Abstract
N6 methylation of adenosine (m6A) was recently discovered to play a role in regulating the life cycle of various viruses by modifying viral and host RNAs. However, different studies on m6A effects on the same or different viruses have revealed contradictory roles for m6A in the viral life cycle. In this study, we sought to define the role of m6A on infection by rice black streaked dwarf virus (RBSDV), a double‐stranded RNA virus, of its vector small brown planthopper (SBPH). Infection by RBSDV decreased the level of m6A in midgut cells of SBPHs. We then cloned two genes (LsMETTL3 and LsMETTL14) that encode m6A RNA methyltransferase in SBPHs. After interference with expression of the two genes, the titre of RBSDV in the midgut cells of SBPHs increased significantly, suggesting that m6A levels were negatively correlated with virus replication. More importantly, our results revealed that m6A modification might be the epigenetic mechanism that regulates RBSDV replication in its insect vector and maintains a certain virus threshold required for persistent transmission.
Keywords: N6 methylation of adenosine (m6A), persistent transmission, replication, rice black streaked dwarf virus, small brown planthopper (Laodelphax striatellus), virus titre
N6 methylation of adenosine modification might be the epigenetic mechanism that regulates rice black streaked dwarf virus replication in its insect vector and maintains a certain virus threshold for persistent transmission.
1. INTRODUCTION
Rice black streaked dwarf virus (RBSDV) (species Rice black streaked dwarf virus, genus Fijivirus, family Reoviridae) is transmitted by the small brown planthopper (SBPH, Laodelphax striatellus) in a persistent propagative manner (Hibino, 1996). Since RBSDV was first identified in Japan in 1952 (Kuribayashi & Shinkai, 1952), it has caused serious yield losses of rice and maize in East Asian countries, including several major outbreaks in China, Japan, and South Korea (Hibino, 1996; Wu et al., 2020). In nature, higher population densities of viruliferous SBPHs have largely contributed to RBSDV epidemics in rice and maize fields, especially at early stages of crop development (Chen & Zhang, 2005; Zhang, Wu, et al., 2021). At present, the best economical and effective method for managing RBSDV‐induced diseases is controlling vector insects due to the lack of disease‐resistant plant varieties. Therefore, understanding the transmission mechanism is crucial for accurate forecasting and disease control.
The genome of RBSDV contains 10 double‐stranded RNA (dsRNA) segments, S1 to S10, numbered in decreasing order of molecular weight (Milne et al., 2005). Most RBSDV genomic segments contain one open reading frame, but S5, S7, and S9 have two (Azuhata et al., 1993; Firth & Atkins, 2009; Isogai et al., 1998). Like other persistent and propagative viruses, RBSDV must initially infect, replicate, and produce viral mRNAs, which are translated to produce the viral proteins, then assemble novel virions in the epithelial cells of the midgut of the insect vector (Coombs, 2008; Mainou & Dermody, 2012). The major outer capsid protein of RBSDV, P10, plays key roles in establishing primary infection in the midgut epithelium (Than et al., 2016). Its nonstructural proteins, P5‐1, P6, and P9‐1, are components of viroplasms, the site of virus replication to generate sufficient titres for successful transmission (Jia, Chen, Zheng et al., 2012; Mao et al., 2013). Once the insects become viruliferous, they retain and transmit virus throughout their life, without visible effects on the behaviour and physiology of the insect (Hajano et al., 2015; Hughes et al., 2008). Although numerous studies of viral replication, differential expression of mRNAs, and protein functions during RBSDV infection have been carried out, the reasons that vector insects can retain and transmit virus in their lifetime have been unclear.
The most abundant internal modification of eukaryotic mRNA, the N6‐methyladenosine (m6A) modification, was first identified in the 1970s and has been found to regulate various biological processes in mammals, insects, plants, and yeasts through modulating many aspects of RNA life such as pre‐mRNA processing, promoting mRNA nuclear export, altering mRNA stability, increasing translation efficiency, and facilitating noncanonical translation initiation (He & He, 2021; Saletore et al., 2012; Zhou et al., 2015). The m6A mRNA methyltransferase complex was purified in the 1990s, with METTL3 identified as a key component (Bokar et al., 1997). Subsequently, METTL3 was found to form a heterodimer with METTL14 to convert A to m6A on mRNA (Liu et al., 2014). METTL3 is the catalytically active methyltransferase, and METTL14 is structurally essential to facilitate catalysis (Liu et al., 2014; Wang et al., 2014). Among invertebrates, BmMETTL3, BmMETTL14, and cytoplasmic YTH‐domain family 3 (BmYTHDF3) have been identified in Bombyx mori (Zhang et al., 2020). Mutants of METTL3, METTL14, and YTHDC1 in Drosophila exhibit a common suite of molecular and phenotypic defects (Kan et al., 2017). However, no orthologs of METTL3 or METTL14 have been found in Caenorhabditis elegans, but F33A8.4 and C38D4.9 have been identified as orthologs of ZC3H13 and METTL5 (Sendinc et al., 2020).
Transcriptome‐wide profiling of m6A through immunoprecipitation coupled with next‐generation sequencing has demonstrated that m6A modification plays a role in regulating the life cycle of many viruses (Dang et al., 2019; Williams et al., 2019). Viral gene expression, replication, and assembly of progeny virions are influenced by m6A modifications in viral RNAs during virus infection (Gokhale et al., 2016; Lichinchi, Gao, et al., 2016). In addition, modifications of host mRNAs by m6A can enhance viral infection or host resistance (Lichinchi, Gao, et al., 2016; Lichinchi, Zhao, et al., 2016). However, different reports on m6A effects in the same or different viruses have revealed contradictory roles for m6A in the viral life cycle. In this study, we sought to define the role of m6A on RBSDV infection in its vector SBPHs. We found that RBSDV infection decreased the level of m6A in the midgut cells of SBPHs. We cloned two genes (LsMETTL3 and LsMETTL14) that encode m6A RNA methyltransferase in the SBPH. Furthermore, after interference with the expression of LsMETTL3 and Ls METTL14, the m6A level was negatively correlated with RBSDV titre in the midgut cells of SBPHs, indicating that m6A modification might be the epigenetic mechanism for regulating RBSDV replication in its vector insect.
2. RESULTS
2.1. RBSDV‐viruliferous SBPHs have a decreased level of m6A modification
To clarify the changes to the m6A modification in SBPHs after RBSDV acquisition, we first quantified the m6A level of nonviruliferous SBPHs and viruliferous SBPHs after a 3‐day acquisition access period (AAP) and a 6‐day circulative period. In nonviruliferous SBPHs, the mean level of m6A was 0.14 ng/200 ng total RNA, significantly higher (p < 0.01) than in RBSDV‐viruliferous SBPHs (0.11 ng/200 ng total RNA) (Figure 1a). Because the midgut epithelium is the main location for replication of RBSDV, individual midguts of SBPHs after the 6‐day circulative period were excised and incubated with anti‐m6A antibody, anti‐RBSDV P10 antibody, and DyLight 633 phalloidin to label actin, then observed with a confocal laser‐scanning microscope. Compared with the m6A fluorescence intensity from the midgut epithelial cells of nonviruliferous SBPHs (mean 18.10 relative fluorescence units [RFU]/10 cells), the m6A fluorescence intensity from the cells of RBSDV‐viruliferous SBPHs was significantly lower (mean 10.10 RFU/10 cells) (Figure 1b,c), suggesting that the level of m6A modifications was lower in RBSDV‐viruliferous SBPHs.
FIGURE 1.
