Abstract
Aberrant C-C motif chemokine ligand 5 (CCL5) is associated with disease progression, poor prognosis and chemotherapy resistance in human malignancy. The tumor microenvironment (TME) contributes to chemotherapy resistance. However, the role of cancer-associated fibroblasts (CAFs)-derived CCL5 is not well documented. Hence, the present study aimed to investigate the effects of CAFs on chemotherapy resistance in A549 non-small cell lung cancer (NSCLC) cells and the underlying mechanism. Primary CAFs isolated from patients with NSCLC were found to express and secrete elevated levels of CCL5, which attenuated cisplatin (DDP)-induced apoptosis, as indicated by flow cytometry analysis. In addition, CCL5 upregulated the expression levels of long non-coding RNA (lncRNA) HOX transcript antisense RNA (HOTAIR) in the tumor cells, and silencing HOTAIR in tumor cells enhanced the cytotoxic effect of cisplatin, characterized by decreased cell viability and increased apoptotic rate. Mechanistically, HOTAIR was found to inactivate the caspase-3/BCL-2 signaling pathway in A549 NSCLC cells. Collectively, the current study demonstrated that CAFs in the TME may serve a crucial role in the higher expression levels of CCL5 in tumors and that CAF-derived CCL5 may promote cisplatin resistance via upregulating lncRNA HOTAIR expression.
Keywords: C-C motif chemokine ligand 5, tumor microenvironment, non-small cell lung cancer, HOX transcript antisense RNA
Introduction
Lung cancer remains the leading cause of tumor-associated death in the world. Globally, 2.09 million newly diagnosed cases (accounting for 11.6% of the global cancer burden) and 1.76 million deaths (18.4% of total cancer deaths) were estimated in 2018 (1). Non-small cell lung cancer (NSCLC) represents a heterogeneous group of tumors that affects >80% of all patients with lung cancer. The majority of patients with advanced lung cancer are treated with chemotherapy (2). Platinum-based drugs, especially cisplatin, are the standard first-line chemotherapy for numerous types of cancer, including NSCLC (3). However, cancer cells commonly acquire resistance against cisplatin treatment resulting in high recurrence rates (4–6). Thus, there is a need to clarify the molecular mechanisms involved in cisplatin resistance to develop more effective chemotherapy for NSCLC and other types of cancer.
Regulated upon Activation Normal T cell Expressed and Secreted (RANTES), also known as C-C motif chemokine ligand 5 (CCL5), which is now widely recognized as an inflammatory chemokine, modulates cancer cell migration, metastasis and chemotherapy resistance in human malignancy (7,8). Increasing evidence has demonstrated that altered CCL5 expression is associated with disease progression, aggressiveness, survival, prognosis and cisplatin resistance in patients with melanoma, breast, lung and pancreatic cancer (9–11). The CCL5/C-C motif chemokine receptor 5 (CCR5) axis promotes tumor progression through a variety of mechanisms: i) CCL5 promotes tumor growth in an autocrine or paracrine manner (12); ii) CCL5 facilitates cancer cell migration and invasion by remodeling the tumor microenvironment (TME) through collagen degradation, integrin activation and actin polarization (13); iii) CCL5 enhances chemotherapy resistance of cancer cells by increasing repair responses to DNA damage (14); and iv) CCL5/CCR5 interaction promotes cancer stem cell expansion (15). Additionally, CCL5 facilitates cisplatin (DDP) resistance of cancer cells in various human malignancies, including cervical, breast and oral cancer (10,16,17). CCL5 can be produced and secreted by tumors themselves or stromal cells in the TME (15).
Tumor progression depends on tumor-stromal crosstalk (18). Cancer-associated fibroblasts (CAFs) are one of the vital components in the TME. CAFs can remodel and reprogram the tumoral extracellular matrix (ECM) to facilitate the growth, metastasis and invasion of the neoplastic lesion, generating a permissive niche for the invasive cancer cells (19). CAFs secrete elevated levels of ECM proteins, such as fibronectin and type I collagen, express higher level of caveolin-1, which can remodel fibronectin matrix, and increase the expression and activation of matrix metallopeptidases (20). These processes contribute to remodeling the ECM biochemically and mechanically. Among all CAF functions, the current study focused on CAFs as important regulators of chemotherapy resistance in NSCLC.
Long non-coding RNAs (lncRNAs) are described as mRNA-like, non-protein coding RNA >200 nucleotides in length (21). lncRNAs regulate diverse processes, including chromatin dynamics, genomic reprogramming (22), gene imprinting (23), transcription and post-transcriptional processing (24,25). Increasing evidence has revealed that lncRNAs serve a crucial role in the processes of tumor cell proliferation, differentiation, apoptosis and metastasis (26,27). HOX transcript antisense RNA (HOTAIR) is one of the few most deeply-studied lncRNAs (28,29). HOTAIR is aberrantly expressed in numerous types of cancer, including lung cancer, and has been recognized as an oncogene in cancer development (28). Higher HOTAIR expression has been associated with tumor metastasis and poor prognosis in lung cancer (30–32). Recently, HOTAIR has been reported to be involved in cisplatin resistance of cancer cells (33,34). However, it is currently unclear whether HOTAIR serves a part in cisplatin resistance in NSCLC and its respective mechanism.
The present study aimed to investigate whether CAFs isolated from patients with NSCLC created a supportive TME that affected the sensitivity of NSCLC cells to cisplatin.
Materials and methods
Isolation of primary fibroblasts
The present study was approved by the medical ethical committee of Hanchuan People's Hospital (Hanchuan, China). Written informed consent was obtained from patients. Human CAFs were isolated from primary tumor tissues, and normal fibroblasts (NFs) were isolated from adjacent normal tissues (5 cm away from the tumor tissues), respectively, from two patients with NSCLC at pT2N0M0 (female, aged 61 years) and pT1N0M0 (male, aged 63 years) stage undergoing surgical resection at Hanchuan People's Hospital between February 2019 and October 2019 with informed consent from the patients. Primary fibroblasts were isolated within 2 h after excision according to a previously published study (35). Briefly, the fresh tissues were cut into small pieces ~1×1×1 mm in size and were digested with 1.5 mg/ml collagenase IV (Roche Applied Science) and 20 mg/ml trypsin (Sigma-Aldrich; Merck KGaA) at 37°C for 1 h. Subsequently, the mixture was filtered through a 40-µm mesh (Falcon; Corning Life Sciences) and the cells were maintained in DMEM/F12 medium containing 10% FBS (both Gibco; Thermo Fisher Scientific, Inc.), 100 U/ml penicillin and 100 µg/ml streptomycin at 37°C in 5% CO2. The first passage was conducted when cells reached ~80% confluency. The cells (after 2–3 passages) were analyzed by immunofluorescence, quantitative (q)PCR and western blotting for α-smooth muscle actin (α-SMA), fibroblast activation protein (FAP) and fibroblast specific protein 1 (FSP1), which are highly expressed in fibroblasts, to confirm the homology of fibroblasts.
To prepare conditioned medium of CAFs (CAF-CM) and NFs (NF-CM) for subsequent in vitro experiments, CAFs and NFs at a density of 2×105 were plated into a 25-cm2 culture flask in 5 ml RPMI-1640 medium (Gibco; Thermo Fisher Scientific, Inc.) supplemented with 10% FBS at 37°C in 5% CO2 and cultured for 24 h. Subsequently, the medium was replaced with RPMI-1640 with 0.5% FBS for another 24 h, after which the culture medium was collected and centrifuged at 3,000 × g at 4°C for 10 min. The supernatant was collected as CM and stored at −80°C until further use.
Cell lines and cultivation
NSCLC A549 (lung adenocarcinoma) and H1299 (lung large cell carcinoma) cell lines were purchased from the China Center for Type Culture Collection, and cultivated in RPMI-1640 medium supplemented with 10% FBS, 100 U/ml penicillin and 100 µg/ml streptomycin in a humidified incubator with 5% CO2 at 37°C. Cells in the logarithmic growth phase were used for all experiments.
For cell treatment, cancer cells were incubated with CAF-CM or NF-CM in combination with either anti-CCL5 antibody (0.1 µg/ml; cat. no. MAB678-SP; R&D Systems, Inc.), CCR5 antagonist (Met-RANTES; 0.1 µg/ml; cat. no. 335-RM-025; R&D Systems, Inc.) or recombinant human CCL5 (3 ng/ml; cat. no. 300-06; PeproTech, Inc.) for 6 h, followed by treatment with 50 µM DDP (Sigma-Aldrich; Merck KGaA) in the presence of CM for another 48 h.
