Abstract
Enzyme immunoassays (EIA) are commonly utilized for the evaluation of androgens in biological fluids; however, careful consideration must be given to cross-reactivity with other endogenous sex-steroid hormones. Our purpose was to determine the validity of a commonly-utilized commercially-available dihydrotestosterone (DHT) EIA. Serum samples obtained from older hypogonadal men who participated in a 12-month randomized controlled trial evaluating the effects of testosterone-enanthate (125 mg/week) or vehicle in combination with finasteride (5 mg/day) or placebo were assayed for DHT via EIA and using a validated gold-standard LC–MS/MS approach. Additionally, commercially-available (DHT-free) buffer containing graded testosterone doses was evaluated by DHT immunoassay. DHT concentrations measured via EIA were 79% to >1000% higher than values obtained by LC–MS/MS (p < 0.05), with the largest differences (415–1128%) occuring in groups receiving finasteride. Both LC–MS/MS and EIA indicated that testosterone-enanthate increased serum DHT to a similar magnitude. In contrast, finasteride-induced reductions in DHT were detected by LC–MS/MS, but not EIA (p < 0.05). No significant associations were present for DHT concentrations between measurement techniques. Cross-reactivity of testosterone with the immunoassay ranged from 18% to 99% and DHT concentrations measured by EIA were highly associated with the spiked testosterone concentrations in DHT-free buffer (r = 0.885, p < 0.001). In conclusion, we provide evidence invalidating a commonly-utilized commercially-available DHT immunoassay because significant cross-reactivity exists between testosterone and the EIA and because the changes in DHT observed via EIA were not associated with a validated gold-standard measurement technique. The cross-reactivity of testosterone is particularly concerning because testsoterone is present in 100-fold greater concentrations than is DHT within the circulation.
Keywords: Testosterone, Dihydrotestosterone, 5α-Reductase, Finasteride
1. Introduction
Testosterone is the primary circulating androgen in humans. Testosterone undergoes conversion to its more potent metabolite 5α-dihydrotestosterone (DHT) in tissues expressing any of the three known 5α-reductase isoenzymes [1]. Concentrations of testosterone in the circulation exceed those of DHT by nearly 100-fold [2], while intraprostatic concentrations of DHT are approximately double that of testosterone due to high 5α-reductase expression within the prostate [3]. DHT produces potent local androgenic effects due to its ability to bind to the androgen receptor (AR) with approximately three times the affinity of testosterone [4]. The myotrophic and bone protective effects of testosterone are primarily AR-mediated and do not require 5α-reduction to DHT [5,6]. Conversely, androgenic responses associated with testosterone (e.g., prostate enlargement and male pattern baldness) are primarily mediated by the localized tissue-specific 5α-reduction of testosterone to DHT [7,8].
Administration of exogenous testosterone increases circulating DHT in a dose-dependent manner [9], which underlies the prostate enlargement that accompanies testosterone administration [10]. Conversely, finasteride (an FDA approved type II 5α-reductase inhibitor) has been used clinically for over 15 years to treat benign prostate hyperplasia (BPH) [7] and male pattern baldness [8] because it reduces tissue [3] and circulating DHT concentrations by 50–70% [2]. Finasteride also ablates the increase in circulating DHT that results from exogenous testosterone administration, thus limiting prostate enlargement resulting from testosterone administration [10].
Analysis of circulating androgen concentrations are commonly performed using enzyme immunoassays (EIA), which function through specific antibody–antigen reactions [11]. EIAs are typically validated against one or more gold-standard techniques, such as radioimmunoassay (RIA) or a chromatography approach combined with mass spectrometry (MS). However, a common pitfall associated with EIAs is cross-reactivity between structurally similar molecules and the antibody used for detection. Cross-reactivity is of particular concern when those similar molecules are present in much higher abundance than the antigen of interest, as is the case with testosterone in relation to DHT [2]. The purpose of this study was to evaluate the validity of a commonly-utilized commercially-available DHT EIA that we suspected of exhibiting a high degree of cross-reactivity with testosterone.
2. Methods and materials
2.1. Study design
This study is a supplementary analysis of data resulting from a 12 month double-blind randomized controlled trial that was approved by the Institutional Review Board of the University of Florida. Only a brief description of the trial design is included herein because the primary findings from this study will be presented elsewhere. Participants provided written informed consent prior being randomized into four study groups: vehicle-placebo (n = 16), testosterone-enanthate (TE)-placebo (n = 14), vehicle-finasteride (n = 13), or TE-finasteride (n = 17) using a 2 × 2 factorial design. Treatment consisted of oral Proscar® (5 mg/day finasteride) or placebo and intramuscular injection of 125 mg/week Delatestryl® (TE) or vehicle. Proscar® and matching placebo were donated by Merck & Co. Delatesteryl was donated by Novartis and matching vehicle was prepared by WestLab Pharmacy, Gainesville, FL. Participants underwent health screenings and other testing at baseline and 3-month intervals throughout the study, during which blood was acquired. Eligibility criteria included men ≥60 years of age, with serum testosterone ≤300 ng/dL or a bioavailable testosterone ≤70 mg/dL. Participants were excluded if they had a history of active prostate cancer or breast cancer, severe benign prostatic hypertrophy, AUA/IPSS score ≥25, class 3 or 4 congestive heart failure, sleep apnea syndrome, hematocrit >49%, prostate-specific antigen (PSA) ≥ 2.6 ng/ml, body mass index (BMI) > 35, orthopedic limitations, or had received testosterone during the previous 4 weeks or finasteride/dutasteride during the previous 6 months.
