Abstract
ARGONAUTE (AGO) proteins play crucial roles in plant defence against virus invasion. To date, the role of OsAGO2 in rice antiviral defence remains largely unknown. In this study, we determined that the expression of OsAGO2 in rice was induced upon rice black‐streaked dwarf virus (RBSDV) infection. Using transgenic rice plants overexpressing OsAGO2 and Osago2 mutants generated through transposon‐insertion or CRISPR/Cas9 technology, we found that overexpression of OsAGO2 enhanced rice susceptibility to RBSDV infection. Osago2 mutant lines exhibited strong resistance to RBSDV infection through the elicitation of an early defence response, including reprogramming defence gene expression and production of reactive oxygen species (ROS). Compared to Nipponbare control, the expression level of OsHXK1 (HEXOKINASE 1) increased significantly, and the methylation levels of its promoter decreased in the Osago2 mutant on RBSDV infection. The expression profile of OsHXK1 was the opposite to that of OsAGO2 during RBSDV infection. Overexpression of OsHXK1 in rice also induced ROS production and enhanced rice resistance to RBSDV infection. These results indicate that OsHXK1 controls ROS accumulation and is regulated by OsAGO2 through epigenetic regulation. It is noteworthy that the Osago2 mutant plants are also resistant to southern rice black‐streaked dwarf virus infection, another member of the genus Fijivirus. Based on the results presented in this paper, we conclude that OsAGO2 modulates rice susceptibility to fijivirus infection by suppressing OsHXK1 expression, leading to the onset of ROS‐mediated resistance. This discovery may benefit future rice breeding programmes for virus resistance.
Keywords: epigenetics, OsAGO2, OsHXK1, rice, Rice black‐streaked dwarf virus, Southern rice black‐streaked dwarf virus
Expression of OsAGO2 is induced on rice black‐streaked dwarf virus (RBSDV) infection, which represses OsHXK1 expression via DNA methylation and controls reactive oxygen species‐mediated resistance in an inactivated mode to facilitate RBSDV infection.

1. INTRODUCTION
Rice (Oryza sativa) is one of the most important staple foods for more than half of the world's population (Chen & Ronald, 2011). Rice black‐streaked dwarf virus is a member of the genus Fijivirus, family Reoviridae, and is the causal agent of rice black‐streaked dwarf disease (RBSDD). Rice black‐streaked dwarf virus (RBSDV) is a double‐stranded RNA (dsRNA) virus that is transmitted by small brown planthoppers (SBPH, Laodelphax striatellus) in a persistent manner (Shikata & Kitagawa, 1977). RBSDV often causes severe damage to rice production, leading to approximately 50% yield losses (Zhou et al., 2017). In addition to rice, RBSDV can also infect wheat, maize, barley, and other cereal crops. In recent years, RBSDV has become a severe cereal viral disease in Asia, Europe, and South America. Southern rice black‐streaked dwarf virus, another member of the genus Fijivirus, was first identified in China in 2001 (Zhou et al., 2013). Southern rice black‐streaked dwarf virus (SRBSDV) can cause similar disease symptoms in rice to those of RBSDV, and it is more common in Asian countries. Occasionally, these two fijiviruses have been reported to cause complete loss of rice production in some areas. Consequently, elucidation of the mechanisms controlling rice resistance to these two viruses has great scientific and economic significance.
Under optimal growth conditions, the production of cellular reactive oxygen species (ROS) is low. Under abiotic or biotic stress (e.g., drought, salinity, air pollutants, and pathogen attack), the production of ROS is quickly induced (Padh, 1990), leading to severe cellular damage or cell death. It has been reported that the production and detoxification of ROS are controlled by plant protective mechanisms (Munne‐Bosch et al., 2013). Numerous studies have shown that the ROS burst caused by abiotic or biotic stress has a protective role. For example, ROS can function as secondary messengers during the signal transduction that regulates host defence against pathogens or programmed cell death (Mittler et al., 2011). Owing to plant–pathogen coevolution, pathogen‐associated molecular pattern (PAMP)‐triggered immunity (PTI) is established, inducing a ROS burst around the infection site to prevent pathogen invasion (Boller & He, 2009).
Accumulating evidence has shown that ARGONAUTE (AGO) proteins are involved in antiviral defence responses in plants. Many reports have shown that the expression of AtAGO1 is upregulated during virus infections, and virus‐derived small interfering (si)RNAs can interact with AGO1 to posttranscriptionally promote viral RNA degradation (Carbonell & Carrington, 2015; Guo et al., 2019; Yang & Li, 2018). Wu and colleagues recently reported that OsAGO18 is a core RNA silencing component that can promote rice antiviral defence by sequestering miR168 and miR528 (Wu et al., 2015, 2017). In 2020, Yang et al. showed that rice stripe virus coat protein (CP) can trigger jasmonic acid (JA)‐OsAGO18‐mediated antiviral defence (Yang et al., 2020). Atago2 mutant plants have been shown to be more susceptible to potato virus X infection than wildtype Arabidopsis (Brosseau & Moffett, 2015; Jaubert et al., 2011). To date, the function of OsAGO2 in plant antiviral defence has not been reported.
Epigenetic mechanisms have been shown to modulate gene expression through DNA or histone modifications or via alterations of noncoding RNA expression (Kota & Feil, 2010). Proteins of the AGO family are major players in the epigenetic regulation of gene expression (Hutvagner & Simard, 2008; Song et al., 2004). A recent report by Zheng et al. indicated that OsAGO2 can negatively regulate ROS production and the time of tapetal programmed cell death (PCD) through epigenetic regulation of OsHXK1 expression (Zheng et al., 2019). Other authors showed that OsAGO2 can positively regulate rice responses to salt and abscisic acid (ABA) stress through the activation of BIG GRAIN3 (BG3) expression via epigenetic modification (Yin et al., 2020). Although OsAGO2 plays an important role in rice gene silencing via DNA methylation, whether it participates in rice antiviral defence is largely unknown.
In this study, we provide evidence that OsAGO2 is a negative regulator of rice defence against RBSDV infection through the epigenetic regulation of OsHXK1 expression. Our results show that the expression of OsAGO2 is highly induced in rice on RBSDV infection. Osago2 mutant plants are more resistant to RBSDV than the parental Nipponbare (NPB) plants because of the activation of an early defence response, upregulation of defence‐related gene expression, and the ROS burst in rice cells. OsHXK1 overexpression lines showed an increase in ROS production, leading to resistance to RBSDV infection, whereas OsAGO2 can repress OsHXK1 expression through methylation of its promoter region during RBSDV infection. It is noteworthy that Osago2 mutant lines are also resistant to SRBSDV infection. We speculate that the suppression of OsHXK1 expression by OsAGO2 is an evolutionarily conserved mechanism in favour of infection with the dsRNA virus of Fijivirus.
2. RESULTS
2.1. Overexpression of OsAGO2 enhances rice sensitivity to RBSDV infection
The rice genome is predicted to encode 19 AGO proteins, and the functions of several rice AGO proteins have now been characterized. To further investigate the function of rice AGO2 protein in RBSDV infection, we first compared the expression pattern of OsAGO2 gene in RBSDV‐infected NPB plants with that of noninfected (mock‐inoculated) NPB plants using quantitative reverse transcription PCR (RT‐qPCR). The results showed that the expression of OsAGO2 in the RBSDV‐infected plants was significantly upregulated immediately after RBSDV inoculation (0 days postinoculation [dpi]), and its expression level remained high at 8 and 19 dpi (Figure 1a), suggesting that OsAGO2 may play a role in the rice response to RBSDV infection.
FIGURE 1.

Overexpression of OsAGO2 enhances rice susceptibility to RBSDV infection. (a) Quantitative reverse transcription PCR (RT‐qPCR) analyses of OsAGO2 expression in the mock‐inoculated (Mock) and RBSDV‐inoculated (RBSDV) Nipponbare (NPB) rice plants using total RNA isolated from various assayed plants at 0, 8, and 19 days postinoculation (dpi). Mock: plants were inoculated with RBSDV‐free small brown planthoppers. (b) The effect of OsAGO2 on RBSDV infection was determined through inoculation of NPB, Osago2, Crispr‐Osago2 (lines #6 and #13), and OE‐OsAGO2 (lines #1 and #2) plants. The RBSDV‐inoculated NPB, Osago2, Crispr‐Osago2, and OE‐OsAGO2 plants were photographed at 30 dpi. Scale bar, 4 cm. (c) Incidence of RBSDV disease in different assayed plants at 30 dpi. The numbers of infected plants of each treatment were determined through RT‐PCR using RBSDV coat protein (CP) gene‐specific primers at 30 dpi. (d) RT‐qPCR analysis of RBSDV RNA accumulation in various assayed plants at 19 dpi. The expression of rice 18S‐rRNA was used as an internal control. RBSDV incidence and RBSDV RNA accumulation in NPB was used as a control to compare with that in other lines. Error bars indicate the standard deviation (SD) of individual treatments from three biological replicates per treatment. (e) Accumulation of RBSDV CP in different samples was determined through western blot assay using a RBSDV CP‐specific antibody at 0 and 19 dpi. Ponceau‐stained RuBisCO large subunit is used to show sample loadings. Statistical significance of difference between the mock‐inoculated and RBSDV‐inoculated NPB plants or among the NPB and other assayed lines was determined using Student's t test. **p < 0.01
To validate this hypothesis, we generated two stable transgenic lines overexpressing OsAGO2 with N‐FLAG in NPB calli (referred to as OE‐OsAGO2#1 and OE‐OsAGO2#2) (Figure S1a). We also obtained an OsAGO2 transposon‐insertion mutant (Osago2, NG8158) with NPB background from the rice Tos17 insertion mutant database (https://tos.nias.affrc.go.jp/). In addition, we constructed two CRISPR/Cas9 vectors targeting the OsAGO2 gene and generated two CRISPR/Cas9 knockout lines in NPB calli (referred to as Crispr‐Osago2#6 and Crispr‐Osago2#13) (Figure S1d,e,h). The expression of OsAGO2 RNA transcripts and proteins in these lines was determined by RT‐qPCR and western blot assays, respectively (Figure S1b,c). Compared with the NPB wildtype control, expression of OsAGO2 RNA transcript was significantly repressed in the T2 generation of the Osago2 mutant (Figure S1b). We also verified mutations in the Crispr‐Osago2 homozygous lines (#6 and #13) through DNA sequencing (Figure S1f,i). The deletion and insertion of one base resulted in premature termination of OsAGO2 translation (Figure S1g,j). Because OsAGO2 shares high sequence similarity with OsAGO3 (Figure S2a), we also examined the expression levels of OsAGO3 in Osago2, Crispr‐Osago2#6, and Crispr‐Osago2#13 plants by RT‐qPCR. OsAGO3 expression was not altered in the Osago2 transposon‐insertion or CRISPR/Cas9 mutants (Figure S2b). DNA sequencing revealed that the OsAGO3 sequence was not altered in the Crispr‐Osago2 mutants (Figure S2c). Therefore, the Osago2 transposon‐insertion and CRISPR/Cas9‐knockout mutants showed considerable suppression of OsAGO2, whereas no obvious effects were verified on its highly similar homolog, OsAGO3.