Cell transfection
The small interfering RNA (siRNA) against HOTAIR (siHOTAIR) and non-targeting control siRNA (siNC) were purchased from Shanghai GenePharma Co., Ltd.. The sequences of the two siRNAs were as follows: siHOTAIR forward, 5′-AUUGAUUAGCUGUUUGUUCCC-3′ and reverse, 5′-AAAGUCUAGACAAUAGAUGGC-3′; siNC forward, 5′-CUAUUGUCUAGACUUUUAUCU-3′ and reverse, 5′-GAAAUCUGGUACAAAGGAAAG-3′. Cells were seeded into 6-well plates to 40–60% confluence and then transfected with siHOTAIR or siNC at a concentration of 60 nM using Lipofectamine 2000® reagent (Invitrogen; Thermo Fisher Scientific, Inc.) and Opti-MEM I Reduced Serum Medium (Thermo Fisher Scientific, Inc.) following the manufacturer's protocol. At 36 h after transfection, cells were collected for subsequent experiments.
Cell viability analysis
Cell viability was determined using the MTT assay. Briefly, A549 or H1299 cells were grown in 96-well plates at a density of 5×103 cells/well and treated with 50 µM DDP for 48 h. Subsequently, 20 µl MTT (5 µg/µl) was added to each well at 37°C for 4 h. The reaction was stopped by the addition of DMSO, and the optical density (OD) was detected at 490 nm by Multiscan Spectrum (Bio-Tek Instruments, Inc.; Agilent Technologies, Inc.). All experiments were repeated three times, and the average OD for each experiment was calculated.
Analysis of apoptosis by flow cytometry
Apoptosis was determined using an Annexin V-FITC/PI apoptosis detection kit (BD Pharmingen; BD Biosciences) according to the manufacturer's protocol. Briefly, 6×105 cells were seeded into 6-well plates and incubated with DDP (50 µM) for 24 h. Subsequently, cells were harvested and incubated with FITC-Annexin V and propidium iodide (PI) at room temperature for 15 min in the dark. The apoptotic rate was analyzed using BD FACScan flow cytometer (Becton-Dickinson and Company). The data were analyzed using the CellQuest software (version 5.1; Becton-Dickinson and Company) Cells positive for Annexin V-FITC alone (early apoptosis) and Annexin V-FITC/PI (late apoptosis) were calculated. All samples were examined in triplicate.
ELISA
The quantity of CCL5 in CM of CAFs, NFs, A549 and H1299 cells was determined using a commercial ELISA kit. Briefly, ~1×106 cells in 3 ml serum-free RPMI 1640 medium were seeded in a 25-cm2 flask for 48 h. Subsequently, CM was collected and CCL5 quantity was assessed using human RANTES ELISA Kit (CCL5) following the manufacturer's instructions (cat. no. ab174446; Abcam). CCL5 quantities were normalized to the cell number.
Western blotting
The cells were lysed with RIPA buffer containing 1 mM PMSF and protease inhibitor cocktail according to the manufacturer's instructions (Beyotime Institute of Biotechnology). Protein concentrations were quantified using a BCA kit (Beyotime Institute of Biotechnology). Total protein (30 µg/lane) was separated by 10% SDS-PAGE and transferred to polyvinylidene difluoride membranes. After blocking with 5% skimmed milk in TBS containing 0.5% Tween-20 (TBST) for 1 h at room temperature, the membranes were incubated with the following primary antibodies overnight at 4°C: β-actin (1:1,000; cat. no. ab8226), E-cadherin (1:5,000; cat. no. ab40772), Vimentin (1:1,000; cat. no. ab92547), FAP (1:500; cat. no. ab53066), FSP1 (1:1,000; cat. no. ab197896) (all Abcam), α-SMA (1:1,000; cat. no. 19245), Bcl-2 (1:1,000; cat. no. 15071), cleaved-poly (ADP-ribose) polymerase (PARP; 1:1,000; cat. no. 5625), PARP (1:1,000, cat. no. 9532), cleaved-caspase-3 (1:1,000, cat. no. 9661) and caspase-3 (1:1,000; cat. no. 9662) (all Cell Signaling Technology, Inc.). After washing three times for 5 min each with TBST, the membranes were incubated with the appropriate HRP-conjugated secondary antibodies (1:2,000; cat. nos. 7076 and 7074, Cell Signaling Technology, Inc.) for 1 h at room temperature. The signal was visualized using chemiluminescent reagents according to the manufacturer's protocols (MilliporeSigma), and the protein bands were quantified using ImageJ software (Version 1.51; National Institutes of Health).
Reverse transcription (RT)-qPCR
For the RNA expression analysis, TRIzol® (Invitrogen; Thermo Fisher Scientific, Inc.) was used to extract total RNA in A549 cells, and the RNA level of each group was examined. Subsequently, 5 µg RNA was reverse transcribed to cDNA using an RT kit (Promega Corporation) following the manufacturer's protocol. qPCR was performed with the GoTaq qPCR Master Mix (Promega Corporation) on an Mx3005P Real-Time PCR System (Stratagene; Agilent Technologies, Inc.). The thermocycling conditions were as follows: 95°C for 10 min, followed by 40 cycles of 95°C for 15 sec, 60°C for 30 sec and 72°C for 30 sec. The primer sequences were as follows: HOTAIR forward, 5′-GATCAGATGCCTGGGTCGAA-3′ and reverse, 5′-AATGATTCTTGCTGGGGGCA-3′; α-SMA forward, 5-TGCCTGCTCTCTGATGTTGG-3 and reverse, 5-GCTACCGAGCCCTGAGTTAC-3; FAP forward, 5-ATGAGCTTCCTCGTCCAATTCA-3 and reverse, 5-AGACCACCAGAGAGCATATTTTG-3; FSP1 forward, 5-GATGAGCAACTTGGACAGCAA-3 and reverse, 5-CTGGGCTGCTTATCTGGGAAG-3; CCL5 forward, 5-CCAGCAGTCGTCTTTGTCAC-3 and reverse, 5-CTCTGGGTTGGCACACACTT-3; CCR5 forward, 5-TTCTGGGCTCCCTACAACATT-3 and reverse, 5-TTGGTCCAACCTGTTAGAGCTA-3 and GAPDH forward, 5′-GGAGCGAGATCCCTCCAAAAT-3′ and reverse, 5′-GGCTGTTGTCATACTTCTCATGG-3′. Each experiment was performed at least three times. The gene expression levels were calculated using the 2−ΔΔCq method (36) GAPDH was used as an internal reference.
Immunofluorescence staining
Cells were washed three times in cold PBS, and then fixed with 4% paraformaldehyde for 10 min at room temperature. After permeabilization with PBS supplemented with 0.25% of Triton X-100 for 10 min at room temperature, the cells were washed three times in PBS and blocked with 1% BSA in PBS for 60 min at room temperature. The following primary antibodies were used for immunofluorescence staining, overnight at 4°C: anti-E-cadherin (1:100, cat. no. 14472), anti-Vimentin (1:100, cat. no. 5741) and anti-α-SMA (1:200, cat. no. 48938) (all from Cell Signaling Technology, Inc.). The cells were washed again with PBS and incubated with Alexa Fluor® 488- or Alexa Fluor® 594-conjugated secondary antibodies (1:500, cat. no. 4408, and 1:500 cat. no. 8890 (respectively), Cell Signaling Technology, Inc.). DAPI (1:1,000, Beyotime Institute of Biotechnology) was used for counterstaining. The images were captured by a fluorescence microscope (Olympus Corporation).
Statistical analysis
Each experiment was performed at least three times independently. All data were presented as the mean ± SD. SPSS 20.0 software (IBM Corp.) was used for data analysis. Comparisons between two groups were performed using unpaired Student's t-test, and one-way ANOVA followed by Bonferroni's post-hoc test was used to examine differences among ≥3 groups. P<0.05 was considered to indicate a statistically significant difference.
