Abstract
Aims
Treatment with sodium–glucose co‐transporter 2 (SGLT2) inhibitors improves outcomes in patients with chronic heart failure (HF) with reduced ejection fraction. There is limited experience with the in‐hospital initiation of SGLT2 inhibitors in patients with acute HF (AHF) with or without diabetes. EMPULSE is designed to assess the clinical benefit and safety of the SGLT2 inhibitor empagliflozin compared with placebo in patients hospitalized with AHF.
Methods
EMPULSE is a randomized, double‐blind, parallel‐group, placebo‐controlled multinational trial comparing the in‐hospital initiation of empagliflozin (10 mg once daily) with placebo. Approximately 500 patients admitted for AHF with dyspnoea, signs of fluid overload, and elevated natriuretic peptides will be randomized 1:1 stratified to HF status (de‐novo and decompensated chronic HF) to either empagliflozin or placebo at approximately 165 sites across North America, Europe and Asia. Patients will be enrolled regardless of ejection fraction and diabetes status and will be randomized during hospitalization and after stabilization (between 24 h and 5 days after admission), with treatment continued up to 90 days after initiation. The primary outcome is clinical benefit at 90 days, consisting of a composite of all‐cause death, HF events, and ≥5 point change from baseline in Kansas City Cardiomyopathy Questionnaire total symptom score (KCCQ‐TSS), assessed using a ‘win‐ratio’ approach. Secondary outcomes include assessments of safety, change in KCCQ‐TSS from baseline to 90 days and change in natriuretic peptides from baseline to 30 days.
Conclusion
The EMPULSE trial will evaluate the clinical benefit and safety of empagliflozin in patients hospitalized for AHF.
Keywords: Heart failure, Sodium–glucose co‐transporter 2 inhibitors, Trial design
Introduction
Heart failure (HF) is one of the most prevalent chronic diseases associated with high mortality and morbidity, and one of the most important reasons for hospital admission.1 After discharge, up to 40% of patients are readmitted within 6 months, and 1‐year post‐discharge mortality is high.2 The cost burden of treating patients with HF is substantial, and approximately 80% of costs are related to hospital admission.3 Unfortunately, previous trials investigating treatment options in patients hospitalized for acute HF (AHF) did not reduce post‐discharge mortality or readmission rates.4
Sodium–glucose co‐transporter 2 (SGLT2) inhibitors reduced the risk of cardiovascular (CV) death in patients with type 2 diabetes (T2D) at increased CV risk.5, 6, 7 These effects were accompanied by a mean 23% relative risk reduction in hospitalizations for heart failure (HHF), both in those with and without a history of HF.8 However, these trials primarily included patients with T2D, most without HF at baseline, and those with a history of HF were not well phenotyped. Two large randomized clinical trials (DAPA‐HF and EMPEROR‐Reduced) in patients with stable chronic HF and reduced ejection fraction (HFrEF) provided definitive evidence that treatment with the SGLT2 inhibitors dapagliflozin and empagliflozin reduced the composite of CV death or HHF, both in patients with and without T2D.9, 10, 11 The SOLOIST‐WHF trial showed that the SGLT1/SGLT2 inhibitor sotagliflozin reduced a primary outcome of death from CV causes or total HHF in patients with T2D before or shortly after hospital discharge following an episode of worsening HF that required hospitalization.12
The mechanisms by which SGLT2 inhibitors reduce CV death and HHF are likely multifactorial and may include among others possible direct effects on the myocardium,13 nephroprotection, improvements in cardiac metabolism and cardiac adenosine triphosphate (ATP) production.14, 15, 16 In addition, the early benefit of SGLT inhibitors seen in DAPA‐HF, EMPEROR‐Reduced and SOLOIST‐WHF are thought to be (partly) caused by its diuretic effects. The diuretic effects of empagliflozin in patients started early after a HF hospital admission were demonstrated in a small pilot study.17 In addition, SOLOIST‐WHF included patients during a HF hospital admission, but was limited to patients with T2D. However, whether in‐hospital initiation leads to clinical benefit and is safe in patients with and without diabetes and irrespective of left ventricular ejection fraction (LVEF) remains unclear. If in‐hospital initiation of empagliflozin is proven to be safe and will improve clinical outcome, this might lead to easier and better clinical adoption of these highly efficacious agents and benefiting this vulnerable patient group that has high burden of debilitating symptoms and is at very high risk of recurrent admissions and death. We therefore designed and initiated the EMPULSE trial. The aim of this paper is to describe the rationale and design of this trial.
Methods
Trial structure and oversight
EMPULSE is a multinational, multicentre, randomized, double‐blind superiority trial to evaluate the effects of once daily oral empagliflozin 10 mg compared to placebo on clinical benefit, safety, and tolerability in patients hospitalized for AHF after initial stabilization. The trial is registered at ClinicalTrials.gov identifier NCT04157751, and is being conducted in accordance with the Declaration of Helsinki and the International Conference on Harmonization Good Clinical Practice guidelines. The institutional review board, ethics committee or relevant national competent authority of each participating centre has to approve the study, and all participants provide written informed consent prior to study entry. EMPULSE was designed jointly and trial oversight is provided by the Executive Committee consisting of Academic members and representatives of Boehringer Ingelheim.
Study participants
Participants in the EMPULSE trial are men and women aged ≥18 years (≥21 years in Japan, being the age of legal consent) hospitalized with a primary diagnosis of AHF, regardless of LVEF (Table 1 and Figure 1). Full inclusion and exclusion criteria are provided in online supplementary Table S1. Patients are required to have dyspnoea with at least two of the following signs of decompensation: congestion on chest X‐ray, rales on chest auscultation; clinically relevant oedema, or elevated jugular venous pressure. Patients will be enrolled during hospitalization (following stabilization between 24 h and 5 days after admission). Patients are considered stabilized if they have: a systolic blood pressure ≥100 mmHg and no symptoms of hypotension in the preceding 6 h; no increase in the intravenous (i.v.) diuretic dose for 6 h prior to randomization; no i.v. vasodilators including nitrates within the last 6 h, and no i.v. inotropic drugs for 24 h. In addition, patients are required to have elevated natriuretic peptides of N‐terminal pro‐B‐type natriuretic peptide (NT‐proBNP) ≥1600 pg/mL or B‐type natriuretic peptide (BNP) ≥400 pg/mL. Patients in atrial fibrillation at inclusion must have a concentration of NT‐proBNP ≥2400 pg/mL or BNP ≥600 pg/mL. Finally, all patients must have been treated with a minimum dose of 40 mg (20 mg for Japanese patients) of i.v. furosemide or equivalent (Table 1).
