Abstract
Objective
Bipolar disorder (BD) is a chronic mental health disorder with significant morbidity and mortality. Age at onset (AAO) may be a key variable in delineating more homogeneous subgroups of BD patients. However, no known research has systematically assessed how BD age‐at‐onset subgroups should be defined.
Methods
We systematically searched the following databases: Cochrane Central Register of Controlled Trials, PsycINFO, MEDLINE, Embase, CINAHL, Scopus, Proquest Dissertations and Theses, Google Scholar and BIOSIS Previews. Original quantitative English language studies investigating AAO in BD were sought.
Results
A total of 9454 unique publications were identified. Twenty‐one of these were included in data analysis (n = 22981 BD participants). Fourteen of these studies (67%, n = 13626 participants) found a trimodal AAO distribution: early‐onset (µ = 17.3, σ = 1.19, 45% of sample), mid‐onset (µ = 26.0, = 1.72, 35%), and late‐onset (µ = 41.9, = 6.16, 20%). Five studies (24%, n = 1422 participants) described a bimodal AAO distribution: early‐onset (µ = 24.3, σ = 6.57, 66% of sample) and late‐onset (µ = 46.3, σ = 14.15, 34%). Two studies investigated cohort effects on BD AAO and found that when the sample was not split by cohort, a trimodal AAO was the winning model, but when separated by cohort a bimodal distribution fit the data better.
Conclusions
We propose that the field conceptualises bipolar disorder age‐at‐onset subgroups as referring broadly to life stages. Demarcating BD AAO groups can inform treatment and provide a framework for future research to continue to investigate potential mechanisms of disease onset.
Keywords: admixture analysis, age at onset, bipolar disorder, systematic review
1. INTRODUCTION
Bipolar disorder (BD) is a chronic mental health disorder with significant morbidity and mortality that affects between 1‐4% of the population.1 Age at onset in BD has been recognised as being important in the course and outcome of the disorder. Meta‐analytic results suggest that an early (compared to late) age at onset in bipolar disorder is associated with a longer delay to treatment, greater severity of depression and higher levels of comorbid anxiety and substance abuse.2, 3 Given this differing clinical trajectory between early‐ versus late‐onset BD, it has been proposed that age at onset (AAO) may be a key variable in delineating more homogeneous subgroups of BD patients.4 To date no research has systematically validated what the various AAO subgroups should be, and there is no concurrence across studies regarding what is meant by ‘early onset’.5, 6
In recent years, it has been acknowledged that AAO in bipolar disorder is not a simple unimodal distribution, but can better be explained by a mixture of distributions. Evidence has suggested that BD aggregates either into a bimodal distribution with two subgroups (early vs. late AAO), or a trimodal distribution with three subgroups (early vs. mid vs. late AAO).3, 4, 7, 8, 9, 10 However, it is not known which of these distribution modalities are more reliable and consistent. A better understanding of the distribution of age at onset in BD could provide an insight into the causes and mechanisms of illness, anticipate disease trajectory, and guide appropriate timeframes for primary and secondary prevention.11 Understanding the age‐at‐onset distribution of bipolar disorder over the life course also has implications relating to the conduct of clinical and epidemiological research, and health service provision and planning.
1.1. Objective
The aim of this systematic review was to investigate age‐at‐onset distributions in bipolar disorder, and correspondingly what constitutes an early age at onset. Only studies that use a data‐driven approach to define AAO groups were included in data synthesis, as segregating BD into AAO groups using pre‐defined cut‐offs is an inherently biased approach. One of the most popular analysis approaches for determining AAO groups is admixture analysis, as it explores the theoretical model that best fits the observed distribution of a continuous variable.
2. METHODS
2.1. Eligibility criteria
This study was pre‐registered via PROSPERO (https://bit.ly/333fs2V). All studies had to meet four criteria: 1) include participants who were recruited with a primary diagnosis of BD I, II or not otherwise specified (NOS); 2) report on the distribution of bipolar disorder AAO using a data‐driven analysis approach (e.g. admixture analysis); 3) be an original article including epidemiological, cohort, longitudinal, cross‐sectional, survey or observational studies and 4) be an English language article. Animal research, single case studies, duplicates, conference abstracts or articles with unobtainable missing data were excluded.
2.2. Search strategy
In February 2019 we carried out searches of the following databases: Cochrane Central Register of Controlled Trials (CENTRAL; Wiley interface), PsycINFO, MEDLINE (OVID interface, 1948 onwards), Embase (OVID interface, 1980 onwards), Cumulative Index of Nursing and Allied Health Literature (CINAHL) and Scopus. We also searched grey literature via Proquest Dissertations and Theses, BIOSIS Previews and Google Scholar.
Search strategies were developed using medical subject headings (MeSH) and text words related to bipolar disorder, age at onset and study type (Supplement S1). The syntax and subject headings of the search strategies were adapted for each database, and Boolean operators and truncation were used to extend the search terms. No date limits were imposed on the searches.
2.3. Study selection
The web‐based systematic review software, DistillerSR,12 was used to complete screening and data extraction. Two reviewers (SB, JW) independently screened titles and abstracts. A third reviewer (KS) resolved any eligibility conflicts. Following title and abstract screening, we conducted full‐text reviews of eligible articles. Where necessary, we sought additional information from study authors to resolve questions about eligibility and obtain missing data. We did not quality assess included studies as accepted standards of quality assessing non‐randomised studies are lacking,13 and our articles employed a broad range of study designs with diverging methodologies and reporting standards.
2.4. Data analysis
We extracted data from eligible studies using a standardised data extraction form. This included data on diagnoses, recruitment strategies, demographics and details of age‐at‐onset groups, including means, standard deviations (SDs) and age ranges. From our extracted data, we computed summary statistics for each study. These summary statistics related to participant characteristics (sample size, age range and gender ratio); diagnostic criteria used; age‐at‐onset definition; recruitment settings (clinic, community and hospital) and study locations. Two studies14, 15 recruited mixed samples which included participants with schizoaffective disorder; where possible, we only included participants with a BD diagnosis in analyses and samples with schizoaffective disorder participants were excluded.
We separated the studies into those reporting a trimodal AAO distribution, a bimodal distribution and those investigating cohort effects on AAO. For each study, we then extracted the average AAO per subgroup—for those studies reporting a trimodal AAO distribution we extracted the mean and SD for the early‐, mid‐ and late‐onset groups, and for those studies reporting a bimodal AAO distribution, we extracted means and SDs for the early‐ and late‐onset groups. These averages were used to plot probability density functions and boxplots for each AAO group in studies reporting a trimodal versus bimodal AAO distribution. To do this, we used the ggplot216 data visualisation package in RStudio (version 1.2.1335)17—the analysis code can be found on our Open Science Framework (OSF) webpage (https://osf.io/5c89s/). Our data are also openly available via the OSF.18
3. RESULTS
Our search produced 14,129 results. After duplicates were removed, we screened the titles and abstracts of 9454 articles for relevance and considered 74 eligible for full‐text review (PRISMA Diagram Figure 1; see Supplement S2 for a list of studies excluded at full‐text review). Twenty‐four articles met full‐text eligibility criteria, and 21 articles were included in data synthesis. We excluded 3 of the 24 eligible studies19, 20, 21 due to missing data, which were unobtainable after contacting the authors.
Figure 1.
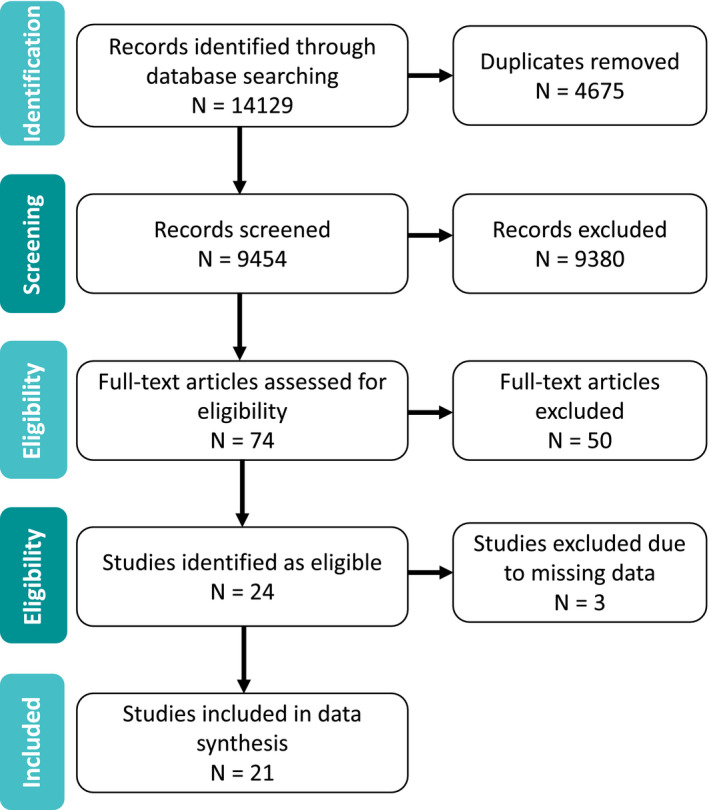
PRISMA flowchart of included studies
3.1. Study characteristics
All included studies were conducted from 2001 to 2017, with the majority (n = 15, 71%) published from 2009 onwards.
