Abstract
After two decades free of Newcastle disease, Belgium encountered a velogenic avian orthoavulavirus type 1 epizootic in 2018. In Belgium, 20 cases were diagnosed, of which 15 occurred in hobby flocks, 2 in professional poultry flocks and 3 in poultry retailers. The disease also disseminated from Belgium towards the Grand Duchy of Luxembourg by trade. Independently, the virus was detected once in the Netherlands, almost simultaneously to the first Belgian detection. As such Newcastle disease emerged in the entire BeNeLux region. Both the polybasic sequence of the fusion gene cleavage site and the intracerebral pathotyping assay demonstrated the high pathogenicity of the strain. This paper represents the first notification of this specific VII.2 subgenotype in the North‐West of Europe. Time‐calibrated full genome phylogenetic analysis indicated the silent or unreported circulation of the virus prior to the emergence of three genetic clusters in the BeNeLux region without clear geographical or other epidemiological correlation. The Dutch strain appeared as an outgroup to the Belgian and Luxembourgian strains in the time‐correlated genetic analysis and no epidemiological link could be identified between the Belgian and Dutch outbreaks. In contrast, both genetic and epidemiological outbreak investigation data linked the G.D. Luxembourg case to the Belgian outbreak. The genetic links between Belgian viruses from retailers and hobby flocks only partially correlated with epidemiological data. Two independent introductions into the professional poultry sector were identified, although their origin could not be determined. Animal experiments using 6‐week‐ old specific pathogen‐free chickens indicated a systemic infection and efficient transmission of the virus. The implementation of re‐vaccination in the professional sector, affected hobby and retailers, as well as the restriction on assembly and increased biosecurity measures, possibly limited the epizootic and resulted in the disappearance of the virus. These findings emphasize the constant need for awareness and monitoring of notifiable viruses in the field.
Keywords: BeNeLux, epizootic, hobby, Newcastle disease, professional poultry
1. INTRODUCTION
The 20 genotypes of formerly classified avian paramyxoviruses have now been divided into 3 genera of ortho, meta and para avian avulaviruses (ICTV, 2019). The avian orthoavulavirus 1 (AOAV‐1), formerly known as avian avulavirus 1 (AAvV‐1) or avian paramyxovirus 1 (APMV‐1), is member of the Paramyxoviridae family of the Avulavirinae subfamily (Adams et al., 2017; ICTV, 2019; King et al., 2018). The AOAV‐1 genome is a single‐stranded polycistronic RNA of around 15 kb comprising 6 genes, encoding the nucleocapsid (N), the phosphoprotein (P), the matrix protein (M), the fusion protein (F), the haemagglutinin‐neuraminidase protein (HN) and the large polymerase (L). Based on the F‐protein gene, AOAV‐1 viruses are phylogenetically divided into classes I and II, each containing defined genotypes and subgenotypes. The class II AOAV‐1 can be further subdivided into 21 genotypes (I‐XXI, genotype XV consisting solely of recombinant genomes) (Dimitrov, Lee et al., 2016; Dimitrov et al., 2019; Snoeck, et al., 2013). These class II AOAV‐1 viruses have a large avian host range of over 200 species, in both domestic and wild birds, comprising both lentogenic and velogenic strains (Dimitrov, Ramey et al., 2016). Velogenic or virulent AOAV‐1 (vAOAV‐1) is the causative agent of Newcastle disease (ND), and notifiable to the world organization of animal health (OIE) (OIE & May, 2012). ND is a devastating, highly contagious infectious poultry disease with important economic impact and global distribution, despite of large‐scale vaccine implementation (Dimitrov et al., 2017). Virulent strains are defined by an intracerebral pathogenicity index above 0.7 in day‐old specific pathogen‐free (SPF) chicks and a fusion precursor protein cleavage site with multiple basic amino acids and a phenylalanine amino acid at position 117 (Nagai et al., 1976).
Since 1993, Belgium, like many other European countries, implements a compulsory ND vaccination programme for all flocks with more than 100 poultry heads and for all hobby poultry taking part in competitions, assemblies or being sold on markets, regardless of numbers (Ministerial Decree 4 May 1992 and 25 January 1993). In Belgium, all birds kept and raised in captivity for food production or trade are considered poultry. The implementation of vaccination and re‐vaccination in all other hobby flocks is neither regulated nor controlled. In addition, Belgium conducts a clinical surveillance programme based on the compulsory notification by veterinarians and poultry/bird owners of symptoms suggestive for avian influenza (AI) or ND infection, such as reduced feed/water consumption, increased mortality, typical clinical symptoms such as respiratory or neurological symptoms and reduced egg production (Belgian Royal Decree 3 February 2014 and 28 November 1994). All suspected cases are subject to further investigations by the Federal Agency for the Safety of the Food Chain (FASFC) and samples are automatically sent to the Belgian National Reference Laboratory (NRL), Sciensano, for AI and ND exclusion.
In Spring 2018, Belgium, the Netherlands and the Grand Duchy (G.D.) of Luxembourg (jointly referred to as the BeNeLux region) encountered vAOAV‐1 introductions. All of the isolates could be assigned to the VII.2 subgenotype, that emerged in 2010 in the Middle East and India, demonstrating a fast Westward spread and a large host spectrum, and therefore considered as a plausible candidate for the next panzootic (Miller et al., 2015). The present study elaborates on the diagnosis, the biological and genetic characterization of this West‐European VII.2 vAOAV‐1 2018 virus, as well as the high‐resolution genetic phylogeny analyses combined with epidemiologic data collected during the outbreak.
2. MATERIAL AND METHODS
2.1. Sample collection
The national reference laboratory for AI and ND at Sciensano received swab or organ samples from suspected hobby birds, retailers and professional poultry flocks, sent in by field veterinarians, from different geographical locations within the country following clinical manifestations or epidemiological investigation.
In the Netherlands, Wageningen Bioveterinary Research received organ samples from suspected pheasants following clinical symptoms on a hobby holding located in the centre of the country.
Samples from Luxembourg were submitted to Sciensano for confirmation of ND after necropsy at the Laboratory of Veterinary Medicine and positive primary molecular diagnostics done at the Luxembourg Institute of Health. The hobby chickens originated from a town in western Luxembourg and had been purchased at a Belgian live bird market near the Belgium‐Luxembourg border.
An overview of the positive materials is provided in Table 1.
Table 1.
Overview of the diagnostic and epidemiological data of the 20 Belgian vAOAV‐1 cases
| Name | Sector type | Species | Sampling date dd/mm/yyyy | Number of birds present | Number of birds affected | Number of birds death | Number of birds killed | % mortality |
|---|---|---|---|---|---|---|---|---|
| 4096_Milmort | Hobby | Chicken | 17/04/2018 | 18 | 8 | 10 | 0 | 55.56 |
| 5573_Soignies | Hobby | Chicken | 04/06/2018 | 53 | 7 | 32 | 21 | 60.38 |
| 5619_Kessel | Hobby | Chicken | 07/06/2018 | 29 | 0 | 13 | 0 | 44.83 |
| 5630_Verviers | Hobby | Chicken | 04/06/2018 | 9 | 0 | 8 | 0 | 88.89 |
| 5876_Villers‐La‐Ville | Hobby | Chicken | 13/06/2018 | 13 | 0 | 7 | 0 | 53.85 |
| 5927_Hélécine | Hobby | Guinea fowl | 30/05/2018 | NA | NA | NA | NA | NA |
| 6011_Oeselgem | Hobby | Chicken | 19/06/2018 | NA | NA | NA | NA | NA |
| 6047_Retailera | Retailer | Chicken | 21/06/2018 | NA | 0 | 0 | 0 | 0 |
| 6083_Retailera | Retailer | Chicken | 20/06/2018 | NA | 0 | 0 | 0 | 0 |
| 6057_Erpe Mere | Hobby | Chicken | 21/06/2018 | NA | NA | NA | NA | NA |
| 6295_Retailer_Professionala | Retailer_Professional | Chicken | 29/06/2018 | 3,648 | 0 | 0 | 3,648 | 0 |
| Turkey | ||||||||
| 6369_Professional | Professional | Chicken | 03/07/2018 | 57,820 | 4,680 | 6,095 | 51,725 | 10.54 |
| 6372_Morlanwelz | Hobby | Chicken | 03/07/2018 | 121 | 80 | 79 | 42 | 65.29 |
| 6496_Sint‐Genesius‐Rode | Hobby | Chicken | 06/07/2018 | NA | NA | NA | NA | NA |
| 6527_Eeklo | Hobby | Pheasant | 09/07/2018 | 8 | 4 | 4 | 0 | 50 |
| 6525_Appelterre | Hobby | Chicken | 09/07/2018 | 180 | 36 | 10 | 0 | 5.56 |
| 6604_Professional | Professional | Chicken | 13/07/2018 | 39,517 | 4,500 | 2,000 | 0 | 5.06 |
| 6617_Robelmont | Hobby | Chicken | 12/07/2018 | 74 | 18 | 18 | 0 | 24.32 |
| 6643_Lessines | Hobby | Chicken | 12/07/2018 | 48 | 28 | 20 | 0 | 41.67 |
| 6930_Macon | Hobby | Chicken | 20/07/2018 | 34 | 24 | 24 | 0 | 70.59 |
| 88367‐368_Luxembourg | Hobby | Chicken | 22/05/2018 | 13 | 13 | 4 | 9 | 30.77 |
| X18009166_Netherlands | Hobby | Pheasant | 13/04/2019 | NA | NA | NA | NA | NA |
The epidemiological data for Belgium was taken from the European Animal Disease Notification System and provided by the Federal Agency for the Safety of the Food Chain.