RBSDV disease symptom observations showed that, at 30 dpi, the RBSDV disease incidence of Osago2 and Crispr‐Osago2 mutant lines was significantly lower than that of the parental NPB plants. In contrast, the incidence of RBSDV disease in OE‐OsAGO2#1 and OE‐OsAGO2#2 lines was significantly higher than that in the NPB plants (Figure 1b,c), confirming that OsAGO2 plays an important role during RBSDV infection in rice.
To determine the effect of OsAGO2 on RBSDV replication, we analysed the accumulation of RBSDV RNA through RT‐qPCR using RBSDV coat protein (CP) gene‐specific primers and CP through western blot assay. The results showed that, compared with NPB plants, the accumulation levels of RBSDV RNA in the Osago2, Crispr‐Osago2#6, and Crispr‐Osago2#13 plants were significantly reduced at 19 dpi, whereas the accumulation levels of RBSDV RNA in the OE‐OsAGO2#6 and OE‐OsAGO2#13 plants were significantly increased (Figure 1d). Similar results were observed for RBSDV CP in the assayed plants using western blot assay (Figure 1e). Further analyses showed that overexpression or knockout of OsAGO2 in rice had no significant effect on SBPH infestation (Figure S3a,b). Based on these results, we conclude that OsAGO2 can regulate rice susceptibility to RBSDV infection, but not the rice response to SBPH infestation.
Because AGO1‐induced gene silencing plays an important role in host antiviral immunity, we investigated OsAGO1 expression during RBSDV infection. We analysed the expression of OsAGO1a, OsAGO1b, OsAGO1c, and OsAGO1d in NPB and Osago2 mutant plants at 0, 8, and 19 dpi through RT‐qPCR. The results showed that RBSDV infection in NPB or in Osago2 mutant plants did not change the expression patterns of these AGO1 genes (Figure S4a–d), indicating that OsAGO2 does not affect the transcriptional expression of OsAGO1a/b/c/d.
2.2. Mutation of OsAGO2 causes early activation of defence responses
To investigate how OsAGO2 regulates rice resistance against RBSDV infection, we examined the expression levels of defence‐related genes during RBSDV infection. Key components or reporter genes involved in plant defence responses were selected and analysed through RT‐qPCR using rice seedlings inoculated with RBSDV or mock‐inoculated. OsNPR1 is the rice homolog of Arabidopsis NPR1, the master regulator of plant innate immunity (Chern et al., 2005, 2016). In this study, the expression levels of OsNPR1 in mock‐inoculated NPB, Osago2, and Crispr‐Osago2 plants were similar at 0 and 19 dpi. Compared with the NPB control, the expression levels of OsNPR1 had no significant difference in the RBSDV‐inoculated Osago2 and Crispr‐Osago2 plants at 0 and 19 dpi (Figure 2a). OsPR1a and OsPR1b, two pathogenesis‐related genes, are involved in the plant defence response (van Loon et al., 2006). Compared with the mock‐inoculated control, the expression of OsPR1a and OsPR1b was significantly induced in the RBSDV‐inoculated Osago2 and Crispr‐Osago2 mutant plants at 0 and 19 dpi. However, there was no significant difference in the expression of OsPR1a and OsPR1b in NPB between mock‐inoculated and RBSDV inoculation treatments (Figure 2b,c).
FIGURE 2.
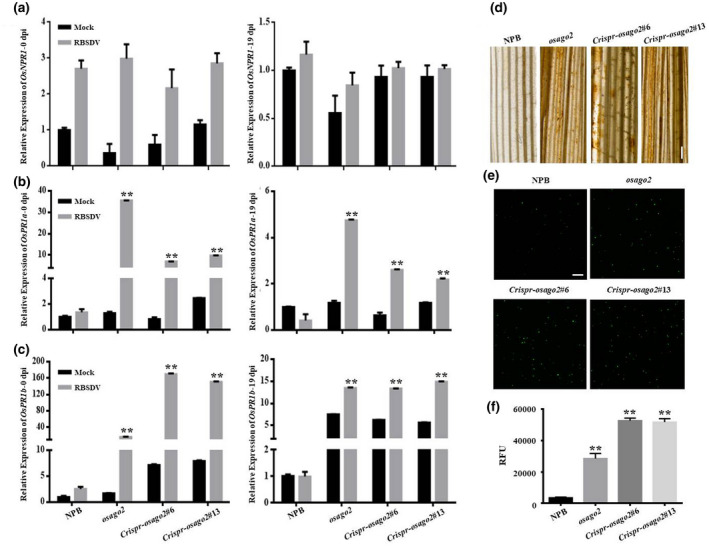
OsAGO2 mutation leads to an early activation of defence response to RBSDV infection. Nipponbare (NPB), Osago2, and Crispr‐Osago2 plants were inoculated with RBSDV and then analysed for early defence responses. (a–c) Quantitative reverse transcription PCR (RT‐qPCR) analyses of OsNPR1, OsPR1a, and OsPR1b expression in the mock‐inoculated (Mock) and RBSDV‐inoculated (RBSDV) NPB, Osago2, and Crispr‐Osago2 plants at 0 and 19 days postinoculation (dpi). The expression of rice 18S‐rRNA was used as an internal control for RT‐qPCR. Error bars indicate the SD of individual treatments with three biological replicates. (d) Leaf tissues were collected from NPB, Osago2, and Crispr‐Osago2 (line #6 and #13), and analysed for H2O2 accumulation through 3,3′‐diaminobenzidine staining at 48 hr postinoculation (hpi). Dark brown colour in the stained leaf tissues indicates the accumulation of H2O2. Scale bar, 1 mm. (e, f) Accumulation of reactive oxygen species (ROS) in the RBSDV‐inoculated NPB or Osago2 mutant line protoplasts was detected using the DCFH‐DA method followed by confocal microscopy or measurements using a microtitre plate reader at 48 hpi. Scale bar, 100 μm. Statistical significance of difference between the mock‐inoculated and RBSDV‐inoculated plants was determined using Student's t test. **p < 0.01
ROS accumulation is an indication of host early defence response against pathogen invasion. When leaves of RBSDV‐inoculated NPB, Osago2, and Crispr‐Osago2 plants were collected and stained with 3,3′‐diaminobenzidine (DAB), we verified that ROS accumulated to high levels in the Osago2 and Crispr‐Osago2 plant leaves (Figure 2d). We then analysed ROS accumulation in the RBSDV‐inoculated rice sheath protoplasts through confocal microscopy and a microplate reader using the DCFH‐DA method (Figure 2e,f). These results showed that, compared with the NPB plants, the level of ROS in the protoplasts of the Osago2 and Crispr‐Osago2 mutant plants was significantly increased.
2.3. OsAOGO2 suppresses Os HXK1 expression via DNA methylation on RBSDV infection
OsHXK1 is a known target gene of OsAGO2 (Zheng et al., 2019). To determine whether OsHXK1 affects RBSDV infection in rice, we performed RT‐qPCR using total RNA isolated from RBSDV‐inoculated NPB, Osago2, Crispr‐Osago2, and OE‐OsAGO2 plants. Compared with NPB plants, the expression of OsHXK1 was significantly induced in the Osago2, Crispr‐Osago2#6, and Crispr‐Osago2#13 plants, whereas its expression in the OE‐OsAGO2#1 and OE‐OsAGO2#2 plants was strongly suppressed (Figure 3a). We then checked the expression of OsHXK1 in the RBSDV‐inoculated or mock‐inoculated NPB plants, and found that expression of OsHXK1 in the RBSDV‐inoculated plants was significantly reduced at 0, 8, and 19 dpi compared with that of mock‐inoculated plants (Figure 3b). This expression profile is the opposite of that found for OsAGO2 in the RBSDV‐inoculated plants (Figure 1b), further confirming that OsHXK1 might be a target of OsAGO2 under RBSDV infection.
FIGURE 3.

