Abstract
As the interface between the cell and its environment, the cell cortex must be able to respond to a variety of external stimuli. This is made possible in part by cortical excitability, a behavior driven by coupled positive and negative feedback loops that generate propagating waves of actin assembly in the cell cortex. Cortical excitability is best known for promoting cell protrusion and allowing the interpretation of and response to chemoattractant gradients in migrating cells. It has recently become apparent, however, that cortical excitability is involved in the response of the cortex to internal signals from the cell-cycle regulatory machinery and the spindle during cell division. Two overlapping functions have been ascribed to cortical excitability in cell division: control of cell division plane placement, and amplification of the activity of the small GTPase Rho at the equatorial cortex during cytokinesis. Here, we propose that cortical excitability explains several important, yet poorly understood features of signaling during cell division. We also consider the potential advantages that arise from the use of cortical excitability as a signaling mechanism to regulate cortical dynamics in cell division.
Introduction
The cell cortex, classically defined as the plasma membrane and the thin layer of cytoplasm just beneath it, is the responsive interface between the cell and its surroundings1. Because the information received by the cell assumes many guises — soluble signals, insoluble signals, contacts with neighboring cells, and contacts with the extracellular matrix, to name but a few — the cortex has a correspondingly diverse repertoire of behavioral responses, including extension or retraction of protrusions, formation of endocytic structures such as coated pits or macropinosomes, and construction of cell–cell and cell–substrate adhesions.
Even in the absence of external inputs, the cortex displays complex dynamic behaviors. Among the most intriguing of these is the propensity to generate propagating waves of assembling actin filaments (F-actin) and actin regulators, including small GTPases, phosphoinositides, and their various targets and regulators. This behavior can be loosely termed ‘cortical excitability’ and was originally described 20 years ago in motile cells of the soil amoeba Dictyostelium discoideum2. Improvements in live-cell imaging and molecular probes have revealed that cortical excitability is a feature of not only motile cells3, but also nonmotile cells4,5, embryos6,7, and tissues8.
Cortical waves display several consistent features: the waves can propagate without losing amplitude; waves auto-annihilate, meaning that colliding wavefronts snuff each other out; and waves can assume complex forms, including spirals and bull’s-eye patterns2,3,4,5,7,9. Such behaviors are attributes of excitable media, although they can also be observed in oscillatory systems. Excitable media are continuous excitable systems with the capacity to respond locally to a suprathreshold stimulus by transitioning from a state of low activity (the ground state) to a state of high activity (excitation). Local excitation can then induce the transition to the excited state in neighboring parts of the medium. In this way, excitation spreads across the excitable medium as a traveling wave. After participating in a wave of excitation, the system returns to the ground state, where it remains for a characteristic period of time (the latent or refractory period) before it can be re-excited. At the mechanistic level, excitable systems are underpinned by fast positive feedback coupled to delayed negative feedback (Figure 1). Positive feedback rapidly drives the system into the excited state in an ‘all-or-none’ fashion and, from the standpoint of the waves, operates at their leading edges, advancing them; delayed negative feedback limits the duration of the excited state and, from the standpoint of the waves, operates at their trailing edge, shutting them off.
Figure 1. The basics of excitable media.
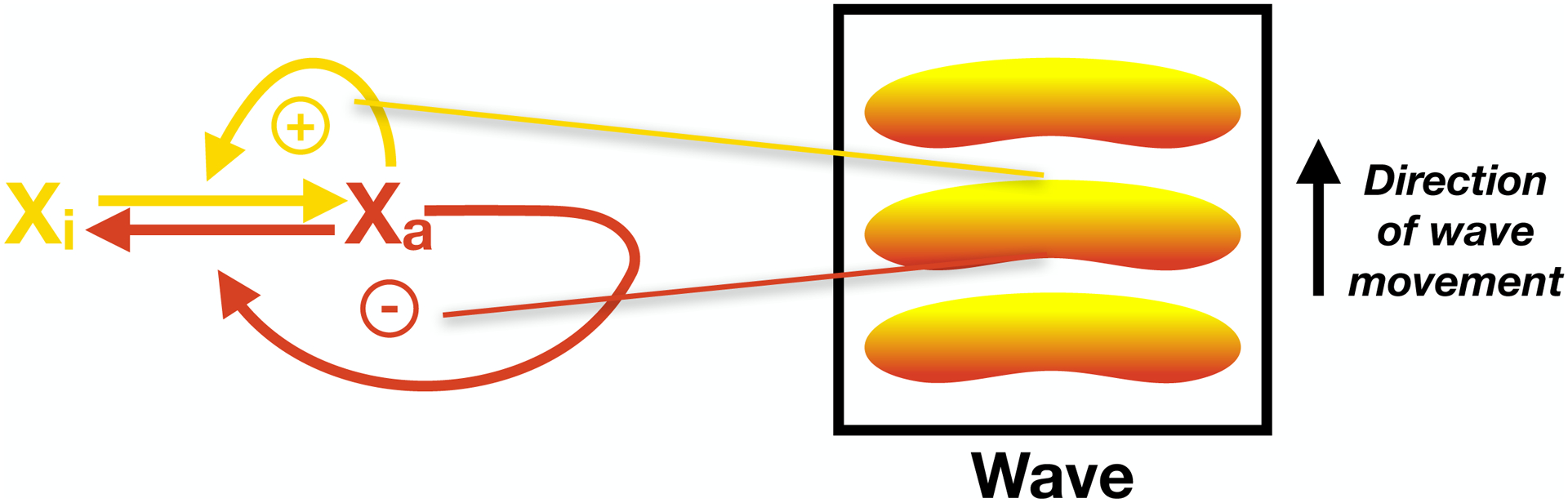
A schematic diagram of coupled positive feedback and delayed negative feedback. Player X transitions between inactive (Xi) and active (Xa) forms. The active player (Xa) engages in positive feedback, promoting more of its own formation. Xa also engages in delayed negative feedback, promoting its own inactivation. The positive feedback (yellow) dominates at the front of waves, driving the wave forward; the negative feedback (red) dominates at the back of the wave, terminating the wave.