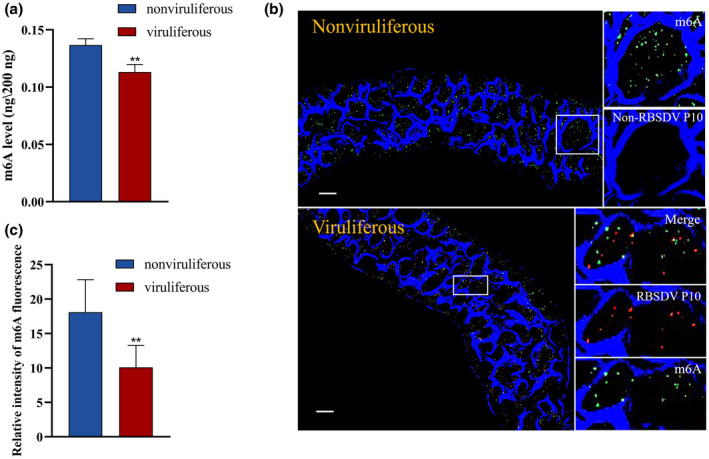
N6 methylation of adenosine (m6A) level in rice black streaked dwarf virus (RBSDV)‐viruliferous small brown planthopper (SBPHs) was lower than that in nonviruliferous SBPHs. (a) m6A levels of nonviruliferous and viruliferous SBPHs detected by an EpiQuik m6A RNA Methylation Quantification Kit (Colorimetric). (b) Fluorescence of m6A and RBSDV in midgut epithelial cells of nonviruliferous and viruliferous SBPHs. Excised midguts were incubated with anti‐m6A (green label) and anti‐RBSDV (red label) antibodies and DyLight 633 phalloidin (blue), then observed with a confocal laser‐scanning microscope. Scale bars, 20 μm. (c) Relative intensity of m6A fluorescence. Ten midgut epithelial cells each from nonviruliferous SBPHs and viruliferous SBPHs were considered. Significant differences between mean values for relative intensity were determined by Student's t test using Prism 8 (**p < 0.01)
2.2. Cloning of two genes (LsMETTL3 and LsMETTL14) encoding methyltransferases in SBPH
To further study the role of m6A in RBSDV infection, we first identified two genes encoding m6A methyltransferase (METTL3 and METTL14) in SBPH. Based on the sequences of NlMETTL3 and NlMETTL14, which encoded methyltransferases in the brown planthopper (Nilaparvata lugens), the coding sequences (CDSs) of METTL3 and METTL14 in SBPH were cloned and designated as LsMETTL3 and LsMETTL14. The sequences comprised 1,809 bp and 1,188 bp and were predicted to encode 602 amino acid and 395 amino acid sequences, respectively (GenBank accessions MW589259 and MW589260). The structure analysis using the NCBI conserved domain search (https://www.ncbi.nlm.nih.gov/Structure/cdd/wrpsb.cgi) revealed that the two proteins have a common MT‐A70 domain (Figure 2a), which was originally identified as the S‐adenosylmethionine‐binding subunit of human N6‐adenosine methyltransferase, which sequence‐specifically methylates adenines in pre‐mRNAs (Bokar et al., 1997).
FIGURE 2.
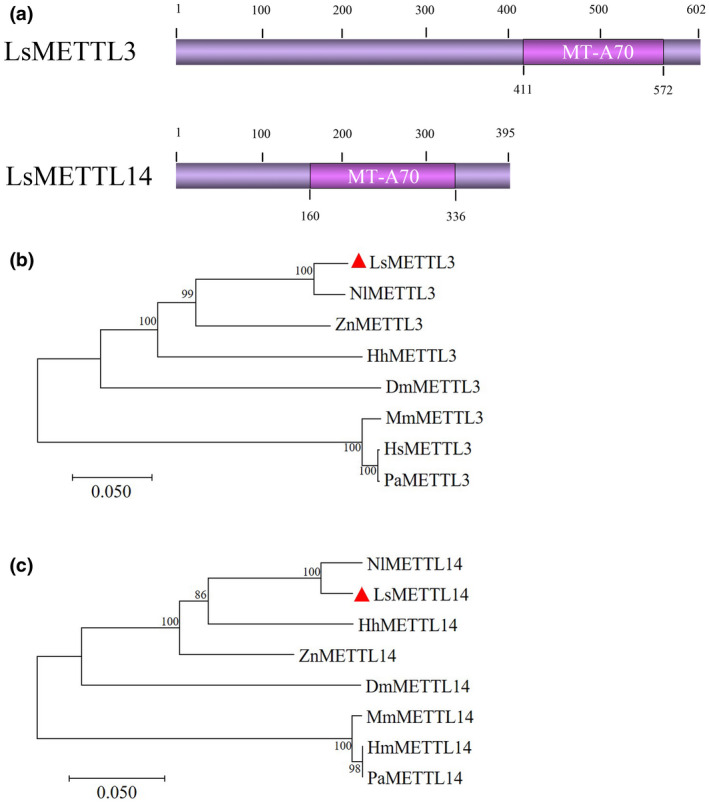
Analysis of LsMETTL3 and LsMETTL14. (a) Conserved domains MT‐A70 of LsMETTL3 and LsMETTL14 determined by a NCBI conserved domain search. (b) Phylogenetic tree of METTL3 of various species. Amino acid sequences for orthologs of LsMETTL3 and LsMETTL14 from other species were downloaded from NCBI. (c) Phylogenetic tree of METTL14 of various species. Phylogenetic trees were constructed using the neighbour‐joining method with 1,000 bootstrap replicates in MEGA 7.0. Nl, Nilaparvata lugens; Dm, Drosophila melanogaster; Ls, Laodelphax striatellus; Zn, Zootermopsis nevadensis; Hh, Halyomorpha halys; Hs, Homo sapiens; Mm, Mus musculus; Pa, Pongo abelii
2.3. Evolutionary conservation of m6A methyltransferases
To evaluate the relationship of m6A methyltransferase among SBPH and other species, we used the NCBI blastp search to compare METTL3 and METTL14 deduced amino acid sequences of SBPH with those of 15 other insect species belonging to Hemiptera, Blattaria, Thysanoptera, Coleoptera, Lepidoptera, and Hymenoptera, and determined sequence identities (Tables S1 and S2). The comparisons showed that LsMETTL3 was more similar to METTL3 from insects belonging to Hemiptera, Blattaria, and Thysanoptera, with the highest identity (88.56%) shared with NlMETTL3. LsMETTL14 was also more similar to METTL14 from insects belonging to Hemiptera, Coleoptera, and Blattaria, with the highest identity (95.00%) shared with NlMETTL14. We selected the METTL3 amino acid sequences from four insects (N. lugens, Drosophila melanogaster, Zootermopsis nevadensis, Halyomorpha halys) and from three mammals (Homo sapiens, Mus musculus, Pongo abelii) to construct the METTL3 phylogenetic tree (Figure 2b). The result showed that LsMETTL3 was much closer to NlMETTL3, and the METTL3 of Mammalia and Insecta were, respectively, clustered, which is consistent with the evolutionary relationship of species; and the result was the same as obtained for the phylogenetic tree of METTL14 (Figure 2c). Subsequent multiple sequence alignments of the deduced amino acid sequences of METTL3 and METTL14 from L. striatellus, N. lugens, D. melanogaster, and H. sapiens suggested that they have highly conserved MT‐A70 domains (Figure 3a,b). These results illustrate that m6A methyltransferases are evolutionarily conserved among species.
FIGURE 3.
