Abstract
Background:
Failed back surgery syndrome (FBSS) is a cause of significant morbidity for up to 40% of patients following spine surgery, and is estimated to cost almost $20 billion. Treatment options for these patients currently include conventional medical management (CMM), repeat operation, or spinal cord stimulation (SCS). Much of the published data regarding cost-effectiveness of SCS comprise smaller scale RCTs rather than large databases capturing practices throughout the US. SCS has been shown to have superior outcomes to CMM or repeat spinal operation in several landmark studies, yet there are few large studies examining its long-term economic impact.
Objectives:
This study compares healthcare utilization for SCS compared to other management in patients with FBSS.
Study Design:
Retrospective.
Setting:
Inpatient and outpatient sample.
Methods:
Patients with a history of FBSS from 2000–2012 were selected. We compared those who received SCS to those who underwent conventional management. A longitudinal analysis was used to model the value of log(cost) in each one year interval using a generalized estimating equations (GEE) model to account for the correlation of the same patient’s cost in multiple years. Similarly, a Poisson GEE model with the log link was applied to correlated count outcomes.
Results:
We identified 122,827 FBSS patients. Of these, 5,328 underwent SCS implantation (4.34%), and 117,499 underwent conventional management. Total annual costs decreased over time following implantation of the SCS system, with follow-up analysis at 1, 3, 6 and 9 years. The longitudinal GEE model demonstrated that placement of an SCS system was associated with an initial increase in total costs at the time of implantation (cost ratio [CR]: 1.74; 95% CI: 1.41, 2.15, p <0.001), however there was a significant and sustained 68% decrease in cost in the year following SCS placement (CR: 0.32; 95% CI: 0.24, 0.42, p <0.001) compared to CMM. There was also an aggregate time trend that for each additional year after SCS, cost decreased on average 40% percent annually (CR: 0.60; 95% CI: 0.55, 0.65, p<0.001), with follow-up up to 1, 3, 6 and 9 years post-procedure.
Limitations:
Costs are not correlated with patient outcomes, patients are not stratified in terms of complexity of prior back surgery, as well as inherent limitations of a retrospective analysis.
Conclusions:
We found that from 2000–2012, only 4.3% of patients across the United States with FBSS were treated with SCS. Long-term total annual costs for these patients were significantly reduced compared to patients with conventional management. Although implantation of an SCS system results in a short-term increase in costs at one year, the subsequent annual cumulative costs were significantly decreased long-term in the following 9 years after implantation. This study combines the largest group of FBSS patients studied to date along with the longest follow-up interval ever analyzed. Since SCS has repeatedly been shown to have superior efficacy to CMM in randomized clinical trials, the current study demonstrating improved long-term health economics at 1, 3, 6 and 9 years supports the long-term cost utility of SCS in the treatment of FBSS patients.
Keywords: Failed back surgery syndrome, spinal cord stimulation, back pain, leg pain, neuromodulation, FBSS, SCS
Introduction:
In an era of soaring health care costs and healthcare reform, there is a growing emphasis on creating cost-effective therapies for chronic conditions. Chronic low back pain (LBP) is rapidly becoming one of the most expensive conditions to treat, with an approximately 37% adult incidence and a lifetime prevalence of 60–85%. LBP is estimated to cost 12.2–90.6 billion health care dollars each year (1–3). Patients who continue to experience pain after surgical treatment for LBP are identified as having Failed Back Surgery Syndrome (FBSS), with a reported incidence rate of 10–40% (4). FBSS is an umbrella term encompassing many pathologic conditions, with common diagnoses including foraminal stenosis (25–29%), painful disc (20–22%), pseudoarthrosis (14%), neuropathic pain (10%), recurrent disc herniation (7–12%), facet joint pain (3%) and sacroiliac joint (SIJ) pain (2%) (5).
Treatment options for FBSS patients include repeat spinal operation or conventional medical management (CMM), with spinal cord stimulation (SCS) serving as an option for select patients in which CMM has failed and reoperation is undesirable (6–8). In practice, SCS is underutilized and has been shown to have lower complication rates and hospital charges than spinal reoperation. For example, in a previous study of 16,455 FBSS patients, we demonstrated that patients with SCS implantation had significantly lower 90-day complication rates (6.5% vs 14.4%, p < 0.0001) and hospital charges ($31,210 vs $40,433, p = 0.02) than patients undergoing spinal reoperation (9).
Previous randomized trials have compared SCS to repeat spinal operation and CMM. Kumar et al. found FBSS patients treated with SCS experienced improved leg and back pain relief, quality of life, functional capacity, and treatment satisfaction compared to CMM alone (10). Moreover, North et al. found that SCS was more effective than reoperation for persistent radicular pain following lumbosacral spine surgery, obviating the need for reoperation in most patients (7). More recent evidence by Kapural et. al. demonstrates improved back and leg pain relief with updated SCS therapies (11). However, despite evidence for improved outcomes with SCS, there has been disagreement with regard to its impact on long-term healthcare utilization and economic impacts. While some studies have found SCS to be cost-effective (12–14), others have suggested that the high procedure costs associated with SCS are not offset by decreased costs over time (15). The aim of the current study was to compare the healthcare utilization and associated costs of SCS and CMM in patients with FBSS.
