Abstract
Background
Posttraumatic olfactory dysfunction is a clinical challenge due to refractory characteristics and limited therapeutic options. Olfactory training has been proved to be effective for olfactory dysfunction with varied etiologies. We pooled existing studies to evaluate the effects of olfactory training in patients with posttraumatic olfactory dysfunction.
Methods
A systematic literature review using PubMed, Embase, Cochrane Library, and Web of Science was conducted to identify studies assessing olfactory change in patients with posttraumatic olfactory dysfunction after olfactory training.
Results
Of the initial 812 abstracts reviewed, 13 full‐text articles were included. Clinically significant results after olfactory training were defined as an improvement of threshold, discrimination, and identification (TDI) score ≥6 or University of Pennsylvania Smell Identification Test (UPSIT) score ≥4. Six studies were included in the meta‐analysis, 36.31% (95% confidence interval [CI], 0.28 to 0.45) of posttraumatic patients would achieve clinically significant results after olfactory training with a mean increase of TDI score of 4.61.
Conclusion
Olfactory training might be a promising modality for the treatment of posttraumatic olfactory dysfunction. More high‐quality studies with controls are needed to clarify the effect of olfactory training on total olfactory performance and subcomponents of olfaction.
Keywords: olfactory dysfunction, olfactory training, meta‐analysis, posttraumatic olfactory dysfunction, systematic review
Posttraumatic olfactory dysfunction is a frequent olfactory disorder, accounting for 16% to 39% of patients seeking consultation from specialized smell and taste clinics.1, 2, 3 It has been reported that up to 60% of patients with traumatic brain injury presented with olfactory dysfunction.4, 5 The frequency of anosmia ranges from 8% to 34% and increases with the severity of traumatic brain injury.6, 7, 8, 9 The quality of life in patients with posttraumatic olfactory dysfunction is significantly impaired, especially in terms of the perception of smell changes, adapting to smell changes, and fear of hazardous substance exposure.10 Furthermore, patients with posttraumatic olfactory dysfunction performed worse on tests of affect recognition, emotional inference, empathy, and memory and executive functioning compared to posttraumatic patients with normosmia.11, 12
Three specific mechanisms have been proposed to describe the possible pathophysiology of posttraumatic olfactory dysfunction including sinonasal tract disruption, direct shearing or stretching of olfactory nerve fibers at the cribriform plate, and focal contusion or hemorrhage within the olfactory bulb and cortex.13, 14 Pharmacologic management and surgical treatment for patients with posttraumatic olfactory dysfunction were minimal and the prognosis is worse than olfactory dysfunction secondary to sinonasal disease or viral infection.15, 16 Anti‐inflammatory drugs including steroids were effective in improving olfactory outcomes during the acute phase of head injury and early treatment with systemic steroids was highly associated with better olfactory outcomes in patients with posttraumatic olfactory dysfunction.16, 17 Animal studies showed that there was a time limit for starting anti‐inflammatory treatment in patients with posttraumatic olfactory dysfunction and patients might not benefit from anti‐inflammatory therapy 14 days or later after head injury.18 Although there are limited therapeutic options for patients with posttraumatic olfactory dysfunction, about 16.8% to 27% of patients may experience some degree of spontaneous recovery, which is mainly due to the high degree of neuroplasticity of the olfactory system.19, 20, 21 Additionally, olfactory bulb integrity has been identified as the sole prognostic factor for posttraumatic olfactory recovery.21
There are accumulating studies demonstrating the therapeutic effect of olfactory training on non‐sinonasal‐related olfactory dysfunction, especially for patients with postviral olfactory dysfunction.22, 23 It has been proved that olfactory training increases the electrophysiological activity at the level of the olfactory epithelium and also increases the gray matter volume and regional functional connectivity within chemosensory processing networks.24, 25, 26 Although several studies have explored the effects of olfactory training on posttraumatic olfactory dysfunction, the efficacy of olfactory training on olfaction among patients with posttraumatic olfactory dysfunction is not well known.
The purpose of the study was to perform a systematic review with a meta‐analysis of the efficacy of olfactory training on the olfactory recovery among patients with posttraumatic olfactory dysfunction.
1. MATERIALS AND METHODS
1.1. Information sources and search strategy
This systematic review of meta‐analysis was performed according to the Preferred Reporting Items for Systematic Reviews and Meta‐Analysis (PRISMA) statement. A comprehensive systematic literature review was performed using the PubMed, Embase, Cochrane Library, and Web of Science databases. We searched the full text with the following keywords: trauma, traumatic, posttraumatic, traumatic brain injury, post traumatic olfactory loss, posttraumatic olfactory dysfunction, post injury, or brain injury, and olfactory training, smell training, or olfactory rehabilitation. The comprehensive search strategy is shown in Table 1.
TABLE 1.
Database search strategy applied for the systematic review of olfactory training for posttraumatic olfactory dysfunction
| Search terms |
|---|
| ALL Fields: trauma OR traumatic OR posttraumatic OR traumatic brain injury OR TBI OR post traumatic olfactory loss OR PTOL OR post injury OR brain injury |
| AND |
| ALL Fields: (olfactory training) OR ALL Fields: (olfactory rehabilitation) OR ALL Fields: (smell training) |
PTOL = posttraumatic olfactory loss; TDI = threshold, discrimination, identification.
