Abstract
Plants alter their morphology and cellular homeostasis to promote resilience under a variety of heat regimes. Molecular processes that underlie these responses have been intensively studied and found to encompass diverse mechanisms operating across a broad range of cellular components, timescales and temperatures. This review explores recent progress throughout this landscape with a particular focus on thermosensing in the model plant Arabidopsis. Direct temperature sensors include the photosensors phytochrome B and phototropin, the clock component ELF3 and an RNA switch. In addition, there are heat‐regulated processes mediated by ion channels, lipids and lipid‐modifying enzymes, taking place at the plasma membrane and the chloroplast. In some cases, the mechanism of temperature perception is well understood but in others, this remains an open question. Potential novel thermosensing mechanisms are based on lipid and liquid–liquid phase separation. Finally, future research directions of high temperature perception and signalling pathways are discussed.
Keywords: biomolecular condensate, ELF3, heat stress, phospholipase, phytochrome B, PIF7, stress granules, thermomorphogenesis, thermotolerance
Short abstract
Knowledge of how plants sense elevated temperatures and initiate protective responses has greatly increased in recent years. Diverse mechanisms, involving changes in proteins, RNA and lipids, function in thermosensing across a range of timescales, locations and temperatures.
1. INTRODUCTION
Heat stress is an increasingly prevalent environmental constraint for plants. A meta‐analysis of 1.700 simulations suggested that, from the 2030s onward, the negative impact of a warming climate on crop yields will be increasingly severe (Challinor et al., 2014). In future scenario's, more frequent temperature extremes are expected to be particularly detrimental (Battisti & Naylor, 2009). Warm temperatures can lead to heat stress, that is, they may impair plant growth, fertility, development, metabolism, photosynthesis and immunity (Hatfield & Prueger, 2015; Howarth & Ougham, 1993; Janda et al., 2019; Wolf, Marani, & Rudich, 1991; Xu, Paulsen, Guikema, & Paulsen, 1995). In natural environments, plants experience daily and seasonal temperature fluctuations that vary in range, rate and duration. Natural populations of Arabidopsis thaliana exhibit differences in sensitivity and responses to temperature extremes that vary in a manner consistent with adaptation to local temperature patterns (Zhang, Belsterling, Raszewski, & Tonsor, 2015). Whether a temperature becomes stressful depends on these variables, as well as coincident stress factors such as drought and salinity. At the cellular level, heat stress perturbs protein folding, membrane fluidity, cytoskeletal organization, transport and enzymatic reactions, which leads to metabolic imbalances and pernicious accumulation of by‐products such as reactive oxygen species (ROS) (Wahid, Gelani, Ashraf, & Foolad, 2007). It is therefore of primary interest for plants to sense temperature alterations and initiate timely adaptive strategies to preserve cell function and viability. Plants respond to different temperature ranges with widely divergent physiological and developmental responses. However much less is known about the sensing mechanisms involved.
At ambient warm temperatures, that is, above the optimum growth temperature, but still within the physiological range (i.e. between around 24 and 30°C, for Arabidopsis), many plants undergo a process known as thermomorphogenesis, in which they alter morphology and development (e.g. through expanded leaf structure, deeper roots and early flowering) to reduce exposure to potentially damaging temperatures (Figure 1; Casal & Balasubramanian, 2019; Crawford, McLachlan, Hetherington, & Franklin, 2012; Park & Park, 2019). At higher temperatures, that is, 30–37°C for Arabidopsis, there is still some growth, but several adverse effects of heat stress become visible. Reproductive development and photosynthesis are affected, and root and shoot growth rates are compromised. At these temperatures, plants employ a variety of acclimation strategies to enhance temperature tolerance, including the activation of molecular chaperones within minutes, and modulating the composition of cell membranes over a period of days (Falcone, Ogas, & Somerville, 2004; Osteryoung & Vierling, 1994). As temperatures rise above 40°C, severe heat stress is experienced, which can result in global injury, malfunction and ultimately, cell death (Figure 1; Vacca et al., 2004).
FIGURE 1.
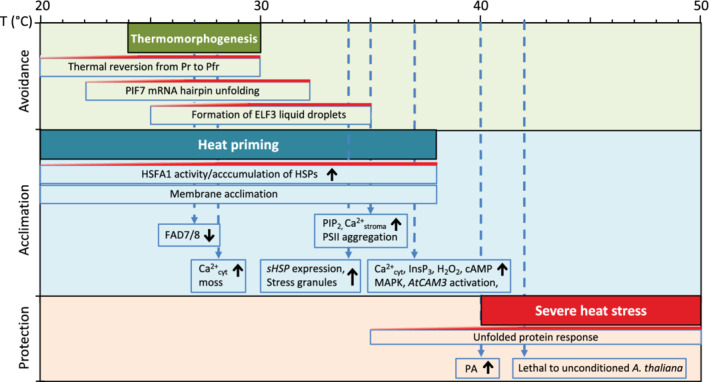
Schematic overview of responses in different warm temperature ranges. Plants display a wide array of responses when they experience above optimal temperatures. At warm ambient temperatures, up to 30°C, Arabidopsis responds by changes in morphology and development, called thermomorphogenesis, which could aid in avoidance of future heat stress. Thermomorphogenesis features the temperature‐sensitive function of phyB. Furthermore in this temperature range, there is thermosensitive regulation of PIF7 mRNA translation. Warm temperatures alter the structure of a hairpin structure in the mRNA of PIF7, which promotes its translation. ELF3 undergoes temperature‐dependent phase separation. High temperatures promote condensation of ELF3, and the inhibition of ELF3‐DNA binding. Under mild heat stress, that is, at temperatures of 30–38°C, Arabidopsis initiates acclimation responses that counteract damage to proteins and membranes, and maintain cellular homeostasis. The suite of physiological changes associated with acclimation enhances plant thermotolerance. This involves the activity of HSFA1 master transcriptional factors (Liu & Charng, 2013). HSPs/sHSP accumulate to limit misfolding of proteins, and stress granules, biomolecular condensates in the cytosol and chloroplast, form to sequester mRNAs and proteins. The membranes' lipid compositions are adjusted so as to prevent disruption of the bilayer structure due to uncontrolled increases in membrane fluidity. The heat sensors that activate acclimation are unknown. The accumulation, within the first ±15 min of heat stress, of putative signalling components, such as Ca2+, H2O2, PIP2, PA and cAMP suggests their function in heat perception, closely tied to the sensor. Temperatures above 40°C are damaging to Arabidopsis, and all responses in this range are devoted to immediate protection or controlled breakdown of cellular structures. Mechanisms of clearance and rescue of unfolded proteins, including the UPR, are important for survival of severe heat stress. These heat stress responses partly rely on the recognition of unfolded proteins in the ER, the cytosol and diverse organelles
Molecular plant scientists have long questioned how heat is actually perceived and converted into a cellular signal. Since macromolecules are generally affected by heat, many have the potential to serve as thermosensors. The concept of thermosensor not only needs to incorporate processes akin to ligand‐receptor binding coupled to downstream signalling, but also includes less well‐demarcated processes such as heat‐induced increases in membrane fluidity followed by changes in membrane structure and function. This makes it difficult to identify macromolecules that actually perceive temperature and elicit specific signalling events. In recent years, several potential thermosensors and sensing mechanisms have emerged, both of ambient warm and stressful hot temperatures. Here, we summarize that knowledge, focussing on their mode of action, and provide a perspective for future research in this exciting field.
2. SENSING MECHANISMS OF WARM AMBIENT TEMPERATURES
2.1. Phytochrome B as temperature sensor
In recent years, there has been a growing realization that the action of some photosensors is temperature‐sensitive. This property allows the fine tuning of growth and differentiation in response to moderate temperature changes. Here, the photo‐/thermosensor acts as a receptor for a change in temperature, and in doing so initiates downstream signalling processes.
There is extensive cross‐talk between light and temperature signalling in plants (Hayes, 2020). Plants perceive light conditions with at least five types of photoreceptors, that is, (a) phytochromes, (b) cryptochromes, (c) phototropins, (d) zeitlupes and (e) UV Resistance Locus 8 (UVR8) (Voitsekhovskaja, 2019). Of these photoreceptors, the temperature‐sensitive activity of phytochromes is the most well characterized, in particular phytochrome B (phyB; Figure 2). Phytochromes control many aspects of thermomorphogenesis, especially architectural changes, accelerated flowering and senescence (Jung et al., 2016; Kim, 2020; Quint et al., 2016).
FIGURE 2.
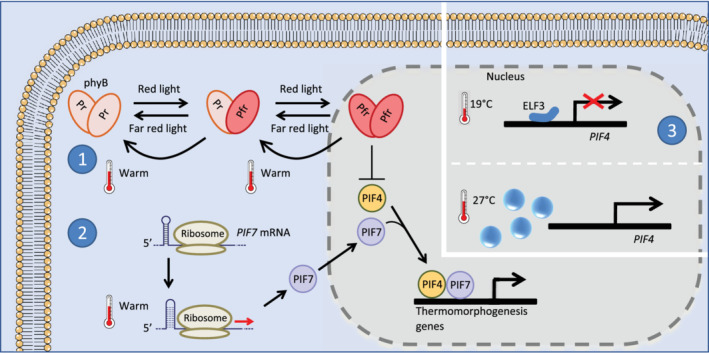
Schematic overview of thermomorphogenic pathways in Arabidopsis. 1, Under red light, phyB is converted to a Pfr homodimer that is translocated to the nucleus where it blocks PIF4 and PIF7 activity. High temperatures promote the reversion of phyB back to its inactive state, leaving PIF4 and PIF7 free to transcribe thermomorphogenesis promoting genes. 2, PIF7 mRNA contains a hairpin near its 5′‐UTR sequence. Upon an increase in temperature, this hairpin structure changes. In warm temperature state, PIF7 mRNA is more easily translated and PIF7 protein levels are increased. 3, At cooler temperatures, ELF3 (as part of the evening complex) represses the expression of PIF4. As temperatures rise, a PrLD in ELF3 promotes its phase separation and the formation of liquid droplets, thus relieving the transcriptional repression of PIF4
Phytochromes are red/far red (R/FR) sensitive photoreceptors that absorb light through a phytochromobilin chromophore. The absorption of light by phytochromobilin induces its isomerisation, and this translates to conformational changes in phytochrome structure. R light promotes a shift to the active form of phytochrome (Pfr), whereas FR light promotes reversion to the inactive form (Pr) (Hayes, 2020). Importantly, Pfr can also spontaneously revert to Pr, and this process is temperature‐dependent. The rate of thermal reversion from Pfr to Pr is accelerated at warm temperatures (Legris et al., 2016), resulting in a reduced pool of active phytochrome (Figure 2).
When activated by R light and cool temperatures, phytochromes promote the degradation of a family of bHLH transcription factors known as PHYTOCHROME INTERACTING FACTORS (PIFs).When phytochrome function is reduced by FR light or warm temperatures, PIFs accumulate and promote hypocotyl elongation through the enhanced expression of auxin biosynthesis genes (Jung et al., 2016; Koini et al., 2009; Legris et al., 2016). Hypocotyl elongation at warm temperatures is largely driven by PIF4, PIF7 and to some extent PIF5 (Chung et al., 2020; Fiorucci et al., 2020; Koini et al., 2009). PIF‐mediated elongation and hyponasty at warm temperatures results in an open architecture and enhances leaf cooling (Crawford et al., 2012; Park & Park, 2019).
2.2. Other photosensors: Phototropin, cryptochrome and UVR8
Phytochrome B is not the only plant photosensor that is temperature‐sensitive. The phototropin of liverwort (Marchantia polymorpha) (MpPHOT) also displays temperature‐dependent changes in activity (Fujii et al., 2017). Phototropins are membrane bound blue light receptors that respond to positional light cues. In Arabidopsis, they regulate phototropism, leaf flattening and chloroplast positioning. In Marchantia, an important phototropin regulated process is the cold avoidance response. At 22°C, blue light induces the movement of chloroplasts to the cell surface, which functions to maximize photosynthesis. In contrast, at 5°C blue light induces the movement of chloroplasts to the periclinal cell walls. This process is known as cold avoidance and is thought to protect the photosynthetic machinery in suboptimal temperature conditions (Fujii et al., 2017).
MpPHOT contains two LOV (light, oxygen or voltage) domains that are responsible for light sensing. In darkness, each LOV domain contains a non‐covalently bound flavin mononucleotide (FMN) chromophore. Blue light absorption by FMN triggers its covalent attachment to the LOV domain. This in turn causes structural re‐arrangement of the phototropin molecule into its active form. Importantly, the covalent bond that links FMN and the LOV domain spontaneously degrades over time, resulting in inactivation of MpPHOT. This degradation rate increases with temperature, meaning that MpPHOT remains more active at cooler temperatures. As a result, MpPHOT promotes the relocation of chloroplasts to the cell periphery, along the anticlinal cell wall, when temperatures are too low for efficient photosynthesis (Fujii et al., 2017). The phototropins of Arabidopsis were also recently implicated in temperature signalling (Kostaki et al., 2020). Warm temperatures promote guard cell movement in a cell autonomous manner. Curiously, warm temperature‐induced stomatal opening is dependent on blue light and phototropins (Kostaki et al., 2020). This seems to hint that in Arabidopsis guard cells, warm temperatures promote phototropin action. Arabidopsis chloroplasts do however still exhibit cold avoidance, in a phot‐dependent manner (Fujii et al., 2017). It is therefore presently unclear whether Arabidopsis phototropin action is enhanced by cool or warm temperatures, or if this is dependent on cellular context. More research into the temperature dependency of phototropin mediated responses in Arabidopsis should help to resolve this point.
