Abstract
Major advances in the field of genomic technologies have led to an improvement in cancer diagnosis, classification and prognostication. However, many cancers remain incurable due to the development of drug resistance, minimal residual disease (MRD) and disease relapse, highlighting an incomplete understanding of the mechanisms underlying these processes. In recent years, the impact of non‐genetic factors on neoplastic transformations has increasingly been acknowledged, and growing evidence suggests that low oxygen (O2) levels (ie hypoxia) in the tumour microenvironment play a critical role in the development and treatment of cancer. As a result, there is a growing need to develop research tools capable of reproducing physiologically relevant O2 conditions encountered by cancer cells in their natural environments in order to gain in‐depth insight into tumour cell metabolism and function. In this review, the authors highlight the importance of hypoxia in the pathogenesis of malignant diseases and provide an overview of novel engineering tools that have the potential to further drive this evolving, yet technically challenging, field of cancer research.
1. INTRODUCTION
Oxygen (O2) tension in the body varies greatly, depending on the location and the physiological condition of the specific tissue.1 The level of tissue oxygenation plays a critical role in both healthy and diseased physiological processes, such as ischaemia, tumours and inflammation.2 In healthy tissues, O2 concentration drops from 20% in the lungs to ~13% in the alveoli, and ~5% in the circulation.3 The O2 content in multicellular structures can further decrease to below 5%. Other tissue‐specific O2 levels include 5% in the venous blood, 1%‐7% in the bone marrow, 0.5%‐7% in the brain and 1% in the cartilage (Figure 1A).4, 5 Increasing lines of evidence suggest that hypoxia is an innate facet of cancer, as the proliferation of malignant cells quickly exceeds the diffusion limit of O2 (100‐200 µm) resulting in inadequate oxygenation.6 The vascular, metabolic and oncogenic adaptations that ensue are known to be critical to the biology of various cancers. Representing a therapeutic liability, tumour hypoxia is increasingly being explored for the development of personalized treatment approaches to influence tumour growth, metastatic potential and drug resistance. However, hypoxia‐targeted strategies have only yielded limited success to date. Part of this unsatisfactory outcome may be attributed to the lack of appropriate experimental methods, which often involve the manipulation and study of cells exposed to non‐physiologic O2 concentrations and gradients that poorly reflect the physiologic conditions encountered by tumour cells in their natural environments. Hence, generating hypoxic conditions and hypoxic gradients in the in vitro setting has received increasing attention because hypoxia is capable of inducing, via hypoxia‐inducible factor α (HIF‐1α), a host of cell survival responses (eg autophagy).7 In this review, we highlight several fundamental concepts of hypoxia, its metabolic adaptation and impact on tumour biology. We also discuss the need and recent progress of novel engineering tools and methodologies required to generate hypoxia and O2 gradients, which are needed to further drive progress in this emerging field of research.
FIGURE 1.
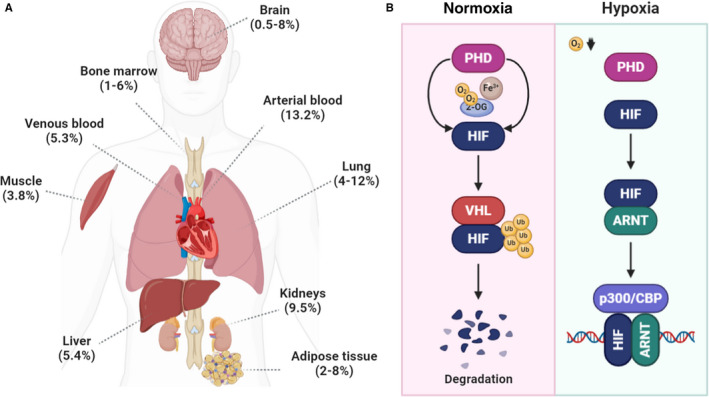
Tissue oxygenation and HIF regulation. (A) O2 concentrations as measured in selected mammalian tissues. (B) Regulation of HIF stability by O2
2. HYPOXIA‐INDUCIBLE FACTORS
The transcription factor hypoxia‐inducible factor (HIF‐1) is a key mediator for transmitting changes in O2 tension into changes in genetic transcription allowing for cellular adaptation.8, 9, 10 The level of HIF‐1 ultimately regulates the expression of a wide range of adaptive processes, including the conversion from oxidative to glycolytic metabolism and angiogenesis.11 Structurally, HIF‐1 is a heterodimeric complex comprised of a stable beta subunit and O2‐sensitive alpha subunits. Under normoxic conditions, prolyl hydroxylases (PHD) hydroxylate the alpha subunits of HIF, leading to ubiquitylation by the von Hippel Lindau (VHL) complex and subsequent proteasomal degradation (Figure 1B).12, 13, 14 Factor inhibiting HIF (FIH) also hydroxylates an asparagine residue of HIF‐1α when O2 is available, blocking its interaction with the transcriptional coactivator protein p300 and preventing transactivation of certain HIF target genes.15, 16 Hypoxia inactivates PHD and FIH, resulting in the accumulation of HIF‐1 and its translocation to the nucleus where it interacts with HIF‐1β and binds to hypoxia‐response elements.17, 18 Notably, these regulatory mechanisms are also affected by the severity and duration of hypoxia.19, 20 In addition, it has been shown that HIF‐1 is stabilized by an acidic intracellular pH, which often develops as a result of hypoxic metabolic changes.21 Beyond being functionally important for the adaptation of normal and malignant cells to hypoxic conditions, HIF has been implicated in promoting genetic instability, immune evasion, migration and metastasis and stem cell maintenance.22, 23, 24, 25 Accordingly, elevated levels of HIF‐1 have been demonstrated in some studies to be an independent negative prognostic indicator portending increased risk of metastasis, mortality and other adverse features in a variety of cancers including breast, lung and pancreas.26, 27 There is also evidence that HIF interacts with key tumour suppressor and proto‐oncoproteins such as p53 and MYC.28, 29 However, for a minority of cancers, such as cervical cancer for example, it does not appear to have any prognostic significance.30, 31
3. METABOLIC ADAPTATIONS TO HYPOXIA
When O2 availability decreases, cellular metabolism shifts from oxidative phosphorylation to the less efficient glycolysis. To maintain this process, pyruvate oxidizes NADH and is reduced to lactate via lactate dehydrogenase (LDH). As lactate accumulates, the cytoplasm becomes increasingly acidic which inhibits glycolysis, so lactate is excreted from cells by monocarboxylate transporters.32 HIF‐1 up‐regulates the production of many glycolytic genes including isozymes of LDH that favour pyruvate reduction, lactate transporters and multiple other enzymes including hexokinase 1 and 3, aldolase A and C and pyruvate dehydrogenase kinase 1.33, 34, 35, 36 HIF‐1 also up‐regulates COX4‐2, a subunit of complex IV of the electron transport chain which appears to be more efficient under hypoxic conditions and potentially generates less reactive oxygen species (ROS).37, 38 When the demand for intracellular glucose increases, cancer cells can utilize glycogen to remain viable and proliferate.39 This too limits the production of ROS, avoiding senescence.40 Hypoxic cells can also utilize glutamine via both oxidative metabolism and reductive carboxylation.41, 42, 43 Finally, in addition to glucose, glycogen and glutamine, there is evidence that hypoxic cancer cells may use other carbon sources such as exogenous acetate to produce acetyl‐CoA, and perhaps nutrients released from organelles as a consequence of autophagy.44, 45 Furthermore, it has been shown that there is metabolic interplay between hypoxic and normoxic tumour regions. For example, tumour vascular endothelial cells have been noted to be highly glycolytic, thus allowing more O2 to reach further into the tumour.44 Another study has shown that a symbiotic relationship can exist between normoxic tumour regions that oxidize lactate to spare glucose and hypoxic tumour regions which metabolize glucose into lactate, thus providing a metabolic substrate for the normoxic regions.45
4. THE ROLE OF HYPOXIA IN SOLID MALIGNANCIEs
In solid cancers, hypoxic tumour cells respond by producing angiogenic factors, but this pathologically induced process yields new vessels that are structurally and functionally suboptimal compared with vessels produced by well‐coordinated physiologic angiogenesis.6 Chaotic non‐laminar blood flow, leakiness, and vascular remodelling lead to dynamic changes in O2 delivery, with hypoxia lasting from seconds to days or of a cyclical nature.46 The result of the high metabolic demands of malignant cells combined with limited O2 delivery due to abnormal vasculature is that even highly vascularized cancers or tumour regions can contain areas of severe hypoxia.11 Similarly, regardless of the degree of hypoxia, the pO2 level of a tumour is always lower than corresponding normal tissue, resulting in hypoxia relative to physioxia.
