This cohort study examines the association between alcohol abstinence and posttransplant survival in patients with alcohol-associated liver disease.
Key Points
Question
Are 6 months of alcohol abstinence a factor in poor posttransplant outcomes in patients with alcohol-associated liver disease?
Findings
In this cohort study of 163 patients, early liver transplant was considered for select transplant candidates with less than 6 months of alcohol abstinence. Compared with patients with more than 6 months of abstinence, those with less than 6 months of abstinence showed no significant difference in 1-year patient survival, allograft survival, or relapse-free survival.
Meaning
Adherence to the 6-month abstinence rule was not associated with superior survival outcomes, suggesting that patients with alcohol-associated liver disease with less than 6 months of abstinence should not be categorically excluded from liver transplant.
Abstract
Importance
Traditionally, liver transplant (LT) for alcohol-associated liver disease (ALD) requires 6 months of abstinence. Although early LT before 6 months of abstinence has been associated with decreased mortality for decompensated ALD, this practice remains controversial and concentrated at a few centers.
Objective
To define patient, allograft, and relapse-free survival in early LT for ALD, and to investigate the association between these survival outcomes and early vs standard LT.
Design, Setting, and Participants
This cohort study analyzed all patients with ALD who underwent their first LT at a single academic referral center between October 1, 2012, and November 13, 2020. Patients with known pretransplant hepatocellular carcinoma, hepatitis B or C, or an alternative cause of liver failure were excluded. Follow-up period was defined as the time from LT to the most recent encounter with a transplant center or death.
Exposures
The exposure of interest was early LT, which was defined as less than 180 days of pre-LT abstinence. Standard LT was defined as 180 days or more of pre-LT abstinence. Patients were separated into early LT and standard LT by time from abstinence to LT.
Main Outcomes and Measures
The outcomes were patient, allograft, relapse-free, and hazardous relapse–free survival for patients who underwent early LT or standard LT. These groups were compared by log-rank testing of Kaplan-Meier estimates. Hazardous relapse was defined as binge, at-risk, or frequent drinking. Abstinence was reassessed at the most recent follow-up visit for all patients.
Results
Of the 163 patients with ALD included in this study, 88 (54%) underwent early LT and 75 (46%) underwent standard LT. This cohort had a mean (SD) age at transplant of 52 (10) years and was predominantly composed of 108 male patients (66%). Recipients of early LT vs standard LT were younger (median [interquartile range (IQR)] age, 49.7 [39.0-54.2] years vs 54.6 [48.7-60.0] years; P < .001) and had a higher median (IQR) Model for End-stage Liver Disease score at listing (35.0 [29.0-39.0] vs 20.0 [13.0-26.0]; P < .001). Both recipients of early LT and standard LT had similar 1-year patient survival (94.1% [95% CI, 86.3%-97.5%] vs 95.9% [95% CI, 87.8%-98.7%]; P = .60), allograft survival (92.7% [95% CI, 84.4%-96.7%] vs 90.5% [95% CI, 81.0%-95.3%]; P = .42), relapse-free survival (80.4% [95% CI, 69.1%-88.0%] vs 83.5% [95% CI, 72.2%-90.6%]; P = .41), and hazardous relapse–free survival (85.8% [95% CI, 75.1%-92.2%] vs 89.6% [95% CI, 79.5%-94.9%]; P = .41).
Conclusions and Relevance
Adherence to the 6-month rule was not associated with superior patient survival, allograft survival, or relapse-free survival among selected patients. This finding suggests that patients with ALD should not be categorically excluded from LT solely on the basis of 6 months of abstinence, but rather alternative selection criteria should be identified that are based on need and posttransplant outcomes.
Introduction
Alcohol-associated liver disease (ALD) is the leading indication for both waiting-list additions and liver transplant (LT).1 Alcohol-associated liver disease exists on a spectrum that ranges from steatosis to acute liver failure and cirrhosis.2 Although alcohol-associated cirrhosis is a common indication for LT, patients with other forms of ALD are often denied access to LT. Largely because of pre-LT abstinence requirements,3,4 presentations such as severe alcoholic hepatitis (SAH) and alcohol-induced acute-on-chronic liver failure are contraindications to LT at many transplant centers despite their high mortality, which exceeds 70% at 6 months in some reports.4,5,6,7,8,9
Recent alcohol use is a defining factor of SAH and is a frequent precipitant of acute-on-chronic liver failure.4,10,11 Although abstinence may result in full or partial recovery in mild cases, nonresponse to medical treatment portends a poor prognosis.12,13,14,15 Approximately 40% of patients with SAH do not respond to steroids,9 and no proven medical alternatives exist.11,16 For these patients, LT is the only remaining treatment.17,18 Although longer abstinence may be associated with lower rates of alcohol relapse, the association of a 6-month cutoff with post-LT relapse in patients with ALD is neither validated nor applicable for patients who are unlikely to survive 6 months without LT.19,20,21,22,23
The survival benefit of LT in patients with SAH and acute-on-chronic liver failure has been established.24,25,26 Work by Mathurin et al27 reported 6-month patient survival for 77% of patients with SAH who underwent LT compared with 23% of those who did not (77 [8%] vs 23 [8%]; P < .001). Preliminary results from the early LT program at one center, which removed the 6-month abstinence requirement for patients with decompensated ALD, showed outcomes that were comparable to those of standard LT.28,29 Although the rates of LT for SAH have increased in recent years, early LT remains limited and concentrated at a few centers in the United States.30,31,32 Before early LT can be expanded, it is vital to understand the long-term survival and the factors associated with poor transplant outcomes.
Using the largest single-center cohort of early LT for ALD to date (to our knowledge), we conducted this cohort study to define patient, allograft, and relapse-free survival. Using standard LT as a comparison group, we also investigated the association of early LT with these survival outcomes.
Methods
This retrospective cohort study was approved by the Johns Hopkins Hospital Institutional Review Board. This board waived the requirement for informed consent for medical record review because participation involved minimal risk and was limited to breach of confidentiality.
Study Population and Data Collection
We studied all patients from an academic referral center who underwent their first LT for ALD (n = 208) from initiation of the early LT program through the end of the study period (October 1, 2012, to November 13, 2020). The follow-up period was defined as the time from LT to the most recent encounter with a transplant center, or death. To create a uniform sample for comparison of early LT to standard LT, we excluded patients from the observational cohort with known pretransplant hepatocellular carcinoma (n = 7), hepatitis B or C (n = 33), or a concomitant alternative cause of liver failure (n = 5).