Table 1.
Key inclusion and exclusion criteria
| Key inclusion criteria | Key exclusion criteria |
|---|---|
|
|
AF, atrial fibrillation; BNP, B‐type natriuretic peptide; eGFR, estimated glomerular filtration rate; HF, heart failure; i.v., intravenous; NT‐proBNP, N‐terminal pro‐B‐type natriuretic peptide; PCI, percutaneous coronary intervention; SBP, systolic blood pressure; TAVI, transcatheter aortic valve implantation.
Figure 1.
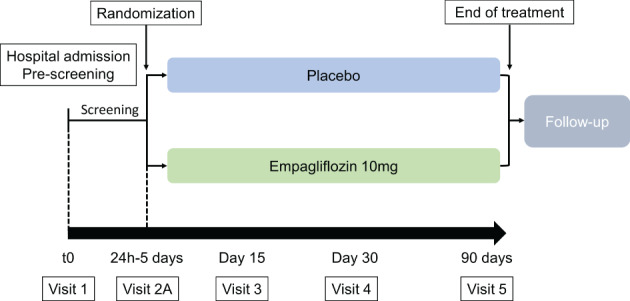
Study design.
Key exclusion criteria include cardiogenic shock, current hospitalization for AHF primarily caused by acute myocardial infarction, major cardiac surgery or interventions planned during the study or in the prior 30 days, or an estimated glomerular filtration rate (eGFR) <20 mL/min/1.73 m2 during hospitalization or patients requiring dialysis. Key exclusion criteria are listed in Table 1. Additional exclusion criteria are listed in online supplementary Table S1 and include current or prior treatment with SGLT1 or SGLT2 inhibitors in the 90 days prior to enrolment, and patients who previously received a cardiac transplant, are expected to receive a transplant during the course of the trial, have planned palliative care for HF, or currently using or planning to use a left ventricular assist device or intra‐aortic balloon pump, or outpatient inotropic support.
Patients should receive usual care per current relevant local and regional guidelines, as defined by their clinician. Patients can be enrolled regardless of T2D status or ejection fraction. Enrolment is stratified according to patients with de‐novo HF and worsening chronic HF.
Study visits and follow‐up
Screening for the study will start when patients are admitted (Visit 1), where informed consent is signed (Figure 1). Patients are subsequently randomized between 24 h and 5 days after admission (Visit 2a) to double‐blind treatment via an IRT system with an equal number of patients (1:1) planned in each group. Visit 2b (day 3) and 2c (day 5) will occur only if patients are still hospitalized. Patients will return to the study site for regularly scheduled visits at 15, 30, and 90 days after randomization. A detailed schedule of assessments is provided in online supplementary Table S2. These on‐site visits will assess the occurrence of safety and efficacy outcomes, and will include measurement of renal function (eGFR), natriuretic peptides, HF severity [New York Heart Association (NYHA) class], and health status using the Kansas City Cardiomyopathy Questionnaire (KCCQ). The selected dose of empagliflozin was 10 mg based on previous evidence of efficacy in improving HF outcomes in large scale trials of patients with ambulatory HF and in trials of patients with T2D.10
Primary and secondary outcomes
The primary outcome of EMPULSE is clinical benefit at 90 days (Table 2). Clinical benefit is defined as a hierarchical composite outcome of time to all‐cause death, the number of HF events (HFE), time to first HFE and a ≥ 5 point increase from baseline in KCCQ total symptom score (KCCQ‐TSS) after 90 days of treatment. HFEs include HHF, urgent HF visits, and unplanned outpatient visits. The definition of a HFE includes the presence of symptoms of HF, signs or laboratory findings corroborating diagnosis, and intensification of therapy (augmentation of either oral diuretics, i.v. diuretics, vasoactive agent, or mechanical or surgical intervention). The full definition is provided in online supplementary Table S3.
Table 2.
Primary and secondary outcomes of the EMPULSE trial
| Primary outcome |
| Clinical benefit, a composite of death, number of heart failure events (including HHFs, urgent heart failure visits and unplanned outpatient visits), time to first heart failure event and change from baseline in KCCQ‐TSS after 90 days of treatment assessed by the win ratio. |
| Secondary outcomes |
|
eGFR, estimated glomerular filtration rate; HHF, hospitalization for heart failure; KCCQ‐TSS, Kansas City Cardiomyopathy Questionnaire total symptom score; NT‐proBNP, N‐terminal pro‐B‐type natriuretic peptide.
Secondary outcomes include an improvement in KCCQ‐TSS of ≥10 points after 90 days of treatment, change from baseline in log‐transformed NT‐proBNP levels over 30 days, days alive and out of hospital until 30 days (after initial hospital discharge) and 90 days (after randomization), time to first occurrence of CV death or HFE until end of trial visit, and change in KCCQ‐TSS between baseline and 90 days. The remainder of the secondary outcomes are listed in Table 2.
To analyse the primary outcome, the ‘win ratio’ will be used (online supplementary Table S4). The efficacy and safety analyses will follow the intention‐to‐treat principle, assigning patient to treatment groups as randomized. The win ratio compares each patient in the trial to every other patient within each stratum (new‐onset HF vs. decompensated chronic HF) in a pairwise hierarchical fashion.18 The win ratio is calculated as the total number of wins in the empagliflozin group across all strata divided by the total number of losses. No adjustment for multiple comparisons is planned. HF status (de‐novo vs. decompensated chronic HF) will be included as a fixed effect. Safety parameters include volume depletion, hypotension and worsening renal function during follow‐up.
Sample size calculations and study conduct
Under a set of assumptions outlined in online supplementary Table S5, including a hazard ratio for death of 0.8, and 0.7 for HFE, we estimated a sample size of 500 (250 per treatment group) randomized patients for a power of 87% and a one‐sided significance level of 0.025. The full details on the power calculations are presented in the online supplementary Appendix.