Zeitlupes are another class of blue light photoreceptor, which act to accelerate the pace of the circadian clock. Zeitlupes contain a LOV domain with a similar activation mechanism to phototropins (Pudasaini et al., 2017). If the rate of zeitlupe inactivation is increased at warm temperatures, this could potentially reduce the pace of the clock at warm temperatures (a process known as temperature compensation) (Hayes, 2020). Several years ago, zeitlupe was identified as a quantitative trait locus for natural variation in temperature compensation in Arabidopsis (Edwards, Lynn, Gyula, Nagy, & Millar, 2005) and so it would be interesting to experimentally test this hypothesis. Other plant photosensors, such as the blue light sensing cryptochrome and UV‐B sensing UVR8, also be temperature‐sensitive (Figure 2). Cryptochromes undergo thermal reversion in a similar manner to phytochromes and phototropins. If cryptochrome thermal reversion is enhanced at warm temperatures, it is feasible that cryptochrome would also exhibit higher activity at cool temperatures (Hayes, 2020). UVR8 exists as a homodimer in the dark, but undergoes monomerization after absorbing UV‐B. The active UVR8 monomer then reverts back to the inactive dimer, in a process that is mediated by Repressor of UV‐B Photomorphogenesis 1 (RUP1) and RUP2. The RUP‐mediated reversion of the UVR8 monomer to the UVR8 dimer seems to be influenced by temperature (Findlay & Jenkins, 2016), but the details of this process are currently unclear. Whether zeitlupe, cryptochrome and UVR8 functions are truly temperature‐sensitive remains to be investigated.
2.3. Temperature‐dependent action of the evening complex
A large proportion of the genome is regulated by the circadian clock. To ensure robust rhythmicity, the clock is entrained to daily cycles of light and temperature. The evening complex (EC) is a group of core clock components that show peak expression in the early evening. In recent years, it has become apparent that the EC is one of the key points at which light and temperature signals enter the clock (Ezer et al., 2017). The EC consists of three components, the scaffold protein EARLY FLOWERING 3 (ELF3), the transcription factor LUX ARRYTHMO (LUX) and a protein of unknown function, ELF4. Together they act as a transcriptional repressor that directly binds DNA (Ezer et al., 2017; Huang et al., 2016; Nusinow et al., 2011). Besides its regulation of the clock, the EC also represses the expression of thermomorphogenesis promoting genes such as PIF4, limiting the period of temperature‐induced growth (Box et al., 2015).
Consistent with its importance in conveying temperature information to the clock, binding of the EC to DNA is temperature‐dependent. At cool temperatures, the EC binds to DNA much more strongly than at warm temperatures (Ezer et al., 2017). Phytochromes play an important role in regulating the EC (Ezer et al., 2017; Huang et al., 2016) and so temperature sensitivity of DNA binding could potentially be ascribed to increased thermal reversion of phyB (Legris et al., 2016). Intriguingly however, EC DNA binding is also temperature‐dependent in vitro, implying that EC activity is directly modulated by temperature (Silva et al., 2020).
ELF3 contains a prion‐like domain (PrLD) with a high proportion of glutamine residues (polyQ region) (Jung et al., 2020). The PrLD shows variable length between species, with A. thaliana ELF3 (AtELF3) containing a PrLD of 180 amino acids, and Brachypodium distachyon ELF3 (BdELF3) lacking similar sequences. Replacing the PrLD of AtELF3 with the corresponding region from BdELF3 abolished the temperature‐dependent DNA binding of AtELF3 (Jung et al., 2020). At high temperatures, AtELF3 forms speckles within the nucleus and this is also dependent on the PrLD (Figure 2). Speckles are biomolecular condensates, or liquid droplets, that assemble through the process of liquid–liquid phase separation (Cuevas‐Velazquez & Dinneny, 2018). Importantly, PrLD‐dependent speckles also form at high temperatures when AtELF3 is expressed in yeast cells, in the absence of other evening complex components. Furthermore, the purified PrLD from AtELF3 spontaneously and reversibly self‐associates and forms condensates when in solution, in a temperature‐dependent manner (Jung et al., 2020). It appears that this region contributes to phase separation of ELF3, and likely plays a role in temperature sensing.
Direct temperature sensing by ELF3 may help to explain a curious observation about phyB at warm temperatures. Under R light, active phyB accumulates in several large subnuclear foci known as photobodies (Hahm, Kim, Qiu, & Chen, 2020). In R + FR light, phyB is inactivated and disperses to numerous smaller foci. Warm temperature also inactivates phyB and so we might expect it to lead to a similar change in phyB photobodies. However, in direct contrast to FR light, warm temperature appears to promote the aggregation of phyB into fewer, larger photobodies (Hahm et al., 2020). Whether there is a link between phyB aggregation and ELF3 PrLD‐mediated condensation at warm temperatures remains to be investigated. However if this is the case, it presents an attractive mechanism whereby plant cells could distinguish between FR and warm temperature signals, based on the inactivation of phyB alone or the inactivation of phyB and ELF3 in conjunction.
2.4. RNA thermosensors
Temperature sensing based on the temperature responsiveness of RNA secondary structures has previously been shown in bacteria and animals (Vu, Gevaert, & De Smet, 2019). Recently, a distinct RNA‐based temperature ‘switch’ was also identified in plants. Chung et al. (2020) used ribosome profiling to identify genes that show an increase in translational efficiency at warm temperatures (27°C). Interestingly, one of the genes that showed enhanced translation was PIF7. PIF7 is one of the key transcription factors regulating hypocotyl and petiole elongation at warm temperature (Chung et al., 2020; Fiorucci et al., 2020). A proportion of the mRNAs identified by Chung et al. (2020) contain a hairpin structure in their 5′UTR, shortly upstream of their AUG. Importantly, the structure of the PIF7 5′UTR hairpin changes between 22 and 27°C. It has been proposed that this change in hairpin structure at warm temperature enhances PIF7 translation (Chung et al., 2020; Figure 2). It is however important to note that regulation of PIF7 translation is not simply due to the presence or absence of a hairpin. Mutations that strengthen the hairpin do indeed block translation, but surprisingly, mutations that disrupt the hairpin also reduce translation. The authors propose that structural information in the hairpin is required for dynamic regulation of translation in response to temperature (Chung et al., 2020).
2.5. Epigenetic regulation of thermomorphogenesis
In a screen for mutants with enhanced temperature response, Kumar and Wigge (2010) discovered ARP6, a component of a chromatin remodelling complex. Mutants lacking ARP6 show constitutive warm temperature phenotypes. ARP6 is required for the deposition of a specific histone variant H2A.Z. It was shown that at warm temperatures, there is a reduction in H2A.Z occupancy at the promoters of warm temperature‐induced genes and it was initially proposed that H2A.Z could play a role in temperature sensing (Kumar & Wigge, 2010). More recent evidence has however cast doubt on this hypothesis. It was shown that at warm temperatures, the binding of HSFA1a to heat‐responsive genes precedes the H2A.Z eviction and the activation of transcription (Cortijo et al., 2017). The eviction of H2A.Z is facilitated by HISTONE DEACETYLASE 9 (HDAC9), and is required for the full transcriptional response to elevated temperatures (van der Woude et al., 2019), but it appears that this occurs mostly downstream of temperature perception.
Epigenetic mechanisms have also been implicated in the establishment of transcriptional memory following an inducing sublethal period of heat stress (Bäurle & Trindade, 2020). Although hypermethylation of histone H3 lysine 4 has been found to prime heat stress‐induced genes for rapid and enhanced expression in the face of a second heat stress episode (Liu et al., 2018), the upstream signalling and heat sensing steps that initiate priming and the onset of transcription in primed plants remain unknown.
3. SENSING MECHANISMS OF MODERATE TO SEVERE HEAT STRESS
3.1. The heat stress response is partly based on unfolded protein sensing
Heat stress triggers adaptive responses which protect macromolecular structures, restore cellular homeostasis and prevent damage. The composite response likely involves the action of multiple parallel sensors that activate signalling pathways in different cellular compartments with different dynamics. Cellular defense mechanisms are activated by monitoring protein, DNA and membrane damage (Balogh et al., 2013; Ding, Shi, & Yang, 2020; Niu & Xiang, 2018). In eukaryotes and also prokaryotes, elevated temperatures induce the expression of heat shock proteins (HSPs). HSPs act as molecular chaperones to promote the correct folding and counteract aggregation of proteins, and thus are crucial for tolerance to high temperatures in plants (Vierling, 1991). In plants, HSPs can also contribute to the thermomorphogenic response. For example, HSP90 promotes the stability of the auxin receptor TIR1 and in doing so, promotes root and shoot elongation at warm temperatures (Wang et al., 2016). HSP90 was also recently shown to be required for the warm temperature mediated induction of HIGH EXPRESSION OF OSMOTICALLY RESPONSIVE GENES1 (HOS1) (Han, Park, & Park, 2019). HOS1 is a protein with E3 ubiquitin ligase activity that also acts as a transcriptional regulator. Stabilization of HOS1 allowed for HOS1‐mediated upregulation of DNA repair and improved thermotolerance (Han et al., 2019). As HOS1 has also been shown to negatively regulate the transcriptional activity of PIF4 (Kim, Lee, Jung, Lee, & Park, 2017), it could also act to repress thermomorphogenic growth under heat stress.
Heat stress triggers the rapid assembly of different kinds of stress granules and other cytosolic foci, that contain misfolded proteins and chaperones, and/or untranslated mRNAs, elongation initiation factors, transcription factors, mRNA‐binding and decay proteins (Chantarachot & Bailey‐Serres, 2018; Kosmacz et al., 2019). Some of these structures contain small HSPs (sHSPs), which promote solubilization of aggregated proteins, and, at the stress granule surface, HSP101, which harbours disaggregase activity and can mediate protein breakdown through its association with the 26S proteasome (McLoughlin et al., 2016; McLoughlin, Kim, Marshall, Vierstra, & Vierling, 2019). Through their dynamic interactions with stress granules, sHSPs/HSPs function not only in preventing permanent cytosolic protein aggregates, but also in the repression and restoration of mRNA translation during and after heat stress, respectively (Merret et al., 2017).
At present there is only limited information on how the expression of HSPs is gated by temperature in plants. Heat shock factors (HSFs) are a class of well conserved transcription factors. HSFs bind to heat shock elements in the promoters of HSP genes and promote HSP expression. It was recently shown that the mRNA of HSFA2 undergoes increased translation at warm temperatures, in a similar manner to PIF7. Like PIF7, the temperature regulation of HSFA2 translation was dependent on the structure of a hairpin in the 3′UTR (Chung et al., 2020). It is possible that warm temperature‐induced translation of HSFA2 contributes to HSP expression in response to heat.
Another possible mechanism for temperature induction of HSP expression is through autoregulatory feedback. In yeast, HSP70 binds to HSF1 and inhibits its transcriptional activity (Zheng et al., 2016). At warm temperatures, misfolded proteins accumulate and out‐titrate HSF1 for binding to HSP70. This triggers the release of HSF1, which then promotes the expression of HSP70. The newly synthesized HSP70 sequesters HSF1 and a new equilibrium is established.
Currently it is unclear whether the HSP:HSF module functions in the same manner in plants, but similar mechanisms are known to activate the unfolded protein response (UPR). When a plant experiences heat stress, unfolded and misfolded proteins can accumulate to such levels that they overload the protein quality control system, leading to ER stress. When unfolded proteins accumulate in the ER, they are bound by binding protein (BiP), an HSP70 chaperone. In the absence of unfolded proteins, BiP binds to the ER membrane‐tethered transcription factors bZIP17 and bZIP28. When unfolded proteins sequester BiP, bZIP17 and bZIP28 are activated by translocation to the Golgi and proteolytic cleavage of their membrane anchors. These transcription factors then travel to the nucleus to promote the expression of chaperones and foldases to assist in protein folding. Together this process is known as the unfolded protein response (UPR). Unfolded proteins also interact with the lumenal domain of the transmembrane sensor IRE1b (inositol‐requiring enzyme1), inducing unconventional splicing of bZIP60, which then activates the ER stress response genes. IRE1b splicing activity is induced by heat (Deng et al., 2011). IRE1 was also found to be induced by lipid bilayer stress in yeast (Ernst, Ballweg, & Levental, 2018; Halbleib et al., 2017), and to regulate the degradation of specific mRNA's, shaping the stress transcriptome. Interestingly, the transcription factor ELONGATED HYPOCOTYL 5 (HY5) was recently found to compete with bZIP17 and bZIP28 to repress the UPR (Nawkar et al., 2017). Warm temperatures lead to the activation of the E3 ligase CONSTITUTIVELY PHOTOMORPHOGENIC 1, which promotes HY5 degradation (Park, Lee, Ha, Kim, & Park, 2017). Reduced HY5 abundance in warm temperatures may help to promote the UPR under heat stress.