OsAGO2 represses OsHXK1 expression via DNA methylation on RBSDV infection. (a) Quantitative reverse transcription PCR (RT‐qPCR) analyses of OsHXK1 expression in RBSDV‐inoculated Nipponbare (NPB) and mutant rice lines or (b) in the mock‐inoculated (mock) and RBSDV‐inoculated NPB plants. The expression of rice 18S‐rRNA was used as an internal control. Error bars indicate the SD of individual treatments with three biological replicates each. Statistical significance of difference between the mock‐inoculated and RBSDV‐inoculated plants was determined using Student's t test. **p < 0.01. (c) Distribution of cytosine DNA methylation in three contexts in 650 bp of the OsHXK1 promoter region in the mock‐ and RBSDV‐inoculated NPB, Osago2, and Crispr‐Osago2 plants as measured by bisulphite sequencing. Sequencing data were analysed using Kismeth software. CpG, lilac; CpHpG, blue; CpHpHp, green. (d) Methylation levels of CpG, CpHpG, and CpHpHp in the mock‐ and RBSDV‐inoculated NPB, Osago2, and Crispr‐Osago2 plants
OsAGO2 directly regulates OsHXK1 expression via DNA methylation in rice spikelets (Zheng et al., 2019). Because OsHXK1 is not a target of any known rice miRNA, it appears that OsAGO2 does not regulate OsHXK1 at the posttranscriptional level. Through genomic sequence analysis, we found that OsHXK1 contains a GC‐rich promoter. Therefore, we hypothesized that DNA methylation may be responsible for the interaction between OsAGO2 and OsHXK1 during RBSDV infection. To explore the interaction of OsAGO2 with OsHXK1, we analysed the methylation level of the OsHXK1 promoter region. In plants, cytosine methylation primarily occurs in three sequence contexts: CG, CHG, and CHH (H = A, T, or C). We performed a bisulphite conversion reaction using a sequence starting 650 bp upstream of the transcriptional start site of OsHXK1 to analyse the methylation status of the OsHXK1 promoter region (Figure S5a). The CG, CHG, and CHH methylation levels in the promoter region were lower in Osago2 mutant plants than in NPB control plants (Figure 3c,d). Compared with the mock control, the levels of CG, CHG, and CHH methylation in the OsHXK1 promoter region were significantly increased in NPB plants on RBSDV infection. However, the methylation levels of the OsHXK1 promoter did not differ significantly between Osago2 and Crispr‐Osago2 plants during RBSDV infection. These results suggest that OsAGO2 regulates the methylation status of the OsHXK1 promoter in rice defence against RBSDV infection. Therefore, OsAGO2 regulates OsHXK1 expression in answer to RBSDV infection.
2.4. OsHXK1 enhances ROS‐mediated basal resistance to RBSDV infection
PCD is a defence response hallmark of biotrophic or hemibiotrophic pathogens (Mukhtar et al., 2016; Zebell & Dong, 2015). It is known that OsHXK1 can initiate early PCD and cause defective anther development in rice (Bruggeman et al., 2015; Zheng et al., 2019). Therefore, we examined PCD in Nicotiana benthamiana leaves transiently overexpressing OsHXK1, OsAGO2, and PcINF1. Leaves overexpressing OsHXK1 could not induce cell death at 5 days postinfiltration; however, leaf tissue expressing PcINF1, an oomycete effector, showed cell death (Figure S6a).
We analysed the role of OsHXK1 in the rice response to RBSDV infection using two stable transgenic rice lines, OE‐OsHXK1#3 and OE‐OsHXK1#5 (Figure S7a,b). The results showed that, similar to Osago2 mutant plants, OE‐OsHXK1#3 and OE‐OsHXK1#5 plants were resistant to RBSDV infection, as evidenced by the much lower disease incidence, milder disease symptoms, and lower RBSDV RNA and CP accumulation at 30 dpi (Figure 4a–d) when compared with the RBSDV‐inoculated NPB plants. In the same experiment, the RBSDV‐inoculated Crispr‐Oshxk1#1 and Crispr‐Oshxk1#2 plants showed more severe disease symptoms than those of the RBSDV‐inoculated NPB plants (Figures 4a–d and S7c–h). Further analyses showed that the Crispr‐Oshxk1 and OE‐OsHXK1 transgenic plants were as susceptible to SBPH infestation as NPB plants (Figure S8a,b). Thus, we conclude that the RBSDV resistance conferred by OsHXK1 is independent of SBPH resistance.
FIGURE 4.

Overexpression of OsHXK1 in rice enhances the reactive oxygen species (ROS)‐mediated basal resistance to RBSDV infection. To determine the role of OsHXK1 in RBSDV infection in rice, we generated two OsHXK1 knockout lines (Crispr‐Oshxk1 #1 and #2) and two overexpressing transgenic lines (OE‐OsHXK1 #3 and #5), and then inoculated them with RBSDV. (a) Disease incidence of RBSDV‐inoculated Nipponbare (NPB), Crispr‐Oshxk1 (lines #1 and #2), and OE‐OsHXK1 (lines #3 and #5) plants were determined at 30 days postinoculation (dpi). (b) Representative mock‐ or RBSDV‐inoculated NPB and various mutant plants were photographed at 30 dpi. Scale bar, 4 cm. (c) The accumulation of RBSDV coat protein (CP) RNA transcripts in different assayed plant samples was analysed by quantitative reverse transcription PCR at 19 dpi. The expression of rice 18S‐rRNA was used as an internal control. Error bars are SD of different treatments with three biological replicates. (d) Expression levels of RBSDV CP in different samples were measured by western blot assay at 0 and 19 dpi. Ponceau‐stained RuBisCO large protein is used to show sample loading. (e) Leaf tissues were collected from NPB and OsHXK1 overexpression (lines #3 and #5) plants, and analysed for H2O2 accumulation through 3,3′‐diaminobenzidine staining at 48 hr postinoculation. Dark‐brown colour in the stained leaf tissues indicates the accumulation of H2O2. Scale bar, 100 µm. (f, g) Accumulation of ROS in different protoplast samples was determined using the DCFH‐DA method followed by confocal microscopy or measurements using a microtitre plate reader. Scale bar, 100 μm. Statistical significance of difference between NPB and other mutant lines was calculated using Student's t test. *p < .05, **p < .01
AtHXK1 can contribute to the generation of ROS (Bruggeman et al., 2015). When leaves of RBSDV‐inoculated NPB and OE‐OsHXK1 plants were collected and stained with DAB, ROS accumulated to high levels in OsHXK1‐overexpressing plant leaves (Figure 4e). We also analysed ROS accumulation in the RBSDV‐infected OE‐OsHXK1#3, OE‐OsHXK1#5, Crispr‐Oshxk1#1, and Crispr‐Oshxk1#2 protoplasts (Figure 4f,g). The results showed that overexpression of OsHXK1 in rice increased ROS production, whereas knockout of OsHXK1 did not induce apparent ROS accumulation, confirming that OsHXK1 is a positive regulator of the ROS burst in rice cells against RBSDV infection. To further clarify the contribution of OsHXK1 to OsAGO2‐mediated rice resistance to RBSDV, OsHXK1 was knocked out using CRISPR/Cas9 in an Osago2 transposon‐insertion mutant background to generate an Osago2/Oshxk1 double mutant (Figure S9a–g). Two homozygous lines were selected for further resistance analysis (DM#17 and DM#30). To examine whether the defence response generated by OsAGO2 mutation could be affected by OsHXK1, we assessed the level of ROS in the double mutant upon RBSDV infection. Compared with the Osago2 control, the Osago2/Oshxk1 double mutant did not induce apparent ROS accumulation in rice leaves and protoplasts (Figure S9h,i,j). This confirmed that OsAGO2 mutation‐induced resistance could be blocked by disruption of OsHXK1. These results suggest that OsHXK1 is regulated by OsAGO2.
We propose a model to describe the function of OsAGO2 response to RBSDV infection, as shown in Figure S10. According to this model, OsAGO2 can repress OsHXK1 expression by regulating the methylation status of the OsHXK1 promoter upon RBSDV infection, leading to rice susceptibility in NPB. In the Osago2 mutant, upregulated expression of OsHXK1 resulted in an increase in ROS production, leading to resistance to RBSDV infection (Figure S10a).
2.5. Induction of OsAGO2 expression enhances rice susceptibility to SRBSDV infection through suppressing OsHXK1 expression
Because OsHXK1 can induce the ROS burst in rice cells, we speculated that OsHXK1 could mediate general antiviral resistance. To validate this hypothesis, we used another typical Fijivirus, SRBSDV, to test the antiviral resistance by inoculating SRBSDV into the indicated rice lines. The results showed that, compared with the mock‐inoculated NPB plants, the expression of OsAGO2 in the SRBSDV‐infected NPB plants was significantly upregulated, indicating that SRBSDV infection can also induce OsAGO2 expression (Figure 5a). In contrast, the expression of OsHXK1 in the SRBSDV‐infected NPB plants was significantly suppressed compared with that in noninfected NPB plants (Figure 5b), and the expression profile showed a significant reverse pattern to the increased expression of OsAGO2 on SRBSDV infection.
FIGURE 5.