Modifications due to the COVID‐19 pandemic
Due to the substantial challenges for conduct of clinical trials stemming from the COVID‐19 pandemic,19 several adjustments to the study protocol have been made, outlined in Table 3. First, in exceptional cases where the patient is unable to come to the study site for a study visit, the visits may be performed as home (physical) or remote (virtual) visits or a combination of home and remote visits. Assessments that can be performed during these visits include NYHA class, parts of the congestion score (dyspnoea, orthopnoea, fatigue), adverse events, concomitant therapy, and the Patient Global Impression of Severity of Heart Failure Symptoms (PGI‐S). The KCCQ can be completed by the patient at home. If blood sample collection for the central lab is not possible, blood analysis for safety labs can be done locally. Urine measurements will not be done in a local lab. Lastly, if the investigator judges it as favourable and safe to continue trial medication, this can be shipped from the site to the patient if the patient is unable to come to the site.
Table 3.
Protocol adjustments due to the COVID‐19 pandemic
|
KCCQ‐TSS, Kansas City Cardiomyopathy Questionnaire total symptom score; NYHA, New York Heart Association.
Discussion
Acute HF is the leading cause of hospitalizations in the United States and Western Europe. Patients with AHF are at high risk for readmission and death following discharge.2 There is a clear unmet need in this patient population for effective treatment options to improve post‐discharge clinical outcomes. The ongoing EMPULSE trial is assessing the clinical benefit and safety of empagliflozin in patients with or without diabetes hospitalized with AHF. Results of this trial will add important evidence regarding the use of empagliflozin in patients not included in previous trials with empagliflozin or other SGLT2 inhibitors. Unique aspects of the EMPULSE trial include: (i) enrolling patients during hospitalization for AHF at the very beginning of what is often called ‘the vulnerable phase’ of HF20; (ii) a follow‐up of 90 days with continuous treatment of empagliflozin throughout the post‐discharge period; (iii) inclusion of patients with and without T2D, and with both HFrEF and HF with preserved ejection fraction (HFpEF); and (iv) the use of a win ratio for determining the overall clinical benefit of empagliflozin. A number of these aspects merit further discussion.
Rationale for starting a sodium–glucose co‐transporter 2 inhibitor in patients hospitalized for acute decompensated heart failure
The potential modes of action of SGLT2 inhibition suggest their early use may be beneficial for patients with acute decompensated HF. SGLT2 inhibition improves myocardial energetics, and has direct positive effects on the cardiomyocyte and kidney.13, 14, 21, 22, 23 In addition, empagliflozin might promote ketogenesis, which has a favourable effect on the heart and kidney by effectively increasing ‘fuel efficiency’.14 SGLT2 inhibitors block reuptake of glucose and sodium in the proximal tubule, and can thus cause glucosuria‐mediated osmotic diuresis and possibly (at least transiently) natriuresis.24, 25 In patients with T2D and HFrEF, empagliflozin increased haematocrit, caused haemoconcentration and weight loss.24, 26 While current evidence primarily from patients with HFrEF points towards an increase in osmotic diuresis,24, 25, 26, 27 data on natriuresis are not conclusive.24, 25, 26, 27, 28 Unlike traditional loop diuretics, the increased diuresis seen in treatment with SGLT2 inhibitors is not paralleled by increased renin–angiotensin–aldosterone system activation.24, 25, 26 Haemodynamically, this causes an increase in electrolyte free water clearance, and decrease in plasma volume. The increase in electrolyte free water clearance can explain the greater reported reduction in interstitial fluid rather than intravascular volume compared to traditional diuretics.26 This suggests that SGLT2 inhibitors might specifically target tissue congestion29 rather than intravascular congestion. Yet, while this can explain the short‐term effects of SGLT2 inhibition, this might not explain the long‐term positive effects. In the EMBRACE‐HF study, empagliflozin reduced pulmonary artery pressure, which was not explained by the diuretic effect of empagliflozin alone.30 Thus, other processes including direct effects on the cardiomyocyte, nephroprotection, and improvements in cardiac metabolism and cardiac adenosine triphosphate (ATP) production may be responsible for long‐term benefits.14, 31
Patient selection
EMPULSE includes patients regardless of diabetic status, unlike the prematurely terminated SOLOIST‐WHF trial, which only included patients hospitalized for AHF with T2D.12 Diabetes status did not modify the efficacy or safety of SGLT2 inhibition in either the DAPA‐HF or EMPEROR‐REDUCED trials.9, 10 Secondly, EMPULSE includes patients with no limitation of LVEF. An analysis from the DECLARE‐TIMI 58 trial suggested that dapagliflozin was equally effective in reducing the risk for CV death or hospitalization for HF in patients with HFrEF and HFpEF at baseline.6 The recent SOLOIST‐WHF trial suggests that the mixed SGLT1/SLGT2 inhibitor sotagliflozin effectively reduced the primary outcome of CV death and total HHF in patients with diabetes with HFrEF and HFpEF,12 however it remains unclear if these results can be extrapolated to empagliflozin in those with and without diabetes. Finally, patients with HFrEF and HFpEF present with a comparable state of venous congestion,32 and previous trials showing successful decongestion did not show a difference in effect between HFrEF and HFpEF.33, 34 Together, preliminary data from previous SGLT2 trials suggest equal efficacy across the severity spectrum– and a greater absolute risk reduction as a result in sicker patients. Importantly, inclusion of patients with HFrEF and HFpEF allows us to assess efficacy of empagliflozin in patients across the LVEF spectrum. The absolute risk is very high in patients with AHF; thus, these patients might experience an even greater absolute benefit.
Study design
Two unique aspects of the EMPULSE design are the window of inclusion and duration of follow‐up. Unlike previous trials with SGLT2 inhibitors, EMPULSE targets patients hospitalized for AHF (Figure 2 ) 35, 36 within the first 5 days of hospitalization and continues treatment only during ‘the vulnerable post‐discharge phase’. Treatment initiation in EMPULSE is even earlier than in the SOLOIST‐WHF and PIONEER‐HF trials, which enrolled patients up to a maximum of 5 and 10 days post‐discharge respectively,35 and exclusively targets an in‐hospital population as compared to the recent VICTORIA (<3–6 months after discharge)37 and GALACTIC‐HF36 (from admission to 1 year post‐discharge) trials (Figure 2).
Figure 2.