High temperature and light conditions can also activate UPR responses through a signal from the chloroplast, methyl‐d‐erythritol 2,4‐cyclodiphosphate (MEcPP, Figure 3). MEcPP is a retrograde signalling metabolite (Rivasseau et al., 2009; Walley et al., 2015) and accumulates as a direct consequence of a stress‐induced metabolic bottleneck in the chloroplasts. Accumulation of MEcPP induces the nuclear transcription of IRE1a and bZIP60 and other genes with a rapid stress response element (Benn et al., 2016).
FIGURE 3.
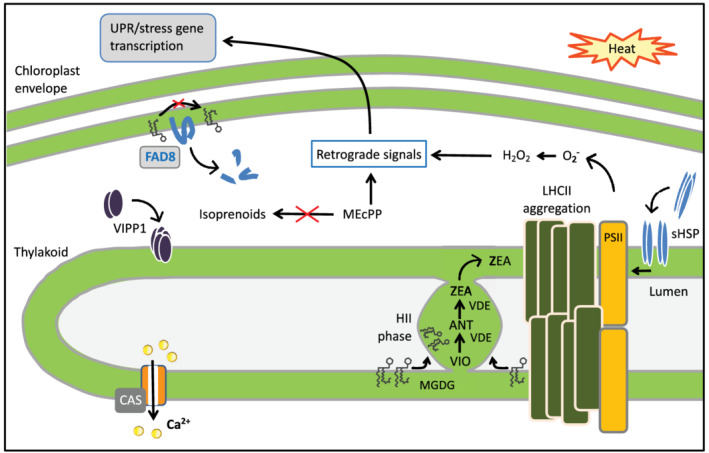
Sensing and signalling of heat stress at the chloroplast. Exposure to moderate heat has various direct consequences for chloroplast proteins and membranes, which trigger rescue pathways. In the thylakoidal membrane, LHCII proteins aggregate, which leads to an excess of its major lipid constituent, the non‐bilayer prone glycerolipid MGDG. As a consequence, a non‐bilayer structure (the HII phase) emerges, which consists of MGDG organized in hexagonally stacked tubules. The xanthophyll cycle enzyme, violaxanthin de‐epoxidase (VDE), recruits specifically to the HII phase in the thylakoid lumen, catalysing the synthesis of zeaxanthin (ZEA) from its precursors, violaxanthin (VIO) and antheraxanthin (ANT). The HII phase remains attached, which allows for free diffusion of the photoprotective xanthophylls to the thylakoid. VIPP1 and sHSP recruit to the thylakoid membrane under heat stress, as they recognize membrane packing defects. They protect thylakoid membrane and PSII integrity. Heat stress is signalled in the chloroplast by a rapid Ca2+ increase in the stroma which depends on the activity of the calcium sensor CAS. Furthermore, heat induces breakdown of the envelope desaturase FAD8, responsible for synthesis of polyunsaturated fatty acids. This causes an adaptive decrease in membrane desaturation. The isoprenoid biosynthesis intermediate MEcPP accumulates due to a high light/temperature‐induced bottleneck in the pathway. MEcPP, together with H2O2 resulting from excess excitation energy, and other stress‐induced molecules, serve as retrograde signals to regulate heat stress response genes in the nucleus
Under heat stress, unfolded proteins also accumulate in chloroplasts. The sHSP of Chlamydomonas, HSP22E/F, forms high‐molecular weight complexes with unfolded chloroplastic proteins to prevent proteotoxicity (Rütgers et al., 2017). In Arabidopsis, short term heat stress (10 min, 42°C) induces the rapid and reversible assembly of stress granules in the chloroplasts. The composition of these granules is similar to their cytosolic counterparts, reflecting translational repression: mRNAs, RNA‐binding proteins with self‐associating PrLDs, RNA editing proteins, HSPs and translation elongation factors (Chodasiewicz et al., 2020). Sequestration of proteins and RNA into stress granules appears to have a double role: It protects these molecules against degradation and also flexibly regulates translation, metabolism and signalling processes in response to heat. It remains to be shown whether the assembly of cytosolic and chloroplast stress granules is primarily driven by heat‐induced phase separation of constituent PrLD‐containing proteins. The particular sensitivity of chloroplasts to heat stress means that retrograde signals are important regulators of the response to high temperature. For an in‐depth review on retrograde signals in heat stress, we direct the reader to (Sun & Guo, 2016).
3.2. Regulation based on physical changes in the membrane
The membrane is the most thermally sensitive macromolecular structure in the cell (Balogh et al., 2013; Niu & Xiang, 2018). With increasing temperature, the rotational motion, lateral diffusion and fatty acid disorder of the lipid bilayer increase, while the headgroup packing density decreases. These four parameters are different aspects of the commonly used term ‘membrane fluidity’. Changes in membrane fluidity affect the folding, mobility and activity of membrane proteins. These changes can have deleterious effects on cell functions, but at moderate levels can also serve as a basis for thermosensing. Plants, like other non‐homeothermic organisms, actively maintain an almost constant membrane fluidity upon shifts in temperature (Higashi, Okazaki, Myouga, Shinozaki, & Saito, 2015; Los & Murata, 2004). The increased fluidity under heat stress is counteracted by the incorporation of fluidity‐decreasing saturated fatty acids, a process known as homeoviscous adaptation. In bacteria, membrane thickness is measured through the membrane protein DesK and in yeast, lipid packing density is measured by Mga2. These sensors support membrane homeostasis by the transcriptional activation of lipid desaturases when temperature drops (Ballweg et al., 2020; Covino et al., 2016; Cybulski, Martín, Mansilla, Fernández, & de Mendoza, 2010).
In plants, such integral membrane property sensors have not been identified. Instead, heat was found to directly inhibit the activity of critical desaturases, simply by virtue of their heat‐instability. The plastidial FAD8 enzyme is responsible for the synthesis of α‐linolenic acid (18:3), a component of the main thylakoid lipid, monogalacosyldiacylglycerol (MGDG). FAD8 contains a labile autoregulatory domain, that destabilizes the protein upon a temperature shift from 22 to 27°C (Matsuda, Sakamoto, Hashimoto, & Iba, 2005; Figure 3). Reduced FAD8 stability resulted in decreased accumulation of 18:3 and reduced membrane fluidity. The importance of this is clear from the finding that mutants with low 18:3 content in their MGDG showed improved heat tolerance (Murakami, 2000). This may be because 18:3‐MGDG is prone to oxidative damage and can destabilize membranes. The ER desaturases FAD2 and FAD3 also display thermolability. Upon transfer to warm temperatures, FAD2 and FAD3 are targeted for ubiquitin‐mediated or ER‐associated degradation, respectively (O'Quin et al., 2010; Tang, Novitzky, Carol Griffin, Huber, & Dewey, 2005). Currently, the mechanism by which these desaturases are inactivated upon heat stress is unknown; they may either be directly temperature‐sensitive or act downstream of temperature perception.
Adjustment of membrane fluidity through changes in membrane desaturation is a slow process and can take several days (Falcone et al., 2004). In the case of acute heat stress, alternative mechanisms are employed to secure bilayer integrity. These sense‐and‐respond mechanisms are based on heat‐induced, biophysical changes in membrane properties. In thylakoid membranes, heat induces packing defects in the lipid headgroups. These defects provide a spatial cue for docking of proteins with membrane‐protecting functions, such as sHSP (Heckathorn, Downs, Sharkey, & Coleman, 1998) and vesicle‐inducing protein in plastids 1 (VIPP1, Figure 3; Theis et al., 2019; Zhang, Kondo, Kamikubo, Kataoka, & Sakamoto, 2016). The inducible association of these proteins with membranes likely follows the sensing of the membrane status through their amphipathic α‐helices.
Acute heat also induces the aggregation of light‐harvesting complex II (LHCII) proteins in the thylakoid membranes. MGDG is normally associated with LHCII, and upon aggregation of LHCII, excess MGDG gets extruded to the lumen (Jahns, Latowski, & Strzalka, 2009; Schaller et al., 2010; Figure 3). Due to MGDG's non‐bilayer propensity, extruded MGDG forms a so‐called inverted hexagonal phase (HII) (Garab et al., 2017; Krumova et al., 2008). Thylakoid membranes are always close to HII phase transition and, as HII phases emerge, they must be controlled to avoid damage, which involves the function of sHSPs (Tsvetkova et al., 2002). HII phases are however key to chloroplast heat acclimation because when they emerge under stress, they recruit and activate the xanthophyll cycle enzyme, violaxanthin de‐epoxidase (VDE) (Dlouhý et al., 2020). VDE synthesizes zeaxanthin which quenches excess excitation energy and enhances membrane stability. The HII phases serve to sequester excess MGDG and promote the diffusion of xanthophylls (Latowski et al., 2002; Figure 3).
Another membrane feature that can undergo rapid stress‐induced modification are microdomains. Most lipids within a membrane exist in liquid‐disordered phase, often envisioned as a two‐dimensional fluid. Lipids can however also exist in the liquid‐ordered phase known as nano‐ and microdomains (Jaillais & Ott, 2020; Saenz, Sezgin, Schwille, & Simons, 2012). Microdomains form coherent, dynamic platforms for proteins with functions in sensing, signalling, membrane integrity maintenance and transport. Even mild changes in temperature can result in altered microdomain fluidity and consequently, redistribution and modified activity of these proteins (Török et al., 2014). Based on studies of membranes and Molecular Dynamics simulation, microdomains are speculated to act as dynamic reservoirs of fluidity‐decreasing lipids. Heat may trigger increased partitioning of these lipids from microdomains to the bulk fluid phase (Nickels et al., 2019). This simple buffering effect that can occur in complex membranes is based on thermodynamics of phase separation and could be far more responsive than the metabolic responses of homeoviscous adaptation (Ernst et al., 2018).
Some plasma membrane microdomains are tethered to the underlying cortical ER at so‐called ER‐plasma membrane contact sites (EPCSs) through synaptotagmins (SYT1 and SYT3) (Ruiz‐Lopez et al., 2020). SYT1/3 are ER proteins that bind (via C2‐domains) to phosphatidylinositolphosphate (PIP)‐containing microdomains of the plasma membrane (Figure 4). The close proximity of the two membranes allows for exchange/removal of detrimental lipids, for example, diacylglycerol that is formed at the plasma membrane during phospholipase C (PLC) signalling (see below). In yeast, EPCSs are important for plasma membrane integrity maintenance under heat stress (Collado et al., 2019), and they appear to function similarly under stresses in plants (Ruiz‐Lopez et al., 2020; Yan et al., 2017).
FIGURE 4.
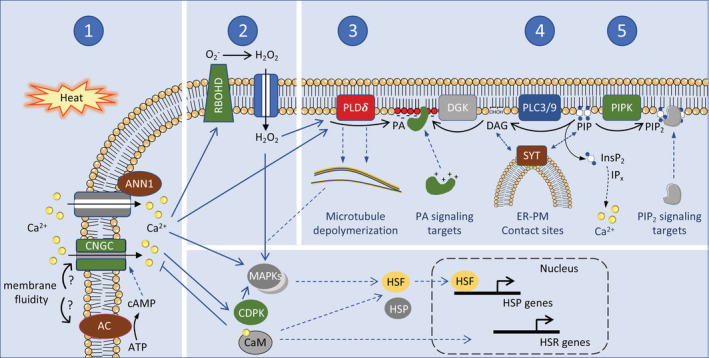
Sensing and primary signalling events of heat stress at the plasma membrane. In response to heat stress (37–45°C, 3–20 min), several plasma membrane‐linked protein activities are triggered which lead to intracellular signals that collectively regulate the heat stress response in plants. 1, Heat perception gives rise to increases in Ca2+, which can enter the cytosol from the apoplast through channels such as CNGC6. This channel might be activated by cAMP, which likely accumulates under heat stress through a membrane‐associated adenylyl cyclase (AC). The latter could be activated as membrane fluidity increases. Through association with calmodulin (CaM), Ca2+ can negatively regulate CNGC6, and promote the function of HSFs. HSFs are the primary regulators of the heat response leading to transcriptional induction of HSPs and other genes. Apart from CNGC6, Annexin 1 (ANN1) may function to increase cytosolic Ca2+. 2, The second major factor in the heat stress response is H2O2, which is generated by the plasma membrane microdomain NADPH oxidase, RBOHD, whose activity is modulated by several factors, including Ca2+ and PA. After H2O2 enters the cell, it modifies the PLDδ protein such that it becomes sensitive to activation by Ca2+. 3, PLDδ generates PA, which has a myriad of signalling functions that are mediated by its interaction with cytosolic target proteins. PLDδ is attached to microtubules and its activity leads to microtubule depolymerization. Moreover, H2O2 can activate HSFs through MAPK signalling. 4, PLC3 and PLC9 are required for sHSP induction and thermotolerance. Most likely, they hydrolyze PIP to generate DAG, releasing the inositol‐bisphosphate (IP2) headgroup. DAG can be phosphorylated to PA by diacylglycerol kinase (DGK). In plants, rather than IP2 or IP3, inositol's more highly phosphorylated derivatives are the likely inducers of cytosolic release of Ca2+. DAG could associate with synaptotagmin (SYT) in the ER at ER‐PM contact sites, which may function to stabilize the plasma membrane under stress, and facilitate the exchange of lipids between the plasma membrane and the cortical ER. 5, Besides PA, also PIP2 accumulates under heat stress, through PIP kinase (PIPK) activity, first only in the plasma membrane, later also in internal structures, including the nucleolus and cytosolic foci. PIP2 regulates effector proteins through specific lipid‐binding domains
The biophysical changes in membranes under heat stress can be sensed by altered protein activity and/or location. Moreover, the alternative lipid phases allow for a prompt response to temperature, thereby providing structural and functional flexibility that is of vital importance under heat stress. Notably, this suggests that homeoviscous adaptation does not necessarily involve a sensor of membrane fluidity. Whether fluidity sensing underlies other heat stress responses remains unknown. Many studies have attempted to probe the effect of membrane fluidization using pharmacological and genetic interventions, but it is becoming clear that these techniques have inadvertent effects on proteins and gene expression (Rütgers et al., 2017; Vu et al., 2019).