The classic biological example of an excitable medium is the neuron, wherein an electrochemical wave — the action potential — propagates down the axon. Here, the positive feedback is provided by membrane-depolarization-dependent opening of voltage-gated Na+ channels that, upon opening, let more Na+ into the neuron, thus further depolarizing the membrane. If the initial stimulus pushes the membrane potential past the threshold, this positive feedback elicits complete, rapid depolarization, initiating an action potential. Negative feedback is provided by the delayed, membrane-depolarization-dependent opening of voltage-gated K+ channels, which let K+ out of the neuron, thereby promoting repolarization and inhibiting the propagation of subsequent action potentials until a resting state is reached. The role of excitability in neurons is well established: it is harnessed both to send information (in the form of the action potential) and to decide whether information should be sent. That is, the dendrites and cell body integrate stimulatory and inhibitory input from other cells and, if the membrane potential of the cell body reaches the threshold, an action potential is generated.
Experimental and modeling approaches have revealed that excitable dynamics play an important role in the behavior of the cortical waves of F-actin assembly and the corresponding waves of its regulators3,5,7,10. However, in contrast to neurons, where excitability is carried by ions and ion channels, cortical excitability is carried by the cortical cytoskeleton and its regulators. There are, of course, other differences between neuronal excitability and cortical excitability. First, axons are essentially one-dimensional, meaning that the waves of membrane depolarization within axons are likewise one-dimensional. The waves that characterize cortical excitability, however, are two-dimensional, allowing them to assume the complex forms mentioned above. Second, signal interpretation by the dendrites and cell body of the neuron results in action potentials that arise consistently at the junction between the cell body and the axon, an arrangement that ensures that the action potential moves in one direction only. In contrast, the cortical waves can potentially arise anywhere and move in any direction, a behavior that leads to auto-annihilation as colliding waves move into the cortex in the latent state. Third, cortical excitability waves are distinctly less all-or-none than action potentials, displaying variation in amplitude in different parts of the cell and variation in response to different stimuli3,4,7,9.
The nonlinearity and two-dimensionality of cortical excitability and the presence of multiple feedback loops (Figure 1) collectively defy intuition, rendering computational modeling an essential tool for further understanding of the process Excitable dynamics are often modeled as reaction–diffusion systems, in which an activator stimulates more of its own production via positive feedback, while also stimulating the production of an inhibitor that is responsible for negative feedback. The activator and inhibitor vary with respect to their diffusivity, with the inhibitor typically being more diffusible than the activator. Historically, activator–inhibitor systems were first proposed to explain static patterns that arise during development11 and, independently, to describe chemical oscillations12. More recently, it has become apparent that changes to the reaction mechanism and diffusion parameters in reaction–diffusion systems can produce a broad spectrum of static and moving phenomena, including stable patterns, excitable waves, and a great variety of oscillatory patterns13. Below, we first discuss the established role of cortical excitability in driving chemotactic cell migration; then we highlight newly revealed roles for cortical excitability during mitosis and cytokinesis and relate the advantages afforded by cortical excitability in chemotaxis to those in cell division.
Cortical excitability and cell locomotion
Cortical excitability is best known from studies of D. discoideum10,14 and neutrophils3. In D. discoideum, waves of F-actin and F-actin-binding proteins move throughout the cell cortex, apparently under the control of complementary waves of signals, such as the small GTPase Ras and phosphatidylinositol 3,4,5-trisphosphate (PIP3)14,15. Similarly, in neutrophils, cortical waves of F-actin and F-actin-regulatory proteins are associated with activation of their upstream regulators, such as the small GTPase Rac3. The feedback interactions among these various players in motile cells are extremely complex16 and, because they are inherently cyclic, delineating their interactions requires time-resolved manipulations17. Consequently, many models of cell locomotion subsume the interlocking subsystems (modules) into a single excitable network, to render modeling more tractable10,16.
What is the benefit of cortical excitability in cell movement? Cortical excitability is harnessed by motile cells to generate cell extensions: waves of actin assembly, upon reaching the cell edge, transform into structures such as pseudopodia and lamellipodia that push the cell forward3,14. One of the virtues of using an excitable, wave-based mechanism for cell protrusion is that it allows cells to migrate around obstacles3. That is, because excitable waves are normally extinguished when they are prevented from moving forward (due to the negative feedback catching up with the positive feedback), a wave-based mechanism provides the cell with the ability to ‘sense’ immovable barriers and crawl around them.
Excitability also, in effect, makes the cortex smart. That is, excitability is intimately linked to decision-making in locomoting cells, the key decision being in which direction to crawl16. In the absence of a chemoattractant, locomoting cells can extend the pseudopodia that arise from excitable dynamics in any direction, a behavior that results in random migration. However, in the presence of a gradient of chemoattractant, excitability becomes polarized, such that the front of the cell (i.e. the side facing the highest concentration of chemoattractant) generates more and higher-amplitude waves than the back of the cell3,18. This results in preferential extension of pseudopods toward the source of chemoattractant and preferential suppression of pseudopod extension at the sides and rear of the cell.
Excitability polarization in response to a chemoattractant is extraordinarily sensitive, such that the cells can persistently migrate up gradients that are as shallow as 1% (i.e. a 1% difference in exposure to chemoattractant from the front to the back of the cell16). Strikingly, the degree of excitability polarization is high, regardless of the steepness of the chemoattractant gradient19. In D. discoideum, this and other features of the chemotactic response are explained by the LEGI-BEN (local excitation-global inhibition biased excitable network) model. This model has been covered in several reviews (e.g. Iglesias and Devreotes10 and Devreotes et al.16) but the basic idea is that the excitable network is throttled by a response regulator that is under the control of chemoattractant–receptor binding. Binding of the chemoattractant to the receptor results in rapid production of a slowly diffusing stimulator of the response regulator and slower production of a rapidly diffusing inhibitor of the response regulator. This results in a higher stimulator:inhibitor ratio where receptor occupancy is high (i.e. at the front of the cell) and a lower stimulator:inhibitor ratio where receptor occupancy is low (i.e. at the sides and back of the cell). The consequence of this is that a shallow gradient of receptor occupancy is converted into sharp differences in local excitability, with high excitability at the front of the cell and low excitability at the sides and back16 (Figure 2).