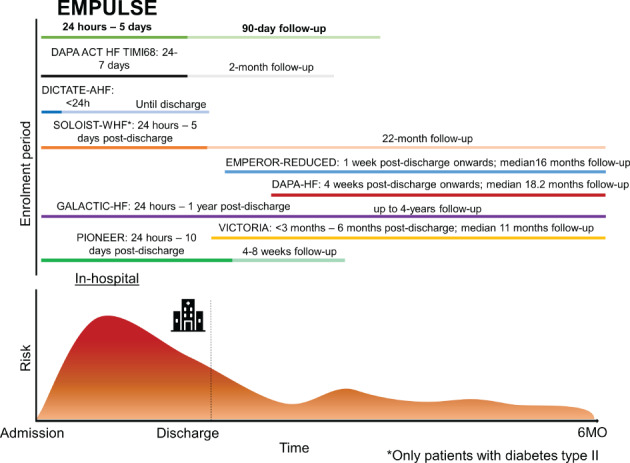
Timeline of EMPULSE for enrolment vs. timelines of enrolment for other recent trials targeting patients with heart failure. The timeline for follow‐up is shown in lighter colour. When follow‐up and inclusion overlap, a darker colour is used.
Previous interventions targeting an AHF population showed no improvement in long‐term outcomes.38, 39, 40, 41, 42 The common theme amongst these earlier studies was that treatment was given only for a short time in‐hospital,39, 40, 41, 42 or for up to 60 days38 post‐discharge. EMPULSE is unique, because it targets patients with AHF in the ‘vulnerable’ phase of HF. While there is much discussion on the duration of this phase,20 most reports suggest a period between 60–90 days post‐discharge.20 Due to the mode of action of SGLT2 inhibitors, we expect an early in‐hospital benefit on congestion relief and outcomes that will transition into the efficacy seen in EMPEROR‐Reduced. An early benefit on outcomes was also observed in the DAPA‐HF, EMPEROR‐Reduced, and EMPA‐RESPONSE‐AHF studies and supports the choice of time frame for inclusion and follow‐up of EMPULSE.9, 10, 17 In both the DAPA‐HF and EMPEROR‐Reduced trials, there was an early benefit in reducing CV death or HHF, observed within days of randomization. Similar trials targeting hospitalized patients with AHF are DICTATE‐AHF43 and DAPA ACT HF‐TIMI 68.44 However, the DICTATE‐AHF randomizes only patients with T2D within 24 h, and continues treatment until discharge.43 Importantly, DICTATE‐AHF is an open‐label study comparing dapagliflozin to usual care. The DAPA ACT HF‐TIMI 68 is currently enrolling patients with HFrEF (LVEF ≤40%) both with and without T2D, but excludes patients with HFpEF.44
Choice of outcome and statistical considerations
EMPULSE utilizes a composite outcome analysed using a stratified win ratio. In the EMPEROR‐Reduced trial, empagliflozin significantly reduced the combined outcome of all‐cause death and HFE compared to placebo as soon as 12 days following randomization.45 Similarly, in a sub‐analysis of patients hospitalized for AHF in the EMPA‐REG OUTCOME trial, empagliflozin significantly reduced the rates of 90‐day post‐discharge all‐cause death or HHF (12.7% vs. 23.2% for placebo).46 Thus, given these data and the efficacy of empagliflozin for improving both HF and non‐HFE, we feel incorporating all‐cause death into the win ratio more appropriately captures its impact. The use of the win ratio was first proposed by Finkelstein and Schoenfeld in 1999.47 The benefit of using a win ratio over a conventional time‐to‐event analysis of a composite outcome is that it gives higher priority to more clinically important events, i.e. mortality. Secondly, it gives a more holistic measure of the improvement of the individual patient, which is very flexible and can be tailored to specific disease areas. There are very few studies using the win ratio as the pre‐specified primary analysis.48, 49 The ATTR‐ACT study used a primary outcome consisting of all‐cause mortality and the frequency of CV hospitalizations, which was analysed using the win ratio.49 In a re‐analysis of 16 large CV trials, hazard ratio and win ratio estimates showed similar treatment effects.48 However, as the win ratio prioritizes fatal outcomes, it may lead to smaller P‐values in trials that show a large effect on fatal events, but larger P‐values in trials without difference in fatal events. In totality, usage of the win ratio enables more proportional weighting of hard outcomes such as mortality over non‐fatal events, which are often weighted equally in conventional approaches. Furthermore, the win ratio allows incorporation of both unfavourable events (death, HHF) and favourable outcomes (improvement in health status/KCCQ‐TSS). The choice for the KCCQ‐TSS rather than the overall summary score or physical domains of the KCCQ, reflects the short nature of the trial, where changes in quality of life are expected earlier than changes in physical domains of the KCCQ or social limitations.
Conclusion
The EMPULSE trial is well positioned to determine the clinical benefit and safety of empagliflozin in a population hospitalized for AHF with continuation of treatment throughout the vulnerable post‐discharge phase. Results of EMPULSE will provide insight as to whether positive results observed in earlier trials performed in patients with chronic HFrEF can be extended to hospitalized patients with HFrEF and HFpEF, both with and without diabetes.
Funding
The EMPULSE trial is funded by the Boehringer Ingelheim & Eli Lilly and Company Diabetes Alliance.
The sponsor of the EMPULSE trial (Boehringer Ingelheim) is committed to responsible sharing of clinical study reports, related clinical documents, and patient level clinical study data. Researchers are invited to submit inquiries via the following website: https://trials.boehringer‐ingelheim.com.
Conflict of interest: J.T. has been a consultant for Roche Diagnostics, and eKo.ai, and has received personal fees from Olink Proteomics, and is shareholder of eKo.ai. C.E.A. has received research/grant support and/or has been a consultant for Abbott, Boehringer Ingelheim, Medtronic, Novartis, ResMed, Thermo Fisher, Vifor and German Federal Ministry of Education and Research. S.P.C. is a consultant for Ortho Clinical, Bristol‐Myers Squibb and Boehringer Ingelheim and receives research support from the NIH, PCORI and Astra Zeneca. J.P.F. is a consultant for Boehringer Ingelheim and receives research support form Astra Zeneca. J.B., A.S., C.G., and M.B. are employees of Boehringer Ingelheim. M.K. has received research grants from AstraZeneca and Boehringer Ingelheim, and has served as a consultant for AstraZeneca, Amgen, Applied Therapeutics, Bayer, Boehringer Ingelheim, Eli Lilly, Janssen, Merck (Diabetes and Cardiovascular), Novo Nordisk, Sanofi and Vifor. M.E.N. has received speaking honoraria from Abbott, and is a consultant for Vifor, Roche and Amgen. J.R.T. has received research support and/or has been a consultant for Amgen, AstraZeneca, Bayer AG, Boehringer Ingelheim, Bristol‐Myers Squibb, Cytokinetics, Medtronic, Merck, Novartis, Servier, and Windtree Therapeutics. A.A.V. has received research support and/or has been a consultant for Amgen, AstraZeneca, Bayer AG, Boehringer Ingelheim, Cytokinetics, Merck, Myokardia, NovoNordisk, Novartis, and Roche Diagnostics.
