Abstract
Background:
Clinical measures in multiple sclerosis (MS) face limitations that may be overcome by utilising smartphone keyboard interactions acquired continuously and remotely during regular typing.
Objective:
The aim of this study was to determine the reliability and validity of keystroke dynamics to assess clinical aspects of MS.
Methods:
In total, 102 MS patients and 24 controls were included in this observational study. Keyboard interactions were obtained with the Neurokeys keyboard app. Eight timing-related keystroke features were assessed for reliability with intraclass correlation coefficients (ICCs); construct validity by analysing group differences (in fatigue, gadolinium-enhancing lesions on magnetic resonance imaging (MRI), and patients vs controls); and concurrent validity by correlating with disability measures.
Results:
Reliability was moderate in two (ICC = 0.601 and 0.742) and good to excellent in the remaining six features (ICC = 0.760–0.965). Patients had significantly higher keystroke latencies than controls. Latency between key presses correlated the highest with Expanded Disability Status Scale (r = 0.407) and latency between key releases with Nine-Hole Peg Test and Symbol Digit Modalities Test (ρ = 0.503 and r = −0.553, respectively), ps < 0.001.
Conclusion:
Keystroke dynamics were reliable, distinguished patients and controls, and were associated with clinical disability measures. Consequently, keystroke dynamics are a promising valid surrogate marker for clinical disability in MS.
Keywords: Multiple sclerosis, disability evaluation, upper extremity, cognition, smartphone, touch typing, ambulatory monitoring, ecological momentary assessment
Introduction
Multiple sclerosis (MS) is a heterogeneous immune-mediated disease characterised by inflammatory disease activity and progression of disability over time.1 Among the many clinical manifestations, fatigue is one of the most common symptoms in MS with its severity often negatively impacting quality of life, work and social participation.2 Clinical deficits underlying ongoing inflammatory activity can be subtle or even absent, while responsive and standardised assessment tools for disability worsening and fatigue are lacking.3,4 Hence, the assessment of disease activity, disability and fatigue relies heavily on magnetic resonance imaging (MRI), Expanded Disability Status Scale (EDSS) and multiple patient-reported fatigue outcomes, respectively.5 However, the current clinical measures are limited in assessment frequency, are obtrusive, are momentary and in the case of the EDSS are faced with inter- and intra-rater variability.5
Alternatively, remote health monitoring through devices with sensors allows continuous, objective and potentially more time- and cost-efficient assessment.6 Of such devices, smartphones are increasingly used in healthcare and widely available;7 87% of patients with MS aged 20–72 years own a smartphone.8 Typing is a common user–device interaction requiring coordinated, repetitive motor skills (e.g. coordination and manual dexterity) and higher order cognitive functions (e.g. information processing and attention), both of which tend to be affected in MS.9 Quantitative analysis of press and release keyboard interactions – keystroke dynamics – allows the identification of typing behaviour (e.g. amount, speed and error rate of typing) in a real-world setting which can reflect motor and non-motor functioning.10,11 We, therefore, hypothesise that keystroke dynamics can be utilised as a biomarker in MS in relation to disease activity, clinical disability and fatigue.
Our objective is to investigate (1) the feasibility and reliability of real-world keystroke dynamics collected by smartphone technology and (2) its validity to assess fatigue, MRI disease activity and clinical disability in MS.
Methods
Study design
This is a prospective observational cohort study at Amsterdam University Medical Centers, location VU University Medical Center. The study comprises five clinical visits with 3-month intervals during which keyboard interaction data were remotely obtained from the everyday environment of the participants. Reported here are the results of the preplanned analysis of the first clinical visit (M0), which included patient-reported outcomes 2 weeks later (M0+2wk). Participants with missing clinical assessments or less than 11 days (i.e. <75%) of keystroke data between M0 and M0+2wk were excluded from the analysis. Study approval was granted by the local institutional ethics review board and the institutional data protection officer conforming to the European General Data Protection Regulation. In compliance with Dutch legislation regarding clinical research involving medical devices, the Dutch healthcare inspectorate has been notified of the study. Written informed consent was obtained from all participants.
Participants
Patients with MS and healthy controls (HC) were included from August 2018 to December 2019. Inclusion criteria were regular use of a smartphone with Android or iOS, age between 18 and 65 years and, for the patient group, a definite diagnosis of MS.12 Exclusion criteria were EDSS score of 7.5 or higher, clinical disease activity or changes in disease-modifying drugs in the past 2 months, significant visual or upper extremity deficit affecting the ability to type on a smartphone, and clinically significant mood, sleep or behavioural disorder based on medical history-taking by a screening physician.
Main outcomes
Clinical measures spanning physical and mental domains were assessed at M0: MRI, EDSS, Nine-Hole Peg Test (NHPT) and Symbol Digit Modalities Test (SDMT). MRI of the brain included T1-weighted images after administration of gadolinium (Gd). The MRI images were assessed by a neuroradiologist for the presence of Gd-enhancing demyelinating lesions, indicative of inflammatory disease activity. The EDSS is a measure of severity of disability due to MS and was performed by trained physicians.13 The NHPT and SDMT were used as measures for manual dexterity and information processing speed, respectively.14,15 At M0+2wk, the Fatigue Severity Scale (FSS) and Checklist Individual Strength Fatigue subscale (CIS-F) were assessed. The FSS and CIS-F measures self-reported fatigue over the past 7 and 14 days, respectively, with higher scores corresponding with more severe fatigue.16,17 The CIS-F cutoff of ⩾35 was used to indicate severe fatigue.18
Keystroke dynamics
A smartphone app (Neurokeys, Neurocast B.V., Amsterdam) was developed for Android and iOS to measure health status through regular typing on the smartphone. The Neurokeys keyboard was installed on the participants’ personal smartphone and replaced the default keyboard. During regular typing, keyboard interactions of interest were logged and timestamped in the background: alphanumeric keys, backspaces, space bars and punctuation keys. Based on these timestamped key types, the manner and rhythm of typing can be discerned by analysing keystroke features. Keyboard interactions were continuously collected and stored per typing session, defined as one successive period of activation followed by inactivation of the keyboard. When a typing session starts, keystroke data from the previous typing session are sent and removed from the smartphone. The collection of keystroke data through Neurokeys did not require additional action from the participants.