In general, all LT candidates for ALD underwent careful evaluation by a multidisciplinary transplant committee, which consisted of transplant surgeons, hepatologists, licensed social workers, and nurse coordinators. Patients who underwent standard LT had at least 6 months of abstinence and participated in alcohol use disorder treatment before the transplant. Early LT was considered when patients were deemed unlikely to survive 6 months without LT. Inclusion on the transplant list was determined by consensus of the committee after evaluating the failure of medical management, strength of social support, insight into hazardous drinking history, and commitment to abstinence. Although previous knowledge of ALD and failed attempts at abstinence were considered, they were not absolute contraindications to early LT.
Data were abstracted from the electronic medical record of Johns Hopkins Hospital and augmented by data from the Scientific Registry of Transplant Recipients. The Scientific Registry of Transplant Recipients data system includes data on all donor, wait-listed candidates, and transplant recipients in the US as submitted by the members of the Organ Procurement and Transplantation Network. The Health Resources and Services Administration of the US Department of Health and Human Services provides oversight of the activities of the Organ Procurement and Transplantation Network and the Scientific Registry of Transplant Recipients contractors. This data set has been described elsewhere.33
Exposure and Outcomes
The exposure of interest was early LT, which was defined as less than 180 days of pre-LT abstinence. Standard LT was defined as 180 days or more of pre-LT abstinence. Date of abstinence was determined by patient interview; if only 1 month was cited, the 15th day was used.
The outcomes of interest were patient survival, allograft survival, relapse-free survival, and hazardous relapse–free survival. Relapse was defined as the first instance of post-LT alcohol use as identified from the patient follow-up visit or laboratory screening. Hazardous relapse was characterized by binge drinking (≥5 drinks for men or ≥4 drinks for women per occasion),34 at-risk drinking (≥14 drinks for men or ≥7 drinks for women per week),35 or frequent drinking (≥4 days of drinking per week)22 using the standard drink definitions of the National Institute on Alcohol Abuse and Alcoholism.36 If alcohol quantity or frequency was not documented (early LT group: n = 1; standard LT group: n = 1), hazardous drinking was subjectively imputed from the electronic medical record. Patient survival, allograft survival, relapse-free survival, and hazardous relapse–free survival were compared between patients who underwent early LT and patients who underwent standard LT by log-rank testing of Kaplan-Meier estimates.
Early relapse was defined as the return to alcohol use within 90 days after LT. If relapse occurred, abstinence was reassessed at the most recent follow-up visit; patients were considered abstinent if there was no suspicion for relapse and if abstinence was maintained for at least 1 month. A Cox proportional hazards regression model was used to estimate the association of early LT with alcohol relapse, hazardous relapse, and patient survival. A multivariable model adjusting for age at transplant, sex, and race was used to evaluate relapse and hazardous relapse. When evaluating the association of early LT with patient survival, early relapse was included because it was significant on univariable analysis. Age was treated as an ordinal variable with 10-year bins.
Definitions of Patient Characteristics
Patient race was evaluated to compare the distribution between groups. Race was ascertained from the electronic medical record and reflected clinician-defined data. The clinical presentation of alcoholic hepatitis was defined using the American Association for the Study of Liver Diseases criteria37: at-risk alcohol use for 6 months or more with less than 60 days of abstinence before the onset of jaundice; aspartate aminotransferase level higher than 50; aspartate aminotransferase to alanine aminotransferase ratio higher than 1.5, with both values lower than 400; and total bilirubin level greater than 3 mg/dL (to convert to micromoles per liter, multiply by 17.104), with the onset of jaundice within the past 8 weeks. When the timing of jaundice onset was not explicit, it was imputed from the electronic medical record. Severity was guided by a Model for End-Stage Liver Disease score of 21 or higher or Maddrey discriminant function of 32 or higher. The following variables for alcohol history were documented only for a portion of patients: age at onset of drinking (early LT group: n = 69; standard LT group: n = 65), years of heavy drinking (early LT group: n = 85; standard LT group: n = 70), and preabstinence weekly drinks (standard LT group: n = 73). No differences in reported characteristics were observed for patients with missing data (eTable 1 in the Supplement).
Liver transplant explant pathology reports were evaluated for histological features supporting cirrhosis and SAH; these features included fibrosis, lobular inflammation, steatohepatitis, ballooning hepatocytes, and Mallory hyaline. Infection was defined as any positive culture results requiring treatment.
Statistical Analysis
Analyses were performed with Stata, version 16.1/MP for Linux (StataCorp LLC), and R, version 4.0.3 (R Foundation for Statistical Computing). Categorical variables were compared using Fisher exact test, and continuous variables were compared using Wilcoxon rank sum test. Continuous variables are reported as median (interquartile range [IQR]), univariable results are presented as hazard ratios (HRs), and multivariable results are presented as adjusted hazard ratios (aHRs). P values and 95% CIs were 2-sided, and α = .05 was used to indicate statistical significance.
Results
Study Population
Of the 163 patients who underwent LT for ALD, 88 (54%) received early LT and 75 (46%) received standard LT. The early LT group represented 88 of 772 first LTs (11%) among adults during the study period. Median (IQR) follow-up was 701 (345-1311) days. The study cohort was composed of 108 male (66%) and 55 female (34%) patients, with a mean (SD) age at transplant of 52 (10) years. No difference in the proportion of female sex was found between the early LT group and standard LT group (33% [29 patients] vs 35% [26 patients]; P = .87). Patients who underwent early LT vs standard LT were younger at the time of transplant (median [IQR] age, 49.7 [39.0-54.2] years vs 54.6 [48.7-60.0] years; P < .001) and were more frequently transferred from an outside institution (63 patients [72%] vs 17 [23%]; P < .001) (Table 1). In the final year of the study, 27 of 40 committee reviews (68%) for early LT were for transferred patients (eFigure in the Supplement).