2.2. Blood acquisition and hormone analysis
At each participant visit, blood was acquired twice between 08:00 AM and 10:00 AM and at least 30 min apart, as per guidelines from the Endocrine Society [12]. Blood was acquired from an antecubital vein, allowed to clot in serum separator tubes at room temperature, centrifuged at 3000g, and aliquoted for storage at −80 °C for future analysis, according to immunoassay manufacturer’s recommendations. Serum samples were analyzed in duplicate and on a single plate using a commercially available DHT EIA (ALPCO, Salem, NH, product #11-DHTU-E01) with a reported sensitivity of 6 pg/ml and an intra-assay covariance of 5.4%. The instructions provided by the manufacturer reported an 8.7% cross-reactivity for testosterone with this DHT immunoassay (using the Abraham method) [13].
The assay was performed in strict compliance with the manufacturer’s instructions. Samples that were grossly hemolyzed, lipemic, or icteric were not analyzed. A calibrator curve was established for every run (using standards provided by the manufacturer) and assay reliability was verified using low and high control buffers on every run (unless specified, all chemicals were provided by ALPCO Diagnostics, Salem, NH). The specific steps followed are listed below:
Immediately prior to beginning the assay, DHT-horseradish peroxidase conjugate concentrate was diluted 1:100 in assay buffer to produce conjugate working solution and wash buffer concentrate was diluted 1:10 in deionized water to produce wash buffer solution.
50 μl of calibrators (Calibrator A = 0 pg/ml, B = 25 pg/ml, C = 100 pg/ml, D = 500 pg/ml, E = 1000 pg/ml, F = 2500 pg/ml), controls (low and high), and samples were pipetted into wells coated in rabbit anti-DHT antibody, in duplicate.
100 μl of conjugate working solution was pipetted into each well using a multichannel pipette.
The 96 well plate was incubated on a plate shaker at 200 rpm at room temperature for 1 h.
300 μl of wash buffer solution was added to each well a total of three times using an automated plate washer and the plate was firmly tapped against an absorbent towel to ensure all fluid was removed.
150 μl of TMB (tetramethylbenzidine and hydrogen peroxide) substrate solution was pipetted into each well using a multichannel pipette.
The 96 well plate was incubated on a plate shaker at 200 rpm at room temperature until the well containing Calibrator A attained a dark blue color (~10–15 min).
50 μl stop solution (containing 1 M sulfuric acid) was pipetted into each well using a multichannel pipette.
The plate was read on a spectrophotometer at 450 nm immediately following addition of stop solution.
Additionally, six calibrators with known DHT concentrations (ranging from 0 pg/ml to 2500 pg/ml) were assayed three to five separate times on a single plate and two controls (low and high) with known DHT concentrations were assayed in duplicate on nine separate plates in order to establish intra- and inter-assay covariance values, which (in our laboratory) were 5.5% and 7.6%, respectively.
Following analysis by EIA, serum samples were analyzed for DHT by Laboratory Corp of America (Calabasas Hills, CA) using high pressure liquid chromatography with tandem mass spectroscopy detection after liquid–liquid extraction with deuterated DHT as an internal standard. DHT was extracted from serum with hexane: ethyl acetate and duplicate sets of serum calibrators were run with each batch. Samples were injected into the ARIA Transcend HPLC system and analytes eluted by means of a solvent gradient. An MDS-SCIEX AP15000 triple quadripole mass spectrometer, operating in electrospray ionization (ESI) mode was used for detection. The assay has an intra-assay coefficient of variance (CV) of 8.6% and an inter-assay CV of 9.9%.
2.3. Verification of finasteride and placebo
The presence of finasteride in study medication provided by Merck & Co. was verified in the University of Florida Forensic Toxicology Laboratory. Approximately 1 mg of the study medication was dissolved in 0.5 mL of methanol and a 2 μL aliquot was analyzed by gas chromatography–mass spectrometry utilizing an Agilent 6890 gas chromatograph interfaced with an Agilent 5973 mass selective detector operated in full scan mode (40–450 amu). A peak of MW 372 with a retention time of 12.94 min was detected in finasteride tablets provided to study participants and in purchased Proscar® tablets, but not in placebo tablets given to participants (Fig. 1).
Fig. 1.