Hypoxic conditions lead to elevated genomic instability, the selection of cells that have diminished DNA repair (down‐regulated MLH1, MSH2, RAD51) and apoptotic potential (TP53 mutations), and a dampening of the antitumour immune response.47, 48, 49, 50, 51 It also leads to the development of protective stem cell niches and enhanced expression of multidrug resistance proteins.52, 53, 54 Furthermore, the lower rate of proliferation of hypoxic cancer cells decreases the effectiveness of cytotoxic chemotherapeutics that work best in actively dividing cells. Finally, in addition to resistance to chemotherapy and radiotherapy, hypoxia has been shown to contribute to resistance to immunotherapy via a variety of mechanisms including down‐regulation of MHC‐I and up‐regulation of immune checkpoints.55, 56 The net result of which is that low O2 levels in solid cancers can generate a more mutagenic and treatment‐resistant phenotype. As a result, tumour hypoxia has been linked to unfavourable cancer outcomes. In prostate cancer, for example, hypoxia has been associated with biochemical relapse independent of factors such as Gleason score, prostate‐specific antigen (PSA) levels or T‐category.57 Another study found an association between HIF‐1 and vascular endothelial growth factor (VEGF) expression on diagnostic tumour biopsies and biochemical relapse following radiotherapy or radical prostatectomy, although it has been acknowledged that factors unrelated to hypoxia may up‐regulate HIF‐1.58 Patients with head and neck cancer treated with radiation alone were found to have an association between tumour hypoxia, as measured with electrodes, and inferior overall survival (OS) and higher rates of local recurrence.59, 60 Studies have also shown that hypoxia seems to increase the propensity for metastatic disease across multiple cancer types, such as cervical and breast cancer.61, 62, 63 A limitation of the available clinical data is that a variety of different techniques were used to measure hypoxia, each with their attendant advantages and disadvantages, which have been reviewed elsewhere.2
There have been various attempts to therapeutically exploit hypoxia as a differentiating metabolic characteristic of malignant cells. These have included radiosensitizers, antiangiogenics and hypoxia‐activated pro‐drugs amongst others. For example, one approach involving a combination of accelerated radiotherapy, the inhalation of carbogen (98% O2 and 2% CO2) and the vasoactive compound nicotinamide (ARCON) was compared with accelerated radiotherapy alone in a phase III trial of patients with laryngeal cancer.64 The combined approach resulted in a statistically significant improvement in regional control, albeit without improvement in local control. Another phase II trial of radiation, carbogen and nicotinamide compared to radiation alone in patients with locally advanced bladder cancer resulted in a significant improvement in OS and local relapse rates with the hypoxia‐directed treatment.65 An example of a radiosensitizer that has been studied is nimorazole, which was tested in combination with radiation in a phase III trial of patients with supraglottic laryngeal and pharyngeal cancers versus placebo and radiation, and demonstrated improved locoregional control.66 However, a number of hypoxia‐activated pro‐drugs demonstrating promising early activity in phase I and II trials ultimately led to negative phase III trials. These include tirapazamine in head and neck cancer and evofosfamide (TH‐302) in advanced pancreatic cancer and soft tissue sarcomas.67, 68
5. THE MOLECULAR HALLMARKS OF HYPOXIA
One major challenge in directly measuring the extent of hypoxia in malignant tissues is the significant amount of both intratumoral heterogeneity and intertumoral heterogeneity in O2 status for each cancer type, which can change over time. There has therefore been a growing effort to understand the molecular hallmarks of hypoxia, ultimately using diagnostic tumour biopsies as both an indirect reflection of the broader O2 microenvironment over time and to deduce a given tumour's dependence on hypoxia for its proliferation. To this end, single‐nucleotide variants (SNVs) and copy number aberrations (CNAs) of TP53, MYC and PTEN have consistently been associated with hypoxia in multiple cancer types.69 There was, however, a notable degree of variation in SNV hypoxia signatures between tumour types, which emphasizes the need for further, in‐depth studies in each malignancy. Another study identified a genomic signature of the metabolic shift associated with tumour hypoxia across multiple cancer types.70 In addition to protein and mRNA, microRNA expression has been associated with hypoxia.71, 72 For example, miR‐210 abundance was associated with hypoxia across 18 tumour types in one study, although more studies are needed to determine their precise regulatory role.69 Some limitations of the clinical utility of hypoxia biomarkers include a degree of dependence on adequate sampling of the tumour to account for special heterogeneity and that many markers are regulated by both hypoxia‐dependent and hypoxia‐independent mechanisms.
It is becoming increasingly clear that microenvironmental pressures such as hypoxia may be shaping the mutational architecture of cancer, selecting for subclones with aggressive features.69 A major challenge is to identify those tumours with a “hypoxic driver” molecular or genetic phenotype in which hypoxia is a primary driver of the cancer's behaviour, as this subgroup will be enriched in predictive value for response to hypoxia‐targeted treatments.73 For example, a retrospective analysis of the aforementioned nimorazole trial examined a 15‐gene hypoxia panel in pre‐treatment biopsies and found that only patients with hypoxic tumours as determined by the panel had improved local control and survival.74 It has also been hypothesized that hypoxic niches in tissues such as the bone marrow may provide shelter to cancer stem cells and are at least partially responsible for treatment resistance in leukaemia and other diseases.75 Therefore, a greater understanding of the key molecular pathways underpinning hypoxic cancer cells’ resistance to treatments may promote the development of novel targets and therapies. However, a major reason why a significant knowledge gap persists in our understanding of the role of hypoxia is the difficulty of studying cancers ex vivo, which often involves the use of un‐physiologic cell culture techniques carried out in ambient air or in chambers that maintain a constant level of hypoxia. Therefore, progress in our understanding of the molecular characteristics of hypoxia, as well as its therapeutic exploitation, will likely require a tandem progress in experimental models.
6. PATHOPHYSIOLOGICAL EFFECTS OF OXYGEN GRADIENTS
Increasing lines of evidence suggest that O2 gradients might play an important role in the process of drug resistance and cancer cell survival, potentially by providing “escape routes” along which neoplastic cells migrate when a cell death signal is activated by cytotoxic therapy. In fact, cellular migration along gradients, including chemokine,76 cytokine77 or growth factor78 gradients, has long been recognized as a fundamental process in cellular adaption. Based on previously reported computer simulation data by Cristini et al, which indicated that tumour cells follow O2 concentration gradients, Mosadegh and colleagues utilized three dimensional paper‐based invasion assays to investigate whether gradients of O2 direct tumour cell migration.79, 80 Using the human adenocarcinoma cell line A549 and three independently derived cell lines, the authors observed that fractions of tumour cells undergo chemotaxis towards higher levels of O2, concluding that migratory responses to O2 gradients might represent a distinctive feature to identify cellular subgroups within complex populations.80 In line with these findings, Lin et al demonstrated that cervical cancer cells migrate faster and over longer distances compared with human umbilical cord vein endothelial cells under hypoxic conditions in a microfluidic cell co‐culturing system device.81 Similarly, but in contrast to the observations in A549 cells, Sleeboom et al described that breast cancer cells and their respective cancer stem cells migrate towards low O2 regions in a microfluidic gradient device.82 Ceradini and colleagues reported that in the process of tissue repair and regeneration, CXCR4+ stem/progenitor cell recruitment to injured tissues is mediated by SDF‐1 and hypoxic gradients. Taking into account that i.) the CXCR4/SDF‐1 axis has previously been shown to play a critical role in tumour cell trafficking in a broad range of malignancies and ii.) neoplastic states are frequently characterized by hypoxic conditions, tumour‐associated microenvironments might utilize the same mechanisms to recruit circulating cancer cells to O2‐deprived niches, thereby potentially providing a sanctuary to facilitate the development of drug resistance and disease relapse.83, 84 Intriguingly, the chemotactic migration of leukemic cancer cells was significantly enhanced when treated with doxorubicin and daunorubicin in a microfluidic microcirculation mimetic device.85 Overall, the concept of O2 gradient‐directed migration is highly relevant from a clinical and translational standpoint as current strategies to decrease tumour vascularity augment tumour hypoxia with the associated risk of promoting tumour cell survival. To date, the detailed mechanisms involved in O2‐directed migration remain to be elucidated. As a deeper understanding of these processes will likely open new avenues in cancer therapy, novel technologies providing O2 gradients in vitro are urgently needed.