3.1.1. Participants
Across all studies, there were a total of 22,904 bipolar disorder participants, with an average sample size of 1,094 participants per study. In total, there were 22,165 (96.78% of total) participants with a diagnosis of BDI, 653 (2.85%) with BDII, 12 (0.05%) with BD‐NOS and 74 (0.32%) with schizoaffective disorder. There were more female than male participants, with an average of 59.9% female participants across all studies.
3.1.2. Age of participants at study entry
Several studies did not report age ranges or average age of their samples; those that did (n = 15) had an overall average age of 43.2 years.
3.1.3. Diagnostic criteria
Thirteen studies (62%, n = 13) used DSM‐IV criteria alone to determine a bipolar disorder diagnosis.5, 29 Two studies used DSM‐IV or ICD‐10 criteria,30, 31 one used DSM‐IV or Research Diagnostic Criteria (RDC),32 one used DSM‐IV or DSM‐III‐R criteria,15 one used both DSM‐III‐R and RDC,33 two used RDC only34, 35 and one used case records only.36
3.1.4. Age‐at‐onset definitions
Heterogeneous definitions of AAO were used across studies including: age at which diagnostic criteria for an affective episode was first met according to medial case notes, interviews or self‐report5, 30, 32, 33, 34, 35; age at first impairment due to an affective episode according to self‐report8; age at first contact with psychiatric services for symptoms of mania14, 22; age at first treatment for an affective disorder28 and age at first psychiatric hospitalisation.36 Across all studies, AAO was determined retrospectively using information gathered from medical records and/or interviews with participants and their relatives.
3.1.5. Recruitment setting
Seven studies recruited patients from a clinic setting only,9, 15, 23, 27, 29, 34, 35 two from community settings only8, 32 and two from inpatient hospital settings only.25, 36 Three studies recruited from both the clinic and the community,24, 30, 31 three from both the clinic and hospital setting5, 28, 33 and four studies recruited from the hospital, clinic and the community.7, 14, 22, 26
3.1.6. Study locations
The largest of the included studies collected data on 4037 bipolar patients across 36 collection sites in 23 countries throughout Asia, Africa, Europe, North and South America and Australia.24 Of the remaining studies, eleven were conducted in Europe,5, 7, 8, 22, 23, 25, 28, 29, 31, 34, 35 six in North America,9, 14, 15, 32, 33, 36 one in Australia27 and two articles combined data from collection sites in both North America and Europe.26, 30
3.2. Age‐at‐onset distributions
There were three separate types of distributions found for bipolar disorder age at onset across the 21 articles. Fourteen studies showed a trimodal distribution,5, 32, 33, 34, 35 five a bimodal distribution9, 14, 22, 23, 36 and two studies examined cohort effects on AAO.24, 31
3.2.1. Trimodal age‐at‐onset distribution
Fourteen (67%) of the included 21 articles, including 59% (n = 13,549) of all participants, reported a trimodal age‐at‐onset distribution with three subgroups: early onset, mid‐onset and late onset (Table 1). Eight of these studies were conducted in Europe, three in America, two in both North America and Europe, and one in Australia. Of the fourteen studies, nine included participants with a diagnosis of BDI only, three with a diagnosis of BDI, BDII or BD‐NOS, and two with a diagnosis of BDI, BDII or schizoaffective disorder.
Table 1.
Details of the studies which report a trimodal age‐at‐onset distribution in bipolar disorder
| Study | N | Country | Diagnosis | Recruitment | Definition of age at onset | Method of determining AAO | Mean age of sample at study entry (SD) | Early onset | Mid‐onset | Late onset | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Upper age limit | Mean (SD), % | Lower and upper age limits | Mean (SD), % |
Lower age limit |
Mean (SD), % | ||||||||
| Azorin et al (2013) | 1082 | France | DSM‐IV BPI | The EPIMAN II Mille study, a multi‐centre naturalistic study conducted in 19 French medical centres | Age at which the patient first met the Research Diagnostic Criteria for an affective episode |
Medical records. Structured interviews with patients and relatives. |
42.9 (13.7) | 20 | 18.6 (2.1), 19% | 21‐29 | 24.3 (5.3), 38.9% | 30 | 36.7 (10.8), 42% |
|
Bellivier et al (2001) |
211 | France | DSM‐IV BPI | Consecutive inpatients and outpatients in France | Age at which DSM‐IV criteria for an affective episode was first met |
Medical records. Diagnostic Interview for Genetic Studies |
42.4 (14.8) | 16.9 (2.7), 41.4% | 26.9 (5.0), 41.8% | 46.2 (8.0), 16.6% | |||
| Bellivier et al (2003) | 579 |
France, Switzerland Germany Ireland |
DSM‐IV BPI |
Inpatients and outpatients across four countries |
Age at which DSM‐IV criteria for an affective episode was first met |
Medical records. Diagnostic Interview for Genetic Studies |
Not reported | 17.4 (2.3), 27.9% | 25.1 (6.2), 50.1% | 40.4 (11.3), 21.9% | |||
| Bellivier et al (2014) | 5891 |
Europe and USA |
DSM‐IV BPI |
Recruited for genetic, pharmacological and observational studies across 18 sites in Europe (N = 3616, incl. participants from the EMBLEM study) and from the Stanley Centre Bipolar Registry in the USA (N = 2275) |
Age at which DSM‐IV criteria for an affective episode were first met | Semi‐structured interview | 44.0 (13.2) | Europe | |||||
| 19 (2.7), 24.8% | 27.2 (6.3), 50.7% | 41.8 (10.7), 24.5% | |||||||||||
| 40.8 (11.7) | USA | ||||||||||||
| 14.5 (4.9), 63.0% | 26.5 (7.6), 28.5% | 39.5 (12.5), 8.5% | |||||||||||
| Biffin et al (2009) | 162 | Australia | DSM‐IV BPI | Recruited as part of the Bipolar Comprehensive Outcome Study (BCOS) in Melbourne, Australia | Self‐reported age at which episode of mania or depression first met diagnostic criteria | Questionnaire developed by the research team |
Early:38.7 (12.6) Mid: 43.7 (12.6) Late: 58.9 (11.5) |
15.5 (2.7), 44.4% | 26.1 (4.8) 48.1% | 50.6 (9.0), 7.4% | |||
| González‐Pinto et al (2009) | 169 | Spain | DSM‐IV BPI | Inpatients and outpatients who were receiving treatment in Alava, a Spanish province. | The age at first treatment for an affective disorder | Medical records. Semi‐structured SCID‐P interview. Emergency service records. Interviews with relatives. | 46.0 (16.0) | 18.2 (2.0), 34.0% | 26.1 (5.5), 44.0% | 50.9 (9.1), 22.0% | |||
| Hamshere et al (2009) | 1369 | UK | DSM‐IV BPI | Large‐scale genetic epidemiological study. Recruited via community mental health teams, general practitioner surgeries and patient support organisations across the UK. | Age at first impairment due to an affective episode according to self‐report | Medical records. Schedules for Clinical Assessment in Neuropsychiatry (SCAN). | Age range: 6 to 73 years | 22 | 18.7 (3.7), 47.1% | 25‐37 | 28.3 (5.5), 38.8% | 40 | 43.3 (9.1), 14.3% |
| Lin et al (2006) | 211 | USA |
DSM‐III‐R BP‐I |
NIMH Genetics Initiative for Bipolar Disorder 85 | Self‐reported age at which episode of (hypo)mania or depression first met diagnostic criteria | Medical records. Diagnostic Interview for Genetic Studies. | Age range: 0 to >61 years | 21 | 16.6 (5.1), 79.7% | 22‐28 | 26.0 (1.4), 7.2% | 28 | 34.7 (6.6), 13.1% |
|
Manchia et al (2008) |
181 | Sardinia |
RDC‐BPI |
Recruited from the Lithium Clinic of the Clinical Psychopharmacology Centre, University of Cagliari, Italy |
Age at first reliably diagnosed (hypo)manic or depressive episode |
Medical records. Semi‐structured interview | 42.8 (14.8) | 20 |