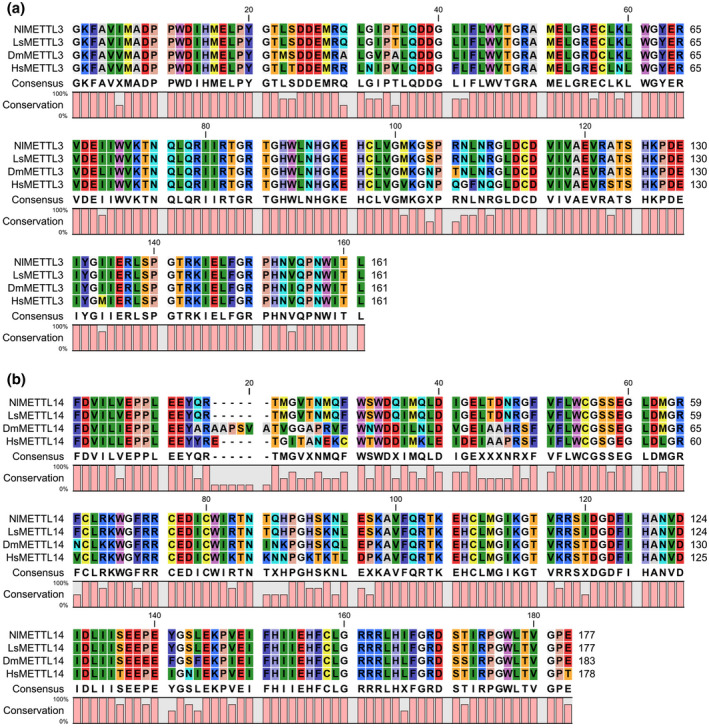
Multiple sequence alignments of MT‐A70 domain of (a) METTL3 and (b) METTL14 from various species. Alignments were done with CLC Sequence Viewer7. Nl, Nilaparvata lugens; Dm, Drosophila melanogaster; Ls, Laodelphax striatellus; Hs, Homo sapiens
2.4. RNA interference of LsMETTL3 and LsMETTL14 reduces m6A methylation levels in SBPH
To determine whether LsMETTL3 and LsMETTL14 are involved in the modification of m6A, RNA interference (RNAi) was used to interfere with the expression of LsMETTL3 and LsMETTL14 in SBPH. The dsRNAs of LsMETTL3 and LsMETTL14 were synthesized and quality confirmed using agarose gel electrophoresis (Figure S1). When a mixture of dsRNAs of dsLsMETTL3 and dsLsMETTL14 (dsLsMETTL3+14) was injected into the nonviruliferous third‐instar nymphs, the mRNA level of LsMETTL3 and LsMETTL14 was between 61% to 79% lower in the injected insects than in the controls at 0.5 to 9 days (Figure 4a,c). In addition, after insects were injected with dsLsMETTL3, dsLsMETTL14 or dsLsMETTL3+14, the average level of m6A in SBPHs decreased, respectively, to 0.132, 0.134, and 0.127 ng/200 ng total RNA compared with the level in the SBPHs injected with dsGFP (0.161) (Figure 4b; respective p values of 0.04, 0.08 and 0.01 were determined using Student's t test).
FIGURE 4.
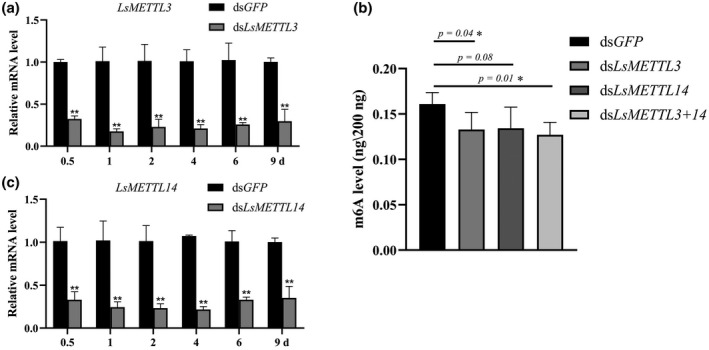
Interference of LsMETTL3 and LsMETTL14 expression after dsRNAs injection and negative effect on N6 methylation of adenosine (m6A) level. (a) Relative expression of LsMETTL3 after small brown planthopper (SBPH) nymphs were microinjected with dsLsMETTL3+14 or dsGFP. (b) Changes in m6A level in insects after injection with dsGFP, dsLs METTL3, dsLs METTL14, or dsLs METTL3+14 (dsLsMETTL3+ dsLsMETTL14). dsGFP was used as an RNA interference (RNAi) control. (c) Relative expression of LsMETTL14 after SBPH nymphs were microinjected with dsLsMETTL3+14 or dsGFP. Significant differences between the mean values were determined using Student's t test (*p < 0.05, **p < 0.01) in Prism 8
We then asked whether the RNAi of LsMETTL3 and LsMETTL14 impacted the expression of other genes that have similar function with LsMETTL3 and LsMETTL14, the expression level of orthologues of Fl(2)d and virilizer that also encode m6A methyltransferases in SBPHs were quantified. After the injection of dsLsMETTL3+14, the mRNA level of Fl(2)d began to rise at 4 days, peaked (at 60% increase) at 6 days, then declined (Figure S2a), but the mRNA level of virilizer did not show the same tendency (Figure S2b).
2.5. Effect of m6A level reduction on virus replication
To analyse the relationship between m6A level and virus replication in SBPHs, we injected dsLsMETTL3+14 or dsGFP into third‐instar nymphs that had been fed on RBSDV‐infected rice plants for a 3‐day AAP, then quantified RBSDV titres in treated insects at 6 days using quantitative reverse transcription PCR (RT‐qPCR) and quantitative PCR (qPCR) primers (Table S3). The results showed an average of 429.9 RBSDV copies in SBPHs injected with dsLsMETTL3+14, significantly more than in SBPHs injected with dsGFP (190.3) (Figure 5a). Total protein was also extracted for western blots probed with either anti‐RBSDV P10 antibody or anti‐actin antibody, and the RBSDV P10 band from SBPHs injected with dsLsMETTL3+14 was distinctly less intense than that of SBPHs injected with dsGFP (Figure 5b). Compared with the fluorescence from dsGFP‐injected SBPHs (11.22 RFU/10 cells) observed with a confocal laser‐scanning microscope, the fluorescence intensity for m6A in the midguts of dsLsMETTL3+14‐injected SBPHs was significantly less intense (5.22 RFU/10 cells), but RBSDV titres were higher in SBPHs injected with dsLsMETTL3+14 than in those injected with dsGFP based on the fluorescence intensity for P10 from RSBDV (12.22 vs 7.33 RFU/10 cells) (Figure 5c,d).
FIGURE 5.