1.2. Selection criteria and data extraction
Two investigators (T.H. and D.W.) checked the titles and abstracts of all identified studies and then reviewed the full articles. We included studies that evaluated olfactory training on patients with olfactory dysfunction due to trauma and excluded the studies focused on other causes of olfactory dysfunction or studies without olfactory training. Some studies included patients with olfactory dysfunction of multiple etiologies, such as postinfectious, posttraumatic, and idiopathic. These studies were also included as long as the data on olfactory training in patients with posttraumatic olfactory dysfunction was available. Animal experiments, case reports, and conference abstracts were excluded. The selection process was illustrated in Figure 1. Data extracted by 2 independent authors (T.H. and D.W.) from each study included sample size, patients, interventions, groups, outcomes, study design, olfactory training duration, the definition of clinically significant results, percentage of patients with clinically significant results, and threshold, discrimination, and identification (TDI) score before and after olfactory training. Then we checked the data and articles to eliminate the errors.
FIGURE 1.
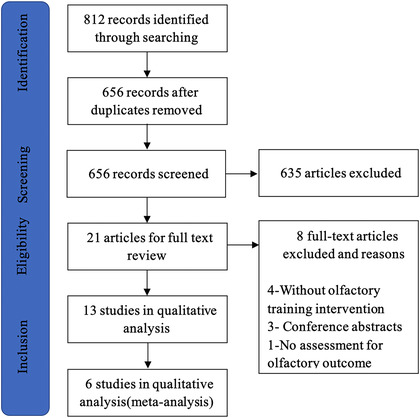
Article selection process for systematic literature review
1.3. Quality assessment
The Cochrane Collaboration's tool was used to assess the risk of bias in randomized controlled trials, which includes random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting, and other bias.27 Each of the 7 areas needs to be assigned to high, low, or unclear risk of bias after assessments. The Newcastle‐Ottawa Scale (NOS) was used to assess the quality of nonrandomized studies. The NOS judges a study on 3 broad perspectives: the selection of the study groups; the comparability of the groups; and the ascertainment of either the exposure or outcome of interest for case‐control or cohort studies, respectively.28 A star system is applied to allow a semiquantitative assessment of study quality, which ranges between 0 and 9 stars (highest quality).
1.4. Statistical analysis
Five studies included in the meta‐analysis reported the proportion of clinically significant results in olfactory training.29, 30, 31, 32, 33 Some studies included patients with olfactory dysfunction of multiple etiologies, and we extracted the data on olfactory training in patients with posttraumatic olfactory dysfunction. According to the extracted information, the total number of patients and the number of patients with clinically significant results were sorted out, and the RevMan 5.3 software (Cochrane Collaboration, London, UK) was used for meta‐analysis. In this study, the incidence of p (proportion of clinically significant results) did not meet the normal distribution, and the ratio type data was used to calculate the incidence.34 The formula was as follows:
where x is the number of patients needed to achieve clinically significant results and n is the total number of patients. We generated a Forest plot and used a fixed‐effects model to calculate the odds ratio (OR). The OR value needs to be calculated by the following formula to get the proportion and 95% confidence interval (CI): P = OR/(1+OR), LL (lower limit) = LLOR/(1+ LLOR), UL (upper limit) = ULOR/(1+ ULOR).34, 35 Two studies with TDI scores before and after olfactory training were included in another meta‐analysis to calculate the mean improvement of TDI score.32, 36 Pellegrino et al.36 divided posttraumatic patients into anosmia group and hyposmia group. In the second meta‐analysis, Robert(a) represented the anosmia group and Robert(b) represented the hyposmia group; there was no combined data.
2. RESULTS
2.1. Characteristics of studies
The completed literature search retrieved a total of 656 articles. After screening, 21 articles were selected for full text review and 8 articles were excluded; the most common reasons were the intervention without olfactory training, conference abstracts and no assessment for the olfactory outcome (Fig. 1). Thirteen articles met the criteria for qualitative analysis29, 30, 31, 32, 33, 36, 37, 38, 39, 40, 41, 42, 43 and 6 of these were included in the meta‐analysis.29, 30, 31, 32, 33, 36 All 13 articles reported the distribution of sex, a total of 565 females and 403 males, skewing toward female patients. Patients were adults with posttraumatic olfactory dysfunction and the duration of trauma ranged from 1 month to 20 years. The main features of the study were presented in Table 2. Studies in the meta‐analysis involved a total of 134 patients with posttraumatic olfactory dysfunction. Because most studies lacked control groups or appropriate controls, we only used the data of the experimental group in studies.
TABLE 2.