18.1 (2.3), 36.0% |
21‐33 |
24.3 (5.3), 39.0% |
34 |
41.0 (11.5), 25.0% |
| Nowrouzi et al (2016) | 194 | Canada |
DSM‐III‐R or DSM‐IV BPI BPII |
Recruited from four clinical sites across Ontario, Alberta and British Columbia, Canada | Unknown | Unknown |
25.2 (9.51), range 14‐65 years |
18.0 (2.9), 69.0% | 28.7 (3.5), 22.0% | 47.3 (7.8), 9.0% | |||
| Ortiz et al (2011) | 379 | Canada |
DSM‐IV or RDC BPI BPII |
Recruited through the Maritime Bipolar Registry, a community‐based project in the Maritime Provinces of Canada 86 |
Age at which DSM‐IV criteria for an affective episode was first met (according to medial case notes and interviews) | Medical records. Schedule for Affective Disorders and Schizophrenia, Lifetime version | 50.1 (12.7) | 19 |
15.5 (2.0), 29.0% |
20‐31 | 22.8 (4.6), 37.1% | 32 | 36.1 (10.1), 33.4% |
| Severino et al (2009) | 300 | Italy |
RDC BPI BPII Schizoaffective bipolar manic type |
Outpatients at the Lithium Clinic of the Clinical Psychopharmacology Centre, University of Cagliari, Italy | Age at first reliably diagnosed (hypo)mania or depression according to RDC criteria (using medical records) | Medical records. Semi‐structured interview | 42.9 (14.8) | 22 | 18.5 (2.6), 43.0% | 23‐37 | 27.5 (6.1), 42.0% | 38 | 43.0 (10.8), 15.0% |
| Tozzi et al (2011) | 964 | UK and Canada |
DSM‐IV or ICD‐10 BP‐I BP‐II |
Recruited across three sites: Toronto (Canada) at the Centre for Addiction and Mental Health, London (UK), at the Institute of Psychiatry, and Dundee (UK) at the University of Dundee | Self‐reported age at which episode of mania or depression first met diagnostic criteria | Schedules for Clinical Assessment in Neuropsychiatry (SCAN) interview |
47.2 (12.1), range 18‐84 years |
24 | 16.1 (4.2), 64.0% | 25 | 25.4 (2.5), 6.0% | 26 | 32.2 (9.5), 30.0% |
| Grigoroiu‐Serbanescu et al (2014)a | 1857 |
Germany Poland Romania |
DSM‐IV BPI | Consecutive inpatients recruited at three sites: | Age at which DSM‐IV criteria for an affective episode were first met | Medical records. Semi‐structured interview with patients and relatives | 43.4 (13.4) | Romania | |||||
| 17.6 (3.2), 43.0% | N/A | 20‐21 | 29.9 (8.2), 57.0% | ||||||||||
| 17.3 (2.8) 33.0% | 25.6 (6.3), 46.0% | 40.9 (5.3), 21.0% | |||||||||||
| 44.0 (13.4) | Germany | ||||||||||||
| 20.7 (6.0), 67.0% | N/A | 25 | 38.4 (6.5), 33.0% | ||||||||||
| 19.3 (5.5), 46.0% | 28.5 (7.1), 41.0% | 45.4 (4.7) 13.0% | |||||||||||
| 45.0 (14.1) | Poland | ||||||||||||
| 20.47 (3.91), 65% | N/A | 24‐25 | 33.57 (9.12), 35% | ||||||||||
| 20.7 (3.7), 44.0% | 33.0 (6.1), 45.0% | 49.0 (5.3), 11.0% | |||||||||||
Age bounds for the subgroups are provided. Numbers reported to one decimal place.
Two component and three component models fitted the data equally well.
Of the two studies including schizoaffective disorder patients,14, 35 Javaid et al (2011) report their findings including and excluding participants with schizoaffective disorder. We use the results of the ‘bipolar only’ sample in our analyses.
Two of these fourteen studies had a partial overlap in their samples34, 35 (Table 1). Manchia et al (2008) recruited 181 BDI participants from the Lithium Clinic of the Clinical Psychopharmacology Centre, University of Cagliari, Italy. Severino et al. (2009) used these same BDI participants, and additionally recruited 45 participants with BDII and 74 participants with a diagnosis of schizoaffective disorder. To account for this sample overlap and the inclusion of participants with schizoaffective disorder, we excluded these papers one by one from analyses. Excluding these studies did not make a significant difference to results (Supplement S3: Supplementary Table S1).
Across these fourteen studies the average age of early, mid‐ and late onset was as follows: 17.3 years (SD = 1.19); 26.0 years (SD = 1.72) and 41.9 years (SD = 6.16). Results suggest that the majority of BD cases occurred in the early‐onset range, with an average of 45% of a total 13626 participants displaying early onset, compared to 35% mid‐onset and 20% late onset (Figure 2).
Figure 2.
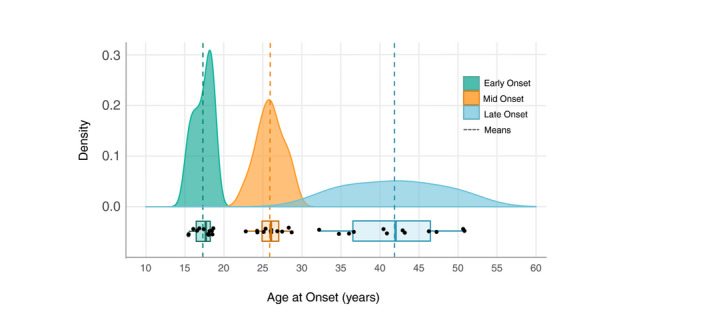
Trimodal age‐at‐onset (AAO) distribution in bipolar disorder. This figure displays the density function for each AAO group across all 14 studies, with the mean AAO per group depicted as dashed vertical lines. Under each density plot, boxplots display interquartile ranges (coloured boxes), medians (solid vertical lines) and the minima and maxima (whiskers: coloured horizontal lines)
3.2.2. Bimodal age‐at‐onset distribution
Five studies (24%), representing 6% (n = 1422) of all participants, described a bimodal age‐at‐onset distribution with two subgroups: early onset and late onset (Table 2). Two of these studies were conducted in Europe and three in North America. Three of the studies included participants with a diagnosis of BDI only, and two included those with a diagnosis of BDI, BDII or BD‐NOS.
Table 2.
Details of the studies which report a bimodal age‐at‐onset distributions in bipolar disorder.
| Study | N | Country | Diagnosis | Recruitment | Definition of age at onset | Method of determining AAO | Mean age of sample at study entry (SD) | Early onset | Late onset | |
|---|---|---|---|---|---|---|---|---|---|---|
| Upper age limit | Mean (SD), % | Mean (SD), % | ||||||||
|
Bauer et al (2010) |
270 | USA |
DSM‐IV BPI BPII |
Consecutive outpatients recruited from US clinics | Age at which episode of (hypo)mania or depression first occurred | Semi‐structured interview | Age range:≤12 to ≥30 years |
15.1 (4.7), 68.1% |
27.5 (10.2), 31.9% |
|
| Javaid et al (2011) | 353 | Canada | DSM‐IV BP or schizoaffective disorder | Recruited through newspaper advertisements and hospital clinic referrals from the Toronto region. | Age at first diagnosis of a major mood episode or mood‐related psychotic symptoms | Medical records. Structured Clinical Interview. Interviews with relatives |
Whole sample: Males: 35 (10.7) Females: 36 (10.7) |
22 | Incl. schizoaffective disorder (n = 353) | |
| 16.9 (3.6) | 24.4 (9.2) | |||||||||
| Bipolar only (n = 318) | ||||||||||
| 16.5 (3.1) | 23.7 (8.9) | |||||||||
| Kennedy et al (2005) | 246 | UK | DSM‐IV BPI, first manic episode | Inpatient and outpatient cases of first‐episode mania presenting to psychiatric services in Camberwell, southeast London, between 1965 and 1999 were identified | Age at which first contact with psychiatric services was sought for mania | Medical records | Age range: 16 to ≥76 | 40 |
25.6 (6.0), 78.0% |
51.0 (16.3), 22.0% |
|
Lehmann & Rabins (2006) |
73 | USA | BPI | Inpatients aged >65 admitted to Johns Hopkins Hospital psychiatric service, 1990‐1995 | Age at first psychiatric hospitalisation | Medical records | ≥65 | 45 |
33.2 (7.4), 52.0% |
64.4 (10.8), 48.0% |
| Manchia et al (2017) | 515 | Italy |
DSM‐IV BPI BPII BP‐NOS |
Recruited at two sites in Italy: Anxiety and Mood Disorders Unit, University of Turin, and at the Department of Psychiatry, University of Naples | Age at which DSM‐IV criteria for an affective episode was first met |
Medical records. Semi‐structured interviews with patients and first‐degree relatives |
47.2 (13.0) | BPI | ||
| 32 | 22.6 (4.8), 67% | 35.1 (10.1), 33.0% | ||||||||
| BPII | ||||||||||
| 28 | 20.9 (4.1), 44.0% | 38.2 (11.8), 56.0% | ||||||||
| Whole Sample | ||||||||||
| 30 | 21.9 (4.6), 55.0% | 37.6 (11.5), 45.0% | ||||||||
Age bounds for the subgroups are provided. Numbers reported to one decimal place.