Methods:
Patients were retrospectively queried from the Truven Reuters MarketScan® database, containing individual patient data from more than 200 million patients in the United States. The MarketScan database encompasses patient-specific data on clinical utilization, including inpatient, outpatient, medication, and laboratory information from insurance enrollment and costs. We queried patients with a history of FBSS from 2000 to 2012.
International Classification of Diseases, Ninth Revision, [ICD-9] codes were used to select patients with a diagnosis of FBSS (72283, 3382, and 3384). FBSS patients were defined as having the ICD-9 code 77283, or a chronic pain diagnosis code of 3382 or 3384 with a prior lumbar spine surgery procedure code of 63005, 63012, 63017, 63030, 63042, or 63047. Patients with a history of SCS were defined by the procedure code 63685 and one of the following codes: 63650 or 63655. Only patients with a minimum of one year of continuous data were included.
Baseline characteristics were collected for all patients, including patient age, gender, race, employment status, geographical region, and date of claim. Cost data were collected for all patients one year prior to the first diagnosis of FBSS, as well as annual costs following the initial diagnosis. Descriptive statistics were reported at the time of SCS implantation, and at 1, 3, 6, and 9-years post-implantation for SCS patients. Negative and extremely large values were removed by excluding the highest and lowest 1% of values to account for outliers. The data included total costs of all medical expenses, pain-related procedure costs, pain prescription costs, medication costs, in-patient admissions, number of pain-related procedures, and number of pain prescriptions.
A longitudinal analysis was used to model the value of log(cost) in each one year interval using a generalized estimating equations (GEE) model to account for the correlation of the same patient’s cost in multiple years. A Poisson generalized model with the log link was applied to counts outcomes. This regression method uses the count of a procedure type in a given year as the dependent variable. Each model includes sex, age, employment status, insurance, prior Charlson score, an indicator of SCS implantation year, procedure calendar year, follow-up years after FBSS diagnosis, an indicator of post-SCS year, and the interaction between indicator of post-SCS year and follow-up years after SCS as the independent variables, among which sex, employment status, and insurance were evaluated as categorical variables. All the GEE models assumed an exchangeable correlation structure for patients with multiple years of data. All analyses and data processing were conducted using SAS software, V9.4, SAS Institute Inc., Cary, NC, USA.
Results:
Patient Demographics
A total of 122,827 FBSS patients were identified, of which 5,328 (4.34%) patients underwent SCS implantation. Overall, there were more females (57.3%) than males, the median interquartile range [IQR] age was 58.8 years old [48.0 – 70.0], and 20,712 (16.9%) patients were employed full-time. Overall, just under 42% of patients had commercial insurance, with 19.7% insured by Medicaid and 38.5% insured by Medicare (Table 1).
Table 1.
Baseline characteristics of matched populations
| Total | Non-SCS patients | SCS Patients | p value | |
|---|---|---|---|---|
| Group - no. (%) | 122,827 (100.0%) | 117,499 (95.66%) | 5,328 (4.34%) | |
| Gender of Patient | <0.0001 | |||
| Male | 52416 (42.7%) | 50292 (42.8%) | 2124 (39.9%) | |
| Female | 70411 (57.3%) | 67207 (57.2%) | 3204 (60.1%) | |
| Age of Patient | <0.0001 | |||
| mean (SD) | 58.8 (14.5) | 58.8 (14.6) | 57.7 (13.7) | |
| median (IQR) | 59.0 (48.0, 70.0) | 59.0 (48.0, 70.0) | 57.0 (47.0, 68.0) | |
| Insurance | 0.1862 | |||
| Commerical | 51354 (41.8%) | 49075 (41.8%) | 2279 (42.8%) | |
| Medicaid | 24183 (19.7%) | 23180 (19.7%) | 1003 (18.8%) | |
| Medicare | 47290 (38.5%) | 45244 (38.5%) | 2046 (38.4%) |
Cost Utility at Baseline, one year prior to SCS-Implantation
Comparing both cohorts (n=122,827) at baseline, patients who went on to receive SCS required greater overall costs, total cost of pain encounters, cost of pain prescriptions, and cost of all medications than CMM patients prior to their SCS procedure (Table 2). The median [IQR] total costs (dollars) between the cohorts were CMM: 8308.3 (3611.2 – 16225.2) vs pre-SCS: 9290.1 (4340.2 – 17198.0) (p = <0.0001); costs (dollars) of pain prescriptions between the cohorts were CMM: 226.2 (32.0 – 840.5) vs pre-SCS: 383.2 (62.9 – 1191.8) (p = <0.0001); and overall medication costs were CMM: 2362.4 (721.4 – 4828.6) vs pre-SCS: 3090.2 (1033.7 – 5881.2) (p < 0.0001).