Characteristics of studies included in the systematic review and meta‐analysis
| Study | Design | Patients | Intervention | OT duration | Outcome | Conclusion |
|---|---|---|---|---|---|---|
| Langdon et al.37 (2018) | Randomized controlled trial |
OT (n = 21) vs Control (n = 21) All posttraumatic |
OT: 6‐odor training set. Control: no intervention |
3 months |
An increase in n‐BTt ≥30% OT group: 26% Control group: 5% |
12 Weeks of OT mildly improves the olfactory threshold in TBI |
| Jiang et al.29 (2019) | Randomized controlled trial |
4‐odorant group(n = 45) vs PEA group(n = 45) All posttraumatic |
4‐Odor training or PEA training | 6 months |
An increase in UPSIT score ≥4 4‐odorant group: 40% (n = 18) PEA group: 46.7% (n = 21) |
OT can slightly improve the odor threshold in patients with traumatic anosmia but did not improve the odor identification |
| Nguyen et al.38 (2018) | Randomized controlled trial | OT + budesonide irrigation (n = 66, posttraumatic = 9) VS OT + saline irrigation (n = 67, posttraumatic = 7) | 4‐Odor training with budesonide irrigation or saline irrigation | 6 months |
An increase in UPSIT score ≥5 OT+ budesonide irrigation: 43% OT + saline irrigation: 26.9% |
OT with budesonide irrigation improves olfaction better than OT with saline irrigation |
| Jiang et al.39 (2017) | Randomized controlled trial |
PEA(n = 42) vs Mineral oil (n = 39) All posttraumatic |
PEA: PEA training. Mineral oil: mineral oil training | 3 months |
An increase in UPSIT score ≥4 PEA group: 14.3% (n = 6) Mineral oil group: 15.4% (n = 6) |
OT with PEA can improve PEA odor threshold levels in patients with traumatic anosmia |
| Hummel et al.30 (2009) | Prospective cohort study | OT (n = 40, posttraumatic = 5) vs Control (n = 16, posttraumatic = 2) |
OT: 4‐odor training set. Control: no intervention |
3 months |
An increase in TDI score ≥6 OT group: 28% (n = 10) Control group: 6% (n = 1) |
Training patients experienced an increase in the Sniffin’ Sticks test score and thresholds |
| Fleiner et al.31 (2012) | Retrospective cohort study | OT (n = 28, Posttraumatic = 4,) vs OT+topical corticosteroids (n = 18, Posttraumatic = 3) | 4‐Odor training with or without topical corticosteroids | 8 months |
An increase ≥6 in TDI score OT: 10.7% (n = 3) OT+topical corticosteroids: 33% (n = 6) |
Olfactory discrimination and identification can be enhanced by the addition of a topical corticosteroid to olfactory training |
| Konstantinidis et al.32 (2013) | Prospective cohort study |
Postinfectious: OT (n = 49) vs Control (n = 32) Posttraumatic: OT (n = 23) vs Control (n = 15) |
OT: 4‐odor training Control: no intervention |
3 months |
An increase ≥6 in TDI score Posttraumatic: OT: 33% (n = 8) Control: 13% (n = 2) |
OT may increase olfaction in patients with postinfectious and posttraumatic OD |
| Poletti et al.40 (2017) | Prospective cohort study | OT with HWM (n = 48, Posttraumatic15) vs OT with LWM (n = 48, Posttraumatic11) | OT with heavy weight molecules (> 150 g/mol) or light weight molecule (< 150 g/mol) | 5 months |
An increase ≥5.5 in TDI score OT with HWM: 38% OT with LWM: 36% |
OT was associated with olfactory improvement. There were no significant differences between LWM and HWM groups. |
| Fornazieri et al.41 (2020) | Prospective cohort study | COT (n = 12, posttraumatic = 1) vs MOT (n = 13, posttraumatic = 5) |
COT: 4‐odor training. MOT: 6 odors of commercial products |
6 months |
An increase ≥5 in UPSIT score COT: 33.3% (n = 2) MOT: 18.2% (n = 2) |
Adherence to treatment was high until the third month but declined significantly by the end of 6 months. |
| Pelligrino et al.36 (2019) | Prospective cohort study |
Anosmia (n = 23) vs Hyposmia (n = 14) All posttraumatic |
4‐Odor training | 6 months | Training increased overall TDI, threshold scores and identification scores | OT improved olfactory performance and there was no change in OB volumes |
| Bratt et al.42 (2020) | Prospective cohort study | 22 Patients with persistent OD after trauma | Treatment for 10 days with oral corticosteroids and thereafter for 3 months with 4‐odor OT | 3 months |
An increase ≥6 in TDI score All patients: 50% (n = 11) |
Treatment with corticosteroids and OT was promising in persistent OD after TBI |
| Yan et al.33 (2018) | Prospective cohort study | OD patients (n = 86, posttraumatic = 37) | 4‐Odor training | 4 months |
An increase ≥6 in TDI score Posttraumatic: 32.4% (n = 11) Postinfectious: 45.7% (n = 21) |
OT can achieve better therapeutic effects at the early stage |
| Jung et al.43 (2019) | Prospective cohort study | Patients with OD (n = 134, posttraumatic = 18) | 4‐Odor training with fluticasone nasal spray | 3 months |
An increase ≥6 in TDI score All patients: 55.2% (n = 74) Posttraumatic: 33.3% (n = 6) |
OT with intranasal corticosteroid was beneficial to improve olfactory function |
COT = classical olfactory training; HWM, heavy weight molecules; LWM, light weight molecule; MOT = modified olfactory training; n‐BTt = n‐butanol threshold; OB = olfactory bulb; OD = olfactory dysfunction; OT = olfactory training; PEA = phenyl ethyl alcohol; TBI = traumatic brain injury; TDI = threshold, discrimination, identification; UPSIT = University of Pennsylvania Smell Identification Test.