Across these five studies, the average age of early onset was 22.5 years (SD = 7.32) and late onset was 40.8 years (SD = 16.89). Results indicated that an average of 63% out of a total of 1422 participants across the five studies displayed early onset, compared to 37% late onset (Figure 3).
Figure 3.
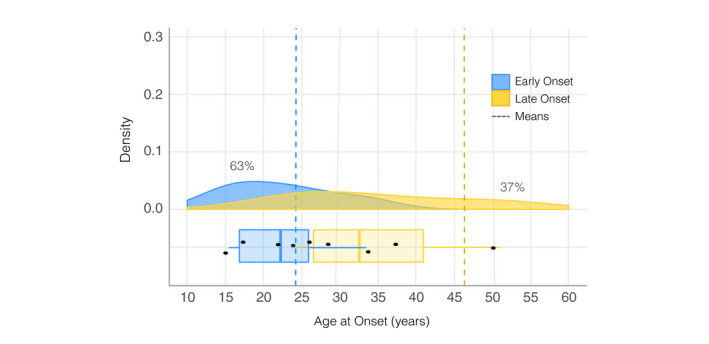
Bimodal age‐at‐onset (AAO) distribution in bipolar disorder. This figure displays the density function for each AAO group across all five studies, with the mean AAO per group depicted as dashed vertical lines. Under each density plot, boxplots display interquartile ranges (coloured boxes), medians (solid vertical lines) and the minima and maxima (whiskers: coloured horizontal lines)
3.2.3. Effect of birth cohort
The remaining 2 of the 21 included articles examined cohort effects on AAO (Table 3). Both studies examined the effect of birth cohorts on age at onset in samples of BDI patients (total n = 7,933) recruited from clinical and community settings.
Table 3.
Details of the studies investigating cohort effects on age‐at‐onset distributions in bipolar disorder.
| Study | N | Country | Diagnosis | Recruitment | Definition of age at onset | Method of determining AAO | Mean age of sample at study entry (SD) | Cohort | Early onset | Mid‐onset | Late onset |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean (SD), % | Mean (SD), % | Mean (SD), % | |||||||||
|
Bauer et al. (2015) |
4037 | 23 countries across Asia, Africa, Australia, Europe, North and South America |
DSMI‐IV BPI |
Data obtained retrospectively from 36 collection sites for a study of the impact of solar insolation on the age of onset of bipolar disorder | Age at first episode of depression, mania or hypomania meeting DSM‐IV criteria (according to medical case notes and interviews). | Medical records and semi‐structured interviews | 48.1 (14.5) |
Whole sample without birth cohorts (n = 4037) With birth cohorts incl. in model (n = 4037): born <1940, 1940‐1959, >1959 Youngest cohort, born >1959 (n = 2550) |
17.2 (3.2), 41.7% 20.7 (5.8), 62.1% 18.1 (3.7), 56.9% |
23.9 (5.1), 24.7% N/A N/A |
32.20 (12.0), 33.6% 30.1 (10.4), 37.9% 25.8 (8.4), 43.1% |
| Golmard et al. (2016) | 3896 |
Belgium, Denmark, Finland, France, Germany, Greece, Ireland, Italy, The Netherlands, Norway, Portugal, Spain, Switzerland and the UK |
DSM‐IV or ICD−10 BPI | Inpatients and outpatients recruited for participation in genetic studies, and patients recruited for the EMBLEM study, a multicentre study conducted in 14 different European countries between 1993 and 2008 | Age at which DSM‐IV criteria for an affective episode was first met (according to medial case notes and interviews) | Medical records. And semi‐structured clinical interviews | 44.0 (13.3) |
Whole sample born >1960 Whole sample born ≤1960 Matched for age at interview (n = 125): Born >1960 Born ≤1960 |
20.6 (3.7), 65% 19.3 (3.0), 49.7% 18.2 (2.5), 48% 16.9 (0.9), 16% |
26.8 (1.7), 26% 25.9 (1.8), 32.8% |
29.8 (0.5), 9% 29.8 (0.5), 17.6% 30.9 (5.3), 52% 27.1 (6.9), 84% |
When the effect of birth cohort was not modelled, both studies found a trimodal bipolar disorder AAO distribution. When birth cohort was adjusted for, both studies reported that a bimodal distribution fit the data better. Across all cohorts in both studies, the overall mean ages for early, mid and late onset were 18.7 (SD = 1.52), 25.5 (SD = 1.47) and 29.4 (SD = 2.21) years, representing an average of 48.5%, 12.0% and 39.5% respectively.
3.2.4. Age‐at‐onset distributions by study location and diagnostic criteria
Prior research has suggested that study location and BD diagnosis may influence AAO distributions.37, 38, 39, 40, 41, 42
Location
Of the eleven studies conducted in Europe, eight found a trimodal AAO distribution, two found a bimodal distribution and one reported cohort effects. There was an even split between studies reporting bi‐ and tri‐modal distributions (3 vs. 3) in North American samples. Studies conducted in both Europe and North America found a trimodal AAO distribution. The one study conducted in Australia reported a trimodal distribution.
Diagnosis
Two thirds of studies included participants with a diagnosis of BDI only (n = 14, 67%). Nine of these studies (64%) found a trimodal AAO distribution, compared to three reporting a bimodal distribution (21%). Five studies (25%) recruited samples with BDI, BDII and BD‐NOS. Three of these studies reported a trimodal distribution and two a bimodal distribution. Two studies included schizoaffective disorder as a diagnostic category, and both of these studies reported a trimodal AAO distribution.
The impact of diagnosis and study location does not appear to affect AAO in a meaningful way (see Supplement S3: Supplementary Table S2).
4. DISCUSSION
This is the first systematic review of age at onset (AAO) in bipolar disorder. The aim of this review was to provide a more reliable understanding of the AAO distribution in BD, including how ‘early‐onset’ should be defined. Our results demonstrate that a trimodal AAO distribution (early‐, mid‐ and late‐onset subgroups), compared to a bimodal distribution (early‐ vs. late‐onset), is found across a broader range of bipolar disorder diagnoses (BDI, BDII and schizoaffective disorder) and a greater number of patients (59% vs. 6% of all participants—excluding cohort studies). This provides compelling evidence to suggest that bipolar disorder onsets during early, mid or late life, with the majority (45%) of participants displaying an average age at onset of 17.3 years (SD = 1.91).
4.1. Defining early‐onset
Our findings offer a more robust understanding of when bipolar disorder is likely to manifest across the life course, and correspondingly provide a benchmark for what can be considered ‘early‐onset’ bipolar. We propose that a distinction should be made between ‘early‐life onset’ and ‘early‐onset’. Our results indicate that the majority of BD cases onset in early‐life, from the ages of 14‐21 years, with an average onset of 17.3 years. As it is customarily used, the term ‘early‐onset’ implies an ‘earlier than expected AAO’, whereas throughout our included studies the ‘early‐onset’ group is the most common age range for the onset of BD. We therefore recommend that the term early onset should be reconceptualised to represent life stage rather than as a comparator. ‘Early‐onset’ in the sense it is traditionally referred to is, thus, best described as onset before the age of 14 years. This distinction has the potential to aid the interpretation of existing treatment guidelines, which currently offer recommendations for treating ‘early‐onset and early‐stage’ BD without providing corresponding definitions.43
The diagnosis of pre‐pubertal bipolar disorder, which is prevalent in North America,41, 44 has long been viewed as contentious due to high rates of comorbidities and elevated levels of symptom overlap with other juvenile psychiatric disorders.45, 46 Our findings do not directly refute the diagnosis of paediatric bipolar disorder, but they do suggest that pre‐pubertal onset is rare. This assertion is strengthened as the included studies used samples from both Europe and North America, and is concordant with a recent meta‐analysis reporting no differences in rates of youth BD between North American and European samples.47 The lack of support for childhood onset may reflect the low diagnostic stability associated with very‐early‐onset BD. Evidence from longitudinal studies of high‐risk offspring suggests that manic‐like symptoms in very young children without a confirmed history of BD are not predictive of a later BD diagnosis.48 Additionally, epidemiological findings suggest that individuals diagnosed with BD‐NOS in childhood do go on to receive an adult BD diagnosis.49, 50 As our included studies assessed AAO retrospectively in adult samples with a confirmed BD diagnosis, any individuals who received a diagnosis of childhood BD which did not persist into adulthood will have been overlooked.