Definition and aggregation of keystroke features
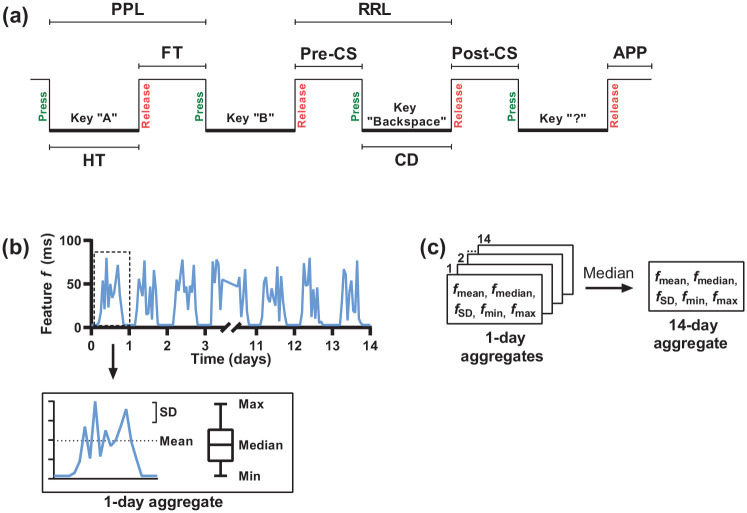
Between M0 and M0+2wk, general typing characteristics were obtained: total number of interactions, typing session length, word length, number of backspaces and number of space bars. From the keyboard interactions, eight timing-related keystroke features were derived (Figure 1). Features based on alphanumeric keys were latency between successive key presses (Press-Press Latency) and releases (Release-Release Latency), time between a key press and subsequent release (Hold Time) and time between a key release and the next key press (Flight Time). Features related to the backspace key were time before (Pre-Correction Slowing), during (Correction Duration) and after a backspace key press (Post-Correction Slowing). Feature based on punctuation marks was time between a punctuation mark and a subsequent alphanumeric key (After Punctuation Pause).
Figure 1.
Schematic representation of the definition and aggregation of the timing-related keystroke features. (a) Keystroke features were derived from timestamped keyboard interactions. (b) For each keystroke feature, five summary statistics (i.e. vectors) were calculated to aggregate all typing sessions per day. (c) The five vectors for each feature were then aggregated into 14 days by taking the median value.
PPL: Press-Press Latency; RRL: Release-Release Latency; FT: Flight Time; Pre-CS: Pre-Correction Slowing; Post-CS: Post-Correction Slowing; APP: After Punctuation Pause; HT: Hold Time; CD: Correction Duration.
To match the keystroke features gathered per typing session with the clinical measures, aggregation into 14 days (and 7 days for comparison with the FSS) was performed (see Figure 1). Typing sessions were first aggregated per day. To aggregate the high sample rate data while retaining meaningful information, for each feature typing sessions were aggregated by calculating five summary statistics (i.e. vectors): mean and median (indicators of central tendency), standard deviation (SD; indicator of dispersion) and minimum and maximum (indicators of range). Then, the 14-day aggregate was obtained by taking the median value of the daily aggregates. Finally, to limit the number of tests, one composite score was computed for each 14-day aggregated keystroke feature by normalising the five vectors v into z-scores with and averaging the five z-scores. In this formula, is the z-score, is a value of one participant, and and are the mean and SD, respectively, of all participants.
Statistical analysis
Keystroke features were assessed for normality using the Kolmogorov–Smirnov test. Non-normally distributed features were transformed using the 10th logarithm, natural logarithm or Box-Cox transformation.19 Test–retest reliability of the keystroke features was assessed using 14-day keystroke aggregates prior to and after M0+2wk, as no substantial change was expected between the two time windows. Intraclass correlation coefficients (ICCs) for consistency were calculated using a two-way random-effects model. Estimates between 0.5 and 0.75, 0.75 and 0.9, and 0.9 or higher were indicative for moderate, good and excellent reliability, respectively.20 The standard error of measurement (SEM) was quantified by .21 Agreement between the test and retest period was visually assessed by constructing Bland–Altman plots with the mean differences and the 95% limits of agreement (mean difference ± 1.96 SD).22
Construct validity of the composite keystroke features was analysed through group comparisons with independent t-tests: HC and patients with MS, patients with non-severe (MS-NF) and severe fatigue (MS-F), and patients with and without Gd-enhancing lesions.23 Subsequently, composite keystroke features with significant group differences were further explored to determine which of the five vectors were different between the groups. Concurrent validity was assessed by calculating Pearson’s and Spearman’s correlation coefficients between the composite keystroke features and clinical measures.23 The ‘static’ clinical measures, EDSS, NHPT and SDMT, were correlated with 14-day keystroke aggregates. The retrospective FSS (past 7 days) and CIS-F (past 2 weeks) assessed at M0+2wk were correlated with 7- and 14-day keystroke aggregates, respectively. As recall bias in self-report measures tends to emphasise recent or more severe events, sensitivity analyses were performed post hoc to explore whether the fatigue measures correlated better with recent (i.e. 2 days prior to M0+2wk) and extreme (i.e. maximum values across the 7- and 14-day aggregates) keystroke events compared to the overall 7- and 14-day aggregates.24 The group and correlation analyses were performed with permutations to increase robustness of the results.25 Permutation-adjusted p-values are reported, and values <0.05 were considered statistically significant.