Identified via epidemiological investigation; NA: Not available
2.2. Diagnostic assays
First‐line diagnostic testing was performed by generic real‐time reverse transcriptase polymerase chain reaction (rRT‐PCR) detection of a conserved region of the M‐gene allowing the detection of both all AOAV‐1 (Wise et al., 2004) and avian influenza (Spackman et al., 2002) viruses. Viral RNA extraction of field samples was performed by the use of the High Pure viral Nucleic acid (Roche Life Science) or the total MagMax (Thermo Fisher Scientific) kit, following the manufacturer's instructions. For the rRT‐PCR, the one‐step AgPath kit (Thermo Fisher Scientific) was used, according to the manufacturer's instructions. The cycling conditions consist of a 30‐min reverse transcription step at 50°C, followed by a 10‐min denaturation and 50 cycles of denaturation (95°C for 15 s), hybridization (54°C for 34 s) and 30 s of elongation at 72°C. The Cp cut‐off value was determined at 38 during validation with a 99% detection limit of 103,20 viral RNA copies/ml swabs. The mean viral excretion was calculated per group and per time point. Non‐excreting birds were assigned a value of 10 (=1 log10) viral RNA copies/ml swabs for statistical analysis, corresponding to a value below the 95% detection limit of the experimental method.
AOAV‐1 virus was isolated from rRT‐PCR‐positive samples to allow further characterization of the strain. Viral isolation was achieved by inoculation into SPF chicken eggs 9–11 days of embryonation, following standard procedures (OIE & May, 2012). Subsequently, the collected haemagglutinating allantoic fluid was identified by haemagglutination inhibition (HI) testing with a panel of poly‐ and monoclonal antisera (Meulemans et al., 1987), allowing differentiation of vaccine and pigeon specific AOAV‐1 strains.
Complementary to HI‐testing, the pathotype of the AOAV‐1 was determined by Sanger sequencing of the fusion gene cleavage site (Kant et al., 1997). The cleavage site sequencing was mostly performed directly on the field sample RNA extract. For allantoic fluid, RNA was extracted by the High Pure viral Nucleic Acid kit. The one‐step RT‐PCR was performed using the OneStep RT‐PCR kit (Qiagen), following the manufacturer's instructions. The cycling protocol described previously by Kant et al. was performed with slight modification (Kant et al., 1997). Briefly, a reverse transcription step of 45 min at 45°C, followed by the PCR‐step initiated by a 2 min denaturation at 94°C, and 40 cycles under following conditions: denaturation for 2 min at 94°C, hybridization with NDV A and B oligo's for 1 min at 55°C and elongation for 1 min at 72°C. The cycle concludes with a final elongation step of 10 min at 72°C. The 363 base pair PCR amplicons were processed on 1% agarose gel for size selection and purified from the gel using the High Pure PCR product purification kit, following the manufacturer's instructions. Sanger sequencing using the BigDye™ Terminator v3.1 Cycle Sequencing Kit (Thermo Fischer Scientific), purification, and capillary electrophoresis on a 3,500 Series Genetic Analyzer (Thermo Fisher Scientific) were performed at the Transversal and Applied Genomics unit of Sciensano.
2.3. Virus
Lung tissue homogenate from the Bassette chicken of the Belgian index case 4,096 was injected into 9‐day‐old embryonated SPF chicken eggs, which died within 2 days. Allantoic fluid was harvested and inoculated as a 10‐fold dilution series into 9‐day‐old embryonated chicken eggs to determine the viral titre of the AOAV‐1/Bassette_Chicken/Belgium/4096/2018 viral stock, determined at a fifty per cent egg infectious dose (EID50) of 109.66 per ml (Reed & Muench, 1938).
2.4. Whole genome sequencing
The 20 Belgian virus isolates and the isolate from G.D. of Luxembourg were filtered through a 0.45 µM size‐selective disc filter (Millipore) and viral nucleic acids were extracted using the Nucleospin RNA Virus kit (Macherey‐Nagel) according to the manufacturer's instructions with the following modification: carrier RNA was substituted by the use of 5 µl of GenElute‐LPA neutral carrier (Sigma‐Aldrich) per sample. DNA was digested from the extracted nucleic acids using Baseline‐ZERO DNase (Lucigen) followed by RNA cleanup and concentration using the RNA Clean & Concentrator‐5 kit (Zymo Research) according to the manufacturer's instructions. cDNA was synthesized using a final concentration of 2.5 µM random hexamer primer and SuperScript IV (Thermo Fisher Scientific) according to the manufacturer's instructions, followed by second strand cDNA synthesis using the NEBNext® Ultra II Non‐Directional RNA Second Strand Synthesis Module (New England Biolabs). Double‐stranded cDNA was purified using the DNA Clean & Concentrator‐5 kit (Zymo Research), followed by fluorometric quantification using the Quantifluor dsDNA system (Promega). Sequencing libraries were generated using the Nextera XT kit (Illumina) and standard Nextera XT indices, equimolarly pooled after quantification using a KAPA library quantification kit (Kapa Biosystems, Roche) and finally sequenced using a MiSeq reagent kit version 3 (Illumina) with 2x300‐bp paired‐end sequencing according to the manufacturer's instructions. Raw sequence datasets were trimmed using Trim Galore! (q = 30, l = 50, paired; https://www.bioinformatics.babraham.ac.uk/projects/ trim_galore/). A random paired subset (2 × 50,000 reads) was used for de novo assembly using SPAdes v3.9.0 (Bankevich et al., 2012) and IVA v1.0.0. (Hunt et al., 2015). Contigs were manually verified and assembled using BioEdit 7.2.5 (Hall, 1999).
Viral RNA of the Dutch virus isolate was extracted from 10% suspensions of organ tissues using the High Pure Viral RNA kit (Roche). Sequence libraries were prepared using the ScriptSeq v2 kit (Illumina), and the products were purified using SPRI beads (Beckman). The double‐stranded cDNA was quantified using the ClarioStar and Quant‐it kit (Thermo Fisher). Fragment lengths were analysed using Tapestation 2200 (Agilent). The libraries were sequenced using Illumina MiSeq paired‐end 150 base pairs sequencing (Illumina). Quality control‐passed sequence reads of high quality were iteratively mapped on resulting consensus sequences using Bowtie2 starting against the genome sequence of the Belgian index case.
All complete virus genome sequences (n = 22) were submitted to GenBank, and are available under the accession numbers MH432252 (‘4096_Milmort’, Belgian index case), MN547973–MN547991 (Belgium), MN547992 (G.D. of Luxembourg) and MN701080 (Netherlands) (Table S1).
2.5. Phylogenetic sequence analysis
To determine the taxonomic classification according to the new Dimitrov et al. (2019) nomenclature, the complete F gene coding sequences of the Belgian index case, the viral isolates of G.D. of Luxembourg and the one from the Netherlands were aligned with 771 representative complete F sequences of genotype VII (curated complete fusion gene dataset distributed by Dimitrov et al., 2019) and one root sequence of genotype VI (KC205479.1; 2011/Ethiopia/ETHMG1C) using Muscle in Mega v7.0.21. The phylogenetic analysis was performed after nucleotide substitution model selection (Mega v7.0.21) with neighbour joining (maximum composite likelihood, gamma‐distributed rates G = 0.95, pairwise removal of gaps and missing data, 1,000 bootstrap replicates) and maximum likelihood (GTR + G + I, 500 bootstrap replicates, identical findings, not shown) methods (Mega v7.0.21). For visual simplification, only the branch leading to the identified subgenotype was displayed, while some branches were compressed.
A high‐resolution, time‐calibrated full genome sequence phylogenetic analysis was undertaken to study the molecular dynamics within the outbreak in the BeNeLux region. The full genome virus sequences from Belgium (n = 20, i.e., all cases), the single sequences of the G.D. of Luxembourg (n = 1) and the Netherlands (n = 1) were aligned (Muscle, Mega v7.0.21 5.01), together with the most similar (NCBI Nucleotide database accessed 2019.06.01) full genome sequences (MG871466.1; chicken/Iran/PCR‐UT/2017) and an additional outgroup of sequences from the Middle East (MF437287.1, KY967611.1, KX791186.1). TempEst v.1.5.3 (Rambaut et al., 2016) was used to evaluate the most optimal use of outgroup sequences and root placement (residual mean square optimization) and to evaluate the clocklikeness of the corresponding datasets using a maximum likelihood tree (Mega v7.0.21; GTR + G + I; 100 bootstrap replicates). The optimal dataset for further phylogenetic analysis included two outgroup sequences (MG871466.1 and KX791186.1) and showed an excellent correlation between the genetic data and the time information (R 2 0.9853; correlation coefficient 0.9926). Time‐resolved phylogenetic trees were estimated using BEAST v1.10.4 (http://github.com/beast‐dev/beast‐mcmc). The field sampling dates, expressed as decimal years, were assigned to the tree tips for time calibration purposes. The selection of priors (nucleotide substitution model, molecular clock, tree prior/population growth) was guided by Bayes factor optimization (Kass & Raftery, 1995) of model comparisons using Path Sampling/Stepping Stone Sampling estimation of marginal likelihoods (Baele et al., 2012). The final analysis employed the GTR + G substitution model for sequence evolution along with gamma‐distributed rate variation among sites and two partitions (codon positions 1 + 2, 3). A strict molecular clock was imposed, and exponential population growth (Laplacian) was used as a coalescent tree prior. The Markov Chain Monte Carlo (MCMC) model was run for 50 million steps sampling trees every 5,000 steps. Convergence of the MCMC model was verified using Tracer v1.7.1 (http://github.com/beast‐dev/tracer/), indicating a suitable burn‐in of 10% of the chain. Estimates had an effective sample size (ESS) of 3,000 at the minimum and most had ESS greater than 10,000. A maximum clade credibility tree displaying median node heights was calculated using TreeAnnotator v1.10.4. Phylogenetic trees were visualized using FigTree v1.4.3 (http://figtree.googlecode.com/).