Table 2.
Baseline healthcare utilization between the cohorts (one year prior to SCS implantation)
| Total | Non-SCS patients | SCS Patients | p value | |
|---|---|---|---|---|
| Total Costs | <0.0001 | |||
| mean (SD) | 12390.6 (14388.4) | 12367.7 (14468.9) | 12895.6 (12471.8) | |
| median (IQR) | 8359.3 (3641.5, 16283.1) | 8308.3 (3611.2, 16225.2) | 9290.1 (4340.2, 17198.0) | |
| Pain Encounters Count | <0.0001 | |||
| mean (SD) | 0.3 (3.2) | 0.3 (3.2) | 0.4 (3.2) | |
| median (IQR) | 0.0 (0.0, 0.0) | 0.0 (0.0, 0.0) | 0.0 (0.0, 0.0) | |
| Total Cost for Pain-Related Encounters | <0.0001 | |||
| mean (SD) | 27.7 (504.4) | 27.0 (508.7) | 44.0 (397.4) | |
| median (IQR) | 0.0 (0.0, 0.0) | 0.0 (0.0, 0.0) | 0.0 (0.0, 0.0) | |
| Pain Prescriptions Cost | <0.0001 | |||
| mean (SD) | 736.9 (1318.7) | 727.3 (1311.2) | 947.6 (1457.9) | |
| median (IQR) | 231.3 (32.8, 853.1) | 226.2 (32.0, 840.5) | 383.2 (62.9, 1191.8) | |
| Medications Cost | <0.0001 | |||
| mean (SD) | 3346.2 (3460.4) | 3317.7 (3442.1) | 3973.5 (3789.4) | |
| median (IQR) | 2391.5 (729.7, 4874.4) | 2362.4 (721.4, 4828.6) | 3090.2 (1033.7, 5881.2) |
Cost Utility Post-SCS Implantation
Cost utility comparisons over time are displayed in Table 3. Values for averaged annual costs for non-SCS patients are shown. Moreover, costs at the time of initial SCS implantation as well as at one, three, six, and nine years post-implantation are displayed. Overall, SCS patients had increased total costs at the time of initial implantation. The median [IQR] annual total cost for the initial SCS implantation year was 13216.8 [8296.4 – 21286.4] compared to 5934.0 [1768.5 – 13360.5] for the averaged non-SCS costs.
Table 3.
Healthcare utilization over time
| Averaged Non-SCS annual cost (N=429590) |
Initial SCS Implantation year (N=5328) | 1 year post SCS (N=4014) | 3 years post SCS (N=2004) | 6 years post SCS (N=733) | 9 years post SCS (N=50) |
|
|---|---|---|---|---|---|---|
|
| ||||||
| Total Cost | ||||||
| N | 429590 | 5328 | 4014 | 2004 | 424 | 50 |
| Mean (SD) | 10103.9 (13695.0) | 16712.3 (13594.4) | 9881.8 (16214.4) | 7866.9 (12193.4) | 7533.5 (11376.0) | 5057.7 (6585.9) |
| Median | 5934.0 | 13216.8 | 5642.8 | 4121.5 | 3053.2 | 2433.0 |
| Q1, Q3 | 1768.5, 13360.5 | 8296.4, 21286.4 | 1588.3, 12891.0 | 302.4, 10832.1 | 166.3, 9355.3 | 461.3, 7185.4 |
| Pain encounters | ||||||
| N | 429590 | 5328 | 4014 | 2004 | 424 | 50 |
| Mean (SD) | 4.8 (11.8) | 24.0 (22.2) | 6.2 (14.0) | 4.3 (11.2) | 5.8 (28.3) | 5.6 (10.7) |
| Median | 0.0 | 19.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Q1, Q3 | 0.0, 4.0 | 8.0, 34.0 | 0.0, 6.0 | 0.0, 2.0 | 0.0, 3.0 | 0.0, 7.0 |
| Total cost for pain encounters | ||||||
| N | 429590 | 5328 | 4014 | 2004 | 424 | 50 |
| Mean (SD) | 683.6 (2422.5) | 4607.5 (4990.8) | 752.7 (2383.5) | 478.5 (1913.5) | 634.0 (3194.6) | 282.2 (935.1) |
| Median | 0.0 | 3032.7 | 0.0 | 0.0 | 0.0 | 0.0 |
| Q1, Q3 | 0.0, 321.9 | 1148.8, 6592.6 | 0.0, 320.0 | 0.0, 87.8 | 0.0, 75.7 | 0.0, 111.1 |
| IP admissions | ||||||
| N | 429590 | 5328 | 4014 | 2004 | 424 | 50 |