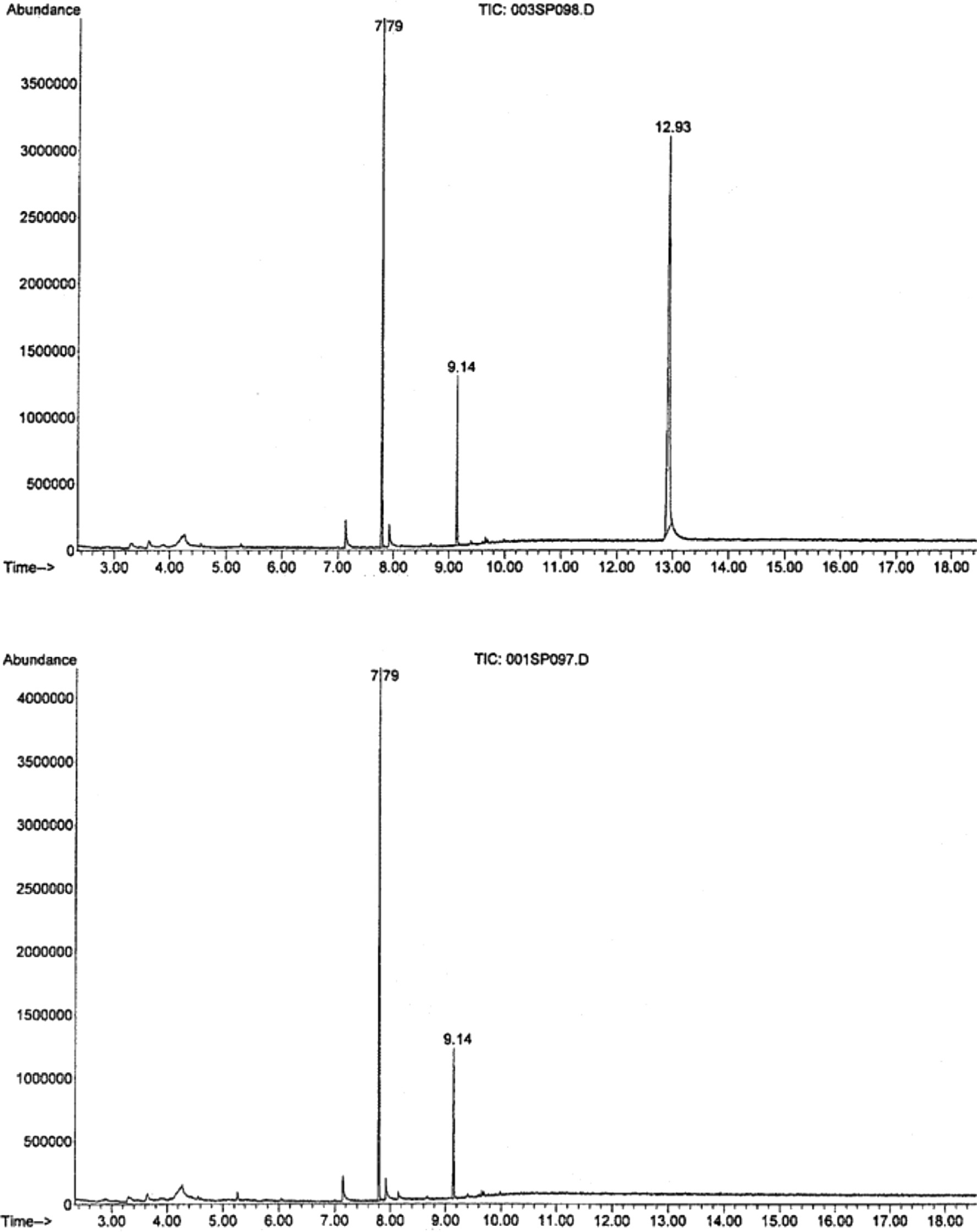
GC-MS verification for finasteride content of the study medication. Top: study medication demonstrating expected peak with amu of 372 and retention time of 12.94 min, indicating the presence of finasteride. Bottom panel: Finasteride is absent in placebo tablet.
2.4. Identification of interfering substance
In order to further investigate the possibility that testosterone might interfere with this commercially-available DHT immunoassay, we assayed several solutions containing graded doses of testosterone that ranged from subphysiologic to supraphysiologic concentrations. Specifically, the following solutions were analyzed in triplicate using the commercially-available DHT EIA described above: (1) buffer containing graded doses of testosterone from a commercially-available testosterone EIA (ALPCO, Salem, NH, product #11-TESHU-E01 Calibrators A–F) and (2) buffer that was stripped of sex-steroid hormones (ALPCO, Salem, NH, product #11-DHTU-E01 Calibrator A) spiked with and without graded concentrations of testosterone (Sigma–Aldrich, St. Louis, MO, product #T1500). Briefly, the testosterone spikes were prepared by combining powdered testosterone (Sigma–Aldrich, St. Louis, MO, product #T1500) 1:1 with 100% ethanol (to ensure solubility).
The testosterone/ethanol solution was serial diluted in assay buffer stripped of sex-steroid hormones (ALPCO, Salem, NH, product #11-DHTU-E01 Calibrator A) to produce solutions ranging from 1550 ng/dL to 11.5 ng/dL of testosterone. In addition, we assayed buffer that was absent of testosterone, but that contained known concentrations of DHT, in order to evaluate whether this immunoassay accurately detects DHT in the absence of interfering substances.
For all samples containing testosterone, the EIA indicated the presence of DHT in concentrations that greatly exceeded the expected concentrations (Tables 1 and 2), demonstrating significant cross-reactivity with testosterone. Cross-reactivity of testosterone was calculated with the following formula:
Table 1.