In addition, the clustering in the Bayesian analysis described above was confirmed using full genome maximum likelihood analysis (GTR + G; 500 bootstrap replicates, complete deletion of missing data and gaps), following an initial maximum likelihood‐based nucleotide substitution model selection (optimizing BIC and AIC criteria) in Mega v7.0.21.
2.6. Outbreak description and epidemiological investigation
After the first cases detected in captive hobby birds, the virus was also detected in two professional poultry flocks in Belgium. Finally, a total of 20 Belgian cases was diagnosed, of which 15 in hobby flocks, 2 in professional farms, both layer‐breeders, and 3 in poultry retailers. Purchase of birds by a Luxembourg private bird owner from a Belgian live bird market resulted in the dissemination of the virus from Belgium to the G.D. of Luxembourg. Almost simultaneously, a single introduction occurred in Dutch pheasants, at a hobby holding in the centre of the country.
The Belgian epidemiological investigation to trace back the origin of, and links between cases was performed by the FASFC. In this context, outbreak sites were visited to map them for wild bird contact, recent purchases, contacts including personnel, recent vaccine implementations, etc. In parallel, the FASFC enforced restriction measures to control the disease spread. Initially, a general ban on gathering, exhibiting and trade of professional and hobby poultry throughout Belgium was established (Ministerial Decree of 24 July 2018). A 500‐metre protection zone around the ND hobby outbreak sites was implemented. Chickens at the outbreak site itself were either stamped out or vaccinated against ND, depending on FASFC risk analysis, and subsequently, sites had to remain empty for 21 days or quarantined for 60 days, respectively. Within the 500‐metre protection zone around these sites, additional protection measures were imposed on all poultry, hobby poultry, and pigeon keepers, all of whom were obliged to draw up inventories and re‐vaccinate against ND, unless vaccination certificates could be presented. Restriction measures were lifted 21 days after re‐vaccination or earlier if a vaccination certificate was presented.
Each affected professional poultry holding was declared to the OIE World Animal Health Information System and stamped out. After culling, the outbreak sites were immediately decontaminated, cleaned, and disinfected. For the affected professional holdings, the FASFC set up 3‐km (protection) and 5‐km (surveillance) restriction zones. In the protection zone, all poultry had to be confined or protected to avoid wild bird contact. Both in the protection and surveillance zones, each professional poultry owner was obliged to draw up an inventory of all poultry and birds kept. All professional poultry operations had to undergo a weekly clinical examination and a headcount by the operation's veterinarian (Royal Decree 28 November 1994). A 30‐day fallow period was imposed on each professional outbreak site after cleaning and disinfection, before lifting the 3‐km and 10‐km restriction zones, after 21 and 30 days, respectively. Strict conditions were imposed on poultry traders for purchasing poultry and hobby poultry (Ministerial Decree 24 July 2018).
2.7. Viral characterization
The viral pathotype of the index case was studied by the intracerebral pathogenicity index (ICPI) in ten one‐day‐old SPF chicks (OIE & May, 2012). Briefly, the ten inoculated birds were scored daily for clinical manifestations after intracerebral injection of the tenfold diluted viral stock during an 8‐day period: score 0 for the absence of clinical manifestation, 1 for clinical symptoms and 2 to indicate mortality. The average score throughout this observation period determines the ICPI‐value. Animals were housed in biosecurity level 3 (BSL3) isolator units in negative pressure (BSL3+) with water and feed ad libitum. Bio‐ethical and biosafety regulations were strictly implemented and approval from official committees was acquired (20170117‐01 and NATREFLAB_ICPI_APMV1_Bassette chicken 4096 2018, respectively).
2.8. Transmission study
Birds were hatched in house from commercially purchased SPF eggs (Lohmann Valo). All birds were blood‐sampled the day before infection to confirm the AOAV‐1 free‐status. To study the virus in more detail six 6‐week‐old SPF white leghorn layer chicken were inoculated oculonasal with a 106 EID50/chicken dose of the virus isolated from the Belgian index case (biosafety and ethical permission references 20180222‐01 and NRL_vAPMV1). To evaluate the transmission potential of the strain, the day after infection of the six SPF birds, six uninfected SPF sentinel contact layer chickens were added. All birds were monitored daily for clinical manifestations or mortality. At regular time‐points, 1, 2, 3, 7 and 9 days post‐infection (dpi), cloacal and oropharyngeal swabs were taken to evaluate the replicative potential of the virus. Besides, the viral tropism was studied by sampling organs (lung/trachea, intestine and brain) and feathers at the time of death or at 14 dpi when the surviving bird was killed. Swabs and organs were analysed by rRT‐PCR as described above. Quantification was done using a standard curve of tenfold diluted synthetic Matrix RNA. Results were expressed as the number of viral RNA copies per millilitre of swabs (log10).
2.9. Statistical analyses
Statistical analysis of survival, viral tropism, and virus shedding results were performed using Graph Pad Prism version 8.00 for Windows. Differences were considered significant at p < .05 (*). Neither normality of distribution of the criterion for each group nor homogeneity of the within groups’ variances were confirmed by the Shapiro–Wilk's test and the Barlett's test, respectively. Therefore, the organ's viral load of infected and sentinel groups were compared for each organ by using the non‐parametric test Mann–Whitney. The comparison of viral excretion was done by the same way at each timing. Friedman paired test, followed by the Dunn's multiple comparisons test, were used to evaluate the viral tropism of both groups, as well as the kinetic of their viral excretion. The survival comparison of infected and sentinel birds was done by using a log‐rank test (Mantel–Cox).
3. RESULTS
3.1. Detection of ND in Belgium
In the framework of the AI/ND clinical surveillance protocol, lung and intestine samples from a hobby flock located at the municipality of Milmort were received mid‐April 2018 for AI/ND exclusion diagnostics. The samples were analysed by the first line generic M AI and ND rRT‐PCRs and tested ND positive. Viral inoculation resulted in the recovery of a haemagglutinating agent within 48 hr, identified by HI test as a non‐vaccinal, non‐pigeon specific, AOAV‐1. Sequence analyses of the cleavage site, RRQKRF, in combination with HI and virus isolation information, pointed towards a ‘velogenic’ or ‘virulent’ AOAV‐1 isolate.
From the first positive detection of AOAV‐1 in April 2018 until the end of July 2018, additional vAOAV‐1 outbreaks were diagnosed in 14 hobby flocks, three retailers and two professional poultry flocks. Hobby flocks demonstrated mortality from 0 up to 89%, while in professional holdings the maximum morbidity and mortality remained limited to about 10% (Table 1). Professional holdings are defined by the number of birds on‐site (>200 birds). Retailers interact with hobby owners, and no cross‐trading with the professional poultry sector is authorized in Belgium. A geographic view of the distribution of the Belgian outbreaks is shown in Figure 1. The first two retailers were investigated in the framework of the live bird market surveillance. Subsequently, early July 2018, an outbreak was confirmed at the premises of a third, large, poultry retailer (East Flanders), who was considered part of the sector, professional, due to its capacity. Two additional outbreaks in professional poultry flocks occurred during the two following weeks, both in layer‐breeder operations, located in the provinces of East and West Flanders, respectively, which are the most densely populated Belgian poultry areas.
Figure 1.
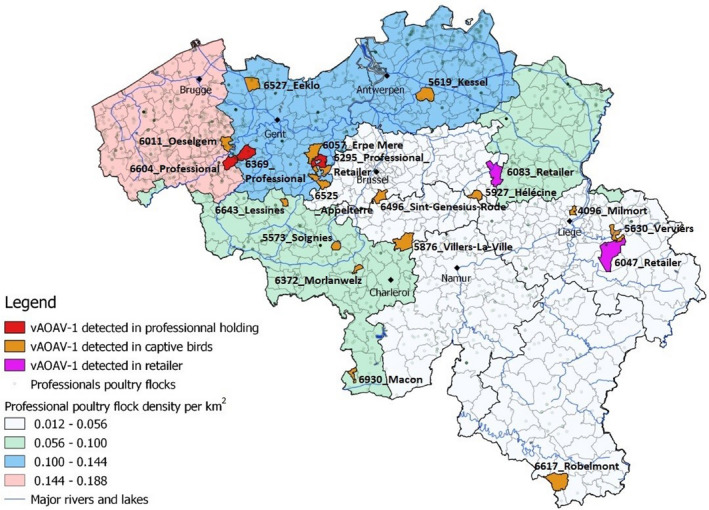
Geographic distribution of the 20 Belgian vAOAV‐VII.2 outbreaks
During this epidemic, Belgium implemented a strict policy to stop the disease propagation, eradicate the virus and decontaminate the professional outbreak sites (EU Directive 92/66/EEC), resulting in the slaughter and destruction of about 93,000 birds.