Figure 2. Cortical excitability and chemotaxis.
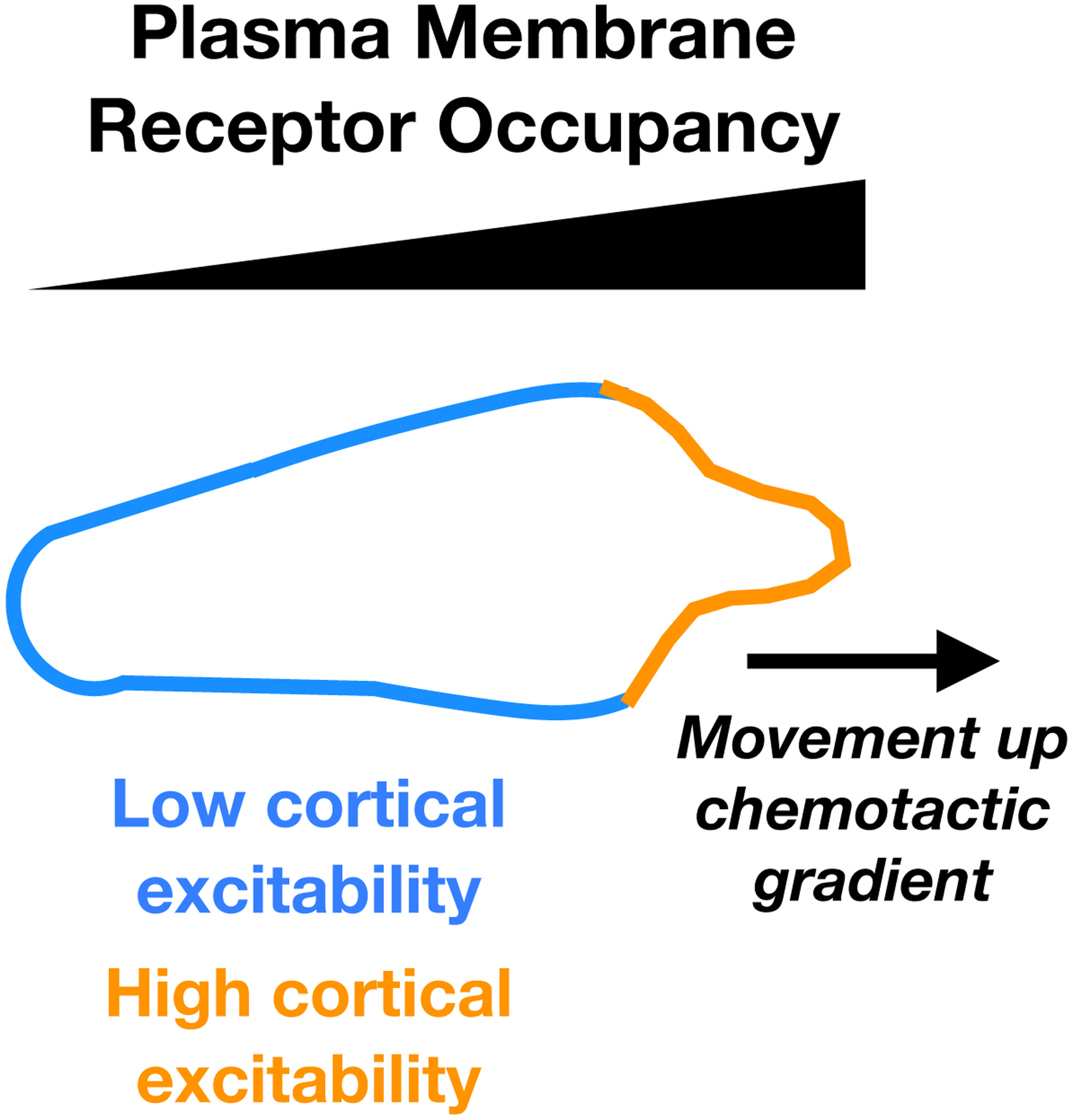
A schematic diagram of the relationship between the occupancy of plasma membrane chemoattractant receptors, cortical excitability, and cell protrusions. A gradual decline in receptor occupancy (black triangle) is converted to a sharp bias in cortical excitability such that the side of the cell facing the gradient has high excitability (orange), while the sides and back of the cell have low cortical excitability (blue). The high cortical excitability at the front of the cell results in movement of the cell up the chemoattactant gradient.
Cortical excitability may also endow locomoting cells with the flexibility needed to generate a variety of different dynamic behaviors. For example, modeling studies indicate that excitable dynamics can be converted into bistable dynamics, meaning that the cortical palette of F-actin behavior can be considerably broadened to include the co-existence of standing F-actin waves and traveling F-actin waves20. Further, manipulation of wave dynamics via experimental interventions that impact the positive and negative feedback can result in profound alterations in cortical dynamics, such that cells can be driven from amoeboid motility to motile states that more closely resemble those of keratinocytes, involving cell locomotion via continuous extension of stable lamellipodia21.
Cortical excitability and mitosis
Cortical excitability is therefore an intrinsic feature of motile cells that can be modulated by external signals in the form of chemoattractants. Because other external signals can also significantly impact cortical excitability4,5, it seems likely that external modulation of cortical excitability will prove to be a commonly utilized mechanism. However, a growing body of evidence indicates that cortical excitability is also responsive to internal signals, particularly during cell division7,9,22. A recent study of mast cells revealed that around 5 minutes after nuclear envelope breakdown, a subset (~27%) of mitotic cells developed striking cortical waves of Cdc42 activity9. These ‘metaphase’ Cdc42 activity waves were accompanied by waves of cortical recruitment of the F-BAR protein FBP17 as well as waves of F-actin, and these waves assumed both bull’s-eye and spiral patterns (Figure 3A). The authors noticed that metaphase waves were more common in cells that were more adherent in mitosis. This correlation was strengthened by demonstrating that experimental upregulation of cell–substrate adhesions resulted in a doubling of the fraction of cells that displayed metaphase waves. Moreover, pharmacological inhibition of the metaphase waves with a Cdc42 inhibitor resulted in increased rounding of cells that had displayed waves prior to treatment, but not of cells without waves, implying feedback between the waves and cell–substrate adhesion.