OsAGO2 suppresses OsHXK1 expression to enhance rice susceptibility to SRBSDV infection. In this study, the mock‐inoculated (mock) or SRBSDV‐inoculated (SRBSDV) Nipponbare (NPB) plants were analysed by quantitative reverse transcription PCR (RT‐qPCR) to determine the effect of OsAGO2 on OsHXK1 expression. In a later experiment, we inoculated NPB, Osago2, Crispr‐Osago2 (lines #6 and #13), OE‐OsAGO2 (lines #1 and #2), Crispr‐Oshxk1 (lines #1 and #2), and OE‐OsHXK1 (lines #3 and #5) plants with SRBSDV to determine their resistance to SRBSDV infection at various days postinoculation (dpi). (a, b) Expression of OsAGO2 or OsHXK1 in the mock‐ and SRBSDV‐inoculated NPB plants was determined by RT‐qPCR at 0, 8, and 19 dpi. The expression of rice 18S‐rRNA was used as an internal control. (c, f) SRBSDV disease incidence in NPB and other mutant lines was determined at 30 dpi. The number of infected plants in each treatment was determined by RT‐PCR at 30 dpi. (d, g) The accumulation of SRBSDV RNA in the assayed plants was determined by RT‐qPCR at 19 dpi using SRBSDV coat protein (CP) gene‐specific primers. The expression of rice 18S‐ rRNA was used as an internal control. The accumulation of SRBSDV RNA in NPB was compared with that in the other mutant lines. (e, h) Accumulation of SRBSDV CP in various SRBSDV‐inoculated plants was measured by western blot assay at 0 and 19 dpi. Ponceau‐stained RuBisCO large subunit is used to show sample loading. Error bars indicate the SD of individual treatments with three biological replicates per treatment. Statistical significance of difference between the treatments was determined using Student's t test. Similar results were obtained in three independent experiments. **p < 0.01
In this study, the SRBSDV incidence and disease symptoms in OE‐OsAGO2 plants were much higher than those in NPB plants; however, the SRBSDV incidence and disease symptoms in Osago2 and Crispr‐Osago2 plants were much lower than those in NPB plants (Figures 5c and S11a). These observations are supported by the results of RT‐qPCR and western blot assays (Figure 5d,e). We also found that the Crispr‐Oshxk1 plants showed much higher SRBSDV incidence and more severe disease symptoms than NPB plants and OE‐OsHXK1 plants (Figure 5f and S11b). Similar results were obtained through RT‐qPCR and western blot assays (Figure 5g,h). Taken together, these data suggest that OsAGO2 enhances rice susceptibility to SRBSDV by repressing OsHXK1 expression.
3. DISCUSSION
To date, several AGO members of dicotyledonous plants, such as AGO1, AGO2, AGO4, AGO5, and AGO7, have been shown to function in host defence against positive‐sense single‐stranded RNA (ssRNA) viruses. Different AGO proteins have specific antiviral activities. For example, AGO1 has been shown to control host resistance against turnip crinkle virus (TCV) and cucumber mosaic virus (CMV) infections and is a key player in siRNA biogenesis (Harvey et al., 2011). Loss of AGO4 function increases host susceptibility to tobacco rattle virus (TRV) infection (Ma, Nicole, et al., 2015). In Arabidopsis and N. benthamiana, AGO5 has been shown to control host resistance against potato virus X (PVX) infection (Brosseau & Moffett, 2015). AtAGO7 is involved in Arabidopsis resistance to TCV (Qu et al., 2008). In 2011, Scholthof et al. determined that AGO2 can regulate Arabidopsis resistance to TCV and CMV infection, and N. benthamiana resistance to tomato bushy stunt virus (TBSV) infection (Scholthof et al., 2011).
In a previous report, AtAGO2 was shown to be a major player in Arabidopsis nonhost resistance to PVX infection (Jaubert et al., 2011). However, Brosseau et al. determined that AGO2 protein from Arabidopsis Col‐0 effectively targets PVX, whereas AGO2 from N. benthamiana does not, suggesting that interspecific differences in AGO2 contribute to distinct outcomes of PVX infection in these species (Brosseau et al., 2020). Unlike the previous report, 27 of 63 Arabidopsis accessions analysed were susceptible to PVX, and this susceptibility was determined by polymorphisms of AtAGO2 protein (Brosseau et al., 2020). Plant–virus interactions may influence natural variation in RNA‐silencing components, thus AtAGO2 protein shows a high incidence of polymorphisms with selective pressure and exhibits a particular antiviral activity. Through molecular and genetic analyses, we determined that monocotyledonous rice AGO2 is a negative regulator of rice resistance to RBSDV, a dsRNA virus. Our study revealed the role of monocotyledonous AGO2 as a factor favouring dsRNA virus infection in rice, in contrast to the widely studied dicotyledonous AGO2, which opposes ssRNA virus infection. Sequence differences in AGO2 can have a significant impact on antiviral activity. The AtAGO2 and OsAGO2 proteins are only 41% identical, which may explain their differential effects on the virus. This maybe another reason that AGO2 proteins have different roles against different viruses. Therefore, we propose that an increase in OsAGO2 expression is probably a specific strategy employed by RBSDV to suppress rice resistance to its infection.
DNA methylation was proposed to be a new modulator of plant responses to biotic and abiotic stress (Baulcombe & Dean, 2014; Dowen et al., 2012). Although several studies of DNA methylation have been conducted, most have involved analysis at the whole‐genome level rather than at specific‐gene level (He et al., 2011). AGO proteins play essential roles in epigenetic gene‐silencing pathways guided by several 24‐nucleotide siRNAs (Qi et al., 2006; Zhang et al., 2016). In rice, OsAGO4 and OsAGO6 are both required for DNA methylation at most of their target loci (Duan et al., 2015). Rice genome methylation has been reported to be a new layer of epigenetic changes in response to RBSDV infection (Li et al., 2020). Zheng and colleagues have demonstrated that OsAGO2 represses OsHXK1 expression in rice spikelets through methylation of its promoter (Zheng et al., 2019). In this study, OsHXK1 expression in RBSDV‐infected rice plants was suppressed, probably due to RBSDV infection‐induced expression of OsAGO2 (Figures 1a and 3b). To determine if OsHXK1 gene expression is associated with its DNA methylation state on RBSDV infection, we performed bisulphite sequencing analysis of the OsHXK1 promoter region. Our results showed that OsAGO2 enhanced the methylation levels of the OsHXK1 promoter region on RBSDV infection (Figure 3c,d). We propose that OsHXK1 expression is regulated epigenetically by OsAGO2‐mediated transcriptional gene silencing against RBSDV infection. Our results revealed RBSDV‐responsive target genes that can be modified by DNA methylation and provide an insight into the antiviral mechanism of rice.
The expression of defence‐responsive genes in plants is an indication of early defence responses elicited by viral infection (Lu et al., 2020). Compared with NPB plants, the expression of OsPR1a and OsPR1b in the Osago2 mutant plants was significantly induced by RBSDV infection (Figure 2), indicating that suppressing OsAGO2 can activate the expression of OsPR1a and OsPR1b. It was reported that the basal ROS plays an important role in plant antiviral resistance (Wu et al., 2017; Xie et al., 2018; Zhang et al., 2019). In this regard, we found that Osago2 and Crispr‐Osago2 plants produced more ROS and showed stronger resistance to RBSDV infection than the NPB control plants did (Figure 2). We speculate that OsAGO2 has a direct or indirect effect on ROS production and rice resistance to RBSDV infection. Because OsAGO2 is a regulator of OsHXK1 expression, we investigated the role of OsHXK1 in ROS production. Our results indicated that an increase in OsHXK1 expression in rice induces ROS production, leading to an enhanced basal ROS‐mediated resistance to RBSDV infection (Figure 4 and S10). Thus, we propose that OsAGO2 is a negative regulator of RBSDV resistance, and keeping the ROS‐mediated resistance in an inactivated mode is a strategy used by RBSDV to ensure a successful infection.
SRBSDV is another fijivirus and causes disease symptoms similar to those of RBSDV. In this study, we found that both RBSDV and SRBSDV infection in rice elevated OsAGO2 expression, resulting in the suppression of OsHXK1 expression, whereas the OsAGO2 knockout mutant lines, Osago2 and Crispr‐Osago2, were resistant to both viruses (Figures 1 and 5). These findings indicate that OsAGO2 may confer a broad susceptibility to RBSDV and SRBSDV. Therefore, we propose that the OsAGO2/OsHXK1 regulatory module may be a universal immune regulator of dsRNA viruses of the genus Fijivirus. This discovery could enable the engineering of genetic resistance against fijiviruses. Given that RBSDV can be transmitted to rice, maize, barley, and wheat in a persistent propagative manner by the SBPH, identification of the host resistance‐related factor OsAGO2 in rice provides a valuable tool for screening for similar genes in other cereal crops and the creation of an artificial gene via genome editing.
4. EXPERIMENTAL PROCEDURES
4.1. Plasmid construction
Full‐length OsAGO2 and OsHXK1 sequences were amplified by PCR, and the resulting PCR products were cloned individually behind a CaMV 35S promoter in the Gateway pEarleyGate vector (with FLAG tag) for transient expression in N. benthamiana. These two vectors were agroinfiltrated into N. benthamiana leaves for transient expression assays.
For rice stable transformation, full‐length OsAGO2 and OsHXK1 sequences were PCR amplified and cloned individually into the pCambia 1300 vector to produce overexpression transgenic rice. For gene editing, we used clustered regularly interspaced short palindromic repeats/CRISPR‐associated protein 9 (CRISPR/Cas9) technology. Briefly, the OsAGO2 and OsHXK1‐specific target sequences were PCR amplified and inserted into a pYLgRNA‐OsU3, pYLgRNA‐OsU6a, or pYLgRNA‐OsU6b vector, as previously described (Ma, Zhang, et al., 2015). All constructs were confirmed by DNA sequencing, introduced into Agrobacterium tumefaciens EHA105, and transformed into NPB by Agrobacterium‐mediated transformation. The primers used in this study are listed in Table S1.