3.2. Whole genome characterization and phylogenetic sequence analysis of the BeNeLux genotype VII.2 strains
The complete genome of the sequenced isolates presented a length of 15,192 nucleotides in agreement with standard length and the rule of six (Czegledi et al., 2006; Huang et al., 2004), encoding an HN protein of 571 amino acids, typical for velogenic AOAV‐1 viruses (Munir, et al., 2012; Wang et al., 2013). The whole genome pairwise nucleotide identity of the index cases from Belgium, the Netherlands and Luxembourg ranged from 0.997 to 0.998 (BE‐NL: 0.998; BE‐LUX: 0.997; NL‐LUX: 0.998), while this ranged from 0.996 to 1.00 for samples from Belgian cases. Following the classification criteria and nomenclature proposed by Dimitrov et al. (2019), the index case ‘Bassette Chicken/Belgium/4096/2018’ (‘4096_Milmort’), belongs to subgenotype VII.2 with 99.5% complete fusion gene nucleotide sequence identity, only 9 single nucleotide polymorphisms, with the F gene of the most similar publically available complete genome, chicken/Iran/PCR‐UT/2017 (MG871466.1) (Figure 2). The publically available South‐East European VII.2 isolate sequence from Bulgaria (2013, MK005972.1) was more distantly related.
Figure 2.
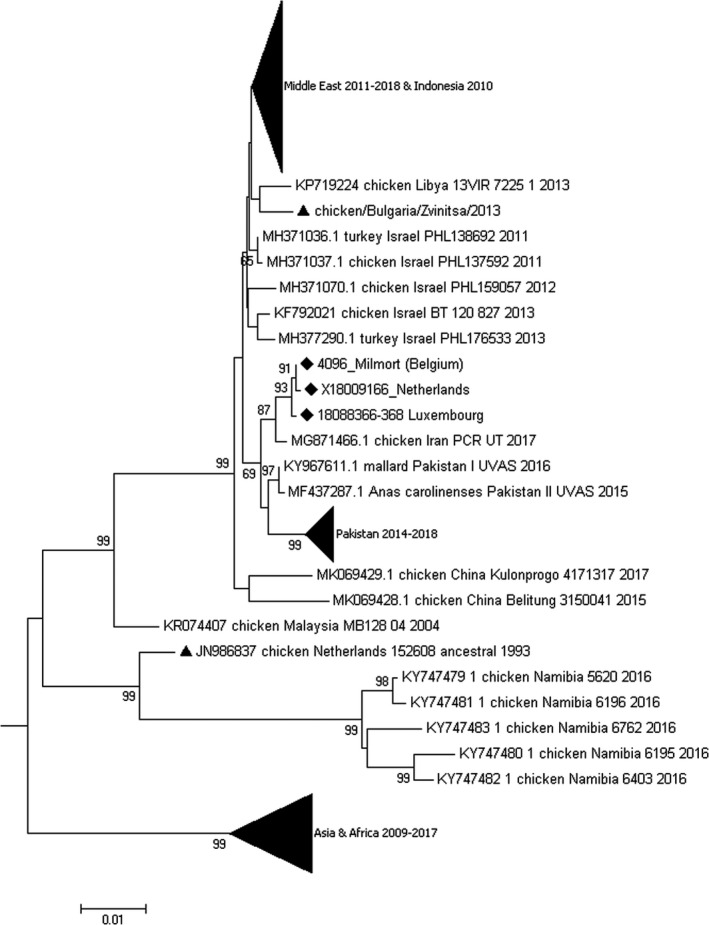
Neighbour joining phylogenetic analysis to determine the subgenotype of the Belgian index case and the cases from the Netherlands and Luxembourg, based on an alignment of 775 full F genotype VII coding sequences. A subtree is shown, depicting subgenotype VII.2 (Mega 7.0, maximum composite likelihood model, gamma‐distributed rates among sites) ‘▲’ indicates previous introductions of VII.2 on the European continent; ‘♦’ indicates viruses sequenced in the present study (complete strain names and GenBank accession numbers available in Table S1)
The time‐resolved Bayesian analysis (Figure 3; Table S2) indicates multiple introduction events, clusters, in poultry in the BeNeLux region, with a common ancestor dating back to January 4, 2018 (95% Highest Posterior Density Interval, 95% HPD 8‐Nov‐2017 to 17‐Feb‐2018). The sole strain from the Netherlands appears as an outgroup linked with high posterior probability (p) support (p = 1.0) to the viruses from Belgium and G.D. Luxembourg. Three main clusters were further identified. The TMRCA node (time to most recent common ancestor) for the viral strains within cluster I dates back to 24 January 2018 (95% HPD 7‐Dec‐2017 to 3‐Mar‐2018). However, the association of the Belgian index case 4096_Milmort to cluster I is only supported by a low node posterior probability (p = .31). In cluster I, a second, well supported node (p = 1) was identified dating back to March 30, 2018 (95% HPD 15‐Mar‐2018 to 15‐May‐2018) (6930_Macon, 6057_Erpe‐Mere, 5619_Kessel, 6011_Oeselgem) (Cluster I). Two additional molecular clusters with high posterior probability support (p = 1) were identified with TMRCA (time to most recent common ancestor) estimates around March 30, 2018 (5% HDP 23‐Apr‐2018 to 25‐Feb‐2018). Cluster II (April 8, 2018; 95% HDP 8‐Mar‐2018 to 1‐May‐2018), contains 5630_Verviers, 6047_Retailer, 5927_Hélécine and 6083_Retailer and a statistically unsupported (p = .4805) link with a professional poultry holding, 6604_Professional (Cluster II). Excluding the unsupported link with 6604_Professional, Cluster II is supported by a high posterior probability (p = 1.0). The third molecular cluster dates back to April 17, 2018 (p = 1; 95% HDP 22‐Mar‐2018 to 7‐May‐2018) containing the majority of Belgian cases, included the third identified retailer (6295_Retailer_Professional), the second professional poultry flock (6369_Professional), the case from the G.D. of Luxembourg and 8 Belgian captive bird cases (Cluster III). Although significantly (p = 1) set within cluster III, the exact position of the cases from the G.D. of Luxembourg and the professional retailer relative to other outbreak viruses in the cluster was less confident due to the low posterior probabilities of 0.36 and 0.43, respectively. The whole genome maximum likelihood analysis (Figure S1, Table S2) confirms the observed clusters and the unclear position of the index cases from Belgium and the Netherlands (4096_Milmort and X18009166_Netherlands, respectively) and one of two professional poultry holdings, 6604_Professional.
Figure 3.
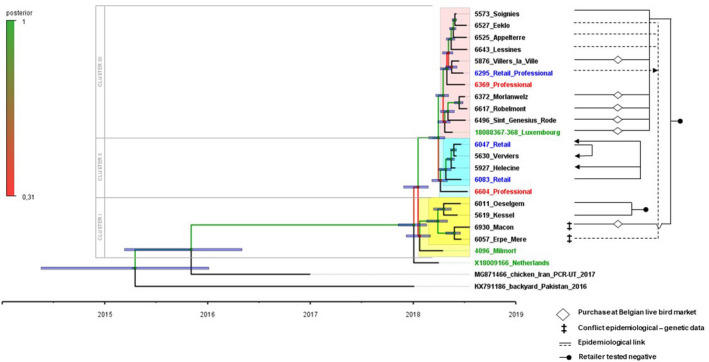
Left side: Time‐resolved Bayesian phylogenetic analysis of the complete genomes of the 22 cases from the AOAV‐1 VII.2 BeNeLux‐outbreak, demonstrating 3 clusters. Both the age of the ‘tips’ (= sampling dates) and the predicted age of the nodes (with a purple node bare representing a 95% credibility interval from the MCMC model) can be read using the time scale below. Exact values of supporting posterior probability and divergence dates + their 95% HDP intervals are available in Table S2. Branches and nodes are colour coded according to their posterior probability support as indicated on the vertical scale. Certain taxon names are colour coded (unrelated to the posterior probability scale): Blue: cases from retailers; Red: cases from professional poultry holdings; Green: index cases in each affected country. Clusters are highlighted, where a lighter shade indicates taxa that are linked to a cluster with low posterior probability: Cluster I: yellow; Cluster II: blue; Cluster III: pink. Right side: Line diagram depicting the potential links between outbreaks (and connected retailers) identified in the epidemiological tracing investigations. Mismatches between genetic and epidemiologic data are identified with a ‡ symbol
3.3. Epidemiological field investigation and the correlation with phylogenetic sequence analysis
For the Belgian index case (4096_Milmort), the purchase of ornamental birds at a local exhibition from an unidentified private owner could not be further traced back. By retrospective sampling of retailers identified as a potential link with other positive hobby cases (Table 1), some were confirmed virus‐positive, while for others no virus could be detected (Figure 3). The retrospective analysis allowed the virus‐positive identification of three retailers (6295_Retailer_Professional, 6083_Retailer, 6047_Retailer), elucidating the introduction into six hobby flocks (5630_Verviers, 5927_Hélécine, 6057_Erpe Mere, 6525_Appelterre, 6527_Eeklo, 6643_Lessines) and the transfer of the virus from one of the retailers to another (6083_Retailer and 6047_Retailer). For three other cases (5619_Kessel, 6011_Oeselgem and 5573_Soignies) purchased directly from a retailer, the analysis could not confirm these purchases as the origin of the introduction as no viral presence could be demonstrated in birds present on the retailer site. In addition, for five Belgian cases (6930_Macon, 5876_Villers‐La‐Ville, 6496_Sint‐Genesius‐Rode, 6617_Robelmont and 6372_Morlanwelz) and the case from the G.D. of Luxembourg originating from purchases at live bird markets in Belgium, no link to a specific retailer could be confirmed during retrospective analysis. Epidemiological field investigations pointed out the purchase of birds at live bird markets or directly from retailers as the major transmission pathway for the Belgian hobby cases and the Luxembourg‐incursion (Figure 3). However, no links with the hobby or retailer cases could be identified for the two professional poultry holdings. Furthermore, epidemiological field investigation did not allow the identification of a link between the two professional cases nor identify the source of infection, for which transports, birds or eggs, food, personnel, vaccines and vaccination teams were evaluated. No link with Belgian live bird markets, retailers or affected poultry holdings was identified for the Dutch pheasants.