Results
Characterization of primary NFs and CAFs
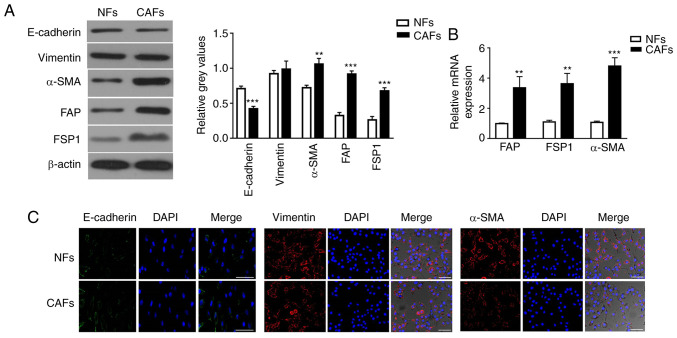
Human CAFs were isolated from primary tumor tissues, and normal fibroblasts (NFs) were isolated from adjacent normal tissues, respectively, from two patients with NSCLC. However, the specimen from the patient at stage pT1N0M0 was contaminated due to improper operation during isolation of the NFs and CAFs. Therefore, the NFs and CAFs used in subsequent experiments were all from the other patient at stage pT2N0M0. The mesenchymal marker vimentin and the epithelial marker E-cadherin were used to identify homogeneity of isolated cells. Isolated CAFs highly expressed vimentin, but expressed lower levels of E-cadherin compared with NFs, as measured by western blot analysis (Fig. 1A). Additionally, the expression levels of α-SMA, FAP and FSP1 in CAFs were 1.47-, 2.84- and 2.60-fold higher than those in NFs (Fig. 1A). It is commonly agreed that CAFs are activated fibroblasts that express mesenchyme-specific proteins α-SMA, FAP and FSP1 (37,38). RT-qPCR analysis confirmed that the expression levels of α-SMA, FAP and FSP1 were significantly higher in CAFs than in NFs (Fig. 1B). Furthermore, immunocytochemistry staining confirmed that CAFs and NFs both expressed vimentin, and that α-SMA expression was higher in CAFs than in NFs (Fig. 1C).
Figure 1.
Characterization of primary isolated and cultivated NFs and CAFs. (A) Protein expression levels of E-cadherin, vimentin, α-SMA, FAP and FSP1 in NFs and CAFs were detected by western blotting. The intensity of each experimental band was normalized to that of the loading control (β-actin). Each bar represents the mean ± SD of three relative intensity arbitrary units. (B) mRNA expression levels of CAF-specific genes, including α-SMA, FAP and FSP1, in NFs and CAFs were detected by reverse transcription-quantitative PCR using GAPDH gene as the internal control. Results were expressed as the mean ± SD, and the means were calculated from ≥3 independent experiments. (C) Immunofluorescence staining showed the subcellular location and the expression of E-cadherin, vimentin and α-SMA in NFs and CAFs. Scale bar, 10 µm. **P<0.01 and ***P<0.001 vs. NFs. CAF, cancer-associated fibroblast; NF, normal fibroblast; α-SMA, α-smooth muscle actin; FAP, fibroblast activation protein; FSP1, fibroblast specific protein 1.
CAF-CM promotes NSCLC cell resistance to cisplatin
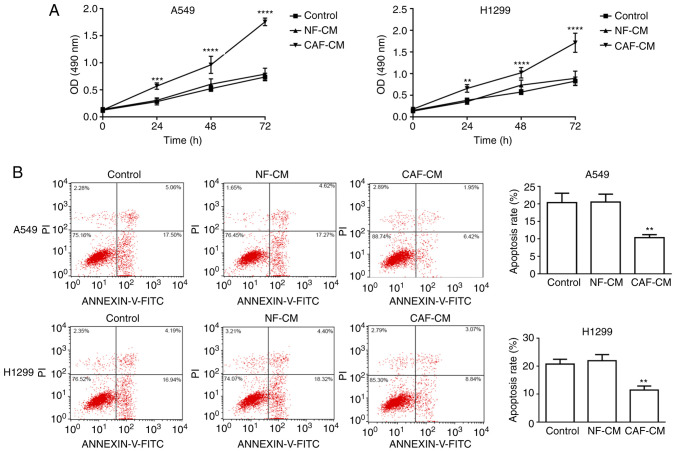
Considering the crucial role of the tumor stroma on tumor growth and metastasis, the present study assessed whether factors secreted by CAFs could affect the viability and apoptosis of A549 cells. First, the role of CAF-CM on the viability and apoptosis of A549 cells was analyzed. A549 and H1299 cells were pre-incubated with CAF-CM or NF-CM for 24 h, and then treated with DDP in the presence of CM for another 48 h. MTT assay revealed that A549 and H1299 cells exhibited significantly higher cell viability with CAF-CM treatment than with NF-CM treatment (Fig. 2A). To demonstrate the effect of CAF-CM on apoptosis, A549 and H1299 cells were pre-treated with CAF-CM or NF-CM for 24 h before the addition of DDP in the presence of CM for another 48 h, and the percentage of apoptotic cells was analyzed using Annexin V-FITC/PI apoptosis staining. The apoptotic rates of A549 cells treated with control, NF-CM and CAF-CM were 20.35±2.71, 20.52±2.27 and 10.35±0.85%, respectively, while the apoptotic rates of H1299 cells treated with control, NF-CM and CAF-CM were 20.71±1.72, 21.93±2.19 and 11.43±1.48%, respectively, revealing that apoptosis was significantly decreased by CAF-CM (Fig. 2B). Overall, these data indicated that CAF-CM attenuated the pro-apoptotic effect induced by chemotherapy in NSCLC cells.
Figure 2.
CAF-CM promotes non-small cell lung cancer cell resistance to cisplatin. (A) MTT assay was conducted to detect cell viability at 0, 24, 48 and 72 h in A549 and H1299 cells pre-treated with CAF-CM or NF-CM for 24 h before the treatment of DDP. (B) Flow cytometry analysis was used to assess apoptosis of A549 and H1299 cells pre-treated with CAF-CM or NF-CM for 24 h before the treatment of DDP. Results were expressed as the mean ± SD, and the means were calculated from ≥3 independent experiments. **P<0.01, ***P<0.001 and ****P<0.0001 vs. control. CAF, cancer-associated fibroblast; NF, normal fibroblast; CM, conditioned medium; DDP, cisplatin; OD, optical density.
Paracrine effect of CCL5 in CAF-CM
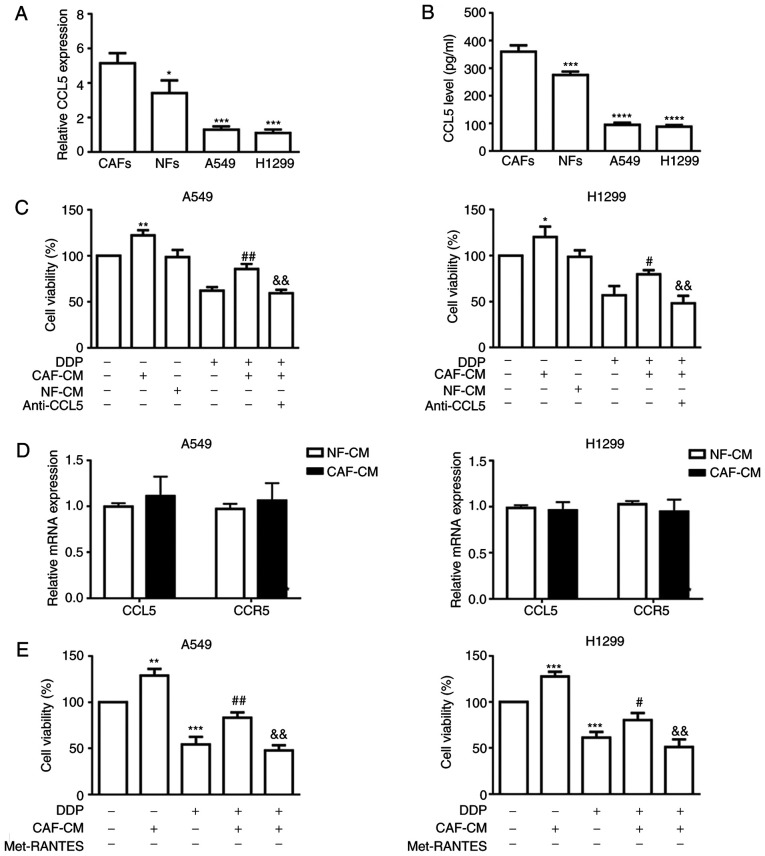
In order to determine whether CAF-secreted CCL5 may induce cisplatin resistance in NSCLC cells, RT-qPCR was performed to assess CCL5 mRNA expression in NFs, CAFs and the two NSCLC cell lines, A549 and H1299, revealing that CCL5 expression was significantly higher in CAFs compared with in all other tested cells (Fig. 3A). Additionally, ELISA results confirmed significantly higher levels of CCL5 in CAF-CM compared with in NF-CM (359.8±23.47 vs. 276±11.77 pg/ml, respectively) and in A549 and H1299 cells (Fig. 3B). In addition, incubation with CAF-CM induced resistance to DDP in NSCLC cells, which was blocked by the anti-CCL5 antibody (Fig. 3C).