4.2. Treatment and diagnosis
A corollary to forming a more robust definition of ‘early‐onset’ BD is that clinical trajectory can be better anticipated, as early‐life‐onset is thought to confer a more severe and remitting course.2, 3 For instance, early‐onset BD is associated with comorbid anxiety disorders and substance abuse2, 51; clinicians should be mindful of this when assessing and treating early‐onset patients. Demarcating these AAO groups, thus, has implications for treatment provision, with the potential to guide appropriate junctures for intervention across the lifespan.
We found no substantive impact of diagnosis (BDI vs. BDII) on AAO distribution. However, most of the included studies (67%) recruited samples of BDI participants only (97% of total sample), while only 25% of studies included samples with BDII participants (3% of total sample). In light of this disparity, our findings must be interpreted with caution, especially as prior meta‐analytic research found evidence partially supporting an earlier AAO in BDI compared to BDII patients. As hypomanic episodes are not always recognised clinically, the true AAO in BDII is likely to be more difficult to determine and less reliable than BDI. Prospective follow‐up of youth at high‐risk of BD may therefore be the most reliable way to measure AAO of BDII (and/or BD‐NOS). Such longitudinal monitoring would facilitate early identification of incipient or sub‐threshold (hypo)manic and depressive symptoms.
4.3. Late life onset
In contrast to the finding that 45% of cases onset in the ‘early’ subgroup, only 20% of cases were deemed ‘late‐life‐onset’ (>40 years of age; Figure 2). This indicates that these two subgroups may be aetiologically distinct forms of the same disorder, as suggested by prior research.37, 52 However, late‐onset BD may have been underreported in the included studies as there was a sizeable skew towards younger samples (with an average age at study entry of 43.2 years). Additionally, a BD diagnosis in older age may be masked or missed in favour of more prevalent later‐life disorders with psychiatric symptoms (e.g. frontotemporal dementia), thus, obscuring the true rate of late‐onset BD.
4.4. Putative mechanisms
Our results indicate that a three‐component model (early, mid‐ and late onset) best describes the AAO distribution of bipolar disorder. As with the majority of psychiatric disorders, the interaction between genes and environment is likely to underpin the manifestation of this trimodal distribution in bipolar disorder AAO.
There is strong evidence for a genetic predisposition in bipolar disorder, with heritability estimates ranging from 60% to 85%, but the influence of genetics on AAO in bipolar is comparatively under‐studied.53, 54 Initial evidence suggests that there is genetic homogeneity within AAO subgroups and heterogeneity between groups.55, 56, 57 It is thought that early onset may be a more heritable form of BD than late onset, with studies demonstrating differences in transmission patterns and more pronounced familial aggregation in early‐ compared to late‐onset BD.4, 6, 8, 54, 57, 58
Genetics does not explain the whole picture, however, and there are environmental and neurobiological factors that are thought to interact with various susceptibility genes to influence the AAO of BD.59 It is thought that exposure to childhood trauma interacts with genes that are involved in pathways relating to neuroplasticity, inflammation and calcium signalling to influence AAO.60, 61, 62, 63 Epigenetic modifications in gene function may play an important role in the mechanism underlying the relationship between childhood trauma and a younger AAO of BD.64, 65, 66, 67, 68, 69 Childhood trauma is also associated with AAO independent of these genetic factors. Evidence suggests a dose effect of exposure to childhood trauma on the AAO of BD, with physical and sexual abuse, as well as verbal abuse, family conflict and emotional and physical neglect being significantly associated with an earlier AAO.2, 51, 70, 71, 72
Other candidate environmental risk factors for the subsequent onset of BD include substance abuse, decreased socioeconomic status, sleep disturbances and comorbid vascular conditions.6, 73, 74 Unlike childhood trauma, these factors are not unique to early life and therefore may contribute to the manifestation of mid‐ and late‐onset groups. Perhaps most relevant to the mid‐onset subgroup (onset in 20 s to early 30 s) is the phenomenon of post‐partum BD. During this time women are at increased risk for mood episodes compared with non‐postpartum periods, and childbirth has been reported as one of the most potent triggers for mania or hypomania.75, 76 It is not yet understood why childbirth is a specific trigger for manic onset, but it has been suggested that immune system dysregulation, puerperal hormones and genetic factors may activate disease pathways.75, 77 Late‐onset bipolar disorder is associated with increased rates of cerebrovascular disease, more medical and psychiatric comorbidities, and a weaker family history of psychiatric problems.78, 79 However, without employing detailed prospective longitudinal methodologies, it is unclear whether all of these environmental factors are a cause or consequence (or both) of incipient BD.
5. STRENGTHS AND LIMITATIONS
This is the only known systematic review investigating age at onset in bipolar disorder. To ensure we captured all relevant studies on bipolar disorder AAO, we used a search strategy with broad criteria and included several different databases and grey literature searches. We were unable to assess risk of bias due to the broad range of reporting standards and methodologies used in our included studies.
Several limitations must be considered when interpreting our findings. It has been suggested that admixture analysis is sensitive to the sample size and the characteristics of the data.80 The studies that reported a bimodal AAO distribution had smaller sample sizes on average compared to those reporting a trimodal AAO distribution. Interestingly, both cohort studies found a bimodal AAO distribution when they included birth cohorts in their models, but a trimodal AAO distribution when analysing the whole sample. This may be due to the fact that including birth cohorts in AAO analysis can artificially truncate the data, making the results of admixture analysis unreliable.
Inter‐study differences in findings may further be attributed to the inconsistency in the definitions used for AAO of BD, as research has suggested that admixture analysis is further sensitive to the criterion used to define groups.80 It has been proposed that the most valid definition for bipolar disorder AAO is the ‘first affective episode meeting diagnostic criteria’, as it does not preclude relevant episodes of depression prior to manic onset.4 However, this does not overcome the limitation of recall bias, which was mitigated in some of the included studies by referring to case notes and interviews with family members rather than relying solely on self‐report. Yet, BD patients may be more likely to recall depressive compared to manic episodes or even fail to recognise hypomanic episodes pre‐diagnosis as pathological.81, 82 Deciding what constitutes pathology in retrospective studies is further distorted by the fact that potential symptoms in youth are viewed through the lens of an adult diagnosis. As a gold standard, therefore, future research investigating AAO in bipolar disorder should aim to employ prospective longitudinal methodologies, using the age at ‘first affective episode meeting diagnostic criteria’ as the standardised definition for the point of disease onset.
Results will also have been influenced by factors including inter‐ and intra‐country differences in diagnostic practices, evolving diagnostic criteria, varying degrees of stigma surrounding mental illness and availability of healthcare provision. Yet, the fact that the clear majority of studies displayed a trimodal AAO despite this heterogeneity suggests that we can consider it a robust finding.
6. FUTURE DIRECTIONS
Notably, one of the largest international BD cohorts—the Systematic Treatment Enhancement Program for Bipolar Disorder cohort (STEP‐BD83)—was not included in this systematic review. This is because the STEP‐BD articles identified by our search strategy used pre‐defined cut‐offs to define AAO groups (e.g. <13, 13‐18, >18 years old) and, therefore, did not meet our eligibility criteria. The field would benefit from a data‐driven approach (such as admixture analysis) to defining BD AAO in this well‐characterised cohort. Similar analyses in other large, phenotypically detailed cohorts should also be prioritised in future research (e.g. the Flourish Canadian prospective high‐risk offspring cohort84).