The epidemiological field tracing and genetic data are generally in agreement (Figure 3). However, two discrepancies (indicated with ‡) between genetic and epidemiological field data were identified. Two hobby cases, 6057_Erpe Mere and 6930_Macon, were located in the molecular Cluster I while the epidemiological field investigation suggested a common retailer as a point of purchase with cases from genetic cluster II.
3.4. Virological characterization of the Belgian genotype AOAV‐1 VII.2 strain
The standard pathogenicity index, ICPI, for AOAV‐1 viruses was determined for the index case 4096_Milmort strain (Bassette Chicken/Belgium/4096/2018 isolate). At 1 dpi, 3 out of 10 chicks demonstrated clinical symptoms in the form of lethargy, resulting in 100% mortality at 2 dpi. An ICPI of 1.79, on a maximum scale of 2, was obtained, confirming the velogenic pathotype of the strain (ICPI > 0.7).
An in vivo viral transmission study was performed in 6‐week‐old SPF chicken to characterize the virus into more detail. Birds infected with the vAOAV‐1 Bassette Chicken/Belgium/4096/2018 demonstrated early clinical symptoms from 2 dpi (3/6) with ruffled feathers, lethargy and turbid eyes, reducing up to only 1 bird with turbid eyes at 3 dpi, increasing again up to 5 out of 6 at 4 dpi, and resulting in death for 3 birds at 5 dpi and for two additional at 6 dpi. The last infected bird died at 7 dpi after showing neurological symptoms, in the form of loss of balance, at 6 dpi (Figure 4a). For the sentinel birds, co‐housed with the infected birds from 1 dpi, the first clinical symptoms, ruffled feathers, were detected at 5 days after contact (6 dpi) for half of the birds. The first sentinel bird died at 7 dpi while the remaining birds only demonstrated minor clinical symptoms with ruffled feathers. On day 7 after contact, corresponding to 8 dpi, 4 more sentinels died, while the sole survivor demonstrated lethargy, apathy, ruffled feathers and loss of balance up to 10 dpi, after which this bird recovered (Figure 4a).
Figure 4.
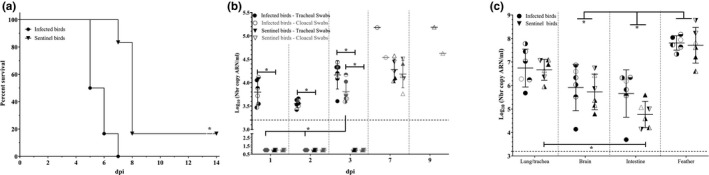
Survival percentages (a), viral excretion (b) and viral tropism (c) after 106 EID50/bird AOAV‐1/Bassette_Chicken/Belgium/4096/2018 inoculation of both infected and sentinel birds. Viral titres are expressed as log10(number of viral RNA copies/ml). Statistical significance is indicated with * (p < .05). Swabs (b) were taken at 1, 2, 3, 7 and 9 dpi, while organs (c) (lung/trachea, intestine and brain) and feathers were sampled at the time of death or at 14 dpi on the day of killing. The surviving sentinel bird is identified with an open triangle (∆) in b and c
Viral excretion (Figure 4b), was detected from the respiratory tract of infected birds from 1 dpi (1 dpi: 3.79 ± 0.26; 2 dpi: 3.55 ± 0.086; 3 dpi: 4.17 ± 0.30 and 7 dpi: 5.18), while excretion in the digestive tract could be observed from 3 dpi onwards (3 dpi: 3.81 ± 0.23 and 7 dpi: 4.54). The sentinel birds started excreting comparable amounts of virus by both routes from 7 dpi onwards (6 days after contact) (7 dpi: 4.28 ± 0.23 (respiratory tract) _ 4.18 ± 0.29 (digestive tract) and 9 dpi: 5.18 (respiratory tract) _ 4.63 (digestive tract)). Once infected and sentinel birds started excreting, viral excretion continued until death. Respiratory and digestive viral excretion of infected birds increased significantly from 2 to 3 dpi, and demonstrated a further increase up to 7 dpi. The amount of virus excreted demonstrated a tendency to increase from 7 up to 9 dpi. For the surviving sentinel bird, viral excretion was comparable to that of the other sentinel birds.
The viral detection in the organs (Figure 4c) demonstrated systemic infection and comparable viral genome copy numbers in the respiratory tract (infected: 6.74 ± 0.81, sentinel: 6.67 ± 0.44), the brain (infected: 5.91 ± 0.99, sentinel: 5.73 ± 0.76) and the gastro‐intestinal tract (infected: 5.66 ± 1.01, sentinel: 4.77 ± 0.56) of infected and sentinel birds at the time of death, corresponding to the end of the 14 day observation period of the study for the surviving sentinel bird. Minimally invasive diagnostic sampling by evaluation of viral presence in feather pulp demonstrated viral RNA at 3 dpi for the infected birds (5.74 ± 0.69, data not shown) while at this time‐point, corresponding to 2 days after contact for the sentinel birds, no virus could be detected in the feather pulp. The viral presence in the feather pulp was highly increased by the time of death (7.81 ± 0.31) (Figure 4c). At the time of death, no significant differences in viral presence in the feather pulp between infected and sentinel birds could be observed (infected: 7.81 ± 0.31, sentinel: 7.71 ± 0.76) (Figure 4c). The viral titre in the feather pulp was significantly higher than in the brain and the gastro‐intestinal tract in both infected and sentinel birds.
The viral presence demonstrated in the different organs of the surviving sentinel was comparable to the average overall value for sentinel birds (lung/trachea 6.64 vs. 6.67 ± 0.44; Brain 6.44 vs. 5.73 ± 0.76; Intestine 5.07 vs. 4.77 ± 0.56 and Feather pulp 7.83 vs. 7.71 ± 0.76).
4. DISCUSSION
Newcastle disease continues to spread around the world despite massive vaccination efforts (Cardenas‐Garcia et al., 2015; Dortmans et al., 2012; Miller et al., 2009). Unlike the pigeon specific AOAV‐1, which is regularly detected in the Belgian racing pigeon population, the last detection of velogenic AOAV‐1 in Belgium goes back 20 years (Meulemans & van den Berg9‐10/12/, 1997). The implementation of compulsory vaccination in 1993 has resulted in the absence of vAOAV‐1‐detection in professional holdings from 1993 onwards, while the last vAOAV‐1 in hobby birds was detected in 1998.
The close genetic relationship between the Belgian index case and the Iranian chicken isolate from 2017 was identified through the analysis of the complete fusion gene and confirmed by performing whole genome sequencing. This Iranian strain, isolated from professional broilers, induced high mortality (70%–80%) as well as severe nervous and enteric clinical signs (Ghalyanchilangeroudi et al., 2018). The Iranian isolate was demonstrated genetically closely related to Pakistani VII.2 strains (Rehmani et al., 2015; Wajid et al., 2017). This VII.2 subgenotype emerged around 2010 in the Middle East and Asia, spreading westward from Indonesia and Pakistan, to Israel and Eastern Europe (Fuller et al., 2017; Miller et al., 2015; Munir, et al., 2012; Rehmani et al., 2015; Shabbir et al., 2013; Xiao et al., 2012). It continued to circulate in the Middle East region (Ghalyanchilangeroudi et al., 2018; Munir et al., 2012; Pandarangga et al., 2016; Wajid et al., 2016). VII.2 vAOAV has a wide host range, including Galliformes species, such as chickens, pheasants and peafowls, as well as parakeets and wild birds, such as ducks, geese and pigeons (Munir, et al., 2012; Shabbir et al., 2012). Our analysis indicated that the present epizootic in Northwestern Europe represented an independent introduction on the European continent distinct from the geographically closest VII.2 ND outbreaks in the South‐East of Europe. The time‐resolved phylogenetic analysis demonstrates a very recent divergence time (January 2018) of the genomes from the BeNeLux outbreak. This suggests a single long‐distance introduction of VII.2 into Western Europe, followed by silent circulation in local poultry or wild birds during the winter months of 2017–2018 prior to the emergence and detection of 3 related genetic clusters in the BeNeLux region in the early spring of 2018. Although we cannot exclude the theoretical possibility of several separate long‐distance introduction events of closely related viruses from the subgenotype VII.2 reservoir in the Middle East, this seems highly unlikely given the recent divergence time of the BeNeLux viral genomes and the short timeframe of the outbreak. The position of the Dutch index case outside the established poultry outbreak clusters (I–III), the low posterior probability only loosely connecting the Belgian index case to outbreak cluster I, and the temporal precedence of both the Belgian and Dutch index case to the other cases in absence of epidemiological links, jointly suggest that these viruses may represent separate introductions from an unsampled reservoir circulating in Western Europe before the establishment of the three outbreak clusters in poultry. Import of hobby birds or migration of wild birds, may have resulted in the long‐distance transmission leading to the 2018 epizootic in the BeNeLux region during the autumn or winter season (Nov 2017 to Feb 2018) as previously suggested for other velogenic AOAV‐1 outbreaks (Cardenas‐Garcia et al., 2015; Diel et al., 2012; Fan et al., 2015; Huang et al., 2004; Liu et al., 2008; Ramey et al., 2017; Snoeck, et al., 2013; Tolf et al., 2013; Vidanovic et al., 2011; Wan et al., 2004). Unfortunately, European countries only perform a very limited active wild bird surveillance for AOAV‐1 circulation, even though such data are essential for a better understanding of the reservoir and dissemination of ND viruses. The epidemiological investigations could not identify the origin of the outbreak, nor the source of introduction or transmission to the professional poultry flocks. The complete genome phylogenetic analysis also indicated low posterior probabilities for the branches connecting to the cases in professional holdings, making it difficult to hypothesize on the introduction events in the professional sector.
