The GRADE-DECIDE evidence-to-decision framework is recommended.
(f). Targeted feedback from key stakeholders and open comment to correct inaccuracies should be part of the process. The final guideline should be published in a peer-reviewed scientific journal with additional modes of dissemination subject to careful consideration. The guideline should be updated on a regular basis when new information becomes available.
Discussion and next steps
Off-label DMTs are used to treat a number of other diseases that have a higher prevalence than MS. Health systems may be more likely to procure these DMTs in the first place and to negotiate a better price for larger orders. This may lead to a more efficient supply chain and a wider distribution within a country. Off-label DMTs for MS are therefore usually more available and affordable than on-label DMTs in national health systems. Affordability of DMTs has been reported by half of the countries surveyed as a major barrier to access,3 but the lack of transparency on negotiated prices makes analysis of this topic challenging.
Azathioprine, rituximab, cyclophosphamide, fludarabine and methotrexate are listed on the World Health Organization (WHO) Essential Medicines List (EML), a list that guides health ministries on the medicines that should be available in all health systems. We analysed the WHO national essential medicine database6 and found that 95% of 137 countries listed at least one treatment that has been known to be used off-label for MS, as opposed to 31% listing at least one treatment that has been known to be used on-label for MS (Table 1). Of note, most national EMLs do not link the medicines to specific indications, so we know only that the medicines are listed, not whether they are used to treat MS.
Table 1.
Number of countries listing DMTs that have been known to be used for MS on their national essential medicine list. Please note that most national medicine lists do not give details of approved indications for use. On-label for MS in (A) and off-label DMTs in (B). The Anatomical Therapeutic Chemical (ATC) codes used for the analysis are included. WHO’s ATC codes classify the active ingredients of drugs according to the organ or system on which they act.
| A | ||
|---|---|---|
| Medicine | ATC code | Number of countries listing medicine |
| Interferon beta | L03AB02 | 39 |
| Peginterferon | L03AB08 | Not listed |
| Glatiramer acetate | L03AX13 | 19 |
| Fingolimod | L04AA27 | 6 |
| Cladribine | L04AA40 | 16 |
| Teriflunomide | L04AA31 | Not listed |
| Dimethyl fumerate | N07XX09 | Not listed |
| Ocrelizumab | L04AA36 | Not listed |
| Alemtuzumab | L04AA34 | 11 |
| Natalizumab | L04AA23 | 9 |
| Total listing at least one medicine | 42 | |
| Not listing any medicine | 95 | |
| (B) | ||
| Medicine | ATC code | Number of countries listing medicine |
| Azathioprine | L04AX01 | 107 |
| Rituximab | L01XC02 | 41 |
| Leflunomide | L04AA13 | 30 |
| Cladribine | L04AA40 | 16 |
| Cyclophosphamide | L01AA01 | 114 |
| Fludarabine | L01BB05 | 38 |
| Methotrexate | L01BA01, L04AX03 | 126 |
| Mitoxantrone | L01DB07 | 37 |
| Total listing at least one medicine | 130 | |
| Not listing any medicine | 7 | |
There is no international law or legal framework for off-label use of medicines and it is considered a matter of national medicines law. Off-label prescribing is rarely illegal by itself, but may have legal consequences for the prescriber. Clinicians should make sure they are aware of the local legislation and requirements and their potential liability. Justification for off-label use and informed consent from the patient are often required. It is imperative that the available options are outlined and explained to people with MS, allowing adequate time and consideration for an informed decision on whether this is the right choice for them.
Providing realistic and affordable treatment options for MS goes beyond the individual. It signals that treating this condition is possible and effective. This encourages early and accurate diagnosis, which in turn highlights the requirement for diagnostic infrastructure within countries. The clinical and patient data needed to underpin such infrastructure encourages the establishment and development of accurate registries. With a better functioning diagnostic, treatment and monitoring infrastructure, there will be a greater drive to provide high-quality care, from people with MS, clinicians and policy makers. Improved awareness of MS, increased need for high quality care and greater availability of high quality clinical and patient data creates markets that are relevant for the pharmaceutical industry, enabling registration of on-label DMTs and allowing effective price-negotiations and sustainable solutions between health systems and industry.
In 2018, MSIF applied for three MS treatments to be added to the WHO EML, using a process that considered all on-label DMTs.1 This application was not successful and the WHO Expert Committee requested a review of all DMTs used for MS, specifically naming two off-label DMTs: azathioprine and rituximab. The WHO request further highlights the need to provide international guidance on whether these off-label DMTs are appropriate to use and under what circumstances.
Crucially, there must be sufficient evidence in place to assess the safety and efficacy of off-label DMTs in MS to enable assessment of the balance between positive and negative health outcomes. The process put forward ensures transparency of the criteria and judgements in making recommendations. This is particularly important when the guidelines will be adopted or adapted into different national or local settings, where parameters may vary. Including areas such as feasibility, resource requirements and equity into decision-making, allows different health systems to consider the implications carefully.
We have taken a collaborative approach and engaged with a number of key international and regional stakeholders to develop the principles and process. We have regularly consulted with the Brain Health Unit, Mental Health and Substance Use Department at WHO. The principles and process have been endorsed by the World Federation of Neurology (WFN), American Academy of Neurology (AAN), European Academy of Neurology (EAN), Americas Committee for Treatment and Research in Multiple Sclerosis (ACTRIMS), European Committee for Treatment and Research in Multiple Sclerosis (ECTRIMS), Middle-East North Africa Committee for Treatment and Research in Multiple Sclerosis (MENACTRIMS) and Pan-Asian Committee for Treatment and Research in Multiple Sclerosis (PACTRIMS).