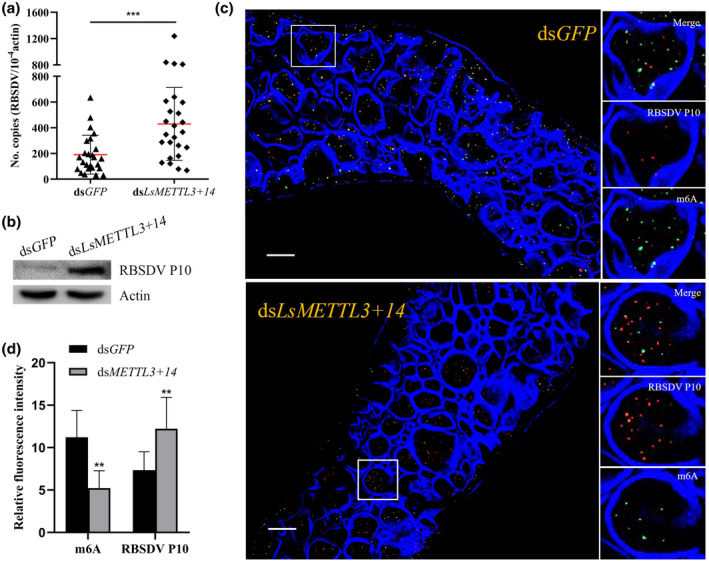
Depletion of N6 methylation of adenosine (m6A) increases the rice black streaked dwarf virus (RBSDV) titres. (a) RBSDV titres in insects injected with either dsLsMETTL3+14 or dsGFP. Each dot represents one RBSDV‐infected small brown planthopper (SBPH) that had been injected with either dsLsMETTL3+14 or dsGFP and collected at 6 days. Data were collected from three independent experiments. dsGFP was used as an RNA interference (RNAi) control. RBSDV titres shown as copies/10−4 actin. Significant differences between mean copies of RBSDV in insects injected with either dsLsMETTL3+14 or dsGFP were determined by Prism 8 using Student's t test (***p < 0.001). (b) Western blots of insects injected with either dsLsMETTL3+14 or dsGFP. Total protein was extracted and treated with anti‐RBSDV P10 antibody and anti‐actin antibody, respectively. (c) Fluorescence intensity of m6A and RBSDV in midgut epithelial cells. Excised midguts were incubated with anti‐m6A (green label), anti‐RBSDV (red label) antibodies, and DyLight 633 phalloidin (blue, labelling actin), then observed with a confocal laser‐scanning microscope. Scale bars, 20 μm. (d) Fluorescence intensity of m6A and RBSDV. Ten midgut epithelial cells of SBPHs injected with dsLsMETTL3+14 or dsGFP were evaluated. Significant differences between mean relative intensity of m6A or RBSDV in epithelial cells of SBPHs injected with dsLsMETTL3+14 or dsGFP were determined using Student's t test (**p < 0.01)
3. DISCUSSION
The m6A modification of eukaryotic RNA is a cotranscriptional modification that can regulate RNA biology (Saletore et al., 2012; Zhou et al., 2015). Identification of the methyltransferase complex for catalysing m6A formation in RNA is essential for understanding the functions of m6A modification. In mammals, the core components of the complex include METTL3, METTL14, Wilms’ tumor 1‐associating protein (WTAP), and others such as METTL5, KIAA1429, and ZC3H13 (Liu et al., 2014; Wang et al., 2014). Several recent studies have shown that m6A modification is also a conserved feature of mRNA in plants and that it plays a critical regulatory role in plant development (Arribas‐Hernandez et al., 2018; Zhang et al., 2019). In Arabidopsis thaliana, orthologs of METTL3, METTL14, and WTAP have been identified as AtMTA, AtMTB, and AtFIP37 (Ruzicka et al., 2017). In rice, OsFIP and OsMTA2 are orthologs of METTL3 and WTAP (Zhang et al., 2019). Among insects, the most studied m6A modification is in the model species D. melanogaster. In Drosophila, the orthologs of METTL3, METTL14, WTAP, and KIAA1429 are Ime4, dMettl14, Fl (2)d, and Virilizer, respectively (Kan et al., 2021). The decrease of Fl(2)d in Drosophila reduces the interaction between Ime4 and dMettl14 (Lence et al., 2016), similar to the function of WTAP in mammals. In addition, the m6A methyltransferase complex has also been studied in B. mori and C. elegans. In Bombyx, BmMETTL3 and BmMETTL14 have been identified as orthologs of METTL3 and METTL14 (Zhang et al., 2020), while in Caenorhabditis, F33A8.4 and C38D4.9 have been identified as the orthologs of ZC3H13 and METTL5 (Sendinc et al., 2020). They all function as methyltransferases. Depletion or overexpression of BmMETTL3 and BmMETTL14 promotes or inhibits the expression of Bombyx mori nuclear polyhedrosis virus (BmNPV) structural protein VP39. Here, similar to BmMETTL3 and BmMETTL14 in Bombyx, we first cloned two genes LsMETTL3 and LsMETTL14 encoding m6A methyltransferases in SBPH. Interfering with the expression of LsMETTL3 and LsMETTL14 decreased m6A mRNA methylation, and virus replication increased in SBPHs. Thus, LsMETTL3 and LsMETTL14 may be the components of the cellular machinery controlling m6A modification for SBPH and RBSDV RNAs. However, unlike BmNPV in Bombyx, based on our long‐term observations, nonviruliferous and RBSDV‐infected SBPHs do not differ significantly in their growth or development (Hajano et al., 2015; Wu et al., 2019). Our study found that m6A on RBSDV might be the epigenetic mechanism that regulates virus replication in its insect vector and thus maintains a certain virus threshold required for persistent transmission. We also found orthologs of Fl(2)d and Virilizer in SBPHs. Interestingly, after injection with dsLsMETTL3+14, there was a striking contrast in the trend in the change between LsMETTL3/14 and Fl(2)d expression. When the expression of LsMETTL3/14 decreased, that of Fl(2)d increased, which might compensate for the decreased expression of LsMETTL3/14; when the expression level of LsMETTL3/14 rose again, expression of Fl(2)d fell. These results indicated that the m6A modification process may be an intricate one. Similar to the situation in mammals and plants, m6A modification can take place after the export of insect mRNAs from the nucleus or after early and late viral mRNA is transcribed from viral dsRNAs in the cytoplasm (Wu et al., 2020).
Recent discoveries showed that m6A is involved in regulating the life cycle of many viruses through the modification of viral and host RNAs (Dang et al., 2019; Williams et al., 2019). Replication and infection by viruses in family Flaviviridae (hepatitis C virus [HCV] and Zika virus [ZIKV]) are negatively regulated by m6A methyltransferase (Gokhale et al., 2016; Lichinchi, Zhao, et al., 2016). During HCV infection, m6A negatively regulates the production of infectious particles (Gokhale et al., 2016). Similar to HCV infection, ZIKV infection is also inhibited by m6A through a combination of direct events regulating viral RNA metabolism (i.e., YTHDF proteins binding to ZIKV RNA) and indirect posttranscriptional regulation of host RNAs, implying that features of m6A regulation may be shared among Flaviviridae members (Lichinchi, Zhao, et al., 2016). Because some Flaviviridae members are transmitted by mosquitoes, m6A might also regulate virus transmission by the insect vector. Regulation of m6A abundance in genomic RNAs of HCV allows the virus to replicate at low rates, evading the host immune system and enabling the establishment of persistent infections (Gokhale et al., 2016). Such a situation may also exist in plants: in the genomes of several single‐stranded RNA plant viruses in family Flexiviridae, a conserved AlkB domain in the replicase polyprotein has been identified that can repair deleterious RNA genome methylation damage, suggesting that this domain may be biologically relevant in preserving the viability of the viral genome (van den Born et al., 2008; Bratlie & Drabløs, 2005). These studies indicate that viruses can affect their hosts through multiple methylation mechanisms. In this study, we found that RBSDV‐viruliferous SBPHs have a lower level of m6A modifications compared with nonviruliferous insects, although there is no significant change in the transcription of genes encoding m6A methyltransferases (Figure S3). Once the m6A modification was inhibited by interfering with the expression of LsMETTL3 and LsMETTL14, RBSDV titres in the midgut cells of SBPH increased significantly compared with the control. These results suggest that m6A may negatively modulate virus replication and prevent severe harm for vector insects, but the virus in turn suppresses m6A modification, which allows the virus to persist in the insect vector.
Our previous studies demonstrated that RBSDV is present at significantly different titres in its host plants and vector insects (Hajano et al., 2015, 2016). The number of RBSDV genome equivalent copies (GEC) in highly susceptible rice cultivars Huaidao 5 and Yuandao 1 is 7,151.7 ± 11 and 15,683.2 ± 35, respectively, yet RBSDV‐infected plants of both cultivars have pronounced stunting, dark‐green and twisted leaves, and white waxy galls and grey streaks on the stem. The RBSDV GEC in seven resistant cultivars with less severe symptoms also varied widely from 200.2 ± 12 to 679.5 ± 98, but was much lower than in the highly susceptible cultivars (Hajano et al., 2016). All this evidence suggests a close correlation between virus titre and symptom expression in host plants. Correspondingly, the RBSDV GEC in the whole body of the vector SBPH ranged from 513.5 ± 88.4 at day 0 to 816.8 ± 110.7 at day 14 after a 3‐day AAP on infected plants (Hajano et al., 2015). Because viruses transmitted in a persistent and propagative manner must infect, multiply, and spread in their insect vector, virus infections would be expected to have multiple effects on the behaviour and physiology of their vector insects, but the reports are contradictory. Feeding of white‐backed planthoppers (WBPHs, Sogatella furcifera) on rice plants infected with southern rice black streaked dwarf virus (SRBSDV) prolongs the duration of nymphal stages and shortens the lifespan, survival rate, and fecundity of adults (Xu et al., 2016). In contrast, another study reported that SRBSDV‐viruliferous WBPHs have increased fecundity and population sizes of macropterous adults (Zhang et al., 2014). Research on RBSDV‐WBPH fitness has shown that nymph survival, sex ratio, female fecundity, and egg hatchability did not significantly differ on the virus‐infected plant compared with the uninfected healthy plant (He et al., 2011). Usually, these adverse or beneficial changes are due to the changes of physiological and metabolic components in host plants caused by virus–plant interactions, rather than direct effects caused by the virus infection in vector insects (Di Feo et al., 2010; Zhang et al., 2014). Our long‐term observations indicate that viruliferous SBPHs reared on healthy rice plants develop well and retain and transmit virus throughout their life (Hajano et al., 2015; Qin et al., 2018; Wang et al., 2019; Wu et al., 2019).