This episode was markedly different from past Belgian epidemics in the professional poultry sector, emphasizing, for the first time, the role of live bird markets and retailers in the epidemiology of NDV in Belgium. All identified infected Belgian hobby flocks could be linked to recent purchases. Moreover, the initial case detected in the G.D. of Luxembourg was linked to a purchase of hobby birds on a Belgian live bird market. For the Dutch case, no direct link with Belgian live bird markets nor retailers was found. Indeed, live bird markets have been demonstrated before as high‐risk factors for the dissemination of avian pathogens such as NDV in Asia, Africa and the United States (Kim et al., 2012; Molia et al., 2016; Ogali et al., 2018; Seal et al., 2005; Wang et al., 2016). Although, the epidemiological field investigation pointed out that purchases and contacts between hobby owners and retailers were the main factors in the spread of the disease, the available epidemiological and genetic data could not identify the origin of introduction nor all outbreak links, suggesting undetected circulation at least in the initial stages of the epidemic.
The spread within hobby flocks was mostly linked to the purchase of new birds from retailers. Birds sold by retailers, on‐site or via live bird markets, are most probably clinically healthy vaccinated birds excreting virus. AOAV‐1 gentotypes I and II have been used as classical ND vaccines in Belgium since the 1960s and have not been updated since then. This absence of update might result in a poor antigenic match with the VII.2 genotype. Indeed, it is reasonable to consider that poorly antigenic matched vaccines might not induce optimal protection against the circulating field strain, which may result in masked circulation by clinically healthy birds, excreting the virus (Brown & Bevins, 2017), and consequent introduction into existing hobby flocks. In addition, although vaccination is compulsory for flocks of more than 100 heads, it may not, or suboptimally, be implemented in retailers and hobby flocks due to the difficult economic‐efficient application for small flocks in the field, introducing heterogeneity in the flock immunity (van Boven et al., 2008; Dimitrov et al., 2017; Jeon et al., 2008). Moreover, interference of maternally derived antibodies reduces vaccination efficacy in newly hatched chickens (Bertran et al., 2018; Rauw et al., 2009). Therefore, hobby flocks can be considered as sentinels for viruses introduced by subclinically infected new purchases or wild birds.
The detailed biological, epidemiological and time‐resolved genetic characterization of the 2018 velogenic AOAV‐1 outbreak clearly pointed out the continuous risk of introduction of viruses with a high economic impact in the professional poultry sector and the importance of active surveillance in wild birds and un‐, or suboptimal, vaccinated sectors, such as retailers and hobbyists.
CONFLICT OF INTEREST
No Conflicts of Interest.
Supporting information
Fig S1
Table S1‐S2
ACKNOWLEDGEMENTS
The sequence information for chicken/Bulgaria/Zvinitsa/2013 (MK005972.1) was kindly shared prior to being publicly available by C. Afonso and K. Dimitrov, Southeast Poultry Research Laboratory, Athens USA. Raw paired‐end sequence data was generated at the Transversal and Applied Genomics unit of Sciensano, as well as at the Laboratory for NGS and Microarray Diagnostics of the Friedrich Loeffler Institut (Germany). We thank Frank Harders and Alex Bossers for NGS facilities at Wageningen Bioveterinary Research (Netherlands). The authors are also grateful to the technical staff involved in the in vivo evaluations, laboratory diagnosis, and molecular characterization: Christophe Delgrange, Alexandre Ausloos, Marc Boschmans, Catherine Rasseneur, Lotte Weckx, Siam Hashem Mahibullah, Emilie Charpentier and Aurélie Sausy. The epidemiological department of Sciensano, Valérie Dewaele and Xavier Simons, provided regular updated maps on the Belgian outbreak sites.
Steensels M, Van Borm S, Mertens I, et al. Molecular and virological characterization of the first poultry outbreaks of Genotype VII.2 velogenic avian orthoavulavirus type 1 (NDV) in North‐West Europe, BeNeLux, 2018. Transbound Emerg Dis.2021;68:2147–2160. 10.1111/tbed.13863
DATA AVAILABILITY STATEMENT
Adherence to Transboundary and Emerging diseases ethics’ policy. All sequence data has been made available by submission to a public database. Other data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- Adams, M. J., Lefkowitz, E. J., King, A. M. Q., Harrach, B., Harrison, R. L., Knowles, N. J.,Kropinski, A. M., Krupovic, M., Kuhn, J. H., Mushegian, A. R., Nibert, M., Sabanadzovic, S., Sanfaçon, H., Siddell, S. G., Simmonds, P., Varsani, A., Zerbini, F. M., Gorbalenya, A. E., Kropinski, A. M., … Davison, A. J. (2017). Changes to taxonomy and the International Code of Virus Classification and Nomenclature ratified by the International Committee on Taxonomy of Viruses (2017). Archives of Virology, 162(8), 2505–2538. 10.1007/s00705-017-3358-5 [DOI] [PubMed] [Google Scholar]
- Baele, G., Lemey, P., Bedford, T., Rambaut, A., Suchard, M. A., & Alekseyenko, A. V. (2012). Improving the accuracy of demographic and molecular clock model comparison while accommodating phylogenetic uncertainty. Molecular Biology and Evolution, 29(9), 2157–2167. 10.1093/molbev/mss084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bankevich, A., Nurk, S., Antipov, D., Gurevich, A. A., Dvorkin, M., Kulikov, A. S., Lesin, V. M., Nikolenko, S. I., Pham, S., Prjibelski, A. D., Pyshkin, A. V., Sirotkin, A. V., Vyahhi, N., Tesler, G., Alekseyev, M. A., & Pevzner, P. A. & Pevzner, P. A. (2012). SPAdes: A new genome assembly algorithm and its applications to single‐cell sequencing. Journal of Computational Biology, 19(5), 455–477. 10.1089/cmb.2012.0021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertran, K., Lee, D. H., Criado, M. F., Balzli, C. L., Killmaster, L. F., Kapczynski, D. R., & Swayne, D. E. (2018). Maternal antibody inhibition of recombinant Newcastle disease virus vectored vaccine in a primary or booster avian influenza vaccination program of broiler chickens. Vaccine, 36(43), 6361–6372. S0264‐410X(18)31264‐7 [DOI] [PubMed] [Google Scholar]
- Brown, V. R., & Bevins, S. N. (2017). A review of virulent Newcastle disease viruses in the United States and the role of wild birds in viral persistence and spread. Veterinary Research, 48(1), 68. 10.1186/s13567-017-0475-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardenas‐Garcia, S., Diel, D. G., Susta, L., Lucio‐Decanini, E., Yu, Q., Brown, C. C., Miller, P. J., & Afonso, C. L. (2015). Development of an improved vaccine evaluation protocol to compare the efficacy of Newcastle disease vaccines. Biologicals, 43(2), 136–145. 10.1016/j.biologicals.2014.11.003 [DOI] [PubMed] [Google Scholar]
- Czegledi, A., Ujvari, D., Somogyi, E., Wehmann, E., Werner, O., & Lomniczi, B. (2006). Third genome size category of avian paramyxovirus serotype 1 (Newcastle disease virus) and evolutionary implications. Virus Research, 120(1–2), 36–48. 10.1016/j.virusres.2005.11.009 [DOI] [PubMed] [Google Scholar]
- Diel, D. G., da Silva, L. H., Liu, H., Wang, Z., Miller, P. J., & Afonso, C. L. (2012). Genetic diversity of avian paramyxovirus type 1: Proposal for a unified nomenclature and classification system of Newcastle disease virus genotypes. Infect Genet Evol, 12(8), 1770–1779. 10.1016/j.meegid.2012.07.012 [DOI] [PubMed] [Google Scholar]