Table 1. Patient Characteristics for Early Liver Transplant (ELT) and Standard Liver Transplant (SLT) Groupsa,b.
| Characteristic | No. (%) | P value | |
|---|---|---|---|
| ELT group (n = 88) | SLT group (n = 75) | ||
| Baseline demographics | |||
| Age at transplant, median (IQR), y | 49.7 (39.0-54.2) | 54.6 (48.7-60.0) | <.001 |
| Female sex | 29 (33) | 26 (35) | .87 |
| Male sex | 59 (67) | 49 (65) | |
| White race | 79 (90) | 63 (84) | .37 |
| Marital status | |||
| Single | 13 (15) | 7 (9) | .06 |
| Married | 47 (53) | 37 (49) | |
| Cohabitation | 6 (7) | 1 (1) | |
| Divorced | 17 (19) | 22 (29) | |
| Divorced and cohabitation | 5 (6) | 3 (4) | |
| Partnered but no cohabitation | 0 (0) | 1 (1) | |
| Widowed | 0 (0) | 4 (5) | |
| Pretransplant employment status | |||
| Employed | 31 (35) | 21 (28) | .46 |
| Unemployed or retired | 37 (42) | 36 (48) | |
| Medical leave | 13 (15) | 8 (11) | |
| Disabled | 7 (8) | 10 (13) | |
| Pretransplant smoking status | |||
| Never | 44 (50) | 26 (35) | .13 |
| Active | 13 (15) | 16 (21) | |
| Former | 31 (35) | 33 (44) | |
| History of psychiatric disorder diagnosis | 24 (27) | 27 (36) | .24 |
| Transplant-related factors | |||
| Transferred from outside the hospital | 63 (72) | 17 (23) | <.001 |
| Time from listing to transplant, median (IQR), d | 7.0 (3.0-17.0) | 77.0 (30.0-399.0) | <.001 |
| Received steroids before transplant | 42 (48) | 1 (1) | <.001 |
| MELD score at listing, median (IQR) | 35.0 (29.0-39.0) | 20.0 (13.0-26.0) | <.001 |
| MELD score at transplant, median (IQR) | 36.0 (30.5-39.5) | 24.0 (16.0-30.0) | <.001 |
| Simultaneous kidney transplant | 7 (8) | 8 (11) | .60 |
| ICU requirement immediately before transplant | 20 (23) | 8 (11) | .06 |
| Pretransplant dialysis | 36 (41) | 15 (20) | .004 |
| Donor type | |||
| Donation after brain death | 85 (97) | 56 (75) | <.001 |
| Donation after cardiac death | 3 (3) | 8 (11) | |
| Living | 0 (0) | 11 (15) | |
| Meets clinical criteria for SAH | 65 (74) | 4 (5) | <.001 |
| Explant pathology | |||
| Cirrhosis | 83 (94) | 73 (97) | .45 |
| Incidental hepatocellular carcinoma | 4 (5) | 5 (7) | .73 |
| SAH | 57 (65) | 23 (31) | <.001 |
Abbreviations: ICU, intensive care unit; IQR, interquartile range; MELD, Model for End-stage Liver Disease; SAH, severe alcoholic hepatitis.
Percentages may not sum to 100% because of rounding.
Categorical variables were compared by using Fisher exact test, and continuous variables were compared by using Wilcoxon rank sum test.
Patients who underwent early LT presented with more severe illness evidenced by a higher median (IQR) Model for End-stage Liver Disease score at listing (35.0 [29.0-39.0] vs 20.0 [13.0-26.0]; P < .001), and shorter median (IQR) waiting-list time (7.0 [3.0-17.0] days vs 77.0 [30.0-399.0] days; P < .001) compared with patients who underwent standard LT. A subgroup analysis of 63 patients who underwent early LT on initial listing hospitalization found a higher median (IQR) Model for End-stage Liver Disease score at listing than among those who were discharged before the transplant (25 patients) (37.0 [34.0-40.0] vs 27.0 [23.0-30.0]) (eTable 2 in the Supplement). All patients who underwent early LT received cadaveric donor allograft, whereas 11 patients (15%) who underwent standard LT received living donor allograft. Although all patients in the early LT group were critically ill, 65 (74%) specifically met the SAH criteria of the American Association for the Study of Liver Diseases. In the standard LT group, 4 patients (5%) initially presented with SAH; however, these patients improved with medical management and achieved more than 180 days of abstinence before the transplant (Table 1).
Liver explant pathology revealed cirrhosis in 83 patients (94%) who underwent early LT and 73 patients (97%) who underwent standard LT (Table 1). Evidence of SAH was present in patients who underwent early LT, which was significantly higher than in patients who underwent standard LT (57 [65%] vs 23 [31%]; P < .001), but SAH was also found in the standard LT group despite the 6 months of abstinence from alcohol reported in this group (Table 1). For recipients with 1 year (46) and 2 years (26) of reported abstinence, a substantial proportion of patients with SAH was found on explant pathology (1 year: 33% [n = 15]; 2 years: 27% [n = 7]).
The early LT group had fewer median (IQR) days of abstinence before the transplant compared with the standard LT group (66.5 [35.0-116.0] days vs 481.0 [280.0-850.0] days; P < .001) (Table 2). No differences were identified in family history of alcohol use disorder (Table 2), personal history of psychiatric disease, tobacco use, or pretransplant employment (Table 1). Patients in the early LT and standard LT groups had a similar median (IQR) age at onset of drinking (18.0 [16.0-21.0] years vs 18.0 [15.0-20.0] years; P = .89) and median (IQR) number of weekly preabstinence drinks (56.0 [29.8-112.0] vs 47.8 [24.5-84.0]; P = .15) (Table 2). Patients who underwent early LT had a shorter median (IQR) duration of heavy drinking compared with those who underwent standard LT (12.0 [6.0-20.0] years vs 20.0 [10.0-29.0] years; P = .009).
Table 2. Pretransplant Alcohol History in Early Liver Transplant (ELT) and Standard Liver Transplant (SLT) Groupsa,b.
| History | ELT group (n = 88) | SLT group (n = 75) | P value |
|---|---|---|---|
| Family history of alcohol use disorder, No. (%) | |||
| None | 51 (58) | 40 (53) | .72 |
| First-degree relative | 20 (23) | 21 (28) | |
| Second-degree relative | 11 (13) | 11 (15) | |
| Unknown | 6 (7) | 3 (4) | |
| Age at onset of drinking, median (IQR), yc | 18.0 (16.0-21.0) | 18.0 (15.0-20.0) | .89 |
| Years of heavy drinking, median (IQR), yc | 12.0 (6.0-20.0) | 20.0 (10.0-29.0) | .009 |
| Weekly preabstinence drinks, median (IQR), No.c | 56.0 (29.8-112.0) | 47.8 (24.5-84.0) | .15 |
| Abstinence at time of transplant, median (IQR), d | 66.5 (35.0-116.0) | 481.0 (280.0-850.0) | <.001 |
| Abstinence at first transplant admission, median (IQR), d | 38.0 (17.5-59.0) | 157.0 (80.0-415.0) | <.001 |
| Patient reported alcohol use despite knowledge of liver disease, No. (%) | 21 (24) | 35 (47) | .003 |
| Previous inpatient alcohol rehabilitation, No. (%) | 18 (21) | 18 (24) | .71 |
Abbreviation: IQR, interquartile range.