4.2. Plant materials and growth conditions
NPB was used as an assay plant and as a parental line for the production of OsAGO2 overexpression (OE‐OsAGO2), OsAGO2 CRISPR/Cas9 knockout (Crispr‐Osago2), OsHXK1 overexpression (OE‐OsHXK1), and OsHXK1 CRISPR/Cas9 knockout (Crispr‐Oshxk1) transgenic lines. The overexpression transgenic lines were generated using an Agrobacterium‐mediated transformation method followed by hygromycin resistance selection. The OsAGO2 silencing mutant line (Osago2) was obtained from the rice Tos17 insertion mutant database (NG8158, https://tos.nias.affrc.go.jp/). All rice lines were grown in a greenhouse maintained at 25 °C with a 12/12 hr (light/dark) photoperiod and 70% relative humidity. These plants were used for various assays at the 1.5‐ to 2‐leaf‐stage. N. benthamiana plants were grown under the same conditions and used for the Agrobacterium infiltration experiments.
4.3. Virus source and virus inoculation
Wheat plants showing typical symptoms of wheat dark‐green dwarf disease were collected from a wheat field in the Jiangsu Province, China, and tested virus by RT‐PCR using RBSDV gene‐specific primers. The RBSDV‐positive plants were grown in a greenhouse, which would be used in the artificial inoculation identification.
SBPH nymphs were from a collection maintained in the laboratory and reared on the RBSDV‐positive rice plants for 7 days. The nymphs were then transferred onto healthy Wuyujing No. 3 rice seedlings for 8 days to allow RBSDV to pass through its circulative period in the SBPHs. The percentage of RBSDV viruliferous SBPHs was determined using dot‐enzyme‐linked immunosorbent assay (dot‐ELISA).
4.4. Evaluation of RBSDV resistance
RBSDV inoculation was performed using the SBPHs as described previously (Zhou et al., 2015). At approximately the 1.5‐leaf‐stage, 30 healthy seedlings were inoculated with RBSDV viruliferous SBPHs (two SBPHs per seedling) for 48 hr. An individual SBPH was then manually removed to ensure a uniform inoculation efficiency. The inoculated seedlings were transplanted into an experimental plot divided with cement pools at the Jiangsu Academy of Agricultural Sciences, Nanjing, China. The seedlings were allowed to grow under a standard farming practice without application of pesticides during the growth period.
One month after transplanting (30 dpi), the incidence of RBSDV was recorded for the NPB and its mutant lines, respectively. Three replicates were used for each treatment and the disease incidence of each assayed line (%) was determined using the formula: number of RBSDV‐infected plants/total number of plants counted × 100.
4.5. Evaluation of rice resistance to SBPH infestation
Two tests (antibiosis test and nonpreference test) were used to evaluate rice resistance to SBPH infestation: (1) Antibiosis test: Seedlings of NPB and mutant lines were infested with first to second instar SBPH nymphs (10 nymphs per seedling). Five days later, the survival rate of SBPHs on each variety was recorded, respectively. (2) Nonpreference test: Seedlings of NPB and mutant lines were transferred into beaker, infested with second to third instar SBPH nymphs (10 nymphs per seedling), and then covered with nylon net. Two days later, the average number of SBPHs on each line was regarded as the value of feeding preferences. Details of the procedures have been described previously (Guo et al., 2018).
4.6. RNA isolation and RT‐qPCR analysis
Total RNA was extracted from collected leaf samples using TRIzol reagent. The resulting RNA samples were treated with DNase prior to reverse transcription. Quantitative PCR was performed using the SYBR Premix mix as instructed (Takara). Relative expression level of RBSDV viral and host defence genes was calculated using the 2−ΔΔ C t method as previously described (Livak & Schmittgen, 2001). The expression of rice 18S‐rRNA gene was used as an internal control during RT‐qPCR. Three biological replicates with three technical replicates each were used for each treatment. The primers used in this study are listed in Table S1.
4.7. Protein transient expression in N . benthamiana
Transient expression assays in N. benthamiana leaves were performed as previously described (Wang et al., 2018). In brief, this was performed by infiltrating 3‐week‐old N. benthamiana plants with A. tumefaciens GV3101 (OD600 = 0.5). OsHXK1 and OsAGO2 coding sequences were cloned into pEarley 202 vectors (with FLAG tags) for expression. The empty vector (EV) pEarley 202 was used as a negative control. PcINF1 was used as a positive control of cell death. After 5 days postinfiltration, the infiltrated leaf tissues were analysed for cell death using a digital camera (Tanon).
4.8. Protein extraction and western blot assay
The harvested plant tissues were ground individually in liquid nitrogen and then homogenized in a protein extraction buffer (Sigma‐Aldrich) supplemented with a protease inhibitor cocktail (Roche; 1 tablet per 50 ml extraction buffer). After 15 min centrifugation at 12,000 × g at 4 °C, the supernatant was collected from each sample and boiled for 8 min. Proteins in each sample were separated on sodium dodecyl sulphate (SDS) polyacrylamide gels by electrophoresis and then transferred onto polyvinylidene fluoride (PVDF) membranes. Antibodies against RBSDV CP or SRBSDV CP used in this study were obtained from Professor Jianxiang Wu (Zhejiang University, Hangzhou, Zhejiang, China). Anti‐FLAG antibody was purchased from Sigma (Sigma‐Aldrich).
4.9. DAB staining assays
H2O2 accumulation in rice leaf tissues was visualized using a DAB staining method. Fragments were cut from rice leaves and placed in containers with a diluted (1 mg/ml) DAB (Sigma‐Aldrich) solution followed by 8 hr staining with shaking at 26 °C in the dark. The stained leaf fragments were decolourized in an ethanol:acetic acid (94:4, vol/vol) solution at 26 °C for 8 hr in the dark. The accumulations of H2O2 in rice leaves were determined by the appearance of a light‐dark brown colour in the tissues. The stained leaf fragments were photographed under a light microscope (Zeiss).
4.10. Protoplast isolation and DCFH‐DA staining assays
Rice protoplasts were isolated from rice sheath as described previously (Zhang et al., 2018). The resulting protoplasts were divided into clean tubes (1 ml each). The accumulation of ROS in rice protoplasts after RBSDV infection was detected using a ROS assay kit as instructed by the manufacturer (Beyotime). DCFH‐DA was added to the rice protoplasts, followed by gentle mixing. After 20 min of incubation at 28 °C, 1 ml of W5 solution (154 mM NaCl, 125 mM CaCl2, 5 mM KCl, 2 mM MES) was added to each tube, followed by gentle mixing. The tubes were centrifuged at 200 × g for 3 min, and the pellets were resuspended individually in 1 ml of W5 solution. Because intracellular ROS can oxidize DCFH to DCF, we measured the fluorescence of DCF in protoplasts to estimate the level of cellular ROS. The fluorescence signal from a specific protoplast sample was recorded using a microtitre plate reader with an excitation wavelength of 488 nm and an emission wavelength of 525 nm, as described previously (Wang et al., 2016).
4.11. Bisulphite sequencing
Genomic DNA was isolated from rice anthers and leaves using the CTAB method. Briefly, 1 μg of genomic DNA was treated with sodium bisulphite using DNA methylation kit according to the manufacturer's instructions (Vazyme). The treated DNA was dissolved in 25 μl distilled water, and 5 μl of this solution was used as the template for PCR in a 150 μl reaction mixture. Specific primers were used to amplify selected regions of the OsHXK1 promoter. The purified PCR products were cloned into the pMD18‐T vector (Takara). For each sample, at least 12 individual clones were sequenced, and the sequencing data were analysed using Kismeth software (http://katahdin.mssm.edu/kismeth/revpage.pl) and CyMATE software (http://www.cymate.org/). The primer sequences used for bisulphite sequencing are listed in Table S1.
CONFLICT OF INTEREST
The authors declare that they have no competing interest.
AUTHOR CONTRIBUTIONS
T.Z. and Z.W. conceived the project and designed the experiments. Z.W. carried out the experiments with assistance from D.C., F.S., W.G., W.W., and X.L. Z.W. analysed the results with assistance from Y.L., L.D., S.L., Y.F., and Y.Z. Z.W., F.S., and H.Z. prepared the plant transgenic materials. Z.W. wrote the manuscript. All authors reviewed and approved the final article.