3.3. Ca2+ and H2O2 as heat stress signals
Heat shock elicits a rapid increase in cytosolic Ca2+, most likely from both extracellular and intracellular sources (Gao et al., 2012; Gong, van der Luit, Knight, & Trewavas, 1998). Ca2+ influx from the apoplast was shown to be required for the induction of HSPs through Ca2+/calmodulin‐dependent kinases and for the acquisition of heat tolerance (Liu et al., 2003, 2007). It has thus been reasoned that Ca2+ channels could function as thermosensors and attempts have been made to identify such channels. Cyclic nucleotide‐gated channels (CNGCs) appeared to be involved in heat‐induced cytosolic Ca2+ increases in Physcomitrium patens and Arabidopsis (Finka, Cuendet, Maathuis, Saidi, & Goloubinoff, 2012; Saidi et al., 2009). In Arabidopsis, heat stress induced an increase in cAMP, leading to a CNGC6‐mediated Ca2+ influx at the plasma membrane (Gao et al., 2012; Figure 4). This rise in cytosolic Ca2+ was required for the induction of HSP expression and thermotolerance. The suggested role of heat‐induced cAMP in triggering the Ca2+ mediated heat response led to the hypothesis that, rather than a Ca2+ channel, an adenylyl cyclase activity could act as membrane‐associated temperature sensor (Thomas, Marondedze, Ederli, Pasqualini, & Gehring, 2013). Interestingly, two proteins with this enzymatic activity were recently identified in maize. These adenyl cyclases were required for heat‐induced cAMP synthesis and full induction of HSP expression (Yang et al., 2020). However, their mode of action has remained unclear. In addition, the potential involvement of ANNEXIN1 (ANN1) in heat‐induced Ca2+ signalling awaits further study (Wang et al., 2015).
Identifying the primary heat‐activated Ca2+ channel remains a challenge. The animal heat‐activated Transient Receptor Potential channel, TRPV1, is a mechanosensitive and voltage‐gated cation channel, that is similar to plant CNGC (Benítez‐Angeles, Morales‐Lázaro, Juárez‐González, & Rosenbaum, 2020). It likely responds to forces transmitted via microtubules (Bavi et al., 2017; Prager‐Khoutorsky, Khoutorsky, & Bourque, 2014). Plants lack TRP channel homologs, but possess other mechanosensitive channel types that may function in heat signalling, including OSCA1 (reduced hyperosmolality‐induced [Ca2+] increase1), MCA (Mid1‐complementing activity), which was implicated in cold sensing (Mori et al., 2018), and Small Conductance Mechanosensitive Ion Channel (MscS)‐Like (MSL) proteins (Ackermann & Stanislas, 2020).
Using Arabidopsis seedlings expressing the Ca2+ reporter aequorin, Lenzoni and Knight (2019) were unable to detect a heat‐induced cytosolic Ca2+ response. Instead, they found a Ca2+ increase in the chloroplast stroma (Lenzoni & Knight, 2019). Temperatures of >35°C induced rapid Ca2+ responses, with higher temperatures provoking higher and faster peaks. The response was not influenced by the rate of warming, but was determined by the absolute temperature. The thylakoid membrane Ca2+ sensor CAS was required for full induction of stromal Ca2+ in response to heat (Figure 3). CAS amplified the Ca2+ signal, but what governs the initial signal is still unknown. Stress‐specific stromal Ca2+ transients could involve the activities of multiple channels and transporters, and are emerging as signals that regulate chloroplast functions and cellular signal transduction (Navazio, Formentin, Cendron, & Szabò, 2020; Nomura et al., 2012; Teardo et al., 2019).
In addition to Ca2+, H2O2 levels at the plasma membrane also rise quickly in response to severe heat stress, a process catalysed by the NADPH oxidase, RbohD (Vacca et al., 2004). The plasma membrane H2O2 signal is required for heat stress gene expression and increase in heat tolerance (Suzuki, Koussevitzky, Mittler, & Miller, 2012; Volkov, Panchuk, Mullineaux, & Schöffl, 2006). There is also accumulation of H2O2 in chloroplasts and mitochondria and this may provide additional priming signals (Sun & Guo, 2016).
3.4. Heat sensing is mediated by lipid signals
In addition to roles as structural components of membranes, lipids also have signalling and regulatory functions, coupling perception of environmental cues to cellular responses (Hou, Ufer, & Bartels, 2015). Lipids such as PA and phosphatidylinositol 4,5‐bisphosphate (PIP2), and their metabolic enzymes, for example, phospholipase C and D (PLC/PLD), diacylglycerol (DAG) kinase (DGK) and phosphatidylinositol‐4‐phosphate 5‐kinase (PIP5K), have a wide range of cellular regulatory functions in environmental stress responses. In Arabidopsis seedlings, heat stress (40°C) triggers increases in PA and PIP2 abundance within 2 min (Mishkind, Vermeer, Darwish, & Munnik, 2009; Figure 4).
This extremely rapid response suggests that the synthesis of these signalling lipids is closely tied to thermosensing, but as of yet it is unknown how increases in temperature activate these lipid‐modifying enzymes. High temperature induction of PA is largely dependent on membrane lipid hydrolysis by PLD (Mishkind et al., 2009; Shiva et al., 2020). The PLD enzyme is localized at the plasma membrane and associated with microtubules, where it regulates their membrane‐anchorage (Andreeva et al., 2009). In heat‐stressed stomatal cells, apoplastic H2O2 enters the cytosol through aquaporins. H2O2 oxidizes cysteine residues in the C2 domain of PLDδ. The modified cysteine residues promoted Ca2+ binding to PLDδ, which resulted in depolymerization of microtubules (Song et al., 2020; Zhang et al., 2017). Blocking microtubule depolymerization by chemical stabilizers inhibited the upregulation of HSP70 and the induction of MAPK activity under heat stress (Sangwan, Orvar, Beyerly, Hirt, & Dhindsa, 2002; Suri & Dhindsa, 2007), which suggests that this pathway acts to promote thermal acclimation. Curiously though, mutants lacking PLDδ were more tolerant to heat stress, which suggests the opposite. The fact that PLDδ requires Ca2+ and H2O2 for its activation hints that, despite its rapid activation, PLDδ signalling occurs downstream of primary thermosensing.
Glyceraldehyde‐3‐phosphate dehydrogenase (GAPC) may also play a role in heat stress signalling. GAPC was shown to translocate to the nucleus under heat stress, where it activates the transcription factor NF‐YC10. Activated NF‐YC10 then promotes the expression of genes that confer thermotolerance (Kim, Guo, & Wang, 2020). The mechanism that promotes GAPC nuclear translocation is as yet unresolved. When in the cytosol, GAPC can directly bind PLDδ and positively regulate its activity (Guo et al., 2012). GAPC has also been shown to bind PA (McLoughlin et al., 2013) but it is unclear how or if these attributes contribute to temperature regulation of GAPC. The involvement of GAPC in PLD/PA signalling raises the possibility that the heat stress response is coordinated with basal cell metabolism.
PLC also appears to have a function in the heat stress response, because PLC9 and PLC3 knock‐out seedlings show severely impaired basal and/or acquired heat tolerance, while overexpression of PLC9 or PLC3 improved heat tolerance (Gao et al., 2014; Zheng et al., 2012; Figure 4). PLCs hydrolyze PIP and PIP2 to generate DAG and inositolphosphates. Inositolphosphates could eventually promote activation of a Ca2+channel (Munnik, 2014). PLC9 and PLC3, both at the plasma membrane, were required for the induction of cytosolic Ca2+ and enhanced expression of sHSPs under heat stress. Acting very early in the response pathway, the PLCs may be physically close to the thermosensor. Regulation of PLCs is complex, involving calcium, G‐proteins and post‐translational modifications (Munnik, 2014). Potential protein interactors of PLC3 and PLC9, including two receptor‐like protein kinases and a cell wall‐associated kinase, could provide interesting clues as to their heat‐responsive mode of activation (Pokotylo et al., 2013).
The heat stress‐induced accumulation of PIP2, revealed by a fluorescent PIP2 biosensor, displayed interesting patterns (Mishkind et al., 2009). During heat exposure, PIP2 accumulated first at the plasma membrane, after which it appeared in cytoplasmic foci, followed by accumulation at the nuclear envelope and nucleolus (Mishkind et al., 2009). PIP2 can function in endocytosis and associates with membrane microdomains (Furt et al., 2010). Microdomains are considered critical in the regulation of early stress signalling, as they contain signalling proteins such as RbohD, HSPs and CNGCs (Dietrich, Moeder, & Yoshioka, 2020; Horvath et al., 1998; Niu & Xiang, 2018). The cytoplasmic PIP2 foci are reminiscent of heat‐induced stress granules, showing similar sizes and conditions of formation. While the possibility that this lipid would associate with membraneless structures like stress granules may seem remote, the apparent occurrence of PIP2 at the nucleolus, another membraneless compartment (Mishkind et al., 2009), and localization to similar structures in mammalian nuclei (Boronenkov, Loijens, Umeda, & Anderson, 1998; Fiume et al., 2019; Sztacho, Sobol, Balaban, Escudeiro Lopes, & Hozák, 2019), could argue for this possibility. The suggested propensity of PIP2 to associate with membraneless compartments under heat stress might shed new light on the potential regulatory functions of this key lipid.
4. CONCLUSIONS AND FUTURE CHALLENGES
Clearly, our knowledge of thermosensory systems of plants has greatly expanded in the past decade. Important discoveries in ambient temperature signalling include the discovery of photo‐/thermosensors, an RNA switch and phase separation of ELF3 into liquid droplets through its PrLD (Figure 2). These three systems unambiguously translate high ambient temperatures into altered gene expression. The reprogramming of development by these factors assists plants to avoid damaging high temperatures. In Arabidopsis, the inhibition of phyB activity by warm temperatures has been shown in detail. It is however still unknown if other photoreceptors function in a similar way. Phototropin plays a role in low temperature signalling in P. patens but it is not known whether this temperature‐dependent activity also stretches to warm temperatures. Other photoreceptors undergo thermal reversion and so could conceptually also function in warm temperature sensing, but this remains to be demonstrated. The finding that PIF7 RNA translation is enhanced at warm temperatures opens the possibility that other RNAs act in a similar way. Indeed, the authors of the PIF7 study show that HSFA2 and WRKY22 RNA may also be regulated through a comparable mechanism (Chung et al., 2020). The finding that ELF3 contains a PrLD that undergoes temperature‐dependent condensation likely has effects beyond just evening complex transcriptional repression. ELF3 acts as a scaffold protein for large protein complexes (Huang et al., 2016) and directly binds to PIF4 to inhibit its transcriptional activity (Nieto, López‐Salmerón, Davière, & Prat, 2015). Both of these functions are likely inhibited at warm temperatures.
Upon moderate temperature increases, plants trigger a heat stress response for acclimation, but the sensing mechanism is still largely unknown. Rather than unfolded proteins, the increased membrane fluidity at high temperatures was speculated to be a molecular basis of sensing. While no fluidity sensor has been found in plants, evidence is accumulating for thermosensory mechanisms based on heat‐induced phase changes in lipids and RNA/protein assemblies.
Under heat stress, thylakoid membranes locally undergo transition to non‐bilayer, HII phases, which are essential for heat acclimation, since they compartmentalize and activate the enzymes of the xanthophyll cycle. At the plasma membrane, dynamic microdomains contain lipids in a liquid‐ordered phase. These domains harbour potential signalling lipids and proteins, including RbohD, which is activated in response to heat stress. Changes in microdomains and HII phases could constitute a basis for thermosensitive regulation of enzymes. Simultaneously, they provide potential avenues for the rapid trafficking of lipids between phases, in order to preserve membrane integrity under heat stress. For the latter function, a membrane fluidity sensor would thus not be required.