Percentages may not sum to 100% because of rounding.
Categorical variables were compared by using Fisher exact test, and continuous variables were compared by using Wilcoxon rank sum test.
Variables were documented only for a portion of the total sample: age at onset of drinking (ELT group n = 69; SLT group n = 65), years of heavy drinking (ELT group n = 85; SLT group n = 70), and weekly preabstinence drinks (SLT group n = 73).
Posttransplant Outcomes
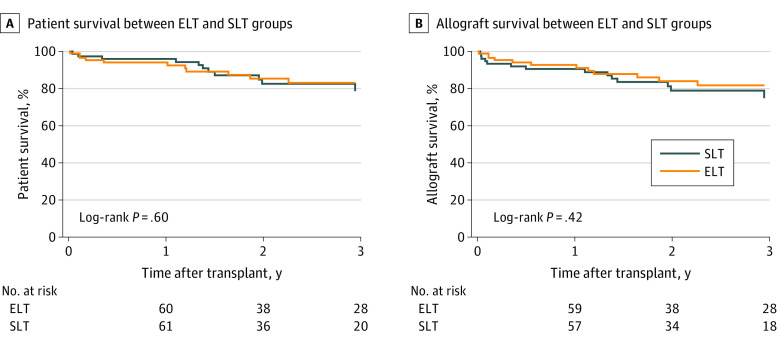
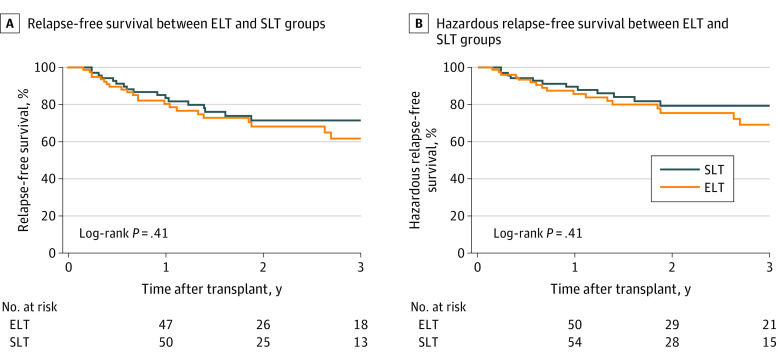
Recipients of early LT and standard LT had similar 1-year (94.1% [95% CI, 86.3%-97.5%] vs 95.9% [95% CI, 87.8%-98.7%]; P = .60) and 3-year (83.0% [95% CI, 70.9%-90.5%] vs 78.6% [95% CI, 63.2%-88.2%]; P = .60) patient survival (Figure 1A) as well as 1-year (92.7% [95% CI, 84.4%-96.7%] vs 90.5% [95% CI, 81.0%-95.3%]; P = .42) and 3-year (81.7% [95% CI, 69.4%-89.4%] vs 74.7% [95% CI, 59.2%-85.0%]; P = .42) allograft survival after the transplant (Figure 1B). Relapse-free survival between early LT and standard LT groups was similar at both 1 year (80.4% [95% CI, 69.1%-88.0%] vs 83.5% [95% CI, 72.2%-90.6%]; P = .41) and 3 years (61.8% [95% CI, 46.4%-73.9%] vs 71.5% [95% CI, 57.7%-81.5%]; P = .41) after the transplant (Figure 2A). Hazardous relapse–free survival was similar between early LT and standard LT groups at 1 year (85.8% [95% CI, 75.1%-92.2%] vs 89.6% [95% CI, 79.5%-94.9%]; P = .41) and 3 years (69.2% [95% CI, 53.9%-80.3%] vs 79.4% [95% CI, 66.1%-88.0%]; P = .41) after the transplant (Figure 2B).
Figure 1. Kaplan-Meier Estimates of Patient Survival and Allograft Survival Among Patients Who Underwent Early Liver Transplant (ELT) vs Standard Liver Transplant (SLT).
Figure 2. Kaplan-Meier Estimates of Relapse-Free Survival and Hazardous Relapse–Free Survival Among Patients Who Underwent Early Liver Transplant (ELT) vs Standard Liver Transplant (SLT).
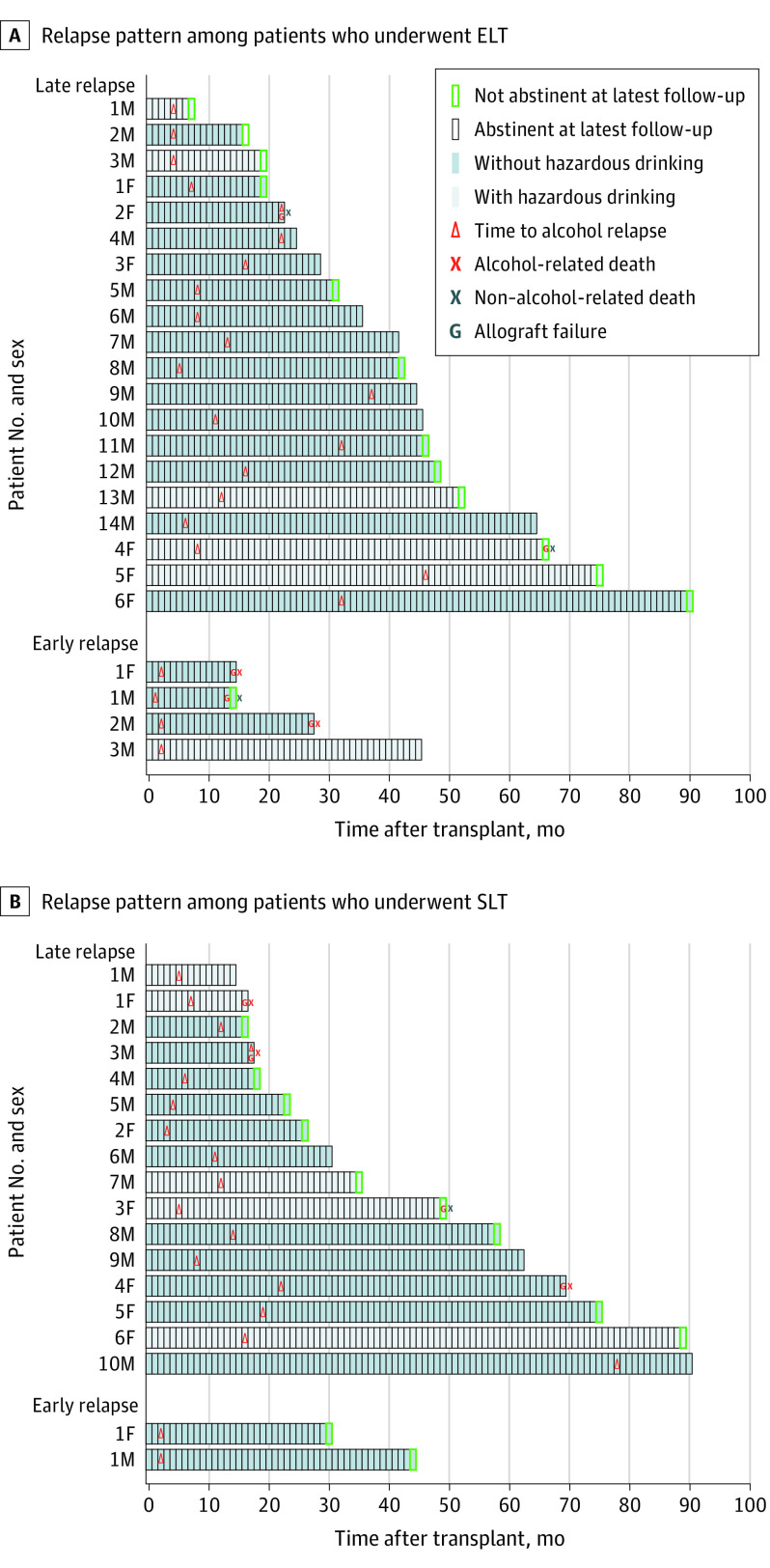
Among those who relapsed, 13 patients in the early LT and 11 patients in the standard LT groups regained abstinence at the most recent follow-up visit, with 77 patients (88%) in the early LT group and 68 patients (91%) in the standard LT group ultimately being abstinent (Figure 3). Of the 6 patients with early relapse, 5 (83%) demonstrated hazardous drinking (Figure 3). Among patients with at least 1 year of follow-up or death before 1 year (early LT group: n = 65; standard LT group: n = 64), no difference was found in 1-year outcomes of infection (35 [54%] vs 36 [56%]; P = .86) or acute rejection (29 [45%] vs 26 [41%]; P = .72).
Figure 3. Posttransplant Relapse Patterns Among Patients Who Underwent Early Liver Transplant (ELT) vs Standard Liver Transplant (SLT).
All patients with relapse are shown as a single bar. Patients were considered to have hazardous drinking on the basis of alcohol use at the time of relapse. Early relapse was defined as relapse within 90 days after the transplant.
Factors Associated with Posttransplant Outcomes
Early LT had no association with relapse on unadjusted (HR, 0.77; 95% CI, 0.42-1.42; P = .41) or adjusted (aHR, 0.87; 95% CI, 0.46-1.63; P = .66) analysis. Furthermore, early LT had no association with hazardous relapse (HR, 0.74 [95% CI, 0.36-1.51; P = .41]; aHR, 0.88 [95% CI, 0.42-1.86; P = .74]). On unadjusted analysis, younger age (21-30 years; n = 5) was associated with relapse (HR, 5.60; 95% CI, 1.32-23.68; P = .02) and hazardous relapse (HR, 6.92; 95% CI, 1.53-31.32; P = .01) compared with recipients who were older than 60 years. This association remained significant for both relapse (aHR, 8.31; 95% CI, 1.73-39.86; P = .008) and hazardous relapse (aHR, 9.02; 95% CI, 1.75-46.44; P = .009) after adjusting for sex, race, and early LT.
Younger age (21-30 years) was not associated with patient survival (HR, 1.84 [95% CI, 0.35-9.54; P = .47]; aHR: 1.11 [95% CI, 0.18-6.90; P = .91]). Early relapse was the only variable associated with patient survival (aHR, 5.46; 95% CI, 1.26-23.65; P = .02). Early LT and standard LT had similar proportions of early relapse (5% [n = 4] vs 3% [n = 2]; P = .69). Early LT was not associated with patient survival (HR, 1.23 [95% CI, 0.57-2.67; P = .60]; aHR, 1.13 [95% CI, 0.50-2.52; P = .77]).