Results
During the recruitment period, 276 people were interested in the study, of whom 132 were not screened after receiving full study information: 91 were unresponsive or declined participation without specification, 31 declined participation due to time or effort constraints, 7 were unwilling to undergo MRI and 3 opted to participate in an intervention study. The remaining 144 people were screened for eligibility. However, 18 were excluded: no conventional use of or typing on the smartphone (n = 6), age above 65 years (n = 5), no definite diagnosis of MS (n = 4) and presence of clinically significant depressive and sleeping disorder, visual impairment or severe tremor of the upper limbs (each n = 1).
A total of 24 HC and 102 patients with MS were included. One HC dropped out, and two HC and one patient did not have complete M0+2wk clinical measurements. Of the remaining participants, 18 (85.7%) HC and 85 (84.2%) patients had sufficient keystroke data as defined and were included in the analysis. There were no significant differences in demographical and clinical characteristics between participants included in the analysis and the participants excluded due to insufficient keystroke data. During the 14 days of follow-up, 96 participants had 14 active days, 10 were active for 13 days, 4 were active for 12 days and 1 had 11 active days of data (see also Supplemental Figure). Demographical and clinical characteristics are summarised in Table 1. Patients with MS had a mean age of 46.4 years, 75.3% were female, 60.0% had relapsing-remitting MS and the median EDSS score was 3.5 (range 1.5–7.0). There were no significant differences observed in age, sex distribution and level of education between HC and patients. HC had a shorter mean typing session length compared to patients with MS (Table 2). The remaining general typing characteristics were not different between HC and patients.
Table 1.
Baseline demographical and clinical characteristics.
| HC (n = 18) | Patients with MS (n = 85) | p value | |
|---|---|---|---|
| Age, years, mean (SD) | 45.2 (13.5) | 46.4 (10.1) | 0.720a |
| Sex, n (%) | 0.146b | ||
| Female | 10 (55.6) | 64 (75.3) | |
| Male | 8 (44.4) | 21 (24.7) | |
| Level of education, n (%) | 0.601b | ||
| Low | 0 (0.0) | 2 (2.4) | |
| Middle | 4 (22.2) | 28 (32.9) | |
| High | 14 (77.8) | 55 (64.7) | |
| MS type, n (%) | n.a. | ||
| Relapsing-remitting | 51 (60.0) | ||
| Secondary progressive | 25 (29.4) | ||
| Primary progressive | 9 (10.6) | ||
| Disease duration, years, median (IQR) | n.a. | ||
| Since diagnosis | 5.7 (3.0–13.5) | ||
| Since onset | 11.3 (5.1–17.7) | ||
| EDSS, median (IQR) | 3.5 (2.5–4.0) | n.a. | |
| NHPT, median (IQR) | 21.0 (19.4–23.9) | n.a. | |
| SDMT, mean (SD) | 54.5 (10.4) | n.a. | |
| FSS, median (IQR) | 2.0 (1.7–2.3) | 4.4 (3.4–5.1) | <0.001c |
| CIS-F, median (IQR) | 17.5 (13.0–24.0) | 35.0 (27.0–42.0) | <0.001c |
HC: healthy controls; MS: multiple sclerosis; EDSS: Expanded Disability Status Scale; NHPT: Nine-Hole Peg Test; SDMT: Symbol Digit Modalities Test; FSS: Fatigue Severity Scale; CIS-F: Checklist Individual Strength Fatigue subscale; SD: standard deviation; IQR: interquartile range.
Independent t-test.
Fisher’s Exact test.
Mann–Whitney U-test.
Table 2.
General typing characteristics and keystroke features.
| HC (n = 18) | Patients with MS (n = 85) | p value | |
|---|---|---|---|
| Total typing events, n | 1373.1 (976.9) | 1653.6 (1515.1) | 0.253a |
| Average typing session duration, ms | 17,343.5 (6376.1) | 21,854.8 (7411.2) | 0.018b |
| Average word length, characters | 4.2 (0.5) | 4.2 (0.4) | 0.757b |
| Ratio of backspaces,c % | 10.4 (4.4) | 9.2 (4.6) | 0.305b |
| Ratio of space bars,c % | 13.0 (1.4) | 13.1 (1.9) | 0.752b |
| Press-Press Latency, ms | −0.388 (0.357) | 0.082 (0.676) | 0.005a |
| Release-Release Latency, ms | −0.349 (0.395) | 0.074 (0.688) | 0.013a |
| Hold Time, ms | −0.172 (0.663) | 0.036 (0.551) | 0.163a |
| Flight Time, ms | −0.360 (0.418) | 0.076 (0.693) | 0.012a |
| Pre-Correction Slowing, ms | −0.511 (0.651) | 0.108 (0.785) | 0.002a |
| Post-Correction Slowing,d ms | 0.370 (0.164) | 0.478 (0.147) | 0.005a |
| Correction Duration,e ms | 0.505 (0.187) | 0.572 (0.221) | 0.234a |
| After Punctuation Pause, ms | −0.452 (0.627) | 0.096 (0.642) | 0.001a |
HC: healthy controls; SD: standard deviation; MS: multiple sclerosis.