MSIF will use this publication, together with subsequent guidelines addressing specific off-label DMTs, to provide tools for national action for MS organisations and health care professionals to discuss off-label DMTs for MS with health authorities, budget holders and payers when making decisions on formularies, national EMLs, national guidelines, budgets and reimbursement systems. The ultimate aim should be for people with MS to have access to a range of safe, effective and affordable DMTs.
Acknowledgments
We would like to acknowledge members of the MSIF Off-Label Treatment panel (MOLT) and other experts for insight, discussion and feedback.
Footnotes
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Multiple Sclerosis International Federation (MSIF) is an alliance of national multiple sclerosis (MS) organisations. MSIF receives income from a wide range of sources, including health care and other companies, individuals, member organisations, campaigns, foundations and trusts. Over the last 5 years, MSIF received funding from the following companies: Biogen, Bristol Myers Squibb (formerly Celgene), MedDay, Merck, Mylan, Novartis, Roche, Sanofi Genzyme and Teva. Our independence and all our donations from the health care industry are governed by our policy: http://www.msif.org/wp-content/uploads/2017/09/Policyand-Practices-in-Relationships-with-the-Healthcare-Industry-2017.pdf. Joanna Laurson-Doube, Nick Rijke, Anne Helme and Peer Baneke have no relevant individual conflicts of interest. Brenda Banwell serves on the Board of ACTRIMS (Americas Committee for Treatment and Research in Multiple Sclerosis), Medical and Scientific Board of the MSIF, Medical Advisory Board of the Canadian Multiple Sclerosis Society, and Board of Directors of the American Academy of Neurology. Dr Banwell has received financial compensation from Novartis for efforts as a central magnetic resonance imaging (MRI) reviewer and as a speaker. Dr Banwell serves as a nonremunerated advisor to Novartis, Biogen-IDEC, Sanofi Aventis, Teva Neuroscience, and Celgene. Dr Banwell has received compensation from Medscape and donations for an educational residency fund, in lieu of personal compensation received from the Corpus for general paediatric MS lectures. Bernhard Hemmer has served on scientific advisory boards for Novartis; he has served as DMSC member for AllergyCare, Polpharma and TG therapeutics; he or his institution have received speaker honoraria from Desitin; his institution received research grants from Regeneron for MS research. He holds part of two patents: one for the detection of antibodies against KIR4.1 in a subpopulation of patients with MS and one for genetic determinants of neutralising antibodies to interferon. Shanthi Viswanathan is the Chair of the Malaysian Clinical Practice Guidelines on the Management of Multiple Sclerosis and has no other conflicts of interest with regard to the content of the current paper. Bassem Yamout has served on advisory boards for Sanofi, Bayer, Roche, Merck and Biogen. He received honoraria as speaker from Sanofi, Bayer, Roche, Merck, Biogen, Pfizer and Lundbeck. He received research grants from Novartis and Biogen. He served on steering committees for Merck.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: MSIF has not received any funding from industry for its access to medicines work in 2019, 2020 or 2021.
ORCID iDs: Joanna Laurson-Doube  https://orcid.org/0000-0001-9619-9170
https://orcid.org/0000-0001-9619-9170
Shanthi Viswanathan  https://orcid.org/0000-0002-0094-0540
https://orcid.org/0000-0002-0094-0540
Bernhard Hemmer  https://orcid.org/0000-0001-5985-6784
https://orcid.org/0000-0001-5985-6784
Contributor Information
Joanna Laurson-Doube, Multiple Sclerosis International Federation, London, UK.
Nick Rijke, Multiple Sclerosis International Federation, London, UK.
Anne Helme, Multiple Sclerosis International Federation, London, UK.
Peer Baneke, Multiple Sclerosis International Federation, London, UK.
Brenda Banwell, Division of Neurology, Children’s Hospital of Philadelphia, Perelman School of Medicine, University of Pennsylvania, Philadelphia, PA, USA.
Shanthi Viswanathan, Department of Neurology, Hospital Kuala Lumpur, Kuala Lumpur, Malaysia.
Bernhard Hemmer, Department of Neurology, Klinikum rechts der Isar, Technische Universität München, Munich, Germany/Munich Cluster for Systems Neurology (SyNergy), Munich, Germany.
Bassem Yamout, Department of Neurology-Nehmeh and Therese Tohmeh Multiple Sclerosis Center, American University of Beirut, Beirut, Lebanon/Neurology Institute, Harley Street Medical Center, Abu Dhabi, United Arab Emirates.
References
- 1.McDonell J, Costello K, Laurson-Doube J, et al. World Health Organization essential medicines list: Multiple sclerosis disease-modifying therapies application. Mult Scler 2020; 26(2): 153–158. [DOI] [PubMed] [Google Scholar]
- 2.Laurson-Doube J, Rijke N, Costello K, et al. Health-care disparities for people with multiple sclerosis. Lancet Neurol 2020; 19(3): 207–208. [DOI] [PubMed] [Google Scholar]
- 3.Atlas of MS. Available at: https://www.atlasofms.org/ (accessed 31 March 2021).
- 4.Marjolein W, Hoebert J, Vervloet M, et al. Study on off-label use of medicinal products in the European Union: Report, 2019. Available at: http://publications.europa.eu/publication/manifestation_identifier/PUB_EW0117413ENN (accessed 6 May 2020).
- 5.Zeineddine MM, Yamout BI.Treatment of multiple sclerosis in special populations: The case of refugees. Mult Scler J Exp Transl Clin 2020; 6(1): 2055217319848466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.EMLs around the world. Available at: https://global.essentialmeds.org/dashboard/countries (accessed 31 March 2021).