| Mean (SD) | 0.7 (1.6) | 0.7 (1.4) | 0.7 (1.5) | 0.6 (1.2) | 0.7 (1.6) | 0.5 (0.8) |
| Median | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Q1, Q3 | 0.0, 1.0 | 0.0, 1.0 | 0.0, 1.0 | 0.0, 1.0 | 0.0, 1.0 | 0.0, 1.0 |
| Pain Prescriptions | ||||||
| N | 429590 | 5328 | 4014 | 2004 | 424 | 50 |
| Mean (SD) | 11.9 (13.3) | 16.4 (14.1) | 14.3 (13.6) | 13.7 (13.1) | 12.2 (13.0) | 9.7 (10.6) |
| Median | 7.0 | 14.0 | 11.0 | 11.0 | 9.0 | 6.0 |
| Q1, Q3 | 1.0, 18.0 | 5.0, 25.0 | 3.0, 22.0 | 3.0, 21.0 | 1.0, 19.5 | 0.0, 16.0 |
| Total Pain Prescriptions cost | ||||||
| N | 429590 | 5328 | 4014 | 2004 | 424 | 50 |
| Mean (SD) | 744.0 (1424.7) | 1114.7 (1659.6) | 1043.5 (1692.1) | 1077.2 (1858.7) | 896.8 (1680.3) | 1249.7 (2437.0) |
| Median | 152.9 | 411.3 | 322.2 | 259.7 | 142.9 | 81.7 |
| Q1, Q3 | 7.3, 805.2 | 52.9, 1443.8 | 15.9, 1323.1 | 10.0, 1248.1 | 2.9, 1030.5 | 0.0, 457.6 |
| Total Medications Costs | ||||||
| N | 429590 | 5328 | 4014 | 2004 | 424 | 50 |
| Mean (SD) | 3430.1 (3630.2) | 4270.7 (4075.6) | 4118.1 (4152.7) | 4143.9 (4354.8) | 3649.7 (4464.9) | 3637.7 (5013.8) |
| Median | 2420.3 | 3309.4 | 3135.3 | 3017.8 | 2283.1 | 965.7 |
| Q1, Q3 | 597.3, 5073.9 | 1020.6, 6400.7 | 699.4, 6207.8 | 499.3, 6292.3 | 152.5, 5525.3 | 52.1, 6355.7 |
Total costs for patients who underwent SCS implantation consistently decreased over time. At one year post-SCS, the average median [IQR] total cost for SCS patients was 5642.8 [1588.3 – 12981.0], while by three years post-SCS costs were reduced even further to 4121.5 [302.4 – 10832.1]. Costs decreased further at six (3053.2 [461.3 – 7185.4] and nine (2433.0 [461.3 – 7185.4]) years post-SCS implantation to less than half of the annualized costs for the non-SCS group.
Total costs associated with pain encounters also followed a similar trend. While significantly higher at initial SCS implantation, these annual costs reduced over time and were less than the costs for the non-SCS cohort at longer follow up. Both total medication costs and pain-related medication costs followed this trend. These costs were initially higher at the time of SCS implantation for the SCS cohort, but decreased over time (Table 3).
Initial and Annual Cumulative Costs following SCS Implantation
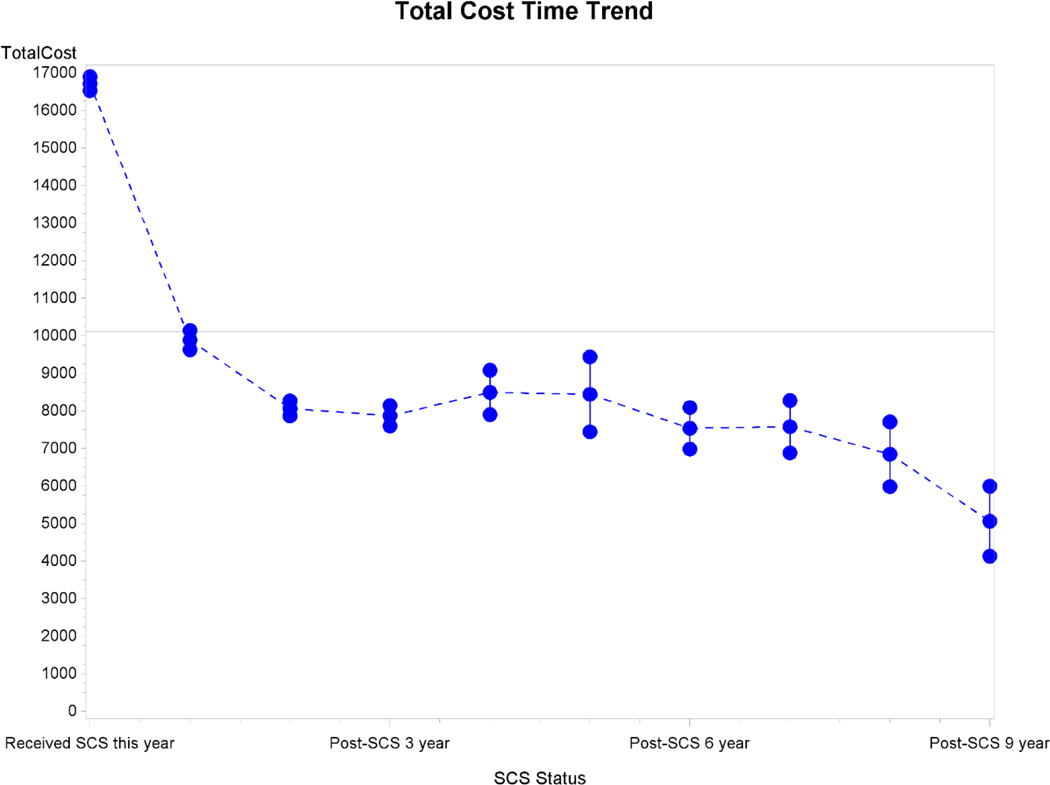
The longitudinal GEE model was used to assess the relative increase in initial costs related to implantation of the SCS system as well as the relative annual change in cost over time for patients who underwent SCS placement. These results are displayed in Table 4. The model demonstrated that placement of an SCS system was associated with an initial 74% increase in total costs (cost ratio [CR]: 1.74; 95% CI: 1.41, 2.15, p <0.001), but in the first year after SCS placement, the total cost decreased by 68% (CR: 0.32; 95% CI: 0.24, 0.42, p <0.001) compared to non-SCS patients. For each additional year after SCS implantation, the total cost decreased further by 40% (CR: 0.60; 95% CI: 0.55, 0.65, p<0.001). The time trend in total costs following SCS implantation is displayed in Figure 1.