Percent cross-reactivity for commercially-available testosterone standards on DHT EIA. Cross-reactivity was determined by dividing the measured DHT concentration by the defined testosterone concentration. Values are reported as pg/ml, to convert to ng/dL divide value by 10. Values reported as >2500 were above highest standard and could not be determined.
| Testosterone concentration (pg/ml) | Normal adult male range | DHT concentration (pg/ml) | Measured DHT concentration (pg/ml) | Percent cross-reactivity (%) |
|---|---|---|---|---|
|
| ||||
| 16,700 | Supraphysiologic | 0 | >2500 | Cannot be determined |
| 5000 | Physiologic/eugonadal | 0 | 923 | 18% |
| 1670 | Hypogonadal | 0 | 397 | 24% |
| 420 | Subphysiologic | 0 | 188 | 45% |
| 80 | Subphysiologic | 0 | 79 | 99% |
| 0 | Subphysiologic | 0 | 44 | Cannot be determined |
| 0 | Subphysiologic | 45 | 49 | N/A |
| 0 | Subphysiologic | 320 | 301 | N/A |
Table 2.
Percent cross-reactivity for testosterone on commercially-available DHT EIA. Testosterone underwent serial dilution in Standard A Buffer that is free of sex-steroid hormones in order to produce testosterone standards ranging from supraphysiologic to subphysiologic concentrations. Cross-reactivity was determined by dividing the measured DHT concentration by the defined testosterone concentration. Values are reported as pg/ml, to convert to ng/dL divide value by 10. Values reported as >2500 were above highest standard and could not be determined.
| Testosterone concentration (pg/ml) | Normal adult male range | DHT concentration (pg/ml) | Measured DHT concentration (pg/ml) | Percent cross reactivity (%) |
|---|---|---|---|---|
|
| ||||
| 15,500 | Supraphysiologic | 0 | >2500 | Cannot be determined |
| 7750 | Physiologic/ eugonadal | 0 | >2500 | Cannot be determined |
| 3850 | Physiologic/ eugonadal | 0 | 2274 | 59% |
| 1900 | Hypogonadal | 0 | 1354 | 71% |
| 950 | Subphysiologic | 0 | 785 | 83% |
| 470 | Subphysiologic | 0 | 400 | 85% |
| 235 | Subphysiologic | 0 | 210 | 89% |
| 115 | Subphysiologic | 0 | 110 | 95% |
| 0 | N/A | 0 | 0 | 0 |
| 0 | N/A | 45 | 40 | N/A |
| 0 | N/A | 320 | 272 | N/A |
correct for potential type I error that can occur when performing multiple comparisons [14]. Data were analyzed with the SPSS v15.0.0 statistical software package.
Using this method, the percent cross-reactivity of testosterone ranged from 18% to 99% using standard commercially-available buffers containing known concentrations of testosterone (Table 1) and from 59% to 98% using buffer that was free of sex-steroid hormones and spiked with varying concentrations of testosterone (Table 2). DHT was very low or completely undetectable in buffer that did not contain testosterone. Cross-reactivity could not be determined for buffers containing supraphysiologic concentrations of testosterone because optical density values were higher than that of the highest standard provided in the EIA, which corresponded to concentrations exceeding >2500 pg/ml DHT. Interestingly, the immunoassay successfully determined DHT concentrations in control buffers that did not contain testosterone, with mean ± standard deviation for the low control equaling 109 ± 8% (range = 89–115%) and for the high control equaling 96 ± 7% (range = 85–108%) across all assays.
2.5. Statistics
The primary statistical focus of this study was to determine differences between assessment techniques (i.e., EIA vs. LC–MS/MS) in order to determine the validity of a commercially-available DHT immunoassay. As such, treatment effects and interactions were not analyzed. Results are reported as Means ± SEM, and p ≤ 0.05 was defined as the threshold of significance. Separate paired samples t-tests were utilized to evaluate differences between EIA and LC–MS/MS values and to evaluate the differences in the DHT percent change from baseline between measurement techniques. Pearson’s correlations were used to determine linear dependence between EIA and LC–MS/MS values for the entire cohort of participants and for the individual groups at each time point and between the known testosterone-spiked concentrations in DHT-free buffer and the DHT concentrations measured by EIA. For all analyses, the Holm–Bonferroni correction was utilized to correct for potential type I error that can occur when performing multiple comparisons [14]. Data were analyzed with the SPSS v15.0.0 statistical software package.
3. Results
3.1. DHT concentrations
A comparison of the results from the commercially-available EIA and LC–MS/MS techniques are reported in Table 3. For all conditions and at every time point, EIA analysis resulted in DHT concentrations that were 79–1128% higher than LC–MS/MS measurements (p < 0.05), with the highest variance appearing in groups receiving finasteride. The percent change in DHT from baseline was not different between EIA and LC–MS/MS at any time point when the entire cohort of individuals was analyzed. However, when the individual groups were analyzed, the percent change from baseline for the TE-finasteride group ranged from an increase of 38% to 129% using EIA (Fig. 2a) to a reduction of −27% to −38% using LC–MS/MS (p < 0.05) (Fig. 2b). The change in DHT from baseline for the vehicle-finasteride group also ranged from −11% to 7% using EIA and from −44% to −78% using LC-MS/MS (p < 0.05). The DHT percent change from baseline was not different between measurement techniques for the TE-placebo or vehicle-placebo groups.