Discussion
To our knowledge, this cohort is the largest single-center cohort of patients who underwent early LT for ALD in the US, with a median follow-up of 701 days. We found that patient, allograft, relapse-free, and hazardous relapse–free survival were similar between the early LT and standard LT groups. We identified an association of young age and early relapse with poor posttransplant outcomes. These results suggest that adherence to the 6-month rule is not associated with improved outcomes and that patients with ALD should not be barred from lifesaving LT solely because they lack 6 months of abstinence.
One-year relapse-free survival of 80.4% for early LT was comparable to published posttransplant relapse rates for ALD of approximately 20%.38,39 The association of patient and allograft survival with relapse is nuanced and affected by the severity of alcohol use.21,40,41 We, therefore, also identified those with hazardous relapse and found that 85.8% of patients who underwent early LT were free of hazardous relapse at 1 year after the transplant, which is similar to that reported for chronic ALD.41,42,43 A pattern was observed toward improved 3-year relapse and hazardous relapse–free survival for standard LT; however, this pattern was not statistically significant nor was it reflected in patient or allograft survival. Patient survival in the present cohort was comparable to that in a multicenter study of LT for SAH (1-year survival: 94%)44 and superior to that in smaller studies of LT for SAH (6-month survival: 89%)45 as well as LT for all indications (1-year survival: 86.0%).46
The 6-month alcohol abstinence rule remains controversial.8,47,48 Although a longer duration of pre-LT abstinence may be associated with a decreased risk of relapse, the validity of this fixed duration is unproven.23,38,49,50,51,52,53 Mandatory abstinence time may erode patient–transplant surgeon rapport and impair access to alcohol use disorder therapy.54 The stringency of this rule is likely affected by the perception that ALD is a self-inflicted behavioral choice. However, other indications for LT, such as nonalcoholic fatty liver disease or intentional drug overdose, can also be viewed in this light but are not subjected to similar selection criteria.24 No national or international mandates exist for the duration of abstinence, and the Organ Procurement and Transplantation Network is clear that past behavior should not be the sole basis for exclusion.54,55
Cirrhosis on explant pathology exceeded 95% in both early LT and standard LT groups, highlighting that chronic liver disease is present in most patients despite an acute presentation. Pathological evidence of SAH was seen in 31% of patients in the standard LT group. Similar findings have been described by other centers, with high rates of SAH on explant pathology despite patients reporting 6 months of abstinence.56 This finding may reflect the heterogeneity in the duration of inflammation associated with alcohol, or it may be secondary to ongoing alcohol use despite patient-reported abstinence.56
As a field, transplantation needs to shift its focus from adherence to this arbitrary time frame to identification of factors associated with poor posttransplant outcomes, posttransplant interventions that minimize relapse, and strategies that treat relapse when it occurs. Ongoing work from our group is dedicated to this effort. Possible alternative factors in relapse that have been identified thus far include psychiatric comorbidities, social support, presence of a life partner, family history of alcohol use in a first-degree relative, previous alcohol rehabilitation, nonalcohol substance use, and age.21,50,52 We found an association between young age and hazardous relapse. Although the early LT group was generally younger than the standard LT group, age did not independently confer a higher risk of relapse. The reason for this association may be the small age difference (approximately 5 years) or that the higher risk of relapse was seen only in the subset of younger patients (aged 20-30 years). With respect to other potential factors in relapse, early LT and standard LT groups were similar.