Table 4.
Longitudinal Generalized Estimating Equation Model
| Outcome | Group | Exp Beta (95% CI) | p value |
|---|---|---|---|
| Total Costs | CMM | reference | |
| SCS- Initial placementa | 1.74 (1.41, 2.15) | <0.001 | |
| One year post-SCSb | 0.32 (0.24, 0.42) | <0.001 | |
| Post-SCS- Time Trendsc | 0.60 (0.55, 0.65) | <0.001 | |
| Medication Costs | CMM | reference | |
| SCS- Initial placement | 1.98 (1.36, 2.88) | <0.001 | |
| One year post-SCS | 0.93 (0.77, 1.13) | 0.466 | |
| Post-SCS- Time Trends | 0.97 (0.89, 1.06) | 0.546 | |
| Pain Prescription Costs | CMM | reference | |
| SCS- Initial placement | 3.40 (2.26, 5.11) | <0.001 | |
| One year post-SCS | 0.69 (0.55, 0.88) | 0.002 | |
| Post-SCS- Time Trends | 0.96 (0.87, 1.06) | 0.456 | |
| Pain Encounters | CMM | reference | |
| SCS- Initial placement | 1.88 (1.67, 2.13) | <0.001 | |
| One year post-SCS | 0.70 (0.55, 0.89) | 0.004 | |
| Post-SCS- Time Trends | 0.94 (0.87, 1.02) | 0.157 | |
| Inpatient Admissions | CMM | reference | |
| SCS- Initial placement | 1.49 (1.20, 1.84) | <0.001 | |
| One year post-SCS | 0.83 (0.74, 0.92) | <0.001 | |
| Post-SCS- Time Trends | 0.97 (0.93, 1.01) | 0.107 |
CMM: conventional Medical Management; SCS: Spinal Cord Stimulation
Represents the relative increase in cost for the initial SCS placement.
Represents the relative change in cost at one year post-SCS implantation for patients who underwent SCS placement
Represents the relative annual change in cost over time for patients who underwent SCS placement
Fig. 1.
Total time cost trend following SCS implantation
At one year post-SCS placement, pain prescription costs were decreased by 31% (CR: 0.69; 95% CI: 0.55, 0.88, p = 0.002). This cost saving was continued, but not significantly different at long-term follow-up. The total number of pain-related encounters (CR: 0.70; 95% CI: 0.55, 0.89, p = 0.004) and inpatient admissions (CR: 0.83; 95% CI: 0.74, 0.92, p <0.001) were also decreased at one year, but not significantly different at long-term follow-up (Table 4).
Discussion:
Prior studies have demonstrated that SCS has superior outcomes to CMM (10,16) or repeat operation (7,9) in FBSS patients, a broad patient cohort that generates significant expenses in the healthcare system given the chronicity and complexity of their symptoms. A 2008 multicenter, prospective, randomized controlled study (PROCESS) evaluated FBSS patients across several quality of life criteria and assessed intervention cost-effectiveness between SCS and CMM arms. Although the 6-month mean total health cost in the SCS group ($14,908) was significantly greater than that of the CMM group ($3,506), this differential could be reasonably attributed to initial high overhead costs associated with the device equipment and procedure. Moreover, although both cohorts began with similar mean baseline health-related quality of life scores, SCS patients displayed significant gains at 3- and 6-months compared to their counterparts randomized to CMM alone, with attendant decreases in opioid, NSAID, antidepressant, and anticonvulsant use (10,17). Kumar et al. extended their mean cost analysis to 5-years post-procedure, and while their results supported the initial high costs in the first 30-months, costs of the CMM cohort ($33,722) significantly exceeded that of the SCS cohort ($24,799) at 5-years (10). SCS patients reported 15% greater improvements in quality of life measures and 88% reported treatment satisfaction (10). A retrospective study in the UK identified cost-neutrality at 5-years, with 64% of FBSS patients discontinuing pain-related drugs following SCS (18).