Figure 3.
CAFs secrete elevated CCL5 to promote cell viability in NSCLC cancer cells via paracrine activation. (A) mRNA expression levels of CCL5 in CAFs, NFs and two NSCLC cell lines by RT-qPCR using GAPDH gene as the internal control. (B) CCL5 in conditioned medium secreted by NFs and CAFs were quantified by ELISA. *P<0.05, ***P<0.001 and ****P<0.0001 vs. CAFs. (C) A549 and H1299 cancer cells were incubated with CAF-CM or NF-CM in combination with anti-CCL5 antibody (0.1 µg/ml) for 6 h followed by treatment with DDP for 48 h. Cell viability was determined by the MTT assay. (D) mRNA expression levels of CCL5 and CCR5 in NSCLC cell lines cultured with CAF-CM were assessed by RT-qPCR using GAPDH gene as the normalization control. (E) A549 and H1299 cancer cells were incubated with CAF-CM or NF-CM in combination with CCR5 inhibitor (Met-RANTES; 0.1 µg/ml) for 6 h followed by treatment with DDP for 48 h. Cell viability was determined by the MTT assay. *P<0.05, **P<0.01 and ***P<0.001 vs. control; #P<0.05 and ##P<0.01 vs. DDP; &&P<0.01 vs. CAF-CM and DDP. Results were expressed as the mean ± SD, and the means were calculated from ≥3 independent experiments. RT-qPCR, reverse transcription-quantitative PCR; CAF, cancer-associated fibroblast; NF, normal fibroblast; CM, conditioned medium; DDP, cisplatin; CCL5, C-C motif chemokine ligand 5; CCR5, C-C motif chemokine receptor 5; NSCLC, non-small cell lung cancer.
To estimate whether the activation of CCL5 signaling in NSCLC cells was mainly caused by paracrine action of CCL5 secreted by CAFs, the expression levels of CCL5 and CCR5 in A549 and H1299 cells, in the presence or absence of CAF-CM, were examined by RT-qPCR. As shown in Fig. 3D, neither CCL5 nor CCR5 expression was markedly altered in the CAF-CM group compared with in the NF-CM group, indicating that CCL5 pathway activation triggered by CAF-CM was not the direct consequence of autocrine or reverse-paracrine mechanisms. Paracrine activation of CCL5 by CAF-CM was further verified by the CCL5 blocking assays using a neutralizing anti-CCL5 antibody or a CCR5 antagonist (Met-RANTES). Cell viability results indicated that either blockade of CCL5 binding to its cognate receptor (Fig. 3C) or inhibition of CCR5 (Fig. 3E) alleviated the CAF-CM-induced cisplatin resistance, indicating that CCL5 alone may be sufficient to facilitate cisplatin resistance in NSCLC cell lines.
CAF-CM-derived CCL5 suppresses DDP sensitivity of NSCLC cells partially by upregulating lncRNA HOTAIR expression
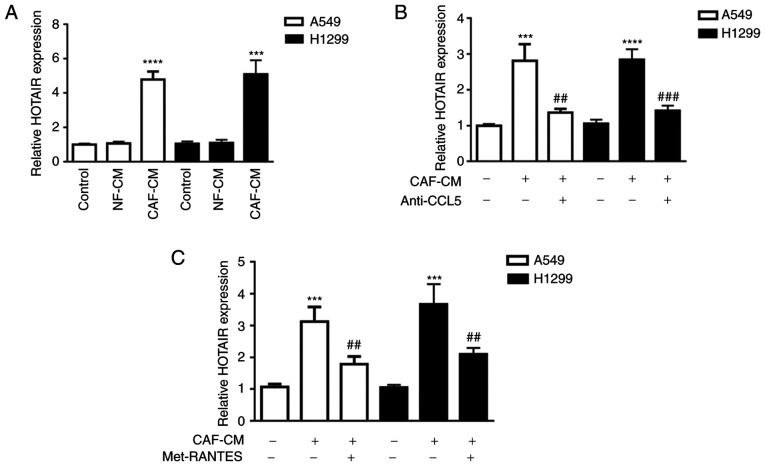
Previous data have shown that HOTAIR is expression is markedly increased in human DDP-resistant NSCLC tissues and cells. Silencing of HOTAIR partially reverses the acquired cisplatin resistance in DDP-resistant NSCLC cells, both in vitro and in vivo (39). Thus, it was hypothesized that CCL5 may induce tumor cell resistance to cisplatin via regulation of lncRNA HOTAIR expression. To verify this, the expression levels of lncRNA HOTAIR in A549 and H1299 cell lines were examined by RT-qPCR. As shown in Fig. 4A, HOTAIR expression in tumor cells was significantly increased when cells were incubated with CAF-CM compared with NF-CM stimulation. Additionally, anti-CCL5 antibody treatment significantly decreased HOTAIR expression by 2.06 and 2.00-fold in A549 and H1299 cells, respectively, compared with CAF-CM treatment alone (Fig. 4B). Similarly, the CCR5 antagonist (Met-RANTES) significantly downregulated HOTAIR expression by 1.74 and 1.75-fold in A549 and H1299 cells, respectively, compared with CAF-CM treatment alone (Fig. 4C). These results demonstrated that CCL5 was at least partially involved in promoting lncRNA HOTAIR expression in both A549 and H1299 cells.
Figure 4.
CAF-CM increases HOTAIR expression in tumor cells in vitro. (A) HOTAIR expression in human NSCLC A549 and H1299 cell lines treated with CAF-CM or NF-CM was determined by qPCR. HOTAIR expression in human NSCLC A549 and H1299 cell lines treated with CAF-CM and (B) CCL5 neutralizing antibody or (C) CCR5 inhibitor (Met-RANTES) was determined by qPCR. ***P<0.001 and ****P<0.0001 vs. control; ##P<0.01 and ###P<0.001 vs. CAF-CM. Results were expressed as the mean ± SD, and the means were calculated from ≥3 independent experiments. qPCR, quantitative PCR; CAF, cancer-associated fibroblast; NF, normal fibroblast; CM, conditioned medium; CCL5, C-C motif chemokine ligand 5; CCR5, C-C motif chemokine receptor 5; NSCLC, non-small cell lung cancer; HOTAIR, HOX transcript antisense RNA.