7. CONCLUSION
The results of this systematic review indicate that bipolar disorder has a trimodal age‐at‐onset distribution, segregating into early‐, mid‐ and late‐onset subgroups with the most common average age at onset being 17.3 years. We propose that the field conceptualises these subgroups as referring broadly to life stage and moves towards a consistent definition of bipolar AAO as ‘the first affective episode meeting diagnostic criteria’. Providing valid evidence for three age‐at‐onset subgroups in BD will help to delineate more homogeneous subgroups of BD. Demarcating bipolar disorder AAO groups in this way can provide a framework for future research to continue to investigate potential mechanisms and, thus, inform treatment targets.
DATA AVAILABLITY STATEMENT
The data that support the findings of this study are openly available via the Open Science Framework (OSF) at http://doi.org/10.17605/OSF.IO/XFJPR.
Supporting information
Supplement S1
Supplement S2
Supplement S3
ACKNOWLEDGEMENTS
This work was supported by the Medical Research Council [grant number MR/N013468/1] and the National Institute for Health Research (NIHR) Oxford Health Biomedical Research Centre.
Bolton S, Warner J, Harriss E, Geddes J, Saunders KEA. Bipolar disorder: Trimodal age-at-onset distribution. Bipolar Disord.2021;23:341–356. 10.1111/bdi.13016
REFERENCES
- 1.Merikangas KR, Jin R, He J‐P, et al. Prevalence and correlates of bipolar spectrum disorder in the World Mental Health Survey Initiative. Arch Gen Psychiatry. 2011;68(3):241‐251. 10.1001/archgenpsychiatry.2011.12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Agnew‐Blais J, Danese A. Childhood maltreatment and unfavourable clinical outcomes in bipolar disorder: a systematic review and meta‐analysis. The Lancet Psychiatry. 2016;3(4):342‐349. 10.1016/S2215-0366(15)00544-1 [DOI] [PubMed] [Google Scholar]
- 3.Joslyn C, Hawes DJ, Hunt C, Mitchell PB. Is age of onset associated with severity, prognosis, and clinical features in bipolar disorder? A meta‐analytic review. Bipolar Disord. 2016;18(5):389‐403. 10.1111/bdi.12419 [DOI] [PubMed] [Google Scholar]
- 4.Leboyer M, Henry C, Paillere‐Martinot ML, Bellivier F. Age at onset in bipolar affective disorders: a review. Bipolar Disord. 2005;7(2):111‐118. 10.1111/j.1399-5618.2005.00181.x [DOI] [PubMed] [Google Scholar]
- 5.Bellivier F, Golmard JL, Henry C, Leboyer M, Schurhoff F. Admixture analysis of age at onset in bipolar I affective disorder. Arch Gen Psychiatry. 2001;58(5):510‐512. [DOI] [PubMed] [Google Scholar]
- 6.Geoffroy PA, Etain B, Scott J, et al. Reconsideration of bipolar disorder as a developmental disorder: Importance of the time of onset. J Physiol Paris. 2013;107(4):278‐285. 10.1016/j.jphysparis.2013.03.006 [DOI] [PubMed] [Google Scholar]
- 7.Bellivier F, Golmard J‐L, Rietschel M, et al. Age at onset in bipolar I affective disorder: Further evidence for three subgroups. Am J Psychiatry. 2003;160(5):999‐1001. 10.1176/appi.ajp.160.5.999 [DOI] [PubMed] [Google Scholar]
- 8.Hamshere ML, Gordon‐Smith K, Forty L, et al. Age‐at‐onset in bipolar‐I disorder: Mixture analysis of 1369 cases identifies three distinct clinical sub‐groups. J Affect Disord. 2009;116(1–2):23‐29. 10.1016/j.jad.2008.10.021 [DOI] [PubMed] [Google Scholar]
- 9.Bauer M, Glenn T, Rasgon N, et al. Association between age of onset and mood in bipolar disorder: Comparison of subgroups identified by cluster analysis and clinical observation. J Psychiatr Res. 2010;44(16):1170‐1175. 10.1016/j.jpsychires.2010.04.009 [DOI] [PubMed] [Google Scholar]
- 10.Coryell W, Fiedorowicz J, Leon AC, Endicott J, Keller MB. Age of onset and the prospectively observed course of illness in bipolar disorder. J Affect Disord. 2013;146(1):34‐38. 10.1016/j.jad.2012.08.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jones PB. Adult mental health disorders and their age at onset. Br J Psychiatry. 2013;202(s54):5‐10. 10.1192/bjp.bp.112.119164 [DOI] [PubMed] [Google Scholar]
- 12.Evidence Partners . DistillerSR: systematic review and literature review software. 2014. [Google Scholar]
- 13.Mueller M, D'Addario M, Egger M, et al. Methods to systematically review and meta‐analyse observational studies: a systematic scoping review of recommendations. BMC Med Res Methodol. 2018;18(1):44. 10.1186/s12874-018-0495-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Javaid N, Kennedy JL, De Luca V. Ethnicity and Age at Onset in Bipolar Spectrum Disorders. CNS Spectr. 2011;16(6):127‐134. 10.1017/S1092852912000296 [DOI] [PubMed] [Google Scholar]
- 15.Nowrouzi B, McIntyre RS, MacQueen G, et al. Admixture analysis of age at onset in first episode bipolar disorder. J Affect Disord. 2016;201:88‐94. 10.1016/j.jad.2016.04.006 [DOI] [PubMed] [Google Scholar]
- 16.Wickham H. ggplot2: Elegant Graphics for Data Analysis. 2016.
- 17.RStudio Team . RStudio: Integrated Development Environment for R. 2018.
- 18.Bolton S. Open Dataset: Bipolar disorder age at onset distribution. 2020. 10.17605/OSF.IO/XFJPR [DOI] [PMC free article] [PubMed]
- 19.Massat I, Lerer B, Souery D, et al. HTR2C (cys23ser) polymorphism influences early onset in bipolar patients in a large European multicenter association study. Mol Psychiatry. 2007;12(9):797‐798. 10.1038/sj.mp.4002018 [DOI] [PubMed] [Google Scholar]
- 20.Holtzman JN, Miller S, Hooshmand F, et al. Gender by onset age interaction may characterize distinct phenotypic subgroups in bipolar patients. J Psychiatr Res. 2016;76:128‐135. 10.1016/j.jpsychires.2016.02.009 [DOI] [PubMed] [Google Scholar]
- 21.Manchia M, Zai CC, Squassina A, Vincent JB, De Luca V, Kennedy JL. Mixture regression analysis on age at onset in Bipolar Disorder patients: Investigation of the role of serotonergic genes. Eur Neuropsychopharmacol. 2010;20(9):663‐670. 10.1016/j.euroneuro.2010.04.001 [DOI] [PubMed] [Google Scholar]
- 22.Kennedy N, Everitt B, Boydell J, van Os J, Jones PB, Murray RM. Incidence and distribution of first‐episode mania by age: results from a 35‐year study. Psychol Med. 2005;35(6):855‐863. 10.1017/S0033291704003307 [DOI] [PubMed] [Google Scholar]
- 23.Manchia M, Maina G, Carpiniello B, et al. Clinical correlates of age at onset distribution in bipolar disorder: a comparison between diagnostic subgroups. Int J Bipolar Disord. 2017;5(1):28. 10.1186/s40345-017-0097-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bauer M, Glenn T, Alda M, et al. Influence of birth cohort on age of onset cluster analysis in bipolar I disorder. Eur Psychiatry. 2015;30(1):99‐105. 10.1016/j.eurpsy.2014.10.005 [DOI] [PubMed] [Google Scholar]
- 25.Azorin J‐M, Bellivier F, Kaladjian A, et al. Characteristics and profiles of bipolar I patients according to age‐at‐onset: findings from an admixture analysis. J Affect Disord. 2013;150(3):993‐1000. 10.1016/j.jad.2013.05.026 [DOI] [PubMed] [Google Scholar]
- 26.Bellivier F, Etain B, Malafosse A, et al. Age at onset in bipolar I affective disorder in the USA and Europe. World J Biol Psychiatry. 2014;15(5):369‐376. 10.3109/15622975.2011.639801 [DOI] [PubMed] [Google Scholar]
- 27.Biffin F, Tahtalian S, Filia K, et al. The impact of age at onset of bipolar I disorder on functioning and clinical presentation. Acta Neuropsychiatr. 2009;21(4):191‐196. 10.1111/j.1601-5215.2009.00399.x [DOI] [PubMed] [Google Scholar]