Supporting information
FIGURE S1 Validation of osago2, Crispr‐osago2, and OE‐OsAGO2 transgenic lines. (a) Schematic representation of the constructs used for OE‐OsAGO2 transgenic line with N‐terminal FLAG. Ubip: ubiquitin promoter. (b) The expression of OsAGO2 in NPB, osago2, Crispr‐osago2, and OE‐OsAGO2 transgenic plants was determined through quantitative reverse transcription PCR. The expression of rice 18s‐rRNA was used as an internal control. Error bars indicate the SD of different treatments with three biological replicates each. **p < .01. This experiment was conducted three times with similar results. (c) The expression of OsAGO2 in the two OE‐OsAGO2 lines was determined through western blot assay using a FLAG‐specific antibody. EV, empty vector. (d) Schematic representation of osago2 (NG8158) transposon‐insertion mutant with NPB background from the rice Tos17 insertion mutant database. Grey block, exon; black inverted triangle, transposon. (e, h) Schematic representation of the constructs used for Crispr‐osago2 transgenic lines (#6 and #13). Crispr‐osago2#6, 1 bp deletion; Crispr‐osago2#13, 1 bp insertion. (i, f) Verification of mutations in the two Crispr‐osago2 lines through PCR amplification and DNA sequencing. Forward and reverse sequencing was unimodal, indicating that Crispr‐osago2#6 and Crispr‐osago2#13 were homozygous mutants. (g, j) Amino acid comparison of OsAGO2 in NPB control and Crispr‐osago2 mutant. The deletion and insertion of one base results in premature termination of OsAGO2 translation
FIGURE S2 Expression of OsAGO3 in wild‐type and osago2 mutant lines. (a), Phylogenetic analysis of 19 rice AGO protein and the phylogenetic trees were draw. (b), Quantitative reverse transcription PCR analysis of the OsAGO3 transcript levels in the wild‐type (NPB), osago2, and Crispr‐osago2 mutant plants. The expression of rice 18s‐rRNA was used as an internal control. Error bars indicate the SD of different treatments with three biological replicates each. (c), The sequencing of homologous regions of OsAGO3 in the NPB and Crispr‐osago2 mutant plants. The sequences in red were the homologous target sits of Crispr‐osago2#6 and Crispr‐osago2#13
FIGURE S3 Effect of OsAGO2 on SBPH infestation. SBPH nymphs were rear on the NPB or individual mutant line seedlings (10 nymphs per seedling). Five days later, the survival rate of SBPH was recorded for each treatment. (a), Survival rates of SBPH on the NPB or other assayed lines are shown. Rice cv. IR50 was used as a SBPH resistant control. Error bars indicate the SD of individual treatments with three biological replicates per treatment. **p < 0.01. This experiment was done three times with similar results. (b), The numbers of SBPH maintained on different assayed lines at two days post SBPH infestation. **p < 0.01. This experiment was conducted three times with similar results
FIGURE S4OsAGO2 does not affect OsAGO1 mRNA expression. (a, b, c, d), Expressions of OsAGO1a, OsAGO1b, OsAGO1c, and OsAGO1d, respectively, in the mock‐inoculated (Mock) and RBSDV‐inoculated (RBSDV) NPB and osago2 plants were analysed through quantitative reverse transcription RT‐PCR at 0, 8, and 19 days postinoculation (dpi). Error bars indicate the SD of three biological replicates. This experiment was done three times with similar results
FIGURE S5 Bisulphite sequencing analysis of methylated cytosine at the OsHXK1 promoter. Bisulphite sequencing data of individual clones were submitted to the CyMATE program to analyse the methylated cytosines. Methylation of CpG, CpHpG, and CpHpHp in the mock‐inoculated and RBSDV‐inoculated NPB, osago2, and Crispr‐osago2 plants at 19 days postinoculation
FIGURE S6OsHXK1 does not induce cell death in Nicotiana benthamiana. N. benthamiana leaves were infiltrated with Agrobacterium tumefaciens carrying an empty vector (EV) or plasmids expressing OsAGO2, OsHXK1 or PcINF1 (a positive regulator of cell death). The leaf was photographed at 5 days postinfiltration. Scale bar, 1 cm
FIGURE S7 Validation of OsHXK1 expression in four transgenic lines. (a), Schematic representation of the constructs used for OE‐OsHXK1 transgenic line with N‐terminal FLAG. Ubip: ubiquitin promoter. (b), The expression of OsHXK1 in OE‐OsHXK1 transgenic lines was analysed through western blot assay using anti‐FLAG antibody. A rice line transformed with the empty vector (EV) was used as control. (c, f), Schematic representation of the constructs used for Crispr‐oshxk1 transgenic line (#1 and #2). Crispr‐oshxk1#1: 1 bp deletion. Crispr‐oshxk1#2: 7 bp deletion. (d, g), Verification of the CRISPR/Cas9 system OsHXK1 knockout lines by PCR‐based DNA sequencing. Forward and reverse sequencing were unimodal, indicating that Crispr‐oshxk1#1 and Crispr‐oshxk1#2 were homozygous mutants. (e, h), Amino acid comparison of OsHXK1 in NPB control and Crispr‐oshxk1 mutant. The deletion of one or several base results in premature termination of OsHXK1 translation
FIGURE S8 Effect of OsHXK1 on SBPH infestation. Survival rates (a) and feeding preference (b) of SBPH on NPB and OsHXK1 knockout or overexpression lines were tested through rearing SBPH nymphs on various seedlings. Rice cv. IR50 was used as a SBPH resistant control. **p < 0.01. This experiment was done three times with similar results
FIGURE S9 Validation of osago2/oshxk1 double mutant line. (a), The expression of OsAGO2 in NPB, osago2/oshxk1 double mutant (DM#17 and DM#30) was determined through quantitative reverse transcription PCR. The expression of rice 18s‐rRNA was used as an internal control. Error bars indicate the SD of different treatments with three biological replicates each. **p < 0.01. (b, d), Schematic representation of the constructs used for double mutant transgenic lines (DM#17 and DM#30). DM#17: 1 bp deletion. DM#30: 2 bp insertion. (c, e), Verification of the CRISPR/Cas9 system OsHXK1 knockout lines by PCR‐based DNA sequencing. Forward sequencing was unimodal, indicating that DM#17 and DM#30 were homozygous mutants. (f, g), Amino acid comparison of OsHXK1 in NPB control and double mutants. The mutation of one or two base results in premature termination of OsHXK1 translation. (h), Leaf tissues were collected from osago2 and DM (#17 and #30) plants, and analysed for H2O2 accumulation through 3.3′‐diaminobenzidine (DAB) staining at 48 hr postinoculation (hpi). Dark brown colour in the stained leaf tissues indicate the accumulation of H2O2. Scale bar, 1 mm. (i,j), Accumulation of reactive oxygen species (ROS) in the RBSDV‐inoculated osago2 or double mutant protoplasts were detected using the DCFH‐DA method followed by confocal microscopy or measurements using a microtitre plate reader at 48 hpi. Scale bar, 100 μm. Statistical significance between the mock‐inoculated and the RBSDV‐inoculated plants were determined using the Student’s t test. **p < 0.01. This experiment was conducted three times with similar results
FIGURE S10 A proposed model of the OsAGO2 regulates rice antiviral response. A proposed model of the OsAGO2‐OsHXK1 that regulates rice defence response against RBSDV infection. In this model, appropriate production of reactive oxygen species (ROS) was controlled by OsHXK1, which negatively regulated by OsAGO2
FIGURE S11 Phenotypes of the mock‐inoculated and SRBSDV‐inoculated OsAGO2 and OsHXK1 transgenic lines. (a, b), NPB, Crispr‐osago2 (Line#6 and #13), OE‐OsAGO2 (Line#1 and #2), Crispr‐oshxk1 (Line#1 and #2) and OE‐OsHXK1 (Line#3 and #5) plants were inoculated with mock‐inoculation or SRBSDV. The inoculated plants were photographed at 30 days postinoculation. Scale bar, 4 cm
TABLE S1 Primers used in the study
ACKNOWLEDGEMENTS
The authors thank Dr Xinshun Ding (Samuel Roberts Nobel Foundation, Oklahoma, USA, retired) for his comments and suggestions during preparation of this manuscript. The authors also thank Professor Jianxiang Wu (Zhejiang University, China) for providing us with the antibodies against RBSDV CP and SRBSDV CP. This work was supported by the National Natural Science Foundation of China (31901959), the National Key R&D Program of China (2017YFD0100400), the Six Talent Peaks project of Jiangsu Province (NY‐056), and the 333 High Level Talent Training project of Jiangsu Province (BRA2018081).
Wang Z, Chen D, Sun F, et al. ARGONAUTE 2 increases rice susceptibility to rice black‐streaked dwarf virus infection by epigenetically regulating HEXOKINASE 1 expression. Mol Plant Pathol. 2021;22:1029–1040. 10.1111/mpp.13091
DATA AVAILABILITY STATEMENT
All data supporting the conclusions of this article are provided within the article (and its Additional files). Sequence data from this study can be found in the Rice Genome Database (http://rice.plantbiology.msu.edu) and GenBank (https://www.ncbi.nlm.nih.gov/genbank) under the accession numbers OsAGO2 (LOC_Os04g52540), OsHXK1 (LOC_Os07g26540), OsNPR1 (LOC_Os01g09800), OsPR1a (LOC_Os07g03710), OsPR1b (LOC_Os01g28450), OsAGO1a (LOC_Os02g45070), OsAGO1b (LOC_Os04g47870), OsAGO1c (LOC_Os02g58490), OsAGO1d (LOC_Os06g51310), RBSDV S10 (AF227205), and SRBSDV S10 (JQ927009).