7. HYPOXIA‐INDUCING CHEMICALS
Intracellular hypoxia‐like responses can be created or mimicked by using chemical reagents, such as sodium sulphite (Na2SO3), cobalt chloride (CoCl2) and the iron chelator desferrioxamine (DFO) (Table 1). Sodium sulphite serves as a O2 scavenging agent by forming sodium sulphate (Na2SO4), resulting in hypoxic conditions (~20 mmHg) after 20‐30 minutes.86, 87 Bhatti et al used sodium sulphite anoxic solution to generate low O2 tension (lower than 20 mmHg) for culturing human dermal neonatal fibroblasts.88 On the other hand, CoCl2 and DFO induce hypoxia‐like responses in the cells by blocking the degradation and thus accumulation of intracellular HIF‐1α.89 Mechanistically, CoCl2 reacts with O2 to form a CoO compound, generating a hypoxia‐like intracellular environment and inhibiting the PHD pathway. CoCl2 acts by either chelating the iron core of HIF‐1α and replacing it with cobalt or taking up the VHL‐binding domain of HIF‐1α, thus rescuing it from degradation.90 Heirani‐Tabasi et al explored the effect of hypoxia‐mimicking agents such as CoCl2 and DFO on several signalling molecules that are involved in migration of adipose‐derived mesenchymal stem cells (Ad‐MSCs) in vitro. On the other hand, DFO inhibits the PHD pathway through chelating iron in the media. DFO could increase both cell migration and expression of genes such as VEGF‐A, VEGF‐C, MAPK4, INPP4B and IL‐8.91 Various studies have exploited this mechanism for inducing HIF‐mediated responses.92, 93 Alternatively, inhibition of PHD through oxoglutarate analogues such as dimethyloxalyglycine (DMOG) can inhibit the hydroxylation of HIF‐1α similar to culturing cells under hypoxic conditions (12.5%‐2.5%).94 While adding chemical reagents to induce hypoxia or mimic intracellular hypoxic response is convenient, the potential cytotoxicity and unintended cellular behaviours (eg cell division and morphology) may occur due to the added chemicals.95, 96
TABLE 1.
Chemical reactions of hypoxia‐mimicking agents
8. ENGINEERING TOOLS FOR CREATING HYPOXIA
8.1. Hypoxic chambers
Various engineering tools are available for controlling O2 content for in vitro cell culture and related experiments, including the bulky glove box and hypoxia workstations. A simple plastic chamber system with controllable gas inets may also be used to create a uniform O2 content within the enclosed chamber, which fits in a conventional incubator (1%‐10%).97 Furthermore, gas cylinders, which usually contain a desired gas mixture, are needed to produce and maintain the conditions of physical hypoxia in the chamber (0.0‐1.5 mg/L, control group showed 7.5 mg/L level of oxygen).98, 99 However, this system cannot reach low levels of hypoxia due to the large volume of air to be exchanged. In addition, O2 concentration would increase drastically even after a short exposure to ambient air for media change, which may significantly influence hypoxia‐related gene expression.95, 100 One common shortfall of glove boxes, hypoxia workstations and simple hypoxic chambers is that only one specific level of O2 content can be maintained at any given time, hencing limiting the study of hypoxia‐related cellular responses to a fixed O2 content.
8.2. Microfluidic devices
To circumvent the disadvantages of hypoxic chamber systems, other sophisticated engineering systems have been developed to generate complex hypoxia patterns for in vitro cell culture, such as microfluidic devices. Microfluidic platforms enable precise controls over the local microenvironmental properties, including flow rate and physicochemical compositions of the media, including O2 content. In general, microfluidic devices are fabricated from polydimethylsiloxane (PDMS) or polystyrene (PS).101 In microfluidic devices, the culture area geometry and flow path can be precisely controlled to permit real‐time imaging of hypoxic cell culture. It is also possible to create complex hypoxia patterns within microfluidic devices.102 To generate O2 gradients inside the microfluidic devices, several methods have been employed, including (i) introducing O2 scavenging agents into the devices and (ii) controlling O2 concentration through gas supply channels. Khan et al designed complex microfluidic chambers using an established soft lithography procedure.102 After manufacturing the chamber, the interior of the chamber was coated with 3‐sided glass to control the permeability of O2. Using this method, O2 gradients of various spatial resolutions (from 0.1 to 19.9 mg/L) can be rapidly and conveniently established (Figure 2A,B) through adjusting the flow rate of medium pre‐equilibrated with lower oxygen tension.95 Shih et al showed that the spatially confined chemical reaction could generate stable O2 gradients within the microfluidic device (21% O2 nomaxia and 1% O2 hypoxia).101 The O2 scavenging chemical reaction between pyrogallol (benzene‐1,2,3‐triol) and NaOH occurred in the chemical reaction chamber (Figure 2C,D). When pyrogallol is added in alkaline solution, it absorbs O2 rapidly and creates a “sink” that induces a unidirectional diffusion of O2 to generate an O2 gradient (Figure 2E). It is possible to alter the range and steepness of the gradient O2 in the same device by changing the composition of the gas mixture fed into the culture areas with different sizes and shapes.103 The disadvantages of microfluidic systems include complicated manufacturing processes, the need of flow control instruments and device set‐up. In addition, it is not suitable for long‐term or large‐scale cell studies.104
FIGURE 2.
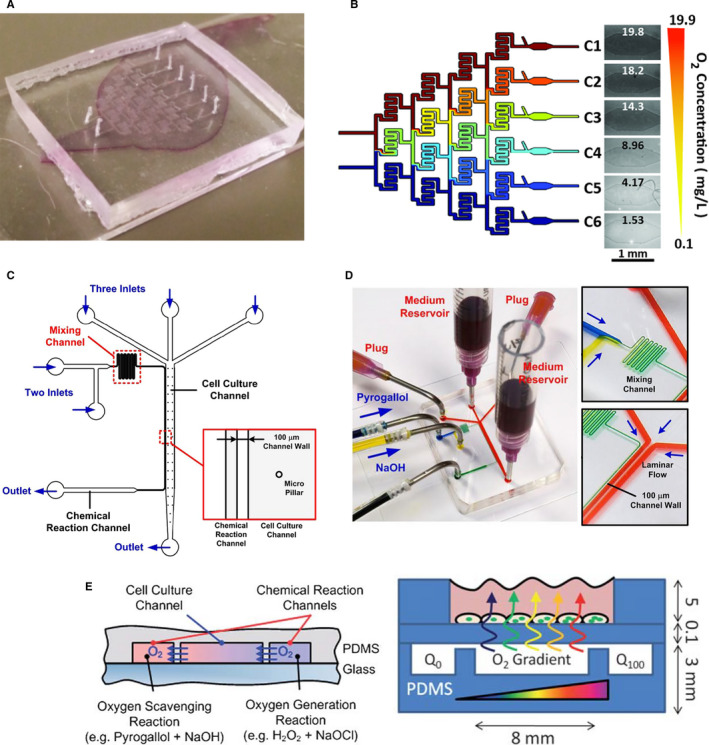
Microfluidic‐based engineering tools for controlling O2 content. (A) Image of a gradient‐generating microfluidic device. (B) Schematic of step O2 concentrations formed in each outlet of the gradient‐generating microfluidic device. O2 diffusion in the chamber is controlled by diffusion through 3‐sided glass coating. (C) Schematic of the microfluidic device capable of performing collective cell migration assays with O2 gradients. (D) Photographs of the fabricated microfluidic devices. O2 scavenging chemical reaction between pyrogallol and NaOH was performed in the reaction channel in order to generate O2 gradient. (E) Schematic illustration of O2 consumption and gradient‐generating mechanism in a microfluidic chamber. Reprint with permission
8.3. Enzymatic reactions
Recently, O2‐consuming enzymes have been exploited as an alternative strategy to create hypoxic culture environments.105 The most widely used O2‐consuming enzyme is glucose oxidase (GOX), which converts glucose, oxygen and water into gluconic acid and hydrogen peroxide (H2O2).106 An endogenous enzyme, GOX, has been used in cancer diagnosis and treatment. For example, the consumption of glucose and oxygen may be exploited for cancer‐starvation and hypoxia‐activated therapy, respectively.107 On the other hand, the reaction product gluconic acid may be employed for pH‐responsive drug release. Finally, H2O2 generated in the reaction can be converted into toxic hydroxyl radicals for cancer cell killing.107 While the reaction of GOX is fast and effective, one significant drawback for its application in cell studies is the production of cytotoxic H2O2, the accumulation of which can lead to undesired cellular toxicity, but can also inactivate GOX.108, 109 To minimize the cytotoxic by‐product of GOX reactions, catalase (CAT) can be used to reduce H2O2 into water. However, this reaction partially offsets hypoxia by producing half an oxygen (Figure 3A). Dawes et al designed GOX immobilized polyethylene glycol diacrylate (PEGDA) hydrogel for extended hypoxic cell cultures (Figure 3B).105 Immobilization of O2‐consuming GOX within covalently cross‐linked hydrogels provides an easy method to control solution O2 tension without using external devices (2.5%‐9%). Furthermore, through the introduction of CAT in cell culture media, duration of hypoxic conditions and concentrations of H2O2 were adjusted to minimize cytotoxicity and enzyme inactivation (decreased from 9 mM to 2 mM). Hudson et al followed up the study with improved processing methods to increase the flexibility and stability of the hypoxia‐inducing hydrogel system.110 While both freshly prepared and lyophilized PEGDA‐GOX hydrogel generated low O2 environments rapidly, lyophilization negatively affected enzyme activity. This could be prevented by using cryoprotectants, such as trehalose and raffinose, during freeze‐drying. Ideally, this approach would not only increase the flexibility of using the enzyme‐immobilized hydrogels but also add commercialization potential. In another experiment, Li et al designed a new approach for O2 tensions using GOX.111 Specifically, GOX/CAT‐containing chitosan coating was applied to the 3D‐printed inserts (Figure 3C). Since O2‐consuming biomaterials were immobilized in the chitosan matrix, O2 consumption only occurs on the surface of biomaterial and hypoxia formed underneath the polymer/enzyme coating (4.7 mmHg to 61.1 mmHg).