Ten studies used a 4‐odor olfactory training strategy (Table 2) including rose, eucalyptus, lemon, and cloves, which is usually defined as classical olfactory training.30 Two studies used phenyl ethyl alcohol as a training reagent. Another 2 studies used 6 odors and 1 study used reagents with different weight molecules. Six articles that were included in the meta‐analysis all evaluated the effect of classical olfactory training. The study reported modified olfactory training showed better results in terms of discrimination and identification scores compared to classical olfactory training.44 The duration of olfactory training ranged from 3 to 8 months with an average of 5 months.
Methods of assessing olfactory performance mainly included Sniffin’ Sticks test and University of Pennsylvania Smell Identification Test (UPSIT), except 1 study used n‐butanol threshold.37 Of 6 articles included in the meta‐analysis, 5 articles used Sniffin’ Sticks test and 1 article used UPSIT. Different methods in assessing olfaction may introduce heterogeneity and studies were divided into 2 subgroups. In these studies, 4 articles defined clinically significant results as an increase of ≥6 in TDI score, 1 defined as an increase of ≥4 in UPSIT score and 1 provided the data of TDI score before and after OT.
2.2. Risk of bias
Four articles were randomized controlled trials,29, 37, 38, 39 assessed by the Cochrane Collaboration's tool, which was shown in Figure 2. Nine articles were cohort studies,30, 31, 32, 33, 36, 40, 41, 42, 43 assessed by the Newcastle‐Ottawa Scale (Table 3). The main sources of bias in these studies were ascertainment of exposure and comparability. Most studies used a written self‐report record to make sure patients complete the smell training on time.
FIGURE 2.
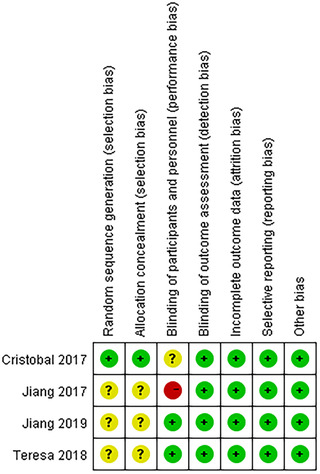
Assessment of 4 randomized controlled trials by Cochrane Collaboration's tool. Plus sign indicates a low risk of bias; the question mark indicates an unclear risk of bias; minus sign indicates a high risk of bias
TABLE 3.
Assessment of 9 cohort studies by Newcastle‐Ottawa Scale
| Parameter | Hummel et al.30 (2009) | Fleiner et al.31 (2012) | Konstantinisis et al.32 (2013) | Poletti et al.40 (2016) | Fornazieri et al.41 (2019) | Pellegrino et al.36 (2019) | Bratt et al.42 (2020) | Yan et al.33 (2018) | Jung et al.43 (2019) |
|---|---|---|---|---|---|---|---|---|---|
| Representativeness of the exposed cohort | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 |
| Selection of the non‐exposed cohort | 1 | 1 | 1 | 1 | 1 | 1 | – | – | – |
| Ascertainment of exposure | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 0 |
| Demonstration that outcome of interest was not present at start of study | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Comparability of cohorts on the basis of the design or analysis | 2 | 0 | 2 | 1 | 0 | 1 | – | – | – |
| Assessment of outcome | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Was follow‐up long enough for outcomes to occur | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Adequacy of follow‐up of cohorts | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 |
| Total score | 8 | 7 | 8 | 7 | 6 | 6 | 5 | 6 | 5 |
2.3. Meta‐analysis
Five articles were included in the meta‐analysis and divided into 2 subgroups according to different olfactory assessments: TDI group30, 31, 32, 33 and UPSIT group.29 In each subgroup and total, low heterogeneity was observed with an I 2 statistic of 0%. The test for subgroup differences showed I 2 was also 0%. Therefore, we used the fixed‐effect model in the forest plot (Fig. 3).
FIGURE 3.
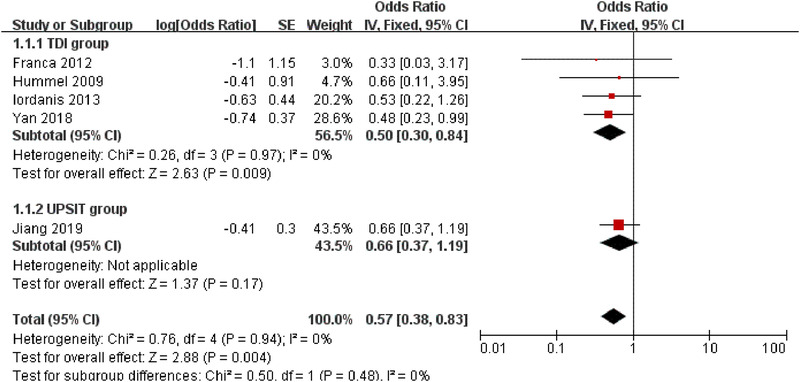
Forest plot for the proportion of clinically significant results in patients with posttraumatic olfactory dysfunction after olfactory training. CI = confidence interval; SE = standard error; TDI = threshold, discrimination, identification; UPSIT = University of Pennsylvania Smell Identification Test
OR needs to be converted to get the proportion of clinically significant results and 95% CI.35 The calculated proportion were 33.33% (95% CI, 0.23 to 0.46) in TDI group, 39.76% (95% CI, 0.27 to 0.54) in UPSIT group, 36.31% (95% CI, 0.28 to 0.45) in total. The final results were very close to each group. About one‐third of patients with posttraumatic olfactory dysfunction experienced clinically significant improvement after olfactory training.