Supporting information
Table S1. Full inclusion and exclusion criteria.
Table S2. Flow chart of study schedule.
Table S3. Definition of a heart failure event.
Table S4. Sequence of the primary outcome for calculating the win ratio.
Table S5. Assumptions for power calculation.
References
- 1.Tromp J, Ferreira JP, Janwanishstaporn S, Shah M, Greenberg B, Zannad F, Lam CS. Heart failure around the world. Eur J Heart Fail 2019;21:1187–1196. [DOI] [PubMed] [Google Scholar]
- 2.Tromp J, Bamadhaj S, Cleland JG, Angermann CE, Dahlstrom U, Ouwerkerk W, Tay WT, Dickstein K, Ertl G, Hassanein M, Perrone SV, Ghadanfar M, Schweizer A, Obergfell A, Lam CS, Filippatos G, Collins SP. Post‐discharge prognosis of patients admitted to hospital for heart failure by world region, and national level of income and income disparity (REPORT‐HF): a cohort study. Lancet Glob Health 2020;8:e411–e422. [DOI] [PubMed] [Google Scholar]
- 3.Heidenreich PA, Albert NM, Allen LA, Bluemke DA, Butler J, Fonarow GC, Ikonomidis JS, Khavjou O, Konstam MA, Maddox TM, Nichol G, Pham M, Piña IL, Trogdon JG. Forecasting the impact of heart failure in the United States: a policy statement from the American Heart Association. Circ Heart Fail 2013;6:606–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Voors AA, Van Veldhuisen DJ. Why do drugs for acute heart failure fail? Eur J Heart Fail 2012;14:955–956. [DOI] [PubMed] [Google Scholar]
- 5.Rådholm K, Figtree G, Perkovic V, Solomon SD, Mahaffey KW, De Zeeuw D, Fulcher G, Barrett TD, Shaw W, Desai M, Matthews DR, Neal B. Canagliflozin and heart failure in type 2 diabetes mellitus: results from the CANVAS Program. Circulation 2018;138:458–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kato ET, Silverman MG, Mosenzon O, Zelniker TA, Cahn A, Furtado RH, Kuder J, Murphy SA, Bhatt DL, Leiter LA, Mcguire DK, Wilding JPH, Bonaca MP, Ruff CT, Desai AS, Goto S, Johansson PA, Gause‐Nilsson I, Johanson P, Langkilde AM, Raz I, Sabatine MS, Wiviott SD. Effect of dapagliflozin on heart failure and mortality in type 2 diabetes mellitus. Circulation 2019;139:2528–2536. [DOI] [PubMed] [Google Scholar]
- 7.Wanner C, Lachin JM, Inzucchi SE, Fitchett D, Mattheus M, George J, Woerle HJ, Broedl UC, Von Eynatten M, Zinman B; EMPA‐REG OUTCOME Investigators . Empagliflozin and clinical outcomes in patients with type 2 diabetes mellitus, established cardiovascular disease, and chronic kidney disease. Circulation 2018;137:119–129. [DOI] [PubMed] [Google Scholar]
- 8.Zelniker TA, Wiviott SD, Raz I, Im K, Goodrich EL, Bonaca MP, Mosenzon O, Kato ET, Cahn A, Furtado RH, Bhatt DL, Leiter LA, McGuire DK, Wilding JPH, Sabatine MS. SGLT2 inhibitors for primary and secondary prevention of cardiovascular and renal outcomes in type 2 diabetes: a systematic review and meta‐analysis of cardiovascular outcome trials. Lancet 2019;393:31–39. [DOI] [PubMed] [Google Scholar]
- 9.McMurray JJ, Solomon SD, Inzucchi SE, Køber L, Kosiborod MN, Martinez FA, Ponikowski P, Sabatine MS, Anand IS, Bělohlávek J, Böhm M, Chiang C‐E, Chopra VK, de Boer RA, Desai AS, Diez M, Drozdz J, Dukát A, Ge J, Howlett JG, Katova T, Kitakaze M, Ljungman CE, Merkely B, Nicolau JC, O'Meara E, Petrie MC, Vinh PN, Schou M, Tereshchenko S, Verma S, Held C, DeMets DL, Docherty KF, Jhund PS, Bengtsson O, Sjöstrand M, Langkilde AM; DAPA‐HF Trial Committees and Investigators . Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med 2019;381:1995–2008. [DOI] [PubMed] [Google Scholar]
- 10.Packer M, Anker SD, Butler J, Filippatos G, Pocock SJ, Carson P, Januzzi J, Verma S, Tsutsui H, Brueckmann M, Jamal W, Kimura K, Schnee J, Zeller C, Cotton D, Bocchi E, Böhm M, Choi DJ, Chopra V, Chuquiure E, Giannetti N, Janssens S, Zhang J, Gonzalez Juanatey JR, Kaul S, Brunner‐La Rocca HP, Merkely B, Nicholls SJ, Perrone S, Pina I, Ponikowski P, Sattar N, Senni M, Seronde MF, Spinar J, Squire I, Taddei S, Wanner C, Zannad F; EMPEROR‐Reduced Trial Investigators . Cardiovascular and renal outcomes with empagliflozin in heart failure. N Engl J Med 2020;383:1413–1424. [DOI] [PubMed] [Google Scholar]
- 11.Zannad F, Ferreira JP, Pocock SJ, Anker SD, Butler J, Filippatos G, Brueckmann M, Ofstad AP, Pfarr E, Jamal W, Packer M. SGLT2 inhibitors in patients with heart failure with reduced ejection fraction: a meta‐analysis of the EMPEROR‐Reduced and DAPA‐HF trials. Lancet 2020;396:819–829. [DOI] [PubMed] [Google Scholar]
- 12.Bhatt DL, Szarek M, Steg PG, Cannon CP, Leiter LA, McGuire DK, Lewis JB, Riddle MC, Voors AA, Metra M, Lund LH, Komajda M, Testani JM, Wilcox CS, Ponikowski P, Lopes RD, Verma S, Lapuerta P, Pitt B; SOLOIST‐WHF Trial Investigators . Sotagliflozin in patients with diabetes and recent worsening heart failure. N Engl J Med 2021;384:117–128. [DOI] [PubMed] [Google Scholar]