- Dimitrov, K. M., Abolnik, C., Afonso, C. L., Albina, E., Bahl, J., Berg, M., & Wong, F. Y. K. (2019). Updated unified phylogenetic classification system and revised nomenclature for Newcastle disease virus. Infection, Genetics and Evolution, 74, 103917. 10.1016/j.meegid.2019.103917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimitrov, K. M., Afonso, C. L., Yu, Q., & Miller, P. J. (2017). Newcastle disease vaccines‐A solved problem or a continuous challenge? Veterinary Microbiology, 206, 126–136. 10.1016/j.vetmic.2016.12.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimitrov, K. M., Lee, D. H., Williams‐Coplin, D., Olivier, T. L., Miller, P. J., & Afonso, C. L. (2016). Newcastle disease viruses causing recent outbreaks worldwide show unexpectedly high genetic similarity to historical virulent isolates from the 1940s. Journal of Clinical Microbiology, 54(5), 1228–1235. 10.1128/JCM.03044-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimitrov, K. M., Ramey, A. M., Qiu, X., Bahl, J., & Afonso, C. L. (2016). Temporal, geographic, and host distribution of avian paramyxovirus 1 (Newcastle disease virus). Infection, Genetics and Evolution, 39, 22–34. 10.1016/j.meegid.2016.01.008 [DOI] [PubMed] [Google Scholar]
- Dortmans, J. C., Peeters, B. P., & Koch, G. (2012). Newcastle disease virus outbreaks: Vaccine mismatch or inadequate application? Veterinary Microbiology, 160(1–2), 17–22. 10.1016/j.vetmic.2012.05.003 [DOI] [PubMed] [Google Scholar]
- Fan, S., Wang, T., Gao, X., Ying, Y., Li, X., Li, Y., Ma, J., Sun, H., Chu, D., Xu, Y., Yang, S., Li, Q., & Gao, Y.& Xia, X. (2015). Phylogenetic analysis of Newcastle disease viruses isolated from wild birds in the Poyang Lake region of China. Journal of Veterinary Medical Science, 77(9), 1143–1149. 10.1292/jvms.14-0080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller, C., Londt, B., Dimitrov, K. M., Lewis, N., van Boheemen, S., Fouchier, R., Coven, F., Goujgoulova, G., Haddas, R., & Brown, I. (2017). An epizootiological report of the re‐emergence and spread of a lineage of virulent newcastle disease virus into Eastern Europe. Transboundary and Emerging Diseases, 64(3), 1001–1007. 10.1111/tbed.12455 [DOI] [PubMed] [Google Scholar]
- Ghalyanchilangeroudi, A., Hosseini, H., Jabbarifakhr, M., Fallah Mehrabadi, M. H., Najafi, H., Ghafouri, S. A., Mousavi, F. S., & Ziafati, Z. & Modiri, A. (2018). Emergence of a virulent genotype VIIi of Newcastle disease virus in Iran. Avian Pathology, 47(5), 509–519. 10.1080/03079457.2018.1495313 [DOI] [PubMed] [Google Scholar]
- Hall, T. A. (1999). BioEdit: A user‐friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. http://www.mbio.ncsu.edu/bioedit.html. Nucleic Acids Symposium Series, 41, 95–98. [Google Scholar]
- Huang, Y., Wan, H. Q., Liu, H. Q., Wu, Y. T., & Liu, X. F. (2004). Genomic sequence of an isolate of Newcastle disease virus isolated from an outbreak in geese: A novel six nucleotide insertion in the non‐coding region of the nucleoprotein gene. Brief Report. Archives of Virology, 149(7), 1445–1457. 10.1007/s00705-004-0297-8 [DOI] [PubMed] [Google Scholar]
- Hunt, M., Gall, A., Ong, S. H., Brener, J., Ferns, B., Goulder, P., Nastouli, E., Keane, J. A., Kellam, P., & Otto, T. D. (2015). IVA: Accurate de novo assembly of RNA virus genomes. Bioinformatics, 31(14), 2374–2376. 10.1093/bioinformatics/btv120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- ICTV (2019). International committee on taxonomy of viruses. Virus Taxonomy: 2018b Release, EC 50, Washington, DC, July 2018; Email ratification February 2019 (MSL #34). [Google Scholar]
- Jeon, W. J., Lee, E. K., Lee, Y. J., Jeong, O. M., Kim, Y. J., Kwon, J. H., & Choi, K. S. (2008). Protective efficacy of commercial inactivated Newcastle disease virus vaccines in chickens against a recent Korean epizootic strain. Journal of Veterinary Science, 9(3), 295–300. 200809295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kant, A., Koch, G., Van Roozelaar, D. J., Balk, F., & Huurne, A. T. (1997). Differentiation of virulent and non‐virulent strains of Newcastle disease virus within 24 hours by polymerase chain reaction. Avian Pathol, 26(4), 837–849. 10.1080/03079459708419257 [DOI] [PubMed] [Google Scholar]
- Kass, R., & Raftery, A. (1995). Bayes factors. Journal of the American Statistical Association, 90(430), 773–795. 10.2307/2291091 [DOI] [Google Scholar]
- Kim, B. Y., Lee, D. H., Kim, M. S., Jang, J. H., Lee, Y. N., Park, J. K., Yuk, S.‐S., Lee, J.‐B., Park, S.‐Y., Choi, I.‐S., & Song, C. S. (2012). Exchange of Newcastle disease viruses in Korea: The relatedness of isolates between wild birds, live bird markets, poultry farms and neighboring countries. Infection, Genetics and Evolution, 12(2), 478–482. 10.1016/j.meegid.2011.12.004 [DOI] [PubMed] [Google Scholar]
- King, A. M. Q., Lefkowitz, E. J., Mushegian, A. R., Adams, M. J., Dutilh, B. E., Gorbalenya, A. E., Harrach, B., Harrison, R. L., Junglen, S., Knowles, N. J., Kropinski, A. M., Krupovic, M., Kuhn, J. H., Nibert, M. L., Rubino, L., Sabanadzovic, S., Sanfaçon, H., Siddell, S. G., Simmonds, P., … Davison, A. J. (2018). Changes to taxonomy and the International Code of Virus Classification and Nomenclature ratified by the International Committee on Taxonomy of Viruses (2018). Archives of Virology, 163(9): 2601–2631. 10.1007/s00705-018-3847-1 [DOI] [PubMed] [Google Scholar]
- Liu, H., Wang, Z., Wang, Y., Sun, C., Zheng, D., & Wu, Y. (2008). Characterization of Newcastle disease virus isolated from waterfowl in China. Avian Diseases, 52(1), 150–155. 10.1637/8030-061507-Reg [DOI] [PubMed] [Google Scholar]
- Meulemans, G., Gonze, M., Carlier, M. C., Petit, P., Burny, A., & Le, L. (1987). Evaluation of the use of monoclonal antibodies to hemagglutinin and fusion glycoproteins of Newcastle disease virus for virus identification and strain differentiation purposes. Archives of Virology, 92(1–2), 55–62. [DOI] [PubMed] [Google Scholar]
- Meulemans, G., & van den Berg, T. (9–10/12/ 1997). Newcastle Disease Situation in Belgium. Paper presented at the Jointh Fourth Annual Meetins of the National Newcastle Disease and Avian Influenza Laboratories of countries of Brussels: the European Union. [Google Scholar]
- Miller, P. J., Estevez, C., Yu, Q., Suarez, D. L., & King, D. J. (2009). Comparison of viral shedding following vaccination with inactivated and live Newcastle disease vaccines formulated with wild‐type and recombinant viruses. Avian Diseases, 53(1), 39–49. 10.1637/8407-071208-Reg.1 [DOI] [PubMed] [Google Scholar]
- Miller, P. J., Haddas, R., Simanov, L., Lublin, A., Rehmani, S. F., Wajid, A., Bibi, T., Khan, T. A., Yaqub, T., Setiyaningsih, S. & Afonso, C. L. (2015). Identification of new sub‐genotypes of virulent Newcastle disease virus with potential panzootic features. Infection, Genetics and Evolution, 29, 216–229. 10.1016/j.meegid.2014.10.032 [DOI] [PubMed] [Google Scholar]
- Molia, S., Boly, I. A., Duboz, R., Coulibaly, B., Guitian, J., Grosbois, V., Fournié, G., & Pfeiffer, D. U. (2016). Live bird markets characterization and trading network analysis in Mali: Implications for the surveillance and control of avian influenza and Newcastle disease. Acta Tropica, 155, 77–88. 10.1016/j.actatropica.2015.12.003 [DOI] [PubMed] [Google Scholar]
- Munir, M., Cortey, M., Abbas, M., Qureshi, Z. U., Afzal, F., Shabbir, M. Z.,Khan, M. T., Ahmed, S., Ahmad, S., Baule, C., Ståhl, K., Zohari, S., Berg, M., & Berg, M. (2012). Biological characterization and phylogenetic analysis of a novel genetic group of Newcastle disease virus isolated from outbreaks in commercial poultry and from backyard poultry flocks in Pakistan. Infection, Genetics and Evolution, 12(5), 1010–1019. 10.1016/j.meegid.2012.02.015 [DOI] [PubMed] [Google Scholar]
- Munir, M., Shabbir, M. Z., Yaqub, T., Shabbir, M. A., Mukhtar, N., Khan, M. R., & Berg, M. (2012). Complete genome sequence of a velogenic neurotropic avian paramyxovirus 1 isolated from peacocks (Pavo cristatus) in a wildlife park in Pakistan. Journal of Virology, 86(23), 13113–13114. 10.1128/JVI.02358-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munir, M., Zohari, S., & Berg, M. (2012). Newcastle disease virus in Pakistan: Genetic characterization and implication in molecular diagnosis. Indian Journal of Virology, 23(3), 368–373. 10.1007/s13337-012-0073-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagai, Y., Klenk, H. D., & Rott, R. (1976). Proteolytic cleavage of the viral glycoproteins and its significance for the virulence of Newcastle disease virus. Virology, 72(2), 494–508. 10.1016/0042-6822(76)90178-1 [DOI] [PubMed] [Google Scholar]