REFERENCES
- Baulcombe, D. & Dean, C. (2014) Epigenetic regulation in plant responses to the environment. Cold Spring Harbor Perspectives in Biology, 6, a01947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boller, T. & He, S.Y. (2009) Innate immunity in plants: an arms race between pattern recognition receptors in plants and effectors in microbial pathogens. Science, 324, 742–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brosseau, C., Bolaji, A., Roussin, C., Zhao, Z.X., Biga, S. & Moffett, P. (2020) Natural variation in the Arabidopsis AGO2 gene is associated with susceptibility to Potato virus X . New Phytologist, 226, 866–878. [DOI] [PubMed] [Google Scholar]
- Brosseau, C. & Moffett, P. (2015) Functional and genetic analysis identify a role for Arabidopsis ARGONAUTE5 in antiviral RNA silencing. The Plant Cell, 27, 1742–1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruggeman, Q., Prunier, F., Mazubert, C., de Bont, L., Garmier, M., Lugan, R. et al. (2015) Involvement of Arabidopsis Hexokinase1 in cell death mediated by myo‐inositol accumulation. The Plant Cell, 27, 1801–1814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbonell, A. & Carrington, J.C. (2015) Antiviral roles of plant ARGONAUTES. Current Opinion in Plant Biology, 27, 111–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, X.W. & Ronald, P.C. (2011) Innate immunity in rice. Trends in Plant Science, 16, 451–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chern, M., Fitzgerald, H.A., Canlas, P.E., Navarre, D.A. & Ronald, P.C. (2005) Overexpression of a rice NPR1 homolog leads to constitutive activation of defense response and hypersensitivity to light. Molecular Plant‐Microbe Interactions, 18, 511–520. [DOI] [PubMed] [Google Scholar]
- Chern, M., Xu, Q.F., Bart, R.S., Bai, W., Ruan, D.L., Sze‐To, W.H. et al. (2016) A genetic screen identifies a requirement for cysteine‐rich‐receptor‐like kinases in rice NH1 (OsNPR1)‐mediated immunity. PLoS Genetics, 12, e1006049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowen, R.H., Pelizzola, M., Schmitz, R.J., Lister, R., Dowen, J.M., Nery, J.R. et al. (2012) Widespread dynamic DNA methylation in response to biotic stress. Proceedings of the National Academy of Sciences of the United States of America, 109, E2183–E2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan, C.G., Zhang, H.M., Tang, K., Zhu, X.H., Qian, W.Q., Hou, Y.J. et al. (2015) Specific but interdependent functions for Arabidopsis AGO4 and AGO6 in RNA‐directed DNA methylation. EMBO Journal, 34, 581–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo, J., Xu, C., Wu, D.i., Zhao, Y., Qiu, Y., Wang, X. et al. (2018) Bph6 encodes an exocyst‐localized protein and confers broad resistance to planthoppers in rice. Nature Genetics, 50, 297–306. [DOI] [PubMed] [Google Scholar]
- Guo, Z., Li, Y. & Ding, S.W. (2019) Small RNA‐based antimicrobial immunity. Nature Reviews Immunology, 19, 31–44. [DOI] [PubMed] [Google Scholar]
- Harvey, J.J.W., Lewsey, M.G., Patel, K., Westwood, J., Heimstädt, S., Carr, J.P. et al. (2011) An antiviral defense role of AGO2 in Plants. PLoS One, 6, e14639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He, X.J., Chen, T.P. & Zhu, J.K. (2011) Regulation and function of DNA methylation in plants and animals. Cell Research, 21, 442–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutvagner, G. & Simard, M.J. (2008) Argonaute proteins: key players in RNA silencing. Nature Reviews Molecular Cell Biology, 9, 22–32. [DOI] [PubMed] [Google Scholar]
- Jaubert, M., Bhattacharjee, S., Mello, A.F.S., Perry, K.L. & Moffett, P. (2011) ARGONAUTE2 mediates RNA‐silencing antiviral defenses against Potato virus X in Arabidopsis . Plant Physiology, 156, 1556–1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kota, S.K. & Feil, R. (2010) Epigenetic transitions in germ cell development and meiosis. Developmental Cell, 19, 675–686. [DOI] [PubMed] [Google Scholar]
- Li, L., He, Y., Zhang, X., Zhang, H., Sun, Z., Li, J. et al. (2020) Alterations of rice (Oryza sativa L.) DNA methylation patterns associated with gene expression in response to Rice black streaked dwarf virus . International Journal of Molecular Sciences, 21, 5753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak, K.J. & Schmittgen, T.D. (2001) Analysis of relative gene expression data using real‐time quantitative PCR and the 2(−ΔΔCt) method. Methods, 25, 402–408. [DOI] [PubMed] [Google Scholar]
- van Loon, L.C., Rep, M. & Pieterse, C.M.J. (2006) Significance of inducible defense‐related proteins in infected plants. Annual Review of Phytopathology, 44, 135–162. [DOI] [PubMed] [Google Scholar]
- Lu, R., Liu, Z., Shao, Y., Su, J., Li, X., Sun, F. et al. (2020) Nitric oxide enhances rice resistance to Rice black‐streaked dwarf virus infection. Rice, 13, 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma, X., Nicole, M.C., Meteignier, L.V., Hong, N., Wang, G. & Moffett, P. (2015) Different roles for RNA silencing and RNA processing components in virus recovery and virus‐induced gene silencing in plants. Journal of experimental botany, 66, 919–932. [DOI] [PubMed] [Google Scholar]
- Ma, X., Zhang, Q., Zhu, Q., Liu, W., Chen, Y., Qiu, R. et al. (2015) A robust CRISPR/Cas9 system for convenient, high‐efficiency multiplex genome editing in monocot and dicot plants. Molecular Plant, 8, 1274–1284. [DOI] [PubMed] [Google Scholar]
- Mittler, R., Vanderauwera, S., Suzuki, N., Miller, G., Tognetti, V.B., Vandepoele, K. et al. (2011) ROS signaling: the new wave? Trends in Plant Science, 16, 300–309. [DOI] [PubMed] [Google Scholar]
- Mukhtar, M.S., McCormack, M.E., Argueso, C.T. & Pajerowska‐Mukhtar, K.M. (2016) Pathogen tactics to manipulate plant cell death. Current Biology, 26, R608–R619. [DOI] [PubMed] [Google Scholar]
- Munne‐Bosch, S., Queval, G. & Foyer, C.H. (2013) The impact of global change factors on redox signaling underpinning stress tolerance. Plant Physiology, 161, 5–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padh, H. (1990) Cellular functions of ascorbic‐acid. Biochemistry and Cell Biology, 68, 1166–1173. [DOI] [PubMed] [Google Scholar]
- Qi, Y.J., He, X.Y., Wang, X.J., Kohany, O., Jurka, J. & Hannon, G.J. (2006) Distinct catalytic and non‐catalytic roles of ARGONAUTE4 in RNA‐directed DNA methylation. Nature, 443, 1008–1012. [DOI] [PubMed] [Google Scholar]
- Qu, F., Ye, X. & Morris, T.J. (2008) Arabidopsis DRB4, AGO1, AGO7, and RDR6 participate in a DCL4‐initiated antiviral RNA silencing pathway negatively regulated by DCL1 . Proceedings of the National Academy of Sciences of the United States of America, 105, 14732–14737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholthof, H.B., Alvarado, V.Y., Vega‐Arreguin, J.C., Ciomperlik, J., Odokonyero, D., Brosseau, C. et al. (2011) Identification of an ARGONAUTE for antiviral RNA silencing in Nicotiana benthamiana . Plant Physiology, 156, 1548–1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shikata, E. & Kitagawa, Y. (1977) Rice black‐streaked dwarf virus – its properties, morphology and intracellular localization. Virology, 77, 826–842. [DOI] [PubMed] [Google Scholar]
- Song, J.J., Smith, S.K., Hannon, G.J. & Joshua‐Tor, L. (2004) Crystal structure of argonaute and its implications for RISC slicer activity. Science, 305, 1434–1437. [DOI] [PubMed] [Google Scholar]
- Wang, Y.W., Zhang, J.H., Yu, Y., Yu, J. & Huang, L. (2016) Inhibition of store‐operated calcium entry protects endothelial progenitor vells from H2O2‐induced apoptosis. Biomolecules & Therapeutics, 24, 371–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, Z., Xia, Y., Lin, S., Wang, Y., Guo, B., Song, X. et al. (2018) Osa‐miR164a targets OsNAC60 and negatively regulates rice immunity against the blast fungus Magnaporthe oryzae . The Plant Journal, 95, 584–597. [DOI] [PubMed] [Google Scholar]
- Wu, J., Yang, R., Yang, Z., Yao, S., Zhao, S., Wang, Y.u. et al. (2017) ROS accumulation and antiviral defence control by microRNA528 in rice. Nature Plants, 3, 16203. [DOI] [PubMed] [Google Scholar]
- Wu, J., Yang, Z., Wang, Y.u., Zheng, L., Ye, R., Ji, Y. et al. (2015) Viral‐inducible Argonaute18 confers broad‐spectrum virus resistance in rice by sequestering a host MicroRNA. eLife, 4, e05733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie, K., Li, L., Zhang, H., Wang, R., Tan, X., He, Y. et al. (2018) Abscisic acid negatively modulates plant defence against Rice black‐streaked dwarf virus infection by suppressing the jasmonate pathway and regulating reactive oxygen species levels in rice. Plant, Cell and Environment, 41, 2504–2514. [DOI] [PubMed] [Google Scholar]
- Yang, Z., Huang, Y.u., Yang, J., Yao, S., Zhao, K., Wang, D. et al. (2020) Jasmonate signaling enhances RNA silencing and antiviral defense in rice. Cell Host & Microbe, 28, 1–15. [DOI] [PubMed] [Google Scholar]
- Yang, Z. & Li, Y. (2018) Dissection of RNAi‐based antiviral immunity in plants. Current Opinion in Virology, 32, 88–99. [DOI] [PubMed] [Google Scholar]
- Yin, W.C., Xiao, Y.H., Niu, M., Meng, W.J., Li, L.L., Zhang, X.X. et al. (2020) ARGONAUTE2 enhances grain length and salt tolerance by activating BIG grain3 to modulate cytokinin distribution in rice. The Plant Cell, 32, 2292–2306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zebell, S.G. & Dong, X.N. (2015) Cell‐cycle regulators and cell death in immunity. Cell Host & Microbe, 18, 402–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, H., He, Y., Tan, X., Xie, K., Li, L., Hong, G. et al. (2019) The dual effect of the brassinosteroid pathway on Rice black‐streaked dwarf virus infection by modulating the peroxidase‐mediated oxidative burst and plant defense. Molecular Plant‐Microbe Interactions, 32, 685–696. [DOI] [PubMed] [Google Scholar]