Much of the published data regarding cost-effectiveness of SCS comprise smaller scale randomized controlled trials (RCTs) including populations ranging from 50 to 200 patients (19) or retrospective analyses ranging from 20 to 400 patients (20). Conflicting data has been presented in the literature. Our results are generated from data on 122,827 FBSS patients, and thus represent practices and resource utilization from across the United States.
Data for patients undergoing SCS procedures demonstrate that this patient group is significantly more expensive to treat at baseline than CMM, with higher medication costs, pain-prescription costs, pain-related encounters and total costs. SCS patients in the United States incurred greater expenses than their CMM counterparts at the initial time of SCS implantation $13,216 [8,296 – 21,286] vs. $5,934 [1,768 – 13,360]. However, total costs for patients who underwent SCS implantation consistently decreased over time, and at nine years post implantation were less than half of the annualized costs for the non-SCS group. Medication costs, pain-prescription costs and pain-related encounters were not significantly different at long-term follow-up. The longitudinal GEE model indicated that the initial placement of the SCS system led to a relative cost increase at the time of implantation, but the subsequent annual cumulative costs were significantly decreased in this group at all future time points. These results suggest that although implantation of the SCS system is more expensive initially, as would be expected for an implanted device, subsequent annual costs for these patients were significantly less and maintained at 1, 3, 6 and 9 years of follow-up. Of note, any costs associated with reoperations for lead migration, revision, or explant should be captured in these total cost values calculated in the SCS cohort.
It has been shown that the efficacy of SCS decreases as the number of prior interventions increases or the length of time from pain onset increases (21). We have previously shown that there is an attendant increase in health care resource utilization as the latency to treatment increases, where every 1-year increase in pain-to-SCS time is associated with 33% increased odds of having medical expenditures of $4,133 or above (22). As our current analysis did not stratify patients based on years of preoperative reported pain, it is possible that these patients with worse baseline pain history are contributing to the greater costs in the SCS cohort. Mekhail et al. followed 222 SCS-treated patients at Cleveland Clinic for more than 3-years and found nearly $18,000 in savings vis-à-vis physician office and emergency department visits, surgical procedures, nerve blocks, imaging, and hospitalizations per patient year in patients with reported pain for 6-years pre-implant (23). A recent multicenter Italian study (PRECISE) concluded that SCS with CMM treatment of FBSS patients is a cost-effective measure from the patient, society, and National Health Service perspective (24). As there are few absolute contraindications to SCS, most of which reference a clear operative neurologic deficit, unacceptable surgical risks, or the patient’s ability to control the device, it is evident that this is a treatment modality in which all pain clinicians and spine surgeons should be educated and patients made aware earlier in the treatment paradigm.
Our study results indicate that although there is an increase in costs at the time of SCS implantation, it is associated with significant cost-savings at 1, 3, 6, and 9 years post-procedure. Thus, it should not preclude serious consideration for patients with neuropathic back and leg pain, for whom SCS has been clearly shown to provide marked symptomatic and functional improvement. Existing reviews of the literature have reported generally impressive statistics supporting the efficacy of SCS; upwards of 80% of patients experience effective pain relief as well as greater patient satisfaction and decreased opiate use compared to CMM at 6-month follow up and reoperation in FBSS patients at mean 3-year follow up (20). Moreover, lumbar back pain is the most common cause of work-related disability in individuals under 45-years of age, a patient demographic that is often otherwise healthy, and pain recurs in 20–40% of patients (20). As such, cumulative costs associated with both pain management and lost productive years produce a significant and long-standing economic burden, and improved efficacy in treatment strategies with long-term cost savings. In particular, the greater initial expenditure in the SCS cohort is likely offset by a decrease in lost work days, disability, etc, which we do not examine here. In addition, given the young age of our patient cohort, the decrease in annual costs predicted by this model may be amplified over the lifetime of these patients.
Of note, our study also demonstrated a general increase in the number of patients that underwent SCS (4.3% of total identified FBSS patients, compared to previously reported 2.4% in the MarketScan® database (9)), suggesting that there is increased awareness of SCS as a viable treatment option. However, SCS is currently treating only a purported <10% of the potential patient population (25). Clearly this number, placed in the context of the enormous number of patients that experience FBSS and chronic LBP, suggests that greater awareness and advocacy for SCS as a treatment modality could be achieved. There are certainly implications regarding the need for uniformity in residency education, fellowship opportunities, and standardization of SCS provider training moving forward. More studies need to be done to better understand how SCS can be used to abrogate rising healthcare costs in the current landscape of increasing spinal procedures, burgeoning health costs and healthcare reform.