- 13.Packer M. Reconceptualization of the molecular mechanism by which sodium‐glucose cotransporter 2 inhibitors reduce the risk of heart failure events. Circulation 2019;140:443–445. [DOI] [PubMed] [Google Scholar]
- 14.Yurista SR, Silljé HH, Oberdorf‐Maass SU, Schouten EM, Pavez Giani MG, Hillebrands JL, van Goor H, van Veldhuisen DJ, de Boer RA, Westenbrink BD. Sodium‐glucose co‐transporter 2 inhibition with empagliflozin improves cardiac function in non‐diabetic rats with left ventricular dysfunction after myocardial infarction. Eur J Heart Fail 2019;21:862–873. [DOI] [PubMed] [Google Scholar]
- 15.McMurray J, Packer M, Desai A, Gong J, Greenlaw N, Lefkowitz M, Rizkala A, Shi V, Rouleau J, Solomon S, Swedberg K, Zile MR, Andersen K, Arango JL, Arnold M, Bělohlávek J, Böhm M, Boytsov S, Burgess L, Cabrera W, Chen CH, Erglis A, Fu M, Gomez E, Gonzalez A, Hagege AA, Katova T, Kiatchoosakun S, Kim KS, Bayram E, Martinez F, Merkely B, Mendoza I, Mosterd A, Negrusz‐Kawecka M, Peuhkurinen K, Ramires F, Refsgaard J, Senni M, Sibulo AS Jr, Silva‐Cardoso J, Squire I, Starling RC, Vinereanu D, Teerlink JR, Wong R; PARADIGM‐HF Committees and Investigators . A putative placebo analysis of the effects of LCZ696 on clinical outcomes in heart failure. Eur Heart J 2015;36:434–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Seferović PM, Fragasso G, Petrie M, Mullens W, Ferrari R, Thum T, Bauersachs J, Anker SD, Ray R, Çavuşoğlu Y, Polovina M, Metra M, Ambrosio G, Prasad K, Seferović J, Jhund PS, Dattilo G, Čelutkiene J, Piepoli M, Moura B, Chioncel O, Ben Gal T, Heymans S, de Boer RA, Jaarsma T, Hill L, Lopatin Y, Lyon AR, Ponikowski P, Lainščak M, Jankowska E, Mueller C, Cosentino F, Lund L, Filippatos GS, Ruschitzka F, Coats AJ, Rosano GM. Sodium‐glucose co‐transporter 2 inhibitors in heart failure: beyond glycaemic control. A position paper of the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail 2020;22:1495–1503. [DOI] [PubMed] [Google Scholar]
- 17.Damman K, Beusekamp JC, Boorsma EM, Swart HP, Smilde TD, Elvan A, van Eck JW, Heerspink HJ, Voors AA. Randomized, double‐blind, placebo‐controlled, multicentre pilot study on the effects of empagliflozin on clinical outcomes in patients with acute decompensated heart failure (EMPA‐RESPONSE‐AHF). Eur J Heart Fail 2020;22:713–722. [DOI] [PubMed] [Google Scholar]
- 18.Pocock SJ, Ariti CA, Collier TJ, Wang D. The win ratio: a new approach to the analysis of composite endpoints in clinical trials based on clinical priorities. Eur Heart J 2012;33:176–182. [DOI] [PubMed] [Google Scholar]
- 19.Anker SD, Butler J, Khan MS, Abraham WT, Bauersachs J, Bocchi E, Bozkurt B, Braunwald E, Chopra VK, Cleland JG, Ezekowitz J, Filippatos G, Friede T, Hernandez AF, Lam CS, Lindenfeld J, McMurray JJ, Mehra M, Metra M, Packer M, Pieske B, Pocock SJ, Ponikowski P, Rosano GM, Teerlink JR, Tsutsui H, Van Veldhuisen DJ, Verma S, Voors AA, Wittes J, Zannad F, Zhang J, Seferovic P, Coats AJ. Conducting clinical trials in heart failure during (and after) the COVID‐19 pandemic: an Expert Consensus Position Paper from the Heart Failure Association (HFA) of the European Society of Cardiology (ESC). Eur Heart J 2020;41:2109–2117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Greene SJ, Fonarow GC, Vaduganathan M, Khan SS, Butler J, Gheorghiade M. The vulnerable phase after hospitalization for heart failure. Nat Rev Cardiol 2015;12:220–229. [DOI] [PubMed] [Google Scholar]
- 21.Tromp J, Collins SP. Dapagliflozin in heart failure: new frontiers. Eur J Heart Fail 2019;21:1412–1414. [DOI] [PubMed] [Google Scholar]
- 22.Packer M, Butler J, Filippatos G, Zannad F, Ferreira JP, Zeller C, Brueckmann M, Jamal W, Pocock SJ, Anker SD; EMPEROR Trial Committees and Investigators . Design of a prospective patient‐level pooled analysis of two parallel trials of empagliflozin in patients with established heart failure. Eur J Heart Fail 2020;22:2393–2398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Anker SD, Butler J, Filippatos G, Khan MS, Ferreira JP, Bocchi E, Böhm M, Brunner‐La Rocca HP, Choi D, Chopra V, Chuquiure E, Giannetti N, Gomez‐Mesa JE, Janssens S, Januzzi JL, Gonzalez‐Juanatey JR, Merkely B, Nicholls SJ, Perrone SV, Piña IL, Ponikowski P, Senni M, Seronde M, Sim D, Spinar J, Squire I, Taddei S, Tsutsui H, Verma S, Vinereanu D, Zhang J, Jamal W, Schnaidt S, Schnee JM, Brueckmann M, Pocock SJ, Zannad F, Packer M; EMPEROR‐Preserved Trial Committees and Investigators . Baseline characteristics of patients with heart failure with preserved ejection fraction in the EMPEROR‐Preserved trial. Eur J Heart Fail 2020;22:2383–2392. [DOI] [PubMed] [Google Scholar]