It is important to note that although we have divided the response to temperatures into three stages (thermomorphogenesis, acclimation and heat stress; Figure 1), there is a degree of overlap between the temperatures that elicit these responses. There are also several examples of overlap in signalling pathways between these stages. Phytochromes are primarily involved with thermomorphogenic responses, but it has been shown that phytochrome signalling can also affect heat shock tolerance (Arico et al., 2019). Chloroplast retrograde signals are important for heat acclimation and tolerance, but recently it was found that chloroplast‐derived MEcPP regulates PIF4/PIF5 expression and phyB abundance (Jiang et al., 2019). More examples of cross‐talk between these stages of heat signalling will likely emerge in the future.
One of the most exciting recent developments in the field of temperature sensing in plants was the identification of ELF3 as a temperature sensor. ELF3 exemplifies a novel type of ambient temperature sensing mechanism, based on the conditional formation of condensed liquid phases within a bulk dilute phase, triggered by the coalescence of proteins through their PrLDs. The resulting biomolecular condensates constitute membraneless compartments that can contain proteins with associated regulatory functions. Heat‐induced stress granules may also be examples of phase‐separated biomolecular condensates, but it is unclear whether their formation is directly triggered by heat, similar to ELF condensates, and whether certain lipids could play a role in their assembly and function.
Could liquid–liquid phase separation also function, at higher temperatures, in the activation of the heat stress response? Such a function was recently proposed for the yeast RNA‐binding protein, Pab1 (poly(A)‐binding protein), which displayed phase separation upon a shift to a temperature that induces the heat shock response (Riback et al., 2017). The extreme thermosensitivity of this process was quantified using the temperature coefficient Q10, the ratio of biological properties measured 10°C apart. With a Q10 of 350, it exceeds by far any other known biological thermosensory process. This indicates the potential of liquid–liquid phase separation of proteins as a thermosensing mechanism. The sharp threshold temperature above which phase separation is triggered, which is determined by the amino acid side chains in the PrLD, allows for precise temperature‐dependent regulation of responses. Pab1 was speculated to activate the heat stress response by sequestering a negative regulator of HSF in liquid droplets, calling into question the requirement of unfolded proteins for activation (Riback et al., 2017). It seems plausible that similar mechanisms could govern heat stress responses in plants.
The stress‐induced clustering of proteins to membrane microdomains could trigger liquid–liquid phase separation in the adjacent cytosol. This could result in coupled lipid and liquid compartments, that assemble selected response components, allowing for specific channelling of sensory signals to downstream responses (Jaillais & Ott, 2020). Similarly, plasma membrane organization could respond to changes in the cell wall, which may also adopt different biophysical states dependent on temperature (Wu, Bulgakov, & Jinn, 2018). As yet, such potential interactions are unexplored territory.
Identifying plant proteins that could act as thermosensors through liquid–liquid phase separation will be challenging. Previously, heat stress was found to induce relocalization of splicing factors with disordered domains, for example, serine/arginine‐rich protein SR45, into enlarged nuclear speckles (Ali, Golovkin, & Reddy, 2003; Reddy, Day, Göhring, & Barta, 2012), which could underlie alternative splicing of pre‐mRNAs. The PrLDs of mRNA‐binding proteins might be critical to trigger thermosensitive condensation into stress granules (Chodasiewicz et al., 2020; Emenecker, Holehouse, & Strader, 2020; Kosmacz et al., 2019). Many of the, approximately, 500 proteins in plants with predicted PrLDs are transcription factors with potential roles in temperature signalling. There are also PrLDs in HSFA1b, several PIFs, auxin response factors and ABRE‐binding factors (Chakrabortee et al., 2016). Investigating the effect of temperature on the coalescence of these factors in vitro could yield interesting results.
In conclusion, thermosensing appears to be a highly distributed capacity, based on a range of mechanisms which are only just beginning to come to light. Most strikingly, the temperature‐dependent behaviour of phyB, the PIF7 RNA hairpin, and both lipid and liquid–liquid phase separations, provide an impressive spectrum of potential heat sensing and responding modes, essential for plants to acclimate and survive.
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
ACKNOWLEDGMENTS
We are grateful to Martin Balcerowicz and Fionn McLoughlin for helpful discussions. The views expressed in this paper do not necessarily reflect those of the U.S. National Science Foundation or the United States Government. Scott Hayes was supported by Wageningen Graduate Schools (WGS) Postdoctoral Talent Programme. Teun Munnik was supported by Netherlands Organisation for Scientific Research (NWO 867.15.020; 711.017.005) and European Research Council (EU‐FET 828753).
Hayes S, Schachtschabel J, Mishkind M, Munnik T, Arisz SA. Hot topic: Thermosensing in plants. Plant Cell Environ. 2021;44:2018–2033. 10.1111/pce.13979
Scott Hayes and Joëlle Schachtschabel should be considered joint first author.
Funding information Netherlands Organisation for Scientific Research, Grant/Award Numbers: EU‐FET 828753, NWO 867.15.020; 711.017.005; Wageningen Graduate Schools, Grant/Award Number: Postdoctoral Talent Programme
REFERENCES
- Ackermann, F., & Stanislas, T. (2020). The plasma membrane—An integrating compartment for mechano‐signaling. Plants, 9(4), 505. 10.3390/plants9040505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali, G. S., Golovkin, M., & Reddy, A. S. N. (2003). Nuclear localization and in vivo dynamics of a plant‐specific serine/arginine‐rich protein. The Plant Journal, 36(6), 883–893. 10.1046/j.1365-313X.2003.01932.x [DOI] [PubMed] [Google Scholar]
- Andreeva, Z., Ho, A. Y. Y., Barthet, M. M., Potocký, M., Bezvoda, R., Žárský, V., & Marc, J. (2009). Phospholipase D family interactions with the cytoskeleton: Isoform delta promotes plasma membrane anchoring of cortical microtubules. Functional Plant Biology, 36(7), 600. 10.1071/FP09024 [DOI] [PubMed] [Google Scholar]
- Arico, D., Legris, M., Castro, L., Garcia, C. F., Laino, A., Casal, J. J., & Mazzella, M. A. (2019). Neighbour signals perceived by phytochrome B increase thermotolerance in Arabidopsis . Plant, Cell & Environment, 42(9), 2554–2566. 10.1111/pce.13575 [DOI] [PubMed] [Google Scholar]
- Ballweg, S., Sezgin, E., Doktorova, M., Covino, R., Reinhard, J., Wunnicke, D., … Ernst, R. (2020). Regulation of lipid saturation without sensing membrane fluidity. Nature Communications, 11, 756. 10.1038/s41467-020-14528-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balogh, G., Péter, M., Glatz, A., Gombos, I., Török, Z., Horváth, I., … Vígh, L. (2013). Key role of lipids in heat stress management. FEBS Letters, 587(13), 1970–1980. 10.1016/j.febslet.2013.05.016 [DOI] [PubMed] [Google Scholar]
- Battisti, D. S., & Naylor, R. L. (2009). Historical warnings of future food insecurity with unprecedented seasonal heat. Science, 323(5911), 240–244. 10.1126/science.1164363 [DOI] [PubMed] [Google Scholar]
- Bäurle, I., & Trindade, I. (2020). Chromatin regulation of somatic abiotic stress memory. Journal of Experimental Botany, 71(17), 5269–5279. 10.1093/jxb/eraa098 [DOI] [PubMed] [Google Scholar]
- Bavi, N., Nikolaev, Y. A., Bavi, O., Ridone, P., Martinac, A. D., Nakayama, Y., … Martinac, B. (2017). Principles of mechanosensing at the membrane interface. In Epand R. M. & Ruysschaert J.‐M. (Eds.), The biophysics of cell membranes (Vol. 19, pp. 85–119). Singapore: Springer. 10.1007/978-981-10-6244-5_4 [DOI] [Google Scholar]
- Benítez‐Angeles, M., Morales‐Lázaro, S. L., Juárez‐González, E., & Rosenbaum, T. (2020). TRPV1: Structure, endogenous agonists, and mechanisms. International Journal of Molecular Sciences, 21(10), 3421. 10.3390/ijms21103421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benn, G., Bjornson, M., Ke, H., De Souza, A., Balmond, E. I., Shaw, J. T., & Dehesh, K. (2016). Plastidial metabolite MEcPP induces a transcriptionally centered stress‐response hub via the transcription factor CAMTA3. Proceedings of the National Academy of Sciences, 113(31), 8855–8860. 10.1073/pnas.1602582113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boronenkov, I. V., Loijens, J. C., Umeda, M., & Anderson, R. A. (1998). Phosphoinositide signaling pathways in nuclei are associated with nuclear speckles containing pre‐mRNA processing factors. Molecular Biology of the Cell, 9(12), 3547–3560. 10.1091/mbc.9.12.3547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Box, M. S., Huang, B. E., Domijan, M., Jaeger, K. E., Khattak, A. K., Yoo, S. J., … Wigge, P. A. (2015). ELF3 controls thermoresponsive growth in Arabidopsis. Current Biology, 25(2), 194–199. 10.1016/j.cub.2014.10.076 [DOI] [PubMed] [Google Scholar]
- Casal, J. J., & Balasubramanian, S. (2019). Thermomorphogenesis. Annual Review of Plant Biology, 70(1), 321–346. 10.1146/annurev-arplant-050718-095919 [DOI] [PubMed] [Google Scholar]
- Chakrabortee, S., Kayatekin, C., Newby, G. A., Mendillo, M. L., Lancaster, A., & Lindquist, S. (2016). Luminidependens (LD) is an Arabidopsis protein with prion behavior. Proceedings of the National Academy of Sciences, 113(21), 6065–6070. 10.1073/pnas.1604478113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Challinor, A. J., Watson, J., Lobell, D. B., Howden, S. M., Smith, D. R., & Chhetri, N. (2014). A meta‐analysis of crop yield under climate change and adaptation. Nature Climate Change, 4(4), 287–291. 10.1038/nclimate2153 [DOI] [Google Scholar]
- Chantarachot, T., & Bailey‐Serres, J. (2018). Polysomes, stress granules, and processing bodies: A dynamic triumvirate controlling cytoplasmic mRNA fate and function. Plant Physiology, 176(1), 254–269. 10.1104/pp.17.01468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chodasiewicz, M., Sokolowska, E. M., Nelson‐Dittrich, A. C., Masiuk, A., Beltran, J. C. M., Nelson, A. D. L., & Skirycz, A. (2020). Identification and characterization of the heat‐induced plastidial stress granules reveal new insight into Arabidopsis stress response. Frontiers in Plant Science, 11, 595792. 10.3389/fpls.2020.595792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung, B. Y. W., Balcerowicz, M., Di Antonio, M., Jaeger, K. E., Geng, F., Franaszek, K., … Wigge, P. A. (2020). An RNA thermoswitch regulates daytime growth in Arabidopsis. Nature Plants, 6, 522–532. 10.1038/s41477-020-0633-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collado, J., Kalemanov, M., Campelo, F., Bourgoint, C., Thomas, F., Loewith, R., … Fernández‐Busnadiego, R. (2019). Tricalbin‐mediated contact sites control ER curvature to maintain plasma membrane integrity. Developmental Cell, 51(4), 476–487.e7. 10.1016/j.devcel.2019.10.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortijo, S., Charoensawan, V., Brestovitsky, A., Buning, R., Ravarani, C., Rhodes, D., … Wigge, P. A. (2017). Transcriptional regulation of the ambient temperature response by H2A.Z nucleosomes and HSF1 transcription factors in Arabidopsis. Molecular Plant, 10(10), 1258–1273. 10.1016/j.molp.2017.08.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Covino, R., Ballweg, S., Stordeur, C., Michaelis, J. B., Puth, K., Wernig, F., … Ernst, R. (2016). A eukaryotic sensor for membrane lipid saturation. Molecular Cell, 63(1), 49–59. 10.1016/j.molcel.2016.05.015 [DOI] [PubMed] [Google Scholar]