Figure 3. Cortical excitability in cell division.
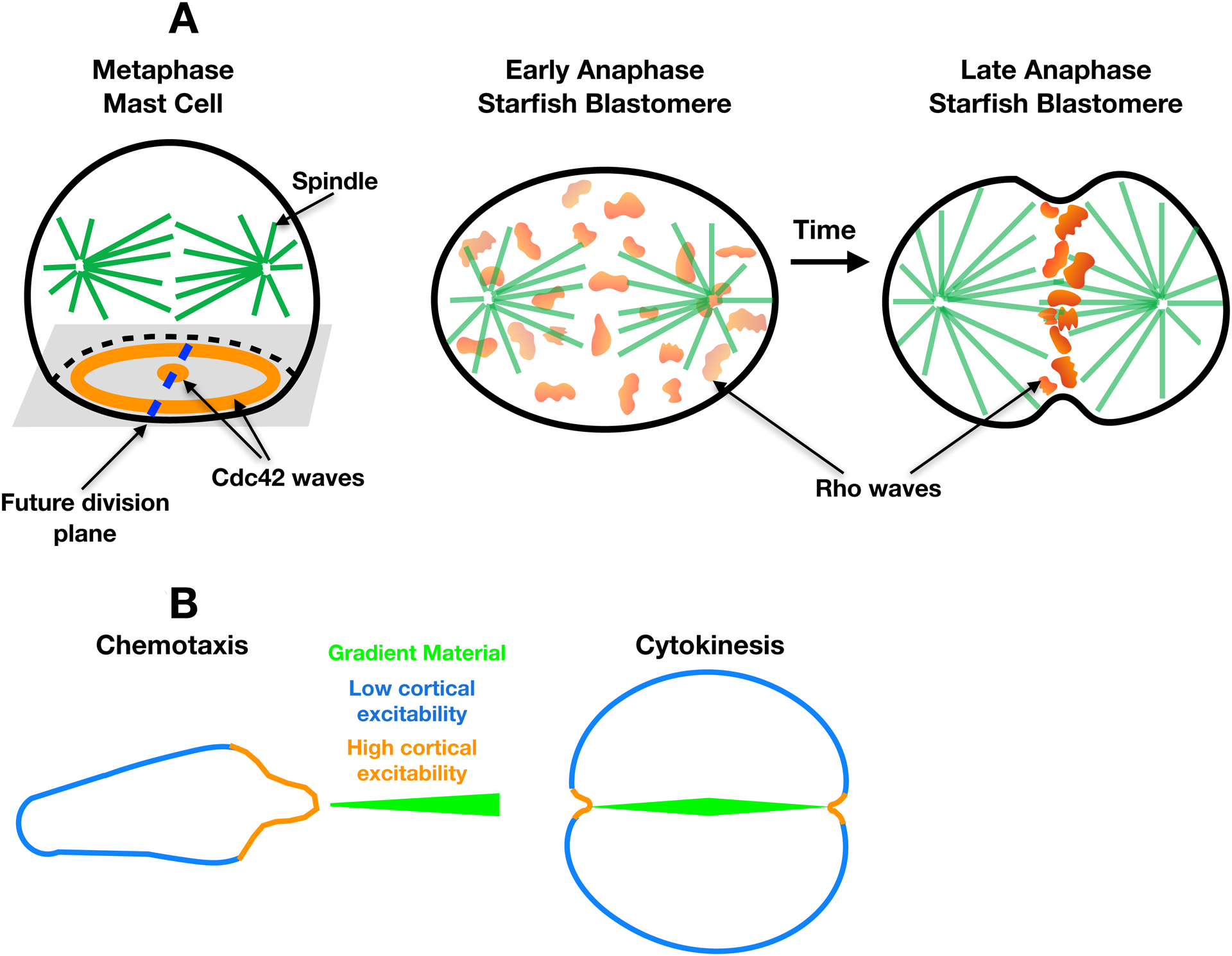
(A) Wave organization in mitotic cells. Left: Schematic diagram of a metaphase mast cell showing the spatial relationships of the Cdc42 waves (orange), the mitotic spindle (green), and the future division plane (dashed blue line). Right: diagram of starfish blastomere showing changes in Rho waves (orange) between early and late anaphase. Over time, the waves are lost from polar cortical regions, while becoming concentrated and amplified (darker orange) at the equatorial cortex. (B) Cytokinesis as inverted chemotaxis. Left: Chemotaxing cell migrating up an external chemotactic gradient (green). As in Figure 2, excitability is high (orange) where the cortex faces the gradient and low (blue) elsewhere. Right: Cell undergoing cytokinesis and ingressing up an internal chemotactic gradient (green). Excitability is high (orange) where the cortex faces the gradient and low (blue) elsewhere.
What is the benefit of metaphase excitability? Strikingly, the center of the bull’s-eye or spiral waves consistently predicted the position of the future cleavage plane of the cells (Figure 3A). While this may not seem surprising, in that the wave centers were usually positioned in the center of the cell, which generally corresponds to the future division plane, this correlation also held in very large cells that underwent multipolar divisions: such cells formed multiple wave cores, each of which predicted a future division plane9.
This study prompts a number of fascinating questions. Firstly, how are the metaphase waves positioned? One possibility is that positioning is mediated by a gradient of Ran-GTP, which has been linked to furrow positioning in cultured cells23. Secondly, how is it possible that the metaphase waves, which disappear at anaphase onset, specify furrow positioning, which occurs well after the start of anaphase? The authors suggested that the cortex retains a memory of the metaphase waves that somehow impacts events in anaphase. Although this point has not been directly tested, the possibility of a cortical memory is intriguing and mirrors ideas developed for crawling cells16. Thirdly, how exactly are the metaphase waves linked to cleavage-plane specification? Because the division plane is dictated by the orientation of the spindle, one possibility is that the Cdc42 waves somehow control spindle rotation. This notion is consistent with the observation that cells displaying metaphase waves showed more extensive rotations in anaphase than cells without metaphase waves9. A second, nonexclusive possibility is that the metaphase waves act more directly on the specification of the cytokinetic apparatus. That is, direct comparison of metaphase waves to anillin, a marker of the cytokinetic apparatus, revealed that, while low-level, peripheral anillin waves were present in metaphase, these were largely excluded from cortical regions where Cdc42 waves dominated. Upon the transition to anaphase, however, anillin accumulated in the cortical regions, coincident with the disappearance of the Cdc42 waves, suggesting that the two wave systems may antagonize each other.