Early relapse was associated with a higher risk of death. Nearly all patients with early relapse (5 [83%]) demonstrated hazardous drinking, suggesting that a phenotype of early relapse with hazardous patterns is extremely detrimental to patient survival. Further work is needed to examine patterns of relapse and mitigate this risk through standardized posttransplant interventions. Future work should also consider the role that social determinants of health play in the development of ALD, access to LT, and posttransplant outcomes.
Limitations
This study has several limitations. First, relapse was detected through patient follow-up visits, with laboratory screening used only as clinically indicated. Although laboratory screening is a more objective measure of relapse, detection of alcohol relapse after the transplant has been done through careful patient interviews,50 and practice at our center has been shifting to routine laboratory screening. Second, the results were subjected to confounding by indication: the selection process and posttransplant alcohol use disorder treatment for patients who underwent early LT or standard LT were different because of illness severity, and the denominator for patients with ALD who were not referred or selected for LT was unknown. However, this limitation in itself supports the argument that alternative selection criteria, rather than the 6-month rule, should be considered when evaluating patients with ALD. We have standardized the cohort by using an objective cutoff of 180 days of pre-LT abstinence.
Conclusions
In this cohort study, adherence to the 6-month alcohol abstinence rule was not associated with superior patient survival, allograft survival, relapse-free survival, or hazardous relapse–free survival. With substantial follow-up at 3 years after the transplant, as well as 8 years of enrolling patients in the early LT program, similar outcomes for patients who underwent early LT and those who underwent standard LT support the continued expansion of early LT in the US. We identified an association between early relapse and patient survival; this modifiable risk factor offers a new target for posttransplant interventions. This study supports the identification of alternative criteria that will allow deliberate selection of patients with ALD on the basis of need and posttransplant outcomes while remaining defensible in the public eye.
eTable 1. Assessment of Patients with Missing Alcohol History Data
eFigure. Alcohol-Associated Liver Disease With Less Than Six-Months Abstinence: Referrals and Committee Review
eTable 2. Hospitalization Characteristics by Liver Transplantation at Initial Listing Hospitalization
References
- 1.Cholankeril G, Ahmed A. Alcoholic liver disease replaces hepatitis C virus infection as the leading indication for liver transplantation in the United States. Clin Gastroenterol Hepatol. 2018;16(8):1356-1358. doi: 10.1016/j.cgh.2017.11.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chacko KR, Reinus J. Spectrum of alcoholic liver disease. Clin Liver Dis. 2016;20(3):419-427. doi: 10.1016/j.cld.2016.02.002 [DOI] [PubMed] [Google Scholar]
- 3.Lucey MR, Brown KA, Everson GT, et al. Minimal criteria for placement of adults on the liver transplant waiting list: a report of a national conference organized by the American Society of Transplant Physicians and the American Association for the Study of Liver Diseases. Liver Transpl Surg. 1997;3(6):628-637. doi: 10.1002/lt.500030613 [DOI] [PubMed] [Google Scholar]
- 4.Gustot T, Jalan R. Acute-on-chronic liver failure in patients with alcohol-related liver disease. J Hepatol. 2019;70(2):319-327. doi: 10.1016/j.jhep.2018.12.008 [DOI] [PubMed] [Google Scholar]
- 5.Hosseini N, Shor J, Szabo G. Alcoholic hepatitis: a review. Alcohol Alcohol. 2019;54(4):408-416. doi: 10.1093/alcalc/agz036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Crabb DW, Bataller R, Chalasani NP, et al. ; NIAAA Alcoholic Hepatitis Consortia . Standard definitions and common data elements for clinical trials in patients with alcoholic hepatitis: recommendation from the NIAAA Alcoholic Hepatitis Consortia. Gastroenterology. 2016;150(4):785-790. doi: 10.1053/j.gastro.2016.02.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Trebicka J, Sundaram V, Moreau R, Jalan R, Arroyo V. Liver transplantation for acute-on-chronic liver failure: science or fiction? Liver Transpl. 2020;26(7):906-915. doi: 10.1002/lt.25788 [DOI] [PubMed] [Google Scholar]
- 8.Fung JY. Liver transplantation for severe alcoholic hepatitis-the CON view. Liver Int. 2017;37(3):340-342. doi: 10.1111/liv.13286 [DOI] [PubMed] [Google Scholar]
- 9.Louvet A, Naveau S, Abdelnour M, et al. The Lille model: a new tool for therapeutic strategy in patients with severe alcoholic hepatitis treated with steroids. Hepatology. 2007;45(6):1348-1354. doi: 10.1002/hep.21607 [DOI] [PubMed] [Google Scholar]
- 10.Duseja A, Singh SP. Toward a better definition of acute-on-chronic liver failure. J Clin Exp Hepatol. 2017;7(3):262-265. doi: 10.1016/j.jceh.2017.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lucey MR, Mathurin P, Morgan TR. Alcoholic hepatitis. N Engl J Med. 2009;360(26):2758-2769. doi: 10.1056/NEJMra0805786 [DOI] [PubMed] [Google Scholar]
- 12.Mathurin P, Mendenhall CL, Carithers RL Jr, et al. Corticosteroids improve short-term survival in patients with severe alcoholic hepatitis (AH): individual data analysis of the last three randomized placebo controlled double blind trials of corticosteroids in severe AH. J Hepatol. 2002;36(4):480-487. doi: 10.1016/S0168-8278(01)00289-6 [DOI] [PubMed] [Google Scholar]