FIGURE 3.
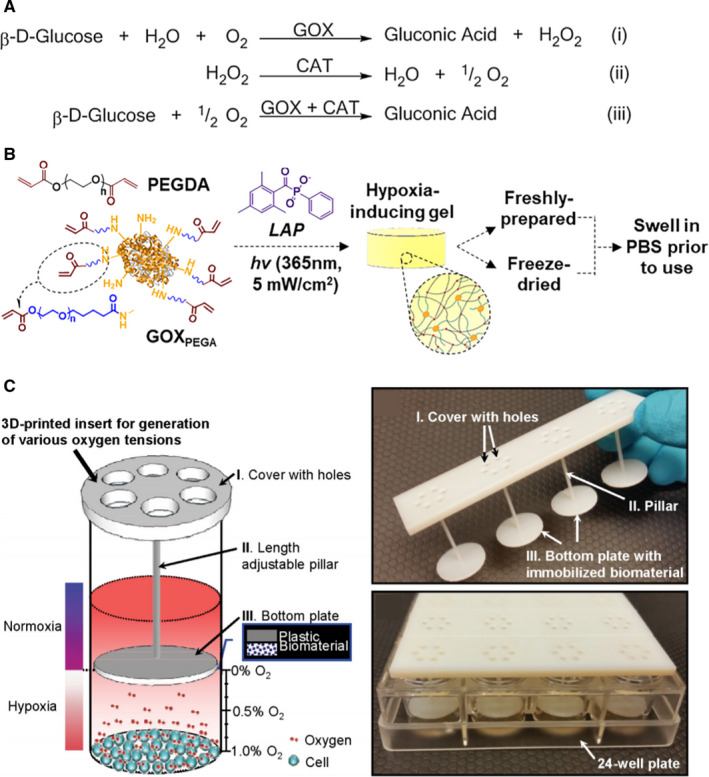
O2‐consuming enzymes for controlling O2 content. (A) The principle of the glucose oxidase/catalase (GOX/CAT) system for independent control of hypoxia and H2O2 level. (B) Reaction scheme of GOX immobilized PEGDA hydrogel. (C) Conceptual illustration and digital images of the 3D‐printed insert with immobilized biomaterial for on demand generation of various O2 tensions for in vitro cell cultures. The biomaterial consisting of glucose oxidase and catalase enzymes consumes O2 in the cell culture media without interfering with the testing environment. Reprint with permission
Another enzyme‐mediated O2‐consuming reaction is through using laccase,112 which also consumes O2 during its catalytic reaction. Park et al developed hypoxia‐inducible (HI) hydrogels by immobilizing laccase substrates (eg ferulic acid [FA] or tyramine [TA]) to the polymer chains. Laccase is a Cu‐containing enzyme that catalyses one‐electron phenolic compounds by transferring four electrons from four substrate molecules to one molecule of molecular oxygen that is then reduced to water.113 Specifically, FA or TA was first immobilized to the polymers (eg gelatin and dextran) via standard carbodiimide chemistry.112, 114 The addition of laccase to the FA/TA‐immobilized polymers resulted in enzymatic cross‐linking of the polymers while the reactions consumed O2 simultaneously (1.8%‐15% according to the thickness of hydrogel). This HI hydrogel technique was used to create a hypoxic microenvironment for a variety of cellular in vitro studies, with potential options in in vivo settings (Figure 4A).115, 116 The duration of hypoxia can be extended by increasing the thickness of the HI hydrogels. As the thickness of the hydrogel increases, the diffusion of O2 in the media or atmosphere decreases and the hypoxic duration in the matrix increases (Figure 4B,C). Laccase‐mediated reactions were shown to be cytocompatible, and the HI hydrogels were supportive of vascular network formation from the encapsulated endothelial colony‐forming cells (ECFCs) owing to the increased secretion of angiogenic growth factors. In principle, this strategy can be easily applied to other natural/synthetic macromers, such as polyethylene glycol (PEG) or hyaluronic acid (HA). Some drawbacks of this approach include the following: (1) the extent of hypoxia is limited by the amount of substrate immobilized to the polymer, and (2) the maintenance of hypoxia relies on limiting diffusion of O2 into the hydrogel network.
FIGURE 4.
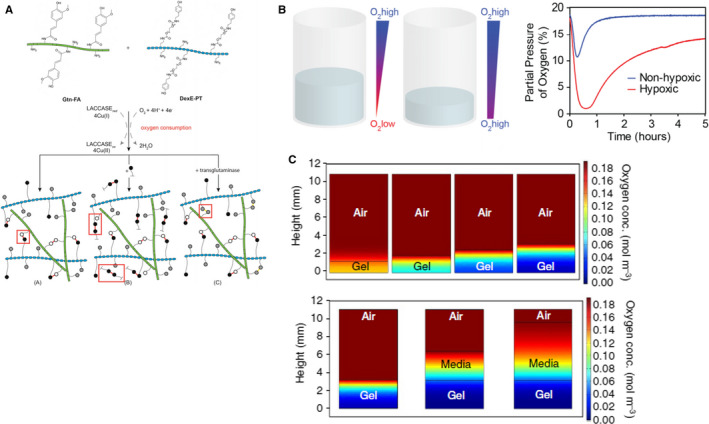
Laccase‐based hypoxia‐inducible hydrogels for controlling O2 content in 3D. (A) Schematic representation of HI hydrogel formation. HI hydrogels are formed via laccase‐mediated dimerization of FA molecules with O2 consumption. (B) Hydrogel height controls O2 gradients. (C) Model predictions of O2 levels and gradients after 30 min of hydrogel formation in the layer model (airgel and air–media‐gel). Reprint with permission
8.4. Diffusion barriers
O2 gradients can be also created and adjusted using a diffusion barrior with different gas permeability. For example, Yi et al designed human glioblastoma (GBM) on a chip model using gas‐permeable polydimethylsiloxane (PDMS) as the diffusion barrior.117 Using a 3D‐printing system, the chip was first fabricated on a non‐permeable glass substrate, followed by printing a ring of endothelial cell‐ladened brain decellularized extracellular matrix (BdECM). Finally, BdECM bioink ladened with GBM cells was printed inside the ring, while the top of the silicon chamber was covered with a glass slip. In this design, O2 was only available to the cells via the gas‐permeable silicone chamber wall. As the cells located in the centre of the device consumed O2, a radial O2 gradient was generated. The combination of cell‐laden matrix with a mechanism for generating O2 gradient using 3D bioprinting provides a novel approach to interrogate the influence of biochemical and biophysical cues on cancer cell progression.
In another study, Campillo et al designed custom‐made co‐culture system based on thin membranes permeable to O2.118 The device consisted of two PDMS well layers separated by a commercially available membrane. O2 concentration over the cell culture can be tightly controlled via direct diffusion from 12% to 1% through the gas‐permeable membrane from a gas source connected to the lower layer. Therefore, cells cultured in the Transwell insert were exposed to either the same or different O2 levels as those cells growing on the chip surface. Another approach to generating O2 gradients through a diffusion barrier is using layered papers.80 For example, Derda et al reported a strategy for controlling the distribution of cultured cells in 3D by fabricating multi‐laminated structures of fibre‐supported hydrogels with each layer composed of paper impregnated with an ECM hydrogel.119 In each layer, Matrigel precursor containing suspended cells was added to a paper support. By stacking and destacking cell‐containing layers, it was possible to manipulate gradients of nutrients and O2 in these constructs and to characterize cells grown in these complex gradients. These diffusion barrier systems are a powerful tool to precisely mimic and control the O2 content in the in vitro cancer models. However, one common disadvantage of diffusion barrier induced O2 gradients is that the process for creating the barriers (eg printing and stacking of paper‐based barrier, printing of silicone chambers) maybe complicated and time‐consuming.