Another fascinating finding is that metaphase excitability scales with cell size. Specifically, the authors demonstrated that the period and wavelength of the metaphase waves of Cdc42 and FBP17 are positively correlated with the basal surface area of the cell9. This finding not only explains why waves might be particularly useful — scaling allows the cell to ensure that furrow specification is normally singular — but also potentially explains the formation of multiple furrows in extremely large cells: once the cells exceed a certain size limit, multiple wave cores develop, resulting in the loss of furrow singularity. Alternatively, it may be that, beyond a certain size, multipolar spindles develop, resulting in multiple Ran-GTP gradients, which give rise to multiple wave cores.
While this study was limited to mast cells, there are hints that other cell types also have metaphase waves: in HeLa cells metaphase waves of cortical and subcortical F-actin have been reported24, and cortical F-actin waves are present throughout the cell cycle in frog embryos7. There is also ample evidence that Cdc42 is important for control of cleavage-plane positioning in epithelial cells25,26, although this has been proposed to reflect a role for Cdc42 in spindle positioning. Finally, from a technical standpoint, it would not be surprising if metaphase waves had been overlooked in previous studies, given that mitotic cells typically round up, making it more difficult to image the cortex at high spatiotemporal resolution.
Cortical excitability and cytokinesis
Cytokinesis in animal cells has long been conceptualized as an essentially linear process, in which the mitotic spindle elicits furrowing activity in the cortical annulus surrounding the spindle midplane. In modern terms this means a set of spindle-derived cues activate the small GTPase Rho via the Rho guanine nucleotide exchange factor (GEF) Ect2, which is concentrated and activated near the equatorial cortex via a collaboration between microtubule geometry and the centralspindlin complex27,28, a motor complex implicated in cell division. Upon patterned activation of Rho at the equator, active Rho promotes actin polymerization, myosin-2 activation, and recruits other components of the cytokinetic apparatus, such as anillin29. After the apparatus has completed constriction, it is believed that Rho is inactivated by a GTPase-activating protein (GAP), and the apparatus disassembles. However, studies of the activated eggs and early embryos of the frog Xenopus laevis and the starfish Patiria miniata revealed distinctly non-linear behavior of active Rho and F-actin during cytokinesis7. In Patiria, low-amplitude cortical waves of Rho activity and F-actin appear shortly after anaphase onset and become progressively concentrated and amplified at the cell equator (Figure 3A). Both concentration and amplification likely result from spindle-mediated redistribution of Ect2, given that depolymerization of microtubules after the concentration of Rho activity at the equator results in the dispersion of the waves and a reduction in their amplitude, and that overexpression of Ect2, which presumably saturates the spindle mechanisms involved in Ect2 redistribution, amplifies the nonequatorial Rho waves as well as those at the equator7. In Xenopus, while the spindle also concentrates and amplifies Rho waves at the equator in anaphase, nonequatorial F-actin waves persist throughout the cell cycle. Nonetheless, as in Patiria, Ect2 overexpression in Xenopus amplifies the nonfurrow Rho waves and drives them into overtly spiral forms. In both species, furrowing commences even while Rho activity and F-actin remain wave-like within the cleavage furrow, although as the starfish blastomeres decrease in size equatorial Rho activity eventually appears as a continuous stripe rather than discrete wavefronts.
Cortical excitability in these cells is negatively regulated by cyclin-dependent kinase 1 (Cdk1) but to different degrees: high Cdk1 activity in prometaphase through metaphase terminates cortical excitability in Patiria, and Cdk1 inactivation at anaphase results in the reappearance of cortical excitability. Artificially arresting cells with high Cdk1 activity via expression of a nondegradable form of cyclin B results in suppression of excitability; this suppression is immediately lifted upon pharmacological inhibition of Cdk1. In Xenopus, cortical excitability is present throughout the cell cycle, but it can nonetheless be terminated by the expression of nondegradable cyclin B7.
In situations where Rho waves have high amplitude, as occurs naturally at the equator or throughout the cortex when Ect2 is overexpressed, Rho waves are ‘chased’ by waves of F-actin, such that where F-actin concentration is highest, Rho activity is waning. Moreover, local reduction of F-actin increases the amplitude of the Rho waves7. In light of these and other findings, a reaction–diffusion model based on Ect2- and Rho-dependent Rho positive feedback and delayed, F-actin-mediated negative feedback was developed. This model captured basic features of anaphase cortical excitability as well as microtubule-dependent concentration and amplification of Rho activity at the equator. The same model also explains the transition of Rho and F-actin waves at the equator to a uniform stripe of overlapping Rho and F-actin30.
What is the benefit of cortical excitability for cytokinesis? Besides inducing furrowing, excitability provides a relatively straightforward way for the cell to ensure Rho flux. That is, there is good reason to think that Rho is not simply activated and left ‘on’ in Rho zones, but instead undergoes constant flux through the GTPase cycle31–34. Cortical excitability accounts for this flux because the time the GTPase remains active is limited by negative feedback. From this standpoint, cortical excitability has the potential to explain two important but poorly understood features of cytokinetic signaling — its sensitivity, and its capacity for error correction. With respect to sensitivity, the induction of a Rho zone and a furrow normally depends on complementary signaling contributions from both the central spindle and the astral microtubules, but cells can nonetheless divide when one or other of these populations is experimentally compromised35. Positive feedback arising from excitability could account for this sensitivity, by amplifying otherwise faint signals at the equatorial cortex, analogous to one of the roles of excitability in chemotaxis16. With respect to error correction, the experimental displacement of the spindle after furrowing onset results in disappearance of the original Rho zone (and furrow regression) and formation of a new Rho zone and furrow over the midplane of the repositioned spindle36. This result can be explained by excitability in that the positive feedback loop between Rho and spindle-provided Ect2 would be lost upon spindle repositioning. Consequently, the negative feedback would rapidly efface the original Rho zone. Meanwhile, a new zone of cortical excitability would form in the newly defined cleavage plane due to the concentration of Ect2 by the spindle.