Moreover, the virus threshold in the insect vector has been demonstrated as a key factor for the transmission of several arboviruses (Hajano et al., 2020; Hardy, 1988). Females of Culex tarsalis do not transmit western equine encephalomyelitis virus when the viral load is low (103 pfu/0.1 ml; Mahmood et al., 2006). Maize fine streak virus titres in its vector black‐faced leafhopper (Graminella nigrifrons) and transmission efficiency increased along with longer AAPs, but no transmission occurred when the AAP was shorter than 4 weeks (Todd et al., 2010). RBSDV content in the gut of SBPHs can reach a higher copy number than in nonvector insects, which may enable the virus to spread from the gut into the haemolymph, fat body, and salivary gland (Jia, Chen, Mao, et al., 2012). As a nonvector of SRBSDV, SBPH had a significantly lower number of SRBSDV GEC in its gut, which may not be high enough for the virus to spread into the haemolymph (Hajano et al., 2015). These reports indicate that due to a special feature, vector insects can tolerate a certain amount of virus without any visible or measurable adverse effects. Our results provide evidence that the vector insect allows the virus to replicate in cells, but a mechanism has evolved by which m6A can restrict excessive virus accumulation.
In summary, our results showed that RBSDV acquisition affected and downregulated the level of m6A modification in its vector insects. After interference of the expression of two genes encoding methyltransferase, RBSDV titres in midgut cells of SBPHs were significantly higher, indicating that the m6A modification might be the epigenetic mechanism that regulates RBSDV replication in its vector insect and allows a certain amount of virus to accumulate in the body for persistent transmission. To gain further mechanistic insights, we need to examine whether the RNAs of RBSDV are methylated or whether the host responds to infection with a posttranscriptional regulation of its own RNAs.
4. EXPERIMENTAL PROCEDURES
4.1. Insects
Nonviruliferous (RBSDV‐free) SBPHs were originally collected in Beijing, China, and maintained in the laboratory for 3 years. The insects were reared individually on a 5–10‐cm tall rice seedling (cv. Huangjinqing) in a glass beaker containing soil at a depth of 2–4 cm. Plants were maintained in an incubator at 27 ± 1 °C, with 80 ± 5% relative humidity and 12‐hr light/12‐hr dark. In these conditions, SBPH development took 7 days for the egg stage, 15–18 days for the nymph stage, and 10–12 days for the adult stage. During the 30–37 days developmental period, the SBPHs were transferred to a fresh seedling every 7–12 days for sufficient nutrition.
4.2. Virus‐infected plants
We brought RBSDV‐infected rice plants from the field to our laboratory and allowed SBPHs to feed on the plants for 3 days. We then transferred these RBSDV‐viruliferous SBPHs to healthy rice plants for transmission in the greenhouse using the following protocol. Two or three rice seeds (cv. Huangjinqing) were planted in a plastic pot containing nutrient soil in a growth chamber (27/24 °C with a 16‐hr light/8‐hr dark cycle). When rice seedlings were 2–3 cm high, more than five RBSDV‐viruliferous SBPHs were placed on each seedling for 5 days. Then the insects were removed and plants were grown for 3 weeks in the growth chamber. After 3 weeks, RT‐PCR (described in section 4.3) was used to test the rice plants for RBSDV when symptoms appeared using primer pair RBSDV‐S10‐F/R (Table S3).
4.3. RT‐PCR
Total RNA was extracted from the rice leaves using TRIzol reagent (Vazyme) according to the manufacturer's protocol, then used for reverse transcription to synthesize cDNA synthesis using 3 µl RNA, 2 µl random primers (2.5 mM), and 7.5 µl double distilled water that was incubated at 95 ℃ for 2 min, then cooled on ice for 2 min. After adding 4 µl MMLV 5× buffer, 2 µl dNTP mixture, 1 μl MMLV reverse transcriptase (Thermo Fisher Scientific), and 0.5 µl recombinant RNase inhibitor (Takara) to the mixture, the mixture was incubated at 37 ℃ for 1 hr and 72 ℃ for 10 min. For the RT‐PCR, the 20‐µl reaction volume (10 µl of PCR Mix [Mei5 Biotechnology, Beijing, China], 0.5 µl of each primer [10 µM], 2 µl of template cDNA, and 7 µl double distilled water) and Biometra advanced PCR amplifier (Analytik Jena) was run in a thermocycler (Biometra Tadvanced, Thermo Fisher Scientific) at 95 °C for 3 min; 35 cycles of 95 °C for 15 s, 55 °C for 30 s, and 72 °C for 1 min; with a final extension of 72 °C for 10 min.
4.4. Quantification of m6A
More than 150 nonviruliferous SBPHs were placed on RBSDV‐infected rice plants for a 3‐day AAP, then transferred to healthy rice plants for a 6‐day circulative period. Total RNA was extracted from individual insects using TRIzol reagent and the manufacturer's protocol and quantified using a NanoDrop 2000 spectrophotometer (Thermo Fisher Scientific), then reverse transcribed into cDNA according to the protocol described in section 4.3 and tested for RBSDV by RT‐PCR using RBSDV‐S10‐F/R primers (Table S3). More than 40 RBSDV‐viruliferous SBPHs were obtained, and 30 were used to quantify m6A levels using the EpiQuik m6A RNA Methylation Quantification Kit (Colorimetric) (Epigentek) as recommended by the manufacturer; 30 nonviruliferous SBPHs were tested as the control. In brief, 200 ng of each RNA sample (1–3 µl) was added to a separate well, and at the same time the standard substances (negative control and diluted positive control with 1× Wash Buffer in six concentrations: 0.01, 0.02, 0.05, 0.1, 0.2, 0.5 ng/µl) were added to separate respective wells, and 80 µl RNA binding solution was added to each well. After 1.5 hr at 37 °C, wells were washed three times with 1× wash buffer, then 50 µl of capture antibody was added, the solution was incubated for a 1 hr at 25 °C, and the wells were washed again three times with the buffer. After 50 µl of detection antibody was added, the solution was incubated for 0.5 hr at 25 °C, then washed four times; 50 µl of enhancer solution was added and the solution was incubated for 0.5 hr at 25 °C, then the wells were washed five times with the buffer. Lastly, 100 μl of developer solution was added and the solution was incubated for 2–15 min at 25 °C, then 100 μl stop solution was added. Absorbance (A) at 450 nm was measured with a microplate reader. The absolute amount of m6A was calculated as m6A (ng) = (ASample – ANegative control)/slope, using the slope calculated from the standard curve generated from A at various concentrations of the standard.
4.5. Cloning of LsMETTL3 and LsMETTL14
The SBPH genome in NCBI and an unpublished transcriptomic database (from sequences previously generated in our laboratory) were searched for orthologues of N. lugens genes of RNA methyltransferases METTL3 and METTL14 (Zhu et al., 2017) to design primers to amplify the CDS of LsMETTL3 and LsMETTL14 (Table S3). Total RNA was isolated from 15 to 20 SBPHs using the method described above, and the quality and concentration of total RNA were determined with the NanoDrop 2000 (Thermo Fisher Scientific). The extracted RNA (500 ng) was subsequently used for reverse transcription in a 20‐µl mixture as described in section 4.3. The CDS of LsMETTL3 and LsMETTL14 was amplified by PCR using the CDS primers (Table S3), and all PCR products were gel purified and ligated into separate pEASY‐T5 Zero cloning vector according to the manufacturer's instructions (TransGen Biotech). These plasmids were then used to transform Escherichia coli DH5α cells. Cells containing the recombinant plasmid were selected using ampicillin (50 mg/ml), and the presence of the plasmid insert was verified by PCR. Subsequently, the CDS of LsMETTL3 and LsMETTL14 in the plasmids were sequenced by Sanger sequencing (Sangon). Finally, the recombinant plasmid DNA with the confirmed sequence was extracted using an Axyprep Plasmid Miniprep Kit (Axygen Biosciences).