Our study examined a large nationwide patient population, thereby reducing variations that are present in single institution studies. MarketScan® collects data from a variety of practices and insurers across the United States. Although it does carry inherent limitations of a retrospective analysis, it does provide a broad view of SCS practices and cost-effectiveness from across the United States. This study was unable to stratify patients in terms of complexity of prior back surgery or surgeries, comorbid status, or the presence of other pain interventions. Single versus multilevel decompression, presence of instrumentation, degree of residual stenosis or herniation, or experience of complications or technical failure are important factors that necessitate varying levels of care and would direct the trajectory of postoperative FBSS management. It may be useful to categorize patients into high and low preoperative healthcare utilizers and to compare the postoperative cost differential across groups. Although this analysis does not correlate costs with patient outcomes, the functional benefits of SCS are reported extensively elsewhere (19). Lastly, our data does not consider more recent developments in SCS technology or software treatment paradigms (HF10 stimulation and BurstDR stimulation) (11), and it is reasonable to hypothesize that improvement in type or efficacy of pain relief provided by recent implant systems would further increase the cost-benefit differential between SCS and CMM cohorts. It will be interesting to examine the long term economic impact of SCS in patients who have received implants beyond 2012, with the advent of novel neurostimulation paradigms.
Conclusions:
In the current study, we found that 4.3% of patients across the United States with FBSS were treated with SCS. Long-term total annual costs in FBSS patients treated with SCS were significantly reduced compared to patients with conventional management. Although implantation of an SCS system results in a short-term increase in costs at one year, the subsequent annual cumulative costs are decreased in the following 9 years after implantation. SCS has been demonstrated to be a superior treatment modality to CMM in FBSS patients in appropriately selected patients. SCS is one of the few operative treatment strategies that is not only reversible, but allows a patient to trial the procedure prior to moving forward with the procedure. This study combines the largest sample of FBSS patients studied to date along with the longest follow-up interval analyzed. Our results definitively support the long-term cost utility of SCS in the treatment of FBSS patients.
Footnotes
Disclosure of Interests Statement
Shivanand Lad, MD, PhD has consulted for or received grant support from Medtronic Inc., Boston Scientific and St. Jude Medical. He serves as Director of the Duke Neuro-Outcomes Center that has received research funding from NIH KM1 CA 156687. Siyun Yang, MS was partially supported by UL1TR001117 from the National Center for Advancing Translational Sciences (NCATS). The remaining authors report no conflicts of interest or financial disclosures.
References:
- 1.Schmidt CO, Raspe H, Pfingsten M, Hasenbring M, Basler HD, Eich W, Kohlmann T. Back pain in the German adult population: prevalence, severity, and sociodemographic correlates in a multiregional survey. Spine (Phila Pa 1976) 2007:32: 2005–2011. [DOI] [PubMed] [Google Scholar]
- 2.Patel AT, Ogle AA. Diagnosis and management of acute low back pain. Am Fam Physician 2000:61: 1779–1786, 1789–1790. [PubMed] [Google Scholar]
- 3.Dagenais S, Caro J, Haldeman S. A systematic review of low back pain cost of illness studies in the United States and internationally. Spine J 2008:8: 8–20. [DOI] [PubMed] [Google Scholar]
- 4.Chan CW, Peng P. Failed back surgery syndrome. Pain Med 2011:12: 577–606. [DOI] [PubMed] [Google Scholar]
- 5.Schofferman J, Reynolds J, Herzog R, Covington E, Dreyfuss P, O’Neill C. Failed back surgery: etiology and diagnostic evaluation. Spine J 2003:3: 400–403. [DOI] [PubMed] [Google Scholar]
- 6.Taylor RS, Ryan J, O’Donnell R, Eldabe S, Kumar K, North RB. The cost-effectiveness of spinal cord stimulation in the treatment of failed back surgery syndrome. Clin J Pain 2010:26: 463–469. [DOI] [PubMed] [Google Scholar]
- 7.North RB, Kidd DH, Farrokhi F, Piantadosi SA. Spinal cord stimulation versus repeated lumbosacral spine surgery for chronic pain: a randomized, controlled trial. Neurosurgery 2005:56: 98–106; discussion 106–107. [DOI] [PubMed] [Google Scholar]
- 8.Van Buyten JP. Neurostimulation for chronic neuropathic back pain in failed back surgery syndrome. J Pain Symptom Manage 2006:31: S25–29. [DOI] [PubMed] [Google Scholar]