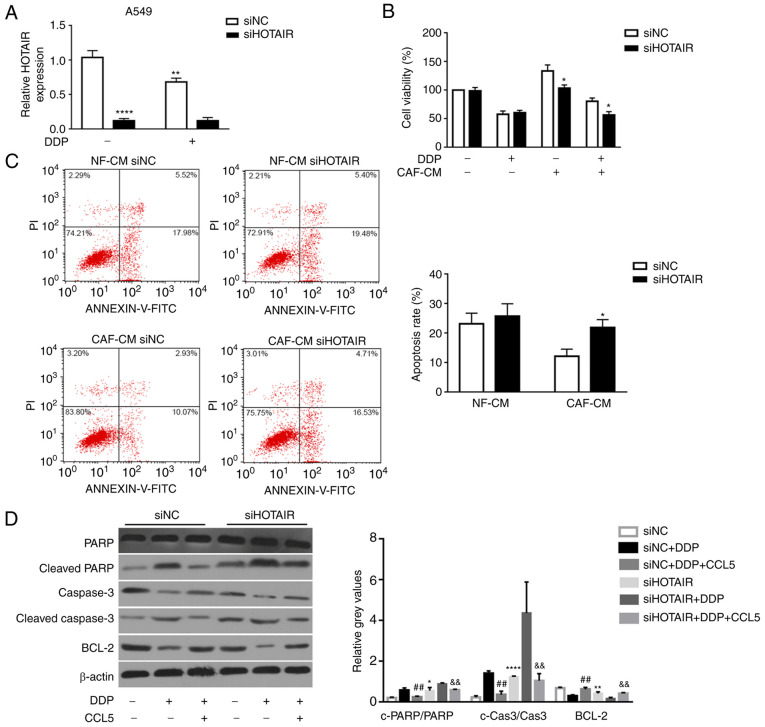
Recently, lncRNA HOTAIR has been found to serve a role in tumor cell proliferation, migration and invasion (30,40,41). Moreover, based on the association of increased lncRNA HOTAIR expression with NSCLC advanced pathological stage, lymph-node metastasis and poor prognosis (42), the present study hypothesized that HOTAIR may serve a role in NSCLC cell resistance to cisplatin. Therefore, its expression was silenced using RNA interference to estimate the effect of HOTAIR on tumor cell sensitivity to cisplatin. qPCR analysis revealed that HOTAIR mRNA expression was significantly downregulated in siHOTAIR-transfected A549 cells compared with in control cells transfected with siNC (Fig. 5A). Additionally, cisplatin treatment significantly decreased HOTAIR expression in siNC-transfected cells compared with siNC-transfected cells without cisplatin treatment (Fig. 5A). Furthermore, the effects of HOTAIR on NSCLC cell sensitivity to cisplatin were assessed by MTT assay. Consistent with the aforementioned hypothesis, silencing HOTAIR in A549 cells significantly sensitized these cells to cisplatin compared with tumor cells transfected with siNC (Fig. 5B). Additionally, the apoptotic rate of A549 cells transfected with siHOTAIR was significantly higher than that in A549 cells transfected with siNC with CAF-CM (21.87±2.70 vs. 12.07±2.45%, respectively), as detected by flow cytometric analysis (Fig. 5C). These results indicated that silencing of lncRNA HOTAIR inhibited NSCLC cell viability and increased apoptosis in vitro.
Figure 5.
HOTAIR-knockdown abrogates CCL5-induced cisplatin resistance via activation of caspase-3-dependent apoptosis. (A) HOTAIR expression in A549 cells transfected with either siNC or siHOTAIR was determined by quantitative PCR. (B) Cell viability and (C) apoptosis of DDP- and CAF-CM-treated A549 cells transfected with either siNC or siHOTAIR was determined by MTT and flow cytometric assays, respectively. *P<0.05, **P<0.01 and ****P<0.0001 vs. siNC. (D) Protein expression levels of PARP, cleaved-PARP, Bcl-2, cleaved caspase-3 and caspase-3 in siNC- or siHOTAIR-transfected A549 cell lines with or without treatment with exogenous CCL5 (100 ng/ml) were determined by western blotting. The intensity of each experimental band was normalized to that of the loading control (β-actin). Each bar represents the mean ± SD of three relative intensity arbitrary units. **P<0.01 vs. siNC; ##P<0.01 vs. siNC+DDP; &&P<0.01 vs. siHOTAIR+DDP. CAF, cancer-associated fibroblast; NF, normal fibroblast; CM, conditioned medium; DDP, cisplatin; CCL5, C-C motif chemokine ligand 5; HOTAIR, HOX transcript antisense RNA; si, small interfering RNA; NC, negative control; PARP, poly (ADP-ribose) polymerase.
HOTAIR-knockdown suppresses CCL5-induced cisplatin resistance through activation of the PARP-dependent apoptotic signaling pathway
Some studies (43,44) have revealed that lncRNA HOTAIR is involved in enhancing cancer cell proliferation by suppressing apoptosis (45). Therefore, the present study investigated whether silencing HOTAIR could abrogate the CCL5-induced cisplatin resistance in NSCLC cell lines through the apoptotic signaling pathway. The expression levels of apoptosis-associated proteins, including cleaved PARP and cleaved caspase-3, in siNC- or siHOTAIR-transfected NSCLC cells upon CCL5 treatment (100 ng/ml) were examined by western blotting. The results revealed that cleaved PARP and cleaved caspase-3 expression was significantly upregulated in A549 cells transfected with siHOTAIR compared with in cells with siNC transfection (Fig. 5D). Moreover, the expression levels of cleaved PARP and cleaved caspase-3 were significantly decreased in siNC transfected-A549 cells, which were pre-treated with CCL5 for 24 h before the treatment of DDP, compared with siNC transfected-A549 cells with DDP treatment alone (Fig. 5D). By contrast, the expression levels of the anti-apoptotic protein Bcl-2 were significantly upregulated in the siNC+DDP+CCL5 groups compared with that in the siNC+DDP group (Fig. 5D). Additionally, Bcl-2 expression was significantly reduced in siHOTAIR transfected-A549 cells compared with that in siNC transfected-A549 cells (Fig. 5D). These results suggested that HOTAIR-knockdown promoted NSCLC cell sensitivity to DDP via the apoptotic signaling pathway.
Overall, the current findings indicated that CAF-derived CCL5 may serve a role in tumor cells by paracrine action, upregulating lncRNA HOTAIR expression in tumor cells and thus upregulating the expression levels of the anti-apoptotic protein Bcl-2, ultimately leading to NSCLC cell resistance to DDP. Therefore, the present study may provide a novel therapeutic strategy for patients with NSCLC with acquired DDP resistance.
Discussion
Cisplatin-based chemotherapy schedule is a first-line treatment against NSCLC, but cisplatin resistance among patients with NSCLC has become increasingly severe, contributing to chemotherapy failure and high recurrence and mortality rates (46). The TME serves a crucial role in tumor progression, metastasis and recurrence. CAFs, a dominant component of the TME, serve a prominent functional role in promoting cancer initiation, progression and therapeutic resistance (47). In the present study, it was demonstrated that CAFs from primary NSCLC tumor tissues had typical myofibroblast characteristics, with high expression levels of α-SMA, FAP and FSP1 (48,49), which were very low or undetectable in NFs. Additionally, it was revealed that CAF-CM could increase the viability of the NSCLC A549 and H1299 cell lines, and inhibit the induction of apoptosis by cisplatin in these cells. However, the specimen from one patient was contaminated during isolation of the NFs and CAFs. The lack of additional CAFs/NFs cell lines to support the findings of the present study is a limitation of the present study. Further studies are necessary to isolate NFs and CAFs from more patients with NSCLC and repeat some key experiments of the present study. A previous study has indicated that the interaction between CAFs and tumor cells affects chemoresistance by inducing the secretion of survival factors (50,51). For instance, elevated secretion of IL-6 from CAFs mediates chemoresistance in NSCLC by promoting epithelial-mesenchymal transition (51). In the present study, higher levels of CCL5 were detected in the CAF-CM. However, CCL5 also has some anticancer properties, since it promotes antitumor immunity by inducing the recruitment of anticancer tumor-infiltrating lymphocytes (52), natural killer cells (53), dendritic cells (54), T helper cell type 1 and type 1 cytotoxic cells (55) to the TME, and therefore enhances the infiltration of the tumor by different types of immune cells. Usually, the elevated expression of a given chemokine can improve the outcome for one type of cancer (56), but promote the progression of other types of cancer. There is no single CC chemokine that facilitates or suppresses the progression of all types of cancer. Thus, a thorough understanding of the pro-cancer and anticancer features of individual chemokines may contribute to a prediction of the outcomes to improve the efficacies of anticancer therapies. Although the role of CCL5 in cancer has been extensively studied, to the best of our knowledge, no such studies have been conducted to date to investigate the role of CAF-secreted CCL5 in chemoresistance of NSCLC. The present study described that CAFs isolated from NSCLC tumor tissues expressed and secreted higher amounts of CCL5 than NFs, and CAF-derived CCL5 enhanced tumor cell resistance to cisplatin via paracrine activation in NSCLC A549 and H1299 cell lines. Notably, CCL5 was substantially expressed by both CAFs and NFs (at least twice higher than A549 cells). However, NF-CM did not induce HOTAIR expression in A549 or H1299 cells. A possible explanation may be that CCL5 is secreted from CAFs and transferred to cancer cells mainly via exosomes rather than via a soluble form, and CAF-derived exosomal CCL5 may be much higher than CCL5 in NF-derived exosomes; thus, NF-CM seemed to have no effects on HOTAIR expression in A549 or H1299 cells in the present study. However, this speculation needs to be confirmed by further research. Li et al (57) has observed a significant upregulation of TGF-β1 in CAF-derived exosomes compared with in normal omentum fibroblasts-derived exosomes, which subsequently activated SMAD2/3 signaling to promote an EMT phenotype in ovarian cancer cells. However, whether CAF-derived CCL5 exerts its effect on A549 or H1299 cells via exosomes requires to be further investigated. Notably, treatment with a neutralizing CCL5 antibody or CCR5 inhibitor, Met-RANTES, reversed CAF-CM-induced cancer cell chemoresistance. No significant upregulation of CCL5 or CCR5 expression was detected in cancer cells under CAF-CM treatment compared with that under NF-CM treatment, further confirming that CCL5 secreted by CAFs is necessary to induce NSCLC cancer cell chemoresistance. Another limitation of the present study is the lack of clinical evidence to confirm the influence of CCL5 expression on the survival and disease-free outcome of patients with NSCLC. Further studies are required to conduct in silico studies evaluating public datasets to evaluate the influence of CCL5 expression on the outcome of patients with NSCLC as evidence to corroborate the current findings.