All data are expressed as mean (SD).
Independent t-tests.
Mann–Whitney U-test.
Ratio to total keystroke events.
Box inverse transformed.
Box square root transformed.
Reliability
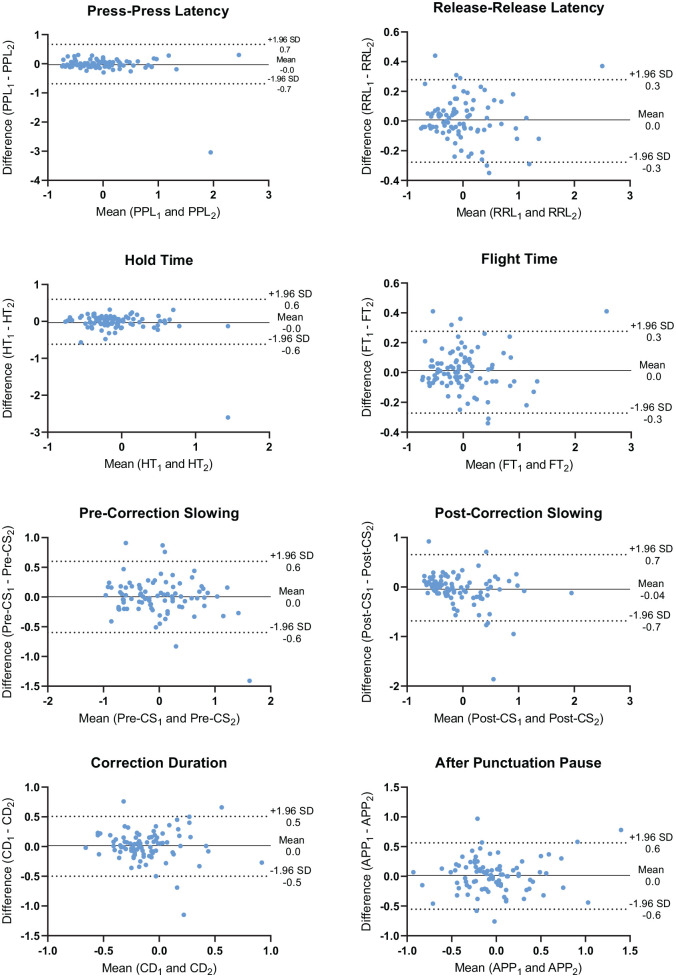
In patients with MS, ICC estimates (and SEM in parentheses) of the keystroke features were as follows: Correction Duration = 0.601 (0.181), indicating moderate reliability; Hold Time = 0.742 (0.219), After Punctuation Pause = 0.760 (0.202), Post-Correction Slowing = 0.787 (0.239), Press-Press Latency = 0.830 (0.241) and Pre-Correction Slowing = 0.865 (0.218), all indicative of good reliability; Flight Time = 0.965 (0.098) and Release-Release Latency = 0.965 (0.099) demonstrated excellent reliability. The SEM for the keystroke features was small compared to the SD of the normalised features. Figure 2 shows the Bland–Altman plots for the eight composite keystroke features. The mean differences (systematic error) between the test and retest period were small and ranged from −0.043 to 0.016. Post-Correction Slowing had the largest limits of agreement (−0.7 to 0.7), in which the differences were slightly more scattered with larger mean values. The limits of agreement of all the other seven keystroke features were relatively small with the magnitude of differences evenly distributed over the whole range of mean values.
Figure 2.
Bland–Altman plots of the keystroke features between the test–retest period. Between the test and retest period, the difference was plotted against the mean for each keystroke feature. The solid line represents the mean difference (systematic error) in keystroke features between the test–retest period, and the two dotted lines represent the 95% limits of agreement (random error).
HC versus patients with MS
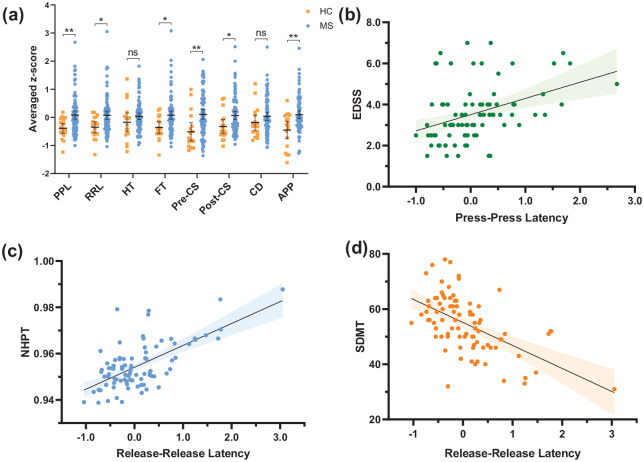
The keystroke features Press-Press Latency, Release-Release Latency, Flight Time, Pre-Correction Slowing, Post-Correction Slowing and After Punctuation Pause were higher in patients with MS compared to HC (Figure 3(a)). Of these six composite features, analyses using the individual statistical vectors revealed that the mean values of all six features and the median values of Press-Press Latency, Release-Release Latency, Pre-Correction Slowing and Post-Correction Slowing were significantly higher in patients than in HC. For Pre-Correction Slowing also, the SD and maximum were higher in patients compared to HC. For After Punctuation Pause, the SD was additionally higher in patients compared to HC. Thus, besides Hold Time and Correction Duration, timing-related keystroke features were significantly higher in patients compared to HC.
Figure 3.