- Zhang, X., Bao, Y., Shan, D., Wang, Z., Song, X., Wang, Z. et al. (2018) Magnaporthe oryzae induces the expression of a microRNA to suppress the immune response in rice. Plant Physiology, 177, 352–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, Z., Liu, X., Guo, X., Wang, X.J. & Zhang, X. (2016) Arabidopsis AGO3 predominantly recruits 24‐nt small RNAs to regulate epigenetic silencing. Nature Plants, 2, 16049. [DOI] [PubMed] [Google Scholar]
- Zheng, S., Li, J., Ma, L., Wang, H., Zhou, H., Ni, E. et al. (2019) OsAGO2 controls ROS production and the initiation of tapetal PCD by epigenetically regulating OsHXK1 expression in rice anthers. Proceedings of the National Academy of Sciences of the United States of America, 116, 7549–7558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou, G., Xu, D., Xu, D. & Zhang, M. (2013) Southern rice black‐streaked dwarf virus: a white‐backed planthopper‐transmitted fijivirus threatening rice production in Asia. Frontiers in Microbiology, 4, 270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou, T., Du, L., Wang, L., Wang, Y., Gao, C., Lan, Y. et al. (2015) Genetic analysis and molecular mapping of QTLs for resistance to rice black‐streaked dwarf disease in rice. Scientific Reports, 5, 10509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou, Y.u., Zhang, L., Zhang, X., Zu, H., Di, H., Dong, L. et al. (2017) Rice black‐streaked dwarf virus genome in China: diversification, phylogeny, and selection. Plant Disease, 101, 1588–1596. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
FIGURE S1 Validation of osago2, Crispr‐osago2, and OE‐OsAGO2 transgenic lines. (a) Schematic representation of the constructs used for OE‐OsAGO2 transgenic line with N‐terminal FLAG. Ubip: ubiquitin promoter. (b) The expression of OsAGO2 in NPB, osago2, Crispr‐osago2, and OE‐OsAGO2 transgenic plants was determined through quantitative reverse transcription PCR. The expression of rice 18s‐rRNA was used as an internal control. Error bars indicate the SD of different treatments with three biological replicates each. **p < .01. This experiment was conducted three times with similar results. (c) The expression of OsAGO2 in the two OE‐OsAGO2 lines was determined through western blot assay using a FLAG‐specific antibody. EV, empty vector. (d) Schematic representation of osago2 (NG8158) transposon‐insertion mutant with NPB background from the rice Tos17 insertion mutant database. Grey block, exon; black inverted triangle, transposon. (e, h) Schematic representation of the constructs used for Crispr‐osago2 transgenic lines (#6 and #13). Crispr‐osago2#6, 1 bp deletion; Crispr‐osago2#13, 1 bp insertion. (i, f) Verification of mutations in the two Crispr‐osago2 lines through PCR amplification and DNA sequencing. Forward and reverse sequencing was unimodal, indicating that Crispr‐osago2#6 and Crispr‐osago2#13 were homozygous mutants. (g, j) Amino acid comparison of OsAGO2 in NPB control and Crispr‐osago2 mutant. The deletion and insertion of one base results in premature termination of OsAGO2 translation
FIGURE S2 Expression of OsAGO3 in wild‐type and osago2 mutant lines. (a), Phylogenetic analysis of 19 rice AGO protein and the phylogenetic trees were draw. (b), Quantitative reverse transcription PCR analysis of the OsAGO3 transcript levels in the wild‐type (NPB), osago2, and Crispr‐osago2 mutant plants. The expression of rice 18s‐rRNA was used as an internal control. Error bars indicate the SD of different treatments with three biological replicates each. (c), The sequencing of homologous regions of OsAGO3 in the NPB and Crispr‐osago2 mutant plants. The sequences in red were the homologous target sits of Crispr‐osago2#6 and Crispr‐osago2#13
FIGURE S3 Effect of OsAGO2 on SBPH infestation. SBPH nymphs were rear on the NPB or individual mutant line seedlings (10 nymphs per seedling). Five days later, the survival rate of SBPH was recorded for each treatment. (a), Survival rates of SBPH on the NPB or other assayed lines are shown. Rice cv. IR50 was used as a SBPH resistant control. Error bars indicate the SD of individual treatments with three biological replicates per treatment. **p < 0.01. This experiment was done three times with similar results. (b), The numbers of SBPH maintained on different assayed lines at two days post SBPH infestation. **p < 0.01. This experiment was conducted three times with similar results
FIGURE S4OsAGO2 does not affect OsAGO1 mRNA expression. (a, b, c, d), Expressions of OsAGO1a, OsAGO1b, OsAGO1c, and OsAGO1d, respectively, in the mock‐inoculated (Mock) and RBSDV‐inoculated (RBSDV) NPB and osago2 plants were analysed through quantitative reverse transcription RT‐PCR at 0, 8, and 19 days postinoculation (dpi). Error bars indicate the SD of three biological replicates. This experiment was done three times with similar results
FIGURE S5 Bisulphite sequencing analysis of methylated cytosine at the OsHXK1 promoter. Bisulphite sequencing data of individual clones were submitted to the CyMATE program to analyse the methylated cytosines. Methylation of CpG, CpHpG, and CpHpHp in the mock‐inoculated and RBSDV‐inoculated NPB, osago2, and Crispr‐osago2 plants at 19 days postinoculation
FIGURE S6OsHXK1 does not induce cell death in Nicotiana benthamiana. N. benthamiana leaves were infiltrated with Agrobacterium tumefaciens carrying an empty vector (EV) or plasmids expressing OsAGO2, OsHXK1 or PcINF1 (a positive regulator of cell death). The leaf was photographed at 5 days postinfiltration. Scale bar, 1 cm
FIGURE S7 Validation of OsHXK1 expression in four transgenic lines. (a), Schematic representation of the constructs used for OE‐OsHXK1 transgenic line with N‐terminal FLAG. Ubip: ubiquitin promoter. (b), The expression of OsHXK1 in OE‐OsHXK1 transgenic lines was analysed through western blot assay using anti‐FLAG antibody. A rice line transformed with the empty vector (EV) was used as control. (c, f), Schematic representation of the constructs used for Crispr‐oshxk1 transgenic line (#1 and #2). Crispr‐oshxk1#1: 1 bp deletion. Crispr‐oshxk1#2: 7 bp deletion. (d, g), Verification of the CRISPR/Cas9 system OsHXK1 knockout lines by PCR‐based DNA sequencing. Forward and reverse sequencing were unimodal, indicating that Crispr‐oshxk1#1 and Crispr‐oshxk1#2 were homozygous mutants. (e, h), Amino acid comparison of OsHXK1 in NPB control and Crispr‐oshxk1 mutant. The deletion of one or several base results in premature termination of OsHXK1 translation
FIGURE S8 Effect of OsHXK1 on SBPH infestation. Survival rates (a) and feeding preference (b) of SBPH on NPB and OsHXK1 knockout or overexpression lines were tested through rearing SBPH nymphs on various seedlings. Rice cv. IR50 was used as a SBPH resistant control. **p < 0.01. This experiment was done three times with similar results
FIGURE S9 Validation of osago2/oshxk1 double mutant line. (a), The expression of OsAGO2 in NPB, osago2/oshxk1 double mutant (DM#17 and DM#30) was determined through quantitative reverse transcription PCR. The expression of rice 18s‐rRNA was used as an internal control. Error bars indicate the SD of different treatments with three biological replicates each. **p < 0.01. (b, d), Schematic representation of the constructs used for double mutant transgenic lines (DM#17 and DM#30). DM#17: 1 bp deletion. DM#30: 2 bp insertion. (c, e), Verification of the CRISPR/Cas9 system OsHXK1 knockout lines by PCR‐based DNA sequencing. Forward sequencing was unimodal, indicating that DM#17 and DM#30 were homozygous mutants. (f, g), Amino acid comparison of OsHXK1 in NPB control and double mutants. The mutation of one or two base results in premature termination of OsHXK1 translation. (h), Leaf tissues were collected from osago2 and DM (#17 and #30) plants, and analysed for H2O2 accumulation through 3.3′‐diaminobenzidine (DAB) staining at 48 hr postinoculation (hpi). Dark brown colour in the stained leaf tissues indicate the accumulation of H2O2. Scale bar, 1 mm. (i,j), Accumulation of reactive oxygen species (ROS) in the RBSDV‐inoculated osago2 or double mutant protoplasts were detected using the DCFH‐DA method followed by confocal microscopy or measurements using a microtitre plate reader at 48 hpi. Scale bar, 100 μm. Statistical significance between the mock‐inoculated and the RBSDV‐inoculated plants were determined using the Student’s t test. **p < 0.01. This experiment was conducted three times with similar results
FIGURE S10 A proposed model of the OsAGO2 regulates rice antiviral response. A proposed model of the OsAGO2‐OsHXK1 that regulates rice defence response against RBSDV infection. In this model, appropriate production of reactive oxygen species (ROS) was controlled by OsHXK1, which negatively regulated by OsAGO2
FIGURE S11 Phenotypes of the mock‐inoculated and SRBSDV‐inoculated OsAGO2 and OsHXK1 transgenic lines. (a, b), NPB, Crispr‐osago2 (Line#6 and #13), OE‐OsAGO2 (Line#1 and #2), Crispr‐oshxk1 (Line#1 and #2) and OE‐OsHXK1 (Line#3 and #5) plants were inoculated with mock‐inoculation or SRBSDV. The inoculated plants were photographed at 30 days postinoculation. Scale bar, 4 cm
TABLE S1 Primers used in the study
Data Availability Statement
All data supporting the conclusions of this article are provided within the article (and its Additional files). Sequence data from this study can be found in the Rice Genome Database (http://rice.plantbiology.msu.edu) and GenBank (https://www.ncbi.nlm.nih.gov/genbank) under the accession numbers OsAGO2 (LOC_Os04g52540), OsHXK1 (LOC_Os07g26540), OsNPR1 (LOC_Os01g09800), OsPR1a (LOC_Os07g03710), OsPR1b (LOC_Os01g28450), OsAGO1a (LOC_Os02g45070), OsAGO1b (LOC_Os04g47870), OsAGO1c (LOC_Os02g58490), OsAGO1d (LOC_Os06g51310), RBSDV S10 (AF227205), and SRBSDV S10 (JQ927009).