9. Conclusion
Cancer continues to represent a leading cause of mortality on a global scale, accounting for approximately 20 million new cases and 10 million deaths each year.120 Despite progress in understanding cancer biology, current treatment strategies generally fail to achieve a cure due to the development of treatment resistance and disease relapse. Growing evidence suggests that the poor outcome is at least in part due to a small fraction of cancer cells (minimal residual disease, MRD) that outlive initial treatments by migrating into specialized, frequently O2‐deprived, niches where they seek protection from therapeutic elimination.121, 122 As a result, tumour hypoxia has moved into the centre of interest as a therapeutically exploitable phenomenon in a wide variety of neoplasms. Efforts to target the multifaceted complex mechanisms underlying MRD, however, have only yielded limited success so far.123, 124, 125 A major contributor to the slow progress in targeting MRD is the difficulty of studying hypoxic cancer cells ex vivo and the use of un‐physiologic cell culture techniques. While O2 levels can vary widely within the human body, it is estimated that most tissues range between 2% and 9% O2. In contrast, rapidly growing, aggressive tumour tissue can be nearly anoxic, particularly in the centre.126, 127 Standard cell culturing techniques carried out in ambient air (normoxia, 21% O2) are thus not consistent with physiological conditions. Current efforts to study cancer cell biology under physiological, hypoxic conditions in vitro utilize specialized incubators and hypoxic chambers to control the O2 tension in cell culture assays. These approaches, however, are technically cumbersome and limited by O2 diffusion and equilibration. In addition, the use of hypoxic incubators and chambers foresees constant levels of hypoxia, whereas recent studies, including mathematical modelling of O2 distribution in tumour tissues, suggest the existence of O2 gradients.80, 128, 129 In order to develop novel strategies to more effectively target cancer cells, a deeper understanding of the molecular mechanisms induced by different levels of hypoxia is needed. This, however, requires innovative, cancer‐relevant technologies to examine therapeutic escape mechanisms that focus on the role of hypoxia and O2 gradients within the tumour tissue. Such technologies may hold the key for the identification and delineation of yet unknown mechanisms underlying drug resistance and MRD, opening new research horizons for the development of novel therapeutic targets and strategies. Yet, the development of experimental methods and tools to study tumour cell biology under controlled O2 conditions has been challenging. As reviewed here, recent efforts with microfluidic devices, enzymatic reactions, hydrogels and 3D‐printing platforms represent innovative solutions to overcome previous limitations and offer opportunities to mimic physiologically relevant O2 dynamics and spatial properties such as encountered by neoplastic cells in their natural habitats. It can be expected that such engineered platforms will help unveil formerly unknown key molecular pathways that act in response to hypoxic stress and O2 gradients. Such new knowledge will likely improve our ability to differentiate and target the metabolism of cancer cells while sparing normal tissues, thereby enhancing the chances of a cure.
CONFLICT OF INTEREST
The authors confirm that there are no conflicts of interest.
AUTHOR CONTRIBUTIONS
Min Hee Kim: Writing‐original draft (equal); Writing‐review & editing (equal). Steven D Green: Writing‐original draft (equal); Writing‐review & editing (equal). Chien‐Chi Lin: Writing‐original draft (equal); Writing‐review & editing (equal). Heiko Konig: Writing‐original draft (equal); Writing‐review & editing (equal).
ACKNOWLEDGEMENTS
This work was supported in part by the National Cancer Institute (R01CA227737, to CL) and IU Simon Comprehensive Cancer Center/Walther Cancer Foundationvia an Oncology Physical Sciences & Engineering Research Embedding Program Award (to CL, HK).
Kim MH, Green SD, Lin C‐C, Konig H. Engineering Tools for Regulating Hypoxia in Tumour Models. J Cell Mol Med. 2021;25:7581–7592. 10.1111/jcmm.16759
Kim and Green equally contributed to this study.
Lin and Konig are Co‐senior authors.
Contributor Information
Chien‐Chi Lin, Email: lincc@iupui.edu.
Heiko Konig, Email: hkonig@iupui.edu.
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
REFERENCES
- 1.Partlow BP, Applegate MB, Omenetto FG, Kaplan DL. Dityrosine Cross‐Linking in Designing Biomaterials. ACS Biomaterials Science & Engineering. 2016;2(12):2108–2121 [DOI] [PubMed] [Google Scholar]
- 2.Carreau A, Hafny‐Rahbi BE, Matejuk A, Grillon C, Kieda C. Why is the partial oxygen pressure of human tissues a crucial parameter? Small molecules and hypoxia. J Cell Mol Med. 2011;15(6):1239‐1253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hung SP, Ho JH, Shih YRV, Lo T, Lee OK. Hypoxia promotes proliferation and osteogenic differentiation potentials of human mesenchymal stem cells. J Orthop Res. 2012;30(2):260‐266 [DOI] [PubMed] [Google Scholar]
- 4.D'Ippolito G, Diabira S, Howard GA, Roos BA, Schiller PC. Low oxygen tension inhibits osteogenic differentiation and enhances stemness of human MIAMI cells. Bone. 2006;39(3):513‐522 [DOI] [PubMed] [Google Scholar]
- 5.Brennan MD, Rexius‐Hall ML, Elgass LJ, Eddington DT. Oxygen control with microfluidics. Lab Chip. 2014;14(22):4305‐4318 [DOI] [PubMed] [Google Scholar]
- 6.Carmeliet P. Jain RK. Angiogenesis in cancer and other diseases. Nature. 2000;407(6801):249‐257 [DOI] [PubMed] [Google Scholar]
- 7.Mazure NM, Pouysségur J. Hypoxia‐induced autophagy: cell death or cell survival? Curr Opin Cell Biol. 2010;22(2):177‐180 [DOI] [PubMed] [Google Scholar]
- 8.Manalo DJ, Rowan A, Lavoie T, et al. Transcriptional regulation of vascular endothelial cell responses to hypoxia by HIF‐1. Blood. 2005;105(2):659‐669 [DOI] [PubMed] [Google Scholar]
- 9.Mole DR, Blancher C, Copley RR, et al. Genome‐wide Association of Hypoxia‐inducible Factor (HIF)‐1α and HIF‐2α DNA Binding with Expression Profiling of Hypoxia‐inducible Transcripts. J Biol Chem. 2009;284(25):16767‐16775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xia X, Lemieux ME, Li W, et al. Integrative analysis of HIF binding and transactivation reveals its role in maintaining histone methylation homeostasis. Proc Natl Acad Sci. 2009;106(11):4260‐4265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Semenza GLOxygen sensing, homeostasis and disease. N Engl J Med. 2011;365(6):537‐547 [DOI] [PubMed] [Google Scholar]
- 12.Bruick RK, McKnight SLJS. A conserved family of prolyl‐4‐hydroxylases that modify HIF. Science. 2001;294(5545):1337‐1340 [DOI] [PubMed] [Google Scholar]
- 13.Maxwell PH, Wiesener MS, Chang G‐W, et al. The tumour suppressor protein VHL targets hypoxia‐inducible factors for oxygen‐dependent proteolysis. Nature. 1999;399(6733):271‐275. [DOI] [PubMed] [Google Scholar]
- 14.Wang GI, Jiang BH, Rue EA, Semenza GL. Hypoxia‐inducible factor 1 is a basic‐helix‐loop‐helix‐PAS heterodimer regulated by cellular O2 tension. Proc Natl Acad Sci. 1995;92(12):5510‐5514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lando D, Peet DJ, Gorman JJ, et al. FIH‐1 is an asparaginyl hydroxylase enzyme that regulates the transcriptional activity of hypoxia‐inducible factor. Genes Dev. 2002;16(12):1466‐1471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McNEILL LA, Hewitson KS, Claridge TD, Seibel JF, Horsfall LE, Schofield CJ. Hypoxia‐inducible factor asparaginyl hydroxylase (FIH‐1) catalyses hydroxylation at the β‐carbon of asparagine‐803. Biochemical Journal. 2002;367(3):571‐575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jiang BH, Rue E, Wang GL, Roe R, Semenza GL. Dimerization, DNA binding, and transactivation properties of hypoxia‐inducible factor 1. J Biol Chem. 1996;271(30):17771‐17778. [DOI] [PubMed] [Google Scholar]