Two articles included in the second meta‐analysis compared the TDI score before and after olfactory training.32, 36 In patients with posttraumatic olfactory dysfunction, the mean improvement of TDI score was 4.61, which was lower than the clinically significant results. It is to be expected based on the fact that only 36.31% of patients achieved clinically significant results and most patients would not achieve an increase of ≥6 in TDI score. Heterogeneity was moderate with an I 2 statistic of 31%.45 Figure 4 showed the mean difference of TDI score before and after olfactory training in posttraumatic patients.
FIGURE 4.
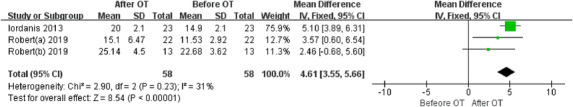
Forest plot for the TDI improvement in patients with posttraumatic olfactory dysfunction after olfactory training. Robert(a) represented the anosmia group and Robert(b) represented the hyposmia group (from Pellegrino et al.36). CI = confidence interval; OT = olfactory training; SD = standard deviation
3. DISCUSSION
This is the first review summarizing the evidence for olfactory training specifically for posttraumatic olfactory dysfunction. Previous meta‐analyses have demonstrated a significantly beneficial effect from olfactory training on olfactory dysfunction with varied etiologies including head trauma.46, 47 This emerging simple and effective protocol has been widely studied in patients with non‐sinonasal‐related olfactory dysfunction. Generally, patients with olfactory loss undergoing olfactory training experienced a significant increase in olfactory function with a mean improvement of 10.3 points on TDI score.30, 46 A recent study by Liu et al.48 showed that olfactory dysfunction with different etiologies presented with a distinct response to olfactory training despite the fact that olfactory training was generally effective for patients with olfactory disorders. It is imperative to define the exact efficacy of olfactory training on patients with posttraumatic olfactory dysfunction. However, meta‐analyses that focused on the efficacy of olfactory training on the olfaction among patients with posttraumatic olfactory dysfunction have not been reported.
Our meta‐analysis demonstrated that 36.31% of posttraumatic patients would achieve clinically significant results after olfactory training within 8 months. It has been reported that up to 27% of patients with posttraumatic olfactory dysfunction experienced spontaneous recovery of olfaction with an increase of ≥6 in TDI score compared to baseline after an average of 74 months follow‐up.19 It should be pointed out that most of the spontaneous recoveries occurred within 6 months and the probability of recovery beyond 2 years was very low.49, 50, 51 Compared to the percentage of spontaneous recovery within 74 months follow‐up, patients with posttraumatic olfactory dysfunction receiving olfactory training had a relatively higher recovery rate within a short‐term treatment duration. It can be speculated that the “excess” olfactory recovery could be attributed to olfactory training, which was beyond what would be expected from spontaneous recovery. Similarly, a study by Konstantinidis et al.32 indicated that 33.2% of patients with posttraumatic olfactory dysfunction achieved an increase of ≥6 in TDI score after 16 weeks of olfactory training. In addition, 13% of patients in the control group who did not receive the olfactory training experienced clinically significant results. It can be inferred that olfactory training promotes olfactory recovery in patients with posttraumatic olfactory dysfunction. It should be pointed out that there is no control group utilized from any of the studies and whether this level of improvement is similar to the placebo effect remains unknown. More studies are needed to define the clinically relevant improvement related to the olfactory training.
Patients with posttraumatic and postinfectious olfactory dysfunction were often studied together, largely because those patients performed relatively well in both odor threshold and discrimination but poorly in odor identification.52 In addition, these 2 types of olfactory disorders are frequently encountered in smell and taste clinics and no curable treatment is available.2, 3 Recent studies showed that patients with postinfectious olfactory dysfunction respond better to olfactory training and had higher rates of olfactory improvement compared to patients with posttraumatic olfactory dysfunction.32, 48 Although olfactory training is a potential treatment for olfactory disorders due to head trauma, the recovery rate is relatively low. The mechanism of olfactory training and reasons for the unsatisfactory effect of olfactory training in patients with posttraumatic olfactory dysfunction remains unclear. It has been proposed that olfactory training improves olfactory function, which seems to be partly driven by top‐down processes rather than bottom‐up processes. Nasal mucosal edema or hematoma, nasal septal deviation, nasal bone fractures, and rhinosinusitis caused by trauma could be solved by surgery or medicine, but patients with the injury of olfactory nerve fibers or olfactory cortex are difficult to treat. In addition, patients with posttraumatic olfactory dysfunction with more severity of the trauma would achieve less olfactory improvement after olfactory training.32 The interruption of the central olfactory pathway, which does not often happen in other types of olfactory disorders, may also be the reason for the poor prognosis and limited effect of olfactory training. In the present study, patients with posttraumatic olfactory dysfunction achieve an average increase of 4.61 in TDI score after olfactory training, which is lower than the change of TDI score in clinically significant results. The heterogeneity of disease severity and relatively poor treatment response among posttraumatic patients might explain the low increase of TDI score after olfactory training. Further studies are needed to explore the mechanism and then improve the prognosis.