- Ogali, I. N., Wamuyu, L. W., Lichoti, J. K., Mungube, E. O., Agwanda, B., & Ommeh, S. C. (2018). Molecular characterization of newcastle disease virus from backyard poultry farms and live bird markets in Kenya. International Journal of Microbiology, 2018, 2368597. 10.1155/2018/2368597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- OIE (May, 2012). Manual of Diagnostic Tests and Vaccines for Terrestrial Animals OIE Terrestrial Manual (Vol. 1 and 2). [Google Scholar]
- Pandarangga, P., Brown, C. C., Miller, P. J., Haddas, R., Rehmani, S. F., Afonso, C. L., & Susta, L. (2016). Pathogenesis of new strains of newcastle disease virus from Israel and Pakistan. Veterinary Pathology, 53(4), 792–796. 10.1177/0300985815622972 [DOI] [PubMed] [Google Scholar]
- Rambaut, A., Lam, T. T., Carvalho, L. M., & Pybus, O. G. (2016). Exploring the temporal structure of heterochronous sequences using TempEst (formerly Path‐O‐Gen). Virus Evolution, 2(1), January 2016, vew007, 10.1093/ve/vew007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramey, A. M., Goraichuk, I. V., Hicks, J. T., Dimitrov, K. M., Poulson, R. L., Stallknecht, D. E., Bahl, J. & Afonso, C. L. (2017). Assessment of contemporary genetic diversity and inter‐taxa/inter‐region exchange of avian paramyxovirus serotype 1 in wild birds sampled in North America. Virology Journal, 14(1), 43. 10.1186/s12985-017-0714-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauw, F., Gardin, Y., Palya, V., van Borm, S., Gonze, M., Lemaire, S., van den Berg, T. & Lambrecht, B. (2009). Humoral, cell‐mediated and mucosal immunity induced by oculo‐nasal vaccination of one‐day‐old SPF and conventional layer chicks with two different live Newcastle disease vaccines. Vaccine, 27(27), 3631–3642. 10.1016/j.vaccine.2009.03.068 [DOI] [PubMed] [Google Scholar]
- Reed, L. J., & Muench, H. (1938). A simple method for estimating fifty endpoints. American Journal of Hygiene, 27, 493–497. [Google Scholar]
- Rehmani, S. F., Wajid, A., Bibi, T., Nazir, B., Mukhtar, N., Hussain, A., Lone, N. A., Yaqub, T., & Afonso, C. L. (2015). Presence of virulent Newcastle disease virus in vaccinated chickens in farms in Pakistan. Journal of Clinical Microbiology, 53(5), 1715–1718. 10.1128/JCM.02818-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seal, B. S., Wise, M. G., Pedersen, J. C., Senne, D. A., Alvarez, R., Scott, M. S.,King, D. J., Yu, Q., & Kapczynski, D. R. (2005). Genomic sequences of low‐virulence avian paramyxovirus‐1 (Newcastle disease virus) isolates obtained from live‐bird markets in North America not related to commonly utilized commercial vaccine strains. Veterinary Microbiology, 106(1–2), 7–16. 10.1016/j.vetmic.2004.11.013 [DOI] [PubMed] [Google Scholar]
- Shabbir, M. Z., Goraya, M. U., Abbas, M., Yaqub, T., Shabbir, M. A., Ahmad, A., Anees, M., Munir, M., & Munir, M. (2012). Complete genome sequencing of a velogenic viscerotropic avian paramyxovirus 1 isolated from pheasants (Pucrasia macrolopha) in Lahore, Pakistan. Journal of Virology, 86(24), 13828–13829. 10.1128/JVI.02626-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shabbir, M. Z., Zohari, S., Yaqub, T., Nazir, J., Shabbir, M. A., Mukhtar, N., Shafee, M., Sajid, M., Anees, M., Abbas, M., Khan, M. T., Ali, A. A., Ghafoor, A., Ahad, A., Channa, A. A., Anjum, A. A., Hussain, N., Ahmad, A., Goraya, M. U., … Munir, M. (2013). Genetic diversity of Newcastle disease virus in Pakistan: A countrywide perspective. Virology Journal, 10, 170. 10.1186/1743-422X-10-170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snoeck, C. J., Adeyanju, A. T., Owoade, A. A., Couacy‐Hymann, E., Alkali, B. R., Ottosson, U., & Muller, C. P. (2013). Genetic diversity of newcastle disease virus in wild birds and pigeons in West Africa. Applied and Environment Microbiology, 79(24), 7867–7874. 10.1128/AEM.02716-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snoeck, C. J., Owoade, A. A., Couacy‐Hymann, E., Alkali, B. R., Okwen, M. P., Adeyanju, A. T., Komoyo, G. F., Nakouné, E., Le Faou, A., & Muller, C. P. (2013). High genetic diversity of Newcastle disease virus in poultry in West and Central Africa: Cocirculation of genotype XIV and newly defined genotypes XVII and XVIII. Journal of Clinical Microbiology, 51(7), 2250–2260. 10.1128/JCM.00684-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spackman, E., Senne, D. A., Myers, T. J., Bulaga, L. L., Garber, L. P., Perdue, M. L., Lohman, K., Daum, L. T., & Suarez, D. L. (2002). Development of a real‐time reverse transcriptase PCR assay for type A influenza virus and the avian H5 and H7 hemagglutinin subtypes. Journal of Clinical Microbiology, 40(9), 3256–3260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolf, C., Wille, M., Haidar, A. K., Avril, A., Zohari, S., & Waldenstrom, J. (2013). Prevalence of avian paramyxovirus type 1 in Mallards during autumn migration in the western Baltic Sea region. Virology Journal, 10, 285. 10.1186/1743-422X-10-285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Boven, M., Bouma, A., Fabri, T. H., Katsma, E., Hartog, L., & Koch, G. (2008). Herd immunity to Newcastle disease virus in poultry by vaccination. Avian Pathology, 37(1), 1–5. 10.1080/03079450701772391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidanovic, D., Sekler, M., Asanin, R., Milic, N., Nisavic, J., Petrovic, T., & Savic, V. (2011). Characterization of velogenic Newcastle disease viruses isolated from dead wild birds in Serbia during 2007. Journal of Wildlife Diseases, 47(2), 433–441. 10.7589/0090-3558-47.2.433 [DOI] [PubMed] [Google Scholar]
- Wajid, A., Dimitrov, K. M., Wasim, M., Rehmani, S. F., Basharat, A., Bibi, T., Arif, S., Yaqub, T., Tayyab, M., Ababneh, M., Sharma, P., Miller, P. J. & Afonso, C. L. (2017). Repeated isolation of virulent Newcastle disease viruses in poultry and captive non‐poultry avian species in Pakistan from 2011 to 2016. Preventive Veterinary Medicine, 142, 1–6. 10.1016/j.prevetmed.2017.04.010 [DOI] [PubMed] [Google Scholar]
- Wajid, A., Rehmani, S. F., Wasim, M., Basharat, A., Bibi, T., Arif, S., & Afonso, C. L. (2016). Complete genome sequence of a virulent newcastle disease virus strain isolated from a clinically healthy duck (Anas platyrhynchos domesticus) in Pakistan. Genome Announcements, 4(4), e00730–16. 10.1128/genomeA.00730-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan, H., Chen, L., Wu, L., & Liu, X. (2004). Newcastle disease in geese: Natural occurrence and experimental infection. Avian Pathology, 33(2), 216–221. 10.1080/0307945042000195803 [DOI] [PubMed] [Google Scholar]
- Wang, J., Lv, Y., Zhang, Y., Zheng, D., Zhao, Y., Castellan, D., Liu, H. & Wang, Z. (2016). Genomic characterizations of a newcastle disease virus isolated from ducks in live bird markets in China. PLoS One, 11(7), e0158771. 10.1371/journal.pone.0158771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, X., Gong, Z., Zhao, L., Wang, J., Sun, G., Liu, Y., Tao, P., Zhang, H., Li, S., Jiang, F., Hu, Y., & Zhang, X. (2013). Complete genome sequences of newcastle disease virus strains isolated from three different poultry species in china. Genome Announcements, 1(4), e00198–12. 10.1128/genomeA.00198-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise, M. G., Suarez, D. L., Seal, B. S., Pedersen, J. C., Senne, D. A., King, D. J., Kapczynski, D. R. & Spackman, E. (2004). Development of a real‐time reverse‐transcription PCR for detection of newcastle disease virus RNA in clinical samples. Journal of Clinical Microbiology, 42(1), 329–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao, S., Paldurai, A., Nayak, B., Samuel, A., Bharoto, E. E., Prajitno, T. Y.,Collins, P. L., & Samal, S. K. (2012). Complete genome sequences of Newcastle disease virus strains circulating in chicken populations of Indonesia. Journal of Virology, 86(10), 5969–5970. 10.1128/JVI.00546-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig S1
Table S1‐S2
Data Availability Statement
Adherence to Transboundary and Emerging diseases ethics’ policy. All sequence data has been made available by submission to a public database. Other data that support the findings of this study are available from the corresponding author upon reasonable request.