Table 3.
Concentrations of serum DHT evaluated by EIA and LC-MS/MS techniques.
| Group | Evaluation method | Baseline DHT (pg/ml) | 3 month DHT (pg/ml) | 6 month DHT (pg/ml) | 9 month DHT (pg/ml) | 12 month DHT (pg/ml) |
|---|---|---|---|---|---|---|
|
| ||||||
| TE-placebo | EIA | 577 ± 109** | 1065 ± 91** | 947±110** | 762 ± 72** | 932 ± 294* |
| LC-MS/MS | 211 ± 19** | 538 ± 123** | 365 ± 60** | 305 ± 57** | 317± 66* | |
| % Difference | 173% | 98% | 159% | 150% | 194% | |
| TE-finasteride | EIA | 638 ± 126** | 952 ± 105** | 1015 ± 155** | 925 ± 170** | 747 ± 241* |
| LC-MS/MS | 219 ± 55** | 120 ± 31** | 127±26** | 90 ± 18** | 145 ± 48* | |
| % Difference | 191% | 693% | 699% | 928% | 415% | |
| Vehicle-placebo | EIA | 450 ± 59** | 461 ± 39** | 467 ± 51** | 406 ± 74** | 468 ± 51** |
| LC-MS/MS | 252 ± 38** | 243 ± 32** | 227 ± 28** | 209 ± 38** | 196±24** | |
| % Difference | 79% | 90% | 106% | 94% | 139% | |
| Vehicle-finasteride | EIA | 491 ± 85** | 528 ± 96** | 450 ± 108** | 486 ± 99** | 446 ± 153* |
| LC-MS/MS | 189±35** | 43 ± 9** | 90 ± 50** | 66 ± 17** | 42 ± 8* | |
| % Difference | 160% | 1128% | 400% | 636% | 962% | |
Percent difference was determined by dividing EIA value by LC-MS/MS value. Indicates difference between measurement techniques at
p < 0.05 or
p < 0.01.
Values represent mean ± SEM in pg/ml of n = 6–17/time point. To convert pg/ml to ng/dL divide value by 10.
Fig. 2.
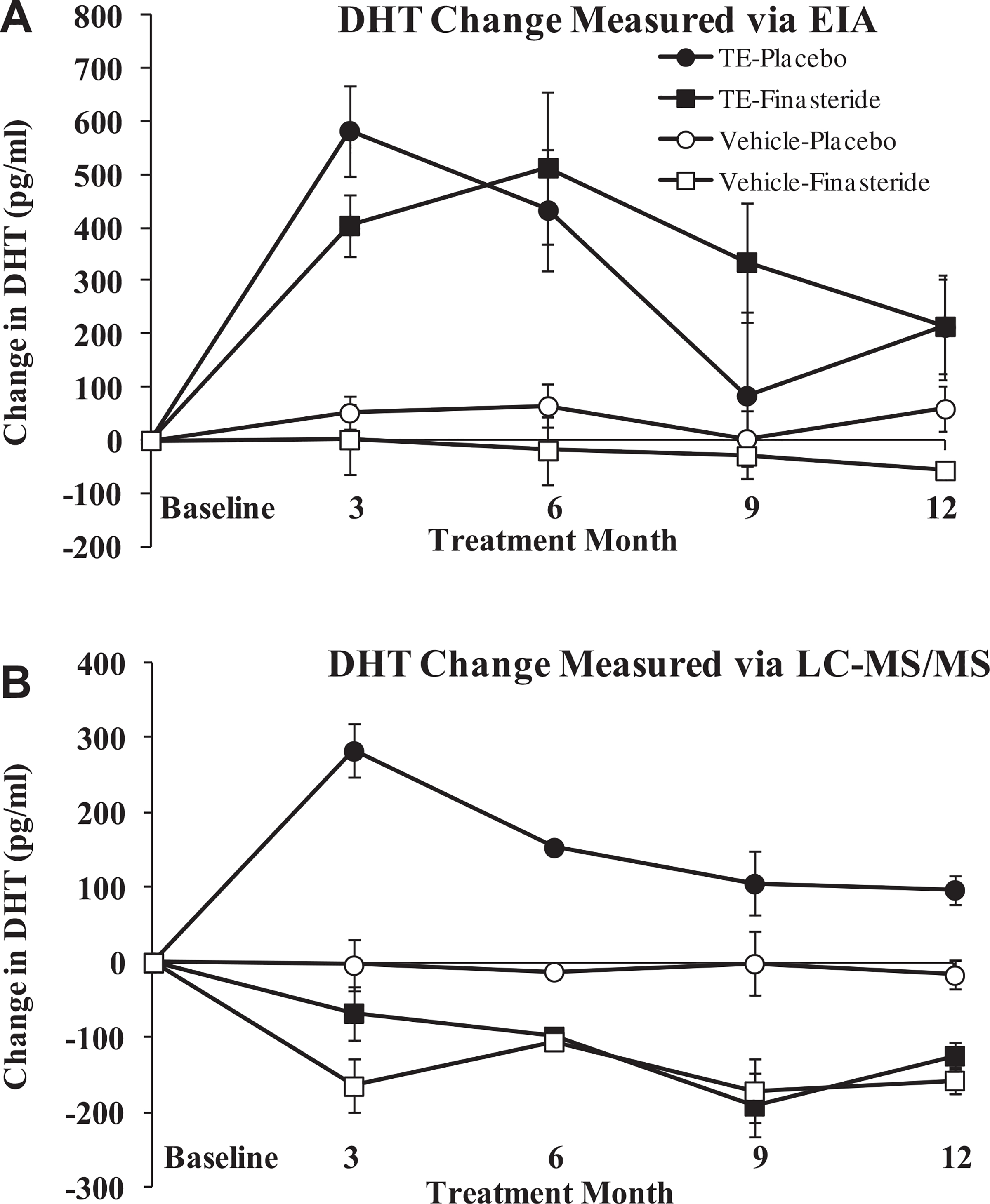
Change in DHT measured via commercially-available EIA (A) and via LC–MS/MS (B). Note the differences in magnitude between measurement techniques and that the change in DHT for groups receiving finasteride were directionally different between measurement techniques. Values are reported as means ± SE. To convert pg/ml to ng/dL divide value by 10.