Scatter plots of timing-related keystroke features. (a) Keystroke features grouped between HC and patients with MS. Horizontal bars represent the mean, and error bars represent the 95% confidence interval of the mean. (b) Press-Press Latency (averaged z-score) and EDSS. (c) Release-Release Latency (averaged z-score) and NHPT (Box-Cox transformed). (d) Release-Release Latency (averaged z-score) and SDMT. HC: healthy controls; MS: patients with multiple sclerosis; PPL: Press-Press Latency; RRL: Release-Release Latency; HT: Hold Time; FT: Flight Time; Pre-CS: Pre-Correction Slowing; Post-CS: Post-Correction Slowing; CD: Correction Duration; APP: After Punctuation Pause; EDSS: Expanded Disability Status Scale; NHPT: Nine-Hole Peg Test; SDMT: Symbol Digit Modalities Test.
nsp ⩾ 0.05, *p < 0.05, **p < 0.01.
Clinical disability
In patients with MS, EDSS was positively correlated with five of the eight timing-related keystroke features, of which latency between key presses showed the highest correlation, r = 0.407, p < 0.001 (Table 3, Figure 3(b)). NHPT was positively correlated with seven of the eight keystroke features, with the highest correlation observed with latency between key releases, ρ = 0.503, p < 0.001 (Table 3, Figure 3(c)). Finally, all eight keystroke features were negatively correlated with SDMT (Table 3). The highest correlation was found between Release-Release Latency and SDMT, r = −0.553, p < 0.001 (Figure 3(d)). Therefore, aside from the duration of backspaces for both NHPT and EDSS, and key hold duration and latency after punctuation marks for EDSS, all timing-related key press and release latencies were significantly associated with EDSS, NHPT and SDMT.
Table 3.
Correlations coefficients between keystroke features and clinical measures in patients with MS (n = 85).
| PPL | RRL | HT | FT | Pre-CS | Post-CS | CD | APP | |
|---|---|---|---|---|---|---|---|---|
| EDSSa | 0.407** | 0.380** | 0.150 | 0.383** | 0.300** | 0.352** | 0.207 | 0.209 |
| NHPTb | 0.455** | 0.503** | 0.251* | 0.457** | 0.386** | 0.441* | 0.179 | 0.408** |
| SDMTa | −0.525** | −0.553** | −0.286** | −0.525** | −0.300** | −0.444** | −0.164* | −0.317** |
| CIS-Fa | −0.056 | −0.030 | 0.038 | −0.025 | −0.067 | −0.086 | −0.012 | −0.085 |
| FSSa | 0.041 | 0.080 | 0.030 | 0.028 | 0.006 | 0.066 | 0.038 | −0.216 |
PPL: Press-Press Latency; RRL: Release-Release Latency; HT: Hold Time; FT: Flight Time; Pre-CS: Pre-Correction Slowing; Post-CS: Post-Correction Slowing; CD: Correction Duration; APP: After Punctuation Pause; NHPT: Nine-Hole Peg Test; SDMT: Symbol Digit Modalities Test; FSS: Fatigue Severity Scale; CIS-F: Checklist Individual Strength Fatigue subscale; EDSS: Expanded Disability Status Scale.
Pearson’s correlation coefficient.
Spearman’s log-rank correlation coefficient.
p < 0.05, **p < 0.01.
Fatigue
In total, 39 (45.9%) patients with MS were stratified as ‘non-severely fatigued’ (MS-NF) and 46 (54.1%) as ‘severely fatigued’ (MS-F) using the CIS-F cutoff of 35. There were no differences in age, sex distribution, disease duration, NHPT and SDMT (all ps > 0.05) between MS-NF and MS-F. EDSS was lower in MS-NF, median (interquartile range (IQR)) = 3.0 (2.5–3.5), compared to MS-F, median (IQR) = 4.0 (3.0–4.5), p = 0.020. No differences in timing-related keystroke features were observed between MS-NF and MS-F (all ps > 0.05). FSS and CIS-F scores did not correlate with the composite keystroke features (Table 3). Hence, the latency between key presses and releases was not different between patients with non-severe compared to severe fatigue, nor associated with level of fatigue. Post hoc sensitivity analyses only revealed significant correlations between FSS and Hold Time for recent (r = 0.313, p = 0.005) and severe (r = 0.338, p = 0.001) keystroke events.
MRI Gd-enhancing lesions
Of the 51 patients with relapsing-remitting MS, 35 (68.6%) had no Gd-enhancing lesions, 12 (23.5%) had at least one Gd-enhancing lesion and 4 (7.8%) were not administered Gd (2 patients had a history of an allergic reaction to Gd, and in 2 patients, Gd administration was omitted). NHPT score was higher in the group with at least one Gd-enhancing lesion, median (IQR) = 21.7 (20.7–25.7), compared to the group without Gd-enhancing lesions, median (IQR) = 19.5 (18.3–21.6), p = 0.003. Besides NHPT, the two groups were similar in age, sex distribution, level of education, disease duration, EDSS and SDMT. There were no differences in timing-related keystroke features between patients without and with Gd-enhancing lesions (all ps > 0.05).
Discussion
The results of this study show reliability and validity of keystroke dynamics to assess health status in MS. Generally, keystroke features collected over a 2-week period were different between patients with MS and HC. Group differences were driven by the mean and median values of latencies between key presses and releases, and the dispersion of latencies prior to backspaces and after punctuation marks, which were all higher in patients compared to HC. The keystroke features were also found to be associated with measures of clinical disability, information processing speed and manual dexterity. These findings together can be seen as a first step towards further clinical validation, showing that keystroke dynamics are a promising monitoring tool for disease status in MS.