Olfactory training together with local or systematic steroid has been utilized for the treatment of posttraumatic olfactory dysfunction.31, 38, 42, 43 The proportion of clinically significant results in patients with combined treatment can reach up to 33% to 50%, which is higher than that in the olfactory training group.38, 42, 43 Anti‐inflammatory treatment with steroids improved neuronal recovery following olfactory nerve transection via suppression of the inflammatory reaction and reduction of glial scar formation.14 This may explain why corticosteroids combined with olfactory training are more effective. Vitamin A, sodium citrate, minocycline, oral zinc, and intranasal insulin were also used in the treatment of olfactory dysfunction.53 A combination of the 2 or more treatments for patients with posttraumatic olfactory dysfunction should be explored in future studies.
Limitations of this study include the lack of a control group and a small number of total patients. Most studies included patients with different causes of olfactory dysfunction and did not have a non‐intervention control group. More research on the effect of olfactory training on posttraumatic olfactory dysfunction, especially randomized controlled trials, is needed. In most cases, the researchers did not have measures and just asked patients to keep a diary to ensure that they finish their olfactory training on time. More reliable and objective monitoring methods, such as remote video, are needed.
4. CONCLUSION
Olfactory training can improve the olfaction in patients with posttraumatic olfactory dysfunction within 8 months with a recovery rate of 36.31%. Our results further point to the consensus that olfactory training might be a promising modality for the treatment of posttraumatic olfactory dysfunction. Additional randomized controlled trials including a combination of olfactory training with other treatments are needed to confirm the therapeutic effect and further improve the recovery of olfaction in patients with posttraumatic olfactory dysfunction.
How to Cite this Article:Huang T, Wei Y, Wu D. Effects of olfactory training on posttraumatic olfactory dysfunction: a systematic review and meta‐analysis. Int Forum Allergy Rhinol. 2021;11:1102–1112. 10.1002/alr.22758
Potential conflict of interest: None provided.
View this article online at wileyonlinelibrary.com.
Contributor Information
Yongxiang Wei, Email: yongxw67@163.com.
Dawei Wu, Email: davidwuorl@163.com.
REFERENCES
- 1.Fokkens WJ, Lund VJ, Hopkins C, et al. EPOS: European position paper on rhinosinusitis and nasal polyps 2020. Rhinology. 2020;58(Suppl S29):1‐464. [DOI] [PubMed] [Google Scholar]
- 2.Hummel T, Whitcroft K, Andrews P, et al. Position paper on olfactory dysfunction. Rhinol Suppl. 2017;54:1‐30. [DOI] [PubMed] [Google Scholar]
- 3.Ciofalo A, Filiaci F, Romeo R, Zambetti G, Vestri AR. Epidemiological aspects of olfactory dysfunction. Rhinology. 2006;44:78‐82. [PubMed] [Google Scholar]
- 4.Drummond M, Douglas J, Olver J. The invisible problem: the incidence of olfactory impairment following traumatic brain injury. Brain Impair. 2015;16:196‐204. [Google Scholar]
- 5.Lecuyer Giguère F, Frasnelli A, De Guise É, Frasnelli J. Olfactory, cognitive and affective dysfunction assessed 24 hours and one year after a mild traumatic brain injury (mTBI). Brain Inj. 2019;33:1184‐1193. [DOI] [PubMed] [Google Scholar]
- 6.Sigurdardottir S, Andelic N, Skandsen T, et al. Olfactory identification and its relationship to executive functions, memory, and disability one year after severe traumatic brain injury. Neuropsychology. 2016;30:98‐108. [DOI] [PubMed] [Google Scholar]
- 7.Sigurdardottir S, Jerstad T, Andelic N, Roe C, Schanke A‐K. Olfactory dysfunction, gambling task performance and intracranial lesions after traumatic brain injury. Neuropsychology. 2010;24:504‐513. [DOI] [PubMed] [Google Scholar]
- 8.Callahan CD, Hinkebein JH. Assessment of anosmia after traumatic brain injury: performance characteristics of the University of Pennsylvania Smell Identification Test. J Head Trauma Rehabil. 2002;17:251‐256. [DOI] [PubMed] [Google Scholar]
- 9.Singh R, Humphries T, Mason S, Lecky F, Dawson J, Sinha S. The incidence of anosmia after traumatic brain injury: the SHEFBIT cohort. Brain Inj. 2018;32:1122‐1128. [DOI] [PubMed] [Google Scholar]
- 10.Ahmedy F, Mazlan M, Danaee M, Abu Bakar MZ. Post‐traumatic brain injury olfactory dysfunction: factors influencing quality of life. Eur Arch Otorhinolaryngol. 2020;277:1343‐1351. [DOI] [PubMed] [Google Scholar]