- 24.Griffin M, Rao VS, Ivey‐Miranda J, Fleming J, Mahoney D, Maulion C, Suda N, Siwakoti K, Ahmad T, Jacoby D, Riello R, Bellumkonda L, Cox Z, Collins S, Jeon S, Turner JM, Wilson FP, Butler J, Inzucchi SE, Testani JM. Empagliflozin in heart failure: diuretic and cardiorenal effects. Circulation 2020;142:1028–1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wilcox CS, Shen W, Boulton DW, Leslie BR, Griffen SC.Interaction between the sodium‐glucose‐linked transporter 2 inhibitor dapagliflozin and the loop diuretic bumetanide in normal human subjects. J Am Heart Assoc 2018;7:e007046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mordi NA, Mordi IR, Singh JS, Mccrimmon RJ, Struthers AD, Lang CC. Renal and cardiovascular effects of SGLT2 inhibition in combination with loop diuretics in patients with type 2 diabetes and chronic heart failure: the RECEDE‐CHF trial. Circulation 2020;142:1713–1724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Boorsma EM, Beusekamp JC, Maaten JM, Figarska SM, Danser AH, Veldhuisen DJ, Meer P, Heerspink HJ, Damman K, Voors AA. Effects of empagliflozin on renal sodium and glucose handling in patients with acute heart failure. Eur J Heart Fail 2021;23:68–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bjornstad P, Laffel L, Tamborlane WV, Simons G, Hantel S, Von Eynatten M, George J, Marquard J, Cherney DZ. Acute effect of empagliflozin on fractional excretion of sodium and eGFR in youth with type 2 diabetes. Diabetes Care 2018;41:e129–e130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Boorsma EM, ter Maaten JM, Damman K, Dinh W, Gustafsson F, Goldsmith S, Burkhoff D, Zannad F, Udelson JE, Voors AA. Congestion in heart failure: a contemporary look at physiology, diagnosis and treatment. Nat Rev Cardiol 2020;17:641–655. [DOI] [PubMed] [Google Scholar]
- 30.Nassif ME, Qintar M, Windsor SL, Jermyn R, Shavelle DM, Tang F, Lamba S, Bhatt K, Brush J, Civitello A, Gordon R, Jonsson O, Lampert B, Pelzel J, Kosiborod M.Empagliflozin effects on pulmonary artery pressure in patients with heart failure: results from the EMBRACE‐HF trial. Circulation 2021;143:1673–1686. [DOI] [PubMed] [Google Scholar]
- 31.Packer M. SGLT2 inhibitors produce cardiorenal benefits by promoting adaptive cellular reprogramming to induce a state of fasting mimicry: a paradigm shift in understanding their mechanism of action. Diabetes Care 2020;43:508–511. [DOI] [PubMed] [Google Scholar]
- 32.Van Aelst LN, Arrigo M, Placido R, Akiyama E, Girerd N, Zannad F, Manivet P, Rossignol P, Badoz M, Sadoune M, Launay JM, Gayat E, Lam CS, Cohen‐Solal A, Mebazaa A, Seronde MF. Acutely decompensated heart failure with preserved and reduced ejection fraction present with comparable haemodynamic congestion. Eur J Heart Fail 2018;20:738–747. [DOI] [PubMed] [Google Scholar]
- 33.Filippatos G, Teerlink JR, Farmakis D, Cotter G, Davison BA, Felker GM, Greenberg BH, Hua T, Ponikowski P, Severin T, Unemori E, Voors AA, Metra M. Serelaxin in acute heart failure patients with preserved left ventricular ejection fraction: results from the RELAX‐AHF trial. Eur Heart J 2014;35:1041–1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Matsue Y, Suzuki M, Torii S, Yamaguchi S, Fukamizu S, Ono Y, Fujii H, Kitai T, Nishioka T, Sugi K, Onishi Y, Noda M, Kagiyama N, Satoh Y, Yoshida K, Goldsmith SR. Clinical effectiveness of tolvaptan in patients with acute heart failure and renal dysfunction. J Card Fail 2016;22:423–432. [DOI] [PubMed] [Google Scholar]
- 35.Velazquez EJ, Morrow DA, DeVore AD, Duffy CI, Ambrosy AP, McCague K, Rocha R, Braunwald E; PIONEER‐HF Investigators . Angiotensin‐neprilysin inhibition in acute decompensated heart failure. N Engl J Med 2019;380:539–548. [DOI] [PubMed] [Google Scholar]
- 36.Teerlink JR, Diaz R, Felker GM, McMurray JJ, Metra M, Solomon SD, Adams KF, Anand I, Arias‐Mendoza A, Biering‐Sørensen T, Böhm M, Bonderman D, Cleland JGF, Corbalan R, Crespo‐Leiro MG, Dahlström U, Echeverria LE, Fang JC, Filippatos G, Fonseca C, Goncalvesova E, Goudev AR, Howlett JG, Lanfear DE, Li J, Lund M, Macdonald P, Mareev V, Momomura SI, O'Meara E, Parkhomenko A, Ponikowski P, Ramires FJ, Serpytis P, Sliwa K, Spinar J, Suter TM, Tomcsanyi J, Vandekerckhove H, Vinereanu D, Voors AA, Yilmaz MB, Zannad F, Sharpsten L, Legg JC, Varin C, Honarpour N, Abbasi SA, Malik FI, Kurtz CE; GALACTIC‐HF Investigators. Cardiac myosin activation with omecamtiv mecarbil in systolic heart failure. N Engl J Med 2021;384:105–116. [DOI] [PubMed] [Google Scholar]
- 37.Armstrong PW, Pieske B, Anstrom KJ, Ezekowitz J, Hernandez AF, Butler J, Lam CS, Ponikowski P, Voors AA, Jia G, McNulty SE, Patel MJ, Roessig L, Koglin J, O'Connor CM; VICTORIA Study Group . Vericiguat in patients with heart failure and reduced ejection fraction. N Engl J Med 2020;382:1883–1893. [DOI] [PubMed] [Google Scholar]
- 38.Konstam MA, Gheorghiade M, Burnett JC, Grinfeld L, Maggioni AP, Swedberg K, Udelson JE, Zannad F, Cook T, Ouyang J, Zimmer C, Orlandi C; Efficacy of Vasopressin Antagonism in Heart Failure Outcome Study With Tolvaptan (EVEREST) Investigators . Effects of oral tolvaptan in patients hospitalized for worsening heart failure: the EVEREST Outcome Trial. JAMA 2007;297:1319–1331. [DOI] [PubMed] [Google Scholar]