- 9.Lad SP, Babu R, Bagley JH, Choi J, Bagley CA, Huh BK, Ugiliweneza B, Patil CG, Boakye M. Utilization of spinal cord stimulation in patients with failed back surgery syndrome. Spine (Phila Pa 1976) 2014:39: E719–727. [DOI] [PubMed] [Google Scholar]
- 10.Kumar K, Taylor RS, Jacques L, Eldabe S, Meglio M, Molet J, Thomson S, O’Callaghan J, Eisenberg E, Milbouw G, Buchser E, Fortini G, Richardson J, North RB. Spinal cord stimulation versus conventional medical management for neuropathic pain: a multicentre randomised controlled trial in patients with failed back surgery syndrome. Pain 2007:132: 179–188. [DOI] [PubMed] [Google Scholar]
- 11.Kapural L, Yu C, Doust MW, Gliner BE, Vallejo R, Sitzman BT, Amirdelfan K, Morgan DM, Yearwood TL, Bundschu R, Yang T, Benyamin R, Burgher AH. Comparison of 10-kHz High-Frequency and Traditional Low-Frequency Spinal Cord Stimulation for the Treatment of Chronic Back and Leg Pain: 24-Month Results From a Multicenter, Randomized, Controlled Pivotal Trial. Neurosurgery 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bala MM, Riemsma RP, Nixon J, Kleijnen J. Systematic review of the (cost-)effectiveness of spinal cord stimulation for people with failed back surgery syndrome. Clin J Pain 2008:24: 741–756. [DOI] [PubMed] [Google Scholar]
- 13.Kumar K, Malik S, Demeria D. Treatment of chronic pain with spinal cord stimulation versus alternative therapies: cost-effectiveness analysis. Neurosurgery 2002:51: 106–115; discussion 115–106. [DOI] [PubMed] [Google Scholar]
- 14.Kumar K, Rizvi S. Cost-effectiveness of spinal cord stimulation therapy in management of chronic pain. Pain Med 2013:14: 1631–1649. [DOI] [PubMed] [Google Scholar]
- 15.Hollingworth W, Turner JA, Welton NJ, Comstock BA, Deyo RA. Costs and cost-effectiveness of spinal cord stimulation (SCS) for failed back surgery syndrome: an observational study in a workers’ compensation population. Spine (Phila Pa 1976) 2011:36: 2076–2083. [DOI] [PubMed] [Google Scholar]
- 16.Coleman SD, Mackey S. Spinal cord stimulation compared with medical management for failed back surgery syndrome. Curr Pain Headache Rep 2009:13: 1–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Manca A, Kumar K, Taylor RS, Jacques L, Eldabe S, Meglio M, Molet J, Thomson S, O’Callaghan J, Eisenberg E, Milbouw G, Buchser E, Fortini G, Richardson J, Taylor RJ, Goeree R, Sculpher MJ. Quality of life, resource consumption and costs of spinal cord stimulation versus conventional medical management in neuropathic pain patients with failed back surgery syndrome (PROCESS trial). Eur J Pain 2008:12: 1047–1058. [DOI] [PubMed] [Google Scholar]
- 18.Budd K. Spinal cord stimulation: cost-benefit study. Neuromodulation 2002:5: 75–78. [DOI] [PubMed] [Google Scholar]
- 19.Grider JS, Manchikanti L, Carayannopoulos A, Sharma ML, Balog CC, Harned ME, Grami V, Justiz R, Nouri KH, Hayek SM, Vallejo R, Christo PJ. Effectiveness of Spinal Cord Stimulation in Chronic Spinal Pain: A Systematic Review. Pain Physician 2016:19: E33–54. [PubMed] [Google Scholar]
- 20.Mekhail N, Wentzel DL, Freeman R, Quadri H. Counting the costs: case management implications of spinal cord stimulation treatment for failed back surgery syndrome. Prof Case Manag 2011:16: 27–36. [DOI] [PubMed] [Google Scholar]
- 21.North R, Shipley J, Prager J, Barolat G, Barulich M, Bedder M, Calodney A, Daniels A, Deer T, DeLeon O, Drees S, Fautdch M, Fehrenbach W, Hernandez J, Kloth D, Krames ES, Lubenow T, North R, Osenbach R, Panchal SJ, Sitzman T, Staats P, Tremmel J, Wetzel T, American Academy of Pain M. Practice parameters for the use of spinal cord stimulation in the treatment of chronic neuropathic pain. Pain Med 2007:8 Suppl 4: S200–275. [DOI] [PubMed] [Google Scholar]
- 22.Lad SP, Petraglia Iii FW, Kent AR, Cook S, Murphy KR, Dalal N, Karst E, Staats P, Sharan A. Longer Delay From Chronic Pain to Spinal Cord Stimulation Results in Higher Healthcare Resource Utilization. Neuromodulation 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mekhail NA, Aeschbach A, Stanton-Hicks M. Cost benefit analysis of neurostimulation for chronic pain. Clin J Pain 2004:20: 462–468. [DOI] [PubMed] [Google Scholar]
- 24.Zucco F, Ciampichini R, Lavano A, Costantini A, De Rose M, Poli P, Fortini G, Demartini L, De Simone E, Menardo V, Cisotto P, Meglio M, Scalone L, Mantovani LG. Cost-Effectiveness and Cost-Utility Analysis of Spinal Cord Stimulation in Patients With Failed Back Surgery Syndrome: Results From the PRECISE Study. Neuromodulation 2015:18: 266–276; discussion 276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cidamon M. Boston Scientific overtakes Medtronic in medical device market for back pain and failed back surgery syndrome. . Nasdaq, GlobeNewswire, 2015. [Google Scholar]