4.6. Analysis of LsMETTL3 and LsMETTL14
The MT‐A70 conserved domain of LsMETTL3 and LsMETTL14 was analysed using the NCBI conserved domain search (https://www.ncbi.nlm.nih.gov/Structure/cdd/wrpsb.cgi). Amino acid sequences for orthologs of LsMETTL3 and LsMETTL14 from other species including D. melanogaster, H. sapiens, and numerous insect species belonging to Hemiptera, Blattaria, Thysanoptera, Coleoptera, Lepidoptera, and Hymenoptera were downloaded from NCBI. An NCBI blastp search was then used to determine the percentage identity shared with LsMETTL3 or LsMETTL14. A multiple sequence alignment of MT‐A70 was then generated by CLC Sequence Viewer 7 (CLC bio), and phylogenetic trees were constructed using the neighbour‐joining (NJ) method with 1,000 bootstrap replicates in MEGA 7.0 (Kumar et al., 2016).
4.7. RNA interference
Using the primers for dsRNA synthesis listed in Table S3 and plasmids containing pEASY‐LsMETTL3, pEASY‐LsMETTL14, or pEASY‐GFP (kept in our laboratory), dsRNAs were synthesized using the T7 RiboMAX Express RNAi System kit using the protocol provided by the manufacturer (Promega), then quality tested by agarose gel electrophoresis. One hundred nonviruliferous instar nymphs of SBPHs were microinjected with 23 nl of 3.0 μg/μl of a 1:1 mixture of dsLsMETTL3 + dsLsMETTL14 or dsGFP using an Auto‐Nanoliter Injector (Drummond) and our laboratory protocol (Qin et al., 2018). After 0.5, 1, 2, 4, 6, and 9 days, the transcript level of LsMETTL3 and LsMETTL14 was quantified by RT‐qPCR (described in section 4.8). Forty nonviruliferous third‐instar nymphs were then injected with dsLsMETTL3, dsLsMETTL14, dsLsMETTL3+14 or dsGFP and after 6 days the level of m6A was quantified as described above. Three hundred RBSDV‐viruliferous third‐instar nymphs that had been fed on RBSDV‐infected plants for a 3‐day AAP were injected with dsLsMETTL3+14 or dsGFP and divided into three groups to quantify changes in levels of RBSDV and m6A at 6 days; each group included SBPHs microinjected with dsLsMETTL3+14 or dsGFP. The first group of SBPHs was used for RT‐qPCR and absolute quantification of the number of copies of RBSDV, the second group of SBPHs was used for western blot analysis of RBSDV and actin, and the midgut of the third group of SBPHs was excised for an immunofluorescence assay of RBSDV and actin (Zhang, Liu, et al., 2021). Each experiment was repeated three times.
4.8. RT‐qPCR with relative quantification analysis
Total RNA was extracted from SBPHs as described above, and first‐strand cDNA was synthesized by the All‐in‐One SuperMix kit as recommended by the manufacturer (TransGen Biotech). The primers used to detect LsMETTL3, LsMETTL14, and LsActin (control) primers were designed based on the respective LsMETTL3, LsMETTL14, and LsActin sequences. RT‐qPCR was conducted using an ABI‐7500 thermocycler (Applied Biosystems) and SYBR Premix Ex Taq (TransGen Biotech) as follows: denaturation for 30 s at 94 °C, followed by 40 cycles at 94 °C for 5 s and 60 °C for 30 s. For LsMETTL3 and LsMETTL14, the relative expression levels for five biological replicates and three technical replicates were calculated using the Livak method (2−ΔΔ C t) (Livak et al., 2001), and expression levels of target genes were normalized to LsActin.
4.9. Absolute quantification using RT‐qPCR
Recombinant plasmid DNA for use in a standard curve was first prepared. Fragments from segment S10 of the RBSDV genome and Actin, amplified by RT‐PCR using the primers listed in Table S3, were cloned in pEASY‐T5 Zero Cloning Vector (TransGen Biotech) using the manufacturer's instructions, then transformed into separate E. coli DH5α cells. Cells containing the recombinant plasmid were selected using ampicillin (50 mg/ml), and the presence of the plasmid was verified by PCR and sequencing of the PCR products. Recombinant plasmid DNA was extracted using the method described above. The concentration of the extracted plasmid DNA was measured using a NanoDrop 2000 spectrophotometer (Thermo Fisher Scientific) and calculated as (Hajano et al., 2015):
Serial dilutions of plasmid DNA of either RBSDV or Actin were run in parallel to prepare a standard curve with dilutions from 101 to 107 copies, and the number of RBSDV or Actin copies in samples was determined using the standard curve. The primers used to detect RBSDV were designed based on the RBSDV‐S10 fragment, and RT‐qPCR was done as described above.
4.10. Confocal laser‐scanning microscopy
Midguts excised from SBPHs were fixed in 4% vol/vol paraformaldehyde in phosphate‐buffered saline for 2 hr at room temperature and incubated in osmotic buffer (2% vol/vol Triton) for 30 min at room temperature. Then the samples were incubated for 16 hr at 4 °C with anti‐m6A antibody (ab151230; Abcam) at 1:50 dilution and RBSDV P10 antibody (a gift from Professor Jianxiang Wu, Zhejiang University) at 1:100 dilution before incubation with DyLight 633 phalloidin (ab143533; Abcam) to label actin and secondary antibodies: Alexa Fluor 488‐labelled secondary goat anti‐rabbit IgG (ab150085; Abcam) and DyLight 549‐labelled secondary goat anti‐mouse IgG (A23310; Abbkine). All samples were visualized with a confocal laser‐scanning microscope (LSM980; Carl Zeiss), and images were saved in ZEN 2011 blue light. Each set of experiments was repeated three times.
4.11. Western blots
Total protein was extracted from SPBHs according to our published protocol with minor modifications (Liu et al., 2015): 0.02 g of SBPH was ground in liquid nitrogen to powder and 200 μl of 50 mM Tris‐HCl was added. After vortexing and vertical shaking for 1 hr at 4 ℃, the mixture was centrifuged at 4 ℃ for 20 min. Then we took 80 μl of supernatant and mixed with 20 μl of 5× sodium dodecyl sulphate (SDS) loading buffer, then subsequently boiled the mixture for 10 min. The denatured proteins were separated by 4%–12% SDS‐polyacrylamide gel electrophoresis (PAGE) and transferred onto polyvinylidene fluoride (PVDF) membranes (GE Healthcare Life Sciences) by electrophoresis at 150 mA for 2 hr. Proteins were then probed with anti‐RBSDV P10 (1:2,500 dilution) or anti‐actin (1:4,000 dilution, 66,008; Proteintech). Immunoreactive bands were detected using a goat anti‐mouse IgG‐conjugated horseradish peroxidase antibody (5220‐0341; SeraCare) at 1:5,000 dilution. Western blots were imaged with a Super ECL Western Blotting Detection Kit (YTHX) and Molecular Imager ChemiDo XRS System (Bio‐Rad). Each western blot was repeated three times.
5. COMPETING INTERESTS
The authors declare that they have no competing interests.