Emerging evidence has revealed that numerous lncRNAs exert crucial functions in tumorigenesis, indicating that they could provide new insights into the biology of this disease. Among these lncRNAs, lncRNA HOTAIR is one of the well-studied lncRNAs in various types of cancer, including lung cancer, and is reported to be dysregulated in various types of cancer. Upregulation of HOTAIR expression has been found in cervical cancer tissues and has been associated with lymph node metastasis and decreased overall survival (58). In diffuse large B-cell lymphoma, high HOTAIR expression is positively associated with a poor prognosis (59). In breast cancer, high expression levels of HOTAIR promote cancer development and progression (60). However, the expression profile of lncRNA HOTAIR in NSCLC and its association with cisplatin resistance are not fully understood. The present study indicated that HOTAIR expression was significantly increased in two NSCLC A549 and H1299 cell lines stimulated with CAF-CM when compared with that in these cells stimulated with NF-CM. Furthermore, silencing of HOTAIR in NSCLC cells partly blocked CCL5-induced cisplatin resistance. Additionally, HOTAIR-knockdown suppressed cell viability and enhanced apoptosis. These data indicated that HOTAIR served a crucial role in cell proliferation and that its high expression was positively associated with cisplatin resistance. Numerous studies (61–63) have indicated that lncRNAs serve a crucial role in the occurrence and progression of several types of cancer, including NSCLC, acting as a reservoir for microRNAs and regulating their downstream target genes (64). It has been reported that HOTAIR targets polycomb repressive complex 2 and regulates H3K27 methylation and gene expression, which ultimately promotes tumor progression and metastasis (65,66). The present study revealed that knockdown of HOTAIR abrogated CCL5-induced cisplatin resistance in NSCLC cells by upregulating cleaved caspase-3 and cleaved PARP levels and suppressing Bcl-2 expression.
In conclusion, the current study demonstrated an association between CAF-derived CCL5, HOTAIR and chemoresistance. CAF-derived CCL5 inhibited cisplatin-induced apoptosis and induced tumor cell cisplatin resistance. Moreover, CCL5 enhanced tumor cell chemoresistance by upregulating lncRNA HOTAIR expression and resulting in increased anti-apoptotic protein Bcl-2 expression in cancer cells. The present results supported CAF-secreted CCL5 as a crucial player in cisplatin-based chemoresistance. Therefore, targeting the CAF-derived CCL5 axis may be of clinical significance for reversing cisplatin resistance in patients with NSCLC.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
XS and ZC made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data, took part in drafting the article or revising it critically for important intellectual content, and agreed to submit to the current journal. Both authors have read and approved the final manuscript, agree to be accountable for all aspects of the work and confirm the authenticity of all the raw data.
Ethics approval and consent to participate
The present study was approved by the medical ethical committee of Hanchuan People's Hospital (Hanchuan, China). Written informed consent was obtained from patients.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Bade BC, Dela Cruz CS. Lung cancer 2020: Epidemiology, etiology, and prevention. Clin Chest Med. 2020;41:1–24. doi: 10.1016/j.ccm.2019.10.001. [DOI] [PubMed] [Google Scholar]
- 2.Siegel R, DeSantis C, Virgo K, Stein K, Mariotto A, Smith T, Cooper D, Gansler T, Lerro C, Fedewa S, et al. Cancer treatment and survivorship statistics, 2012. CA Cancer J Clin. 2012;62:220–241. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 3.Rossi A, Di Maio M. Platinum-based chemotherapy in advanced non-small-cell lung cancer: Optimal number of treatment cycles. Expert Rev Anticancer Ther. 2016;16:653–660. doi: 10.1586/14737140.2016.1170596. [DOI] [PubMed] [Google Scholar]
- 4.Olaussen KA, Dunant A, Fouret P, Brambilla E, André F, Haddad V, Taranchon E, Filipits M, Pirker R, Popper HH, et al. DNA repair by ERCC1 in non-small-cell lung cancer and cisplatin-based adjuvant chemotherapy. N Engl J Med. 2006;355:983–991. doi: 10.1056/NEJMoa060570. [DOI] [PubMed] [Google Scholar]
- 5.Mitsudomi T, Morita S, Yatabe Y, Negoro S, Okamoto I, Tsurutani J, Seto T, Satouchi M, Tada H, Hirashima T, et al. Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): An open label, randomised phase 3 trial. Lancet Oncol. 2010;11:121–128. doi: 10.1016/S1470-2045(09)70364-X. [DOI] [PubMed] [Google Scholar]
- 6.Scagliotti GV, Parikh P, von Pawel J, Biesma B, Vansteenkiste J, Manegold C, Serwatowski P, Gatzemeier U, Digumarti R, Zukin M, et al. Phase III study comparing cisplatin plus gemcitabine with cisplatin plus pemetrexed in chemotherapy-naive patients with advanced-stage non-small-cell lung cancer. J Clin Oncol. 2008;26:3543–3551. doi: 10.1200/JCO.2007.15.0375. [DOI] [PubMed] [Google Scholar]
- 7.Ben-Baruch A. Inflammation-associated immune suppression in cancer: The roles played by cytokines, chemokines and additional mediators. Semin Cancer Biol. 2006;16:38–52. doi: 10.1016/j.semcancer.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 8.Karnoub AE, Dash AB, Vo AP, Sullivan A, Brooks MW, Bell GW, Richardson AL, Polyak K, Tubo R, Weinberg RA. Mesenchymal stem cells within tumour stroma promote breast cancer metastasis. Nature. 2007;449:557–563. doi: 10.1038/nature06188. [DOI] [PubMed] [Google Scholar]
- 9.Luboshits G, Shina S, Kaplan O, Engelberg S, Nass D, Lifshitz-Mercer B, Chaitchik S, Keydar I, Ben-Baruch A. Elevated expression of the CC chemokine regulated on activation, normal T cell expressed and secreted (RANTES) in advanced breast carcinoma. Cancer Res. 1999;59:4681–4687. [PubMed] [Google Scholar]
- 10.Niwa Y, Akamatsu H, Niwa H, Sumi H, Ozaki Y, Abe A. Correlation of tissue and plasma RANTES levels with disease course in patients with breast or cervical cancer. Clin Cancer Res. 2001;7:285–289. [PubMed] [Google Scholar]
- 11.Yaal-Hahoshen N, Shina S, Leider-Trejo L, Barnea I, Shabtai EL, Azenshtein E, Greenberg I, Keydar I, Ben-Baruch A. The chemokine CCL5 as a potential prognostic factor predicting disease progression in stage II breast cancer patients. Clin Cancer Res. 2006;12:4474–4480. doi: 10.1158/1078-0432.CCR-06-0074. [DOI] [PubMed] [Google Scholar]
- 12.Aldinucci D, Borghese C, Casagrande N. The CCL5/CCR5 axis in cancer progression. Cancers (Basel) 2020;12:1765. doi: 10.3390/cancers12071765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aldinucci D, Casagrande N. Inhibition of the CCL5/CCR5 axis against the progression of gastric cancer. Int J Mol Sci. 2018;19:1477. doi: 10.3390/ijms19051477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhou B, Sun C, Li N, han W, Lu H, Guo L, Guo E, Xia M, Weng D, Meng L, et al. Cisplatin-induced CCL5 secretion from CAFs promotes cisplatin-resistance in ovarian cancer via regulation of the STAT3 and PI3K/Akt signaling pathways. Int J Oncol. 2016;48:2087–2097. doi: 10.3892/ijo.2016.3442. [DOI] [PubMed] [Google Scholar]