- 28.González Pinto A, Barbeito S, José Díaz F, et al. Age at onset in bipolar I disorder: two may be better than three subgroups. J Psychiatry Ment Heal. 2009;2(1):29‐34. 10.1016/S1888-9891(09)70711-6 [DOI] [PubMed] [Google Scholar]
- 29.Grigoroiu‐Serbanescu M, Rietschel M, Hauser J, et al. Commingling analysis of age‐of‐onset in bipolar I disorder and the morbid risk for major psychoses in first degree relatives of bipolar I probands. J Affect Disord. 2014;168:197‐204. 10.1016/j.jad.2014.06.054 [DOI] [PubMed] [Google Scholar]
- 30.Tozzi F, Manchia M, Galwey NW, et al. Admixture analysis of age at onset in bipolar disorder. Psychiatry Res. 2011;185(1–2):27‐32. 10.1016/j.psychres.2009.11.025 [DOI] [PubMed] [Google Scholar]
- 31.Golmard J‐L, Scott J, Etain B, et al. Using admixture analysis to examine birth‐cohort effects on age at onset of bipolar disorder. Acta Psychiatr Scand. 2016;133(3):205‐213. 10.1111/acps.12478 [DOI] [PubMed] [Google Scholar]
- 32.Ortiz A, Bradler K, Slaney C, et al. An admixture analysis of the age at index episodes in bipolar disorder. Psychiatry Res. 2011;188(1):34‐39. 10.1016/j.psychres.2010.10.033 [DOI] [PubMed] [Google Scholar]
- 33.Lin P‐I, McInnis MG, Potash JB, et al. Clinical correlates and familial aggregation of age at onset in bipolar disorder. Am J Psychiatry. 2006;163(2):240‐246. 10.1176/appi.ajp.163.2.240 [DOI] [PubMed] [Google Scholar]
- 34.Manchia M, Lampus S, Chillotti C, et al. Age at onset in Sardinian bipolar I patients: evidence for three subgroups. Bipolar Disord. 2008;10(3):443‐446. 10.1111/j.1399-5618.2007.00572.x [DOI] [PubMed] [Google Scholar]
- 35.Severino G, Manchia M, Contu P, et al. Association study in a Sardinian sample between bipolar disorder and the nuclear receptor REV‐ERBα gene, a critical component of the circadian clock system. Bipolar Disord. 2009;11(2):215‐220. 10.1111/j.1399-5618.2009.00667.x [DOI] [PubMed] [Google Scholar]
- 36.Lehmann SW, Rabins PV. Factors related to hospitalization in elderly manic patients with early and late‐onset bipolar disorder. Int J Geriatr Psychiatry. 2006;21(11):1060‐1064. 10.1002/gps.1607 [DOI] [PubMed] [Google Scholar]
- 37.Schürhoff F, Bellivier F, Jouvent R, et al. Early and late onset bipolar disorders: two different forms of manic‐depressive illness? J Affect Disord. 2000;58(3):215‐221. 10.1016/S0165-0327(99)00111-1 [DOI] [PubMed] [Google Scholar]
- 38.James A, Hoang U, Seagroatt V, Clacey J, Goldacre M, Leibenluft E. A comparison of American and english hospital discharge rates for pediatric bipolar disorder, 2000 to 2010. J Am Acad Child Adolesc Psychiatry. 2014;53(6):614‐624. 10.1016/j.jaac.2014.02.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dubicka B, Carlson GA, Vail A, Harrington R. Prepubertal mania: diagnostic differences between US and UK clinicians. Eur Child Adolesc Psychiatry. 2008;17(3):153‐161. 10.1007/s00787-007-0649-5 [DOI] [PubMed] [Google Scholar]
- 40.Post RM, Altshuler LL, Kupka R, et al. More childhood onset bipolar disorder in the United States than Canada or Europe: Implications for treatment and prevention. Neurosci Biobehav Rev. 2017;74:204‐213. 10.1016/j.neubiorev.2017.01.022 [DOI] [PubMed] [Google Scholar]
- 41.Duffy A. Does bipolar disorder exist in children? A Selected Review. Can J Psychiatry. 2007;52(7):409‐417. 10.1177/070674370705200702 [DOI] [PubMed] [Google Scholar]
- 42.Dell'Osso B, Grancini B, Vismara M, et al. Age at onset in patients with bipolar I and II disorder: a comparison of large sample studies. J Affect Disord. 2016;201:57‐63. 10.1016/j.jad.2016.04.009 [DOI] [PubMed] [Google Scholar]
- 43.Chia MF, Cotton S, Filia K, et al. Early intervention for bipolar disorder – Do current treatment guidelines provide recommendations for the early stages of the disorder? J Affect Disord. 2019;257:669‐677. 10.1016/j.jad.2019.07.062 [DOI] [PubMed] [Google Scholar]
- 44.Wozniak J. Pediatric bipolar disorder: The new perspective on severe mood dysfunction in children. J Child Adolesc Psychopharmacol. 2003;13(4):449‐451. 10.1089/104454603322724832 [DOI] [PubMed] [Google Scholar]
- 45.Youngstrom EA, Birmaher B, Findling RL. Pediatric bipolar disorder: validity, phenomenology, and recommendations for diagnosis. Bipolar Disord. 2008;10(1p2):194‐214. 10.1111/j.1399-5618.2007.00563.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Serra G, Uchida M, Battaglia C, et al. Pediatric Mania: The Controversy between Euphoria and Irritability. Curr Neuropharmacol. 2017;15(3):386‐393. 10.2174/1570159X14666160607100403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Van Meter A, Moreira ALR, Youngstrom E. Updated meta‐analysis of epidemiologic studies of pediatric bipolar disorder. J Clin Psychiatry. 2019;80(3):1‐11. 10.4088/JCP.18r12180 [DOI] [PubMed] [Google Scholar]
- 48.Duffy A, Vandeleur C, Heffer N, Preisig M. The clinical trajectory of emerging bipolar disorder among the high‐risk offspring of bipolar parents: current understanding and future considerations. Int J Bipolar Disord. 2017;5(1):37. 10.1186/s40345-017-0106-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Parry P, Allison S, Bastiampillai T. ‘Paediatric bipolar disorder’ rates are lower than claimed – a reexamination of the epidemiological surveys used by a meta‐analysis. Child Adolesc Ment Health. 2018;23(1):14‐22. 10.1111/camh.12231 [DOI] [PubMed] [Google Scholar]
- 50.Stringaris A, Santosh P, Leibenluft E, Goodman R. Youth meeting symptom and impairment criteria for mania‐like episodes lasting less than four days: an epidemiological enquiry. J Child Psychol Psychiatry. 2010;51(1):31‐38. 10.1111/j.1469-7610.2009.02129.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Larsson S, Aas M, Klungsøyr O, et al. Patterns of childhood adverse events are associated with clinical characteristics of bipolar disorder. BMC Psychiatry. 2013;13(1):97. 10.1186/1471-244X-13-97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schouws SNTM, Comijs HC, Stek ML, et al. cognitive impairment in early and late bipolar disorder. Am J Geriatr Psychiatry. 2009;17(6):508‐515. 10.1097/JGP.0b013e31819e2d50 [DOI] [PubMed] [Google Scholar]
- 53.Burmeister M, McInnis MG, Zöllner S. Psychiatric genetics: progress amid controversy. Nat Rev Genet. 2008;9(7):527‐540. 10.1038/nrg2381 [DOI] [PubMed] [Google Scholar]
- 54.Priebe L, Degenhardt FA, Herms S, et al. Genome‐wide survey implicates the influence of copy number variants (CNVs) in the development of early‐onset bipolar disorder. Mol Psychiatry. 2012;17(4):421‐432. 10.1038/mp.2011.8 [DOI] [PubMed] [Google Scholar]
- 55.Etain B, Mathieu F, Rietschel M, et al. Genome‐wide scan for genes involved in bipolar affective disorder in 70 European families ascertained through a bipolar type I early‐onset proband: supportive evidence for linkage at 3p14. Mol Psychiatry. 2006;11(7):685‐694. 10.1038/sj.mp.4001815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mathieu F, Dizier M‐H, Etain B, et al. European collaborative study of early‐onset bipolar disorder: Evidence for genetic heterogeneity on 2q14 according to age at onset. Am J Med Genet Part B Neuropsychiatr Genet. 2010;153B(8):1425‐1433. 10.1002/ajmg.b.31121 [DOI] [PubMed] [Google Scholar]