- Crawford, A. J., McLachlan, D. H., Hetherington, A. M., & Franklin, K. A. (2012). High temperature exposure increases plant cooling capacity. Current Biology, 22(10), R396–R397. 10.1016/j.cub.2012.03.044 [DOI] [PubMed] [Google Scholar]
- Cuevas‐Velazquez, C. L., & Dinneny, J. R. (2018). Organization out of disorder: Liquid–liquid phase separation in plants. Current Opinion in Plant Biology, 45, 68–74. 10.1016/j.pbi.2018.05.005 [DOI] [PubMed] [Google Scholar]
- Cybulski, L. E., Martín, M., Mansilla, M. C., Fernández, A., & de Mendoza, D. (2010). Membrane thickness cue for cold sensing in a bacterium. Current Biology, 20(17), 1539–1544. 10.1016/j.cub.2010.06.074 [DOI] [PubMed] [Google Scholar]
- Deng, Y., Humbert, S., Liu, J.‐X., Srivastava, R., Rothstein, S. J., & Howell, S. H. (2011). Heat induces the splicing by IRE1 of a mRNA encoding a transcription factor involved in the unfolded protein response in Arabidopsis. Proceedings of the National Academy of Sciences, 108(17), 7247–7252. 10.1073/pnas.1102117108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietrich, P., Moeder, W., & Yoshioka, K. (2020). Plant cyclic nucleotide‐gated channels: New insights on their functions and regulation. Plant Physiology, 184, 27–38. 10.1104/pp.20.00425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding, Y., Shi, Y., & Yang, S. (2020). Molecular regulation of plant responses to environmental temperatures. Molecular Plant, 13(4), 544–564. 10.1016/j.molp.2020.02.004 [DOI] [PubMed] [Google Scholar]
- Dlouhý, O., Kurasová, I., Karlický, V., Javornik, U., Šket, P., Petrova, N. Z., … Garab, G. (2020). Modulation of non‐bilayer lipid phases and the structure and functions of thylakoid membranes: Effects on the water‐soluble enzyme violaxanthin de‐epoxidase. Scientific Reports, 10, 11959. 10.1038/s41598-020-68854-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards, K. D., Lynn, J. R., Gyula, P., Nagy, F., & Millar, A. J. (2005). Natural allelic variation in the temperature‐compensation mechanisms of the Arabidopsis thaliana circadian clock. Genetics, 170(1), 387–400. 10.1534/genetics.104.035238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emenecker, R. J., Holehouse, A. S., & Strader, L. C. (2020). Emerging roles for phase separation in plants. Developmental Cell, 55(1), 69–83. 10.1016/j.devcel.2020.09.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst, R., Ballweg, S., & Levental, I. (2018). Cellular mechanisms of physicochemical membrane homeostasis. Current Opinion in Cell Biology, 53, 44–51. 10.1016/j.ceb.2018.04.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ezer, D., Jung, J.‐H., Lan, H., Biswas, S., Gregoire, L., Box, M. S., … Wigge, P. A. (2017). The evening complex coordinates environmental and endogenous signals in Arabidopsis. Nature Plants, 3, 17087. 10.1038/nplants.2017.87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falcone, D. L., Ogas, J. P., & Somerville, C. R. (2004). Regulation of membrane fatty acid composition by temperature in mutants of Arabidopsis with alterations in membrane lipid composition. BMC Plant Biology, 4(1), 17. 10.1186/1471-2229-4-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Findlay, K. M. W., & Jenkins, G. I. (2016). Regulation of UVR8 photoreceptor dimer/monomer photo‐equilibrium in Arabidopsis plants grown under photoperiodic conditions: UVR8 photo‐equilibrium. Plant, Cell & Environment, 39(8), 1706–1714. 10.1111/pce.12724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finka, A., Cuendet, A. F. H., Maathuis, F. J. M., Saidi, Y., & Goloubinoff, P. (2012). Plasma membrane cyclic nucleotide gated calcium channels control land plant thermal sensing and acquired thermotolerance. The Plant Cell, 24(8), 3333–3348. 10.1105/tpc.112.095844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiorucci, A.‐S., Galvão, V. C., Ince, Y. Ç., Boccaccini, A., Goyal, A., Allenbach Petrolati, L., … Fankhauser, C. (2020). Phytochrome interacting factor 7 is important for early responses to elevated temperature in Arabidopsis seedlings. New Phytologist, 226(1), 50–58. 10.1111/nph.16316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiume, R., Faenza, I., Sheth, B., Poli, A., Vidalle, M. C., Mazzetti, C., … Divecha, N. (2019). Nuclear phosphoinositides: Their regulation and roles in nuclear functions. International Journal of Molecular Sciences, 20(12), 2991. 10.3390/ijms20122991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujii, Y., Tanaka, H., Konno, N., Ogasawara, Y., Hamashima, N., Tamura, S., … Kodama, Y. (2017). Phototropin perceives temperature based on the lifetime of its photoactivated state. Proceedings of the National Academy of Sciences, 114(34), 9206–9211. 10.1073/pnas.1704462114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furt, F., König, S., Bessoule, J.‐J., Sargueil, F., Zallot, R., Stanislas, T., … Mongrand, S. (2010). Polyphosphoinositides are enriched in plant membrane rafts and form microdomains in the plasma membrane. Plant Physiology, 152(4), 2173–2187. 10.1104/pp.109.149823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao, F., Han, X., Wu, J., Zheng, S., Shang, Z., Sun, D., … Li, B. (2012). A heat‐activated calcium‐permeable channel – Arabidopsis cyclic nucleotide‐gated ion channel 6 – Is involved in heat shock responses: CNGC6 is a heat‐activated calcium channel. The Plant Journal, 70(6), 1056–1069. 10.1111/j.1365-313X.2012.04969.x [DOI] [PubMed] [Google Scholar]
- Gao, K., Liu, Y.‐L., Li, B., Zhou, R.‐G., Sun, D.‐Y., & Zheng, S.‐Z. (2014). Arabidopsis thaliana phosphoinositide‐specific phospholipase C isoform 3 (AtPLC3) and AtPLC9 have an additive effect on thermotolerance. Plant and Cell Physiology, 55(11), 1873–1883. 10.1093/pcp/pcu116 [DOI] [PubMed] [Google Scholar]
- Garab, G., Ughy, B., de Waard, P., Akhtar, P., Javornik, U., Kotakis, C., … Lambrev, P. H. (2017). Lipid polymorphism in chloroplast thylakoid membranes – As revealed by 31P‐NMR and time‐resolved merocyanine fluorescence spectroscopy. Scientific Reports, 7, 13343. 10.1038/s41598-017-13574-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong, M., van der Luit, A. H., Knight, M. R., & Trewavas, A. J. (1998). Heat‐shock‐induced changes in intracellular Ca 2+ level in tobacco seedlings in relation to thermotolerance. Plant Physiology, 116(1), 429–437. 10.1104/pp.116.1.429 [DOI] [Google Scholar]
- Guo, L., Devaiah, S. P., Narasimhan, R., Pan, X., Zhang, Y., Zhang, W., & Wang, X. (2012). Cytosolic glyceraldehyde‐3‐phosphate dehydrogenases interact with phospholipase Dδ to transduce hydrogen peroxide signals in the Arabidopsis response to stress. The Plant Cell, 24(5), 2200–2212. 10.1105/tpc.111.094946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahm, J., Kim, K., Qiu, Y., & Chen, M. (2020). Increasing ambient temperature progressively disassembles Arabidopsis phytochrome B from individual photobodies with distinct thermostabilities. Nature Communications, 11, 1660. 10.1038/s41467-020-15526-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halbleib, K., Pesek, K., Covino, R., Hofbauer, H. F., Wunnicke, D., Hänelt, I., … Ernst, R. (2017). Activation of the unfolded protein response by lipid bilayer stress. Molecular Cell, 67(4), 673–684.e8. 10.1016/j.molcel.2017.06.012 [DOI] [PubMed] [Google Scholar]
- Han, S.‐H., Park, Y.‐J., & Park, C.‐M. (2019). HOS1 activates DNA repair systems to enhance plant thermotolerance. Nature Plants. 14, 1554469. 10.1080/15592324.2018.1554469 [DOI] [PubMed] [Google Scholar]
- Hatfield, J. L., & Prueger, J. H. (2015). Temperature extremes: Effect on plant growth and development. Weather and Climate Extremes, 10, 4–10. 10.1016/j.wace.2015.08.001 [DOI] [Google Scholar]
- Hayes, S. (2020). Interaction of light and temperature signalling in plants. In ELS (1st ed. Chichester, England: ). John Wiley & Sons Ltd. 10.1002/047001590X [DOI] [Google Scholar]
- Heckathorn, S. A., Downs, C. A., Sharkey, T. D., & Coleman, J. S. (1998). The small, methionine‐rich chloroplast heat‐shock protein protects photosystem II electron transport during heat stress. Plant Physiology, 116(1), 439–444. 10.1104/pp.116.1.439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higashi, Y., Okazaki, Y., Myouga, F., Shinozaki, K., & Saito, K. (2015). Landscape of the lipidome and transcriptome under heat stress in Arabidopsis thaliana . Scientific Reports, 5, 10533. 10.1038/srep10533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvath, I., Glatz, A., Varvasovszki, V., Torok, Z., Pali, T., Balogh, G., … Vigh, L. (1998). Membrane physical state controls the signaling mechanism of the heat shock response in synechocystis PCC 6803: Identification of hsp17 as a “fluidity gene”. Proceedings of the National Academy of Sciences, 95(7), 3513–3518. 10.1073/pnas.95.7.3513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou, Q., Ufer, G., & Bartels, D. (2015). Lipid signalling in plant responses to abiotic stress. Plant, Cell & Environment, 39, 1029–1048. 10.1111/pce.12666 [DOI] [PubMed] [Google Scholar]
- Howarth, C. J., & Ougham, H. J. (1993). Gene expression under temperature stress. New Phytologist, 125(1), 1–26. 10.1111/j.1469-8137.1993.tb03862.x [DOI] [PubMed] [Google Scholar]
- Huang, H., Alvarez, S., Bindbeutel, R., Shen, Z., Naldrett, M. J., Evans, B. S., … Nusinow, D. A. (2016). Identification of evening complex associated proteins in Arabidopsis by affinity purification and mass spectrometry. Molecular & Cellular Proteomics, 15(1), 201–217. 10.1074/mcp.M115.054064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahns, P., Latowski, D., & Strzalka, K. (2009). Mechanism and regulation of the violaxanthin cycle: The role of antenna proteins and membrane lipids. Biochimica et Biophysica Acta (BBA) – Bioenergetics, 1787(1), 3–14. 10.1016/j.bbabio.2008.09.013 [DOI] [PubMed] [Google Scholar]
- Jaillais, Y., & Ott, T. (2020). The nanoscale organization of the plasma membrane and its importance in signaling: A proteolipid perspective. Plant Physiology, 182(4), 1682–1696. 10.1104/pp.19.01349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janda, M., Lamparová, L., Zubíková, A., Burketová, L., Martinec, J., & Krčková, Z. (2019). Temporary heat stress suppresses PAMP‐triggered immunity and resistance to bacteria in Arabidopsis thaliana . Molecular Plant Pathology, 20(7), 1005–1012. 10.1111/mpp.12799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang J., Zeng L., Ke H., De La Cruz B., Dehesh K. (2019). Orthogonal regulation of phytochrome B abundance by stress‐specific plastidial retrograde signaling metabolite. Nature Communications, 10, 2904. 10.1038/s41467-019-10867-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung, J.‐H., Barbosa, A. D., Hutin, S., Kumita, J. R., Gao, M., Derwort, D., … Wigge, P. A. (2020). A prion‐like domain in ELF3 functions as a thermosensor in Arabidopsis. Nature, 585, 256–260. 10.1038/s41586-020-2644-7 [DOI] [PubMed] [Google Scholar]
- Jung, J.‐H., Domijan, M., Klose, C., Biswas, S., Ezer, D., Gao, M., … Wigge, P. A. (2016). Phytochromes function as thermosensors in Arabidopsis . Science, 354(6314), 886–889. 10.1126/science.aaf6005 [DOI] [PubMed] [Google Scholar]
- Kim, C. (2020). High ambient temperature accelerates leaf senescence via PHYTOCHROME‐INTERACTING FACTOR 4 and 5 in Arabidopsis. Molecules and Cells, 43(7), 645–661. 10.14348/MOLCELLS.2020.0117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, J.‐H., Lee, H.‐J., Jung, J.‐H., Lee, S., & Park, C.‐M. (2017). HOS1 facilitates the phytochrome B‐mediated inhibition of PIF4 function during hypocotyl growth in Arabidopsis. Molecular Plant, 10(2), 274–284. 10.1016/j.molp.2016.11.009 [DOI] [PubMed] [Google Scholar]