Supporting information
FIGURE S1 Quality of dsRNA in this study tested by agarose gel electrophoresis
FIGURE S2 Relative expression of (a) Fl(2)d and (b) Virilizer after small brown planthopper nymphs were microinjected with dsLsMETTL3+14 or dsGFP
FIGURE S3 Relative expression of genes coding methyltransferases in nonviruliferous and viruliferous small brown planthoppers
TABLE S1 Percentage identities between LsMETTL3 and its homologs in other insects
TABLE S2 Identities (%) between LsMETTL14 and its homologues in other insects
TABLE S3 Primers used in this study
ACKNOWLEDGEMENTS
The authors thank Dr B. E. Hazen (Willows End scientific editing and writing, USA) for critically reading and revising the manuscript. This work was supported by the National Natural Science Foundation of China (31630058) and the National Key Research and Development Program of China: Inter‐Governmental S&T Cooperation Proposal (2019YFE0108500).
Tian, S., Wu, N., Zhang, L.& Wang, X. (2021) RNA N6‐methyladenosine modification suppresses replication of rice black streaked dwarf virus and is associated with virus persistence in its insect vector. Molecular Plant Pathology, 22, 1070–1081. 10.1111/mpp.13097
DATA AVAILABILITY STATEMENT
Sequence data for this study is available at GenBank (https://www.ncbi.nlm.nih.gov/genbank/) as accessions MW589259 and MW589260.
REFERENCES
- Arribas‐Hernandez, L., Bressendorff, S., Hansen, M.H., Poulsen, C., Erdmann, S. & Brodersen, P. (2018) An m6A‐YTH module controls developmental timing and morphogenesis in Arabidopsis. The Plant Cell, 30, 952–967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azuhata, F., Uyeda, I., Kimura, I. & Shikata, E. (1993) Close similarity between genome structures of rice black‐streaked dwarf and maize rough dwarf viruses. Journal of General Virology, 74, 1227–1232. [DOI] [PubMed] [Google Scholar]
- Bokar, J.A., Shambaugh, M.E., Polayes, D., Matera, A.G. & Rottman, F.M. (1997) Purification and cDNA cloning of the AdoMet‐binding subunit of the human mRNA (N6‐adenosine)‐methyltransferase. RNA, 3, 1233–1247. [PMC free article] [PubMed] [Google Scholar]
- van den Born, E., Omelchenko, M.V., Bekkelund, A., Leihne, V., Koonin, E.V., Dolja, V.V. et al. (2008) Viral AlkB proteins repair RNA damage by oxidative demethylation. Nucleic Acids Research, 36, 5451–5461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bratlie, M.S. & Drabløs, F. (2005) Bioinformatic mapping of AlkB homology domains in viruses. BMC Genomics, 6, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, S.X. & Zhang, Q.Y. (2005) Advance in researches on rice black‐streaked dwarf diseases. Acta Phytophylacica Sinica, 32, 97–103. [Google Scholar]
- Coombs, K.M. (2008) Reoviruses: molecular biology. In: Mahy, B.W.J. & Van Regenmortel, M.H.V. (Eds.) Encyclopedia of virology, 3rd edition. New York: Academic Press, pp. 390–439. [Google Scholar]
- Dang, W., Xie, Y., Cao, P., Xin, S., Wang, J., Li, S. et al. (2019) N6‐methyladenosine and viral infection. Frontiers in Microbiology, 10, 417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Feo, L.D.V., Laguna, I.G. & Biderbost, E.B. (2010) Physiological alterations associated to the Mal de Rio Cuarto virus (MRCV) infection and to vector (Delphacodes kuscheli Fennah) phytotoxicity in wheat. Tropic Plant Pathology, 35, 79–87. [Google Scholar]
- Firth, A.E. & Atkins, J.F. (2009) Analysis of the coding potential of the partially overlapping 3’ ORF in segment 5 of the plant fijiviruses. Virology Journal, 6, 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gokhale, N.S., McIntyre, A.B.R., McFadden, M.J., Roder, A.E., Kennedy, E.M., Gandara, J.A. et al. (2016) N6‐methyladenosine in Flaviviridae viral RNA genomes regulates infection. Cell Host & Microbes, 20, 654–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajano, J.D., Raza, A., Zhang, L., Liu, W. & Wang, X. (2020) Ribavirin targets sugar transporter 6 to suppress acquisition and transmission of rice stripe tenuivirus by its vector Laodelphax striatellus . Pest Management Science, 76, 4086–4092. [DOI] [PubMed] [Google Scholar]
- Hajano, J.D., Wang, B., Ren, Y., Lu, C. & Wang, X. (2015) Quantification of southern rice black streaked dwarf virus and rice black streaked dwarf virus in the organs of their vector and nonvector insect over time. Virus Research, 208, 146–155. [DOI] [PubMed] [Google Scholar]
- Hajano, J.D., Zhang, H., Ren, Y., Lu, C. & Wang, X. (2016) Phenotypic and molecular‐based screening of rice (Oryza sativa) for resistance to rice black‐streaked dwarf disease. Plant Pathology, 65, 1509–1517. [Google Scholar]
- Hardy, J.L. (1988) Susceptibility and resistance of vector mosquitoes. In: Monath, T.P. (Ed.) The arboviruses: epidemiology and ecology. Boca Raton, FL: CRC Press, pp. 87–126. [Google Scholar]
- He, P.C. & He, C. (2021) m6A RNA methylation: from mechanisms to therapeutic potential. The EMBO Journal, 40, e105977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He, X., Xu, H., Zheng, X., Yang, Y., Gao, G., Pan, J. et al. (2011) Effects of rice black streaked dwarf virus on ecological fitness of non‐vector planthopper, Sogatella fucifera . Chinese Journal of Rice Science, 25, 654–658. [Google Scholar]
- Hibino, H. (1996) Biology and epidemiology of rice viruses. Annual Review of Phytopathology, 34, 249–274. [DOI] [PubMed] [Google Scholar]
- Hughes, G.L., Allsopp, P.G., Brumbley, S.M., Johnson, K.N. & O’Neill, S.L. (2008) In vitro rearing of Perkinsiella saccharicida and the use of leaf segments to assay Fiji disease virus transmission. Phytopathology, 98, 810–814. [DOI] [PubMed] [Google Scholar]
- Isogai, M., Uyeda, I. & Lee, B.C. (1998) Detection and assignment of proteins encoded by rice black streaked dwarf fijivirus S7, S8, S9 and S10. Journal of General Virology, 79, 1487–1494. [DOI] [PubMed] [Google Scholar]
- Jia, D., Chen, H., Mao, Q., Liu, Q. & Wei, T. (2012) Restriction of viral dissemination from the midgut determines incompetence of small brown planthopper as a vector of southern rice black‐streaked dwarf virus. Virus Research, 167, 404–408. [DOI] [PubMed] [Google Scholar]
- Jia, D., Chen, H., Zheng, A., Chen, Q., Liu, Q., Xie, L. et al. (2012) Development of an insect vector cell culture and RNA interference system to investigate the functional role of Fijivirus replication protein. Journal of Virology, 86, 5800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kan, L., Grozhik, A.V., Vedanayagam, J., Patil, D.P., Pang, N., Lim, K.‐S. et al. (2017) The m6A pathway facilitates sex determination in Drosophila. Nature Communications, 8, 15737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kan, L., Ott, S., Joseph, B., Park, E.S., Dai, W., Kleiner, R.E. et al. (2021) A neural m6A/Ythdf pathway is required for learning and memory in Drosophila . Nature Communications, 12, 1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar, S., Stecher, G. & Tamura, K. (2016) MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Molecular Biology and Evolution, 33, 1870–1874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuribayashi, K. & Shinkai, A. (1952) On the new disease of rice, black‐streaked dwarf. Annals of the Phytopathological Society of Japan, 16, 41. [Google Scholar]
- Lence, T., Akhtar, J., Bayer, M., Schmid, K., Spindler, L., Ho, C.H. et al. (2016) m6A modulates neuronal functions and sex determination in Drosophila . Nature, 540, 242–247. [DOI] [PubMed] [Google Scholar]