Another fruitful of line thought arises via the comparison of excitability in cytokinesis to excitability in chemotaxis. It was previously noted that some of the same players that adopt polarized distributions in migrating amoeba — phosphoinositide 3-kinase (PI3K), PIP3 and the PIP3 3’-phosphatase PTEN — also adopt polarized distributions during cytokinesis, and this polarization is important for both cytokinesis and directed migration37. If one grants that in both cases excitability drives cortical dynamics, a new idea emerges, proposing furrowt ingression as a form of inward-directed chemotaxis, with the cortex tracking a gradient of diffusible signal toward the center of the cell (Figure 3B). In this model, the microtubules are primarily responsible for shaping the gradient, while the cortex is responsible for interpreting the gradient. This may seem at odds with the manner in which cytokinesis is usually conceptualized, i.e. as the closure of a circumferential contractile ring. The standard conceptualization of cytokinesis can differ significantly from reality, however; for example, highly asymmetric furrow ingression is the rule in many cell types38.
If cortical excitability allows the cortex to track a gradient of diffusible material to the spindle midzone, what is the diffusible signal? One reasonable candidate is Ect2 itself, which normally forms an internal gradient with its maximum at the spindle midzone as a result of its interaction with the centralspindlin component, MgcRacGAP (e.g. Su et al.39). If so, Ect2 would serve both as a critical participant in cortical excitability and as a soluble signal. One objection to this idea is that elimination of the spindle midzone by a variety of approaches eliminates the normal ladder of centralspindlin and Ect2 localization in the cell midplane, but fails to prevent cytokinesis39. However, this objection is less potent than it seems: in the absence of a central spindle, centralspindlin and Ect2 can nonetheless accumulate on cortical, equatorial microtubules, forming a simulacrum of the central spindle just beneath the equatorial cortex and ahead of the ingressing furrow39. Assuming that the furrow keeps pushing the simulacrum inward, the source of Ect2 remains in front of the furrow, analogous to a chemotaxing leukocyte hunting a bacterium and, occasionally, pushing it forward before it finally manages to engulf it. One of the virtues of such an inverted chemotaxis model is that it explains the results of experiments in which furrows that initially form away from the axis defined by the spindle midplane can nonetheless track toward the center of the cell40, as well as experiments in which displacement of the spindle to one side of the cell produces a highly asymmetric furrow and Rho zone that somehow manage to split the cell in half36.
Because the eggs, zygotes, and blastomeres of Xenopus and Patiria are large, and because frogs and starfish develop externally, it might naturally be wondered whether cytokinetic excitability reflects an evolutionary specialization. This point remains to be settled, but the following observations suggest that cortical excitability during cytokinesis may be broadly conserved. Firstly, 8-cell mouse embryos display traveling waves of cortical F-actin that have roughly the same spatiotemporal characteristics as those seen in early Xenopus embryos6. Secondly, in a study of cytokinesis in Ptk1 (rat kangaroo kidney epithelial) cells, depolymerization of microtubules just after the onset of anaphase results in the formation of traveling, Rho-dependent waves of cortical F-actin41. Similarly, in mast cells treated with nocodazole and then driven into anaphase via Cdk1 inhibition, waves of cortical Rho activity and anillin recruitment develop9. We interpret these results to indicate that, as in Xenopus and Patiria, the spindle confines cortical Rho activity waves, but in these smaller cells the microtubules more rapidly reach the cortex, making the initial development of anaphase cortical waves difficult to detect. Depolymerization of microtubules in anaphase thus unmasks the excitability of the cortex.
Future perspectives
Excitable dynamics are fun — indeed, exciting — to observe, and they tempt one to ascribe functional and interesting roles wherever such behaviors emerge: from cortical or electrical excitability in cells, to migrating swarms of soil amoebae, bees on their hives, or soccer fans. Yet as every heart patient or migraine sufferer can likely attest, excitability is not universally welcome; in many contexts, excitability is a potentially disastrous liability of systems that entangle positive and negative feedback for the sake of coordination, sensitivity, or homeostasis. In other cases, excitability may be only an epiphenomenon with no functional role, good or bad. But even core traits of living organisms, like the citric acid cycle or microtubule dynamic instability, were once epiphenomena too; emergent traits of complex systems for which evolution found an adaptive value. In the case of cortical excitability, it may be that in some cells or in some contexts this behavior is irrelevant or even pathological, but in others it has clear functional roles. Direct tests of adaptive significance are thus a high priority.
Regarding the role of cortical excitability in cell division, many critical pieces of mechanistic detail are missing. Currently, we have almost no information on the feedback mechanisms that result in metaphase excitability. Further, for the working model of the cytokinetic excitability circuit, the basis of the proposed positive feedback between Ect2 and Rho is unknown. It could be direct: it was recently shown that Ect2 has a binding site for active Rho independent of its GEF domain42. Upon binding to active Rho, Ect2 autoinhibition is relieved, increasing GEF activity. Similarly, the basis of negative feedback between F-actin and Rho is unknown. A promising candidate is ARHGAP11a (also known as RGA3/4 and MPGAP), which negatively regulates Rho during Caenorhabditis elegans and HeLa cytokinesis34,43, and which is associated with delayed, F-actin-dependent negative feedback during pulsed contractions in C. elegans44.
Based on comparisons to chemotaxis, it also seems certain that the core cytokinetic excitability circuit sketched above is excessively simplistic. Indeed, other feedback loops are thought to exist in cytokinetic signaling45,46; it will be important to determine how they connect to the core circuit. Additionally, it will be useful to consider other well-known cytokinetic proteins such as MgcRacGAP (also known as Cyk-4) through the lens of cortical excitability. That is, the role played by this protein in cytokinesis has proven controversial32,47–50; perhaps this reflects the inherent difficulty in assigning an epistatic role to a participant in what is apparently a cyclical network rather than a linear pathway.