Differences in keystroke features were found between patients and HC even with similarities in general typing characteristics and despite a relatively short disease duration (median of 5.7 years) and mild disease severity (median EDSS of 3.5) of our cohort. This suggests sensitivity of keystroke dynamics to subtle differences. Deficits in cognitive and motor skills are commonly seen and may already be present early in MS and can therefore explain the observed differences between patients and HC.26 This is further supported by the correlations we found between keystroke features and both NHPT and SDMT in our patients, in line with our hypothesis. For NHPT, our highest correlation was observed with the Release-Release Latency, in which the press and release finger motions resemble the grip and release finger movements during the NHPT. Similar results were reported with accelerometers during typing tasks on a physical keyboard and upper limb dysfunction.27 The significant negative correlations found between the keystroke features and SDMT score, indicating longer keystroke delays, were associated with lower information processing speed, in line with a study comparing tactile touchscreen activity (swipe, tap and keystroke events) with neuropsychological constructs.28 In addition to the NHPT and SDMT, a more general measure of clinical disability (the EDSS) and disease duration (data not shown) was also associated with timing-related keystroke features. Altogether, the moderate correlation coefficients between keystroke features and clinical disability measures suggest concurrent validity to some extent. This is intuitive given that typing requires both cognitive and motor skills to perform coordinated, successive finger movements, producing intended words and sentences.27,29
We also hypothesised that fatigue negatively impacts typing. However, the keystroke features did not correlate with the FSS and CIS-F. Prior studies comparing momentarily assessed symptoms, including fatigue, with assessment of the symptom over a longer time period found none to modest correlations, possibly due to recall bias.30,31 As recall bias was shown to be influenced by recent and extreme events, we performed post hoc sensitivity analyses by comparing recent and extreme keystroke data with the fatigue measures.24 This, however, also did not yield consistent significant correlations. Changes in typing behaviour resulting from fatigue may be better described as fatigability, which is found to be a different construct than perceived fatigue.32 In addition, and perhaps above all, our current cross-sectional analyses does not account for the between-subject variability, which is presumably high for a heterogeneous symptom such as fatigue. Hence, the use of keystroke dynamics to measure fatigue in MS needs further investigation in a longitudinal setting using more frequently assessed fatigue or fatigability measures. Similarly, in this cross-sectional analysis no significant differences were observed in keystroke features between patients with relapsing-remitting MS with and without Gd-enhancing lesions on MRI. Improvement in keystroke features may be anticipated in patients in whom disease activity diminishes. Thus, we expect to find within-subject differences over time in our longitudinal analyses for fatigue and for MRI Gd-enhancing lesions.
Smartphone keystroke dynamics have been investigated previously in Parkinson’s disease, mood disorders and cognitive performance.28,33,34 While these studies also showed associations between clinical measures and typing behaviour, most were conducted in an experimental setting by transcribing standardised text excerpts or using a specific type of smartphone. By contrast, keystroke data in our study were gathered during regular use of the participants’ own smartphone. This ability to assess health outcomes more frequently and unobtrusively from a remote distance in real time is an important advantage compared to current clinical measures.35 However, while interpreting our findings, limitations need to be considered. To comply with the assumptions of the statistical tests, data aggregation was performed, resulting in loss of detailed and high-frequency information regarding day-to-day differences. More complex models should be used next to utilise all available data points to determine the temporal association between keystroke features and clinical outcomes. Moreover, we focused on single keystroke features as a first step rather than a combination of keystroke features. Following this study, future directions should focus on overcoming these limitations, which were also among the challenges discussed recently on the use of wearable technology in MS.36 As with all biomarkers, standardisation and reproducibility are required to further this (technological) biomarker into the clinical practice. Keystroke dynamics may then be employed in conjunction with the current clinical measures to supplement monitoring of MS outside of the clinical windows and more closely to the patients’ functioning in the daily setting.
The results of this study show that keyboard interactions can be used as a monitoring tool through the collection of reliable high sample rate data. Based on the derived keystroke features, we were not only able to distinguish between the HC and MS group, but also show moderate correlations between the keystroke measures and clinical disability, manual dexterity and information processing speed in patients with MS. Therefore, keystroke dynamics are a promising potential outcome measure that enables remote and unobtrusive phenotyping of health status in patients with MS and opens up new applications for disease monitoring, patient management and outcomes for clinical trials.
Supplemental Material
Supplemental material, MSJ968797_supplementary_figure for Real-world keystroke dynamics are a potentially valid biomarker for clinical disability in multiple sclerosis by KH Lam, KA Meijer, FC Loonstra, EME Coerver, J Twose, E Redeman, B Moraal, F Barkhof, V de Groot, BMJ Uitdehaag and J Killestein in Multiple Sclerosis Journal
Acknowledgments
The authors thank all the participants who participated in the study, and Ton Schweigmann and his colleagues for performing the MRIs.
Footnotes
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship and/or publication of this article: K.H.L., F.C.L., E.M.E.C., B.M. and V.d.G have no conflicts of interest. K.A.M., J.T. and E.R. are the employees of Neurocast B.V. (industry partner). F.B. acts as a consultant to Biogen Idec, Janssen Alzheimer Immunotherapy, Bayer Schering, Merck Serono, Roche, Novartis, Genzyme and Sanofi-aventis; he has received sponsorship from EU-H2020, NWO, SMSR, EU-FP7, TEVA, Novartis and Toshiba; he is on the editorial board of Radiology, Brain, Neuroradiology, Multiple Sclerosis Journal (MSJ) and Neurology; he is supported by the NIHR biomedical research centre at UCLH. B.M.J.U. received consultancy fees from Biogen Idec, Genzyme, Merck Serono, Novartis, Roche and Teva. J.K. has accepted speaker and consultancy fees from Merck, Biogen, Teva, Genzyme, Roche and Novartis.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship and/or publication of this article: The collaboration project is co-funded by the PPP Allowance made available by Health Holland, Top Sector Life Sciences and Health (Grant No. LSHM16060-SGF) and Stichting MS Research (Grant No. 16-946 MS) to stimulate public–private partnerships and by a contribution from Biogen (unrestricted funding).