- 13.Maddrey WC, Boitnott JK, Bedine MS, Weber FL Jr, Mezey E, White RI Jr. Corticosteroid therapy of alcoholic hepatitis. Gastroenterology. 1978;75(2):193-199. doi: 10.1016/0016-5085(78)90401-8 [DOI] [PubMed] [Google Scholar]
- 14.Rahimi E, Pan JJ. Prognostic models for alcoholic hepatitis. Biomark Res. 2015;3:20. doi: 10.1186/s40364-015-0046-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Srikureja W, Kyulo NL, Runyon BA, Hu KQ. MELD score is a better prognostic model than Child-Turcotte-Pugh score or Discriminant Function score in patients with alcoholic hepatitis. J Hepatol. 2005;42(5):700-706. doi: 10.1016/j.jhep.2004.12.022 [DOI] [PubMed] [Google Scholar]
- 16.Thursz MR, Richardson P, Allison M, et al. ; STOPAH Trial . Prednisolone or pentoxifylline for alcoholic hepatitis. N Engl J Med. 2015;372(17):1619-1628. doi: 10.1056/NEJMoa1412278 [DOI] [PubMed] [Google Scholar]
- 17.Mathurin P, Lucey MR. Liver transplantation in patients with alcohol-related liver disease: current status and future directions. Lancet Gastroenterol Hepatol. 2020;5(5):507-514. doi: 10.1016/S2468-1253(19)30451-0 [DOI] [PubMed] [Google Scholar]
- 18.Marot A, Dubois M, Trépo E, Moreno C, Deltenre P. Liver transplantation for alcoholic hepatitis: A systematic review with meta-analysis. PLoS One. 2018;13(1):e0190823. doi: 10.1371/journal.pone.0190823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chuncharunee L, Yamashiki N, Thakkinstian A, Sobhonslidsuk A. Alcohol relapse and its predictors after liver transplantation for alcoholic liver disease: a systematic review and meta-analysis. BMC Gastroenterol. 2019;19(1):150. doi: 10.1186/s12876-019-1050-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McCallum S, Masterton G. Liver transplantation for alcoholic liver disease: a systematic review of psychosocial selection criteria. Alcohol Alcohol. 2006;41(4):358-363. doi: 10.1093/alcalc/agl033 [DOI] [PubMed] [Google Scholar]
- 21.Lim J, Curry MP, Sundaram V. Risk factors and outcomes associated with alcohol relapse after liver transplantation. World J Hepatol. 2017;9(17):771-780. doi: 10.4254/wjh.v9.i17.771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.DiMartini A, Day N, Dew MA, et al. Alcohol consumption patterns and predictors of use following liver transplantation for alcoholic liver disease. Liver Transpl. 2006;12(5):813-820. doi: 10.1002/lt.20688 [DOI] [PubMed] [Google Scholar]
- 23.Dew MA, DiMartini AF, Steel J, et al. Meta-analysis of risk for relapse to substance use after transplantation of the liver or other solid organs. Liver Transpl. 2008;14(2):159-172. doi: 10.1002/lt.21278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Im GY, Cameron AM, Lucey MR. Liver transplantation for alcoholic hepatitis. J Hepatol. 2019;70(2):328-334. doi: 10.1016/j.jhep.2018.11.007 [DOI] [PubMed] [Google Scholar]
- 25.Artru F, Louvet A, Ruiz I, et al. Liver transplantation in the most severely ill cirrhotic patients: a multicenter study in acute-on-chronic liver failure grade 3. J Hepatol. 2017;67(4):708-715. doi: 10.1016/j.jhep.2017.06.009 [DOI] [PubMed] [Google Scholar]
- 26.Al-Saeedi M, Barout MH, Probst P, et al. Meta-analysis of patient survival and rate of alcohol relapse in liver-transplanted patients for acute alcoholic hepatitis. Langenbecks Arch Surg. 2018;403(7):825-836. doi: 10.1007/s00423-018-1720-z [DOI] [PubMed] [Google Scholar]
- 27.Mathurin P, Moreno C, Samuel D, et al. Early liver transplantation for severe alcoholic hepatitis. N Engl J Med. 2011;365(19):1790-1800. doi: 10.1056/NEJMoa1105703 [DOI] [PubMed] [Google Scholar]
- 28.Lee BP, Chen PH, Haugen C, et al. Three-year results of a pilot program in early liver transplantation for severe alcoholic hepatitis. Ann Surg. 2017;265(1):20-29. doi: 10.1097/SLA.0000000000001831 [DOI] [PubMed] [Google Scholar]
- 29.Weeks SR, Sun Z, McCaul ME, et al. Liver transplantation for severe alcoholic hepatitis, updated lessons from the world’s largest series. J Am Coll Surg. 2018;226(4):549-557. doi: 10.1016/j.jamcollsurg.2017.12.044 [DOI] [PubMed] [Google Scholar]
- 30.Noureddin N, Yang JD, Alkhouri N, et al. Increase in alcoholic hepatitis as an etiology for liver transplantation in the United States: a 2004-2018 analysis. Transplant Direct. 2020;6(11):e612. doi: 10.1097/TXD.0000000000001051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee BP, Vittinghoff E, Dodge JL, Cullaro G, Terrault NA. National trends and long-term outcomes of liver transplant for alcohol-associated liver disease in the United States. JAMA Intern Med. 2019;179(3):340-348. doi: 10.1001/jamainternmed.2018.6536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cotter TG, Sandıkçı B, Paul S, et al. Liver transplantation for alcoholic hepatitis in the United States: excellent outcomes with profound temporal and geographic variation in frequency. Am J Transplant. 2021;21(3):1039-1055. doi: 10.1111/ajt.16143 [DOI] [PubMed] [Google Scholar]
- 33.Massie AB, Kucirka LM, Segev DL. Big data in organ transplantation: registries and administrative claims. Am J Transplant. 2014;14(8):1723-1730. doi: 10.1111/ajt.12777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.National Institute on Alcohol Abuse and Alcoholism. Drinking levels defined. Accessed December 30, 2020. https://www.niaaa.nih.gov/alcohol-health/overview-alcohol-consumption/moderate-binge-drinking
- 35.National Institute on Alcohol Abuse and Alcoholism. Helping Patients Who Drink Too Much : A Clinician’s Guide. Accessed January 26, 2021. https://pubs.niaaa.nih.gov/publications/Practitioner/CliniciansGuide2005/guide.pdf