3.2. Relationship between EIA and LC–MS/MS DHT concentrations and between testosterone-spiked values and DHT measured via EIA
No significant relationship was observed between DHT values evaluated via EIA and LC–MS/MS at baseline or at any time point when the entire cohort of individuals was analyzed or when groups were analyzed separately. Conversely, the spiked testosterone concentration in DHT-free buffer was highly and positively correlated with the DHT value measured by EIA (r = 0.845, p < 0.001, Fig. 3).
Fig. 3.
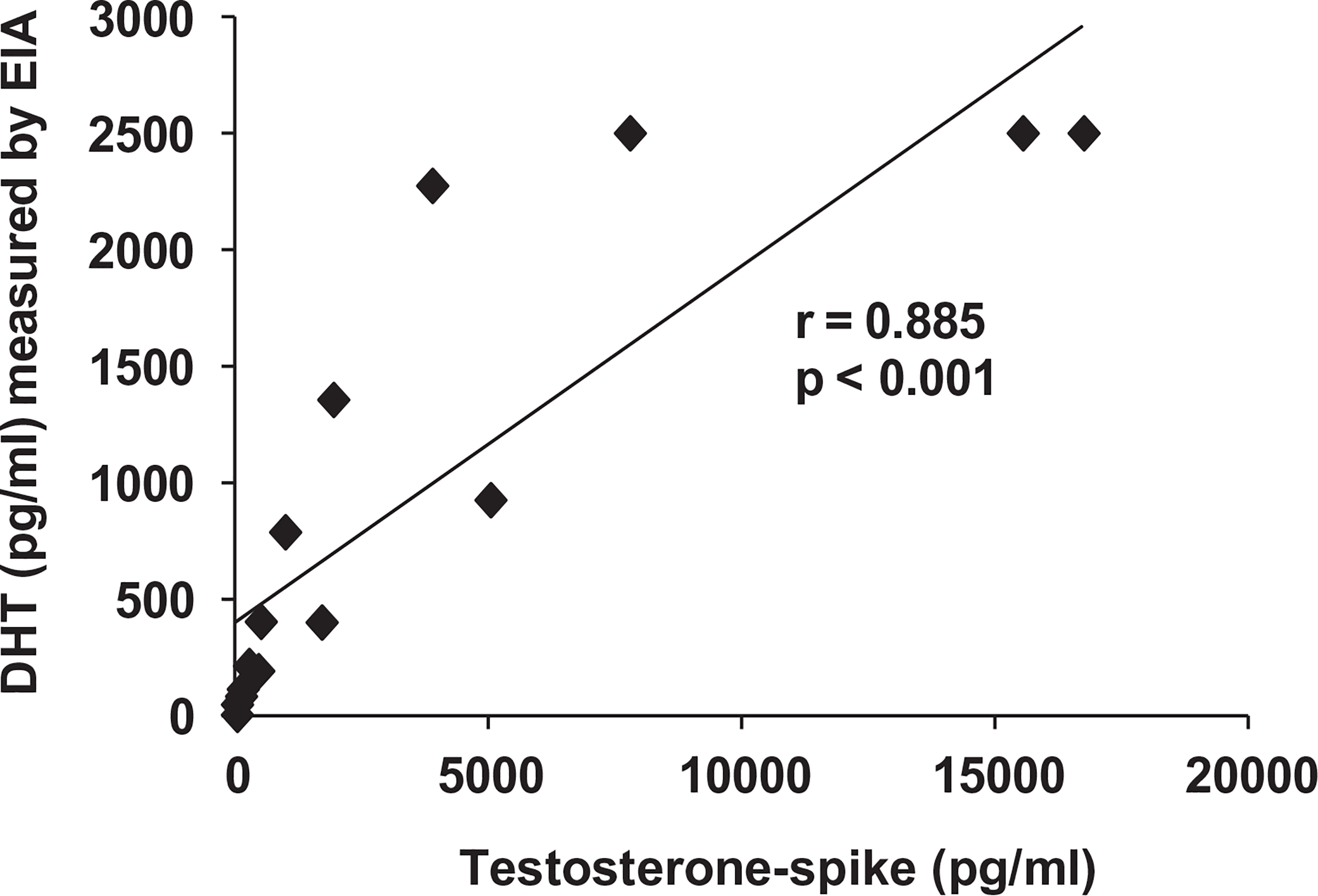
Association between spiked testosterone concentration in DHT-free buffer and DHT concentration measured via commercially-available EIA. DHT = dihydrotestosterone. Values represent individual samples, n = 15.