- 15.Huang R, Wang S, Wang N, Zheng Y, Zhou J, Yang B, Wang X, Zhang J, Guo L, Wang S, et al. CCL5 derived from tumor-associated macrophages promotes prostate cancer stem cells and metastasis via activating β-catenin/STAT3 signaling. Cell Death Dis. 2020;11:234. doi: 10.1038/s41419-020-2435-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chuang JY, Yang WH, Chen HT, Huang CY, Tan TW, Lin YT, Hsu CJ, Fong YC, Tang CH. CCL5/CCR5 axis promotes the motility of human oral cancer cells. J Cell Physiol. 2009;220:418–426. doi: 10.1002/jcp.21783. [DOI] [PubMed] [Google Scholar]
- 17.Velasco-Velázquez M, Jiao X, De La Fuente M, Pestell TG, Ertel A, Lisanti MP, Pestell RG. CCR5 antagonist blocks metastasis of basal breast cancer cells. Cancer Res. 2012;72:3839–3850. doi: 10.1158/0008-5472.CAN-11-3917. [DOI] [PubMed] [Google Scholar]
- 18.Koontongkaew S. The tumor microenvironment contribution to development, growth, invasion and metastasis of head and neck squamous cell carcinomas. J Cancer. 2013;4:66–83. doi: 10.7150/jca.5112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kalluri R, Zeisberg M. Fibroblasts in cancer. Nat Rev Cancer. 2006;6:392–401. doi: 10.1038/nrc1877. [DOI] [PubMed] [Google Scholar]
- 20.Erdogan B, Webb DJ. Cancer-associated fibroblasts modulate growth factor signaling and extracellular matrix remodeling to regulate tumor metastasis. Biochem Soc Trans. 2017;45:229–236. doi: 10.1042/BST20160387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ernst C, Morton CC. Identification and function of long non-coding RNA. Front Cell Neurosci. 2013;7:168. doi: 10.3389/fncel.2013.00168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Loewer S, Cabili MN, Guttman M, Loh YH, Thomas K, Park IH, Garber M, Curran M, Onder T, Agarwal S, et al. Large intergenic non-coding RNA-RoR modulates reprogramming of human induced pluripotent stem cells. Nat Genet. 2010;42:1113–1117. doi: 10.1038/ng.710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gregg C, Zhang J, Weissbourd B, Luo S, Schroth GP, Haig D, Dulac C. High-resolution analysis of parent-of-origin allelic expression in the mouse brain. Science. 2010;329:643–648. doi: 10.1126/science.1190830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guttman M, Rinn JL. Modular regulatory principles of large non-coding RNAs. Nature. 2012;482:339–346. doi: 10.1038/nature10887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Batista PJ, Chang HY. Long noncoding RNAs: Cellular address codes in development and disease. Cell. 2013;152:1298–1307. doi: 10.1016/j.cell.2013.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhao W, Geng D, Li S, Chen Z, Sun M. LncRNA HOTAIR influences cell growth, migration, invasion, and apoptosis via the miR-20a-5p/HMGA2 axis in breast cancer. Cancer Med. 2018;7:842–855. doi: 10.1002/cam4.1353. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 27.Shao TR, Zheng ZN, Chen YC, Wu QQ, Huang GZ, Li F, Zeng WS, Lv XZ. LncRNA AC007271.3 promotes cell proliferation, invasion, migration and inhibits cell apoptosis of OSCC via the Wnt/β-catenin signaling pathway. Life Sci. 2019;239:117087. doi: 10.1016/j.lfs.2019.117087. [DOI] [PubMed] [Google Scholar]
- 28.Zhang J, Zhang P, Wang L, Piao HL, Ma L. Long non-coding RNA HOTAIR in carcinogenesis and metastasis. Acta Biochim Biophys Sin (Shanghai) 2014;46:1–5. doi: 10.1093/abbs/gmt117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wan Y, Chang HY. HOTAIR: Flight of noncoding RNAs in cancer metastasis. Cell Cycle. 2010;9:3391–3392. doi: 10.4161/cc.9.17.13122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhao W, An Y, Liang Y, Xie XW. Role of HOTAIR long noncoding RNA in metastatic progression of lung cancer. Eur Rev Med Pharmacol Sci. 2014;18:1930–1936. [PubMed] [Google Scholar]
- 31.Nakagawa T, Endo H, Yokoyama M, Abe J, Tamai K, Tanaka N, Sato I, Takahashi S, Kondo T, Satoh K. Large noncoding RNA HOTAIR enhances aggressive biological behavior and is associated with short disease-free survival in human non-small cell lung cancer. Biochem Biophys Res Commun. 2013;436:319–324. doi: 10.1016/j.bbrc.2013.05.101. [DOI] [PubMed] [Google Scholar]
- 32.Liu MY, Li XQ, Gao TH, Cui Y, Ma N, Zhou Y, Zhang GJ. Elevated HOTAIR expression associated with cisplatin resistance in non-small cell lung cancer patients. J Thorac Dis. 2016;8:3314–3322. doi: 10.21037/jtd.2016.11.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Guo J, Dou D, Zhang T, Wang B. HOTAIR promotes cisplatin resistance of osteosarcoma cells by regulating cell proliferation, invasion, and apoptosis via miR-106a-5p/STAT3 axis. Cell Transplant. 2020 Aug 6; doi: 10.1177/0963689720948447. (Epub ahead of print). doi: 10.1177/0963689720948447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang Y, Ai H, Fan X, Chen S, Wang Y, Liu L. Knockdown of long non-coding RNA HOTAIR reverses cisplatin resistance of ovarian cancer cells through inhibiting miR-138-5p-regulated EZH2 and SIRT1. Biol Res. 2020;53:18. doi: 10.1186/s40659-020-00286-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li J, Guan J, Long X, Wang Y, Xiang X. mir-1-mediated paracrine effect of cancer-associated fibroblasts on lung cancer cell proliferation and chemoresistance. Oncol Rep. 2016;35:3523–3531. doi: 10.3892/or.2016.4714. [DOI] [PubMed] [Google Scholar]
- 36.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 37.Augsten M. Cancer-associated fibroblasts as another polarized cell type of the tumor microenvironment. Front Oncol. 2014;4:62. doi: 10.3389/fonc.2014.00062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yi Y, Zeng S, Wang Z, Wu M, Ma Y, Ye X, Zhang B, Liu H. Cancer-associated fibroblasts promote epithelial-mesenchymal transition and EGFR-TKI resistance of non-small cell lung cancers via HGF/IGF-1/ANXA2 signaling. Biochim Biophys Acta Mol Basis Dis. 2018;1864:793–803. doi: 10.1016/j.bbadis.2017.12.021. [DOI] [PubMed] [Google Scholar]
- 39.Zhan Y, Abuduwaili K, Wang X, Shen Y, Nuerlan S, Liu C. Knockdown of long non-coding RNA HOTAIR suppresses cisplatin resistance, cell proliferation, migration and invasion of DDP-resistant NSCLC Cells by targeting miR-149-5p/doublecortin-like kinase 1 axis. Cancer Manag Res. 2020;12:7725–7737. doi: 10.2147/CMAR.S246299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Huang J, Ke P, Guo L, Wang W, Tan H, Liang Y, Yao S. Lentivirus-mediated RNA interference targeting the long noncoding RNA HOTAIR inhibits proliferation and invasion of endometrial carcinoma cells in vitro and in vivo. Int J Gynecol Cancer. 2014;24:635–642. doi: 10.1097/IGC.0000000000000121. [DOI] [PubMed] [Google Scholar]
- 41.Pei CS, Wu HY, Fan FT, Wu Y, Shen CS, Pan LQ. Influence of curcumin on HOTAIR-mediated migration of human renal cell carcinoma cells. Asian Pac J Cancer Prev. 2014;15:4239–4243. doi: 10.7314/APJCP.2014.15.10.4239. [DOI] [PubMed] [Google Scholar]