- 36.National Institute on Alcohol Abuse and Alcoholism. What is a standard drink? Accessed December 23, 2020. https://www.niaaa.nih.gov/alcohols-effects-health/overview-alcohol-consumption/what-standard-drink#:~:text=In%20the%20United%20States%2C%20one,which%20is%20about%2040%25%20alcohol
- 37.Crabb DW, Im GY, Szabo G, Mellinger JL, Lucey MR. Diagnosis and treatment of alcohol-associated liver diseases: 2019 practice guidance from the American Association for the Study of Liver Diseases. Hepatology. 2020;71(1):306-333. doi: 10.1002/hep.30866 [DOI] [PubMed] [Google Scholar]
- 38.Pfitzmann R, Schwenzer J, Rayes N, Seehofer D, Neuhaus R, Nüssler NC. Long-term survival and predictors of relapse after orthotopic liver transplantation for alcoholic liver disease. Liver Transpl. 2007;13(2):197-205. doi: 10.1002/lt.20934 [DOI] [PubMed] [Google Scholar]
- 39.Lim JK, Keeffe EB. Liver transplantation for alcoholic liver disease: current concepts and length of sobriety. Liver Transpl. 2004;10(10 suppl 2):S31-S38. doi: 10.1002/lt.20267 [DOI] [PubMed] [Google Scholar]
- 40.Rice JP, Eickhoff J, Agni R, Ghufran A, Brahmbhatt R, Lucey MR. Abusive drinking after liver transplantation is associated with allograft loss and advanced allograft fibrosis. Liver Transpl. 2013;19(12):1377-1386. doi: 10.1002/lt.23762 [DOI] [PubMed] [Google Scholar]
- 41.Pageaux GP, Bismuth M, Perney P, et al. Alcohol relapse after liver transplantation for alcoholic liver disease: does it matter? J Hepatol. 2003;38(5):629-634. doi: 10.1016/S0168-8278(03)00088-6 [DOI] [PubMed] [Google Scholar]
- 42.Lucey MR. Liver transplantation in patients with alcoholic liver disease. Liver Transpl. 2011;17(7):751-759. doi: 10.1002/lt.22330 [DOI] [PubMed] [Google Scholar]
- 43.Björnsson E, Olsson J, Rydell A, et al. Long-term follow-up of patients with alcoholic liver disease after liver transplantation in Sweden: impact of structured management on recidivism. Scand J Gastroenterol. 2005;40(2):206-216. doi: 10.1080/00365520410009591 [DOI] [PubMed] [Google Scholar]
- 44.Lee BP, Mehta N, Platt L, et al. Outcomes of early liver transplantation for patients with severe alcoholic hepatitis. Gastroenterology. 2018;155(2):422-430.e1. doi: 10.1053/j.gastro.2018.04.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Im GY, Kim-Schluger L, Shenoy A, et al. Early liver transplantation for severe alcoholic hepatitis in the United States–a single-center experience. Am J Transplant. 2016;16(3):841-849. doi: 10.1111/ajt.13586 [DOI] [PubMed] [Google Scholar]
- 46.Jain A, Reyes J, Kashyap R, et al. Long-term survival after liver transplantation in 4,000 consecutive patients at a single center. Ann Surg. 2000;232(4):490-500. doi: 10.1097/00000658-200010000-00004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lucey MR. Liver transplantation for severe alcoholic hepatitis-the PRO view. Liver Int. 2017;37(3):343-344. doi: 10.1111/liv.13343 [DOI] [PubMed] [Google Scholar]
- 48.Brown RS Jr. Transplantation for alcoholic hepatitis–time to rethink the 6-month “rule.” N Engl J Med. 2011;365(19):1836-1838. doi: 10.1056/NEJMe1110864 [DOI] [PubMed] [Google Scholar]
- 49.Lim J, Sundaram V. Risk factors, scoring systems, and interventions for alcohol relapse after liver transplantation for alcoholic liver disease. Clin Liver Dis (Hoboken). 2018;11(5):105-110. doi: 10.1002/cld.696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.DiMartini A, Day N, Dew MA, et al. Alcohol use following liver transplantation: a comparison of follow-up methods. Psychosomatics. 2001;42(1):55-62. doi: 10.1176/appi.psy.42.1.55 [DOI] [PubMed] [Google Scholar]
- 51.Addolorato G, Mirijello A, Leggio L, et al. ; Gemelli OLT Group . Liver transplantation in alcoholic patients: impact of an alcohol addiction unit within a liver transplant center. Alcohol Clin Exp Res. 2013;37(9):1601-1608. doi: 10.1111/acer.12117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.De Gottardi A, Spahr L, Gelez P, et al. A simple score for predicting alcohol relapse after liver transplantation: results from 387 patients over 15 years. Arch Intern Med. 2007;167(11):1183-1188. doi: 10.1001/archinte.167.11.1183 [DOI] [PubMed] [Google Scholar]
- 53.Tandon P, Goodman KJ, Ma MM, et al. A shorter duration of pre-transplant abstinence predicts problem drinking after liver transplantation. Am J Gastroenterol. 2009;104(7):1700-1706. doi: 10.1038/ajg.2009.226 [DOI] [PubMed] [Google Scholar]
- 54.Rice JP, Lucey MR. Should length of sobriety be a major determinant in liver transplant selection? Curr Opin Organ Transplant. 2013;18(3):259-264. doi: 10.1097/MOT.0b013e32835fb94b [DOI] [PubMed] [Google Scholar]
- 55.US Department of Health & Human Services. Organ Procurement and Transplantation Network. General considerations in assessment for transplant candidacy. Accessed December 29, 2020. https://optn.transplant.hrsa.gov/resources/ethics/general-considerations-in-assessment-for-transplant-candidacy/
- 56.Wells JT, Said A, Agni R, et al. The impact of acute alcoholic hepatitis in the explanted recipient liver on outcome after liver transplantation. Liver Transpl. 2007;13(12):1728-1735. doi: 10.1002/lt.21298 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Assessment of Patients with Missing Alcohol History Data
eFigure. Alcohol-Associated Liver Disease With Less Than Six-Months Abstinence: Referrals and Committee Review
eTable 2. Hospitalization Characteristics by Liver Transplantation at Initial Listing Hospitalization