- 39.O'Connor CM, Starling RC, Hernandez AF, Armstrong PW, Dickstein K, Hasselblad V, Heizer GM, Komajda M, Massie BM, McMurray JJ, Nieminen MS, Reist CJ, Rouleau JL, Swedberg K, Adams KF, Anker SD, Atar D, Battler A, Botero R, Bohidar NR, Butler J, Clausell N, Corbalán R, Costanzo MR, Dahlstrom U, Deckelbaum LI, Diaz R, Dunlap ME, Ezekowitz JA, Feldman D, Felker GM, Fonarow GC, Gennevois D, Gottlieb SS, Hill JA, Hollander JE, Howlett JG, Hudson MP, Kociol RD, Krum H, Laucevicius A, Levy WC, Méndez GF, Metra M, Mittal S, Oh BH, Pereira NL, Ponikowski P, Tang WH, Tanomsup S, Teerlink JR, Triposkiadis F, Troughton RW, Voors AA, Whellan DJ, Zannad F, Califf RM. Effect of nesiritide in patients with acute decompensated heart failure. N Engl J Med 2011;365:32–43. [DOI] [PubMed] [Google Scholar]
- 40.Massie BM, O'Connor CM, Metra M, Ponikowski P, Teerlink JR, Cotter G, Weatherley BD, Cleland JG, Givertz MM, Voors A, DeLucca P, Mansoor GA, Salerno CM, Bloomfield DM, Dittrich HC; PROTECT Investigators and Committees . Rolofylline, an adenosine A1‐receptor antagonist, in acute heart failure. N Engl J Med 2010;363:1419–1428. [DOI] [PubMed] [Google Scholar]
- 41.Metra M, Teerlink JR, Cotter G, Davison BA, Felker GM, Filippatos G, Greenberg BH, Pang PS, Ponikowski P, Voors AA, Adams KF, Anker SD, Arias‐Mendoza A, Avendaño P, Bacal F, Böhm M, Bortman G, Cleland JG, Cohen‐Solal A, Crespo‐Leiro MG, Dorobantu M, Echeverría LE, Ferrari R, Goland S, Goncalvesová E, Goudev A, Køber L, Lema‐Osores J, Levy PD, McDonald K, Manga P, Merkely B, Mueller C, Pieske B, Silva‐Cardoso J, Špinar J, Squire I, Stępińska J, Van Mieghem W, von Lewinski D, Wikström G, Yilmaz MB, Hagner N, Holbro T, Hua TA, Sabarwal SV, Severin T, Szecsödy P, Gimpelewicz C; RELAX‐AHF‐2 Committees Investigators . Effects of serelaxin in patients with acute heart failure. N Engl J Med 2019;381:716–726.31433919 [Google Scholar]
- 42.Packer M, O'Connor C, McMurray JJ, Wittes J, Abraham WT, Anker SD, Dickstein K, Filippatos G, Holcomb R, Krum H, Maggioni AP, Mebazaa A, Peacock WF, Petrie MC, Ponikowski P, Ruschitzka F, van Veldhuisen DJ, Kowarski LS, Schactman M, Holzmeister J; TRUE‐AHF Investigators . Effect of ularitide on cardiovascular mortality in acute heart failure. N Engl J Med 2017;376:1956–1964. [DOI] [PubMed] [Google Scholar]
- 43.Cox ZL, Collins SP, Aaron M, Hernandez GA, McRae AT 3rd, Davidson BT, Fowler M, Lindsell CJ, Harrell FE Jr, Jenkins CA, Kampe C, Miller KF, Stubblefield WB, Lindenfeld J. Efficacy and safety of dapagliflozin in acute heart failure: rationale and design of the DICTATE‐AHF trial. Am Heart J 2021;232:116–124. [DOI] [PubMed] [Google Scholar]
- 44.Dapagliflozin and Effect on Cardiovascular Events in Acute Heart Failure ‐Thrombolysis in Myocardial Infarction 68 (DAPA ACT HF‐TIMI 68). https://clinicaltrials.gov/show/NCT04363697. 2020. https://www.cochranelibrary.com/central/doi/10.1002/central/CN‐02093765/full (14 December 2020)
- 45.Packer M, Anker SD, Butler J, Filippatos GS, Ferreira JP, Pocock S, Carson PE, Anand IS, Doehner W, Haass M, Komajda M, Miller AB, Pehrson S, Teerlink JR, Brueckmann M, Jamal W, Zeller C, Schnaidt S, Zannad F. Effect of empagliflozin on the clinical stability of patients with heart failure and a reduced ejection fraction: the EMPEROR‐Reduced trial. Circulation 2020;143:326–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Savarese G, Sattar N, Januzzi J, Verma S, Lund LH, Fitchett D, Zeller C, George JT, Brueckmann M, Ofstad AP, Inzucchi SE, Wanner C, Zinman B, Butler J. Empagliflozin is associated with a lower risk of post‐acute heart failure rehospitalization and mortality. Circulation 2019;139:1458–1460. [DOI] [PubMed] [Google Scholar]
- 47.Finkelstein DM, Schoenfeld DA. Combining mortality and longitudinal measures in clinical trials. Stat Med 1999;18:1341–1354. [DOI] [PubMed] [Google Scholar]
- 48.Ferreira JP, Jhund PS, Duarte K, Claggett BL, Solomon SD, Pocock S, Petrie MC, Zannad F, McMurray JJ. Use of the win ratio in cardiovascular trials. JACC Heart Fail 2020;8:441–450. [DOI] [PubMed] [Google Scholar]
- 49.Maurer MS, Schwartz JH, Gundapaneni B, Elliott PM, Merlini G, Waddington‐Cruz M, Kristen AV, Grogan M, Witteles R, Damy T, Drachman BM, Shah SJ, Hanna M, Judge DP, Barsdorf AI, Huber P, Patterson TA, Riley S, Schumacher J, Stewart M, Sultan MB, Rapezzi C; ATTR‐ACT Study Investigators . Tafamidis treatment for patients with transthyretin amyloid cardiomyopathy. N Engl J Med 2018;379:1007–1016. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Full inclusion and exclusion criteria.
Table S2. Flow chart of study schedule.
Table S3. Definition of a heart failure event.
Table S4. Sequence of the primary outcome for calculating the win ratio.
Table S5. Assumptions for power calculation.