- 57.Grigoroiu‐Serbanescu M, Martinez M, Nöthen MM, et al. Different familial transmission patterns in bipolar I disorder with onset before and after age 25. Am J Med Genet. 2001;105(8):765‐773. 10.1002/ajmg.10047 [DOI] [PubMed] [Google Scholar]
- 58.Post RM, Altshuler LL, Kupka R, et al. Age at onset of bipolar disorder related to parental and grandparental illness burden. J Clin Psychiatry. 2016;77(10):e1309‐e1315. 10.4088/JCP.15m09811 [DOI] [PubMed] [Google Scholar]
- 59.Nassan M, Veldic M, Winham S, et al. Methylation of Brain Derived Neurotrophic Factor (BDNF) Val66Met CpG site is associated with early onset bipolar disorder. J Affect Disord. 2020;267:96‐102. 10.1016/j.jad.2020.02.002 [DOI] [PubMed] [Google Scholar]
- 60.Benedetti F, Riccaboni R, Poletti S, et al. The serotonin transporter genotype modulates the relationship between early stress and adult suicidality in bipolar disorder. Bipolar Disord. 2014;16(8):857‐866. 10.1111/bdi.12250 [DOI] [PubMed] [Google Scholar]
- 61.Etain B, Lajnef M, Henrion A, et al. Interaction between SLC6A4 promoter variants and childhood trauma on the age at onset of bipolar disorders. Sci Rep. 2015;5(1):16301. 10.1038/srep16301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Oliveira J, Etain B, Lajnef M, et al. Combined effect of TLR2 gene polymorphism and early life stress on the age at onset of bipolar disorders. Eugenin EA, ed. PLoS One. 2015;10(3):e0119702. 10.1371/journal.pone.0119702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Anand A, Koller DL, Lawson WB, Gershon ES, Nurnberger JI. Genetic and childhood trauma interaction effect on age of onset in bipolar disorder: an exploratory analysis. J Affect Disord. 2015;179:1‐5. 10.1016/j.jad.2015.02.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Perroud N, Zewdie S, Stenz L, et al. Methylation of serotonin receptor 3A in ADHD, Borderline personality, and bipolar disorders: Link with severity of the disorders and childhood maltreatment. Depress Anxiety. 2016;33(1):45‐55. 10.1002/da.22406 [DOI] [PubMed] [Google Scholar]
- 65.Petronis A. Epigenetics and bipolar disorder: new opportunities and challenges. Am J Med Genet. 2003;123C(1):65‐75. 10.1002/ajmg.c.20015 [DOI] [PubMed] [Google Scholar]
- 66.Miller S, Hallmayer J, Wang PW, Hill SJ, Johnson SL, Ketter TA. Brain‐derived neurotrophic factor val66met genotype and early life stress effects upon bipolar course. J Psychiatr Res. 2013;47(2):252‐258. 10.1016/j.jpsychires.2012.10.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Roth TL, Lubin FD, Funk AJ, Sweatt JD. Lasting epigenetic influence of early‐life adversity on the BDNF gene. Biol Psychiatry. 2009;65(9):760‐769. 10.1016/j.biopsych.2008.11.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Duffy A, Goodday SM, Keown‐Stoneman C, et al. Epigenetic markers in inflammation‐related genes associated with mood disorder: a cross‐sectional and longitudinal study in high‐risk offspring of bipolar parents. Int J Bipolar Disord. 2019;7(1):17. 10.1186/s40345-019-0152-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Aas M, Haukvik UK, Djurovic S, et al. Interplay between childhood trauma and BDNF val66met variants on blood BDNF mRNA levels and on hippocampus subfields volumes in schizophrenia spectrum and bipolar disorders. J Psychiatr Res. 2014;59:14‐21. 10.1016/j.jpsychires.2014.08.011 [DOI] [PubMed] [Google Scholar]
- 70.Daruy‐Filho L, Brietzke E, Lafer B, Grassi‐Oliveira R. Childhood maltreatment and clinical outcomes of bipolar disorder. Acta Psychiatr Scand. 2011;124(6):427‐434. 10.1111/j.1600-0447.2011.01756.x [DOI] [PubMed] [Google Scholar]
- 71.Post RM, Altshuler LL, Kupka R, et al. Verbal abuse, like physical and sexual abuse, in childhood is associated with an earlier onset and more difficult course of bipolar disorder. Bipolar Disord. 2015;17(3):323‐330. 10.1111/bdi.12268 [DOI] [PubMed] [Google Scholar]
- 72.Maniglio R. The impact of child sexual abuse on the course of bipolar disorder: a systematic review. Bipolar Disord. 2013;15(4):341‐358. 10.1111/bdi.12050 [DOI] [PubMed] [Google Scholar]
- 73.Ritter PS, Höfler M, Wittchen H‐U, et al. Disturbed sleep as risk factor for the subsequent onset of bipolar disorder – Data from a 10‐year prospective‐longitudinal study among adolescents and young adults. J Psychiatr Res. 2015;68:76‐82. 10.1016/j.jpsychires.2015.06.005 [DOI] [PubMed] [Google Scholar]
- 74.Strakowski S. The co‐occurrence of bipolar and substance use disorders. Clin Psychol Rev. 2000;20(2):191‐206. 10.1016/S0272-7358(99)00025-2 [DOI] [PubMed] [Google Scholar]
- 75.Jones I, Craddock N. Bipolar disorder and childbirth: the importance of recognising risk. Br J Psychiatry. 2005;186(6):453‐454. 10.1192/bjp.186.6.453 [DOI] [PubMed] [Google Scholar]
- 76.Tsuchiya KJ, Byrne M, Mortensen PB. Risk factors in relation to an emergence of bipolar disorder: a systematic review. Bipolar Disord. 2003;5(4):231‐242. 10.1034/j.1399-5618.2003.00038.x [DOI] [PubMed] [Google Scholar]
- 77.Bergink V. Immune mechanisms in postpartum psychosis. Biol Psychiatry. 2016;79(9 SUPPL 1):S141‐S142. 10.1016/j.biopsych.2016.03.1054 [DOI] [Google Scholar]
- 78.Cassidy F, Carroll BJ. Vascular risk factors in late onset mania. Psychol Med. 2002;32(2):359‐362. 10.1017/S0033291701004718 [DOI] [PubMed] [Google Scholar]
- 79.Hays JC, Krishnan KRR, George LK, Blazer DG. Age of first onset of bipolar disorder: demographic, family history, and psychosocial correlates. Depress Anxiety. 1998;7(2):76‐82. [DOI] [PubMed] [Google Scholar]
- 80.Montlahuc C, Curis E, Jonas SF, Bellivier F, Chevret S. Age‐at‐onset subsets of bipolar I disorders: a critical insight into admixture analyses. Int J Methods Psychiatr Res. 2017;26(3): 10.1002/mpr.1536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.de Assis da Silva R, Mograbi DC, Silveira LAS, et al. The reliability of self‐assessment of affective state in different phases of bipolar disorder. J Nerv Ment Dis. 2014;202(5):386‐390. 10.1097/NMD.0000000000000136 [DOI] [PubMed] [Google Scholar]
- 82.Gazalle FK, Frey BN, Hallal PC, et al. Mismatch between self‐reported quality of life and functional assessment in acute mania: a matter of unawareness of illness? J Affect Disord. 2007;103(1–3):247‐252. 10.1016/j.jad.2007.01.013 [DOI] [PubMed] [Google Scholar]
- 83.Sachs GS, Thase ME, Otto MW, et al. Rationale, design, and methods of the systematic treatment enhancement program for bipolar disorder (STEP‐BD). Biol Psychiatry. 2003;53(11):1028‐1042. 10.1016/S0006-3223(03)00165-3 [DOI] [PubMed] [Google Scholar]
- 84.Duffy A, Alda M, Crawford L, Milin R, Grof P. The early manifestations of bipolar disorder: a longitudinal prospective study of the offspring of bipolar parents. Bipolar Disord. 2007;9(8):828‐838. 10.1111/j.1399-5618.2007.00421.x [DOI] [PubMed] [Google Scholar]
- 85.McInnis MG, Lan T‐H, Willour VL, et al. Genome‐wide scan of bipolar disorder in 65 pedigrees: supportive evidence for linkage at 8q24, 18q22, 4q32, 2p12, and 13q12. Mol Psychiatry. 2003;8(3):288‐298. 10.1038/sj.mp.4001277 [DOI] [PubMed] [Google Scholar]
- 86.Hajek T, Slaney C, Garnham J, Ruzickova M, Passmore M, Alda M. Clinical correlates of current level of functioning in primary care‐treated bipolar patients. Bipolar Disord. 2005;7(3):286‐291. 10.1111/j.1399-5618.2005.00182.x [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplement S1
Supplement S2
Supplement S3