ORCID iDs: KH Lam  https://orcid.org/0000-0003-0926-1445
https://orcid.org/0000-0003-0926-1445
FC Loonstra  https://orcid.org/0000-0002-1644-4410
https://orcid.org/0000-0002-1644-4410
EME Coerver  https://orcid.org/0000-0001-5673-9996
https://orcid.org/0000-0001-5673-9996
J Twose  https://orcid.org/0000-0001-8499-0256
https://orcid.org/0000-0001-8499-0256
Frederik Barkhof  https://orcid.org/0000-0003-3543-3706
https://orcid.org/0000-0003-3543-3706
Supplemental material: Supplemental material for this article is available online.
Contributor Information
KH Lam, Department of Neurology, Amsterdam University Medical Centers, Vrije Universiteit Amsterdam, Amsterdam Neuroscience, MS Center Amsterdam, Amsterdam, The Netherlands.
KA Meijer, Neurocast B.V., Amsterdam, The Netherlands.
FC Loonstra, Department of Neurology, Amsterdam University Medical Centers, Vrije Universiteit Amsterdam, Amsterdam Neuroscience, MS Center Amsterdam, Amsterdam, The Netherlands.
EME Coerver, Department of Neurology, Amsterdam University Medical Centers, Vrije Universiteit Amsterdam, Amsterdam Neuroscience, MS Center Amsterdam, Amsterdam, The Netherlands.
J Twose, Neurocast B.V., Amsterdam, The Netherlands.
E Redeman, Neurocast B.V., Amsterdam, The Netherlands.
B Moraal, Department of Radiology and Nuclear Medicine, Amsterdam University Medical Centers, Vrije Universiteit Amsterdam, Amsterdam Neuroscience, MS Center Amsterdam, Amsterdam, The Netherlands.
F Barkhof, Department of Radiology and Nuclear Medicine, Amsterdam University Medical Centers, Vrije Universiteit Amsterdam, Amsterdam Neuroscience, MS Center Amsterdam, Amsterdam, The Netherlands/Queen Square Institute of Neurology and Centre for Medical Image Computing, University College London, London, UK.
V de Groot, Department of Rehabilitation Medicine, Amsterdam University Medical Centers, Vrije Universiteit Amsterdam, MS Center Amsterdam, Amsterdam Neuroscience, Amsterdam, The Netherlands.
BMJ Uitdehaag, Department of Neurology, Amsterdam University Medical Centers, Vrije Universiteit Amsterdam, Amsterdam Neuroscience, MS Center Amsterdam, Amsterdam, The Netherlands.
J Killestein, Department of Neurology, Amsterdam University Medical Centers, Vrije Universiteit Amsterdam, Amsterdam Neuroscience, MS Center Amsterdam, Amsterdam, The Netherlands.
References
- 1.Compston A, Coles A.Multiple sclerosis. Lancet 2008; 372: 1502–1517. [DOI] [PubMed] [Google Scholar]
- 2.Fisk JD, Pontefract A, Ritvo PG, et al. The impact of fatigue on patients with multiple sclerosis. Canadian J Neurol Sci (Le Journal Canadien Des Sciences Neurologiques) 1994; 21: 9–14. [PubMed] [Google Scholar]
- 3.Min M, Spelman T, Lugaresi A, et al. Silent lesions on MRI imaging – Shifting goal posts for treatment decisions in multiple sclerosis. Mult Scler 2018; 24(12): 1569–1577. [DOI] [PubMed] [Google Scholar]
- 4.Uitdehaag BMJ. Disability outcome measures in phase III clinical trials in multiple sclerosis. CNS Drugs 2018; 32(6): 543–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tur C, Moccia M, Barkhof F, et al. Assessing treatment outcomes in multiple sclerosis trials and in the clinical setting. Nat Rev Neurol 2018; 14(2): 75–93. [DOI] [PubMed] [Google Scholar]
- 6.Majumder S, Mondal T, Deen MJ.Wearable sensors for remote health monitoring. Sensors 2017; 17: 130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mosa AS, Yoo I, Sheets L.A systematic review of healthcare applications for smartphones. BMC Med Inform Decis Mak 2012; 12: 67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Griffin N, Kehoe M.A questionnaire study to explore the views of people with multiple sclerosis of using smartphone technology for health care purposes. Disabil Rehabil 2018; 40(12): 1434–1442. [DOI] [PubMed] [Google Scholar]
- 9.Einarsson U, Gottberg K, von Koch L, et al. Cognitive and motor function in people with multiple sclerosis in Stockholm County. Mult Scler 2006; 12(3): 340–353. [DOI] [PubMed] [Google Scholar]
- 10.Obaidat MS, Sadoun B.Verification of computer users using keystroke dynamics. IEEE T Syst Man Cybern B Cybern 1997; 27(2): 261–269. [DOI] [PubMed] [Google Scholar]
- 11.Brizan DG, Goodkind A, Koch P, et al. Utilizing linguistically enhanced keystroke dynamics to predict typist cognition and demographics. Int J Human Comput Stud 2015; 82: 57–68. [Google Scholar]
- 12.Thompson AJ, Banwell BL, Barkhof F, et al. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol 2018; 17: 162–173. [DOI] [PubMed] [Google Scholar]
- 13.Kurtzke JF.Rating neurologic impairment in multiple sclerosis: An expanded disability status scale (EDSS). Neurology 1983; 33(11): 1444–1452. [DOI] [PubMed] [Google Scholar]