Finally, recent studies of motile cells indicate that excitable circuits enable a diversity of motile behaviors that may be selectively expressed depending on the constraints imposed by the cell’s environment20,21. Cell division is also subject to various constraints based on cell size, cell–cell adhesions, tissue mechanics, and spindle orientation, all of which vary dramatically between organisms and over the course of development and disease. It will be of great interest to determine whether specific features of excitability, such as wavelength and period, show consistent variation in different cellular contexts.
Acknowledgements
Work in the authors’ labs was supported by the National Science Foundation Award MCB-1614190 (W.M.B.), MCB-1615338 (A.L.M.), MCB-1614606 (G.v.D.), the National Institutes of Health Award GM052932 (W.M.B.), and Biotechnology and Biological Sciences Research Council of UK grants BB/P01190X and BB/P006507 (A.B.G.). J.L. is supported by an American Cancer Society Postdoctoral Fellowship.
Footnotes
Declaration of Interests
The authors declare no competing interests.
References
- 1.Just EE (1939). The Biology of the Cell Surface (Philadelphia: P. Blakiston’s and Son Inc.). [Google Scholar]
- 2.Vicker MG (2000). Reaction-diffusion waves of actin filament polymerization/depolymerization in Dictyostelium pseudopodium extension and cell locomotion. Biophys Chem. 84, 87–98. [DOI] [PubMed] [Google Scholar]
- 3.Weiner OD, Marganski WA, Wu LF, Altschuler SJ, and Kirschner MW (2007). An actin-based wave generator organizes cell motility. PLoS Biol. 5, e221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wu M, Wu X, and De Camilli P (2013). Calcium oscillations-coupled conversion of actin travelling waves to standing oscillations. Proc. Natl. Acad. Sci. USA 110, 1339–1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Graessl M, Koch J, Calderon A, Kamps D, Banerjee S, Mazel T, Schulze N, Jungkurth JK, Patwardhan R, Solouk D, et al. (2017). An excitable Rho GTPase signaling network generates dynamic subcellular contraction patterns. J. Cell Biol 216, 4271–4285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maître JL, Niwayama R, Turlier H, Nédélec F, and Hiiragi T (2015). Pulsatile cell-autonomous contractility drives compaction in the mouse embryo. Nat. Cell Biol 17, 849–55. [DOI] [PubMed] [Google Scholar]
- 7.Bement WM, Leda M, Moe AM, Kita AM, Larson ME, Golding AE, Pfeuti C, Su KC, Miller AL, Goryachev AB, and von Dassow G (2015). Activator-inhibitor coupling between Rho signalling and actin assembly makes the cell cortex an excitable medium. Nat. Cell Biol 17, 1471–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chanet S, and Huynh JR (2020). Collective cell sorting requires contractile cortical waves in germline cells. Curr. Biol 30, 4213–4226. [DOI] [PubMed] [Google Scholar]
- 9.Xiao S, Tong C, Yang Y, and Wu M (2017). Mitotic cortical waves predict future division sites by encoding positional and size information. Dev. Cell 43, 493–506. [DOI] [PubMed] [Google Scholar]
- 10.Iglesias PA, and Devreotes PN (2012). Biased excitable networks: how cells direct motion in response to gradients. Curr. Opin. Cell Biol 24, 245–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Turing AM (1952). The chemical basis of morphogenesis. Philos. Trans. Roy. Soc. Lond. Series B, Biol. Sci 237, 37–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Prigogine I and Lefever R (1968). Symmetry breaking instabilities in dissipative systems. II J. Chem. Phys 48, 1695–1700. [Google Scholar]
- 13.Vanag VK, and Epstein IR (2009). Cross-diffusion and pattern formation in reaction-diffusion systems. Phys. Chem. Chem. Phys 11, 897–912. [DOI] [PubMed] [Google Scholar]
- 14.Bretschneider T, Anderson K, Ecke M, Müller-Taubenberger A, Schroth-Diez B, Ishikawa-Ankerhold HC, and Gerisch G (2009). The three-dimensional dynamics of actin waves, a model of cytoskeletal self-organization. Biophys. J 96, 2888–2900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fukushima S, Matsuoka S, and Ueda M (2019). Excitable dynamics of Ras triggers spontaneous symmetry breaking of PIP3 signaling in motile cells. J. Cell Sci 132, 224121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Devreotes PN, Bhattacharya S, Edwards M, Iglesias PA, Lampert T, and Miao Y (2017). Excitable signal transduction networks in directed cell migration. Annu. Rev. Cell Dev. Biol 33, 103–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Graziano BR, Gong D, Anderson KE, Pipathsouk A, Goldberg AR, and Weiner OD (2017). A module for Rac temporal signal integration revealed with optogenetics. J. Cell Biol 216, 2515–2531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lange M, Prassler J, Ecke M, Müller-Taubenberger A, and Gerisch G (2017). Local Ras activation, PTEN pattern, and global actin flow in the chemotactic responses of oversized cells. J. Cell Sci 129, 3462–3472. [DOI] [PubMed] [Google Scholar]
- 19.Janetopoulos C, Ma L, Devreotes PN, and Iglesias PA (2004). Chemoattractant-induced phosphatidylinositol 3,4,5-trisphosphate accumulation is spatially amplified and adapts, independent of the actin cytoskeleton. Proc. Natl. Acad. Sci. USA 101, 8951–8956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bhattacharya S, Banerjee T, Miao Y, Zhan H, Devreotes PN, and Iglesias PA (2020). Traveling and standing waves mediate pattern formation in cellular protrusions. Sci. Adv 6, eaay7682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miao Y, Bhattacharya S, Edwards M, Cai H, Inoue T, Iglesias PA, and Devreotes PN (2017). Altering the threshold of an excitable signal transduction network changes cell migratory modes. Nat. Cell Biol 19, 329–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bischof J, Brand CA, Somogyi K, Májer I, Thome S, Mori M, Schwarz US, and Lénárt P (2017). A cdk1 gradient guides surface contraction waves in oocytes. Nat. Commun 8, 849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Beaudet