- 11.Neumann D, Zupan B, Babbage DR, et al. Affect recognition, empathy, and dysosmia after traumatic brain injury. Arch Phys Med Rehabil. 2012;93:1414‐1420. [DOI] [PubMed] [Google Scholar]
- 12.Callahan CD, Hinkebein J. Neuropsychological significance of anosmia following traumatic brain injury. J Head Trauma Rehabil. 1999;14:581‐587. [DOI] [PubMed] [Google Scholar]
- 13.Marin C, Langdon C, Alobid I, Mullol J. Olfactory dysfunction in traumatic brain injury: the role of neurogenesis. Curr Allergy Asthma Rep. 2020;20:1‐11. [DOI] [PubMed] [Google Scholar]
- 14.Howell J, Costanzo RM, Reiter ER. Head trauma and olfactory function. World J Otorhinolaryngol Head Neck Surg. 2018;4:39‐45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rombaux P, Huart C, Deggouj N, Duprez T, Hummel T. Prognostic value of olfactory bulb volume measurement for recovery in postinfectious and posttraumatic olfactory loss. Otolaryngol Head Neck Surg. 2012;147:1136‐1141. [DOI] [PubMed] [Google Scholar]
- 16.Kim DH, Kim SW, Hwang SH, et al. Prognosis of olfactory dysfunction according to etiology and timing of treatment. Otolaryngol Head Neck Surg. 2017;156:371‐377. [DOI] [PubMed] [Google Scholar]
- 17.Kobayashi M, Costanzo RM. Olfactory nerve recovery following mild and severe injury and the efficacy of dexamethasone treatment. Chem Senses. 2009;34:573‐580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kobayashi M, Tamari K, Kitano M, Takeuchi K. A time limit for initiating anti‐inflammatory treatment for improved olfactory function after head injury. J Neurotrauma. 2018;35:652‐660. [DOI] [PubMed] [Google Scholar]
- 19.Welge‐Lüssen A, Hilgenfeld A, Meusel T, Hummel T. Long‐term follow‐up of posttraumatic olfactory disorders. Rhinology. 2012;50:67‐72. [DOI] [PubMed] [Google Scholar]
- 20.Fan LY, Kuo CL, Lirng JF, Shu CH. Investigation of prognostic factors for post‐traumatic olfactory dysfunction. J Chin Med Assoc. 2015;78:299‐303. [DOI] [PubMed] [Google Scholar]
- 21.AbdelBari Mattar M, El Adle H. Prognostic factors for olfactory dysfunction in adult mild head trauma. World Neurosurg. 2020;141:545‐552. [DOI] [PubMed] [Google Scholar]
- 22.Kattar N, Do TM, Unis GD, Migneron MR, Thomas AJ, McCoul ED. Olfactory training for postviral olfactory dysfunction: systematic review and meta‐analysis. Otolaryngol Head Neck Surg. (in press). Epub July 14 2020. 10.1177/0194599820943550. [DOI] [PubMed] [Google Scholar]
- 23.Hura N, Xie DX, Choby GW, et al. Treatment of post‐viral olfactory dysfunction: an evidence‐based review with recommendations. Int Forum Allergy Rhinol. 2020;10:1065‐1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hummel T, Stupka G, Haehner A, Poletti SC. Olfactory training changes electrophysiological responses at the level of the olfactory epithelium. Rhinology. 2018;56:330‐335. [DOI] [PubMed] [Google Scholar]
- 25.Gellrich J, Han P, Manesse C, et al. Brain volume changes in hyposmic patients before and after olfactory training. Laryngoscope. 2018;128:1531‐1536. [DOI] [PubMed] [Google Scholar]
- 26.Kollndorfer K, Fischmeister FP, Kowalczyk K, et al. Olfactory training induces changes in regional functional connectivity in patients with long‐term smell loss. Neuroimage Clin. 2015;9:401‐410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Higgins JP, Altman DG, Gøtzsche PC, et al. Cochrane bias methods group; Cochrane Statistical Methods Group. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stang A. Critical evaluation of the Newcastle‐Ottawa scale for the assessment of the quality of nonrandomized studies in meta‐analyses. Eur J Epidemiol. 2010;25:603‐605. [DOI] [PubMed] [Google Scholar]
- 29.Jiang RS, Twu CW, Liang KL. The effect of olfactory training on odor identification in patients with traumatic anosmia. Int Forum Allergy Rhinol. 2019;9:1244‐1251. [DOI] [PubMed] [Google Scholar]
- 30.Hummel T, Rissom K, Reden J, Hähner A, Weidenbecher M, Hüttenbrink KB. Effects of olfactory training in patients with olfactory loss. Laryngoscope. 2009;119:496‐499. [DOI] [PubMed] [Google Scholar]
- 31.Fleiner F, Lau L, Göktas Ö. Active olfactory training for the treatment of smelling disorders. Ear Nose Throat J. 2012;91:198‐203, 215. [DOI] [PubMed] [Google Scholar]
- 32.Konstantinidis I, Tsakiropoulou E, Bekiaridou P, Kazantzidou C, Constantinidis J. Use of olfactory training in post‐traumatic and postinfectious olfactory dysfunction. Laryngoscope. 2013;123:E85‐E90. [DOI] [PubMed] [Google Scholar]