- 18.Schödel J, Oikonomopoulos S, Ragoussis J, Pugh CW, Ratcliffe PJ, Mole DR. High‐resolution genome‐wide mapping of HIF‐binding sites by ChIP‐seq. Blood. 2011;117(23):e207–e217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ginouves A, Ilc K, Macías N, Pouysségur J, Berra E. PHDs overactivation during chronic hypoxia desensitizes HIFα and protects cells from necrosis. PNAS. 2008;105(12):4745‐4750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jiang B‐H, Semenza GL, Bauer C, Marti HH. Hypoxia‐inducible factor 1 levels vary exponentially over a physiologically relevant range of O2 tension. American Journal of Physiology‐Cell Physiology. 1996;271(4):C1172‐C1180 [DOI] [PubMed] [Google Scholar]
- 21.Chiche J, Brahimi‐Horn MC, Pouysségur J. Tumour hypoxia induces a metabolic shift causing acidosis: a common feature in cancer. J Cell Mol Med. 2010;14(4):771‐794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bristow RG. Hypoxia, DNA repair and genetic instability. Nat Rev Cancer. 2008;8(3):180‐192 [DOI] [PubMed] [Google Scholar]
- 23.Eales K, Hollinshead K, Tennant DA. Hypoxia and metabolic adaptation of cancer cells. Oncogenesis. 2016;5(1):e190–e190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liao D, Johnson RSJC, Reviews M. Hypoxia: A key regulator of angiogenesis in cancer. Cancer Metastasis Rev. 2007;26(2):281‐290 [DOI] [PubMed] [Google Scholar]
- 25.Benita Y, Kikuchi H, Smith AD, Zhang MQ, Chung DC, Xavier RJ. An integrative genomics approach identifies Hypoxia Inducible Factor‐1 (HIF‐1)‐target genes that form the core response to hypoxia. Nucleic Acids Res. 2009;37(14):4587‐4602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vaupel P, Mayer AJC, Reviews M. Hypoxia in cancer: significance and impact on clinical outcome. Cancer Metastasis Rev. 2007;26(2):225‐239 [DOI] [PubMed] [Google Scholar]
- 27.Semenza GL. HIF‐1 mediates metabolic responses to intratumoral hypoxia and oncogenic mutations. Journal of Clinical Investigation. 2013;123(9):3664‐3671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gordan JD, Bertout JA, Hu C‐J, Diehl JA, Simon MCJCc.HIF‐2α Promotes Hypoxic Cell Proliferation by Enhancing c‐Myc Transcriptional Activity. Cancer Cell. 2007;11(4):335‐347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schmid T, Zhou J, Brüne B. HIF‐1 and p53: communication of transcription factors under hypoxia. Journal of Cellular and Molecular Medicine. 2004;8(4):423–431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hutchison GJ, Valentine HR, Loncaster JA, et al. Hypoxia‐inducible factor 1α expression as an intrinsic marker of hypoxia. Clin Cancer Res. 2004;10(24):8405‐8412 [DOI] [PubMed] [Google Scholar]
- 31.Mayer A, Wree A, Höckel M, Leo C, Pilch H, Vaupel P. Lack of Correlation between expression of HIF‐1α protein and oxygenation status in identical tissue areas of squamous cell carcinomas of the uterine cervix. Can Res. 2004;64(16):5876‐5881 [DOI] [PubMed] [Google Scholar]
- 32.Gatenby RA. Gawlinski ET. The glycolytic phenotype in carcinogenesis and tumor invasion: insights through mathematical models. Cancer Res. 2003;63(14):3847‐3854 [PubMed] [Google Scholar]
- 33.Ullah MS, Davies AJ, Halestrap AP. The Plasma Membrane Lactate Transporter MCT4, but Not MCT1, Is Up‐regulated by Hypoxia through a HIF‐1α‐dependent Mechanism*. Journal of Biological Chemistry. 2006;281(14):9030–9037 [DOI] [PubMed] [Google Scholar]
- 34.Semenza GL, Jiang B‐H, Leung SW, et al. Hypoxia response elements in the Aldolase A, enolase 1, and lactate dehydrogenase a gene promoters contain essential binding sites for hypoxia‐inducible factor 1. J Biol Chem. 1996;271(51):32529‐32537 [DOI] [PubMed] [Google Scholar]
- 35.Schofield CJ. Schofield CJ, Ratcliffe PJ. Oxygen sensing by HIF hydroxylases. Nat Rev Mol Cell Biol. 2004;5(5):343‐354 [DOI] [PubMed] [Google Scholar]
- 36.Kaplon J, Zheng L, Meissl K, et al. A key role for mitochondrial gatekeeper pyruvate dehydrogenase in oncogene‐induced senescence. Nature. 2013;498(7452):109‐112. [DOI] [PubMed] [Google Scholar]
- 37.Fukuda R, Zhang H, Kim J‐W, Shimoda L, Dang CV, Semenza GLJC. HIF‐1 Regulates cytochrome oxidase subunits to optimize efficiency of respiration in hypoxic cells. Cell. 2007;129(1):111‐122 [DOI] [PubMed] [Google Scholar]
- 38.Semenza GL. Oxygen‐dependent regulation of mitochondrial respiration by hypoxia‐inducible factor 1. Biochemical Journal. 2007;405(1):1‐9 [DOI] [PubMed] [Google Scholar]
- 39.Pescador N, Villar D, Cifuentes D, et al. Hypoxia Promotes Glycogen Accumulation through Hypoxia Inducible Factor (HIF)‐Mediated Induction of Glycogen Synthase 1. PLoS One. 2010;5(3):e9644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Favaro E, Bensaad K, Chong MG, et al. Glucose Utilization via Glycogen Phosphorylase Sustains Proliferation and Prevents Premature Senescence in Cancer Cells. Cell Metab. 2012;16(6):751‐764 [DOI] [PubMed] [Google Scholar]
- 41.Mullen AR, Wheaton WW, Jin ES, et al. Reductive carboxylation supports growth in tumour cells with defective mitochondria. Nature. 2012;481(7381):385‐388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Metallo CM, Gameiro PA, Bell EL, et al. Reductive glutamine metabolism by IDH1 mediates lipogenesis under hypoxia. Nature. 2012;481(7381):380‐384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wise DR, Ward PS, Shay JE, et al. Hypoxia promotes isocitrate dehydrogenase‐dependent carboxylation of α‐ketoglutarate to citrate to support cell growth and viability Proc Natl Acad Sci. 2011;108(49):19611‐19616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kamphorst JJ, Chung MK, Fan J, Rabinowitz JD. Quantitative analysis of acetyl‐CoA production in hypoxic cancer cells reveals substantial contribution from acetate. Cancer & Metabolism. 2014;2(1):1‐8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Frezza C, Zheng L, Tennant DA, et al. Metabolic profiling of hypoxic cells revealed a catabolic signature required for cell survival. PLoS One. 2011;6(9):e24411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dewhirst MW. Relationships between Cycling Hypoxia, HIF‐1, Angiogenesis and Oxidative Stress. Radiation Research. 2009;172(6):653–665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Graeber TG, Osmanian C, Jacks T, et al. Hypoxia‐mediated selection of cells with diminished apoptotic potential in solid tumours. Nature. 1996;379(6560):88‐91 [DOI] [PubMed] [Google Scholar]
- 48.Kondo A, Safaei R, Mishima M, Niedner H, Lin X, Howell SBJCR. Hypoxia‐induced enrichment and mutagenesis of cells that have lost DNA mismatch repair. Cancer Res. 2001;61(20):7603‐7607 [PubMed] [Google Scholar]
- 49.Greijer A E. The role of hypoxia inducible factor 1 (HIF‐1) in hypoxia induced apoptosis. Journal of Clinical Pathology. 2004;57(10):1009–1014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bindra RS, Schaffer PJ, Meng A, et al. Down‐regulation of Rad51 and decreased homologous recombination in hypoxic cancer cells. Mol Cell Biol. 2004;24(19):8504‐8518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Noman MZ, Hasmim M, Messai Y, et al. Hypoxia: a key player in antitumor immune response. A review in the theme: Cellular responses to hypoxia. American Journal of Physiology‐Cell Physiology. 2015;309(9):C569‐C579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ledoux S, Yang R, Friedlander G. Laouari D. Glucose depletion enhances P‐glycoprotein expression in hepatoma cells: role of endoplasmic reticulum stress response. Cancer Res. 2003;63(21):7284‐7290 [PubMed] [Google Scholar]
- 53.Mohyeldin A, Garzón‐Muvdi T, Quiñones‐Hinojosa A. Oxygen in Stem Cell Biology: A Critical Component of the Stem Cell Niche. Cell Stem Cell. 2010;7(2):150–161 [DOI] [PubMed] [Google Scholar]
- 54.Eliasson P. Jönsson JIJJocp. The hematopoietic stem cell niche: low in oxygen but a nice place to be. 2010;222(1):17‐22. [DOI] [PubMed] [Google Scholar]
- 55.Vito A, El‐Sayes N, Mossman K. Hypoxia‐Driven Immune Escape in the Tumor Microenvironment. Cells. 2020;9(4):992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Noman MZ, Hasmim M, Lequeux A, et al. Improving Cancer Immunotherapy by Targeting the Hypoxic Tumor Microenvironment: New Opportunities and Challenges. Cells. 2019;8(9):1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Milosevic M, Warde P, Ménard C, et al. Tumor hypoxia predicts biochemical failure following radiotherapy for clinically localized prostate cancer. Clin Cancer Res. 2012;18(7):2108‐2114 [DOI] [PubMed] [Google Scholar]