- Kim, S.‐C., Guo, L., & Wang, X. (2020). Nuclear moonlighting of cytosolic glyceraldehyde‐3‐phosphate dehydrogenase regulates Arabidopsis response to heat stress. Nature Communications, 11, 3439. 10.1038/s41467-020-17311-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koini, M. A., Alvey, L., Allen, T., Tilley, C. A., Harberd, N. P., Whitelam, G. C., & Franklin, K. A. (2009). High temperature‐mediated Adaptations in plant architecture require the bHLH transcription factor PIF4. Current Biology, 19(5), 408–413. 10.1016/j.cub.2009.01.046 [DOI] [PubMed] [Google Scholar]
- Kosmacz, M., Gorka, M., Schmidt, S., Luzarowski, M., Moreno, J. C., Szlachetko, J., … Skirycz, A. (2019). Protein and metabolite composition of Arabidopsis stress granules. New Phytologist, 222(3), 1420–1433. 10.1111/nph.15690 [DOI] [PubMed] [Google Scholar]
- Kostaki, K.‐I., Coupel‐Ledru, A., Bonnell, V. C., Gustavsson, M., Sun, P., McLaughlin, F. J., … Franklin, K. A. (2020). Guard cells integrate light and temperature signals to control stomatal aperture. Plant Physiology, 182(3), 1404–1419. 10.1104/pp.19.01528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krumova, S. B., Dijkema, C., de Waard, P., Van As, H., Garab, G., & van Amerongen, H. (2008). Phase behavior of phosphatidylglycerol in spinach thylakoid membranes as revealed by 31P‐NMR. Biochimica et Biophysica Acta (BBA) – Biomembranes, 1778(4), 997–1003. 10.1016/j.bbamem.2008.01.004 [DOI] [PubMed] [Google Scholar]
- Kumar, S. V., & Wigge, P. A. (2010). H2A.Z‐containing nucleosomes mediate the thermosensory response in Arabidopsis. Cell, 140(1), 136–147. 10.1016/j.cell.2009.11.006 [DOI] [PubMed] [Google Scholar]
- Latowski, D., Kruk, J., Burda, K., Skrzynecka‐Jaskier, M., Kostecka‐Gugała, A., & Strzałka, K. (2002). Kinetics of violaxanthin de‐epoxidation by violaxanthin de‐epoxidase, a xanthophyll cycle enzyme, is regulated by membrane fluidity in model lipid bilayers: Violaxanthin de‐epoxidation in liposomes. European Journal of Biochemistry, 269(18), 4656–4665. 10.1046/j.1432-1033.2002.03166.x [DOI] [PubMed] [Google Scholar]
- Legris, M., Klose, C., Burgie, E. S., Rojas, C. C. R., Neme, M., Hiltbrunner, A., … Casal, J. J. (2016). Phytochrome B integrates light and temperature signals in Arabidopsis . Science, 354(6314), 897–900. 10.1126/science.aaf5656 [DOI] [PubMed] [Google Scholar]
- Lenzoni, G., & Knight, M. R. (2019). Increases in absolute temperature stimulate free calcium concentration elevations in the chloroplast. Plant and Cell Physiology, 60(3), 538–548. 10.1093/pcp/pcy227 [DOI] [PubMed] [Google Scholar]
- Liu, H., & Charng, Y. (2013). Common and distinct functions of Arabidopsis class A1 and A2 heat shock factors in diverse abiotic stress responses and development. Plant Physiology, 163(1), 276–290. 10.1104/pp.113.221168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, H., Lämke, J., Lin, S., Hung, M.‐J., Liu, K.‐M., Charng, Y., & Bäurle, I. (2018). Distinct heat shock factors and chromatin modifications mediate the organ‐autonomous transcriptional memory of heat stress. The Plant Journal, 95(3), 401–413. 10.1111/tpj.13958 [DOI] [PubMed] [Google Scholar]
- Liu, H.‐T., Li, B., Shang, Z.‐L., Li, X.‐Z., Mu, R.‐L., Sun, D.‐Y., & Zhou, R.‐G. (2003). Calmodulin is involved in heat shock signal transduction in wheat. Plant Physiology, 132(3), 1186–1195. 10.1104/pp.102.018564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, H.‐T., Li, G.‐L., Chang, H., Sun, D.‐Y., Zhou, R.‐G., & Li, B. (2007). Calmodulin‐binding protein phosphatase PP7 is involved in thermotolerance in Arabidopsis. Plant, Cell & Environment, 30(2), 156–164. 10.1111/j.1365-3040.2006.01613.x [DOI] [PubMed] [Google Scholar]
- Los, D. A., & Murata, N. (2004). Membrane fluidity and its roles in the perception of environmental signals. Biochimica et Biophysica Acta (BBA) – Biomembranes, 1666(1–2), 142–157. 10.1016/j.bbamem.2004.08.002 [DOI] [PubMed] [Google Scholar]
- Matsuda, O., Sakamoto, H., Hashimoto, T., & Iba, K. (2005). A temperature‐sensitive mechanism that regulates post‐translational stability of a plastidial ω‐3 fatty acid desaturase (FAD8) in Arabidopsis leaf tissues. Journal of Biological Chemistry, 280(5), 3597–3604. 10.1074/jbc.M407226200 [DOI] [PubMed] [Google Scholar]
- McLoughlin, F., Arisz, S. A., Dekker, H. L., Kramer, G., de Koster, C. G., Haring, M. A., … Testerink, C. (2013). Identification of novel candidate phosphatidic acid‐binding proteins involved in the salt‐stress response of Arabidopsis thaliana roots. Biochemical Journal, 450(3), 573–581. 10.1042/BJ20121639 [DOI] [PubMed] [Google Scholar]
- McLoughlin, F., Basha, E., Fowler, M. E., Kim, M., Bordowitz, J., Katiyar‐Agarwal, S., & Vierling, E. (2016). Class I and II small heat‐shock proteins protect protein translation factors during heat stress. Plant Physiology, 172, 1221–1236. 10.1104/pp.16.00536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLoughlin, F., Kim, M., Marshall, R. S., Vierstra, R. D., & Vierling, E. (2019). HSP101 interacts with the proteasome and promotes the clearance of ubiquitylated protein aggregates. Plant Physiology, 180(4), 1829–1847. 10.1104/pp.19.00263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merret, R., Carpentier, M.‐C., Favory, J.‐J., Picart, C., Descombin, J., Bousquet‐Antonelli, C., … Charng, Y. (2017). Heat shock protein HSP101 affects the release of ribosomal protein mRNAs for recovery after heat shock. Plant Physiology, 174(2), 1216–1225. 10.1104/pp.17.00269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishkind, M., Vermeer, J. E. M., Darwish, E., & Munnik, T. (2009). Heat stress activates phospholipase D and triggers PIP 2 accumulation at the plasma membrane and nucleus. The Plant Journal, 60(1), 10–21. 10.1111/j.1365-313X.2009.03933.x [DOI] [PubMed] [Google Scholar]
- Mori, K., Renhu, N., Naito, M., Nakamura, A., Shiba, H., Yamamoto, T., … Miura, K. (2018). Ca2+‐permeable mechanosensitive channels MCA1 and MCA2 mediate cold‐induced cytosolic Ca2+ increase and cold tolerance in Arabidopsis. Scientific Reports, 8, 550. 10.1038/s41598-017-17483-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munnik, T. (2014). PI‐PLC: Phosphoinositide‐phospholipase C in plant signaling. In Wang X. (Ed.), Phospholipases in plant signaling (Vol. 20, pp. 27–54). Berlin, Germany: Springer. 10.1007/978-3-642-42011-5_2 [DOI] [Google Scholar]
- Murakami, Y. (2000). Trienoic fatty acids and plant tolerance of high temperature. Science, 287(5452), 476–479. 10.1126/science.287.5452.476 [DOI] [PubMed] [Google Scholar]
- Navazio, L., Formentin, E., Cendron, L., & Szabò, I. (2020). Chloroplast calcium signaling in the spotlight. Frontiers in Plant Science, 11, 186. 10.3389/fpls.2020.00186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nawkar, G. M., Kang, C. H., Maibam, P., Park, J. H., Jung, Y. J., Chae, H. B., … Lee, S. Y. (2017). HY5, a positive regulator of light signaling, negatively controls the unfolded protein response in Arabidopsis . Proceedings of the National Academy of Sciences, 114(8), 2084–2089. 10.1073/pnas.1609844114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nickels, J. D., Smith, M. D., Alsop, R. J., Himbert, S., Yahya, A., Cordner, D., … Rheinstädter, M. C. (2019). Lipid rafts: Buffers of cell membrane physical properties. The Journal of Physical Chemistry B, 123(9), 2050–2056. 10.1021/acs.jpcb.8b12126 [DOI] [PubMed] [Google Scholar]
- Nieto, C., López‐Salmerón, V., Davière, J.‐M., & Prat, S. (2015). ELF3‐PIF4 interaction regulates plant growth independently of the evening complex. Current Biology, 25(2), 187–193. 10.1016/j.cub.2014.10.070 [DOI] [PubMed] [Google Scholar]
- Niu, Y., & Xiang, Y. (2018). An overview of biomembrane functions in plant responses to high‐temperature stress. Frontiers in Plant Science, 9, 915. 10.3389/fpls.2018.00915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomura, H., Komori, T., Uemura, S., Kanda, Y., Shimotani, K., Nakai, K., … Shiina, T. (2012). Chloroplast‐mediated activation of plant immune signalling in Arabidopsis. Nature Communications, 3, 926. 10.1038/ncomms1926 [DOI] [PubMed] [Google Scholar]
- Nusinow, D. A., Helfer, A., Hamilton, E. E., King, J. J., Imaizumi, T., Schultz, T. F., … Kay, S. A. (2011). The ELF4–ELF3–LUX complex links the circadian clock to diurnal control of hypocotyl growth. Nature, 475(7356), 398–402. 10.1038/nature10182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Quin, J. B., Bourassa, L., Zhang, D., Shockey, J. M., Gidda, S. K., Fosnot, S., … Dyer, J. M. (2010). Temperature‐sensitive post‐translational regulation of plant omega‐3 fatty‐acid desaturases is mediated by the endoplasmic reticulum‐associated degradation pathway. Journal of Biological Chemistry, 285(28), 21781–21796. 10.1074/jbc.M110.135236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osteryoung, K. W., & Vierling, E. (1994). Dynamics of small heat shock protein distribution within the chloroplasts of higher plants. The Journal of Biological Chemistry, 269(46), 28676–28682. [PubMed] [Google Scholar]
- Park, Y.‐J., Lee, H.‐J., Ha, J.‐H., Kim, J. Y., & Park, C.‐M. (2017). COP1 conveys warm temperature information to hypocotyl thermomorphogenesis. New Phytologist, 215(1), 269–280. 10.1111/nph.14581 [DOI] [PubMed] [Google Scholar]
- Park, Y.‐J., & Park, C.‐M. (2019). Physicochemical modeling of the phytochrome‐mediated photothermal sensing. Scientific Reports, 9, 10485. 10.1038/s41598-019-47019-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pokotylo, I., Pejchar, P., Potocký, M., Kocourková, D., Krčková, Z., Ruelland, E., … Martinec, J. (2013). The plant non‐specific phospholipase C gene family. Novel competitors in lipid signalling. Progress in Lipid Research, 52(1), 62–79. 10.1016/j.plipres.2012.09.001 [DOI] [PubMed] [Google Scholar]
- Prager‐Khoutorsky, M., Khoutorsky, A., & Bourque, C. W. (2014). Unique interweaved microtubule scaffold mediates osmosensory transduction via physical interaction with TRPV1. Neuron, 83(4), 866–878. 10.1016/j.neuron.2014.07.023 [DOI] [PubMed] [Google Scholar]
- Pudasaini, A., Shim, J. S., Song, Y. H., Shi, H., Kiba, T., Somers, D. E., … Zoltowski, B. D. (2017). Kinetics of the LOV domain of ZEITLUPE determine its circadian function in Arabidopsis. ELife, 6, e21646. 10.7554/eLife.21646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quint, M., Delker, C., Franklin, K. A., Wigge, P. A., Halliday, K. J., & van Zanten, M. (2016). Molecular and genetic control of plant thermomorphogenesis. Nature Plants, 2, 15190. 10.1038/nplants.2015.190 [DOI] [PubMed] [Google Scholar]
- Reddy, A. S. N., Day, I. S., Göhring, J., & Barta, A. (2012). Localization and dynamics of nuclear speckles in plants. Plant Physiology, 158(1), 67–77. 10.1104/pp.111.186700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riback, J. A., Katanski, C. D., Kear‐Scott, J. L., Pilipenko, E. V., Rojek, A. E., Sosnick, T. R., & Drummond, D. A. (2017). Stress‐triggered phase separation is an adaptive, evolutionarily tuned response. Cell, 168(6), 1028–1040.e19. 10.1016/j.cell.2017.02.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivasseau, C., Seemann, M., Boisson, A.‐M., Streb, P., Gout, E., Douce, R., … Bligny, R. (2009). Accumulation of 2‐C‐methyl‐d‐erythritol 2,4‐cyclodiphosphate in illuminated plant leaves at supraoptimal temperatures reveals a bottleneck of the prokaryotic methylerythritol 4‐phosphate pathway of isoprenoid biosynthesis. Plant, Cell & Environment, 32(1), 82–92. 10.1111/j.1365-3040.2008.01903.x [DOI] [PubMed] [Google Scholar]