4. Discussion
One commonly utilized method to analyze circulating DHT concentrations is the EIA; however, care must be taken to ensure valid measurements are obtained with this technique because androgens of similar structure may produce cross-reactivity. Herein, we report that testosterone has significant cross-reactivity with a commonly-utilized and commercially-available DHT immunoassay ranging from 18% to 99% within physiologic to subphysiologic testosterone ranges. This cross-reactivity greatly exceeds that reported by the manufacturer (i.e., 8.7%) and is especially concerning because testosterone is present within the circulation in 100-fold greater concentrations than DHT [2]. As such, the specific immunoassay analyzed in this study appears invalid for the measurement of circulating DHT within biologic samples that contain physiologic or even subphysiologic testosterone concentrations.
When evaluating the validity of this commercially-available DHT EIA, several aberrant findings were noted. In particular, the results of this immunoassay appeared to indicate that finasteride did not reduce circulating DHT in men receiving vehicle injections and that finasteride did not blunt the TE-induced elevation in circulating DHT. These findings are in direct conflict with the well-established ability of finasteride to reduce circulating DHT by 50–70% in older men [2] and inconsistent with previous reports indicating that finasteride administration completely blunts the elevation in circulating DHT associated with testosterone administration [10]. Additionally, the apparent inability of finasteride to reduce DHT (as assessed by EIA) is inconsistent with several outcomes-based variables from our clinical trial that are directly influenced by alterations in DHT, providing further evidence that the EIA is an invalid means of assessing DHT. For example, finasteride completely prevented the increase in prostate volume and elevation in PSA induced by TE administration, without altering the beneficial musculoskeletal or lipolytic effects of TE (unpublished laboratory data).
In order to further examine our preliminary EIA findings we analyzed a sample of the finasteride and placebo tablets provided for the clinical trial via GC-MS and verified that the drug/placebo were provided as indicated. Subsequently, we determined cross-reactivity of testosterone with the DHT EIA by assaying buffer containing known concentrations of testosterone and buffer free of sex-steroid hormones that was spiked with testosterone ranging from subphysiologic to supraphysiologic concentrations. In this regard, the normal physiologic range of testosterone is 270–1070 ng/dL (9–38 nmol/L) for adult men, 15–70 ng/dL (0.52–2.4 nmol/L) for adult women, and 2–20 ng/dL (0.07–0.7 nmol/L) for children. In the above ranges, we observed a significant degree of cross-reactivity for testosterone (ranging from 18% to 99%) that greatly exceeded the value (8.7%) reported by the manufacturer of this assay. Of particular concern was that the cross-reactivity of testosterone appeared to increase towards the low physiologic/hypogonadal and subphysiologic ranges for adult males, which encompasses the range of testosterone concentrations for participants in most clinical trials evaluating the safety and efficacy of testosterone-replacement-therapy. Importantly, the developer of this DHT assay reported that the classic Abraham method [13] was used to evaluate cross-reactivity with testosterone, which is a different than the method we utilized. Specifically, the Abraham method is based on the following:
The Abraham method may be a valid assessment technique for cross-reactivity when the concentration of the antigen exceeds that of the competitor or when antigen/competitor concentrations are nearly equal in concentration. However, this method of assessment dramatically underestimates actual cross-reactivity when the competitor concentration drastically exceeds that of the antigen, as is the case with testosterone which is ubiquitously present in 100-fold higher concentrations than DHT within mammalian biologic fluids. Additionally, concern was raised because DHT concentrations could not be determined for samples that contained testosterone in the high-normal range for adult males (i.e., 775–1100 ng/ml) because the optical density values appeared higher than the highest standard contained in the commercially-available immunoassay (i.e., >2500 pg/ml). These results indicate that testosterone produces significant interfering cross-reactivity on this commercially-available immunoassay across a spectrum of concentrations that encompasses the entire physiologic ranges for adult men, adult women, and children.
In order to directly verify the DHT concentrations contained in our serum samples a validated GC-MS “gold-standard” protocol was subsequently utilized. GC-MS findings directly contradicted what was observed via EIA in several ways. First, DHT concentrations were significantly (79% to >1000%) higher for all groups when measured by EIA, with the largest variance (415–1128% difference) appearing in groups receiving finasteride. Second, GC-MS indicated that finasteride reduced serum DHT by 44–78% in men not receiving testosterone which is similar to the percent reduction reported by others [2]; while the EIA indicated no reduction. Third, GC-MS indicated that finasteride administration completely blocked the elevation in DHT that occurs following TE administration, as others have reported [10]; while the EIA indicated that finasteride did not produce a reduction. Interestingly, when the entire cohort of participants was evaluated, the percent change in DHT from baseline was similar between EIA and LC–MS/MS; however, differences appeared between techniques for the individual groups that were administered finasteride. Specifically, the commercially-available immunoassay was unable to determine reductions in DHT that occurred as a result of finasteride. In contrast to the above data, the immunoassay successfully detected DHT in expected concentrations when assayed in buffer that contained no testosterone, which indicates that the antibody utilized adequately binds DHT in the absence of interfering substances. Despite the ability of the antibody to bind DHT, the validity of this assay remains highly questionable because testosterone is ubiquitous throughout human biological fluids.