- 58.Vergis R, Corbishley CM, Norman AR, et al. Intrinsic markers of tumour hypoxia and angiogenesis in localised prostate cancer and outcome of radical treatment: a retrospective analysis of two randomised radiotherapy trials and one surgical cohort study. Lancet Oncol. 2008;9(4):342‐351 [DOI] [PubMed] [Google Scholar]
- 59.Nordsmark M, Bentzen SM, Rudat V, et al. Prognostic value of tumor oxygenation in 397 head and neck tumors after primary radiation therapy. An international multi‐center study. Radiother Oncol. 2005;77(1):18‐24 [DOI] [PubMed] [Google Scholar]
- 60.Nordsmark M, Overgaard J. A confirmatory prognostic study on oxygenation status and loco‐regional control in advanced head and neck squamous cell carcinoma treated by radiation therapy. Radiotherapy and Oncology. 2000;57(1):39–43 [DOI] [PubMed] [Google Scholar]
- 61.Zhong H, De Marzo AM, Laughner E, et al. Overexpression of hypoxia‐inducible factor 1α in common human cancers and their metastases. Cancer Res. 1999;59(22):5830–5835 [PubMed] [Google Scholar]
- 62.Yamamoto Y, Ibusuki M, Okumura Y, et al. Hypoxia‐inducible factor 1α is closely linked to an aggressive phenotype in breast cancer. Breast Cancer Res Treat. 2008;110(3):465‐475 [DOI] [PubMed] [Google Scholar]
- 63.Sundfør K, Lyng H. Rofstad EK. Tumour hypoxia and vascular density as predictors of metastasis in squamous cell carcinoma of the uterine cervix. Br J Cancer. 1998;78(6):822‐827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Janssens GO, Rademakers SE, Terhaard CH, et al. Accelerated radiotherapy with carbogen and nicotinamide for laryngeal cancer: Results of a Phase III randomized trial. J Clin Oncol. 2012;30(15):1777‐1783 [DOI] [PubMed] [Google Scholar]
- 65.Hoskin P J, Rojas A M, Bentzen S M, Saunders M I. Radiotherapy With Concurrent Carbogen and Nicotinamide in Bladder Carcinoma. Journal of Clinical Oncology. 2010;28(33):4912–4918 [DOI] [PubMed] [Google Scholar]
- 66.Overgaard J, Hansen HS, Overgaard M, et al. A randomized double‐blind phase III study of nimorazole as a hypoxic radiosensitizer of primary radiotherapy in supraglottic larynx and pharynx carcinoma. Results of the Danish Head and Neck Cancer Study (DAHANCA) Protocol 5‐85. Radiother Oncol 1998;46(2):135‐146 [DOI] [PubMed] [Google Scholar]
- 67.Rischin D, Peters LJ, O’Sullivan B, et al. Tirapazamine, cisplatin, and radiation versus cisplatin and radiation for advanced squamous cell carcinoma of the head and neck (TROG 02.02, HeadSTART): a phase III trial of the Trans‐Tasman Radiation Oncology. Group. 2008 [DOI] [PubMed] [Google Scholar]
- 68.Tap WD, Papai Z, Van Tine BA, et al. Doxorubicin plus evofosfamide versus doxorubicin alone in locally advanced, unresectable or metastatic soft‐tissue sarcoma (TH CR‐406/SARC021): an international, multicentre, open‐label, randomised phase 3 trial. Lancet Oncol. 2017;18(8):1089‐1103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bhandari V, Hoey C, Liu LY, et al. Molecular landmarks of tumor hypoxia across cancer types. Nat Genet. 2019;51(2):308‐318 [DOI] [PubMed] [Google Scholar]
- 70.Haider S, McIntyre A, van Stiphout RG , et al. Genomic alterations underlie a pan‐cancer metabolic shift associated with tumour hypoxia. Genome Biol. 2016;17(1):1‐17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kulshreshtha R, Ferracin M, Wojcik SE, et al. A MicroRNA Signature of Hypoxia. Mol Cell Biol. 2007;27(5):1859‐1867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kulshreshtha R, Davuluri RV, Calin GA, Ivan M. A microRNA component of the hypoxic response. Cell Death & Differentiation. 2008;15(4):667–671 [DOI] [PubMed] [Google Scholar]
- 73.Dhani N, Fyles A, Hedley D, Milosevic M. Clinical Significance of Hypoxia in Human Cancers. Semin Nucl Med. 2015;45(2):110‐121 [DOI] [PubMed] [Google Scholar]
- 74.Toustrup K, Sørensen BS, Lassen P, et al. Gene expression classifier predicts for hypoxic modification of radiotherapy with nimorazole in squamous cell carcinomas of the head and neck. Radiother Oncol. 2012;102(1):122‐129 [DOI] [PubMed] [Google Scholar]
- 75.Zhou H‐S, Carter BZ. Andreeff M. Bone marrow niche‐mediated survival of leukemia stem cells in acute myeloid leukemia:Yin and Yang. Cancer Biol Me. 2016;13(2):248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Schumann K, Lämmermann T, Bruckner M, et al. Immobilized Chemokine Fields and Soluble Chemokine Gradients Cooperatively Shape Migration Patterns of Dendritic Cells. Immunity. 2010;32(5):703‐713 [DOI] [PubMed] [Google Scholar]
- 77.Lee E‐J, Hwang C‐M, Baek D‐H, Lee S‐H. Fabrication of microfluidic system for the assessment of cell migration on 3D micropatterned substrates. Annu Int Conf IEEE Eng Med Biol Soc. 2009:6034‐6037 [DOI] [PubMed] [Google Scholar]
- 78.Wu J, Sheibani N. Modulation of VE‐cadherin and PECAM‐1 mediated cell‐cell adhesions by mitogen‐activated protein kinases. J Cell Biochem. 2003;90(1):121–137 [DOI] [PubMed] [Google Scholar]
- 79.Frieboes HB, Zheng X, Sun C‐H, Tromberg B, Gatenby R, Cristini V. An Integrated Computational/Experimental Model of Tumor Invasion. Can Res. 2006;66(3):1597–1604 [DOI] [PubMed] [Google Scholar]
- 80.Mosadegh B, Lockett MR, Minn KT, et al. A paper‐based invasion assay: Assessing chemotaxis of cancer cells in gradients of oxygen. Biomaterials. 2015;52:262‐271 [DOI] [PubMed] [Google Scholar]
- 81.Chen X, Li Y, Lin Q, et al. Tea polyphenols induced apoptosis of breast cancer cells by suppressing the expression of Survivin. Sci Rep. 2014;4:4416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sleeboom JJ, Den Toonder JM, Sahlgren CM. MDA‐MB‐231 Breast Cancer Cells and Their CSC Population Migrate Towards Low Oxygen in a Microfluidic Gradient Device. Int J Mol Sci. 2018;19(10):3047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Gelmini S, Mangoni M, Serio M, Romagnani P, Lazzeri E. The critical role of SDF‐1/CXCR4 axis in cancer and cancer stem cells metastasis. J Endocrinol Invest. 2008;31(9):809–819 [DOI] [PubMed] [Google Scholar]
- 84.Ceradini DJ, Kulkarni AR, Callaghan MJ, et al. Progenitor cell trafficking is regulated by hypoxic gradients through HIF‐1 induction of SDF‐1. Nat Med. 2004;10(8):858–864 [DOI] [PubMed] [Google Scholar]
- 85.Prathivadhi‐Bhayankaram SV, Ning J, Mimlitz M, et al. Chemotherapy impedes in vitro microcirculation and promotes migration of leukemic cells with impact on metastasis. Biochem Biophys Res Commun. 2016;479(4):841‐846 [DOI] [PubMed] [Google Scholar]
- 86.Cheema U, Brown R, Alp B, MacRobert A. Spatially defined oxygen gradients and vascular endothelial growth factor expression in an engineered 3D cell model. Cell Mol Life Sci. 2008;65(1):177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Barmaki S, Jokinen V, Obermaier D, et al. A microfluidic oxygen sink to create a targeted cellular hypoxic microenvironment under ambient atmospheric conditions. Acta Biomater. 2018;73:167‐179 [DOI] [PubMed] [Google Scholar]
- 88.Bhatti M, MacRobert A, Meghji S, Henderson B, Wilson M. A study of the uptake of toluidine blue O by porphyromonas gingivalis and the mechanism of lethal photosensitization. Photochemistry and Photobiology. 1998;68(3):370–376 [PubMed] [Google Scholar]
- 89.Guo M, Song L‐P, Jiang Y, Liu W, Yu Y, Chen G‐Q. Hypoxia‐mimetic agents desferrioxamine and cobalt chloride induce leukemic cell apoptosis through different hypoxia‐inducible factor‐1α independent mechanisms. Apoptosis. 2006;11(1):67‐77 [DOI] [PubMed] [Google Scholar]
- 90.Yuan Y, Hilliard G, Ferguson T, Millhorn DE. Cobalt inhibits the interaction between hypoxia‐inducible factor‐α and von Hippel‐Lindau protein by direct binding to hypoxia‐inducible factor‐α. J Biol Chem. 2003;278(18):15911‐15916 [DOI] [PubMed] [Google Scholar]
- 91.Heirani‐Tabasi A, Mirahmadi M, Mishan MA, et al. Comparison the effects of hypoxia‐mimicking agents on migration‐related signaling pathways in mesenchymal stem cells. Cell Tissue Banking. 2020;21(4):643‐653 [DOI] [PubMed] [Google Scholar]