- Ruiz‐Lopez, N., Pérez‐Sancho, J., del Valle, A. E., Haslam, R. P., Vanneste, S., Catalá, R., Perea‐Resa, C., Van Damme, D., García‐Hernández, S., Albert, A., Vallarino, J., Lin, J., Friml, J., Macho, A. P., Salinas, J., Rosado, A., Napier, J. A., Amorim‐Silva, V., & Botella, M. A. (2020). Synaptotagmins maintain diacylglycerol homeostasis at endoplasmic reticulum‐plasma membrane contact sites during abiotic stress. Molecular Biology 10.1101/2020.07.28.222919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rütgers, M., Muranaka, L. S., Mühlhaus, T., Sommer, F., Thoms, S., Schurig, J., … Schroda, M. (2017). Substrates of the chloroplast small heat shock proteins 22E/F point to thermolability as a regulative switch for heat acclimation in Chlamydomonas reinhardtii . Plant Molecular Biology, 95(6), 579–591. 10.1007/s11103-017-0672-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rütgers, M., Muranaka, L. S., Schulz‐Raffelt, M., Thoms, S., Schurig, J., Willmund, F., & Schroda, M. (2017). Not changes in membrane fluidity but proteotoxic stress triggers heat shock protein expression in Chlamydomonas reinhardtii . Plant, Cell & Environment, 40(12), 2987–3001. 10.1111/pce.13060 [DOI] [PubMed] [Google Scholar]
- Saenz, J. P., Sezgin, E., Schwille, P., & Simons, K. (2012). Functional convergence of hopanoids and sterols in membrane ordering. Proceedings of the National Academy of Sciences, 109(35), 14236–14240. 10.1073/pnas.1212141109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saidi, Y., Finka, A., Muriset, M., Bromberg, Z., Weiss, Y. G., Maathuis, F. J. M., & Goloubinoff, P. (2009). The heat shock response in moss plants is regulated by specific calcium‐permeable channels in the plasma membrane. The Plant Cell, 21(9), 2829–2843. 10.1105/tpc.108.065318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sangwan, V., Orvar, B. L., Beyerly, J., Hirt, H., & Dhindsa, R. S. (2002). Opposite changes in membrane fluidity mimic cold and heat stress activation of distinct plant MAP kinase pathways. The Plant Journal, 31(5), 629–638. 10.1046/j.1365-313X.2002.01384.x [DOI] [PubMed] [Google Scholar]
- Schaller, S., Latowski, D., Jemioła‐Rzemińska, M., Wilhelm, C., Strzałka, K., & Goss, R. (2010). The main thylakoid membrane lipid monogalactosyldiacylglycerol (MGDG) promotes the de‐epoxidation of violaxanthin associated with the light‐harvesting complex of photosystem II (LHCII). Biochimica et Biophysica Acta (BBA) – Bioenergetics, 1797(3), 414–424. 10.1016/j.bbabio.2009.12.011 [DOI] [PubMed] [Google Scholar]
- Shiva, S., Samarakoon, T., Lowe, K. A., Roach, C., Vu, H. S., Colter, M., … Welti, R. (2020). Leaf lipid alterations in response to heat stress of Arabidopsis thaliana . Plants, 9(7), 845. 10.3390/plants9070845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva, C. S., Nayak, A., Lai, X., Hutin, S., Hugouvieux, V., Jung, J.‐H., … Zubieta, C. (2020). Molecular mechanisms of evening complex activity in Arabidopsis . Proceedings of the National Academy of Sciences, 117(12), 6901–6909. 10.1073/pnas.1920972117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song, P., Jia, Q., Chen, L., Jin, X., Su, Y., Zhang, W., & Zhang, Q. (2020). Involvement of phospholipase D δ in regulation of ROS‐mediated microtubule organization and stomatal movement upon heat shock, 35. [DOI] [PubMed] [Google Scholar]
- Sun, A.‐Z., & Guo, F.‐Q. (2016). Chloroplast retrograde regulation of heat stress responses in plants. Frontiers in Plant Science, 7, 398. 10.3389/fpls.2016.00398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suri, S. S., & Dhindsa, R. S. (2007). A heat‐activated MAP kinase (HAMK) as a mediator of heat shock response in tobacco cells: HAMK in heat‐shock response. Plant, Cell & Environment, 31(2), 218–226. 10.1111/j.1365-3040.2007.01754.x [DOI] [PubMed] [Google Scholar]
- Suzuki, N., Koussevitzky, S., Mittler, R., & Miller, G. (2012). ROS and redox signalling in the response of plants to abiotic stress: ROS and redox signalling in plants. Plant, Cell & Environment, 35(2), 259–270. 10.1111/j.1365-3040.2011.02336.x [DOI] [PubMed] [Google Scholar]
- Sztacho, M., Sobol, M., Balaban, C., Escudeiro Lopes, S. E., & Hozák, P. (2019). Nuclear phosphoinositides and phase separation: Important players in nuclear compartmentalization. Advances in Biological Regulation, 71, 111–117. 10.1016/j.jbior.2018.09.009 [DOI] [PubMed] [Google Scholar]
- Tang, G.‐Q., Novitzky, W. P., Carol Griffin, H., Huber, S. C., & Dewey, R. E. (2005). Oleate desaturase enzymes of soybean: Evidence of regulation through differential stability and phosphorylation: Stability and phosphorylation of FAD2 enzymes. The Plant Journal, 44(3), 433–446. 10.1111/j.1365-313X.2005.02535.x [DOI] [PubMed] [Google Scholar]
- Teardo, E., Carraretto, L., Moscatiello, R., Cortese, E., Vicario, M., Festa, M., … Szabo, I. (2019). A chloroplast‐localized mitochondrial calcium uniporter transduces osmotic stress in Arabidopsis. Nature Plants, 5(6), 581–588. 10.1038/s41477-019-0434-8 [DOI] [PubMed] [Google Scholar]
- Theis, J., Gupta, T. K., Klingler, J., Wan, W., Albert, S., Keller, S., … Schroda, M. (2019). VIPP1 rods engulf membranes containing phosphatidylinositol phosphates. Scientific Reports, 9, 8725. 10.1038/s41598-019-44259-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas, L., Marondedze, C., Ederli, L., Pasqualini, S., & Gehring, C. (2013). Proteomic signatures implicate cAMP in light and temperature responses in Arabidopsis thaliana . Journal of Proteomics, 83, 47–59. 10.1016/j.jprot.2013.02.032 [DOI] [PubMed] [Google Scholar]
- Török, Z., Crul, T., Maresca, B., Schütz, G. J., Viana, F., Dindia, L., … Vigh, L. (2014). Plasma membranes as heat stress sensors: From lipid‐controlled molecular switches to therapeutic applications. Biochimica et Biophysica Acta (BBA) – Biomembranes, 1838(6), 1594–1618. 10.1016/j.bbamem.2013.12.015 [DOI] [PubMed] [Google Scholar]
- Tsvetkova, N. M., Horvath, I., Torok, Z., Wolkers, W. F., Balogi, Z., Shigapova, N., … Vigh, L. (2002). Small heat‐shock proteins regulate membrane lipid polymorphism. Proceedings of the National Academy of Sciences, 99(21), 13504–13509. 10.1073/pnas.192468399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vacca, R. A., de Pinto, M. C., Valenti, D., Passarella, S., Marra, E., & De Gara, L. (2004). Production of reactive oxygen species, alteration of cytosolic ascorbate peroxidase, and impairment of mitochondrial metabolism are early events in heat shock‐induced programmed cell death in tobacco bright‐yellow 2 cells. Plant Physiology, 134(3), 1100–1112. 10.1104/pp.103.035956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Woude, L. C., Perrella, G., Snoek, B. L., van Hoogdalem, M., Novák, O., van Verk, M. C., … van Zanten, M. (2019). HISTONE DEACETYLASE 9 stimulates auxin‐dependent thermomorphogenesis in Arabidopsis thaliana by mediating H2A.Z depletion. Proceedings of the National Academy of Sciences, 116(50), 25343–25354. 10.1073/pnas.1911694116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vierling, E. (1991). The roles of heat shock proteins in plants. Annual Review of Plant Physiology and Plant Molecular Biology, 42(1), 579–620. 10.1146/annurev.pp.42.060191.003051 [DOI] [Google Scholar]
- Voitsekhovskaja, O. V. (2019). Phytochromes and other (photo)receptors of information in plants. Russian Journal of Plant Physiology, 66(3), 351–364. 10.1134/S1021443719030154 [DOI] [Google Scholar]
- Volkov, R. A., Panchuk, I. I., Mullineaux, P. M., & Schöffl, F. (2006). Heat stress‐induced H2O2 is required for effective expression of heat shock genes in Arabidopsis. Plant Molecular Biology, 61(4–5), 733–746. 10.1007/s11103-006-0045-4 [DOI] [PubMed] [Google Scholar]
- Vu, L. D., Gevaert, K., & De Smet, I. (2019). Feeling the heat: Searching for plant thermosensors. Trends in Plant Science, 24(3), 210–219. 10.1016/j.tplants.2018.11.004 [DOI] [PubMed] [Google Scholar]
- Wahid, A., Gelani, S., Ashraf, M., & Foolad, M. (2007). Heat tolerance in plants: An overview. Environmental and Experimental Botany, 61(3), 199–223. 10.1016/j.envexpbot.2007.05.011 [DOI] [Google Scholar]
- Walley, J., Xiao, Y., Wang, J.‐Z., Baidoo, E. E., Keasling, J. D., Shen, Z., … Dehesh, K. (2015). Plastid‐produced interorgannellar stress signal MEcPP potentiates induction of the unfolded protein response in endoplasmic reticulum. Proceedings of the National Academy of Sciences, 112(19), 6212–6217. 10.1073/pnas.1504828112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, R., Zhang, Y., Kieffer, M., Yu, H., Kepinski, S., & Estelle, M. (2016). HSP90 regulates temperature‐dependent seedling growth in Arabidopsis by stabilizing the auxin co‐receptor F‐box protein TIR1. Nature Communications, 7, 10269. 10.1038/ncomms10269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, X., Ma, X., Wang, H., Li, B., Clark, G., Guo, Y., … Tang, W. (2015). Proteomic study of microsomal proteins reveals a key role for Arabidopsis annexin 1 in mediating heat stress‐induced increase in intracellular calcium levels. Molecular & Cellular Proteomics, 14(3), 686–694. 10.1074/mcp.M114.042697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf, S., Marani, A., & Rudich, J. (1991). Effect of temperature on carbohydrate metabolism in potato plants. Journal of Experimental Botany, 42(5), 619–625. 10.1093/jxb/42.5.619 [DOI] [Google Scholar]
- Wu, H.‐C., Bulgakov, V. P., & Jinn, T.‐L. (2018). Pectin methylesterases: Cell wall remodeling proteins are required for plant response to heat stress. Frontiers in Plant Science, 9, 1612. 10.3389/fpls.2018.01612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu, Q., Paulsen, A. Q., Guikema, J. A., & Paulsen, G. M. (1995). Functional and ultrastructural injury to photosynthesis in wheat by high temperature during maturation. Environmental and Experimental Botany, 35(1), 43–54. 10.1016/0098-8472(94)00030-9 [DOI] [Google Scholar]
- Yan, Q., Huang, Q., Chen, J., Li, J., Liu, Z., Yang, Y., … Wang, J. (2017). SYTA has positive effects on the heat resistance of Arabidopsis. Plant Growth Regulation, 81(3), 467–476. 10.1007/s10725-016-0224-5 [DOI] [Google Scholar]
- Yang, H., Zhao, Y., Chen, N., Liu, Y., Yang, S., Du, H., … Hu, X. (2020). A new adenylyl cyclase, putative disease resistance RPP13‐like protein 3, participates in abscisic acid‐mediated heat stress resistance in maize. Journal of Experimental Botany, eraa431. 10.1093/jxb/eraa431 [DOI] [PubMed] [Google Scholar]
- Zhang, L., Kondo, H., Kamikubo, H., Kataoka, M., & Sakamoto, W. (2016). VIPP1 has a disordered C‐terminal tail necessary for protecting photosynthetic membranes against stress. Plant Physiology, 171(3), 1983–1995. 10.1104/pp.16.00532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, N., Belsterling, B., Raszewski, J., & Tonsor, S. J. (2015). Natural populations of Arabidopsis thaliana differ in seedling responses to high temperature stress. AoB Plants, 7, plv101. 10.1093/aobpla/plv101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, Q., Song, P., Qu, Y., Wang, P., Jia, Q., Guo, L., … Zhang, W. (2017). Phospholipase Dδ negatively regulates plant thermotolerance by destabilizing cortical microtubules in Arabidopsis: PLD regulation of microtubules and heat shock. Plant, Cell & Environment, 40(10), 2220–2235. 10.1111/pce.13023 [DOI] [PubMed] [Google Scholar]
- Zheng, S.‐Z., Liu, Y.‐L., Li, B., Shang, Z.‐ l., Zhou, R.‐G., & Sun, D.‐Y. (2012). Phosphoinositide‐specific phospholipase C9 is involved in the thermotolerance of Arabidopsis: AtPLC9 plays a role in thermotolerance. The Plant Journal, 69(4), 689–700. 10.1111/j.1365-313X.2011.04823.x [DOI] [PubMed] [Google Scholar]
- Zheng, X., Krakowiak, J., Patel, N., Beyzavi, A., Ezike, J., Khalil, A. S., & Pincus, D. (2016). Dynamic control of Hsf1 during heat shock by a chaperone switch and phosphorylation. ELife, 5, e18638. 10.7554/eLife.18638 [DOI] [PMC free article] [PubMed] [Google Scholar]