- Lichinchi, G., Gao, S., Saletore, Y., Gonzalez, G.M., Bansal, V., Wang, Y. et al. (2016) Dynamics of the human and viral m6A RNA methylomes during HIV‐1 infection of T cells. Nature Microbiology, 1, 16011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichinchi, G., Zhao, B.S., Wu, Y., Lu, Z., Qin, Y., He, C. et al. (2016) Dynamics of human and viral RNA methylation during Zika virus infection. Cell Host & Microbes, 20, 666–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, J., Yue, Y., Han, D., Wang, X., Fu, Y.E., Zhang, L. et al. (2014) A METTL3‐METTL14 complex mediates mammalian nuclear RNA N6‐adenosine methylation. Nature Chemical Biology, 10, 93–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, W., Gray, S., Huo, Y., Li, L., Wei, T. & Wang, X. (2015) Proteomic analysis of interaction between a plant virus and its vector insect reveals new functions of hemipteran cuticular protein. Molecular & Cellular Proteomics, 14, 2229–2242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak, K.J. & Schmittgen, T.D. (2001) Analysis of relative gene expression data using real‐time quantitative PCR and the 2−ΔΔCt method. Methods, 25, 402–408. [DOI] [PubMed] [Google Scholar]
- Mahmood, F., Chiles, R.E., Fang, Y., Green, E.N. & Reisen, W.K. (2006) Effects of time after infection, mosquito genotype, and infectious viral dose on the dynamics of Culex tarsalis vector competence for western equine encephalomyelitis virus. Journal of the American Mosquito Control Association, 22, 272–281. [DOI] [PubMed] [Google Scholar]
- Mainou, B.A. & Dermody, T.S. (2012) Transport to late endosomes is required for efficient reovirus infection. Journal of Virology, 86, 8346–8358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao, Q., Zheng, S., Han, Q., Chen, H., Ma, Y., Jia, D. et al. (2013) New model for the genesis and maturation of viroplasms induced by fijiviruses in insect vector cells. Journal of Virology, 87, 6819–6828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milne, R.G., del Vas, M. , Harding, R.M., Marzachi, R. & Mertens, P.P.C. (2005) Genus fijivirus. In: Fauquet, C.M., Mayo, M.A., Maniloff, J., Desselberger, U. & Ball, L.A. (Eds.) Virus taxonomy: 8th report of the International Committee on Taxonomy of Viruses. San Diego: Elsevier Academic Press, pp. 534–542. [Google Scholar]
- Qin, F., Liu, W., Wu, N., Zhang, L., Zhou, X. & Wang, X. (2018) Invasion of midgut epithelial cells by a persistently transmitted virus is mediated by sugar transporter in its insect vector. PLoS Pathogens, 14, e1007201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruzicka, K., Zhang, M., Campilho, A., Bodi, Z., Kashif, M., Saleh, M. et al. (2017) Identification of factors required for m6A mRNA methylation in Arabidopsis reveals a role for the conserved E3 ubiquitin ligase HAKAI. New Phytologist, 215, 157–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saletore, Y., Meyer, K., Korlach, J., Vilfan, I.D., Jaffrey, S. & Mason, C.E. (2012) The birth of the epitranscriptome: Deciphering the function of RNA modifications. Genome Biology, 13, 175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sendinc, E., Valle‐Garcia, D., Jiao, A. & Shi, Y. (2020) Analysis of m6A RNA methylation in Caenorhabditis elegans . Cell Discovery, 6, 47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Than, W., Qin, F.L., Liu, W.W. & Wang, X. (2016) Analysis of Sogatella furcifera proteome that interact with P10 protein of southern rice black‐streaked dwarf virus. Scientific Reports, 6, 32445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd, J.C., el Ammar, D. , Redinbaugh, M.G., Hoy, C. & Hogenhout, S.A. (2010) Plant host range and leafhopper transmission of Maize fine streak virus . Phytopathology, 100, 1138–1145. [DOI] [PubMed] [Google Scholar]
- Wang, H., Liu, Y., Zhang, L., Kundu, J.K., Liu, W. & Wang, X. (2019) ADP ribosylation factor 1 facilitates spread of wheat dwarf virus in its insect vector. Cellular Microbiology, 21, e13047. [DOI] [PubMed] [Google Scholar]
- Wang, Y., Li, Y., Toth, J.I., Petroski, M.D., Zhang, Z. & Zhao, J.C. (2014) N6‐methyladenosine modification destabilizes developmental regulators in embryonic stem cells. Nature Chemical Biology, 16, 191–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams, D.D., Gokhale, N.S. & Horner, S.M. (2019) Regulation of viral infection by the RNA modification N6‐methyladenosine. Annual Review of Virology, 6, 235–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu, N., Zhang, L., Ren, Y. & Wang, X. (2020) Rice black‐streaked dwarf virus: from multiparty interactions among plant–virus–vector to intermittent epidemics. Molecular Plant Pathology, 21, 1007–1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu, N., Zhang, P., Liu, W., Cao, M., Massart, S. & Wang, X. (2019) Complete genome sequence and characterization of a new iflavirus from the small brown planthopper (Laodelphax striatellus). Virus Research, 272, 197651. [DOI] [PubMed] [Google Scholar]
- Xu, H.X., He, X.C., Zheng, X.S., Yang, Y.J., Zhang, J.F. & Lu, Z.X. (2016) Effects of SRBSDV‐infected rice plants on the fitness of vector and non‐vector rice planthoppers. Journal of Asia‐Pacific Entomology, 19, 707–710. [Google Scholar]
- Zhang, F., Zhang, Y.‐C., Liao, J.‐Y., Yu, Y., Zhou, Y.‐F., Feng, Y.‐Z. et al. (2019) The subunit of RNA N6‐methyladenosine methyltransferase OsFIP regulates early degeneration of microspores in rice. PLoS Genetics, 15, e1008120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, J., Zheng, X., Chen, Y.D., Hu, J., Dong, J.H., Su, X.X. et al. (2014) Southern rice black‐streaked dwarf virus infection improves host suitability for its insect vector, Sogatella furcifera (Hemiptera: Delphacidae). Journal of Economic Entomology, 107, 92–97. [DOI] [PubMed] [Google Scholar]
- Zhang, L., Liu, W. & Wang, X. (2021) Immunofluorescent labeling of plant virus and vector proteins in hemiptera guts. Journal of Visualized Experiments, 171, e62605. [DOI] [PubMed] [Google Scholar]
- Zhang, L., Wu, N., Ren, Y. & Wang, X. (2021) Insights into insect vector transmission and epidemiology of plant‐infecting fijiviruses. Frontiers in Microbiology, 12, 628262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, X., Zhang, Y., Dai, K., Liang, Z.i., Zhu, M., Pan, J. et al. (2020) N6‐methyladenosine level in silkworm midgut/ovary cell line is associated with Bombyx mori nucleopolyhedrovirus infection. Frontiers in Microbiology, 10, 2988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou, J., Wan, J., Gao, X., Zhang, X., Jaffrey, S.R. & Qian, S.B. (2015) Dynamic m6A mRNA methylation directs translational control of heat shock response. Nature, 526, 591–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu, J., Jiang, F., Wang, X., Yang, P., Bao, Y., Zhao, W. et al. (2017) Genome sequence of the small brown planthopper, Laodelphax striatellus . GigaScience, 6, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
FIGURE S1 Quality of dsRNA in this study tested by agarose gel electrophoresis
FIGURE S2 Relative expression of (a) Fl(2)d and (b) Virilizer after small brown planthopper nymphs were microinjected with dsLsMETTL3+14 or dsGFP
FIGURE S3 Relative expression of genes coding methyltransferases in nonviruliferous and viruliferous small brown planthoppers
TABLE S1 Percentage identities between LsMETTL3 and its homologs in other insects
TABLE S2 Identities (%) between LsMETTL14 and its homologues in other insects
TABLE S3 Primers used in this study
Data Availability Statement
Sequence data for this study is available at GenBank (https://www.ncbi.nlm.nih.gov/genbank/) as accessions MW589259 and MW589260.