- 33.Yan XG, Gao X, Sun ZF, et al. Efficacy and associated factors of olfactory training in the treatment of olfactory dysfunction. Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2018;53:815‐819. [DOI] [PubMed] [Google Scholar]
- 34.Cao HH, Wang LL, Geng CK, et al. Therapeutic effects of chimeric antigen receptor T cells (CAR‐T) on relapse/refractory diffuse large B‐cell lymphoma (R/R DLBCL): a meta‐analysis. Eur Rev Med Pharmacol Sci. 2020;24:4921‐4930. [DOI] [PubMed] [Google Scholar]
- 35.Chen YH, Du L, Geng XY, Liu GJ. Implement meta‐analysis with non‐comparative binary data in RevMan software. Chin J Evid‐Based Med. 2014;14(7):889‐896. [Google Scholar]
- 36.Pellegrino R, Han P, Reither N, Hummel T. Effectiveness of olfactory training on different severities of posttraumatic loss of smell. Laryngoscope. 2019;129:1737‐1743. [DOI] [PubMed] [Google Scholar]
- 37.Langdon C, Lehrer E, Berenguer J, et al. Olfactory training in post‐traumatic smell impairment: mild improvement in threshold performances: results from a randomized controlled trial. J Neurotrauma. 2018;35:2641‐2652. [DOI] [PubMed] [Google Scholar]
- 38.Nguyen TP, Patel ZM. Budesonide irrigation with olfactory training improves outcomes compared with olfactory training alone in patients with olfactory loss. Int Forum Allergy Rhinol. 2018;8:977‐981. [DOI] [PubMed] [Google Scholar]
- 39.Jiang RS, Twu CW, Liang KL. The effect of olfactory training on the odor threshold in patients with traumatic anosmia. Am J Rhinol Allergy. 2017;31:317‐322. [DOI] [PubMed] [Google Scholar]
- 40.Poletti SC, Michel E, Hummel T. Olfactory training using heavy and light weight molecule odors. Perception. 2017;46:343‐351. [DOI] [PubMed] [Google Scholar]
- 41.Fornazieri MA, Garcia ECD, Lopes NMD, et al. Adherence and efficacy of olfactory training as a treatment for persistent olfactory loss. Am J Rhinol Allergy. 2020;34:238‐348. [DOI] [PubMed] [Google Scholar]
- 42.Bratt M, Moen KG, Nordgård S, Helvik AS, Skandsen T. Treatment of posttraumatic olfactory dysfunction with corticosteroids and olfactory training. Acta Otolaryngol. 2020;140:761‐767. [DOI] [PubMed] [Google Scholar]
- 43.Jung YD, Kim DS, Kang BJ, Shin SH, Ye MK. The effects of olfactory training with intranasal corticosteroid spray in korean patients with olfactory dysfunction. J Rhinol. 2019;26:106‐122. [Google Scholar]
- 44.Altundag A, Cayonu M, Kayabasoglu G, et al. Modified olfactory training in patients with postinfectious olfactory loss. Laryngoscope. 2015;125:1763‐1766. [DOI] [PubMed] [Google Scholar]
- 45.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ. 2003;327:557‐560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pekala K, Chandra RK, Turner JH. Efficacy of olfactory training in patients with olfactory loss: a systematic review and meta‐analysis. Int Forum Allergy Rhinol. 2016;6:299‐307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sorokowska A, Drechsler E, Karwowski M, Hummel T. Effects of olfactory training: a meta‐analysis. Rhinology. 2017;55:17‐26. [DOI] [PubMed] [Google Scholar]
- 48.Liu DT, Pellegrino R, Sabha M, et al. Factors associated with relevant olfactory recovery after olfactory training: a retrospective study including 601 participants. Rhinology. (in press). Epub September 9, 2020. 10.4193/Rhin20.262. [DOI] [PubMed] [Google Scholar]
- 49.Gudziol V, Hoenck I, Landis B, Podlesek D, Bayn M, Hummel T. The impact and prospect of traumatic brain injury on olfactory function: a cross‐sectional and prospective study. Eur Arch Otorhinolaryngol. 2014;271:1533‐1540. [DOI] [PubMed] [Google Scholar]
- 50.Coelho DH, Costanzo RM. Posttraumatic olfactory dysfunction. Auris Nasus Larynx. 2016;43:137‐143. [DOI] [PubMed] [Google Scholar]
- 51.Reden J, Mueller A, Mueller C, et al. Recovery of olfactory function following closed head injury or infections of the upper respiratory tract. Arch Otolaryngol Head Neck Surg. 2006;132:265‐269. [DOI] [PubMed] [Google Scholar]
- 52.Whitcroft KL, Cuevas M, Haehner A, Hummel T. Patterns of olfactory impairment reflect underlying disease etiology. Laryngoscope. 2017;127:291‐295. [DOI] [PubMed] [Google Scholar]
- 53.Whitcroft KL, Hummel T. Clinical diagnosis and current management strategies for olfactory dysfunction: a review. JAMA Otolaryngol Head Neck Surg. 2019;145:846‐853. 10.1001/jamaoto.2019.1728. [DOI] [PubMed] [Google Scholar]