- 42.Liu XH, Liu ZL, Sun M, Liu J, Wang ZX, De W. The long non-coding RNA HOTAIR indicates a poor prognosis and promotes metastasis in non-small cell lung cancer. BMC Cancer. 2013;13:464. doi: 10.1186/1471-2407-13-464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Qiu JJ, Wang Y, Ding JX, Jin HY, Yang G, Hua KQ. The long non-coding RNA HOTAIR promotes the proliferation of serous ovarian cancer cells through the regulation of cell cycle arrest and apoptosis. Exp Cell Res. 2015;333:238–248. doi: 10.1016/j.yexcr.2015.03.005. [DOI] [PubMed] [Google Scholar]
- 44.Li D, Chai L, Yu X, Song Y, Zhu X, Fan S, Jiang W, Qiao T, Tong J, Liu S, et al. The HOTAIRM1/miR-107/TDG axis regulates papillary thyroid cancer cell proliferation and invasion. Cell Death Dis. 2020;11:227. doi: 10.1038/s41419-020-2416-1. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 45.Li D, Feng J, Wu T, Wang Y, Sun Y, Ren J, Liu M. Long intergenic noncoding RNA HOTAIR is overexpressed and regulates PTEN methylation in laryngeal squamous cell carcinoma. Am J Pathol. 2013;182:64–70. doi: 10.1016/j.ajpath.2012.08.042. [DOI] [PubMed] [Google Scholar]
- 46.Galluzzi L, Vitale I, Michels J, Brenner C, Szabadkai G, Harel-Bellan A, Castedo M, Kroemer G. Systems biology of cisplatin resistance: Past, present and future. Cell Death Dis. 2014;5:e1257. doi: 10.1038/cddis.2013.428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kalluri R. The biology and function of fibroblasts in cancer. Nat Rev Cancer. 2016;16:582–598. doi: 10.1038/nrc.2016.73. [DOI] [PubMed] [Google Scholar]
- 48.Chen WJ, Ho CC, Chang YL, Chen HY, Lin CA, Ling TY, Yu SL, Yuan SS, Chen YJ, Lin CY, et al. Cancer-associated fibroblasts regulate the plasticity of lung cancer stemness via paracrine signalling. Nat Commun. 2014;5:3472. doi: 10.1038/ncomms4472. [DOI] [PubMed] [Google Scholar]
- 49.Wintzell M, Hjerpe E, Åvall Lundqvist E, Shoshan M. Protein markers of cancer-associated fibroblasts and tumor-initiating cells reveal subpopulations in freshly isolated ovarian cancer ascites. BMC Cancer. 2012;12:359. doi: 10.1186/1471-2407-12-359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tao L, Huang G, Wang R, Pan Y, He Z, Chu X, Song H, Chen L. Cancer-associated fibroblasts treated with cisplatin facilitates chemoresistance of lung adenocarcinoma through IL-11/IL-11R/STAT3 signaling pathway. Sci Rep. 2016;6:38408. doi: 10.1038/srep38408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shintani Y, Fujiwara A, Kimura T, Kawamura T, Funaki S, Minami M, Okumura M. IL-6 secreted from cancer-associated fibroblasts mediates chemoresistance in NSCLC by increasing epithelial-mesenchymal transition signaling. J Thorac Oncol. 2016;11:1482–1492. doi: 10.1016/j.jtho.2016.05.025. [DOI] [PubMed] [Google Scholar]
- 52.Lapteva N, Aldrich M, Weksberg D, Rollins L, Goltsova T, Chen SY, Huang XF. Targeting the intratumoral dendritic cells by the oncolytic adenoviral vaccine expressing RANTES elicits potent antitumor immunity. J Immunother. 2009;32:145–156. doi: 10.1097/CJI.0b013e318193d31e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Taub DD, Sayers TJ, Carter CR, Ortaldo JR. Alpha and beta chemokines induce NK cell migration and enhance NK-mediated cytolysis. J Immunol. 1995;155:3877–3888. [PubMed] [Google Scholar]
- 54.Böttcher JP, Bonavita E, Chakravarty P, Blees H, Cabeza-Cabrerizo M, Sammicheli S, Rogers NC, Sahai E, Zelenay S, Reis e Sousa C. NK cells stimulate recruitment of cDC1 into the tumor microenvironment promoting cancer immune control. Cell. 2018;172:1022–1037.e14. doi: 10.1016/j.cell.2018.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Simon PS, Bardhan K, Chen MR, Paschall AV, Lu C, Bollag RJ, Kong FC, Jin J, Kong FM, Waller JL, et al. NF-κB functions as a molecular link between tumor cells and Th1/Tc1 T cells in the tumor microenvironment to exert radiation-mediated tumor suppression. Oncotarget. 2016;7:23395–23415. doi: 10.18632/oncotarget.8246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Araujo JM, Gomez AC, Aguilar A, Salgado R, Balko JM, Bravo L, Doimi F, Bretel D, Morante Z, Flores C, et al. Effect of CCL5 expression in the recruitment of immune cells in triple negative breast cancer. Sci Rep. 2018;8:4899. doi: 10.1038/s41598-018-23099-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Li W, Zhang X, Wang J, Li M, Cao C, Tan J, Ma D, Gao Q. TGFβ1 in fibroblasts-derived exosomes promotes epithelial-mesenchymal transition of ovarian cancer cells. Oncotarget. 2017;8:96035–96047. doi: 10.18632/oncotarget.21635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kim HJ, Lee DW, Yim GW, Nam EJ, Kim S, Kim SW, Kim YT. Long non-coding RNA HOTAIR is associated with human cervical cancer progression. Int J Oncol. 2015;46:521–530. doi: 10.3892/ijo.2014.2758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Oh EJ, Kim SH, Yang WI, Ko YH, Yoon SO. Long non-coding RNA HOTAIR expression in diffuse large B-cell lymphoma: In relation to polycomb repressive complex pathway proteins and H3K27 trimethylation. J Pathol Transl Med. 2016;50:369–376. doi: 10.4132/jptm.2016.06.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yang X, Luo E, Liu X, Han B, Yu X, Peng X. Delphinidin-3-glucoside suppresses breast carcinogenesis by inactivating the Akt/HOTAIR signaling pathway. BMC Cancer. 2016;16:423. doi: 10.1186/s12885-016-2465-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ni W, Yao S, Zhou Y, Liu Y, Huang P, Zhou A, Liu J, Che L, Li J. Long noncoding RNA GAS5 inhibits progression of colorectal cancer by interacting with and triggering YAP phosphorylation and degradation and is negatively regulated by the m6A reader YTHDF3. Mol Cancer. 2019;18:143. doi: 10.1186/s12943-019-1079-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Liu J, Tian W, Zhang W, Jia Y, Yang X, Wang Y, Zhang J. MicroRNA-142-3p/MALAT1 inhibits lung cancer progression through repressing β-catenin expression. Biomed Pharmacother. 2019;114:108847. doi: 10.1016/j.biopha.2019.108847. [DOI] [PubMed] [Google Scholar]
- 63.Lan T, Li H, Zhang D, Xu L, Liu H, Hao X, Yan X, Liao H, Chen X, Xie K, et al. KIAA1429 contributes to liver cancer progression through N6-methyladenosine-dependent post-transcriptional modification of GATA3. Mol Cancer. 2019;18:186. doi: 10.1186/s12943-019-1106-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yang G, Lu X, Yuan L. LncRNA: A link between RNA and cancer. Biochim Biophys Acta. 2014;1839:1097–1109. doi: 10.1016/j.bbagrm.2014.08.012. [DOI] [PubMed] [Google Scholar]
- 65.Gupta RA, Shah N, Wang KC, Kim J, Horlings HM, Wong DJ, Tsai MC, Hung T, Argani P, Rinn JL, et al. Long non-coding RNA HOTAIR reprograms chromatin state to promote cancer metastasis. Nature. 2010;464:1071–1076. doi: 10.1038/nature08975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yao Y, Li J, Wang L. Large intervening non-coding RNA HOTAIR is an indicator of poor prognosis and a therapeutic target in human cancers. Int J Mol Sci. 2014;15:18985–18999. doi: 10.3390/ijms151018985. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.