- 14.Feys P, Lamers I, Francis G, et al. The nine-hole peg test as a manual dexterity performance measure for multiple sclerosis. Mult Scler 2017; 23(5): 711–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Benedict RH, DeLuca J, Phillips G, et al. Validity of the symbol digit modalities test as a cognition performance outcome measure for multiple sclerosis. Mult Scler 2017; 23(5): 721–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Krupp LB, LaRocca NG, Muir-Nash J, et al. The fatigue severity scale: Application to patients with multiple sclerosis and systemic lupus erythematosus. Arch Neurol 1989; 46(10): 1121–1123. [DOI] [PubMed] [Google Scholar]
- 17.Vercoulen JH, Hommes OR, Swanink CM, et al. The measurement of fatigue in patients with multiple sclerosis: A multidimensional comparison with patients with chronic fatigue syndrome and healthy subjects. Arch Neurol 1996; 53(7): 642–649. [DOI] [PubMed] [Google Scholar]
- 18.Worm-Smeitink M, Gielissen M, Bloot L, et al. The assessment of fatigue: Psychometric qualities and norms for the checklist individual strength. J Psychosom Res 2017; 98: 40–46. [DOI] [PubMed] [Google Scholar]
- 19.Sakia RM.The box-cox transformation technique: A review. J Royal Stat Soc Ser D 1992; 41: 169–178. [Google Scholar]
- 20.Koo TK, Li MY.A guideline of selecting and reporting intraclass correlation coefficients for reliability research. J Chiropractic Med 2016; 15: 155–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.de Vet HCW, Terwee CB, Mokkink LB, et al. Measurement in medicine: A practical guide. Cambridge: Cambridge University Press, 2011. [Google Scholar]
- 22.Bland JM, Altman DG.Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1986; 1: 307–310. [PubMed] [Google Scholar]
- 23.Roach KE.Measurement of health outcomes: Reliability, validity and responsiveness. J Prosthet Orthotic 2006; 18: P8–P12. [Google Scholar]
- 24.Kahneman D, Fredrickson BL, Schreiber CA, et al. When more pain is preferred to less: Adding a better end. Psychol Sci 1993; 4: 401–405. [Google Scholar]
- 25.Romano JP, Wolf M.Efficient computation of adjusted p-values for resampling-based stepdown multiple testing. Stat Probab Lett 2016; 113: 38–40. [Google Scholar]
- 26.Benedict RH, Holtzer R, Motl RW, et al. Upper and lower extremity motor function and cognitive impairment in multiple sclerosis. J Int Neuropsychol Soc 2011; 17(4): 643–653. [DOI] [PubMed] [Google Scholar]
- 27.Londral A, Pinto S, de Carvalho M.Markers for upper limb dysfunction in amyotrophic lateral sclerosis using analysis of typing activity. Clin Neurophysiol 2016; 127(1): 925–931. [DOI] [PubMed] [Google Scholar]
- 28.Dagum P.Digital biomarkers of cognitive function. NPJ Digit Med 2018; 1: 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Baus C, Strijkers K, Costa A.When does word frequency influence written production. Front Psychol 2013; 4: 963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Heine M, van den Akker LE, Blikman L, et al. Real-time assessment of fatigue in patients with multiple sclerosis: How does it relate to commonly used self-report fatigue questionnaires. Arch Phys Med Rehabil 2016; 97(11): 1887–1894. [DOI] [PubMed] [Google Scholar]
- 31.Stone AA, Schwartz JE, Broderick JE, et al. Variability of momentary pain predicts recall of weekly pain: A consequence of the peak (or salience) memory heuristic. Pers Soc Psychol Bull 2005; 31(10): 1340–1346. [DOI] [PubMed] [Google Scholar]
- 32.Loy BD, Taylor RL, Fling BW, et al. Relationship between perceived fatigue and performance fatigability in people with multiple sclerosis: A systematic review and meta-analysis. J Psychosom Res 2017; 100: 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Iakovakis D, Hadjidimitriou S, Charisis V, et al. Touchscreen typing-pattern analysis for detecting fine motor skills decline in early-stage Parkinson’s disease. Sci Rep 2018; 8: 7663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zulueta J, Piscitello A, Rasic M, et al. Predicting mood disturbance severity with mobile phone keystroke metadata: A biaffect digital phenotyping study. J Med Intern Res 2018; 20: e241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Morris M, Intille SS, Beaudin JS.Embedded assessment: Overcoming barriers to early detection with pervasive computing. Berlin: Springer, 2005, pp. 333–346. [Google Scholar]
- 36.Brichetto G.We should monitor our patients with wearable technology instead of neurological examination: Commentary. Mult Scler 2020; 26(9): 1028–1030. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, MSJ968797_supplementary_figure for Real-world keystroke dynamics are a potentially valid biomarker for clinical disability in multiple sclerosis by KH Lam, KA Meijer, FC Loonstra, EME Coerver, J Twose, E Redeman, B Moraal, F Barkhof, V de Groot, BMJ Uitdehaag and J Killestein in Multiple Sclerosis Journal