D, Akhshi T, Phillipp J, Law C, and Piekny A (2017). Active Ran regulates anillin function during cytokinesis. Mol. Biol. Cell 28, 3517–3531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mitsushima M, Aoki K, Ebisuya M, Matsumura S, Yamamoto T, Matsuda M, Toyoshima F, and Nishida E (2010). Revolving movement of a dynamic cluster of actin filaments during mitosis. J. Cell Biol 191, 453–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jaffe AB, Kaji N, Durgan J, and Hall A (2008). Cdc42 controls spindle orientation to position the apical surface during epithelial morphogenesis. J. Cell Biol 183, 625–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Qin Y, Meisen WH, Hao Y, and Macara IG (2010). Tuba, a Cdc42 GEF, is required for polarized spindle orientation during epithelial cyst formation. J. Cell Biol 189, 661–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Green RA, Paluch E, and Oegema K (2012). Cytokinesis in animal cells. Annu. Rev. Cell Dev. Biol 28, 29–58. [DOI] [PubMed] [Google Scholar]
- 28.Basant A, and Glotzer M (2018). Spatiotemporal regulation of RhoA during cytokinesis. Curr. Biol 28, R570–R580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Piekny AJ, Maddox AS (2010). The myriad roles of Anillin during cytokinesis. Semin. Cell Dev. Biol 21, 881–891. [DOI] [PubMed] [Google Scholar]
- 30.Goryachev AB, Leda M, Miller AL, von Dassow G, and Bement WM (2016). How to make a static cytokinetic furrow out of traveling excitable waves. Small GTPases 7, 65–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bement WM, Miller AL, and von Dassow G (2006). Rho GTPase activity zones and transient contractile arrays. Bioessays. 28, 983–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Miller AL, and Bement WM (2009). Regulation of cytokinesis by Rho GTPase flux. Nat. Cell Biol 11, 71–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yoshida S, Bartolini S, and Pellman D (2009). Mechanisms for concentrating Rho1 during cytokinesis. Genes Dev. 23, 810–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zanin E, Desai A, Poser I, Toyoda Y, Andree C, Moebius C, Bickle M, Conradt B, Piekny A, and Oegema K (2013). A conserved RhoGAP limits M phase contractility and coordinates with microtubule asters to confine RhoA during cytokinesis. Dev. Cell 26, 496–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.von Dassow G, Verbrugghe KJ, Miller AL, Sider JR, and Bement WM (2009). Action at a distance during cytokinesis. J. Cell Biol 187, 831–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bement WM, Benink HA, and von Dassow G (2005). A microtubule-dependent zone of active RhoA during cleavage plane specification. J. Cell Biol 170, 91–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Janetopoulos C, Borleis J, Vazquez F, Iijima M, and Devreotes P (2005). Temporal and spatial regulation of phosphoinositide signaling mediates cytokinesis. Dev. Cell 8, 467–477. [DOI] [PubMed] [Google Scholar]
- 38.Rappaport R (1996). Cytokinesis in Animal Cells (London: Cambridge University Press; ). [Google Scholar]
- 39.Su KC, Bement WM, Petronczki M, and von Dassow G (2015). An astral simulacrum of the central spindle accounts for normal, spindle-less, and anucleate cytokinesis in echinoderm embryos. Mol. Biol. Cell 25, 4049–4062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lewellyn L, Dumont J, Desai A, and Oegema K (2010). Analyzing the effects of delaying aster separation on furrow formation during cytokinesis in the Caenorhabditis elegans embryo. Mol. Biol. Cell 21, 50–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Murthy K, and Wadsworth P (2008). Dual role for microtubules in regulating cortical contractility during cytokinesis. J. Cell Sci. 121, 2350–2359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chen M, Pan H, Sun L, Shi P, Zhang Y, Li L, Huang Y, Chen J, Jiang P, Fang X, Wu C, and Chen Z (2020). Structure and regulation of human epithelial cell transforming 2 protein. Proc. Natl. Acad. Sci. USA 117, 1027–1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bell KR, Werner ME, Doshi A, Cortes DB, Sattler A, Vuong-Brender T, Labouesse M, and Maddox AS (2020). Novel cytokinetic ring components drive negative feedback in cortical contractility. Mol. Biol. Cell 15, 1623–1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Michaux JB, Robin FB, McFadden WM, and Munro EM (2018). Excitable RhoA dynamics drive pulsed contractions in the early C. elegans embryo. J. Cell Biol 217, 4230–4252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Basant A, Lekomtsev S, Tse YC, Zhang D, Longhini KM, Petronczki M, and Glotzer M (2015). Aurora B kinase promotes cytokinesis by inducing centralspindlin oligomers that associate with the plasma membrane. Dev. Cell 33, 204–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Khaliullin RN, Green RA, Shi LZ, Gomez-Cavazos JS, Berns MW, Desai A, and Oegema K (2018). A positive-feedback-based mechanism for constriction rate acceleration during cytokinesis in Caenorhabditis elegans. eLife 7, e36073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Canman JC, Lewellyn L, Laband K, Smerdon SJ, Desai A, Bowerman B, and Oegema K (2008). Inhibition of Rac by the GAP activity of centralspindlin is essential for cytokinesis. Science 322, 1543–1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bastos RN, Penate X, Bates M, Hammond D, and Barr FA (2012). CYK4 inhibits Rac1-dependent PAK1 and ARHGEF7 effector pathways during cytokinesis. J Cell Biol. 198, 865–880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang D, and Glotzer M (2017). The RhoGAP activity of CYK-4/MgcRacGAP functions non-canonically by promoting RhoA activation during cytokinesis. eLife 7, e08898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhuravlev Y, Hirsch SM, Jordan SN, Dumont J, Shirasu-Hiza M, and Canman JC (2017). CYK-4 regulates Rac, but not Rho, during cytokinesis. Mol. Biol. Cell 28, 1258–1270. [DOI] [PMC free article] [PubMed] [Google Scholar]