- 92.Vengellur A, LaPres J. The role of hypoxia inducible factor 1α in cobalt chloride induced cell death in mouse embryonic fibroblasts. Toxicol Sci. 2004;82(2):638‐646 [DOI] [PubMed] [Google Scholar]
- 93.Ji Z, Yang G, Shahzidi S, et al. Induction of hypoxia‐inducible factor‐1α overexpression by cobalt chloride enhances cellular resistance to photodynamic therapy. Cancer Lett. 2006;244(2):182‐189 [DOI] [PubMed] [Google Scholar]
- 94.Sears JE, Hoppe G, Ebrahem Q, Anand‐Apte B. Prolyl hydroxylase inhibition during hyperoxia prevents oxygen‐induced retinopathy. Proc Natl Acad Sci. 2008;105(50):19898‐19903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Byrne MB, Leslie MT, Gaskins HR, Kenis PJ. Methods to study the tumor microenvironment under controlled oxygen conditions. Trends Biotechnol. 2014;32(11):556‐563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Dai Z‐J, Gao J, Ma X‐B, et al. Up‐regulation of hypoxia inducible factor‐1α by cobalt chloride correlates with proliferation and apoptosis in PC‐2 cells. Journal of Experimental & Clinical Cancer Research. 2012;31(1):28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wu D, Yotnda P. Induction and Testing of Hypoxia in Cell Culture. Journal of Visualized Experiments. 2011;54:e2899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Jiang B, Ren C, Li Y, et al. Sodium sulfite is a potential hypoxia inducer that mimics hypoxic stress in Caenorhabditis elegans. J Biol Inorg Chem. 2011;16(2):267‐274 [DOI] [PubMed] [Google Scholar]
- 99.Allen CB, Schneider BK, White CW. Limitations to oxygen diffusion and equilibration in in vitro cell exposure systems in hyperoxia and hypoxia. American Journal of Physiology‐Lung Cellular and Molecular Physiology. 2001;281(4):L1021‐L1027 [DOI] [PubMed] [Google Scholar]
- 100.Broxmeyer HE, O'Leary HA, Huang X, Mantel C. The importance of hypoxia and extra physiologic oxygen shock/stress for collection and processing of stem and progenitor cells to understand true physiology/pathology of these cells ex vivo. Curr Opin Hematol. 2015;22(4):273‐278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Shih H‐C, Lee T‐A, Wu H‐M, Ko P‐L, Liao W‐H, Tung Y‐C. Microfluidic Collective Cell Migration Assay for Study of Endothelial Cell Proliferation and Migration under Combinations of Oxygen Gradients, Tensions, and Drug Treatments. Sci Rep. 2019;9(1):1–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Khan DH, Roberts SA, Cressman JR, Agrawal N. Rapid Generation and Detection of Biomimetic Oxygen Concentration Gradients In Vitro. Scientific Reports. 2017;7(1):1–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Boyce MW, Simke WC, Kenney RM, Lockett MR. Generating linear oxygen gradients across 3D cell cultures with block‐layered oxygen controlled chips (BLOCCs). Anal Methods. 2020;12(1):18‐24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Lo JF, Sinkala E, Eddington DT. Oxygen gradients for open well cellular cultures via microfluidic substrates. Lab Chip. 2010;10(18):2394‐2401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Dawes CS, Konig H, Lin C‐C. Enzyme‐immobilized hydrogels to create hypoxia for in vitro cancer cell culture. J Biotechnol. 2017;248:25‐34 [DOI] [PubMed] [Google Scholar]
- 106.Zhang R, Feng L, Dong Z, et al. Glucose & oxygen exhausting liposomes for combined cancer starvation and hypoxia‐activated therapy. Biomaterials. 2018;162:123‐131 [DOI] [PubMed] [Google Scholar]
- 107.Fu L‐H, Qi C, Lin J, Huang PJCSR. Catalytic chemistry of glucose oxidase in cancer diagnosis and treatment. Chem Soc Rev. 2018;47(17):6454‐6472 [DOI] [PubMed] [Google Scholar]
- 108.Kornecki JF, Carballares D, Tardioli PW, et al. Enzyme production of D‐gluconic acid and glucose oxidase: successful tales of cascade reactions. Carbohydr Res. 2020;10(17):5740‐5771 [Google Scholar]
- 109.López‐Gallego F, Guisan JM. Betancor L. Immobilization of Enzymes on Supports Activated with Glutaraldehyde:A Very Simple Immobilization Protocol. Immobilization of Enzymes and Cells. Springer. 2020;119‐127 [DOI] [PubMed] [Google Scholar]
- 110.Hudson BN, Dawes CS, Liu H‐Y, et al. Stabilization of enzyme‐immobilized hydrogels for extended hypoxic cell culture. Emergent Materials. 2019;2(2):263‐272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Li C, Chaung W, Mozayan C, Chabra R, Wang P. A New Approach for On‐Demand Generation of Various Oxygen Tensions for In Vitro Hypoxia Models. PLoS One. 2016;11(5):e0155921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Park KM, Gerecht S. Hypoxia‐inducible hydrogels. Nature communications Nat Commun. 2014;5(1):1‐14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Demarche P, Junghanns C, Ardao I, Agathos SN. Dynamic measurement of oxidase activity based on oxygen consumption in open systems. Eng Life Sci. 2015;15(8):804–814 [Google Scholar]
- 114.Blatchley MR, Hall F, Wang S, Pruitt HC, Gerecht S. Hypoxia and matrix viscoelasticity sequentially regulate endothelial progenitor cluster‐based vasculogenesis. Science Advances. 2019;5(3):eaau7518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Park KM, Blatchley MR, Gerecht S. The Design of Dextran‐Based Hypoxia‐Inducible Hydrogels via In Situ Oxygen‐Consuming Reaction. Macromol Rapid Commun. 2014;35(22):1968‐1975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Blatchley M, Park KM, Gerecht S. Designer hydrogels for precision control of oxygen tension and mechanical properties. Journal of Materials Chemistry B. 2015;3(40):7939–7949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Yi H‐G, Jeong YH, Kim Y, et al. A bioprinted human‐glioblastoma‐on‐a‐chip for the identification of patient‐specific responses to chemoradiotherapy. Nature Biomedical Engineering. 2019;3(7):509‐519 [DOI] [PubMed] [Google Scholar]
- 118.Campillo N, Falcones B, Otero J, et al. Differential Oxygenation in Tumor Microenvironment Modulates Macrophage and Cancer Cell Crosstalk: Novel Experimental Setting and Proof of Concept. Frontiers in Oncology. 2019;9:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Derda R, Laromaine A, Mammoto A, et al. supported 3D cell culture for tissue‐based bioassays. Proc Natl Acad Sci U S A. 2009;106(44):18457‐18462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394‐424 [DOI] [PubMed] [Google Scholar]
- 121.Petrova V, Annicchiarico‐Petruzzelli M, Melino G, Amelio I. The hypoxic tumour microenvironment. Oncogenesis. 2018;7(1):1‐13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Cosse J‐P. Michiels C. Tumour hypoxia affects the responsiveness of cancer cells to chemotherapy and promotes cancer progression. Anticancer Agents Med Chem. 2008;8(7):790‐797 [DOI] [PubMed] [Google Scholar]
- 123.Benito J, Zeng Z, Konopleva M. Targeting hypoxia in the leukemia microenvironment. International Journal of Hematologic Oncology. 2013;2(4):279‐288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Konopleva M, Tabe Y, Zeng Z, Andreeff MJDRU. Therapeutic targeting of microenvironmental interactions in leukemia: Mechanisms and approaches. Drug Resist Updates. 2009;12(4–5):103‐113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Xia Y, Choi H‐K, Lee K. Recent advances in hypoxia‐inducible factor (HIF)‐1 inhibitors. Eur J Med Chem. 2012;49:24‐40 [DOI] [PubMed] [Google Scholar]
- 126.Begg K, Tavassoli M. Inside the hypoxic tumour: reprogramming of the DDR and radioresistance. Cell Death Discovery. 2020;6(1):1–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Ameri K, Hammond E, Culmsee C, et al. Induction of activating transcription factor 3 by anoxia is independent of p53 and the hypoxic HIF signalling pathway. Oncogene. 2007;26(2):284‐289 [DOI] [PubMed] [Google Scholar]
- 128.Lewis DM, Park KM, Tang V, et al. Intratumoral oxygen gradients mediate sarcoma cell invasion. Proc Natl Acad Sci. 2016;113(33):9292‐9297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Cristini V, Frieboes HB, Gatenby R, Caserta S, Ferrari M, Sinek JJCCR. Morphologic Instability and Cancer Invasion. Clin Cancer Res. 2005;11(19):6772‐6779 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.