In conclusion, we have presented evidence that raises strong concern regarding the validity of a commonly-utilized commercially-available EIA used to evaluate DHT in biological fluids. In particular, strong cross-reactivity is present between testosterone and the DHT immunoassay which is of particular concern given the high abundance of testosterone within the circulation and most other biological fluids in relation to DHT. We recommend that individuals interested in evaluating DHT concentrations within the circulation or within other biologic fluids of men and women utilize a validated gold-standard approach, such as LC–MS/MS in order to avoid the potential for cross-reactivity with more abundant structurally similar androgens.
Acknowledgements
This study was supported by a Department of Veterans Affairs Merit Award to SEB and CDA-2 (#B7733-W) to JFY. The opinions expressed in this paper are those of the authors and do not necessarily represent those of Merck & Co.
Sources of support
This work was supported by a Veterans Department Merit Award to SEB and CDA-2 (#B7733-W) to JFY.
References
- [1].Yarrow JF, McCoy SC, Borst SE. Intracrine and myotrophic roles of 5alpha-reductase and androgens: a review. Med Sci Sports Exerc 2012;44:818–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Amory JK, Wang C, Swerdloff RS, Anawalt BD, Matsumoto AM, Bremner WJ, et al. The effect of 5alpha-reductase inhibition with dutasteride and finasteride on semen parameters and serum hormones in healthy men. J Clin Endocrinol Metab 2007;92:1659–65. [DOI] [PubMed] [Google Scholar]
- [3].Marks LS, Hess DL, Dorey FJ, Luz Macairan M, Cruz Santos PB, Tyler VE. Tissue effects of saw palmetto and finasteride: use of biopsy cores for in situ quantification of prostatic androgens. Urology 2001;57:999–1005. [DOI] [PubMed] [Google Scholar]
- [4].Bauer ER, Daxenberger A, Petri T, Sauerwein H, Meyer HH. Characterisation of the affinity of different anabolics and synthetic hormones to the human androgen receptor, human sex hormone binding globulin and to the bovine progestin receptor. APMIS 2000;108:838–46. [DOI] [PubMed] [Google Scholar]
- [5].Borst SE, Conover CF, Carter CS, Gregory CM, Marzetti E, Leeuwenburgh C, et al. Anabolic effects of testosterone are preserved during inhibition of 5alpha-reductase. Am J Physiol Endocrinol Metab 2007;293:E507–14. [DOI] [PubMed] [Google Scholar]
- [6].Borst SE, Lee JH, Conover CF. Inhibition of 5alpha-reductase blocks prostate effects of testosterone without blocking anabolic effects. Am J Physiol Endocrinol Metab 2005;288:E222–7. [DOI] [PubMed] [Google Scholar]
- [7].Marks LS. 5alpha-Reductase: history and clinical importance. Rev Urol 2004;6(Suppl 9):S11–21. [PMC free article] [PubMed] [Google Scholar]
- [8].Shapiro J, Kaufman KD. Use of finasteride in the treatment of men with androgenetic alopecia (male pattern hair loss). J Investig Dermatol Symp Proc 2003;8:20–3. [DOI] [PubMed] [Google Scholar]
- [9].Lakshman KM, Kaplan B, Travison TG, Basaria S, Knapp PE, Singh AB, et al. The effects of injected testosterone dose and age on the conversion of testosterone to estradiol and dihydrotestosterone in young and older men. J Clin Endocrinol Metab 2010;95:3955–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Amory JK, Watts NB, Easley KA, Sutton PR, Anawalt BD, Matsumoto AM, et al. Exogenous testosterone or testosterone with finasteride increases bone mineral density in older men with low serum testosterone. J Clin Endocrinol Metab 2004;89:503–10. [DOI] [PubMed] [Google Scholar]
- [11].Christenson RH, Duh SH. Methodological and analytic considerations for blood biomarkers. Prog Cardiovasc Dis 2012;55:25–33. [DOI] [PubMed] [Google Scholar]
- [12].Bhasin S, Cunningham GR, Hayes FJ, Matsumoto AM, Snyder PJ, Swerdloff RS, et al. Testosterone therapy in men with androgen deficiency syndromes: an endocrine society clinical practice guideline. J Clin Endocrinol Metab 2010;95:2536–59. [DOI] [PubMed] [Google Scholar]
- [13].Abraham GE. Solid-phase radioimmunoassay of estradiol-17 beta. J Clin Endocrinol Metab 1969;29:866–70. [DOI] [PubMed] [Google Scholar]
- [14].Holm S. A simple sequentially rejective multiple test procedure. Scand J Stat 1979;6:65–70. [Google Scholar]


